Associations of Ambient Environmental Conditions with Growth and Dissemination of Staphylococcus epidermidis on the Surface of Teatcups from Sheep Milking Parlours
Abstract
1. Introduction
2. Materials and Methods
2.1. Staphylococcal Isolates
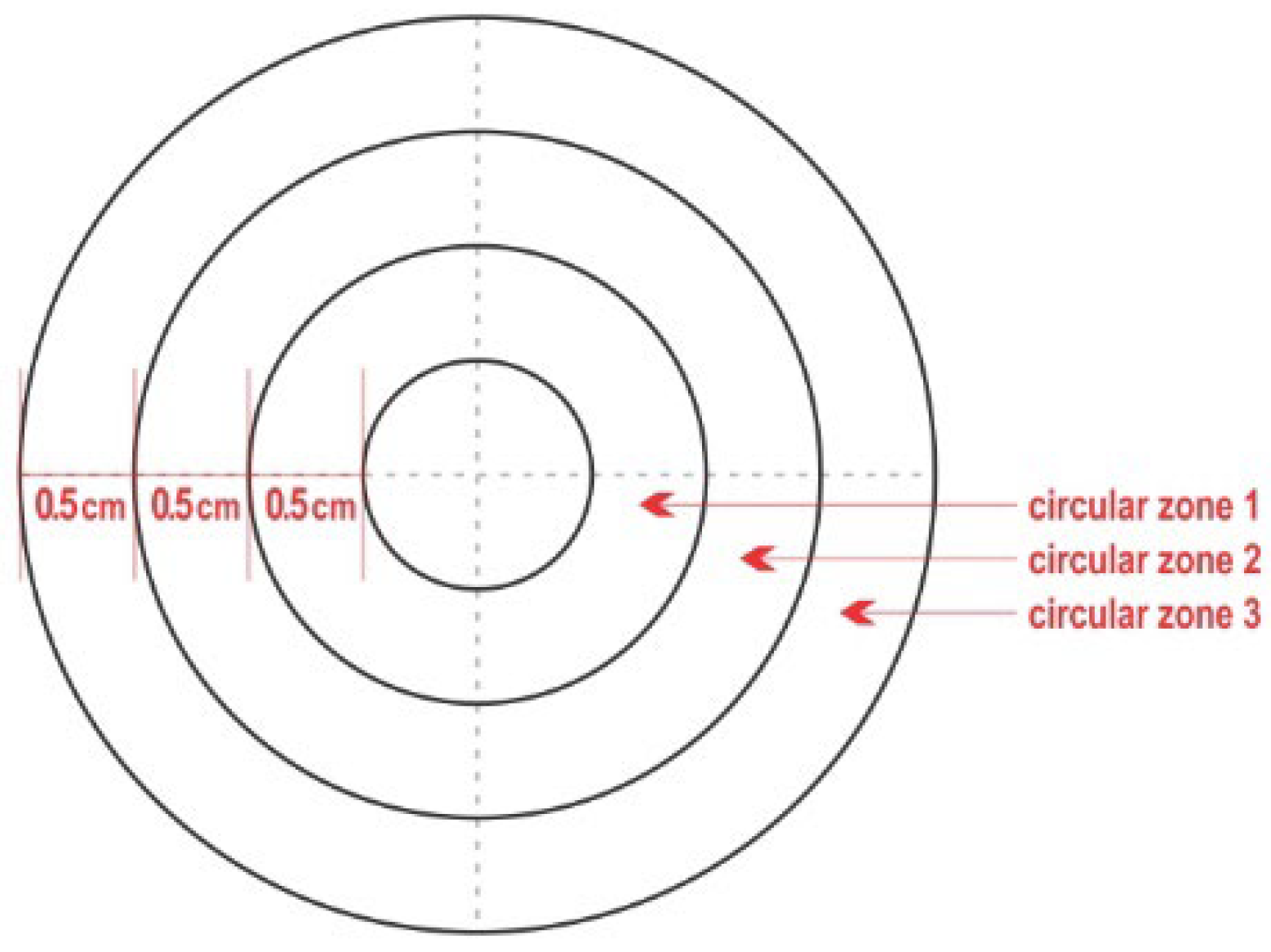
2.2. Test Material
2.3. Experimental Work
2.4. Ambient Conditions
2.5. Data Management and Analysis
3. Results
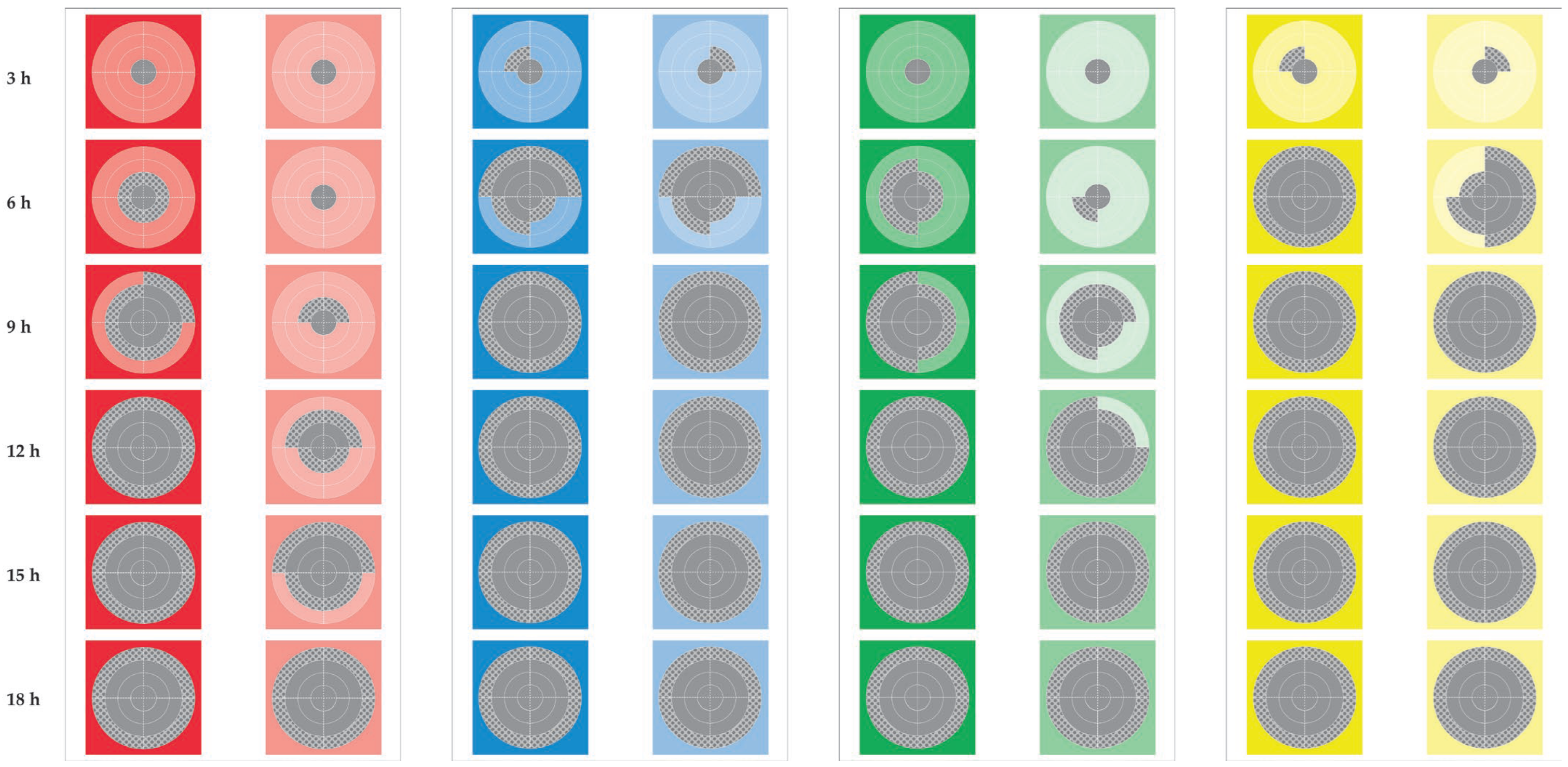
3.1. Frequency of Staphylococcal Recoveries
3.2. Speed of Staphylococcal Dissemination
3.3. Teatcup Surface Covered by Staphylococci
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selvaggi, M.; D’ Alessandro, A.G.; Dario, C. Environmental and genetic factors affecting milk yield and quality in three Italian sheep breeds. J. Dairy Res. 2017, 84, 27–31. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on the welfare risks related to the farming of sheep for wool, meat and milk production. EFSA J. 2014, 12, 3933–4060. [Google Scholar]
- Romero, G.; Peris, C.; Fthenakis, G.C.; Diaz, J.R. Effects of machine milking on udder health in dairy ewes. Small Rumin. Res. 2020, 188, 106096. [Google Scholar]
- Katsarou, E.I.; Katsafadou, A.I.; Karakasidis, T.; Chatzopoulos, D.C.; Vasileiou, N.G.C.; Lianou, D.T.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Growth of Staphylococcus epidermidis on the surface of teatcups from milking parlours. Microorganisms 2021, 9, 852. [Google Scholar] [PubMed]
- Skarbye, A.P.; Thomsen, P.T.; Krogh, M.A.; Svennesen, L.; Østergaard, S. Effect of automatic cluster flushing on the concentration of Staphylococcus aureus in teat cup liners. J. Dairy Sci. 2020, 103, 5431–5439. [Google Scholar] [CrossRef]
- Latorre, A.A.; Pachá, P.A.; González-Rocha, G.; San Martín, I.; Quezada-Aguiluz, M.; Aguayo-Reyes, A.; Bello-Toledo, H.; Oliva, R.; Estay, A.; Pugin, J.; et al. On-farm surfaces in contact with milk: The role of Staphylococcus aureus-containing biofilms for udder health and milk quality. Foodborne Pathog. Dis. 2020, 17, 44–51. [Google Scholar] [CrossRef]
- De Castro Melo, P.; Sousa, C.; Botelho, C.; Oliveira, R.; Nader-Filho, A. NaOCl effect on biofilm produced by Staphylococcus aureus isolated from the milking environment and mastitis infected cows. Pesq. Vet. Bras. 2014, 34, 109–113. [Google Scholar] [CrossRef]
- Manzur, A.G.; Junior, V.S.M.; Morais-Costa, F.; Mariano, E.G.A.; Careli, R.T.; da Silva, L.M.V.; Coelho, S.G.; de Almeida, A.C.; Duarte, E.R. Extract of Mangifera indica L. leaves may reduce biofilms of Staphylococcus spp. in stainless steel and teatcup rubbers. Food Sci. Technol. Int. 2020, 26, 11–20. [Google Scholar] [CrossRef]
- Katsarou, E.I.; Chatzopoulos, D.C.; Giannoulis, T.; Ioannidi, K.S.; Katsafadou, A.I.; Kontou, P.I.; Lianou, D.T.; Mamouris, Z.; Mavrogianni, V.S.; Michael, C.K.; et al. MLST-based analysis and antimicrobial resistance of Staphylococcus epidermidis from cases of sheep mastitis in Greece. Biology 2021, 10, 170. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Cripps, P.J.; Ioannidi, K.S.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Petinaki, E.; Politis, A.P.; Calvo Gonzalez-Valerio, T.; Argyros, S.; et al. Extensive countrywide field investigation of subclinical mastitis in sheep in Greece. J. Dairy Sci. 2018, 101, 7297–7310. [Google Scholar]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. J. Hyg. Camb. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Barrow, G.I.; Feltham, R.K.A. Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Euzeby, J.P. List of bacterial names with standing in nomenclature: A folder available on the Internet. Int. J. Syst. Bacteriol. 1997, 47, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Bergonier, D.; Berthelot, X. New advances in epizootiology and control of ewe mastitis. Liv. Prod. Sci. 2003, 79, 1–16. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Mavrogianni, V.S.; Petridis, I.G.; Vasileiou, N.G.C.; Fthenakis, G.C. Mastitis in sheep—The last 10 years and the future of research. Vet. Microbiol. 2015, 185, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.A.; Bennett, A.M.; Walker, J.T. Aerosol survival of Staphylococcus epidermidis. J. Hosp. Inf. 2011, 78, 216–220. [Google Scholar] [CrossRef]
- Chuaybamroong, P.; Thunyasirinon, C.; Supothina, S.; Sribenjalux, P.; Wu, C.Y. Performance of photocatalytic lamps on reduction of culturable airborne microorganism concentration. Chemosphere 2011, 83, 730–735. [Google Scholar]
- Lin, M.H.; Ke, W.J.; Liu, C.C.; Yang, M.W. Modulation of Staphylococcus aureus spreading by water. Sci. Rep. 2016, 6, 25233. [Google Scholar] [CrossRef]
- Pollitt, E.J.G.; Diggle, S.P. Defining motility in the staphylococci. Cell Mol Life Sci. 2017, 74, 2943–2958. [Google Scholar]
- Schijve, J. Fatigue of structures and materials in the 20th century and the state of the art. Int. J. Fatig. 2003, 25, 679–702. [Google Scholar] [CrossRef]
- Yoda, I.; Koseki, H.; Tomita, M.; Shida, T.; Horiuchi, H.; Sakoda, H.; Osaki, M. Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiol. 2014, 14, 234. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Henrichsen, J. Bacterial surface translocation: A survey and a classification. Bacteriol. Rev. 1972, 36, 478–503. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.K.; Lianou, D.T.; Vasileiou, N.G.C.; Tsilipounidaki, K.; Katsafadou, A.I.; Politis, A.I.; Kordalis, N.G.; Ioannidi, K.S.; Gougoulis, D.A.; Trikalinou, C.; et al. Association of staphylococcal populations on teatcups of milking rarlours with vaccination against staphylococcal mastitis in sheep and goat farms. Pathogens 2021, 10, 385. [Google Scholar] [CrossRef]
- Armit, D.; Vickers, M.; Parr, A.; Van Rosendal, S.; Trott, N.; Gunasena, R.; Parkinson, B. Humidity a potential risk factor for prosthetic joint infection in a tropical Australian hospital. ANZ J. Surg. 2018, 88, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulos, A.; Vasileiou, N.G.C.; Gougoulis, D.A.; Cripps, P.J.; Ioannidi, K.S.; Chatzopoulos, D.C.; Billinis, C.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Use of geographical information system and ecological niche modelling for predicting potential space distribution of subclinical mastitis in ewes. Vet. Microbiol. 2019, 228, 119–128. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Giannakopoulos, A.; Cripps, P.J.; Ioannidi, K.S.; Chatzopoulos, D.C.; Gougoulis, D.A.; Billinis, C.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Study of potential environmental factors predisposing ewes to subclinical mastitis in Greece. Comp. Immunol. Microbiol. Inf. Dis. 2019, 62, 40–45. [Google Scholar] [CrossRef] [PubMed]


| Time after Application by Smearing | Total Recoveries | ||
|---|---|---|---|
| New Teatcups | Used Teatcups | p | |
| Humidity 60% | |||
| 3 h | 1/144 | 0/144 | 0.32 |
| 6 h | 58/144 | 2/144 | <0.0001 |
| 9 h | 109/144 | 30/144 | <0.0001 |
| 12 h | 144/144 | 86/144 | <0.0001 |
| 15 h | 144/144 | 132/144 | 0.0004 |
| 18 h | 144/144 | 144/144 | n/a |
| 24 h | 144/144 | 144/144 | n/a |
| Total | 744/1008 | 538/1008 | <0.0001 |
| Humidity 80% | |||
| 3 h | 5/144 | 10/144 | 0.18 |
| 6 h | 124/144 | 100/144 | 0.0007 |
| 9 h | 144/144 | 143/144 | 0.32 |
| 12 h | 144/144 | 144/144 | n/a |
| 15 h | 144/144 | 144/144 | n/a |
| 18 h | 144/144 | 144/144 | n/a |
| 24 h | 144/144 | 144/144 | n/a |
| Total | 849/1008 | 829/1008 | 0.23 |
| Grand total | 1593/2016 | 1367/2016 | <0.0001 |
| Time after Application by Smearing | Total Recoveries | ||
|---|---|---|---|
| New Teatcups | Used Teatcups | p | |
| Temperature 21 °C | |||
| 3 h | 1/144 | 3/144 | 0.56 |
| 6 h | 82/144 | 46/144 | <0.0001 |
| 9 h | 122/144 | 81/144 | <0.0001 |
| 12 h | 144/144 | 104/144 | <0.0001 |
| 15 h | 144/144 | 132/144 | 0.0004 |
| 18 h | 144/144 | 144/144 | n/a |
| 24 h | 144/144 | 144/144 | n/a |
| Total | 781/1008 | 654/1008 | <0.0001 |
| Temperature 31 °C | |||
| 3 h | 5/144 | 7/144 | 0.56 |
| 6 h | 100/144 | 56/144 | <0.0001 |
| 9 h | 131/144 | 92/144 | <0.0001 |
| 12 h | 144/144 | 126/144 | <0.0001 |
| 15 h | 144/144 | 144/144 | n/a |
| 18 h | 144/144 | 144/144 | n/a |
| 24 h | 144/144 | 144/144 | n/a |
| Total | 812/1008 | 713/1008 | <0.0001 |
| Grand total | 1593/2016 | 1367/2016 | <0.0001 |
| Ambient Conditions 1 | New Teatcups | Used Teatcups | p |
|---|---|---|---|
| 21 °C, 60% | 5.048 ± 0.930 a,b | 2.588 ± 0.459 a,b | 0.039 |
| 21 °C, 80% | 17.170 ± 3.953 a,c | 20.119 ± 3.070 a,c | 0.57 |
| 31 °C, 60% | 9.682 ± 1.061 d | 5.145 ± 0.566 c,d | 0.004 |
| 31 °C, 80% | 27.730 ± 2.460 b,c,d | 25.491 ± 3.271 b,d | 0.60 |
| p | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsarou, E.I.; Petinaki, E.; Fthenakis, G.C. Associations of Ambient Environmental Conditions with Growth and Dissemination of Staphylococcus epidermidis on the Surface of Teatcups from Sheep Milking Parlours. Bioengineering 2023, 10, 81. https://doi.org/10.3390/bioengineering10010081
Katsarou EI, Petinaki E, Fthenakis GC. Associations of Ambient Environmental Conditions with Growth and Dissemination of Staphylococcus epidermidis on the Surface of Teatcups from Sheep Milking Parlours. Bioengineering. 2023; 10(1):81. https://doi.org/10.3390/bioengineering10010081
Chicago/Turabian StyleKatsarou, Eleni I., Efthymia Petinaki, and George C. Fthenakis. 2023. "Associations of Ambient Environmental Conditions with Growth and Dissemination of Staphylococcus epidermidis on the Surface of Teatcups from Sheep Milking Parlours" Bioengineering 10, no. 1: 81. https://doi.org/10.3390/bioengineering10010081
APA StyleKatsarou, E. I., Petinaki, E., & Fthenakis, G. C. (2023). Associations of Ambient Environmental Conditions with Growth and Dissemination of Staphylococcus epidermidis on the Surface of Teatcups from Sheep Milking Parlours. Bioengineering, 10(1), 81. https://doi.org/10.3390/bioengineering10010081







