Polysaccharides and Structural Proteins as Components in Three-Dimensional Scaffolds for Breast Cancer Tissue Models: A Review
Abstract
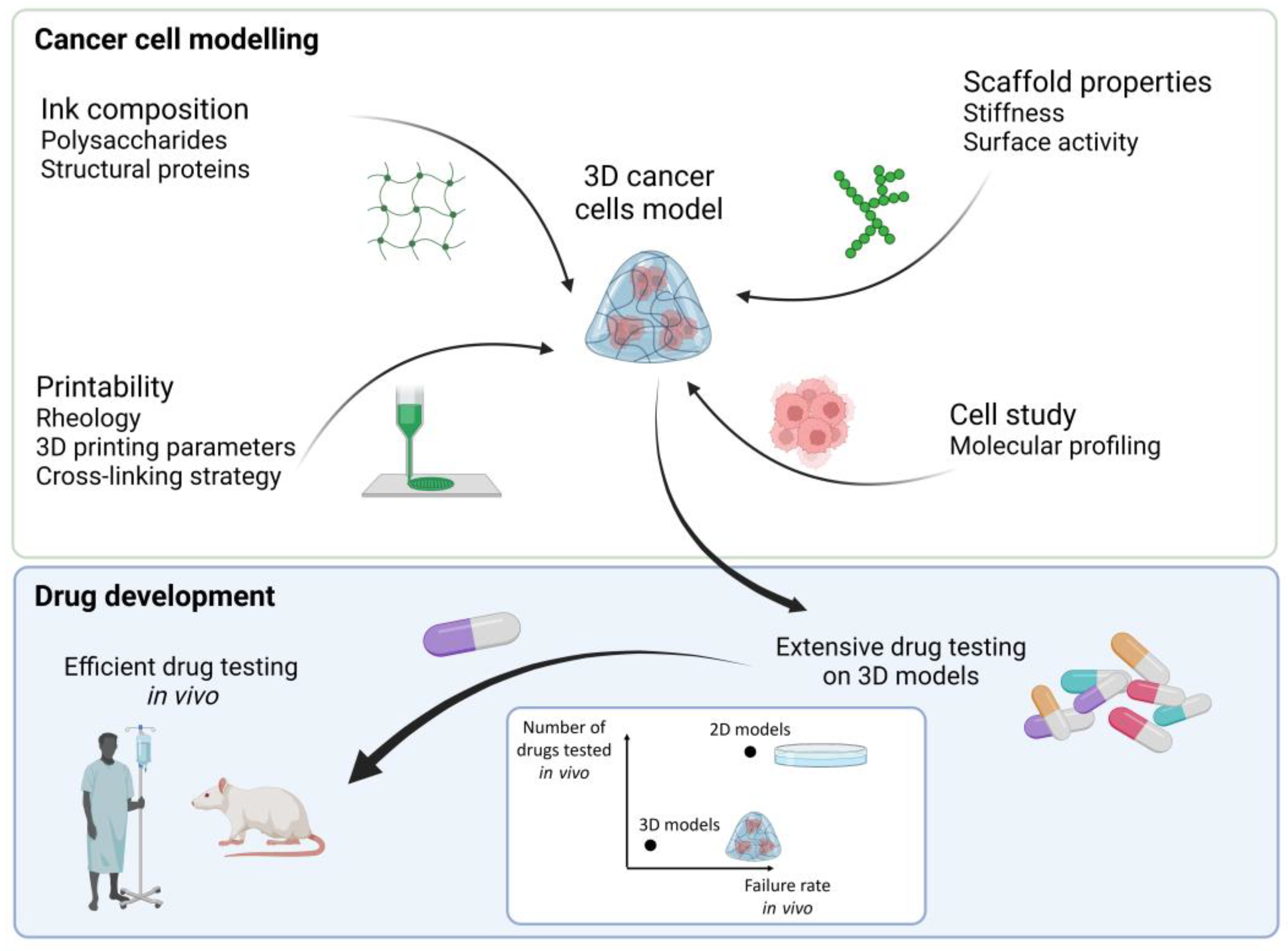
1. 3D Cancer Tissue Models
2. Additive Manufacturing
2.1. Rheology of the Ink
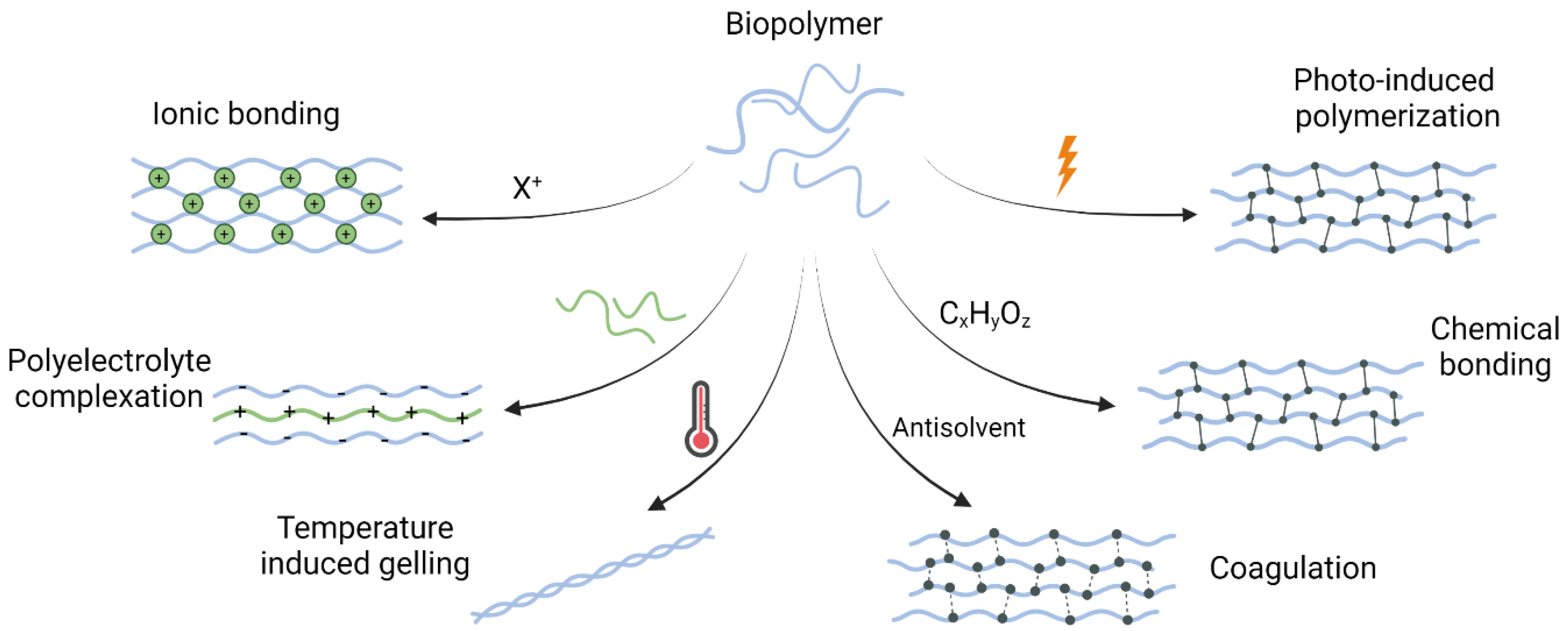
2.2. Cross-Linking Mechanisms
2.3. Bioprinting
| Ink Composition | Cross-Linking Method | Specific Type of Cross-Linker | Results | References | |
|---|---|---|---|---|---|
| 3D printed scaffolds | Ad-MeHA (adamantane-modified and methacrylated hyaluronic acid) and CD-MeHA (cyclodextrin-modified and methacrylated HA) | Guest-host cross-linking before printing (cyclodextrin-adamantane) and photo-induced cross-linking during printing (methacrylated HA) | Irgacure 2959 for photopolymerization of methacrylated HA, 5 min UV at 320−390 nm | Guest-host cross-linking was necessary for stable printing and covalent cross-linking was needed for long term stability. Structures were stable over one month. The methacrylate moieties allowed to chemically attach RGD motifs. | [57] |
| CNF and gelatin | Temperature gelling (gelatin) during printing and chemical cross-linking (gelatin and genipin) post-printing | Genipin | Gelatin gel was mechanically reinforced with CNF; maximum strength was obtained with 10% of CNF. Cross-linking with genipin was completed within 24h and increases with genipin concentration. | [58] | |
| TEMPO CNF | Ionic cross-linking during printing and chemical cross-linking post printing | CaCl2 and, 1,4-butanediol diglycidyl ether (BDDE) | Compression modulus increased with the amount of cross-linker. The scaffold was stable for 3 months in PBS. Higher cross-linker amounts led to higher cell proliferation due to increasing stiffness of the scaffold. | [59] | |
| TEMPO CNF and alginate | Ionic cross-linking | CaCl2 | Alginate reduced the print quality (form and shape factors are reduced). When cross-linked with CaCl2, alginate reinforced the CNF structure post-printing. | [60,61] | |
| Galactoglucomannan methacrylate (GGMMAs) and TEMPO CNF | Photo-induced cross-linking post printing | Irgacure 2959, 5 min UV at 320−390 nm | Compressive modulus was tuned depending on GGMMAs type and concentration. GGMMA was non cytotoxic and supported cell proliferation. | [62] | |
| Collagen and chitosan | Physical gelling (collagen) during printing, ionic and chemical cross-linking post-printing | NaOH and genipin | Degradation rate and mechanical properties were controlled by the chitosan concentration. Chitosan decreased the degradation rate of collagen and increased its mechanical properties. | [63] | |
| Chitosan and TEMPO CNF | Polyelectrolyte complexation during printing and chemical cross-linking post-printing | Glutaraldehyde | Mixture of TEMPO CNF and chitosan was not printable (not homogeneous ink). Multilayers of chitosan/TEMPO CNF and TEMPO CNF were printed in chitosan bath. A maximum of 10% weight loss was obtained after one month. | [45] | |
| Cell-laden scaffolds | Pentenoate-functionalized hyaluronic acid (PHA), rBMSCs and rNSCs cells | Photo-induced and chemical cross-linking | Irgacure 2959 for UV cross-linking (312 nm for 2 min), dithiothreitol (DTT) | The cross-linking chemistry was fast with low amount of photo-initiator. rBMSCs had long term viability while rNSCs viability was affected by the bioprinting. High cell concentrations had minimal effect on the printed shape fidelity, yield stress, and viscosity. | [64] |
| Gelatin, silk fibroin and hTMSCs cells | Enzymatic or physical (sonication) cross-linking | Mushroom tyrosinase or sonication | The swelling of enzymatically crosslinked structure was higher compared to the sonicated structure due to lower amount of β-sheets structure of silk fibroin. Enzymatically crosslinked structure was stable over one month while sonicated structure was stable for 7 days because of gelatin release. | [65] | |
| Gelatin/alginate/fibrinogen (G–A–F) or gelatin/alginate (G–A) and 293FT cells or Hela cells | Physical gelling (gelatin), ionic (alginate), and enzymatic cross-linking (fibrinogen) | CaCl2 and thrombin | Alginate brought time stability to gelatin structure. Ionic cross-link of alginate was more stable than the temperature crosslinking of gelatin. Fibrinogen was added to chemically stabilize the structure. The structure with fibrinogen was stable over 30 days of cell culture. | [31,51,66] | |
| Alginate and U87-MG cells | Ionic cross-linking | CaCl2 before and during printing and BaCl2 post-printing. | The stability of the structure was increased from 3 days to 11 days by adding a post-printing cross-linking with BaCl2. Cell viability was 93% after bioprinting and maintained over 88% after 11 days. | [67] |
3. Biopolymers for Additive Manufacturing
| Hydrogel | Cell Line | 3D Printing Technique | Results | Ref. |
|---|---|---|---|---|
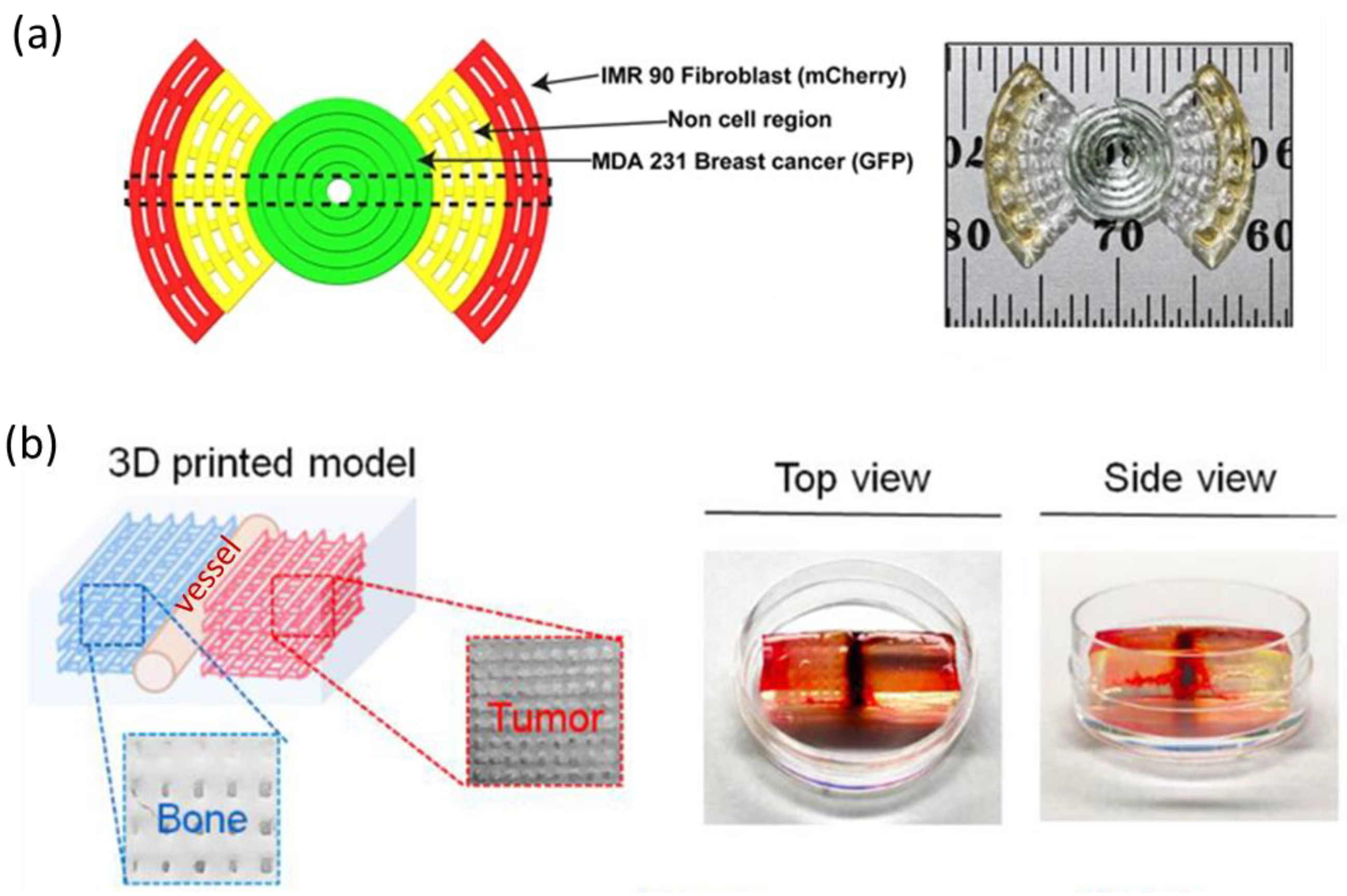
| Alginate/Gelatin | MDA-MB-231 and IMR-90 (fibroblast cells) | Micro-extrusion | 2 cell lines were printed at specific initial locations. Fibroblast migrated, infiltrated the MDA-MB-231 spheroids, and created mix MDA-MB-231/IMR-90 multicellular tumor spheroids. | [32] |
| Alginate/Gelatin | MDA-MB-231 | Micro-extrusion | The hydrogels composition influenced bioprinting and cell adhesion. The rheology of different alginate/gelatin composition was studied. Increase in gelatin concentration led to higher cells proliferation and larger tumor spheroids. | [27] |
| Collagen/Matrigel | MDA-MB-231 and EpH4 | Micro-extrusion | Collagen could not be 3D printed alone, Matrigel was added to improve its rheology. Collagen fiber alignment was controlled during 3D printing. Cancer cells oriented along the collagen fibers direction. | [76] |
| Collagen | MCF-12A, MCF-7 and MDA-MB-468 | Micro-extrusion (injection of cells in collagen gel) | Chimeric (human mammary organoids with cancer cells) structures for cancer cells redirection by a normal microenvironment were formed. Efficiency of chimeric organoid formation was higher using bioprinting process (90% at 14 days) compared to manual matrix embedding procedures (<10%). | [77] |
| Chitosan/gelatin | MCF-7 | Electrodeposition | Chitosan/gelatin 3D structures were coated with alginate to reach 7 days stability. Electrodeposited hydrogels were biocompatible, but cells did not spread. | [78] |
| Alginate, hydroxyapatite and periostin | MCF-7 and MDA-MB-231 | Micro-extrusion | Mechanical properties of the scaffold were tuned depending on the alginate concentration. MCF-7 and MDA-MB-231 showed different cellular adhesion and proliferation behavior. Similar drug response was obtained between 3D printed alginate scaffold and patient derived scaffold. | [24] |
| TEMPO CNF | MCF7 and MDA-MB-231 | Micro-extrusion | TEMPO CNF formed porous 3D structures suitable for cancer cells growth. The cell culture media influenced the scaffold mechanical properties. The expression of genes related to stemness, and migratory properties were increased compared with 2D cultures. | [25] |
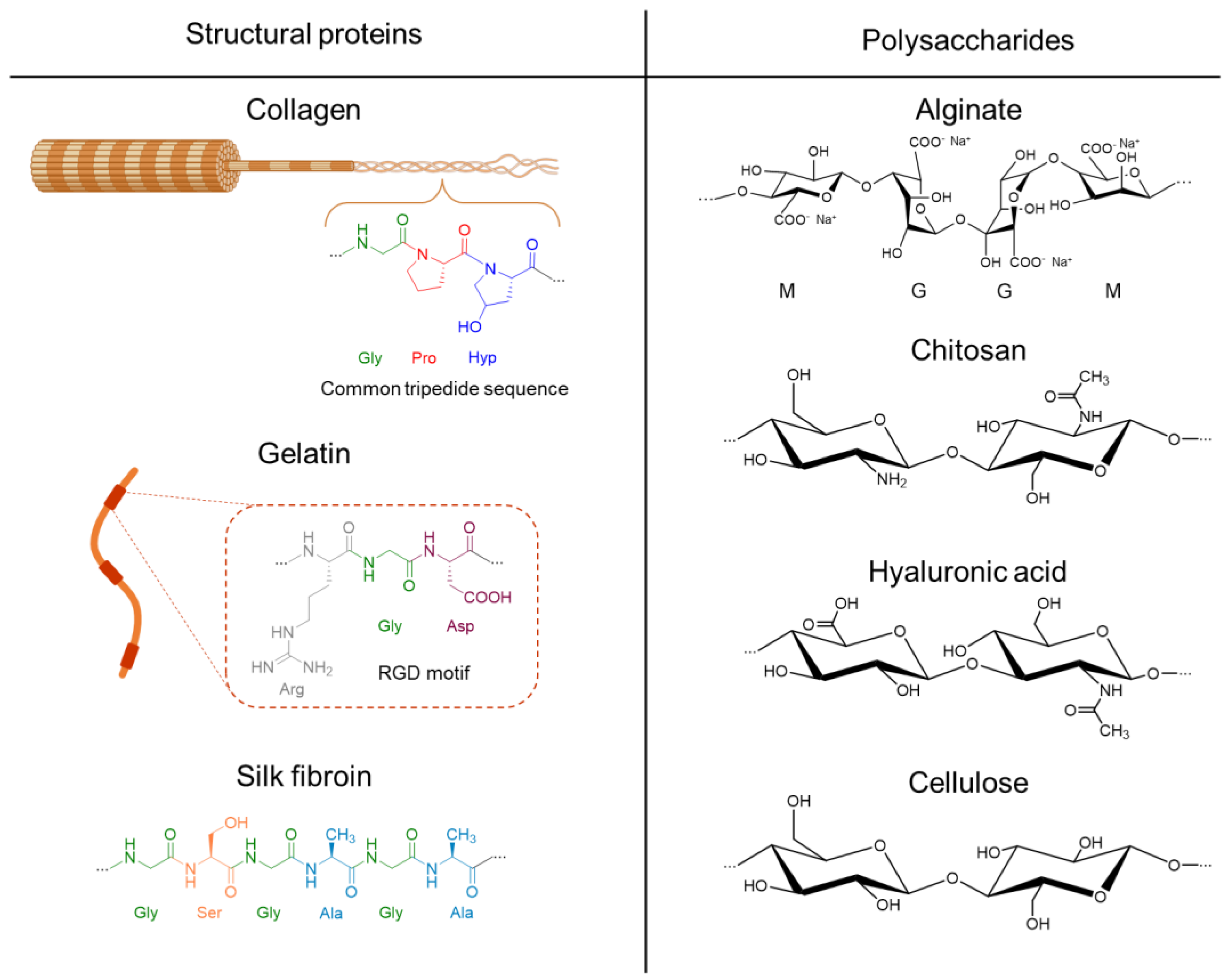
3.1. Collagen
3.2. Gelatin
3.3. Silk Fibroin
3.4. Alginate
3.5. Chitosan
3.6. Hyaluronic Acid
3.7. Cellulose Nanofibers
3.7.1. CNF from Wood
3.7.2. CNF from Macro-Algae
3.7.3. CNF from Tunicate
3.7.4. CNF from Bacteria
4. Scaffolds for Mimicking Breast Cancer Tissue Microenvironment
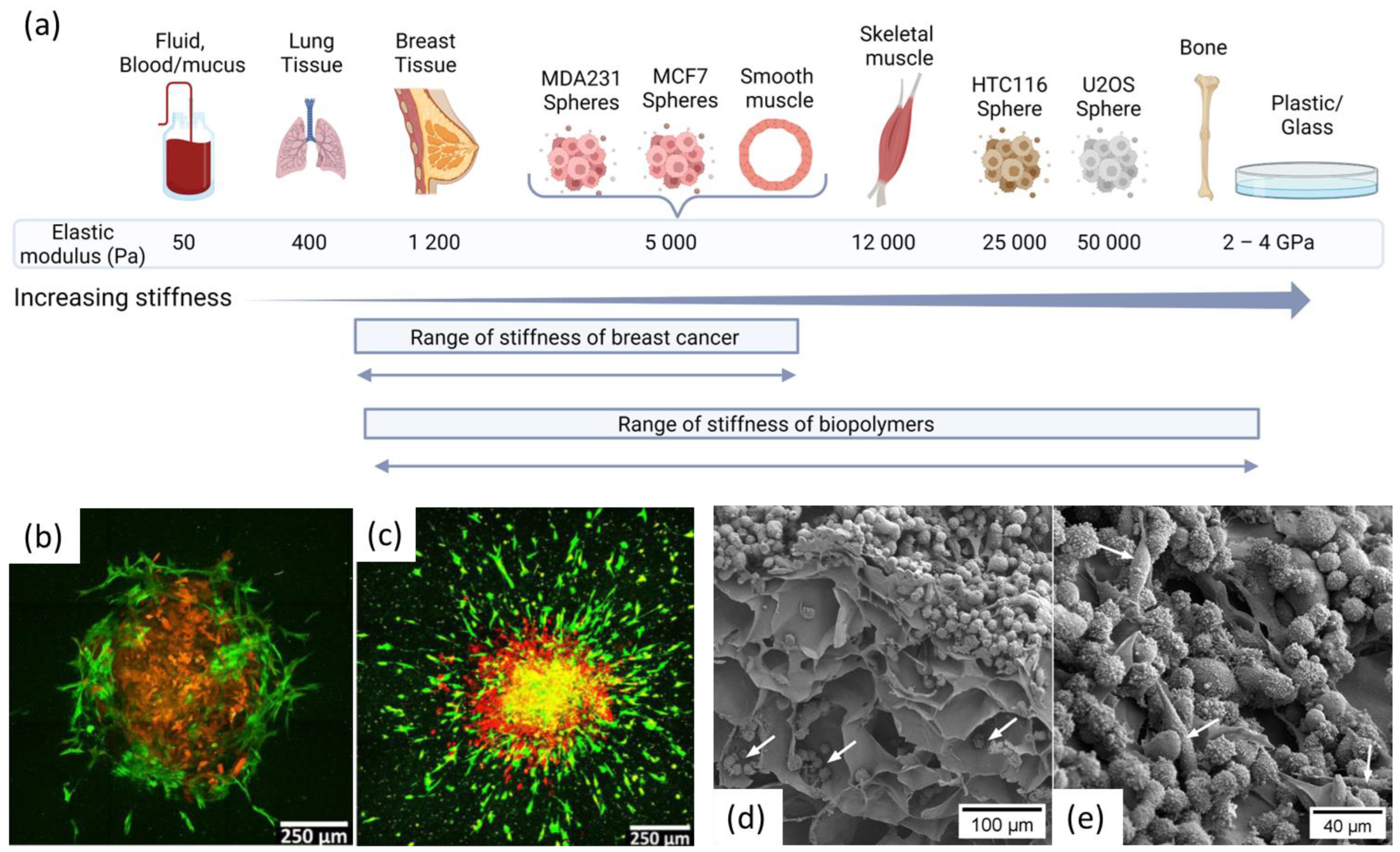
4.1. Biomechanical Properties
4.2. Bioactive Surface for Cell Adhesion, Proliferation, and Differentiation
4.3. Biological Components to Optimize Breast Cancer Tissue Models
5. Molecular Profiling of 3D Cultured Cells
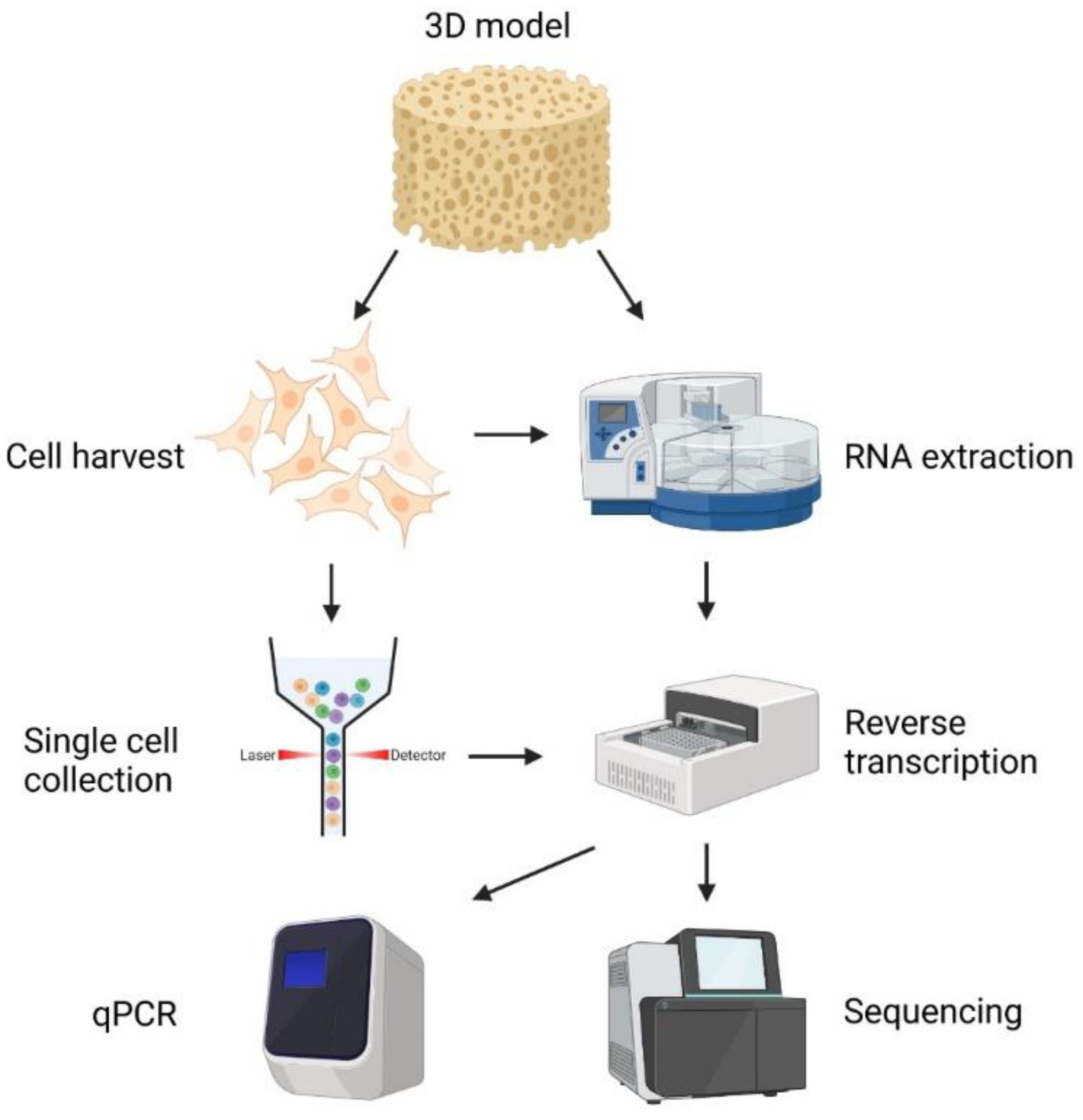
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Lloyd, I. Pharma R&D Annual Review 2019; Pharma Intelligence: London, UK, 2019. [Google Scholar]
- Perrin, S. Preclinical research: Make mouse studies work. Nature 2014, 507, 423–425. [Google Scholar] [CrossRef]
- Hutchinson, L.; Kirk, R. High drug attrition rates—Where are we going wrong? Nat. Rev. Clin. Oncol. 2011, 8, 189–190. [Google Scholar] [CrossRef]
- David, W.; Thomas, J.B.; Audette, J.; Carroll, A.; Dow-Hygelund, C.; Hay, M. Clinical Development Success Rates 2006–2015. BIO Ind. Anal. 2016, 1, 16. [Google Scholar]
- Koenig, J. Does process excellence handcuff drug development? Drug Discov. Today 2011, 16, 377–381. [Google Scholar] [CrossRef]
- Monsma, D.J.; Monks, N.R.; Cherba, D.M.; Dylewski, D.; Eugster, E.; Jahn, H.; Srikanth, S.; Scott, S.B.; Richardson, P.J.; Everts, R.E.; et al. Genomic characterization of explant tumorgraft models derived from fresh patient tumor tissue. J. Transl. Med. 2012, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Cobb, L.M. The Behaviour of Carcinoma of the Large Bowel in Man Following Transplantation into Immune Deprived Mice. Br. J. Cancer 1973, 28, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.A.; Taylor, D.M. Maintenance of biological and biochemical characteristics of human colorectal tumours during serial passage in immune-deprived mice. Br. J. Cancer 1978, 37, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Tentler, J.J.; Tan, A.C.; Weekes, C.D.; Jimeno, A.; Leong, S.; Pitts, T.M.; Arcaroli, J.J.; Messersmith, W.A.; Eckhardt, S.G. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012, 9, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-Derived Xenograft Models: An Emerging Platform for Translational Cancer Research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Mostrag-Szlichtyng, A.; Zaldívar Comenges, J.-M.; Worth, A.P. Computational toxicology at the European Commission’s Joint Research Centre. Expert Opin. Drug Metab. Toxicol. 2010, 6, 785–792. [Google Scholar] [CrossRef]
- Redmond, J.; McCarthy, H.; Buchanan, P.; Levingstone, T.J.; Dunne, N.J. Advances in biofabrication techniques for collagen-based 3D in vitro culture models for breast cancer research. Mater. Sci. Eng. C 2021, 122, 111944. [Google Scholar] [CrossRef] [PubMed]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene expression perturbation in vitro—A growing case for three-dimensional (3D) culture systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Jeffrey, S.S.; van de Rijn, M.; Rees, C.A.; Eisen, M.B.; Ross, D.T.; Pergamenschikov, A.; Williams, C.F.; Zhu, S.X.; Lee, J.C.F.; et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc. Natl. Acad. Sci. USA 1999, 96, 9212–9217. [Google Scholar] [CrossRef] [PubMed]
- Smalley, K.S.M.; Lioni, M.; Herlyn, M. Life ins’t flat: Taking cancer biology to the next dimension. Vitr. Cell. Dev. Biol. Anim. 2006, 42, 242–247. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef]
- Achilli, T.M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert. Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef]
- Akrap, N.; Andersson, D.; Bom, E.; Gregersson, P.; Ståhlberg, A.; Landberg, G. Identification of Distinct Breast Cancer Stem Cell Populations Based on Single-Cell Analyses of Functionally Enriched Stem and Progenitor Pools. Stem Cell Rep. 2016, 6, 121–136. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Braccini, S.; Tacchini, C.; Chiellini, F.; Puppi, D. Polymeric Hydrogels for In Vitro 3D Ovarian Cancer Modeling. Int. J. Mol. Sci. 2022, 23, 3265. [Google Scholar] [CrossRef] [PubMed]
- Svanström, A.; Rosendahl, J.; Salerno, S.; Leiva, M.C.; Gregersson, P.; Berglin, M.; Bogestål, Y.; Lausmaa, J.; Oko, A.; Chinga-Carrasco, G.; et al. Optimized alginate-based 3D printed scaffolds as a model of patient derived breast cancer microenvironments in drug discovery. Biomed. Mater. 2021, 16, 045046. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, J.; Svanström, A.; Berglin, M.; Petronis, S.; Bogestål, Y.; Stenlund, P.; Standoft, S.; Ståhlberg, A.; Landberg, G.; Chinga-Carrasco, G.; et al. 3D Printed Nanocellulose Scaffolds as a Cancer Cell Culture Model System. Bioengineering 2021, 8, 97. [Google Scholar] [CrossRef]
- Svanström, A.; Rosendahl, J.; Salerno, S.; Jonasson, E.; Håkansson, J.; Ståhlberg, A.; Landberg, G. The Effect of Hypoxic and Normoxic Culturing Conditions in Different Breast Cancer 3D Model Systems. Front. Bioeng. Biotechnol. 2021, 9, 711977. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Gu, K.; Bavoux, M.M.; Flores-Torres, S.; Kort-Mascort, J.; Grant, J.; Vijayakumar, S.; De Leon-Rodriguez, A.; Ehrlicher, A.J.; et al. Engineering bioprintable alginate/gelatin composite hydrogels with tunable mechanical and cell adhesive properties to modulate tumor spheroid growth kinetics. Biofabrication 2019, 12, 015024. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Biodegradable Polymers for Biomedical Additive Manufacturing. Appl. Mater. Today 2020, 20, 100700. [Google Scholar] [CrossRef]
- Jeong, H.J.; Nam, H.; Jang, J.; Lee, S.J. 3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs. Bioengineering 2020, 7, 32. [Google Scholar] [CrossRef]
- Hull, S.M.; Brunel, L.G.; Heilshorn, S.C. 3D Bioprinting of Cell-Laden Hydrogels for Improved Biological Functionality. Adv. Mater. 2022, 34, 2103691. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; Leon-Rodriguez, A.D.; Kinsella, J.M. Directing the Self-assembly of Tumour Spheroids by Bioprinting Cellular Heterogeneous Models within Alginate/Gelatin Hydrogels. Sci. Rep. 2017, 7, 4575. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Holmes, B.; Glazer, R.I.; Zhang, L.G. 3D printed nanocomposite matrix for the study of breast cancer bone metastasis. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 69–79. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Dai, X.; Zhang, X.; Zhang, J.; Xu, T.; Lan, Q. Coaxial extrusion bioprinted shell-core hydrogel microfibers mimic glioma microenvironment and enhance the drug resistance of cancer cells. Colloids Surf. B Biointerfaces 2018, 171, 291–299. [Google Scholar] [CrossRef]
- Cui, H.; Esworthy, T.; Zhou, X.; Hann, S.Y.; Glazer, R.I.; Li, R.; Zhang, L.G. Engineering a Novel 3D Printed Vascularized Tissue Model for Investigating Breast Cancer Metastasis to Bone. Adv. Healthc. Mater. 2020, 9, e1900924. [Google Scholar] [CrossRef]
- Loterie, D.; Delrot, P.; Moser, C. High-resolution tomographic volumetric additive manufacturing. Nat. Commun. 2020, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Oudshoorn, M.H.; van Geemen, D.; Hennink, W.E.; Alblas, J.; Dhert, W.J.A. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials 2009, 30, 344–353. [Google Scholar] [CrossRef]
- Chiulan, I.; Heggset, E.B.; Voicu, Ş.I.; Chinga-Carrasco, G. Photopolymerization of Bio-Based Polymers in a Biomedical Engineering Perspective. Biomacromolecules 2021, 22, 1795–1814. [Google Scholar] [CrossRef]
- Noh, S.-H.; Kim, S.-W.; Kim, J.-W.; Lee, T.-H.; Nah, J.-W.; Lee, Y.-G.; Kim, M.-K.; Ito, Y.; Son, T.-I. Preparation of drug-immobilized anti-adhesion agent using visible light-curable alginate derivative containing furfuryl group. Int. J. Biol. Macromol. 2019, 121, 301–308. [Google Scholar] [CrossRef]
- Brennan-Pierce, E.P.; MacAskill, I.; Price, R.B.; Lee, J.M. Riboflavin-sensitized photo-crosslinking of collagen using a dental curing light. Biomed Mater Eng 2014, 24, 1659–1671. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Xu, C. Nanocellulose-Based Inks for 3D Bioprinting: Key Aspects in Research Development and Challenging Perspectives in Applications—A Mini Review. Bioengineering 2020, 7, 40. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.-R.; Kuisma, S.W.; Kanninen, L.; Lille, M.; Corlu, A.; GuGuen-Guillouzo, C.; Ikkala, O.; et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Fato, M.; Peñuela, L.; Beltrame, F.; Raiteri, R.; Scaglione, S. Microenvironment complexity and matrix stiffness regulate breast cancer cell activity in a 3D in vitro model. Sci. Rep. 2016, 6, 35367. [Google Scholar] [CrossRef] [PubMed]
- Syverud, K.; Pettersen, S.R.; Draget, K.; Chinga-Carrasco, G. Controlling the elastic modulus of cellulose nanofibril hydrogels—Scaffolds with potential in tissue engineering. Cellulose 2015, 22, 473–481. [Google Scholar] [CrossRef]
- Ajdary, R.; Reyes, G.; Kuula, J.; Raussi-Lehto, E.; Mikkola, T.S.; Kankuri, E.; Rojas, O.J. Direct Ink Writing of Biocompatible Nanocellulose and Chitosan Hydrogels for Implant Mesh Matrices. ACS Polym. Au 2022, 2, 97–107. [Google Scholar] [CrossRef]
- Rashad, A.; Mustafa, K.; Heggset, E.B.; Syverud, K. Cytocompatibility of Wood-Derived Cellulose Nanofibril Hydrogels with Different Surface Chemistry. Biomacromolecules 2017, 18, 1238–1248. [Google Scholar] [CrossRef]
- Gusmão, A.; Sanjuan-Alberte, P.; Ferreira, F.C.; Leite, M. Design, fabrication, and testing of a low-cost extrusion based 3D bioprinter for thermo-sensitive and light sensitive hydrogels. Mater. Today Proc. 2022, 70, 148–154. [Google Scholar] [CrossRef]
- Olive, P.L.; Vikse, C.; Trotter, M.J. Measurement of oxygen diffusion distance in tumor cubes using a fluorescent hypoxia probe. Int. J. Radiat. Oncol. *Biol. *Phys. 1992, 22, 397–402. [Google Scholar] [CrossRef]
- Tannock, I.F. Oxygen diffusion and the distribution of cellular radiosensitivity in tumours. Br. J. Radiol. 1972, 45, 515–524. [Google Scholar] [CrossRef]
- Thomlinson, R.H.; Gray, L.H. The Histological Structure of Some Human Lung Cancers and the Possible Implications for Radiotherapy. Br. J. Cancer 1955, 9, 539–549. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Coecke, S.; Balls, M.; Bowe, G.; Davis, J.; Gstraunthaler, G.; Hartung, T.; Hay, R.; Merten, O.-W.; Price, A.; Schechtman, L.; et al. Guidance on Good Cell Culture Practice:A Report of the Second ECVAM Task Force on Good Cell Culture Practice. Altern. Lab. Anim. 2005, 33, 261–287. [Google Scholar] [CrossRef]
- Garre, E.; Gustafsson, A.; Leiva, M.C.; Håkansson, J.; Ståhlberg, A.; Kovács, A.; Landberg, G. Breast Cancer Patient-Derived Scaffolds Can Expose Unique Individual Cancer Progressing Properties of the Cancer Microenvironment Associated with Clinical Characteristics. Cancers 2022, 14, 2172. [Google Scholar] [CrossRef] [PubMed]
- Leiva, M.C.; Garre, E.; Gustafsson, A.; Svanström, A.; Bogestål, Y.; Håkansson, J.; Ståhlberg, A.; Landberg, G. Breast cancer patient-derived scaffolds as a tool to monitor chemotherapy responses in human tumor microenvironments. J. Cell. Physiol. 2021, 236, 4709–4724. [Google Scholar] [CrossRef]
- Österberg, K.; Bogestål, Y.; Jenndahl, L.; Gustafsson-Hedberg, T.; Synnergren, J.; Holmgren, G.; Bom, E.; Petronis, S.; Krona, A.; Eriksson, J.S.; et al. Personalized tissue-engineered veins—Long term safety, functionality and cellular transcriptome analysis in large animals. Biomater. Sci. 2023, 11, 3860–3877. [Google Scholar] [CrossRef]
- Jenndahl, L.; Österberg, K.; Bogestål, Y.; Simsa, R.; Gustafsson-Hedberg, T.; Stenlund, P.; Petronis, S.; Krona, A.; Fogelstrand, P.; Strehl, R.; et al. Personalized tissue-engineered arteries as vascular graft transplants: A safety study in sheep. Regen. Ther. 2022, 21, 331–341. [Google Scholar] [CrossRef]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xv, X.; Liu, D.; Yang, Z.; Zhang, Q.; Shi, H.; Zhao, G.; Zhou, J. Preparation of Cellulose Nanofiber-reinforced Gelatin Hydrogel and Optimization for 3D Printing Applications. BioResources 2018, 13, 16. [Google Scholar] [CrossRef]
- Xu, C.; Zhang Molino, B.; Wang, X.; Cheng, F.; Xu, W.; Molino, P.; Bacher, M.; Su, D.; Rosenau, T.; Willför, S.; et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J. Mater. Chem. B 2018, 6, 7066–7075. [Google Scholar] [CrossRef]
- Espinosa, E.; Filgueira, D.; Rodríguez, A.; Chinga-Carrasco, G. Nanocellulose-Based Inks—Effect of Alginate Content on the Water Absorption of 3D Printed Constructs. Bioengineering 2019, 6, 65. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Ehman, N.V.; Filgueira, D.; Johansson, J.; Vallejos, M.E.; Felissia, F.E.; Håkansson, J.; Area, M.C. Bagasse—A major agro-industrial residue as potential resource for nanocellulose inks for 3D printing of wound dressing devices. Addit. Manuf. 2019, 28, 267–274. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Yang, P.; Långvik, O.; Wang, X.; Zhang, Y.; Cheng, F.; Österberg, M.; Willför, S.; Xu, C. Surface Engineered Biomimetic Inks Based on UV Cross-Linkable Wood Biopolymers for 3D Printing. ACS Appl. Mater. Interfaces 2019, 11, 12389–12400. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Zhang, J.; Xu, M.; Wang, L. Low-temperature 3D printing of collagen and chitosan composite for tissue engineering. Mater. Sci. Eng. C 2021, 123, 111963. [Google Scholar] [CrossRef] [PubMed]
- Kiyotake, E.A.; Douglas, A.W.; Thomas, E.E.; Nimmo, S.L.; Detamore, M.S. Development and quantitative characterization of the precursor rheology of hyaluronic acid hydrogels for bioprinting. Acta Biomater. 2019, 95, 176–187. [Google Scholar] [CrossRef]
- Das, S.; Pati, F.; Choi, Y.-J.; Rijal, G.; Shim, J.-H.; Kim, S.W.; Ray, A.R.; Cho, D.-W.; Ghosh, S. Bioprintable, cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015, 11, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Chen, X.; Na, J.; Sun, W. 3D printing of HEK 293FT cell-laden hydrogel into macroporous constructs with high cell viability and normal biological functions. Biofabrication 2015, 7, 015010. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Hermida, M.A.; Leslie, N.R.; Shu, W. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication 2015, 7, 045012. [Google Scholar] [CrossRef]
- Quarta, A.; Gallo, N.; Vergara, D.; Salvatore, L.; Nobile, C.; Ragusa, A.; Gaballo, A. Investigation on the Composition of Agarose–Collagen I Blended Hydrogels as Matrices for the Growth of Spheroids from Breast Cancer Cell Lines. Pharmaceutics 2021, 13, 963. [Google Scholar] [CrossRef]
- Florczyk, S.J.; Kievit, F.M.; Wang, K.; Erickson, A.E.; Ellenbogen, R.G.; Zhang, M. 3D Porous Chitosan-Alginate Scaffolds Promote Proliferation and Enrichment of Cancer Stem-Like Cells. J. Mater. Chem. B 2016, 4, 6326–6334. [Google Scholar] [CrossRef]
- Dai, X.; Ma, C.; Lan, Q.; Xu, T. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 2016, 8, 045005. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Zhang, A.; Huang, Y.; Tavakoli, J.; Tang, Y. Novel Bacterial Cellulose/Gelatin Hydrogels as 3D Scaffolds for Tumor Cell Culture. Polymers 2018, 10, 581. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, B.; Dong, Y.; Wang, W.; Zheng, X.; Zhou, W.; Zhang, K.; Du, Z. Three-dimensional prostate tumor model based on a hyaluronic acid-alginate hydrogel for evaluation of anti-cancer drug efficacy. J. Biomater. Sci. Polym. Ed. 2017, 28, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Chen, W.; Yuan, Z.; You, B.; Liu, Y.; Yang, S.; Li, F.; Qu, C.; Zhang, X. A Novel 3D in Vitro Tumor Model Based on Silk Fibroin/Chitosan Scaffolds To Mimic the Tumor Microenvironment. ACS Appl. Mater. Interfaces 2018, 10, 36641–36651. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Ikram, M.; Subhan, F.; Kang, H.Y.; Lim, Y.; Lee, R.; Jin, S.; Jeong, Y.H.; Kwak, J.-Y.; Na, Y.-J.; et al. Alginate–marine collagen–agarose composite hydrogels as matrices for biomimetic 3D cell spheroid formation. RSC Adv. 2016, 6, 46952–46965. [Google Scholar] [CrossRef]
- An, R.; Strissel, P.L.; Al-Abboodi, M.; Robering, J.W.; Supachai, R.; Eckstein, M.; Peddi, A.; Hauck, T.; Bäuerle, T.; Boccaccini, A.R.; et al. An Innovative Arteriovenous (AV) Loop Breast Cancer Model Tailored for Cancer Research. Bioengineering 2022, 9, 280. [Google Scholar] [CrossRef]
- Nerger, B.A.; Brun, P.T.; Nelson, C.M. Microextrusion printing cell-laden networks of type I collagen with patterned fiber alignment and geometry. Soft Matter. 2019, 15, 5728–5738. [Google Scholar] [CrossRef]
- Reid, J.A.; Palmer, X.-L.; Mollica, P.A.; Northam, N.; Sachs, P.C.; Bruno, R.D. A 3D bioprinter platform for mechanistic analysis of tumoroids and chimeric mammary organoids. Sci. Rep. 2019, 9, 7466. [Google Scholar] [CrossRef]
- Taira, N.; Ino, K.; Ida, H.; Nashimoto, Y.; Shiku, H. Electrodeposition-based rapid bioprinting of 3D-designed hydrogels with a pin art device. Biofabrication 2019, 11, 035018. [Google Scholar] [CrossRef]
- Olegovich Osidak, E.; Igorevich Kozhukhov, V.; Sergeevna Osidak, M.; Petrovich Domogatskiy, S. Collagen as Bioink for Bioprinting: A Comprehensive Review. Int. J. Bioprinting 2020, 6, 270. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, Z.; Meng, Y.; Zhao, Y.; Han, J.; Su, G.; Chen, B.; Dai, J. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials 2012, 33, 1437–1444. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/chitosan/hyaluronic acid-based injectable hydrogels for tissue engineering applications—Design, physicochemical and biological characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef]
- Arya, A.D.; Hallur, P.M.; Karkisaval, A.G.; Gudipati, A.; Rajendiran, S.; Dhavale, V.; Ramachandran, B.; Jayaprakash, A.; Gundiah, N.; Chaubey, A. Gelatin Methacrylate Hydrogels as Biomimetic Three-Dimensional Matrixes for Modeling Breast Cancer Invasion and Chemoresponse in Vitro. ACS Appl. Mater. Interfaces 2016, 8, 22005–22017. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, G.; Yan, S.; Zhang, Q. 3D Printing of Silk Fibroin for Biomedical Applications. Materials 2019, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, C.; Ma, X.; Li, Y. Optimization of macroporous 3-D silk fibroin scaffolds by salt-leaching procedure in organic solvent-free conditions. J. Mater. Sci. Mater. Med. 2012, 23, 315–324. [Google Scholar] [CrossRef]
- Talukdar, S.; Mandal, M.; Hutmacher, D.W.; Russell, P.J.; Soekmadji, C.; Kundu, S.C. Engineered silk fibroin protein 3D matrices for in vitro tumor model. Biomaterials 2011, 32, 2149–2159. [Google Scholar] [CrossRef]
- Painter, T.J. 4—Algal Polysaccharides. In The Polysaccharides; Aspinall, G.O., Ed.; Academic Press: Cambridge, MA, USA, 1983; pp. 195–285. [Google Scholar]
- Gorin, P.A.J.; Spencer, J.F.T. Exocellular alginic acid from Azotobacter vinelandii. Can. J. Chem. 1966, 44, 993–998. [Google Scholar] [CrossRef]
- Govan, J.R.W.; Fyfe, J.A.M.; Jarman, T.R. Isolation of Alginate-producing Mutants of Pseudomonas fluorescens, Pseudomonas putida and Pseudomonas mendocina. Microbiology 1981, 125, 217–220. [Google Scholar] [CrossRef]
- Skják-Bræk, G.; Smidsrød, O.; Larsen, B. Tailoring of alginates by enzymatic modification in vitro. Int. J. Biol. Macromol. 1986, 8, 330–336. [Google Scholar] [CrossRef]
- Smidsrød, O. Molecular basis for some physical properties of alginates in the gel state. Faraday Discuss. Chem. Soc. 1974, 57, 263–274. [Google Scholar] [CrossRef]
- Strand, B.L.; Ryan, L.; Veld, P.I.t.; Kulseng, B.; Rokstad, A.M.; Skjåk-Bræk, G.; Espevik, T. Poly-L-Lysine Induces Fibrosis on Alginate Microcapsules via the Induction of Cytokines. Cell Transplant. 2001, 10, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Wang, J.; Guo, R.; Zhang, Q. The effect of ionic strength on the viscosity of sodium alginate solution. Polym. Adv. Technol. 2001, 12, 740–745. [Google Scholar] [CrossRef]
- Vold, I.M.N.; Kristiansen, K.A.; Christensen, B.E. A study of the chain stiffness and extension of alginates, in vitro epimerized alginates, and periodate-oxidized alginates using size-exclusion chromatography combined with light scattering and viscosity detectors. Biomacromolecules 2006, 7, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lewin Mejia, D.; Chiang, B.; Luker, K.E.; Luker, G.D. Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion. Acta Biomater. 2018, 75, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, E.; Sarvestani, S.K.; Daneshian, L.; Moeinzadeh, S. Optimum 3D Matrix Stiffness for Maintenance of Cancer Stem Cells Is Dependent on Tissue Origin of Cancer Cells. PLoS ONE 2015, 10, e0132377. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Vårum, K.M.; Antohonsen, M.W.; Grasdalen, H.; Smidsrød, O. Determination of the degree of N-acetylation and the distribution of N-acetyl groups in partially N-deacetylated chitins (chitosans) by high-field nmr spectroscopy. Carbohydr. Res. 1991, 211, 17–23. [Google Scholar] [CrossRef]
- Strand, S.P.; Tømmeraas, K.; Vårum, K.M.; Østgaard, K. Electrophoretic light scattering studies of chitosans with different degrees of N-acetylation. Biomacromolecules 2001, 2, 1310–1314. [Google Scholar] [CrossRef]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Water-solubility of partially N-acetylated chitosans as a function of pH: Effect of chemical composition and depolymerisation. Carbohydr. Polym. 1994, 25, 65–70. [Google Scholar] [CrossRef]
- Elviri, L.; Foresti, R.; Bergonzi, C.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R. Highly defined 3D printed chitosan scaffolds featuring improved cell growth. Biomed. Mater. 2017, 12, 045009. [Google Scholar] [CrossRef]
- Wu, Q.; Maire, M.; Lerouge, S.; Therriault, D.; Heuzey, M.C. 3D printing of microstructured and stretchable chitosan hydrogel for guided cell growth. Adv. Biosyst. 2017, 1, 1700058. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chang, T.W.; Peng, T.K. Three-dimensional plotted alginate fibers embedded with diclofenac and bone cells coated with chitosan for bone regeneration during inflammation. J. Biomed. Mater. Res. Part A 2018, 106, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Choi, B.; Wu, B.; Lee, M. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 2013, 5, 045003. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef]
- Delair, T. Colloidal polyelectrolyte complexes of chitosan and dextran sulfate towards versatile nanocarriers of bioactive molecules. Eur. J. Pharm. Biopharm. 2011, 78, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-K.; Wang, Y.-Y.; Qiu, W.-Y.; Wu, J.-Y. Construction and characterization of nanosized curdlan sulfate/chitosan polyelectrolyte complex toward drug release of zidovudine. Carbohydr. Polym. 2017, 174, 209–216. [Google Scholar] [CrossRef]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodriguez-Rodriguez, R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Mohammadi, S.; Mohammadi, S.; Salimi, A. A 3D hydrogel based on chitosan and carbon dots for sensitive fluorescence detection of microRNA-21 in breast cancer cells. Talanta 2021, 224, 121895. [Google Scholar] [CrossRef]
- Wang, K.; Kievit, F.M.; Erickson, A.E.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. Culture on 3D Chitosan-Hyaluronic Acid Scaffolds Enhances Stem Cell Marker Expression and Drug Resistance in Human Glioblastoma Cancer Stem Cells. Adv. Healthc. Mater. 2016, 5, 3173–3181. [Google Scholar] [CrossRef]
- Henriksson, I.; Gatenholm, P.; Hägg, D.A. Increased lipid accumulation and adipogenic gene expression of adipocytes in 3D bioprinted nanocellulose scaffolds. Biofabrication 2017, 9, 015022. [Google Scholar] [CrossRef]
- Carvalho, M.P.; Costa, E.C.; Miguel, S.P.; Correia, I.J. Tumor spheroid assembly on hyaluronic acid-based structures: A review. Carbohydr. Polym. 2016, 150, 139–148. [Google Scholar] [CrossRef]
- Florczyk, S.J.; Wang, K.; Jana, S.; Wood, D.L.; Sytsma, S.K.; Sham, J.G.; Kievit, F.M.; Zhang, M. Porous chitosan-hyaluronic acid scaffolds as a mimic of glioblastoma microenvironment ECM. Biomaterials 2013, 34, 10143–10150. [Google Scholar] [CrossRef]
- Suo, A.; Xu, W.; Wang, Y.; Sun, T.; Ji, L.; Qian, J. Dual-degradable and injectable hyaluronic acid hydrogel mimicking extracellular matrix for 3D culture of breast cancer MCF-7 cells. Carbohydr. Polym. 2019, 211, 336–348. [Google Scholar] [CrossRef]
- Ee, L.Y.; Yau Li, S.F. Recent advances in 3D printing of nanocellulose: Structure, preparation, and application prospects. Nanoscale Adv. 2021, 3, 1167–1208. [Google Scholar] [CrossRef] [PubMed]
- Pennells, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 2020, 27, 575–593. [Google Scholar] [CrossRef]
- Ajdary, R.; Tardy, B.L.; Mattos, B.D.; Bai, L.; Rojas, O.J. Plant Nanomaterials and Inspiration from Nature: Water Interactions and Hierarchically Structured Hydrogels. Adv. Mater. 2021, 33, e2001085. [Google Scholar] [CrossRef]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between Length and Degree of Polymerization of TEMPO-Oxidized Cellulose Nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Nordli, H.R.; Chinga-Carrasco, G.; Rokstad, A.M.; Pukstad, B. Producing ultrapure wood cellulose nanofibrils and evaluating the cytotoxicity using human skin cells. Carbohydr. Polym. 2016, 150, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Polez, R.T.; Morits, M.; Jonkergouw, C.; Phiri, J.; Valle-Delgado, J.J.; Linder, M.B.; Maloney, T.; Rojas, O.J.; Österberg, M. Biological activity of multicomponent bio-hydrogels loaded with tragacanth gum. Int. J. Biol. Macromol. 2022, 215, 691–704. [Google Scholar] [CrossRef]
- Filote, C.; Santos, S.C.R.; Popa, V.I.; Botelho, C.M.S.; Volf, I. Biorefinery of marine macroalgae into high-tech bioproducts: A review. Environ. Chem. Lett. 2021, 19, 969–1000. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Metz, M.; Martínez-Abad, A.; Knutsen, S.H.; Ballance, S.; López-Rubio, A.; Martínez-Sanz, M. Valorization of alginate-extracted seaweed biomass for the development of cellulose-based packaging films. Algal Res. 2022, 61, 102576. [Google Scholar] [CrossRef]
- Wahlström, N.; Edlund, U.; Pavia, H.; Toth, G.; Jaworski, A.; Pell, A.J.; Choong, F.X.; Shirani, H.; Nilsson, K.P.R.; Richter-Dahlfors, A. Cellulose from the green macroalgae Ulva lactuca: Isolation, characterization, optotracing, and production of cellulose nanofibrils. Cellulose 2020, 27, 3707–3725. [Google Scholar] [CrossRef]
- Dunlop, M.J.; Acharya, B.; Bissessur, R. Isolation of nanocrystalline cellulose from tunicates. J. Environ. Chem. Eng. 2018, 6, 4408–4412. [Google Scholar] [CrossRef]
- Apelgren, P.; Sämfors, S.; Säljö, K.; Mölne, J.; Gatenholm, P.; Troedsson, C.; Thompson, E.M.; Kölby, L. Biomaterial and biocompatibility evaluation of tunicate nanocellulose for tissue engineering. Biomater. Adv. 2022, 137, 212828. [Google Scholar] [CrossRef]
- Sacui, I.A.; Nieuwendaal, R.C.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, E.J.; Olsson, R.T.; Gilman, J.W. Comparison of the Properties of Cellulose Nanocrystals and Cellulose Nanofibrils Isolated from Bacteria, Tunicate, and Wood Processed Using Acid, Enzymatic, Mechanical, and Oxidative Methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef] [PubMed]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, E.; Burdiles, P.A.; Quero, F.; Palma, P.; Olate-Moya, F.; Palza, H. 3D Printing of Antimicrobial Alginate/Bacterial-Cellulose Composite Hydrogels by Incorporating Copper Nanostructures. ACS Biomater. Sci. Eng. 2019, 5, 6290–6299. [Google Scholar] [CrossRef]
- Ahn, G.; Park, J.H.; Kang, T.; Lee, J.W.; Kang, H.-W.; Cho, D.-W. Effect of Pore Architecture on Oxygen Diffusion in 3D Scaffolds for Tissue Engineering. J. Biomech. Eng. 2010, 132, 104506. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, H.K.; Ray, A.R.; Panda, A.K. Characterization and evaluation of chitosan matrix for in vitro growth of MCF-7 breast cancer cell lines. Biomaterials 2004, 25, 5147–5154. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Ghajar, C.M.; Bissell, M.J. The need for complex 3D culture models to unravel novel pathways and identify accurate biomarkers in breast cancer. Adv. Drug Deliv. Rev. 2014, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, J.; Zarna, C.; Håkansson, J.; Chinga-Carrasco, G. Gene-Expression Analysis of Human Fibroblasts Affected by 3D-Printed Carboxylated Nanocellulose Constructs. Bioengineering 2023, 10, 121. [Google Scholar] [CrossRef]
- Pathak, A.; Kumar, S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl. Acad. Sci. USA 2012, 109, 10334–10339. [Google Scholar] [CrossRef] [PubMed]
- Asghar, W.; El Assal, R.; Shafiee, H.; Pitteri, S.; Paulmurugan, R.; Demirci, U. Engineering cancer microenvironments for in vitro 3-D tumor models. Mater. Today 2015, 18, 539–553. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Barney, L.E.; Dandley, E.C.; Jansen, L.E.; Reich, N.G.; Mercurio, A.M.; Peyton, S.R. A cell–ECM screening method to predict breast cancer metastasis. Integr. Biol. 2014, 7, 198–212. [Google Scholar] [CrossRef]
- Merrett, K.; Wan, F.; Lee, C.-J.; Harden, J.L. Enhanced Collagen-like Protein for Facile Biomaterial Fabrication. ACS Biomater. Sci. Eng. 2021, 7, 1414–1427. [Google Scholar] [CrossRef]
- Curvello, R.; Kast, V.; Abuwarwar, M.H.; Fletcher, A.L.; Garnier, G.; Loessner, D. 3D Collagen-Nanocellulose Matrices Model the Tumour Microenvironment of Pancreatic Cancer. Front. Digit. Health 2021, 3, 704584. [Google Scholar] [CrossRef]
- Curvello, R.; Kerr, G.; Micati, D.J.; Chan, W.H.; Raghuwanshi, V.S.; Rosenbluh, J.; Abud, H.E.; Garnier, G. Engineered Plant-Based Nanocellulose Hydrogel for Small Intestinal Organoid Growth. Adv. Sci. 2021, 8, 2002135. [Google Scholar] [CrossRef]
- Yang, J.; Bahcecioglu, G.; Zorlutuna, P. The Extracellular Matrix and Vesicles Modulate the Breast Tumor Microenvironment. Bioengineering 2020, 7, 124. [Google Scholar] [CrossRef]
- Ozawa, P.M.M.; Alkhilaiwi, F.; Cavalli, I.J.; Malheiros, D.; de Souza Fonseca Ribeiro, E.M.; Cavalli, L.R. Extracellular vesicles from triple-negative breast cancer cells promote proliferation and drug resistance in non-tumorigenic breast cells. Breast Cancer Res. Treat. 2018, 172, 713–723. [Google Scholar] [CrossRef]
- Khanna, A.; Oropeza, B.P.; Huang, N.F. Engineering Spatiotemporal Control in Vascularized Tissues. Bioengineering 2022, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Landberg, G.; Fitzpatrick, P.; Isakson, P.; Jonasson, E.; Karlsson, J.; Larsson, E.; Svanström, A.; Rafnsdottir, S.; Persson, E.; Gustafsson, A.; et al. Patient-derived scaffolds uncover breast cancer promoting properties of the microenvironment. Biomaterials 2020, 235, 119705. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Ståhlberg, A.; Kubista, M. Technical aspects and recommendations for single-cell qPCR. Mol. Asp. Med. 2018, 59, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, E.; Deng, Q. Single-cell RNA sequencing: Technical advancements and biological applications. Mol. Asp. Med. 2018, 59, 36–46. [Google Scholar] [CrossRef]
- Raj, A.; Peskin, C.S.; Tranchina, D.; Vargas, D.Y.; Tyagi, S. Stochastic mRNA Synthesis in Mammalian Cells. PLOS Biol. 2006, 4, e309. [Google Scholar] [CrossRef]
- Bengtsson, M.; Ståhlberg, A.; Rorsman, P.; Kubista, M. Gene expression profiling in single cells from the pancreatic islets of Langerhans reveals lognormal distribution of mRNA levels. Genome Res. 2005, 15, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Marx, V. Method of the Year: Spatially resolved transcriptomics. Nat. Methods 2021, 18, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Svec, D.; Dolatabadi, S.; Thomsen, C.; Cordes, N.; Shannon, M.; Fitzpatrick, P.; Landberg, G.; Åman, P.; Ståhlberg, A. Identification of inhibitors regulating cell proliferation and FUS-DDIT3 expression in myxoid liposarcoma using combined DNA, mRNA, and protein analyses. Lab. Investig. 2018, 98, 957–967. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquier, E.; Rosendahl, J.; Solberg, A.; Ståhlberg, A.; Håkansson, J.; Chinga-Carrasco, G. Polysaccharides and Structural Proteins as Components in Three-Dimensional Scaffolds for Breast Cancer Tissue Models: A Review. Bioengineering 2023, 10, 682. https://doi.org/10.3390/bioengineering10060682
Pasquier E, Rosendahl J, Solberg A, Ståhlberg A, Håkansson J, Chinga-Carrasco G. Polysaccharides and Structural Proteins as Components in Three-Dimensional Scaffolds for Breast Cancer Tissue Models: A Review. Bioengineering. 2023; 10(6):682. https://doi.org/10.3390/bioengineering10060682
Chicago/Turabian StylePasquier, Eva, Jennifer Rosendahl, Amalie Solberg, Anders Ståhlberg, Joakim Håkansson, and Gary Chinga-Carrasco. 2023. "Polysaccharides and Structural Proteins as Components in Three-Dimensional Scaffolds for Breast Cancer Tissue Models: A Review" Bioengineering 10, no. 6: 682. https://doi.org/10.3390/bioengineering10060682
APA StylePasquier, E., Rosendahl, J., Solberg, A., Ståhlberg, A., Håkansson, J., & Chinga-Carrasco, G. (2023). Polysaccharides and Structural Proteins as Components in Three-Dimensional Scaffolds for Breast Cancer Tissue Models: A Review. Bioengineering, 10(6), 682. https://doi.org/10.3390/bioengineering10060682








