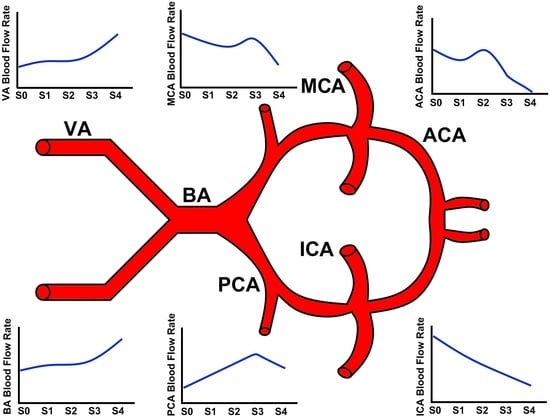
Computational Modelling of Cerebral Blood Flow Rate at Different Stages of Moyamoya Disease in Adults and Children
Abstract
1. Introduction
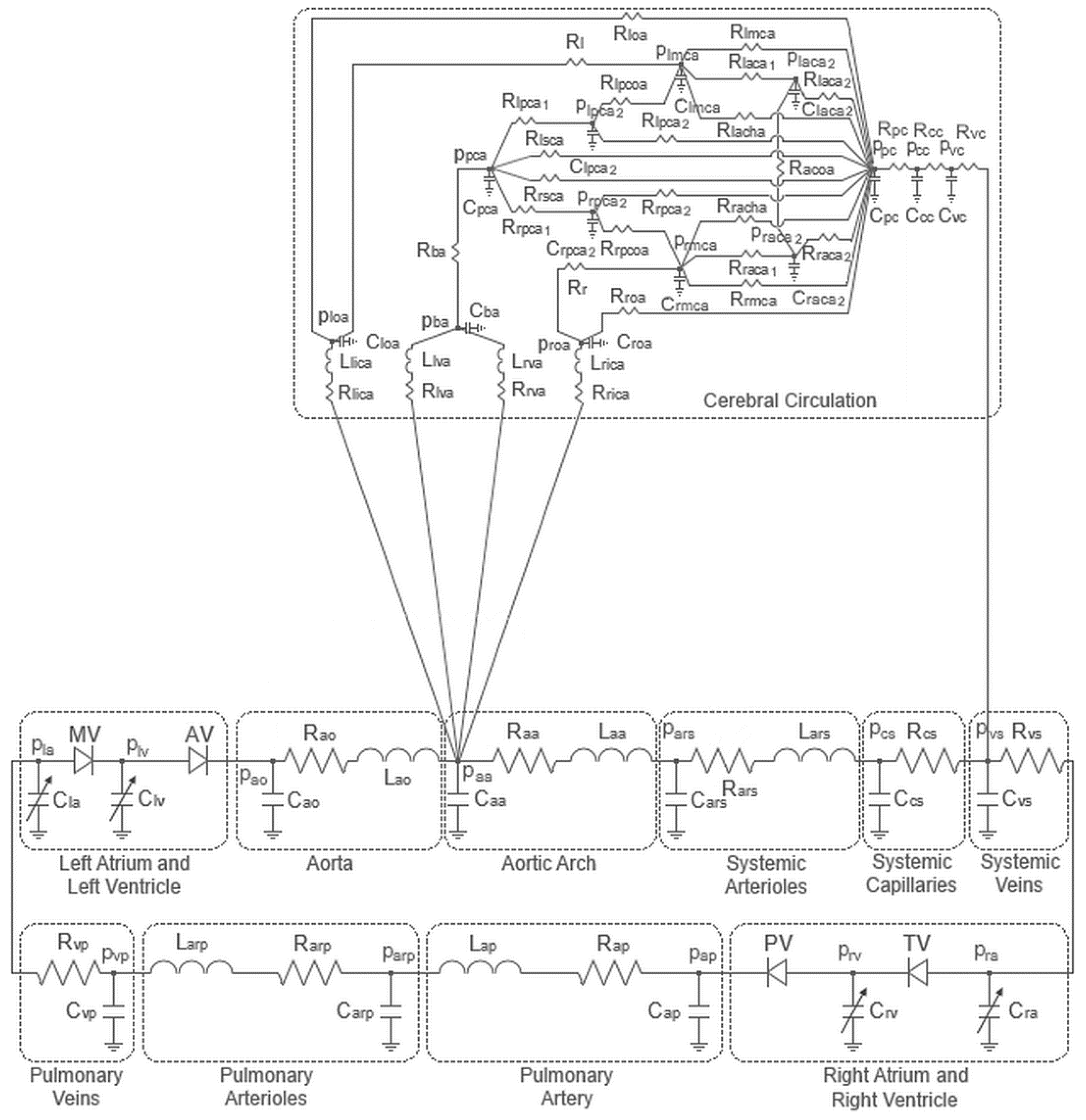
2. Materials and Methods
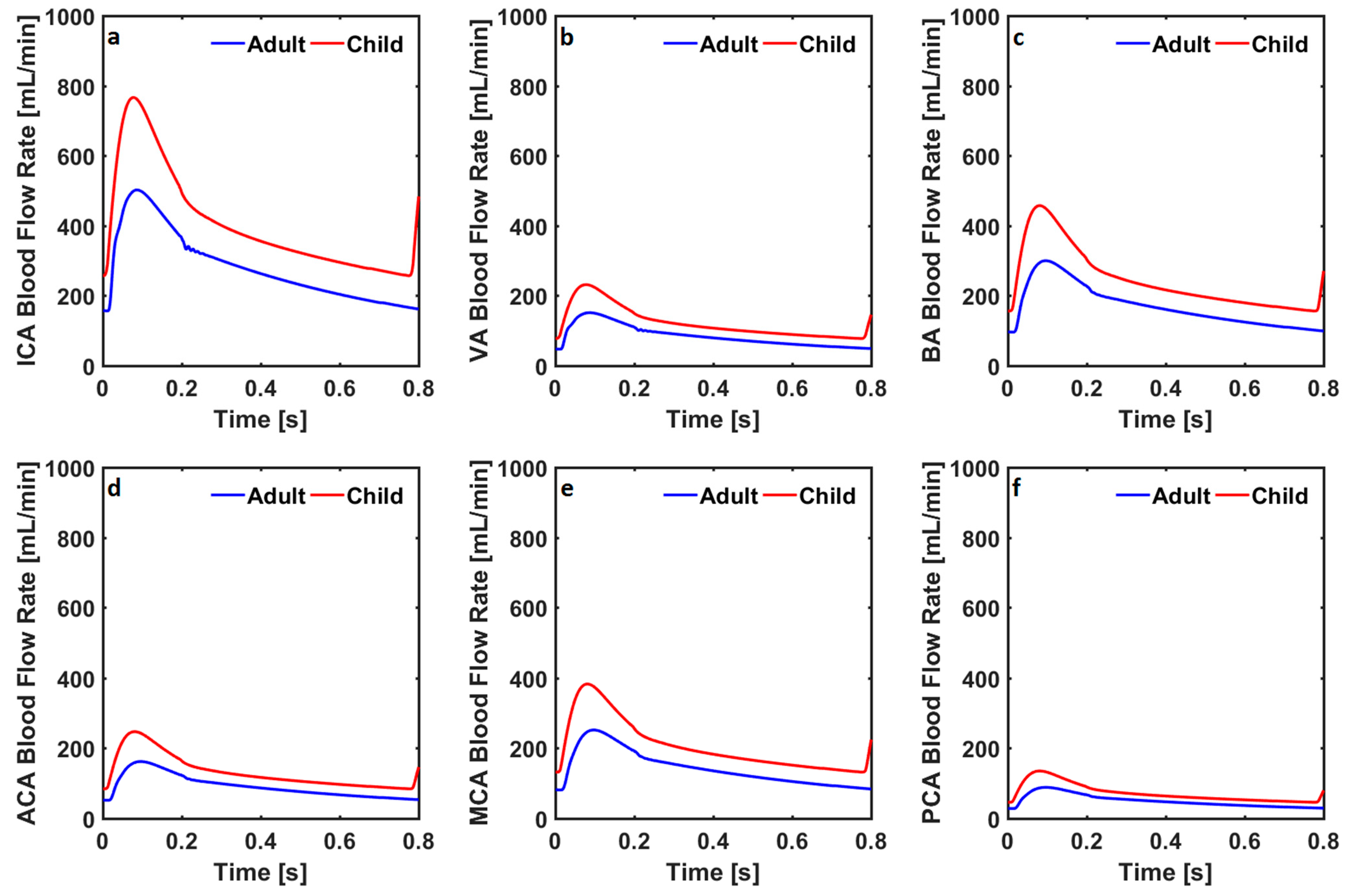
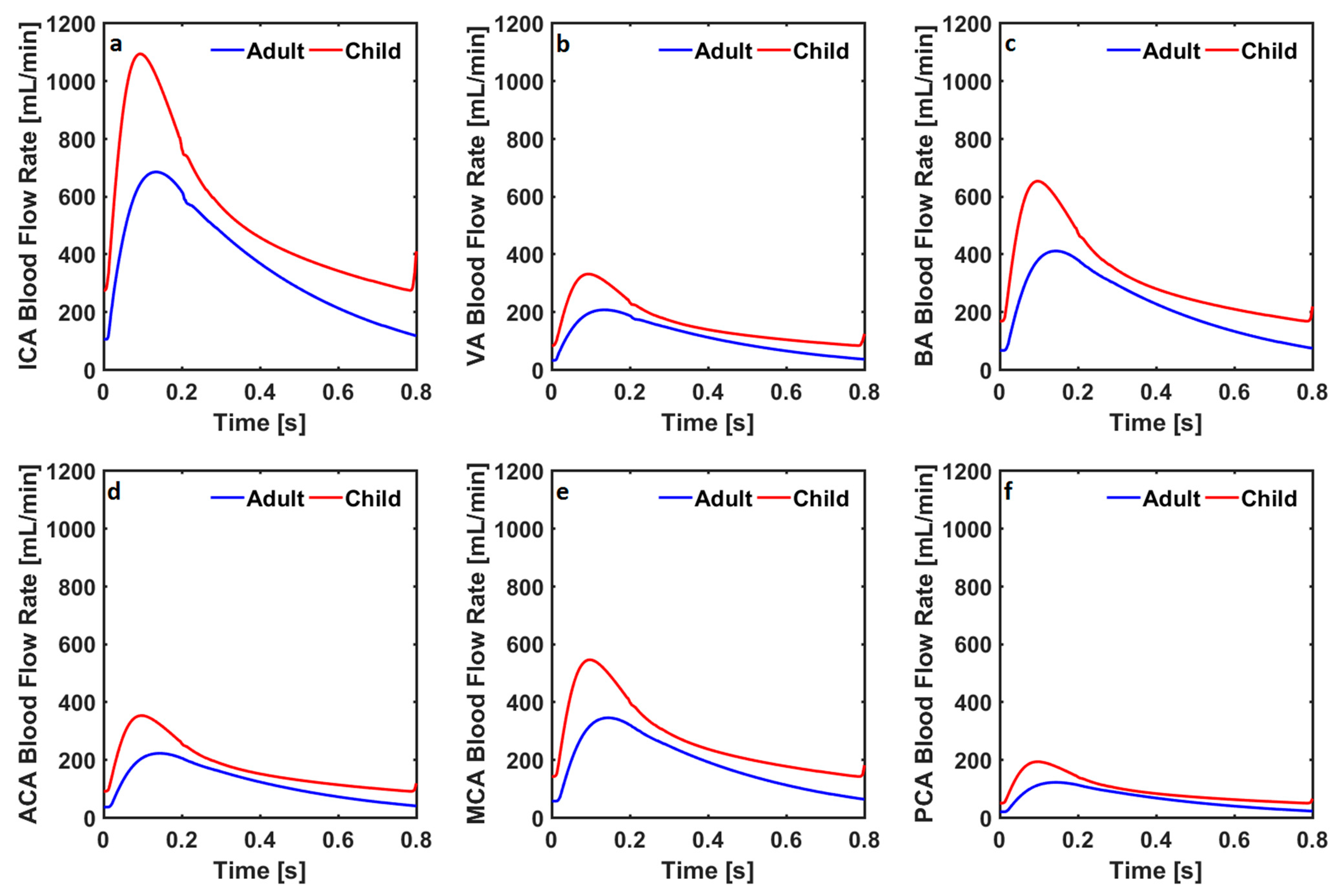
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Z.; Ma, G.; Zhang, D. Haemodynamic Analysis of Adult Patients with Moyamoya Disease: CT Perfusion and DSA Gradings. Stroke Vasc. Neurol. 2021, 6, 41–47. [Google Scholar] [CrossRef]
- Khan, N.; Lober, R.M.; Ostergren, L.; Petralia, J.; Bell-Stephens, T.; Navarro, R.; Feroze, A.; Steinberg, G.K. Measuring Cerebral Blood Flow in Moyamoya Angiopathy by Quantitative Magnetic Resonance Angiography Noninvasive Optimal Vessel Analysis. Neurosurgery 2017, 81, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Togao, O.; Mihara, F.; Yoshiura, T.; Tanaka, A.; Noguchi, T.; Kuwabara, Y.; Kaneko, K.; Matsushima, T.; Honda, H. Cerebral Hemodynamics in Moyamoya Disease: Correlation between Perfusion-Weighted MR Imaging and Cerebral Angiography. Am. J. Neuroradiol. 2006, 27, 391–397. [Google Scholar] [PubMed]
- Appireddy, R.; Ranjan, M.; Durafourt, B.A.; Riva-Cambrin, J.; Hader, W.J.; Adelson, P.D. Surgery for Moyamoya Disease in Children. J. Child Neurol. 2019, 34, 517–529. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Ichiya, Y.; Otsuka, M.; Tahara, T.; Gunasekera, R.; Hasuo, K.; Masuda, K.; Matsushima, T.; Fukui, M. Cerebral Hemodynamic Change in the Child and the Adult with Moyamoya Disease. Stroke 1990, 21, 272–277. [Google Scholar] [CrossRef]
- Nagaraja, D.; Verma, A.; Taly, A.B.; Kumar, M.V.; Jayakumar, P.N. Cerebrovascular Disease in Children. Acta Neurol. Scand. 1994, 90, 251–255. [Google Scholar] [CrossRef]
- Lee, M.; Guzman, R.; Bell-Stephens, T.; Steinberg, G.K. Intraoperative Blood Flow Analysis of Direct Revascularization Procedures in Patients with Moyamoya Disease. J. Cereb. Blood Flow Metab. 2011, 31, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Canavero, I.; Vetrano, I.G.; Zedde, M.; Pascarella, R.; Gatti, L.; Acerbi, F.; Nava, S.; Ferroli, P.; Parati, E.A.; Bersano, A. Clinical Management of Moyamoya Patients. J. Clin. Med. 2021, 10, 3628. [Google Scholar] [CrossRef] [PubMed]
- Korematsu, K.; Yoshioka, S.; Maruyama, T.; Nagai, Y.; Inoue, K.; Yukaya, N.; Baba, H.; Kuratsu, J. Moyamoya Disease Associated with Midaortic Syndrome. Pediatr. Neurosurg. 2007, 43, 54–59. [Google Scholar] [CrossRef]
- Babaoğlu, K.; Demir, T.; Saltık, L.; Kutluğ, Ş.; Işlak, C. Moyamoya Disease and Aortic Coarctation in a Patient with Common Brachiocephalic Trunk. Anatol. J. Cardiol. 2007, 7, 85–87. [Google Scholar]
- Christiaens, F.J.C.; Van den Broeck, L.K.L.; Christophe, C.; Dan, B. Moyamoya Disease (Moyamoya Syndrome) and Coarctation of the Aorta. Neuropediatrics 2000, 31, 47–48. [Google Scholar] [CrossRef]
- Schuster, J.M.; Roberts, T.S. Symptomatic Moyamoya Disease and Aortic Coarctation in a Patient with Noonan’s Syndrome: Strategies for Management. Pediatr. Neurosurg. 1999, 30, 2006–2010. [Google Scholar] [CrossRef]
- Lutterman, J.; Scott, M.; Nass, R.; Geva, T. Moyamoya Syndrome Associated With Congenital Heart Disease. Pediatrics 1998, 101, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Oh, C.W.; Bang, J.S.; Kim, J.E.; Cho, W.-S. Moyamoya Disease: Treatment and Outcomes. J. Stroke 2016, 18, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Gao, F.; Deng, X.; Zhang, D.; Zhang, Y. Results of Conservative Follow-up or Surgical Treatment of Moyamoya Patients Who Present without Hemorrhage, Transient Ischemic Attack, or Stroke. World Neurosurg. 2017, 108, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Charbel, F.T.; Misra, M.; Clarke, M.E.; Ausman, J.I. Computer Simulation of Cerebral Blood Flow in Moyamoya and the Results of Surgical Therapies. Clin. Neurol. Neurosurg. 1997, 99, S68–S73. [Google Scholar] [CrossRef]
- Zhu, F.; Qian, Y.; Xu, B.; Gu, Y.; Karunanithi, K.; Zhu, W.; Chen, L.; Mao, Y.; Morgan, M.K. Quantitative Assessment of Changes in Hemodynamics of the Internal Carotid Artery after Bypass Surgery for Moyamoya Disease. J. Neurosurg. 2017, 129, 677–683. [Google Scholar] [CrossRef]
- Rashad, S.; Saqr, K.M.; Fujimura, M.; Niizuma, K.; Tominaga, T. The Hemodynamic Complexities Underlying Transient Ischemic Attacks in Early-Stage Moyamoya Disease: An Exploratory CFD Study. Sci. Rep. 2020, 10, 3700. [Google Scholar] [CrossRef]
- Seol, H.J.; Shin, D.C.; Kim, Y.S.; Shim, E.B.; Kim, S.-K.; Cho, B.-K.; Wang, K.-C. Computational Analysis of Hemodynamics Using a Two-Dimensional Model in Moyamoya Disease: Laboratory Investigation. J. Neurosurg. Pediatr. 2010, 5, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, S. Mathematical Modeling of Cardiac Function to Evaluate Clinical Cases in Adults and Children. PLoS ONE 2019, 14, e0224663. [Google Scholar] [CrossRef]
- Bozkurt, S.; Volkan Yilmaz, A.; Bakaya, K.; Bharadwaj, A.; Safak, K.K. A Novel Computational Model for Cerebral Blood Flow Rate Control Mechanisms to Evaluate Physiological Cases. Biomed. Signal Process. Control. 2022, 78, 103851. [Google Scholar] [CrossRef]
- Bozkurt, S. Effect of Cerebral Flow Autoregulation Function on Cerebral Flow Rate Under Continuous Flow Left Ventricular Assist Device Support. Artif. Organs 2018, 42, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Sezer, S.S.; Narin, N.; Ozyurt, A.; Onan, S.H.; Pamukcu, O.; Argun, M.; Baykan, A.; Uzum, K. Cardiovascular Changes in Children with Coarctation of the Aorta Treated by Endovascular Stenting. J. Hum. Hypertens. 2014, 28, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W.; Parker, D.E. Association between Arterial Compliance and Age in Participants 9 to 77 Years Old. Angiology 2010, 61, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Ursino, M.; Giannessi, M. A Model of Cerebrovascular Reactivity Including the Circle of Willis and Cortical Anastomoses. Ann. Biomed. Eng. 2010, 38, 955–974. [Google Scholar] [CrossRef]
- Wu, C.; Honarmand, A.R.; Schnell, S.; Kuhn, R.; Schoeneman, S.E.; Ansari, S.A.; Carr, J.; Markl, M.; Shaibani, A. Age-Related Changes of Normal Cerebral and Cardiac Blood Flow in Children and Adults Aged 7 Months to 61 Years. J. Am. Heart Assoc. 2016, 5, e002657. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Yoshimoto, T.; Suzuki, J.; Sakurai, Y. Cerebral Blood Flow in Moyamoya Disease Part 1: Correlation with Age and Regional Distribution. Acta Neurochir. Eur. J. Neurosurg. 1990, 105, 30–34. [Google Scholar] [CrossRef]
- Zarrinkoob, L.; Ambarki, K.; Wahlin, A.; Birgander, R.; Eklund, A.; Malm, J. Blood Flow Distribution in Cerebral Arteries. J. Cereb. Blood Flow Metab. 2015, 35, 648–654. [Google Scholar] [CrossRef]
- Borzage, M.; Blüml, S.; Seri, I. Equations to Describe Brain Size across the Continuum of Human Lifespan. Brain Struct. Funct. 2014, 219, 141–150. [Google Scholar] [CrossRef]
- Iqbal, S. A Comprehensive Study of the Anatomical Variations of the Circle of Willis in Adult Human Brains. J. Clin. Diagn. Res. 2013, 7, 2423–2427. [Google Scholar] [CrossRef]
- Wijesinghe, P.; Steinbusch, H.W.M.; Shankar, S.K.; Yasha, T.C.; De Silva, K.R.D. Circle of Willis Abnormalities and Their Clinical Importance in Ageing Brains: A Cadaveric Anatomical and Pathological Study. J. Chem. Neuroanat. 2020, 106, 101772. [Google Scholar] [CrossRef] [PubMed]
- Paniukov, D.; Lebel, R.M.; Giesbrecht, G.; Lebel, C. Cerebral Blood Flow Increases across Early Childhood. NeuroImage 2020, 204, 116224. [Google Scholar] [CrossRef] [PubMed]
- Domogo, A.A.; Ottesen, J.T. Patient-Specific Parameter Estimation: Coupling a Heart Model and Experimental Data. J. Theor. Biol. 2021, 526, 110791. [Google Scholar] [CrossRef]
- Bozkurt, S.; Paracha, W.; Bakaya, K.; Schievano, S. Patient-Specific Modelling and Parameter Optimisation to Simulate Dilated Cardiomyopathy in Children. Cardiovasc Eng. Tech. 2022, 13, 712–724. [Google Scholar] [CrossRef]
- Fukui, M.; Kono, S.; Sueishi, K.; Ikezaki, K. Moyamoya Disease. Neuropathology 2000, 20, S61–S64. [Google Scholar] [CrossRef]
- Karasawa, J.; Touho, H.; Ohnishi, H.; Miyamoto, S.; Kikuchi, H. Cerebral Revascularization Using Omental Transplantation for Childhood Moyamoya Disease. J. Neurosurg. 1993, 79, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Magee, R.; Marshall, M.; Schaub, M.; Terrio, L. Speech-Language Patterns in a Child with Moya Moya Disease. Percept. Mot. Ski. 1994, 79, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Wu, W.; Yang, Z.; Yu, J. Research Progress of Moyamoya Disease in Children. Int. J. Med. Sci. 2015, 12, 566–577. [Google Scholar] [CrossRef]
- Hallemeier, C.L.; Rich, K.M.; Grubb, R.L.; Chicoine, M.R.; Moran, C.J.; Cross, D.T., III; Zipfel, G.J.; Dacey, R.G., Jr.; Derdeyn, C.P. Clinical Features and Outcome in North American Adults With Moyamoya Phenomenon. Stroke 2006, 37, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Jiang, H.; Xu, B.; Lei, Y.; Yang, H.; Su, J.; Gu, Y.; Mao, Y. Treatment of Aneurysms in Patients with Moyamoya Disease: A 10-Year Single-Center Experience. J. Neurosurg. 2018, 128, 1813–1822. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.-K.; Cheon, J.-E.; Choi, J.W.; Phi, J.H.; Kim, I.-O.; Cho, B.-K.; Wang, K.-C. Posterior Cerebral Artery Involvement in Moyamoya Disease: Initial Infarction and Angle between PCA and Basilar Artery. Childs Nerv. Syst. 2013, 29, 2263–2269. [Google Scholar] [CrossRef]
- Funaki, T.; Takahashi, J.C.; Takagi, Y.; Yoshida, K.; Araki, Y.; Kikuchi, T.; Kataoka, H.; Iihara, K.; Miyamoto, S. Impact of Posterior Cerebral Artery Involvement on Long-Term Clinical and Social Outcome of Pediatric Moyamoya Disease: Clinical Article. J. Neurosurg. Pediatr. 2013, 12, 626–632. [Google Scholar] [CrossRef]
- Wong, R.; Ahmad, W.; Davies, A.; Spratt, N.; Boyle, A.; Levi, C.; Howe, P.; Collins, N. Assessment of Cerebral Blood Flow in Adult Patients with Aortic Coarctation. Cardiol. Young 2017, 27, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Ünver, O.; Kutlubay, B.; Çetiner, N.; Saylan, B.; Baltacioğlu, F.; Ekinci, G.; Akalın, F.; Türkdoğan, D. PP04.13–2780: The Association of Moya Moya Disease with Pseudocoarctation: A Case Report. Eur. J. Paediatr. Neurol. 2015, 19, S45. [Google Scholar] [CrossRef]
- Zheng, Y.-M.; Xie, C.-H.; Gong, F.-Q. Moyamoya Disease Associated with Aortic Coarctation. World J. Pediatr. 2014, 10, 374. [Google Scholar] [CrossRef] [PubMed]

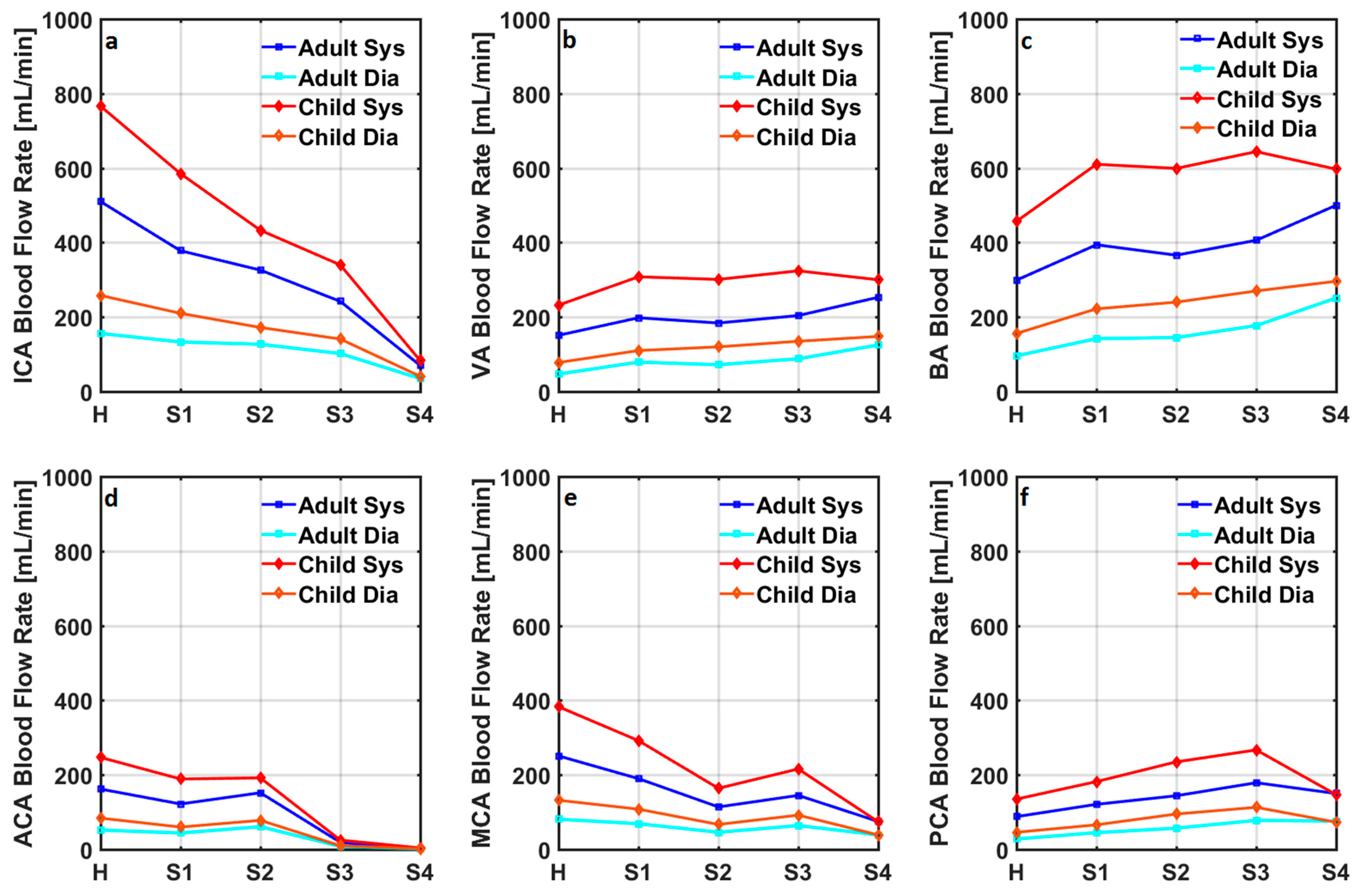
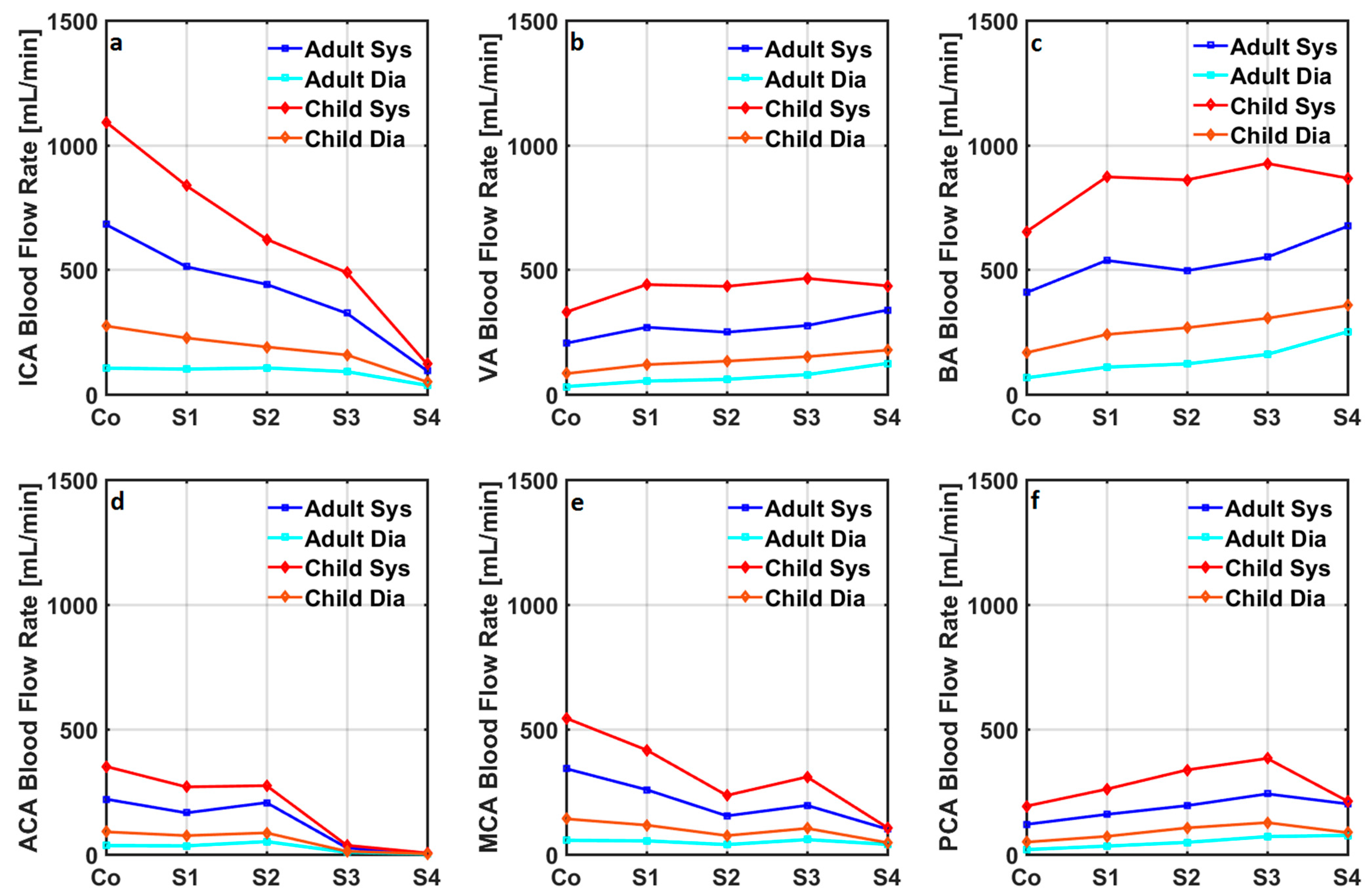
| Healthy | Stage 1 | Stage 2 | Stage 3 | Stage 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adult | Child | Adult | Child | Adult | Child | Adult | Child | Adult | Child | |
| SBP [mmHg] | 119 | 102 | 119 | 103 | 120 | 105 | 121 | 106 | 123 | 110 |
| DBP [mmHg] | 78 | 58 | 79 | 58 | 79 | 60 | 80 | 61 | 82 | 65 |
| CO [L/min] | 5.06 | 4.60 | 5.05 | 4.59 | 5.04 | 4.57 | 5.03 | 4.56 | 5.01 | 4.50 |
| CBF [mL/min] | 724 | 1072 | 676 | 1007 | 627 | 892 | 571 | 831 | 444 | 537 |
| ICA [mL/min] | 278 | 412 | 222 | 330 | 200 | 263 | 154 | 212 | 49 | 58 |
| VA [mL/min] | 84 | 124 | 117 | 174 | 113 | 183 | 131 | 203 | 173 | 210 |
| BA [mL/min] | 168 | 249 | 233 | 347 | 227 | 366 | 263 | 406 | 346 | 420 |
| ACA [mL/min] | 91 | 134 | 72 | 108 | 95 | 118 | 12 | 16 | 0.77 | 2.69 |
| MCA [mL/min] | 141 | 209 | 113 | 168 | 71 | 102 | 94 | 137 | 53 | 54 |
| PCA [mL/min] | 50 | 73 | 70 | 104 | 89 | 144 | 116 | 169 | 104 | 103 |
| SBPCo [mmHg] | 147 | 133 | 148 | 135 | 150 | 138 | 151 | 139 | 154 | 147 |
| DBPCo [mmHg] | 84 | 64 | 84 | 66 | 85 | 68 | 86 | 69 | 89 | 76 |
| COCo [L/min] | 4.79 | 4.58 | 4.78 | 4.56 | 4.76 | 4.53 | 4.75 | 4.52 | 4.71 | 4.44 |
| CBFCo [mL/min] | 926 | 1421 | 867 | 1341 | 806 | 1197 | 735 | 1121 | 576 | 741 |
| ICACo [mL/min] | 356 | 546 | 284 | 439 | 257 | 353 | 198 | 286 | 63 | 81 |
| VACo [mL/min] | 107 | 165 | 149 | 231 | 146 | 246 | 169 | 274 | 225 | 290 |
| BACo [mL/min] | 215 | 330 | 299 | 462 | 291 | 491 | 339 | 548 | 449 | 579 |
| ACACo [mL/min] | 116 | 178 | 93 | 144 | 121 | 158 | 16 | 21 | 0.99 | 3.71 |
| MCACo [mL/min] | 181 | 278 | 145 | 224 | 91 | 137 | 122 | 185 | 69 | 74 |
| PCACo [mL/min] | 63 | 97 | 89 | 138 | 115 | 194 | 149 | 228 | 134 | 142 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozkurt, S.; Bozkurt, S. Computational Modelling of Cerebral Blood Flow Rate at Different Stages of Moyamoya Disease in Adults and Children. Bioengineering 2023, 10, 77. https://doi.org/10.3390/bioengineering10010077
Bozkurt S, Bozkurt S. Computational Modelling of Cerebral Blood Flow Rate at Different Stages of Moyamoya Disease in Adults and Children. Bioengineering. 2023; 10(1):77. https://doi.org/10.3390/bioengineering10010077
Chicago/Turabian StyleBozkurt, Surhan, and Selim Bozkurt. 2023. "Computational Modelling of Cerebral Blood Flow Rate at Different Stages of Moyamoya Disease in Adults and Children" Bioengineering 10, no. 1: 77. https://doi.org/10.3390/bioengineering10010077
APA StyleBozkurt, S., & Bozkurt, S. (2023). Computational Modelling of Cerebral Blood Flow Rate at Different Stages of Moyamoya Disease in Adults and Children. Bioengineering, 10(1), 77. https://doi.org/10.3390/bioengineering10010077