Attenuation of Hyperlipidemia by Medicinal Formulations of Emblica officinalis Synergized with Nanotechnological Approaches
Abstract
1. Introduction
2. Hyperlipidemia
2.1. Causes of Hyperlipidemia
2.2. Types of Hyperlipidemia
2.3. Pathophysiology
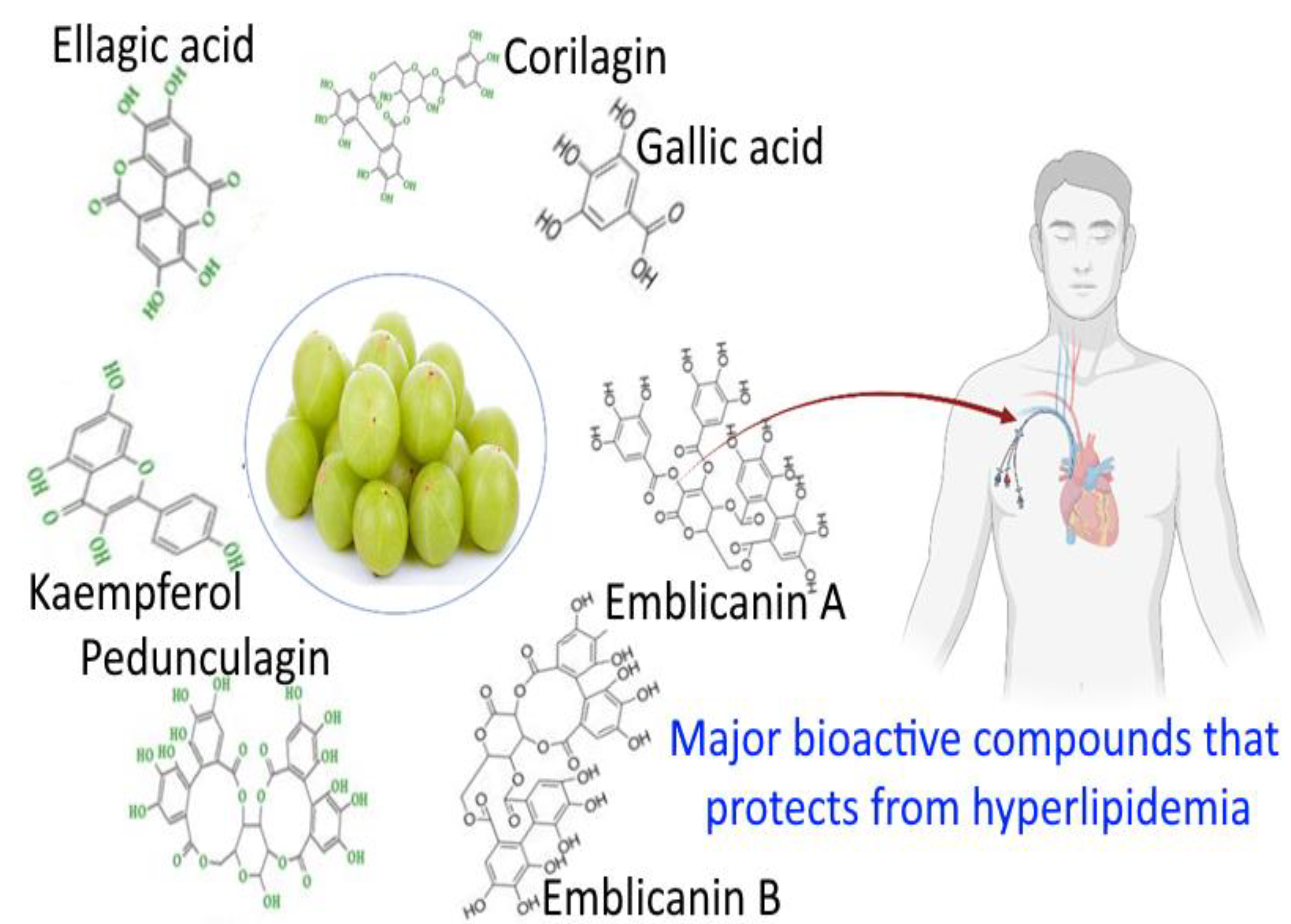
3. Biochemical Profiling of Amla
3.1. Phytochemistry
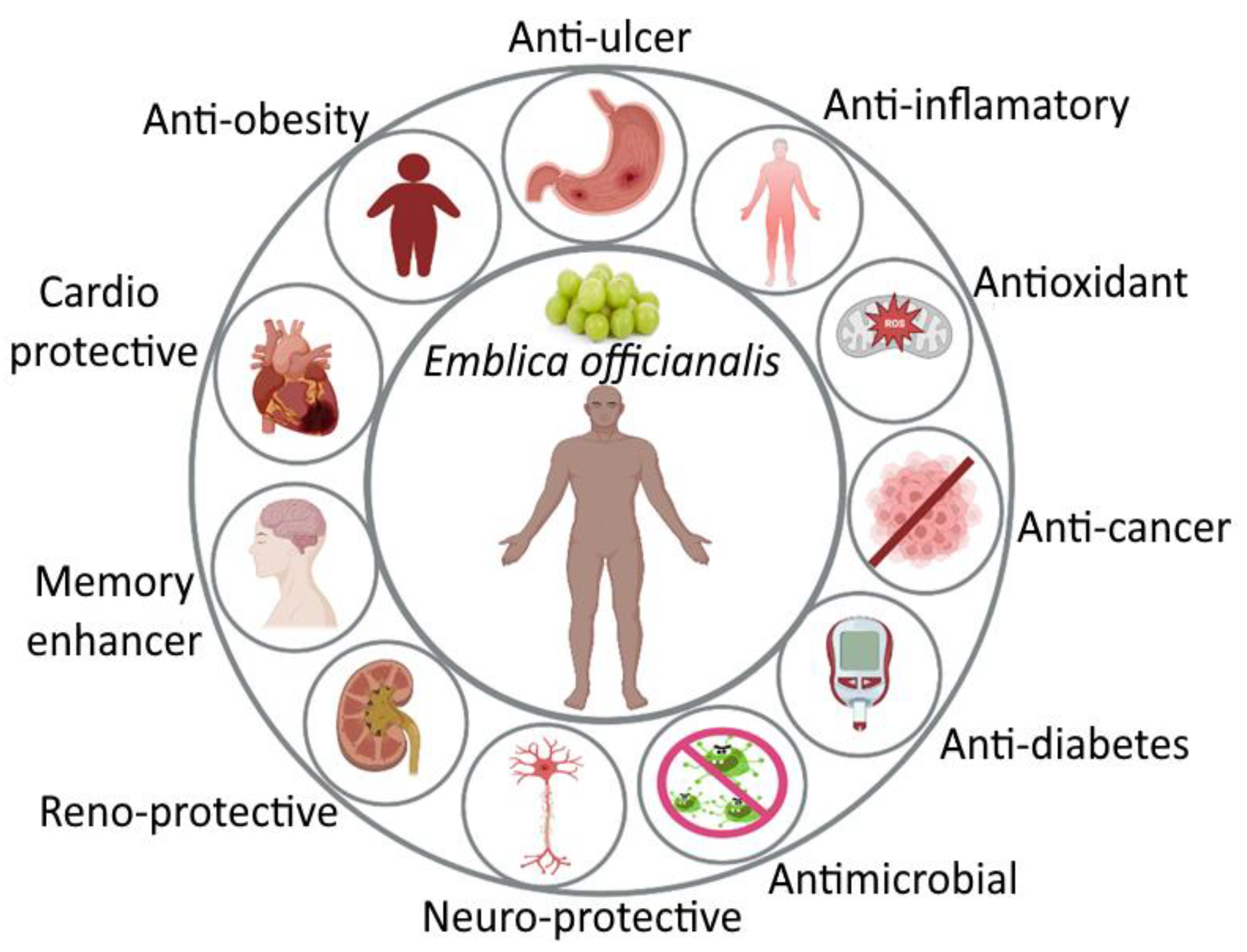
3.2. Pharmacological Activity of Amla
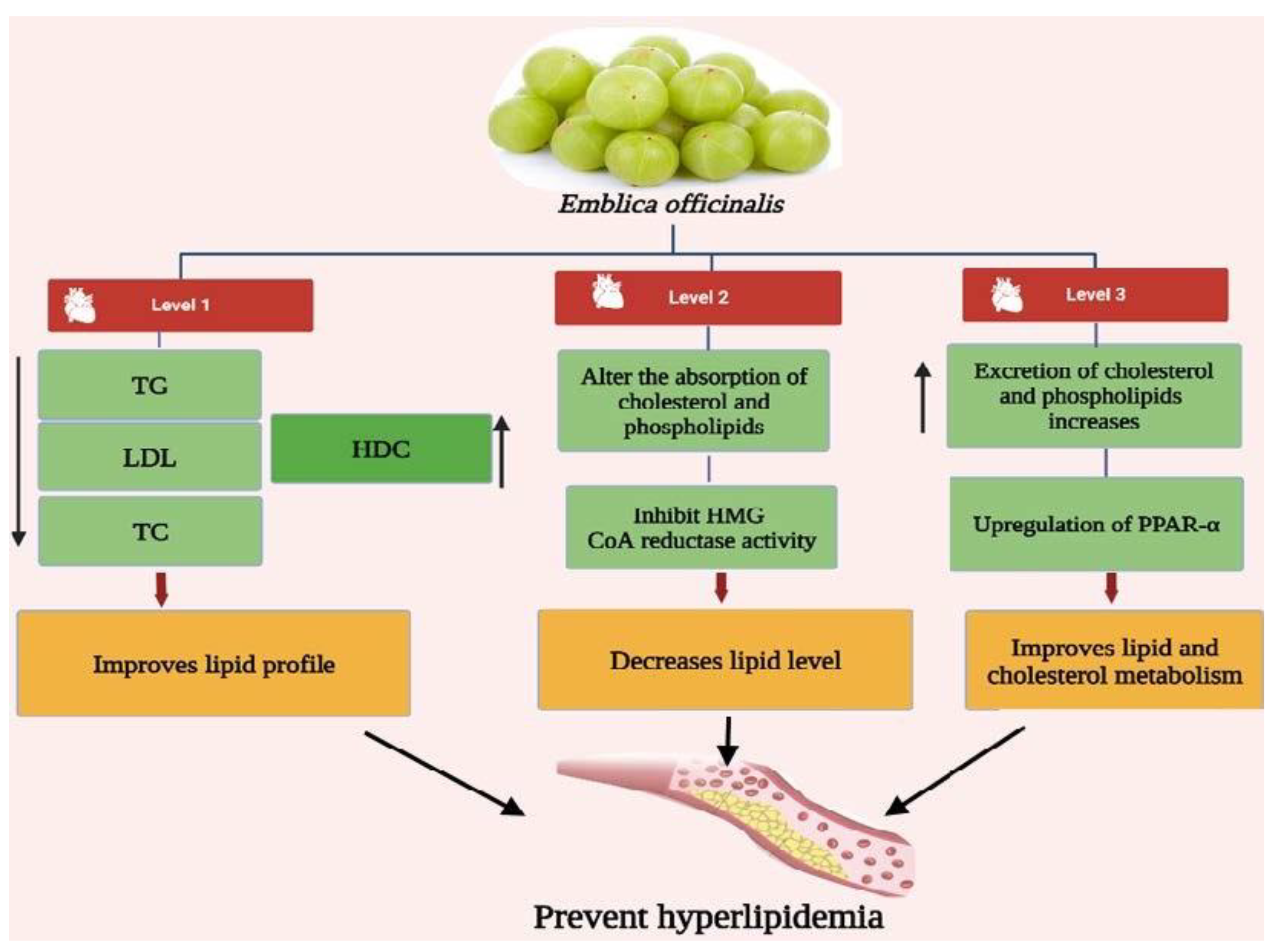
3.3. Antihyperlipidemic Activity of Emblica officinalis
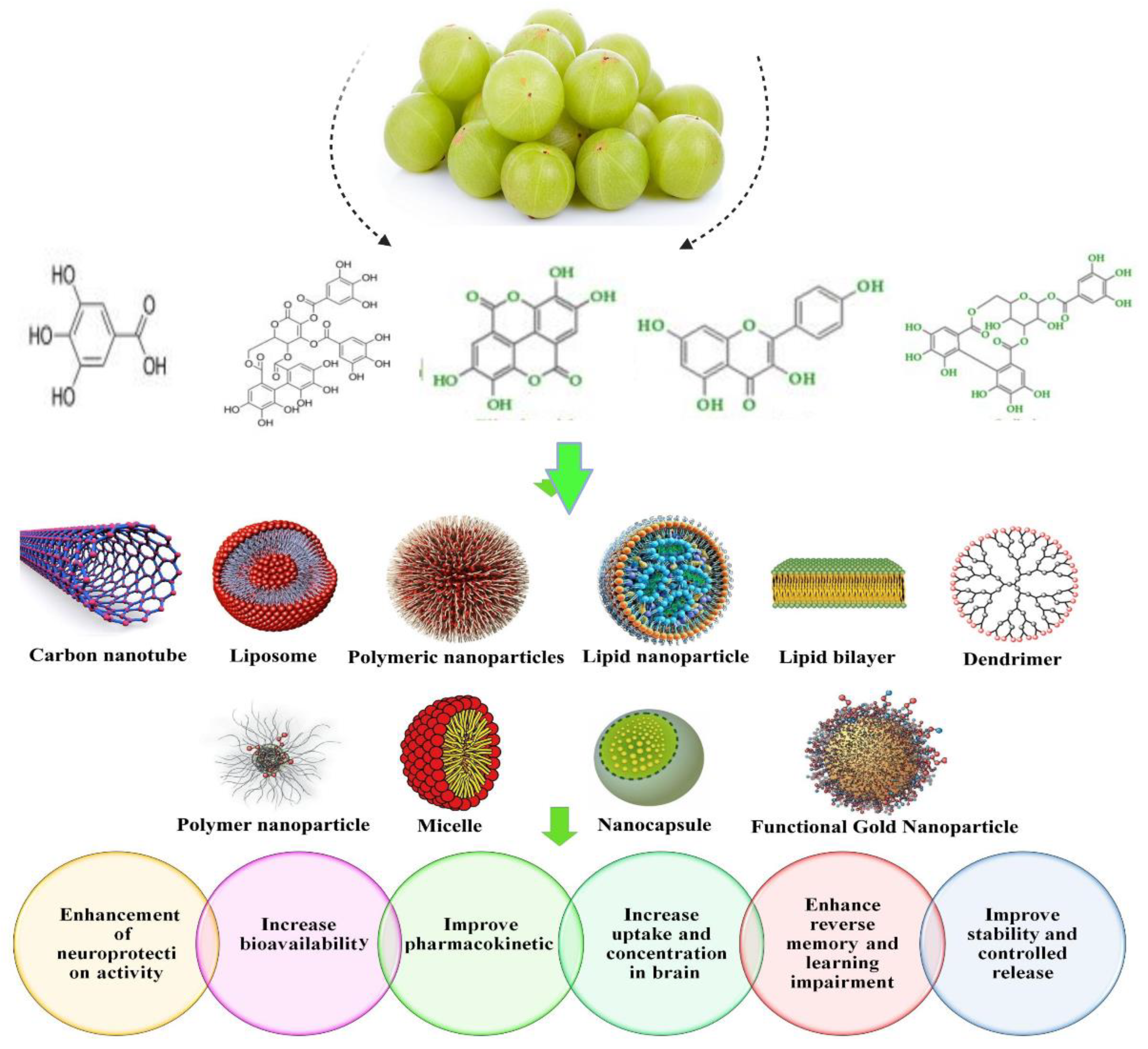
3.4. Nanoparticulate Carrier System for the Treatment of Hyperlipidemia
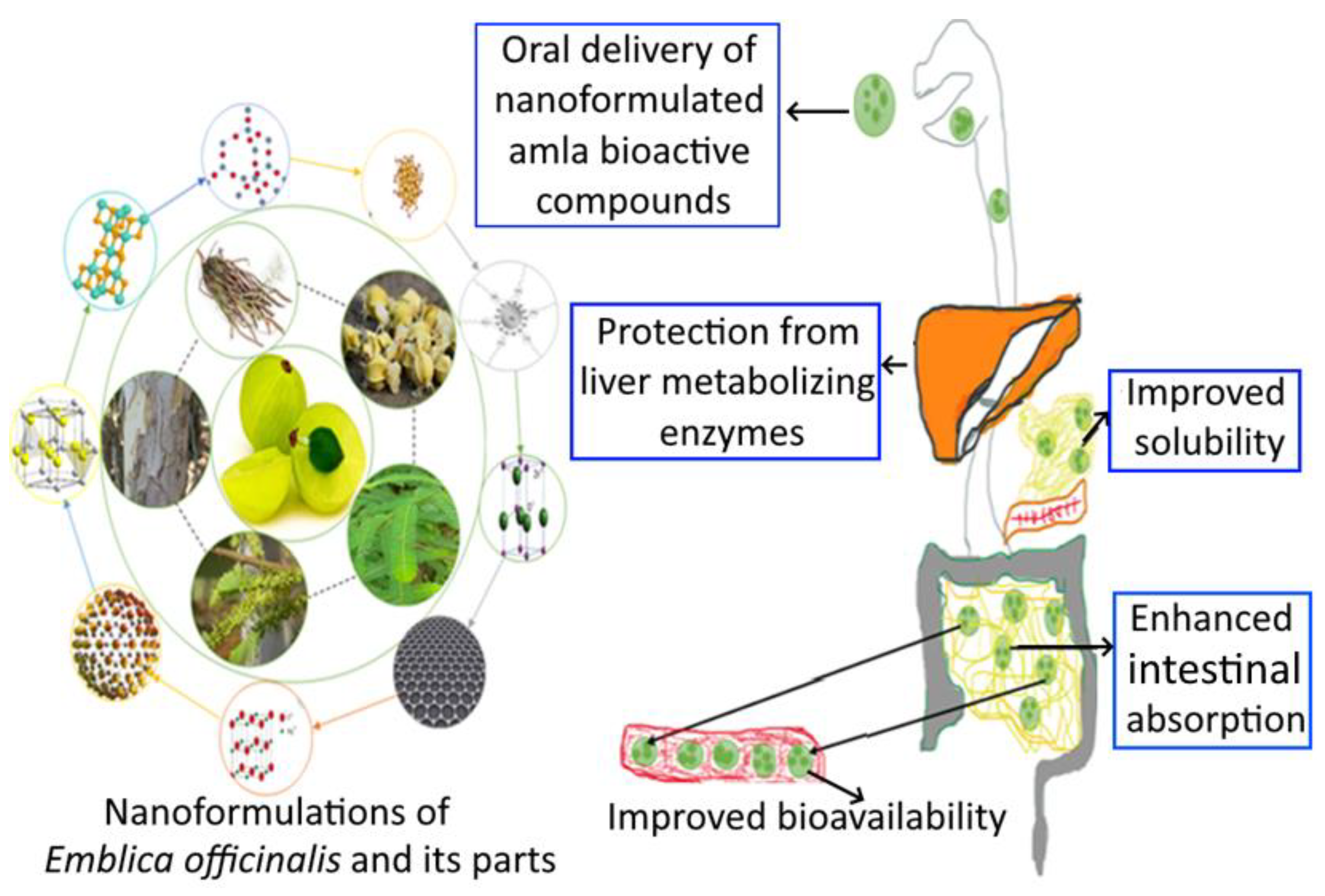
3.5. Nanoformulation of Emblica officinalis and Its Applications
3.6. Health Care Application for Emblicanin-A and Emblicanin-B Nanoformulation
3.7. Adversity and Toxicity of Nanoformulations
3.8. Correlation between Microbiota Bioactivity and Bioavailability of Functional Compounds: Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ezeh, K.J.; Ezeudemba, O. Hyperlipidemia: A Review of the Novel Methods for the Management of Lipids. Cureus 2021, 13. [Google Scholar] [CrossRef]
- El-Tantawy, W.H.; Temraz, A. Natural products for controlling hyperlipidemia: Review. Arch. Physiol. Biochem. 2019, 125, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Mirunalini, S.; Krishnaveni, M. Therapeutic potential of Phyllanthus emblica (amla): The ayurvedic wonder. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 93–105. [Google Scholar] [CrossRef]
- Muzaffar, K.; Sofi, S.A.; Makroo, H.A.; Majid, D.; Dar, B.N. Insight about the biochemical composition, postharvest processing, therapeutic potential of Indian gooseberry (amla), and its utilization in development of functional foods—A comprehensive review. J. Food Biochem. 2022, e14132. [Google Scholar]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Emblica officinalis (Amla): A review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol. Res. 2016, 111, 180–200. [Google Scholar] [CrossRef]
- Brahm, A.J.; Hegele, R.A. Combined hyperlipidemia: Familial but not (usually) monogenic. Curr. Opin. Lipidol. 2016, 27, 131–140. [Google Scholar] [CrossRef]
- Abo-Zalam, H.B.; El-Denshary, E.S.; Abdelsalam, R.M.; Khalil, I.A.; Khattab, M.M.; Hamzawy, M.A. Therapeutic advancement of simvastatin-loaded solid lipid nanoparticles (SV-SLNs) in treatment of hyperlipidemia and attenuating hepatotoxicity, myopathy and apoptosis: Comprehensive study. Biomed. Pharmacother. 2021, 139, 111494. [Google Scholar] [CrossRef]
- Braich, A.K.; Kaur, G.; Singh, A.; Dar, B.N. Amla essential oil-based nano-coatings of Amla fruit: Analysis of morphological, physiochemical, enzymatic parameters, and shelf-life extension. J. Food Process. Preserv. 2022, 46, e16498. [Google Scholar] [CrossRef]
- Panahi, Y.; Ahmadi, Y.; Teymouri, M.; Johnston, T.P.; Sahebkar, A. Curcumin as a potential candidate for treating hyperlipidemia: A review of cellular and metabolic mechanisms. J. Cell Physiol. 2018, 233, 141–152. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, K.; Mishra, N. Nanoparticulate carrier system: A novel treatment approach for hyperlipidemia. Drug Deliv. 2016, 23, 684–699. [Google Scholar] [CrossRef]
- Nirosha, K.; Divya, M.; Vamsi, S.; Sadiq, M. A review on hyperlipidemia. Int. J. Nov. Trends Pharm. Sci. 2014, 4, 81–92. [Google Scholar]
- Hemphill, L.C. Familial hypercholesterolemia: Current treatment options and patient selection for low-density lipoprotein apheresis. J. Clin. Lipidol. 2010, 4, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care Clin. Off. Pract. 2013, 40, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.-H. Recent Guidelines on the Management of Blood Cholesterol: 2013 ACC/AHA Guidelines and 2014 NICE Draft Guidelines. Korean J. Med. 2014, 87, 142–150. [Google Scholar] [CrossRef]
- Van Lennep, J.E.R.; Westerveld, H.T.; Erkelens, D.W.; van der Wall, E.E. Risk factors for coronary heart disease: Implications of gender. Cardiovasc. Res. 2002, 53, 538–549. [Google Scholar] [CrossRef]
- Hegele, R.A.; Ban, M.R.; Hsueh, N.; Kennedy, B.A.; Cao, H.; Zou, G.Y.; Anand, S.; Yusuf, S.; Huff, M.W.; Wang, J. A polygenic basis for four classical Fredrickson hyperlipoproteinemia phenotypes that are characterized by hypertriglyceridemia. Hum. Mol. Genet. 2009, 18, 4189–4194. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Tao, Y.; Guo, J.; Hu, Y.-M.; Su, Z.-Q. Hypolipidemic effects of chitosan nanoparticles in hyperlipidemia rats induced by high fat diet. Int. Immunopharmacol. 2011, 11, 457–461. [Google Scholar] [CrossRef]
- Okerson, T.; Patel, J.; DiMario, S.; Burton, T.; Seare, J.; Harrison, D.J. Effect of 2013 ACC/AHA Blood Cholesterol Guidelines on Statin Treatment Patterns and Low-Density Lipoprotein Cholesterol in Atherosclerotic Cardiovascular Disease Patients. J. Am. Heart Assoc. 2017, 6, e004909. [Google Scholar] [CrossRef] [PubMed]
- Starc, T.J. Management of hyperlipidemia in children. Prog. Pediatr. Cardiol. 2001, 12, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Simons, L.A. Additive effect of plant sterol-ester margarine and cerivastatin in lowering low-density lipoprotein cholesterol in primary hypercholesterolemia. Am. J. Cardiol. 2002, 90, 737–740. [Google Scholar] [CrossRef]
- Harchaoui, K.E.L.; Visser, M.E.; Kastelein, J.J.P.; Stroes, E.S.; Dallinga-Thie, G.M. Triglycerides and Cardiovascular Risk. Curr. Cardiol. Rev. 2009, 5, 216–222. [Google Scholar] [CrossRef]
- Lee, M.-R.; Lim, C.-J.; Lee, Y.-H.; Park, J.-G.; Sonn, S.K.; Jung, I.-H.; Jeong, S.-J.; Lee, M.; Oh, K.S.; Yang, Y.; et al. The adipokine Retnla modulates cholesterol homeostasis in hyperlipidemic mice. Nat. Commun. 2014, 5, 4410. [Google Scholar] [CrossRef]
- Castilla-Guerra, L.; Fernández-Moreno, M.D.C.; Álvarez-Suero, J. Secondary stroke prevention in the elderly: New evidence in hypertension and hyperlipidemia. Eur. J. Intern. Med. 2009, 20, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care. 2017, 23 (Suppl. S9), 139–148. [Google Scholar]
- Merćep, I.; Strikić, D.; Slišković, A.M.; Reiner, Ž. New Therapeutic Approaches in Treatment of Dyslipidaemia—A Narrative Review. Pharmaceuticals 2022, 15, 839. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.F.; Bordoni, B. Hyperlipidemia; StatPearls: Tampa, FL, USA, 2022. [Google Scholar]
- Koshy, S.M.; Bobby, Z.; Jacob, S.E.; Ananthanarayanan, P.H.; Sridhar, M.G.; Paulose, D.T. Amla prevents fructose-induced hepatic steatosis in ovariectomized rats: Role of liver FXR and LXRα. Climacteric 2015, 18, 299–310. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants′ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Hasan, M.R.; Islam, M.N.; Islam, M.R. Phytochemistry, pharmacological activities and traditional uses of Emblica officinalis: A review. Int. Curr. Pharm. J. 2016, 5, 14–21. [Google Scholar] [CrossRef]
- Pria, F.F.; Islam, M.S. Phyllanthus emblica Linn. (Amla)—A Natural Gift to Humans: An Overview. J. Dis. Med. Plants 2019, 5, 1–9. [Google Scholar]
- Rai, N.; Tiwari, L.; Sharma, R.K.; Verma, A.K. Pharmaco-botanical Profile on Emblica officinalis Gaertn.–A Pharmacopoeial Herbal Drug. Res. Rev. J. Bot. 2012, 11, 62. [Google Scholar]
- Gantait, S.; Mahanta, M.; Bera, S.; Verma, S.K. Advances in biotechnology of Emblica officinalis Gaertn. syn. Phyllanthus emblica L.: A nutraceuticals-rich fruit tree with multifaceted ethnomedicinal uses. 3 Biotech 2021, 11, 62. [Google Scholar] [CrossRef]
- Khan, K.H. Roles of Emblica officinalis in medicine—A review. Bot. Res. Int. 2009, 2, 218–228. [Google Scholar]
- Singh, E.; Sharma, S.; Pareek, A.; Dwivedi, J.; Yadav, S.; Sharma, S. Phytochemistry, traditional uses and cancer chemopreventive activity of Amla (Phyllanthus emblica): The Sustainer. J. Appl. Pharma. Sci. 2011, 2, 176–183. [Google Scholar]
- Bhandari, P.; Kamdod, M. Emblica officinalis (Amla): A review of potential therapeutic applications. Int. J. Green Pharm. 2012, 6, 257. [Google Scholar] [CrossRef]
- Fitriansyah, S.N.; Aulifa, D.L.; Febriani, Y.; Sapitri, E. Correlation of total phenolic, flavonoid and carotenoid content of Phyllanthus emblica extract from Bandung with DPPH scavenging activities. Pharmacog. J. 2018, 10, 447–452. [Google Scholar] [CrossRef]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Anola (Emblica officinalis): Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas; Hussain, S.Z., Naseer, B., Qadri, T., Fatima, T., Bhat, T.A., Eds.; Springer: Cham, Switzerland, 2021; pp. 193–206. ISBN 30755027_15. [Google Scholar]
- Kc, Y.; Rayamajhi, S.; Dangal, A.; Shiwakoti, L.D. Phytochemical, Nutritional, Antioxidant Activity and Sensorial Characteristics of Amala (Phyllanthus emblica L.) Chutney. Asian Food Sci. J. 2020, 18, 43–52. [Google Scholar] [CrossRef]
- Bhagat, M. Indian gooseberry (Emblica officinalis): Pharmacognosy review. In Utilisation and Management of Medicinal Plants; Gupta, A., Kaul, V.K., Eds.; Daya Publishing House: New Delhi, India, 2014; pp. 471–487. [Google Scholar]
- Bansal, V.; Sharma, A.; Ghanshyam, C.; Singla, M.L. Rapid HPLC Method for determination of vitamin C, phenolic acids, hydroxycinnamic acid, and flavonoids in seasonal samples of Emblica officinalis juice. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 619–624. [Google Scholar] [CrossRef]
- Zhang, L.-Z.; Zhao, W.-H.; Guo, Y.-J.; Tu, G.-Z.; Lin, S.; Xin, L.-G. Studies on chemical constituents in fruits of Tibetan medicine Phyllanthus emblica. China J. Chin. Mater. Medica 2003, 28, 940–943. [Google Scholar]
- Zhang, Y.-J.; Abe, T.; Tanaka, T.; Yang, C.-R.; Kouno, I. Two New Acylated Flavanone Glycosides from the Leaves and Branches of Phyllanthus emblica. Chem. Pharm. Bull. 2002, 50, 841–843. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Nagao, T.; Tanaka, T.; Yang, C.-R.; Okabe, H.; Kouno, I. Antiproliferative Activity of the Main Constituents from Phyllanthus emblica. Biol. Pharm. Bull. 2004, 27, 251–255. [Google Scholar] [CrossRef]
- Naik, G.H.; Priyadarsini, K.I.; Bhagirathi, R.G.; Mishra, B.; Mishra, K.P.; Banavalikar, M.M.; Mohan, H. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother. Res. 2005, 19, 582–586. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Chatterjee, A.; Ghosal, S.; Bhattacharya, S.K. Antioxidant activity of active tannoid principles of Emblicaofficinalis (amla). Indian J. Exp. Biol. 1999, 37, 676–680. [Google Scholar] [PubMed]
- Habib-ur-Rehman; Yasin, K.A.; Choudhary, M.A.; Khaliq, N.; Atta-ur-Rahman; Choudhary, M.I.; Malik, S. Studies on the chemical constituents of Phyllanthus emblica. Nat. Prod. Res. 2007, 21, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Kumar, V.; Sharma, H.K. Physical and chemical characteristics of different cultivars of Indian gooseberry (Emblica officinalis). J. Food Sci. Technol. 2019, 56, 1641–1648. [Google Scholar] [CrossRef]
- Parveen, K.; Khatkar, B.S. Physico-chemical properties and nutritional composition of aonla (Emblica officinalis) varieties. Int. Food Res. J. 2015, 22, 2358–2363. [Google Scholar]
- Srinivasan, P.; Vijayakumar, S.; Kothandaraman, S.; Palani, M. Anti-diabetic activity of quercetin extracted from Phyllanthus emblica L. fruit: In silico and in vivo approaches. J. Pharm. Anal. 2018, 8, 109–118. [Google Scholar]
- Zhang, Y.-J.; Tanaka, T.; Yang, C.-R.; Kouno, I. New phenolic constituents from the fruit juice of Phyllanthus emblica. Chem. Pharm. Bull. 2001, 49, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Tanaka, T.; Iwamoto, Y.; Yang, C.R.; Kouno, I. Novel sesquiterpenoids from the roots of Phyllanthus emblica. J. Nat. Prod. 2001, 64, 870–873. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Tanaka, T.; Iwamoto, Y.; Yang, C.R.; Kouno, I. Novel norsesquiterpenoids from the roots of Phyllanthus emblica. J Nat. Prod. 2000, 63, 1507–1510. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Tanaka, T.; Iwamoto, Y.; Yang, C.-R.; Kouno, I. Phyllaemblic acid, a novel highly oxygenated norbisabolane from the roots of Phyllanthus emblica. Tetrahedron Lett. 2000, 41, 1781–1784. [Google Scholar] [CrossRef]
- Baliga, M.S.; Shivashankara, A.R.; Thilakchand, K.R.; Baliga-Rao, M.P.; Palatty, P.L.; George, T.; Rao, S. Hepatoprotective effects of the Indian Gooseberry (Emblica officinalis Gaertn): A revisit. In Dietary Interventions in Liver Disease: Foods, Nutrients, and Dietary Supplements; Watson, R.S., Preedy, V.R., Eds.; Academic Press: Amsterdam, The Netherlands, 2019; pp. 193–201. [Google Scholar]
- Qi, W.-Y.; Li, Y.; Hua, L.; Wang, K.; Gao, K. Cytotoxicity and structure activity relationships of phytosterol from Phyllanthus emblica. Fitoterapia 2013, 84, 252–256. [Google Scholar] [CrossRef]
- Chugh, C.A.; Bharti, D. Chemical characterization of antifungal constituents of Emblica officinalis. Allelopath. J. 2014, 34, 155–178. [Google Scholar]
- Nambiar, S.S.; Paramesha, M.; Shetty, N.P. Comparative analysis of phytochemical profile, antioxidant activities and foam prevention abilities of whole fruit, pulp and seeds of Emblica officinalis. J. Food Sci. Technol. 2015, 52, 7254–7262. [Google Scholar] [CrossRef]
- Goyal, R.; Patel, S. Emblica officinalis Geart.: A Comprehensive Review on Phytochemistry, Pharmacology and Ethnomedicinal Uses. Res. J. Med. Plant 2012, 6, 6–16. [Google Scholar] [CrossRef]
- Priya, G.; Parminder, N.; Jaspreet, S. Antimicrobial and antioxidant activity on Emblica officinalis seed extract. Int. J. Res. Ayur. Pharma. 2012, 3, 591–596. [Google Scholar]
- Usha, T.; Middha, S.K.; Goyal, A.K.; Lokesh, P.; Yardi, V.; Mojamdar, L.; Keni, D.S.; Babu, D. Toxicological Evaluation of Emblica officinalis Fruit Extract and its Anti-inflammatory and Free Radical Scavenging Properties. Pharmacogn. Mag. 2015, 11, S427–S433. [Google Scholar] [CrossRef]
- Satish, S.; Mohana, D.C.; Raghavendra, M.P.; Raveesha, K.A. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. J. Agric. Technol. 2007, 3, 109–119. [Google Scholar]
- Saini, R.; Sharma, N.; Oladeji, O.S.; Sourirajan, A.; Dev, K.; Zengin, G.; El-Shazly, M.; Kumar, V. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114570. [Google Scholar] [CrossRef]
- Patil, S.G.; Deshmukh, A.A.; Padol, A.R.; Kale, D.B. In vitro antibacterial activity of Emblica officinalis fruit extract by tube Dilution Method. Int. J. Toxicol. Appl. Pharmacol. 2012, 2, 49–51. [Google Scholar]
- Kamal, R.; Yadav, S.; Mathur, M.; Katariya, P. Antiradical efficiency of 20 selected medicinal plants. Nat. Prod. Res. 2012, 26, 1054–1062. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, S.; Xiang, S.; Guo, C.W.; Ge, F.; Yang, C.R.; Zhang, Y.; Wang, Y.; Kitazato, K. Antiviral activity and possible mechanisms of action of pentagalloylglucose (PGG) against influenza A virus. Arch. Virol. 2011, 156, 1359–1369. [Google Scholar] [CrossRef]
- Usharani, P.; Merugu, P.L.; Nutalapati, C. Evaluation of the effects of a standardized aqueous extract of Phyllanthus emblica fruits on endothelial dysfunction, oxidative stress, systemic inflammation and lipid profile in subjects with metabolic syndrome: A randomised, double blind, placebo controlled clinical study. BMC Complement. Altern. Med. 2019, 19, 97. [Google Scholar]
- Malik, S.; Suchal, K.; Bhatia, J.; Khan, S.I.; Vasisth, S.; Tomar, A.; Goyal, S.; Kumar, R.; Arya, D.S.; Ojha, S.K. Therapeutic potential and molecular mechanisms of Emblica officinalis gaertn in countering nephrotoxicity in rats induced by the chemotherapeutic agent cisplatin. Front. Pharmacol. 2016, 7, 350. [Google Scholar] [CrossRef]
- Purena, R.; Seth, R.; Bhatt, R. Protective role of Emblica officinalis hydro-ethanolic leaf extract in cisplatin induced nephrotoxicity in Rats. Toxicol. Rep. 2018, 5, 270–277. [Google Scholar] [CrossRef]
- Dhingra, D.; Joshi, P.; Gupta, A.; Chhillar, R. Possible Involvement of Monoaminergic Neurotransmission in Antidepressant-like activity of Emblica officinalis Fruits in Mice. CNS Neurosci. Ther. 2012, 18, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Suja, R.S.; Nair, A.M.C.; Sujith, S.; Preethy, J.; Deepa, A.K. Evaluation of immunomodulatory potential of Emblica officinalis fruit pulp extract in mice. Indian J. Anim. Res. 2009, 43, 103–106. [Google Scholar]
- Ansari, A.; Shahriar, S.Z.; Hassan, M.; Das, S.R.; Rokeya, B.; Haque, A.; Haque, E.; Biswas, N.; Sarkar, T. Emblica officinalis improves glycemic status and oxidative stress in STZ induced type 2 diabetic model rats. Asian Pac. J. Trop. Med. 2014, 7, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Ramzan, A.; Ali, A.; Ahmad, M. Effect of Amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int. J. Food Sci. Nutr. 2011, 62, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Gopa, B.; Bhatt, J.; Hemavathi, K.G. A comparative clinical study of hypolipidemic efficacy of Amla (Emblica officinalis) with 3- hydroxy-3-methylglutaryl-coenzyme-a reductase inhibitor simvastatin. Indian J. Pharmacol. 2012, 44, 238. [Google Scholar]
- Variya, B.C.; Bakrania, A.K.; Chen, Y.; Han, J.; Patel, S.S. Suppression of abdominal fat and anti-hyperlipidemic potential of Emblica officinalis: Upregulation of PPARs and identification of active moiety. Biomed. Pharmacother. 2018, 108, 1274–1281. [Google Scholar] [CrossRef]
- Husain, I.; Zameer, S.; Madaan, T.; Minhaj, A.; Ahmad, W.; Iqubaal, A.; Ali, A.; Najmi, A.K. Exploring the multifaceted neuroprotective actions of Emblica officinalis (Amla): A review. Metab. Brain Dis. 2019, 34, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Shubhi, M.; Rohitash, J.; Radhey, S.; Dharmendra, K.M.; Kshipra, M.; Rajashree, P.; De, R.; Mukhopadhyay, A.; Srivastava, A.K.; Shoma, P.N. Anti-Helicobacter pylori and antioxidant properties of Emblica officinalis pulp extract: A potential source for therapeutic use against gastric ulcer. J. Med. Plants Res. 2011, 5, 2577–2583. [Google Scholar]
- Al-Rehaily, A.J.; Al-Howiriny, T.S.; Al-Sohaibani, M.O.; Rafatullah, S. Gastroprotective effects of ‘Amla’Emblica officinalis on in vivo test models in rats. Phytomedicine 2002, 9, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Santoshkumar, J.; Manjunath, S.; Sakhare, P.M. A study of anti-hyperlipedemia, hypolipedemic and anti-atherogenic activity of fruit of Emblica officinalis (amla) in high fat fed Albino rats. Int. J. Med. Res. Health Sci. 2013, 2, 70–77. [Google Scholar]
- Kapoor, M.P.; Suzuki, K.; Derek, T.; Ozeki, M.; Okubo, T. Clinical evaluation of Emblica officinalis Gatertn (Amla) in healthy human subjects: Health benefits and safety results from a randomized, double-blind, crossover placebo-controlled study. Contemp. Clin. Trials Commun. 2020, 17, 100499. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Liu, Z.-W.; Haq, I.U.; Rabail, R.; Faheem, F.; Walayat, N.; Nawaz, A.; Shabbir, M.A.; Munekata, P.E.S.; Lorenzo, J.M.; et al. Functional and Nutraceutical Significance of Amla (Phyllanthus emblica L.): A Review. Antioxidants 2022, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Okubo, T.; Juneja, L.R.; Yokozawa, T. The protective role of amla (Emblica officinalis Gaertn.) against fructose-induced metabolic syndrome in a rat model. Br. J. Nutr. 2010, 103, 502–512. [Google Scholar] [CrossRef]
- Nisar, M.F.; He, J.; Ahmed, A.; Yang, Y.; Li, M.; Wan, C. Chemical components and biological activities of the genus Phyllanthus: A review of the recent literature. Molecule 2018, 23, 2567. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, D.; Singh, H.; Khan, M.U. Emblica officinalis: A meritocratic drug for treating various disorders. Indo Am. J. Pharm. Res. 2013, 3, 2231–6876. [Google Scholar]
- Zahid, S.; Anjum, K.M.; Mughal, M.S.; Yaqub, A.; Yameen, M. Evaluation of antioxidant and antihyperlipidemic activity of Indian gooseberry (Emblica officinalis) fruit in high fat-fed rabbits. JAPS J. Animal Plant Sci. 2018, 28, 1007–1013. [Google Scholar]
- Iyer, U.; Shah, A.; Venugopal, S. Amla (Emblica officinalis) and Guava (Psidium guajava) supplementation: Impact of Low Carbon footprint local seasonal fruits on Lipemic Status of Morning Walkers. Eco. Environ. Cons. 2021, 27, S233–S238. [Google Scholar]
- Sandeep, B.S.; Panda, N.; Sethy, K.; Nath, S. Effect of dietary supplementation of amla (Emblica officinalis) powder and equivalent synthetic vitamin C on growth performance in black rock broiler chicken. Pharma Innov. J. 2022, 11, 2002–2007. [Google Scholar]
- Dabulici, C.M.; Sârbu, I.; Vamanu, E. The Bioactive Potential of Functional Products and Bioavailability of Phenolic Compounds. Foods 2020, 9, 953. [Google Scholar] [CrossRef]
- Taleuzzaman, M.; Mahapatra, D.K.; Gupta, D.K. Emblicanin-A and Emblicanin-B: Pharmacological and Nano-Pharmacotherapeutic Perspective for Healthcare Applications. In Applied Pharmaceutical Practice and Nutraceuticals; Apple Academic Press: Waretown, NJ, USA, 2021; pp. 13–27. [Google Scholar]
- Li, T.; Liang, W.; Xiao, X.; Qian, Y. Nanotechnology, an alternative with promising prospects and advantages for the treatment of cardiovascular diseases. Int. J. Nanomed. 2018, 13, 7349–7362. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Koo, S.Y.; Choi, K.Y. Emerging nanoformulation strategies for phytocompounds and applications from drug delivery to phototherapy to imaging. Bioact. Mater. 2022, 14, 182–205. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.Z.; Momtaz, S.; Bayrami, Z.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of Herbal Extracts in Treatment of Neurodegenerative Disorders. Front. Bioeng. Biotechnol. 2020, 8, 238. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Kapoor-Narula, U.; Lenka, N. Phytochemicals and their nanoformulation in sustained drug delivery and therapy. In Innovations in Fermentation and Phytopharmaceutical Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 181–220. [Google Scholar]
- Enrico, C. Nanotechnology-Based Drug Delivery of Natural Compounds and Phytochemicals for the Treatment of Cancer and Other Diseases. Stud. Nat. Prod. Chem. 2019, 62, 91–123. [Google Scholar] [CrossRef]
- Omran, Z.; Bader, A.; Porta, A.; Vandamme, T.; Anton, N.; Alehaideb, Z.; El-Said, H.; Faidah, H.; Essa, A.; Vassallo, A.; et al. Evaluation of Antimicrobial Activity of Triphala Constituents and Nanoformulation. Evid.-Based Complement. Altern. Med. 2020, 2020, 6976973. [Google Scholar] [CrossRef]
- Rosarin, S.; Fathima, S.; Vadivel, A.; Samuthira, N.; Sankaran, M. Antiproliferative effect of silver nanoparticles synthesized using amla on Hep2 cell line. Asian Pac. J. Trop Med. 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Abitha, S.T.; Rajeshkumar, S.; Lakshmi, T.; Roy, A. Cytotoxic effects of silver nanoparticles synthesized using amla fruit seed. Drug Invent. Today 2019, 1, 11. [Google Scholar]
- Soundarajan, S.; Sankari, M.; Rajeshkumar, S. Antibacterial activity and cytotoxicity of amla seed mediated graphene oxide, silver nanoparticle and go-ag nanoparticle-an in vitro study. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 55–67. [Google Scholar]
- Ranjani, S.; Hemalatha, S. Triphala decorated multipotent green nanoparticles and its applications. Mater. Lett. 2021, 308, 131184. [Google Scholar] [CrossRef]
- Naik, S.; Jarnain, T.; David, N. Phytofabrication of silver and zinc oxide nanoparticles using the fruit extract of Phyllanthus emblica and its potential anti-diabetic and anti-cancer activity. Part Sci. Technol. 2022, 1–13. [Google Scholar] [CrossRef]
- Harakeh, S.; Mohammed, A.; Al Soad, J.; Saad, A.; Saber, H.; Al Turki, A.; Najiah; Azhar, E. Antidiabetic effects of novel ellagic acid nanoformulation: Insulin-secreting and anti-apoptosis effects. Saudi J. Biol. Sci. 2020, 27, 3474–3480. [Google Scholar] [CrossRef]
- Hosny, K.M.; Rizg, W.Y.; Khallaf, R.A. Preparation and Optimization of In Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity. Pharmaceutics 2020, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Suresh, K. Chitosan and glyceryl monooleate nanostructures containing gallic acid isolated from amla fruit: Targeted delivery system. Heliyon 2021, 7, e06526. [Google Scholar] [CrossRef]
- Alfei, S.; Barbara, M.; Guendalina, Z.; Federica, T.; Cinzia, D. Dendrimer nanodevices and gallic acid as novel strategies to fight chemoresistance in neuroblastoma cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]
- Khan, B.A.; Mahmood, T.; Menaa, F.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; Ray, S.D. New perspectives on the efficacy of gallic acid in cosmetics & nanocosmeceuticals. Curr. Pharm. Des. 2018, 24, 5181–5187. [Google Scholar]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Parodi, B.; Zuccari, G.; Pittaluga, A.M.; Boggia, R. Preparation of ellagic acid micro and nano formulations with amazingly increased water solubility by its entrapment in pectin or non-PAMAM dendrimers suitable for clinical applications. New J. Chem. 2019, 43, 2438–2448. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from “oxidative stress”. Drug Deliv. Transl. Res. 2019, 10, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Mady, F.; Ibrahim, S.R.-M. Cyclodextrin-based nanosponge for improvement of solubility and oral bioavailability of Ellagic acid. Pak. J. Pharm. Sci. 2018, 31, 2069–2076. [Google Scholar] [PubMed]
- Rosman, R.; Saifullah, B.; Maniam, S.; Dorniani, D.; Hussein, M.Z.; Fakurazi, S. Improved Anticancer Effect of Magnetite Nanocomposite Formulation of Gallic Acid (Fe3 O4 -PEG-GA) Against Lung, Breast and Colon Cancer Cells. Nanomaterials 2018, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Klooster, S.T.; Villeneuve, P.; Bourlieu-Lacanal, C.; Durand, E.; Schroën, K.; Berton-Carabin, C. Alkyl chain length modulates antioxidant activity of gallic acid esters in spray-dried emulsions. Food Chem. 2022, 387, 132880. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Das, R.J.; Saikia, R.; Sahariah, J.J.; Pathak, H.; Sarma, H.; Das, A. Design and fabrication of gallic acid loaded chitosan nanoformulation. Drug Deliv. Lett. 2022, 12, 135–148. [Google Scholar] [CrossRef]
- Harakeh, S.; Qari, M.; Rajeh, N.; Ali, S.; El-Shitany, N.; Hassan, S.; Abd-Allah, E.A.; Tashkandi, H.; Malik, M.F.A.; Aljabri, F.K.; et al. Ellagic acid nanoparticles attenuate oxidative stress and testicular damage in high fat Diet/Streptozotocin-Induced diabetic rats. J. King Saud Univ. Sci. 2022, 34, 101720. [Google Scholar] [CrossRef]
- Dayar, E.; Pechanova, O. Targeted Strategy in Lipid-Lowering Therapy. Biomedicines 2022, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Chakraborty, P.; Mukherjee, B.; De Feo, V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. Int. J. Mol. Sci. 2020, 21, 2217. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, Y.D.; Hajialyani, M.; Naseri, R.; Hesari, M.; Mohammadi, P.; Stefanucci, A.; Mollica, A.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomed. 2019, 14, 5303–5321. [Google Scholar] [CrossRef]
- Martakov, I.S.; Shevchenko, O.G.; Torlopov, M.A.; Gerasimov, E.Y.; Sitnikov, P.A. Formation of gallic acid layer on γ-AlOOH nanoparticles surface and their antioxidant and membrane-protective activity. J. Inorg. Biochem. 2019, 199, 110782. [Google Scholar] [CrossRef]
- Puttasiddaiah, R.; Lakshminarayana, R.; Somashekar, N.L.; Gupta, V.K.; Inbaraj, B.S.; Usmani, Z.; Raghavendra, V.B.; Sridhar, K.; Sharma, M. Advances in Nanofabrication Technology for Nutraceuticals: New Insights and Future Trends. Bioengineering 2022, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Vamanu, E. Polyphenolic Nutraceuticals to Combat Oxidative Stress Through Microbiota Modulation. Front. Pharmacol. 2019, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Scotti, E.; Boué, S.; Sasso, G.L.; Zanetti, F.; Belcastro, V.; Poussin, C.; Sierro, N.; Battey, J.; Gimalac, A.; Ivanov, N.V.; et al. Exploring the microbiome in health and disease. Toxicol. Res. Appl. 2017, 1, 1–37. [Google Scholar] [CrossRef]
- Guirro, M.; Costa, A.; Gual-Grau, A.; Herrero, P.; Torrell, H.; Canela, N.; Arola, L. Effects from diet-induced gut microbiota dysbiosis and obesity can be ameliorated by fecal microbiota transplantation: A multiomics approach. PLoS ONE 2019, 14, e0218143. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Mileo, A.M.; Nisticò, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Vamanu, E.; Gatea, F. Correlations between Microbiota Bioactivity and Bioavailability of Functional Compounds: A Mini-Review. Biomedicines 2020, 8, 39. [Google Scholar] [CrossRef]
- Yu, J.B.; Zhao, Z.X.; Peng, R.; Pan, L.B.; Fu, J.; Ma, S.R.; Han, P.; Cong, L.; Zhang, Z.W.; Sun, L.X.; et al. GutMicrobiota-Based Pharmacokinetics and the Antidepressant Mechanism of Paeoniflorin. Front. Pharmacol. 2019, 10, 268. [Google Scholar] [CrossRef]
- Frolinger, T.; Sims, S.; Smith, C.; Wang, J.; Cheng, H.; Faith, J.; Ho, L.; Hao, K.; Pasinetti, G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019, 9, 3546. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Luo, Z.; Zhou, J.; Sun, B. Gut Microbiota and Antidiabetic Drugs: Perspectives of Personalized Treatment in Type 2 Diabetes Mellitus. Front. Cell Infect. Microbiol. 2022, 12, 685. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef] [PubMed]

| Type | Lipoprotein Abnormality | Total Cholesterol | LDL Cholesterol | Plasma TGs | Clinical Manifestation | Population Prevalence |
|---|---|---|---|---|---|---|
| Familia chylomicronemia (HLP type 1) | Excess chylomicrons | Elevated | Low or normal | Elevated | Lipemia retinalis, focal neurologic symptoms, failure to thrive, recurrent epigastric pain, hepatosplenomegaly | 1 in 1 million |
| Combined hyperlipidemia (HLP type 2) | Excess LDL and VLDL | Elevated or normal | Elevated | Normal | Physical stigmata such as xanthomas or xanthelasmas are rare | 1 in 40 |
| Dysbetalipidemia (HLP type 3) | Excess IDL elevated chylomicron remnants | Elevated | Low or normal | Elevated | Tuberous and palmar xanthomata elevations in atherogenic IDL leads to increased risk for CVD | 1 in 10,000 |
| Primary simple hyperlipidemia (HLP type 4) | Excess VLDL | Elevated or normal | Normal | Elevated | Associated with increased risk of obesity, CVD, DM2, hypertension, insulin resistance, and hyperuricemia | 1 in 20 |
| Primary mixed hyperlipidemia (HLP type 5) | Excess chylomicron and VLDL | Elevated | Normal | Elevated | Similar clinical manifestation as type 1 but develops in adulthood | 1 in 600 |
| Kingdom | Plantae (Plants) |
|---|---|
| Subkingdom | Tracheobionta (vascular plants) |
| Super division | Spermatophyta (seed plants) |
| Division | Angiospermae (flowering plants) |
| Class | Magnoliopsida |
| Subclass | Rosidae |
| Order | Euphorbiales |
| Family | Euphorbiaceae |
| Genus | Emblica |
| Species | officinalis Geartn |
| Feature | Description |
|---|---|
| Habitat | Central and southern India, Pakistan, Bangladesh, Sri Lanka, Malaysia, southern China, the Mascarene Islands, Southeast Asia, and Uzbekistan. |
| Appearance | Medium sized deciduous tree, 8–18 m height, with thin light gray bark exfoliating in small, thin, irregular flakes. |
| Used parts | Dried fruits, fresh fruit, seed, leaves, root bark, and flowers. |
| Leaves | Simple, subsessile, closely set along the branchlets, light green, having the appearance of pinnate leaves. |
| Fruits | 15–20 mm long and 18–25 mm wide, nearly spherical or globular, wider than long and with a small and slight conic depression on both apexes. Mesocarp is yellow, and endocarp is yellowish brown in ripened condition. |
| Globose, fleshy, pale yellow with six obscure vertical furrows enclosing six trigonous seeds in three 2-seeded crustaceous cocci. | |
| Seedlings start bearing fruits in 7–8 years after planting, while the budded clones will start bearing fruits from the 5th year onward. | |
| Fresh fruits are light green, and ripe fruits turn light brown in color. The average weight of the fruit is 60–70 g. | |
| Flowers | Greenish yellow, in axillary fascicles, unisexual, males numerous on short slender pedicels, females few, subsessile, ovary 3-celled. |
| Seeds | Four to six, smooth, dark brown. |
| Barks | Thick to 12 mm, shining grayish brown or grayish green |
| Flowering and fruiting | February–May and December–January |
| Edible part | Mesocarp and endocarp that forms the hard stone which encages the seed |
| Chemical Components | Amount |
|---|---|
| Fruits: moisture (%) | 81.20 |
| Protein (%) | 0.50 |
| Fat (%) | 0.10 |
| Mineral matter (%) | 0.10 |
| Fiber (%) | 3.40 |
| Carbohydrate (%) | 14.10 |
| Bulk elements (mg/100 g) | Net weight |
| Calcium (%) | 0.05 |
| Phosphorus (%) | 0.02 |
| Iron (mg/100 g) | 1.20 |
| Vitamin C (mg/100 g) | 600 |
| Nicotinic acid (mg/100 g) | 0.20 |
| Source | Main Active Compounds | Biological Activity |
|---|---|---|
| Fruit | Gallic acid | Cardioprotective, anti-hyperlipidemia |
| Fruit | Ellagic acid | Cardioprotective, anti-hyperlipidemia |
| Fruit | Emblicanin A and B | Cardioprotective, anti-hyperlipidemia |
| Fruit | Myricetin and Kaempferol | Cardioprotective, anti-hyperlipidemia |
| Fruit | Punigluconin pedunculagin | Cardioprotective, anti-hyperlipidemia |
| Fruit | Chebulagic acid | Cardioprotective, anti-hyperlipidemia |
| Fruit | Geraniin and corilagin | Cardioprotective, anti-hyperlipidemia |
| Fruit | Quercetin and rutin | Anti-inflammatory |
| Fruit | Tannins and gallic acid | Gastrointestinal activity |
| Fruit | Flavonoids | Antidiabetic activity |
| Fruit | Polyphenols | Neuroprotective |
| Fruit | Gallic acid | Anticancer activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachitha, P.; Krishnaswamy, K.; Lazar, R.A.; Gupta, V.K.; Inbaraj, B.S.; Raghavendra, V.B.; Sharma, M.; Sridhar, K. Attenuation of Hyperlipidemia by Medicinal Formulations of Emblica officinalis Synergized with Nanotechnological Approaches. Bioengineering 2023, 10, 64. https://doi.org/10.3390/bioengineering10010064
Rachitha P, Krishnaswamy K, Lazar RA, Gupta VK, Inbaraj BS, Raghavendra VB, Sharma M, Sridhar K. Attenuation of Hyperlipidemia by Medicinal Formulations of Emblica officinalis Synergized with Nanotechnological Approaches. Bioengineering. 2023; 10(1):64. https://doi.org/10.3390/bioengineering10010064
Chicago/Turabian StyleRachitha, Puttasiddaiah, Krupashree Krishnaswamy, Renal Antoinette Lazar, Vijai Kumar Gupta, Baskaran Stephen Inbaraj, Vinay Basavegowda Raghavendra, Minaxi Sharma, and Kandi Sridhar. 2023. "Attenuation of Hyperlipidemia by Medicinal Formulations of Emblica officinalis Synergized with Nanotechnological Approaches" Bioengineering 10, no. 1: 64. https://doi.org/10.3390/bioengineering10010064
APA StyleRachitha, P., Krishnaswamy, K., Lazar, R. A., Gupta, V. K., Inbaraj, B. S., Raghavendra, V. B., Sharma, M., & Sridhar, K. (2023). Attenuation of Hyperlipidemia by Medicinal Formulations of Emblica officinalis Synergized with Nanotechnological Approaches. Bioengineering, 10(1), 64. https://doi.org/10.3390/bioengineering10010064











