The Role of Hydrodynamics on the Sustainable Mussels’ Culture Activity. The Case of the Chalastra Basin (NW Gulf of Thessaloniki)
Abstract
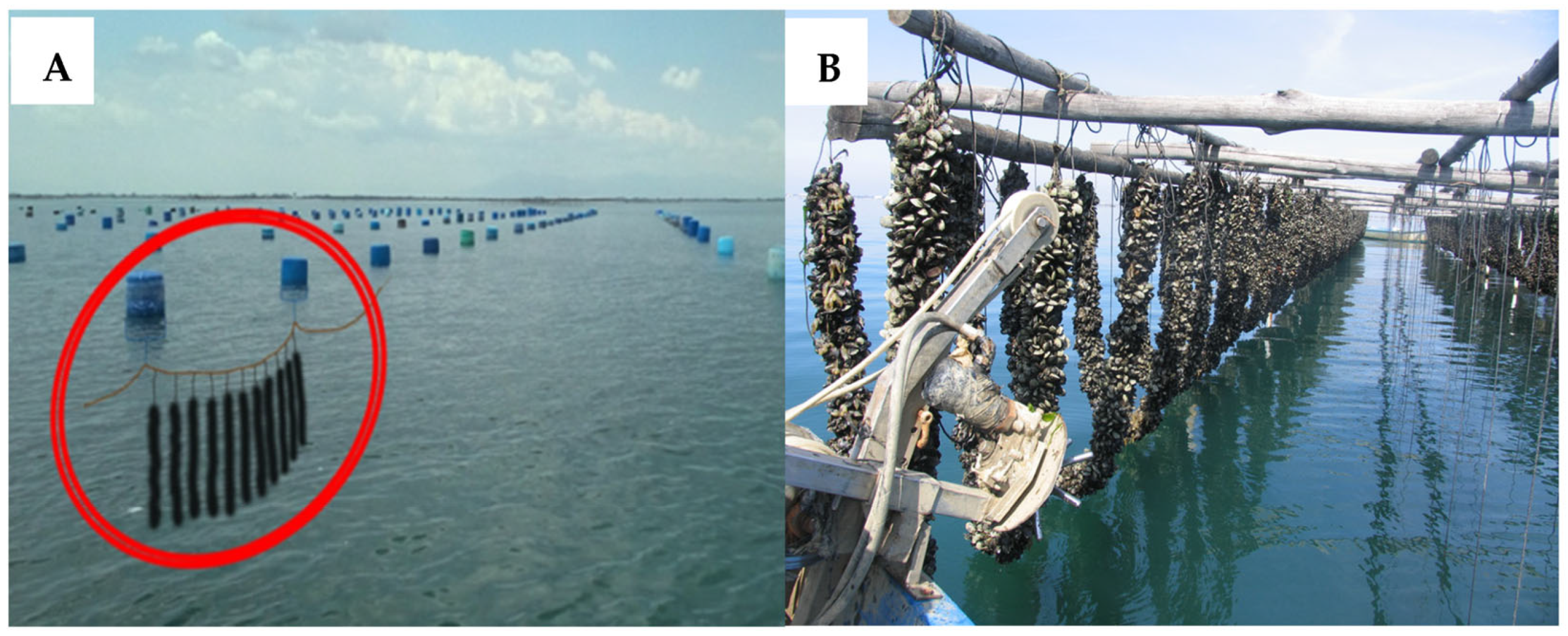
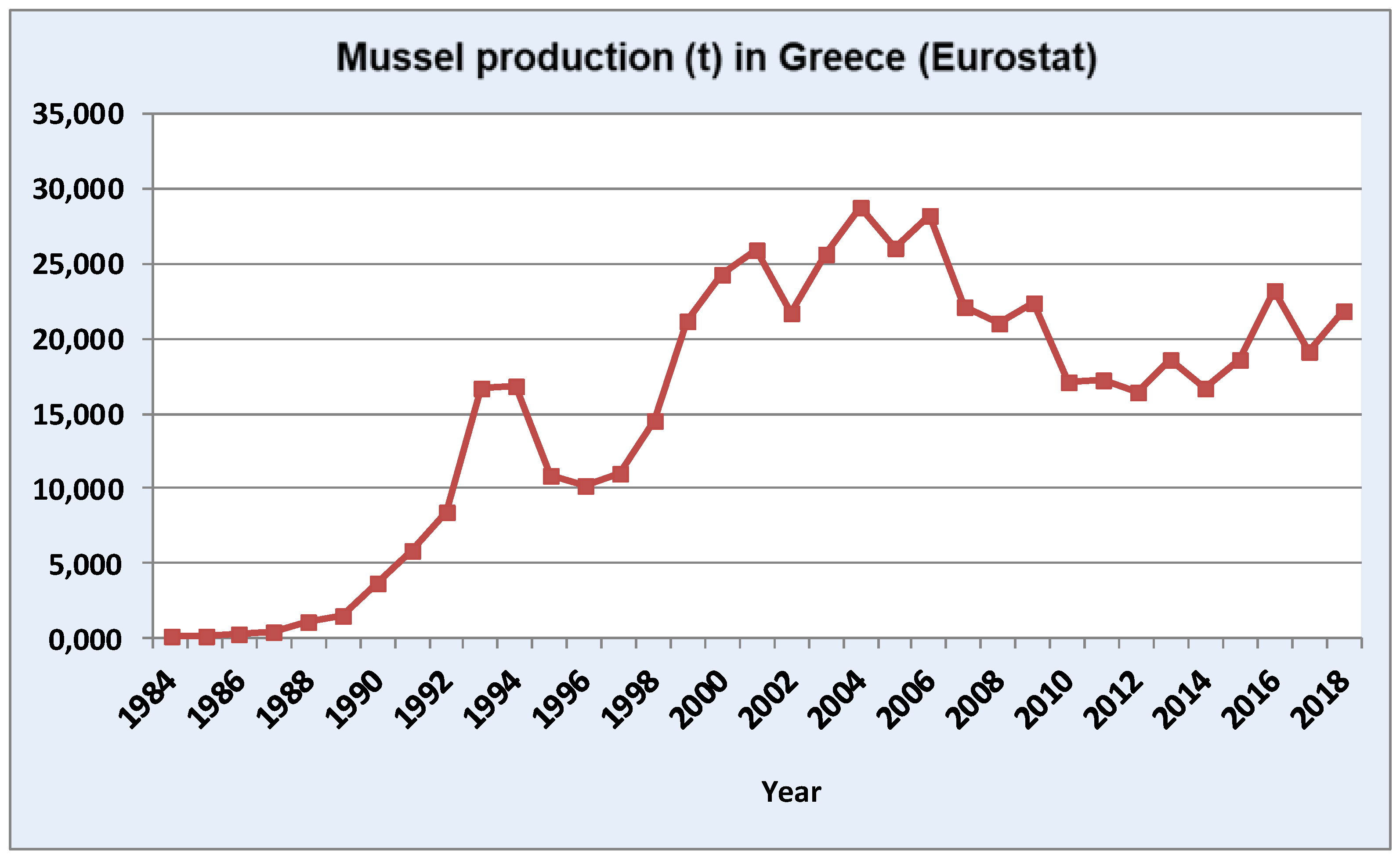
:1. Introduction
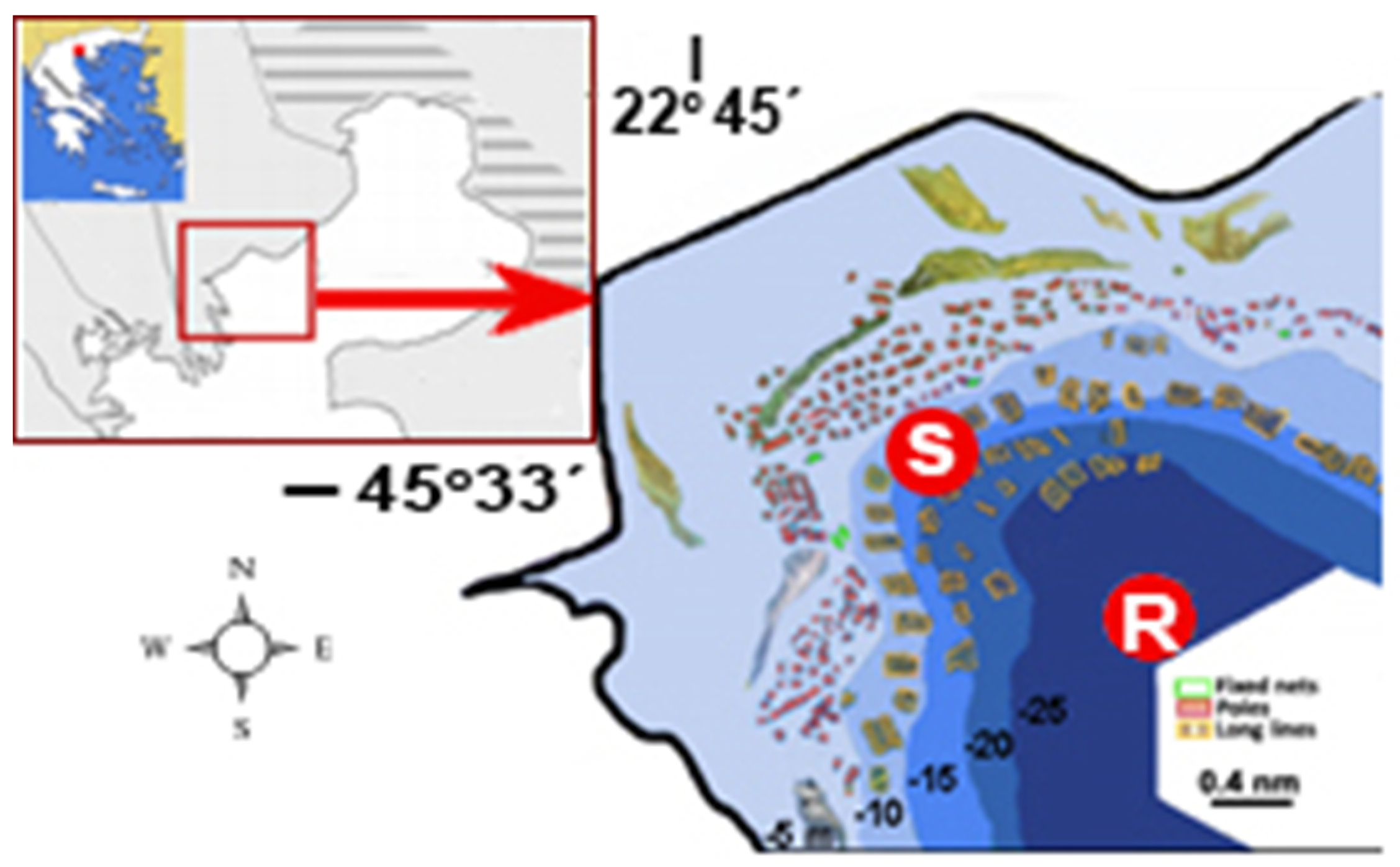
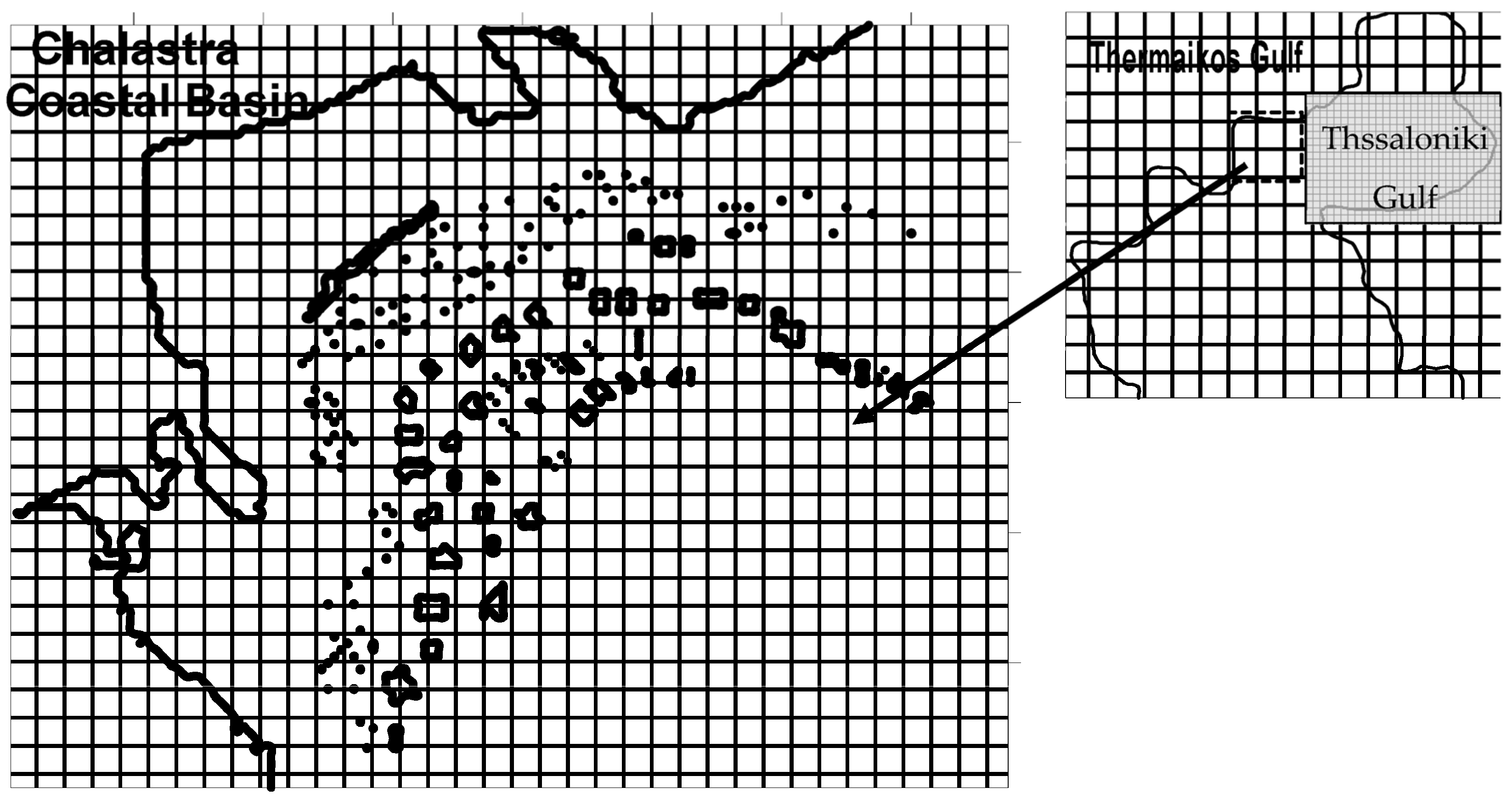
2. Materials and Methods
2.1. Hydrodynamic Model and Simulation
- h: the depth of the water column,
- U, V: vertically averaged horizontal current velocities,
- ζ: surface elevation,
- f: Coriollis parameter,
- τsx, τsy: wind surface shear stresses,
- τbx, τby: bottom shear stresses,
- νh: dispersion coefficient (Smagorinski, 1963),
- ρ: seawater density, and
- g: gravity acceleration.
2.2. Monitoring of Nutrients and Chlorophyll-a
3. Results
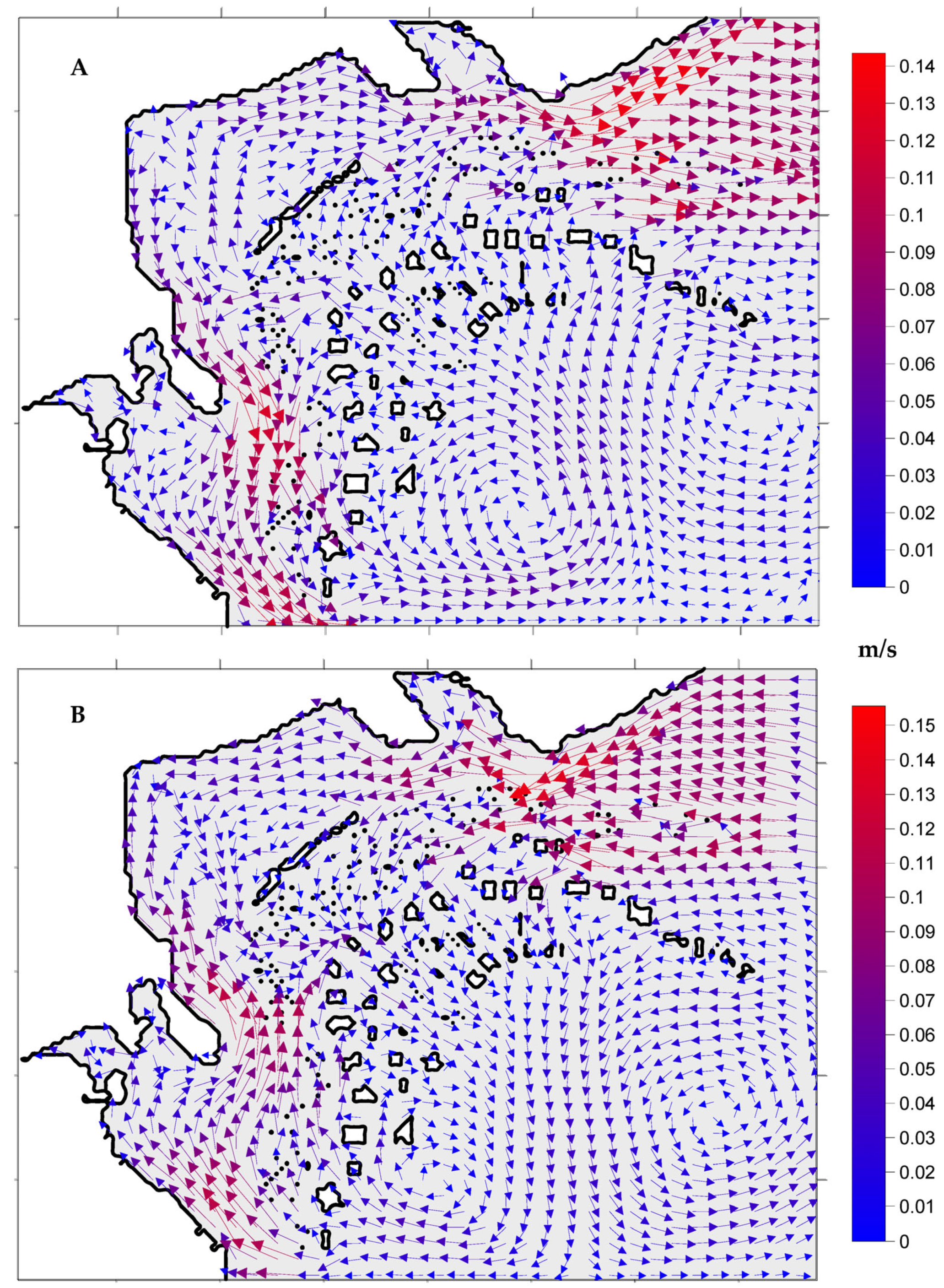
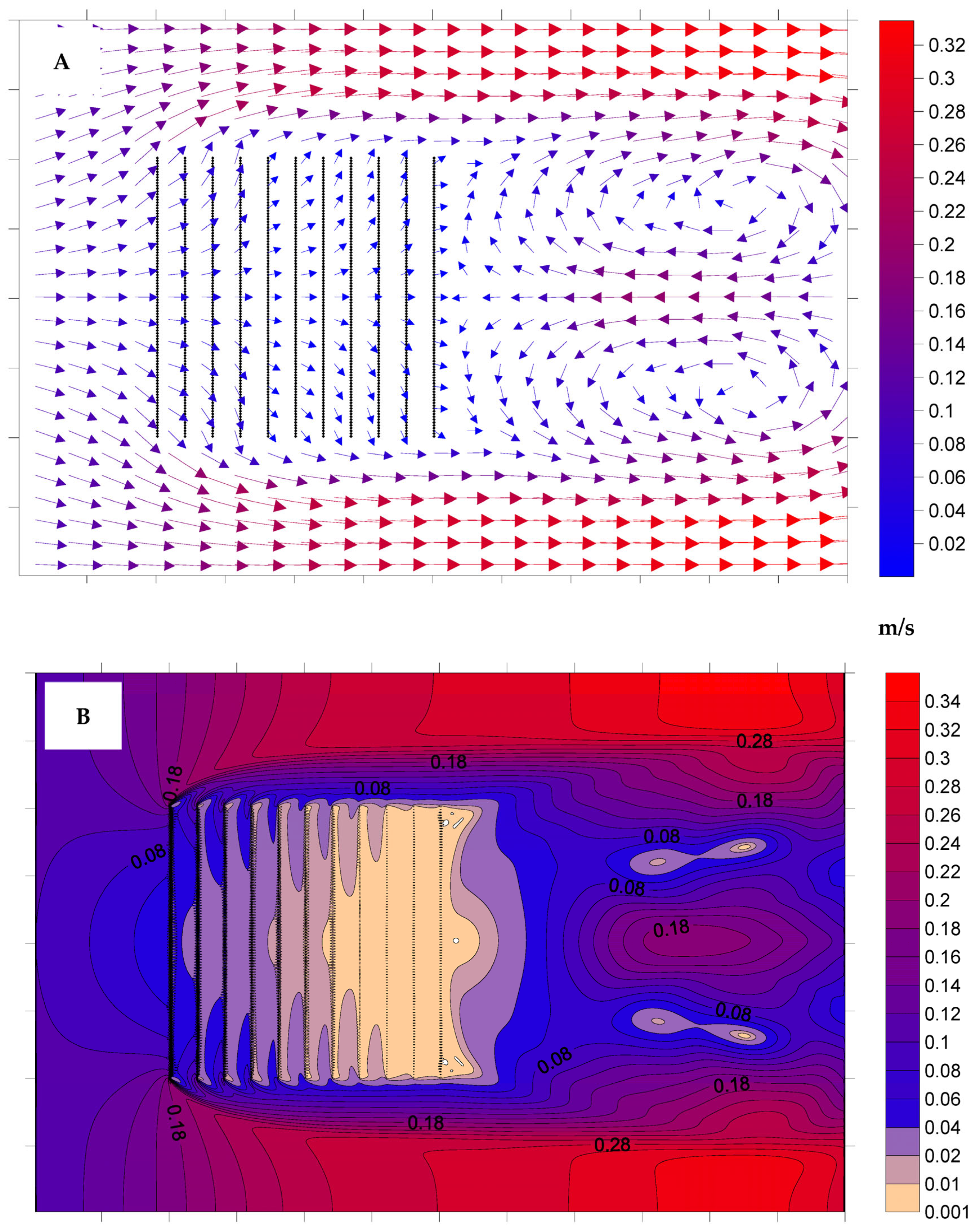
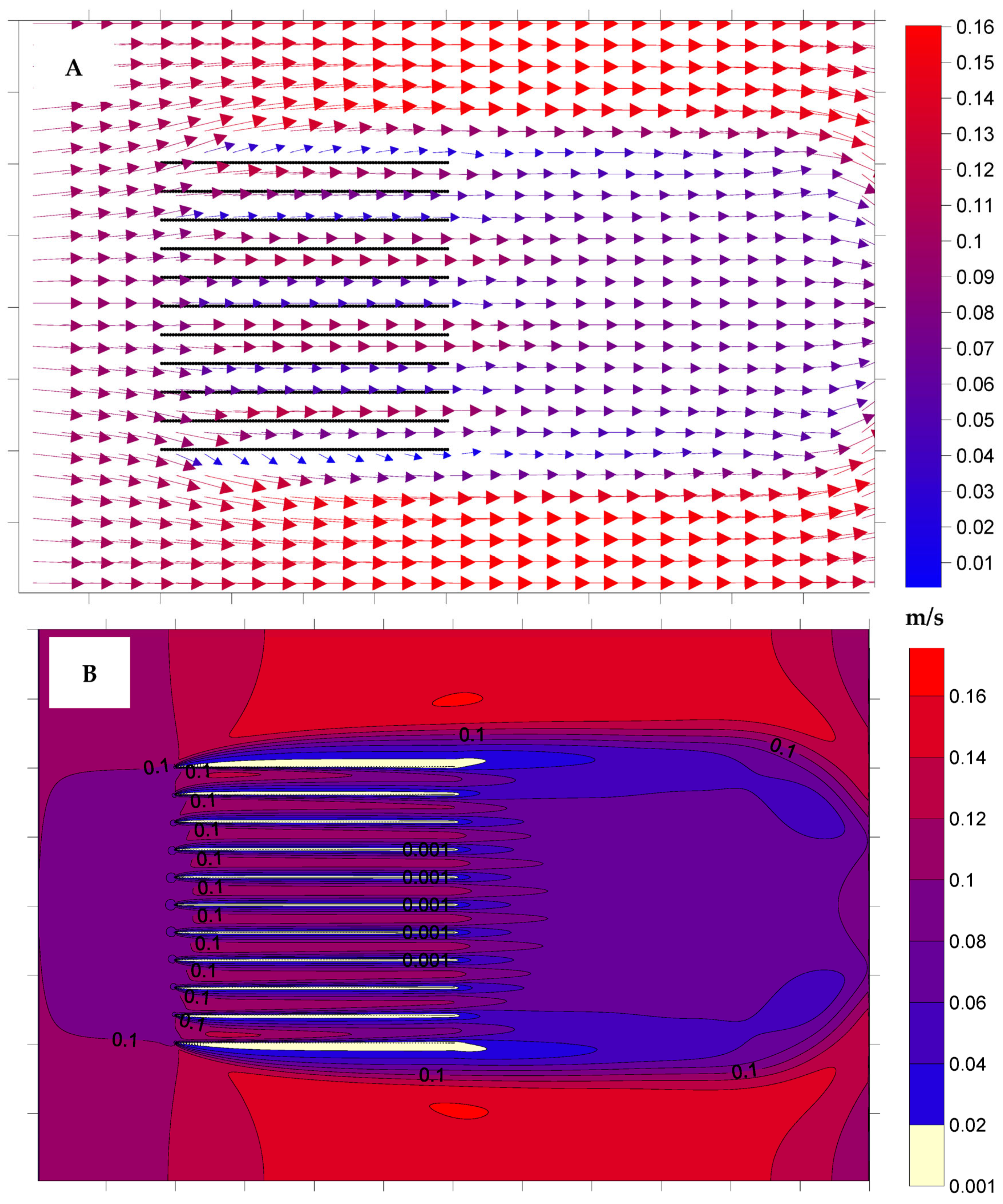
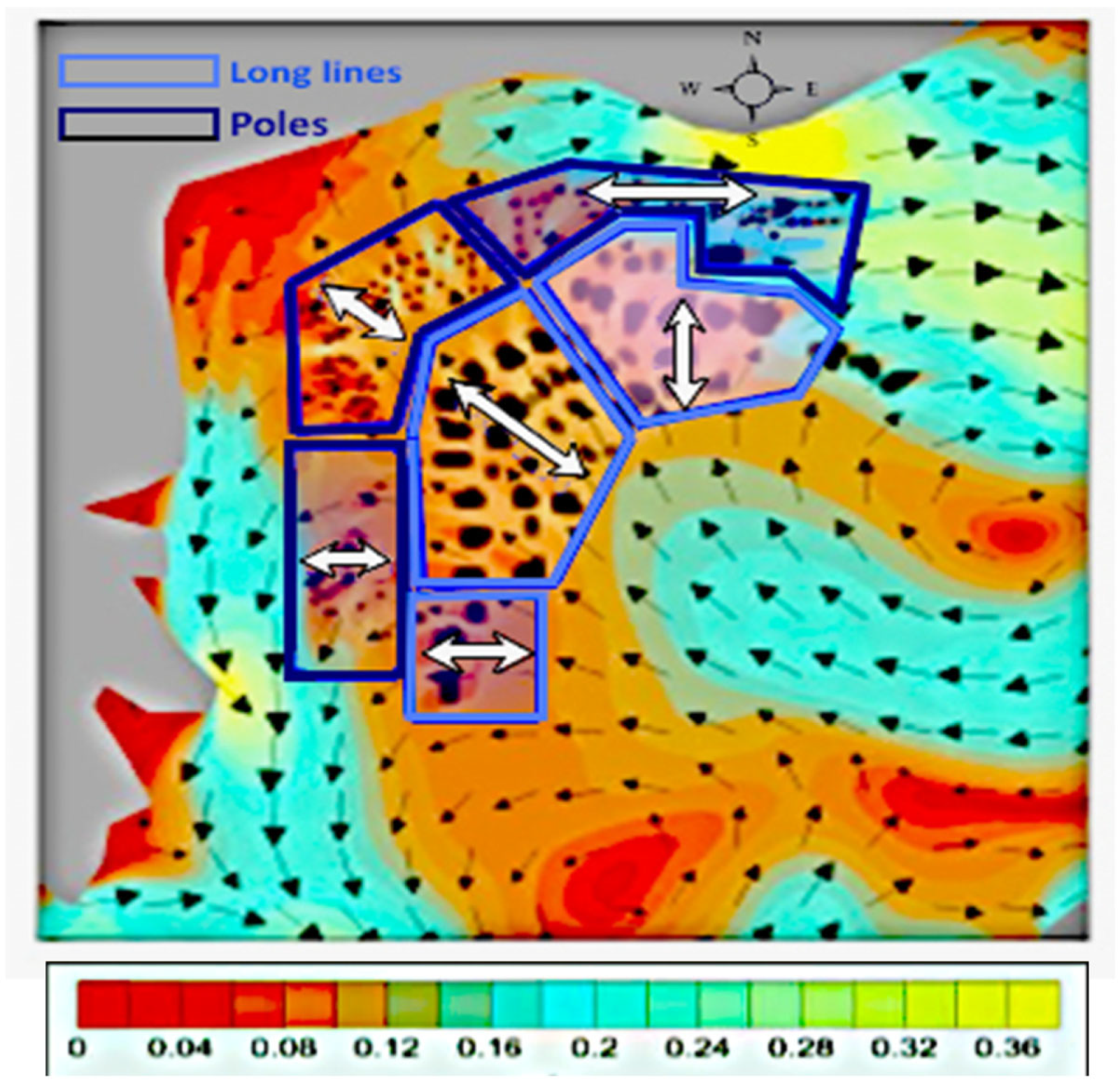
3.1. Hydrodynamic Model Simulations
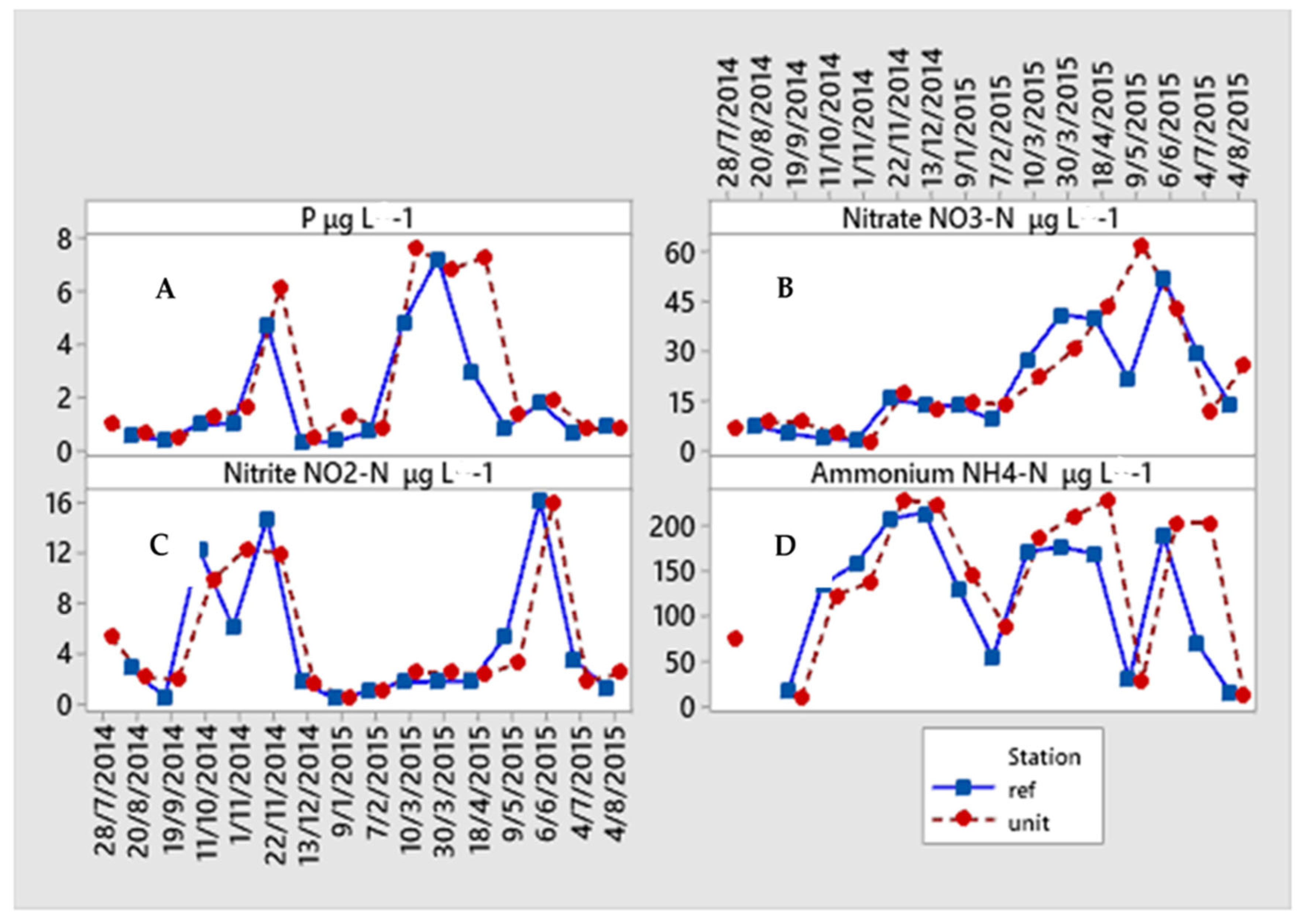
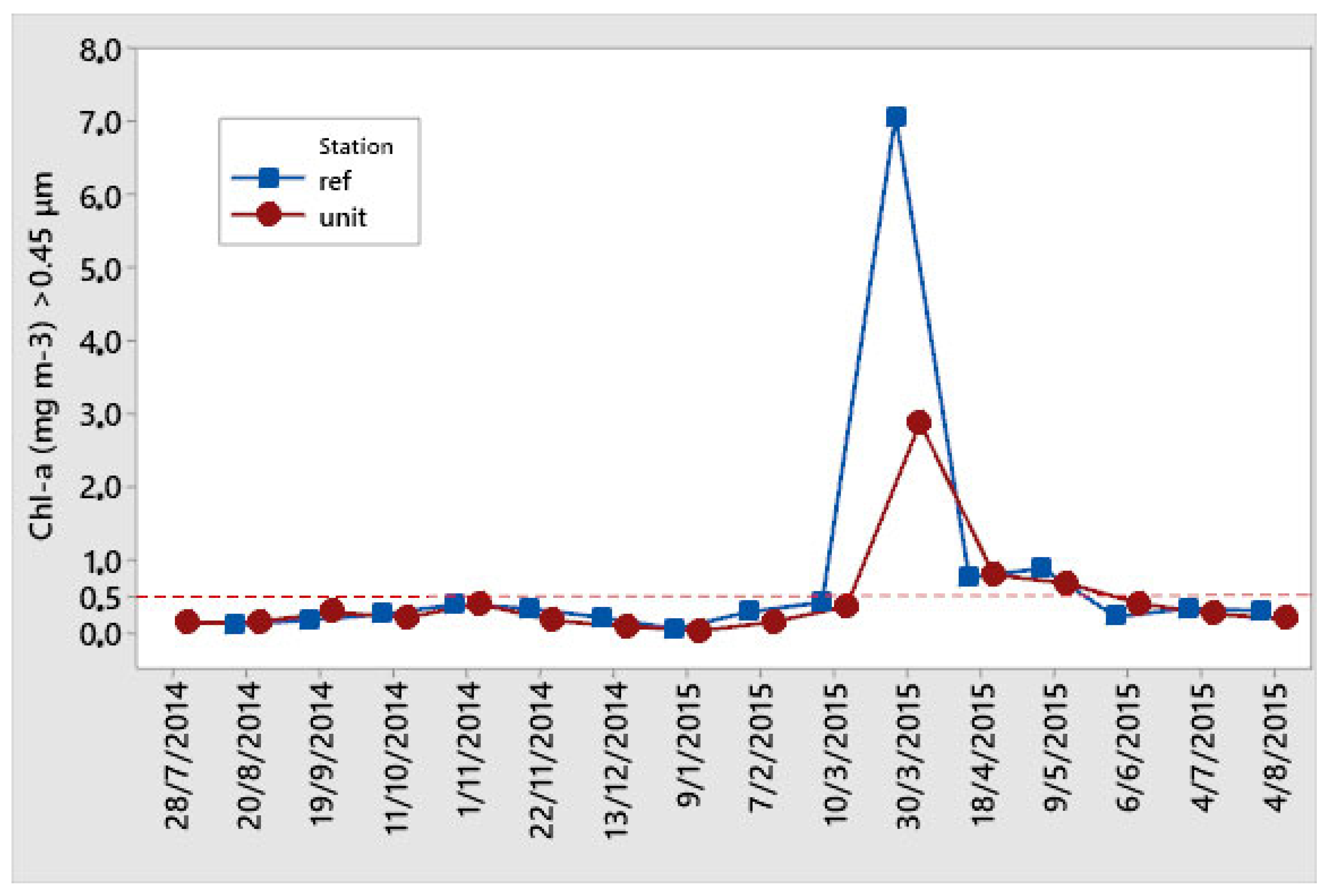
3.2. Monitoring of Nutrients and Chlorophyll–a
4. Discussion
4.1. Hydrodynamic Model Simulations
4.2. Chlorophyll–a and Nutrient Availability
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eurostat. Landings of Fishery Products. Edible Crab (Cancer Pagurus). Available online: http://ec.europa.eu/eurostat/web/fisheries/data/database (accessed on 15 March 2021).
- Pouvreau, S.; Bourles, Y.; Lefebvre, S.; Gangnery, A.; Alunno-Bruscia, M. Application of a dynamic energy budget model to the Pacific oyster, Crassostrea gigas, reared under various environmental conditions. J. Sea Res. 2006, 56, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.; Hawkins, A.; Bricker, S. Management of productivity, environmental effects and profitability of shellfish aquaculture—The Farm Aquaculture Resource Management (FARM) model. Aquaculture 2007, 264, 160–174. [Google Scholar] [CrossRef]
- Barillé, L.; Lerouxel, A.; Dutertre, M.; Haure, J.; Barillé, A.L.; Pouvreau, S.; Alunno-Bruscia, M. Growth of the Pacific oyster (Crassostrea gigas) in a high-turbidity environment: Comparison of model sim-ulations based on scope for growth and dynamic energy budgets. J. Sea Res. 2011, 66, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Filgueira, R.; Rosland, R.; Grant, J. A comparison of scope for growth (SFG) and dynamic energy budget (DEB) models applied to the blue mussel (Mytilus edulis). J. Sea Res. 2011, 66, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, A.J.S.; Pascoe, P.L.; Parry, H.; Brinsley, M.; Cacciatore, F.; Black, K.D.; Fang, J.G.; Jiao, H.; Mcgonigle, C.; Moore, H.; et al. Comparative Feeding on Chlorophyll-Rich Versus Remaining Organic Matter in Bivalve Shellfish. J. Shellfish. Res. 2013, 32, 883–897. [Google Scholar] [CrossRef]
- Hawkins, A.J.S.; Pascoe, P.L.; Parry, H.; Brinsley, M.; Black, K.D.; McGonigle, C.; Moore, H.; Newell, C.R.; O’Boyle, N.; O’Carroll, T.; et al. ShellSIM: A generic model of growth and environmental effects validated across contrasting habitats in bivalve shell-fish. J. Shellfish. Res. 2013, 32, 237–253. [Google Scholar] [CrossRef]
- Stevens, C.; Plew, D.; Hartstein, N.; Fredriksson, D. The physics of open-water shellfish aquaculture. Aquac. Eng. 2008, 38, 145–160. [Google Scholar] [CrossRef]
- Duarte, P.; Alvarez-Salgado, X.A.; Fernández-Reiriz, M.J.; Piedracoba, S.; Labarta, U. A modeling study on the hydrodynamics of a coastal embayment occupied by mussel farms (Ria de Ares-Betanzos, NW Iberian Peninsula). Estuar. Coast. Shelf Sci. 2014, 147, 42–55. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Li, C.; Zhang, S. Hydrodynamic effect of a large offshore mussel suspended aquaculture farm. Aquaculture 2016, 451, 147–155. [Google Scholar] [CrossRef]
- Konstantinou, Z.I.; Kombiadou, K.; Krestenitis, Y.N. Effective mussel-farming governance in Greece: Testing the guidelines through models, to evaluate sustainable management alternatives. Ocean. Coast. Manag. 2015, 118, 247–258. [Google Scholar] [CrossRef]
- Grant, J.; Bacher, C. A numerical model of flow modification induced by suspended aquaculture in a Chinese bay. Can. J. Fish. Aquat. Sci. 2001, 58, 1003–1011. [Google Scholar] [CrossRef]
- Plew, D.R. The Hydrodynamic Effects of Long-line Mussel Farms. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2005; 356p. [Google Scholar]
- Plew, D.R.; Stevens, C.L.; Spigel, R.H.; Hartstein, N.D. Hydrodynamic implications of large off shore mussel farms. IEEE J. Ocean. Engin. 2005, 30, 95–108. [Google Scholar] [CrossRef]
- Delaux, S.; Stevens, C.L.; Popinet, S. High-resolution computational fluid dynamics modelling of suspended shellfish structures. Environ. Fluid Mech. 2011, 11, 405–425. [Google Scholar] [CrossRef] [Green Version]
- Filgueira, R.; Grant, J.; Petersen, J.K. Identifying the optimal depth for mussel suspended culture in shallow and turbid environments. J. Sea Res. 2018, 132, 15–23. [Google Scholar] [CrossRef] [Green Version]
- ATEITH. Water Circulation in Organized Areas of Aquaculture Development and Land Planning and Environmental Management Interventions Final Technical Report; Alexander Technological Educational Institute of Thessaloniki: Moudania, Greece, 2007. (In Greek) [Google Scholar]
- Savvidis, Y.G.; Koutitas, C.G.; Krestenitis, Y.N. Modelling the water mass exchange through navigational channels connecting adjacent coastal basins—Application to the Channel of Potidea (North Aegean Sea). Ann. Geophys. 2005, 23, 231–238. [Google Scholar] [CrossRef]
- Galinou-Mitsoudi, S.; Savvidis, Y.; Moriki, A. Mussel culture in Greece, fifty years of experience and future perspectives. In Proceedings of the 3rd Hellenic Congress on Technology of Animal Production, Thessaloniki, Greek, 17–19 Μarch 2004. [Google Scholar]
- ATEITH. Innovative Practices for a Sustainable and Environmental Friendly Mussel Culture’ Co-Financed by the Greek State and the European Union in the context of the Operational Program of Fiesheries 2007–2013. 2015. Available online: http://www.mussel.teithe.gr/en/?fbclid=IwAR0c_Vu8338KZxRTeYlQ3ZUfhN2vteo18-frZCvmkC7CAQ1AjzMc-Gus9Bc (accessed on 15 March 2021).
- Koutitas, C. Three-Dimensional Models of Coastal Circulation: An Engineering View-point. In Three-Dimensional Coastal Ocean Models; American Geophysical Union: Washington, DC, USA, 1987; pp. 107–123. [Google Scholar]
- Hyder, P.; Simpson, J.H.; Christopoulos, S.; Krestenitis, Y. The seasonal cycles of stratification and circulation in the Thermaikos gulf region of freshwater influence (ROFI), north-West Aegean. Cont. Shelf. Res. 2002, 22, 2573–2597. [Google Scholar] [CrossRef]
- Kourafalou, V.H. River plume development in semi-enclosed Mediterranean regions: North Adriatic Sea and north western Aegean Sea. J. Mar. Syst. 2001, 30, 181–205. [Google Scholar] [CrossRef]
- Tsiaras, K.P.; Petihakis, G.; Kourafalou, V.H.; Triantafyllou, G. Impacts of the river nutrient load variability on the North Aegean ecosystem functioning over the last decades. J. Sea Res. 2014, 86, 97–109. [Google Scholar] [CrossRef]
- Nikolaidis, N.P.; Karageorgis, A.P.; Kapsimalis, V.; Marconis, G.; Drakopoulou, P.; Kontoyiannis, H.; Krasakopoulou, E.; Pavlidou, A.; Pagou, K. Circulation and nutrient modeling of Thermaikos Gulf, Greece. J. Mar. Syst. 2006, 60, 51–62. [Google Scholar] [CrossRef]
- Parsons, R.; Maita, Y.; Lalli, M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; 173p. [Google Scholar]
- Solórzano, L. Determination of ammonia in natural waters by the phenolhypochlorite method 11 this research was fully supported by U.S. Atomic Energy Commission Contract No. ATS (11-1) GEN 10, P.A. 20. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- Inglis, G.J.; Hayden, B.J.; Ross, A.H. An Overview of Factors Affecting the Carrying Capacity of Coastal Embayments for Mussel Culture; Ministry for the Environment, NIWA: Auckland, New Zealand, 2000. [Google Scholar]
- Savvidis, Y.; Antoniou, A.; Dimitriadis, X.; Koutitas, C.; Moriki, A.; Galinou-Mitsoudi, S.; Petridis, D.; Alvanou, L. Hydrodynamics in a mussel culture area in Thermaikos Gulf. In Proceedings of the 8th International Conference MEDCOAST, Alexandria, Egypt, 13–17 November 2007. [Google Scholar]
- Moriki, A.; Antoniou, A.; Savvidis, Y.; Papadimitriou, C.; Stoilas, V.O. Nutrient Limitation in a Coastal System Influenced by Mussel Farming, River Outflow and On-shore Circulation of Waters. Environ. Process. 2019, 6, 1019–1029. [Google Scholar] [CrossRef]
- Konstantinou, Z.I. Integrated Coastal Zone Management Utilizing Conceptualand Numerical Models. Ph.D. Thesis, Department of Civil Engineering, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2013. [Google Scholar]
- Savvidis, Y.; Antoniou, A.; Stoilas, V.O.; Galinou-Mitsoudi, S. Hydrodynamics in longline mussel culture layouts. In Proceedings of the 12th International Conference MEDCOAST 2011, Varna, Bulgaria, 6–10 October 2015. [Google Scholar]
- Konstantinou, Z.I.; Kombiadou, K. Rethinking suspended mussel-farming modelling: Combining hydrodynamic and bio-economic models to support integrated aquaculture management. Aquaculture 2020, 532, 735179. [Google Scholar] [CrossRef]
- Moriki, A.; Antoniou, A.; Savvidis, Y.; Papadimitriou, C.A.; Stoilas, V.O.; Nikolaidis, N.; Karageorgis, A.; Kapsimalis, V.; Drakopoulou, P.; Skoulikidis, N.; et al. Management of nutrient emissions of Axios River catchment: Their effect in the coastal zone of Thermaikos gulf. Greece Ecol. Modell. 2009, 220, 383–396. [Google Scholar]
- Moriki, A.; Savvidis, Y.; Antoniou, A.; Stoilas, V.O. Τhe influence of hydrodynamic circulation in the trophic status of western Thessaloniki gulf coastal waters. In Proceedings of the 5th International Conference on Small and De-centralized Water and Wastewater Treatment Plants, Thessaloniki, Greece, 26–29 August 2018. [Google Scholar]
- Cranford, P.J. Relationship between food quantity and quality and absorption efficiency in sea scallops Placopecten magellacinus (Gmelin). J. Exp. Mar. Biol. Ecol. 1995, 189, 123–142. [Google Scholar] [CrossRef]
- Trottet, A.; Roy, S.; Tamigneaux, E.; Lovejoy, C.; Tremblay, R. Impact of suspended mussels (Mytilus edulis L.) on plankton communities in a Magdalen Islands lagoon (Québec, Canada): A mesocosm approach. J. Exp. Mar. Biol. Ecol. 2008, 365, 103–115. [Google Scholar] [CrossRef]
- Giles, H.; Broekhuizen, N.; Bryan, K.R.; Pilditch, C.A. Modelling the dispersal of biodeposits from mussel farms: The importance of simulating biodeposit erosion and decay. Aquaculture 2009, 291, 168–178. [Google Scholar] [CrossRef]
- Hartstein, N.D.; Rowdena, A.A. Effect of biodeposits from mussel culture on macroinvertebrate as-semblages at sites of different hydrodynamic regime. Mar. Environ. Res. 2004, 57, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, F.; Berto, D.; Giani, M.; Brigolin, D.; Covelli, S.; Cacciatore, F.; Brusà, R.B.; Bellucci, L.G.; Pastres, R. Impact of mussel farming on sedimentary geochemical properties of a Northern Adriatic area in-fluenced by freshwater inflows. Estuar. Coast. Shelf Sci. 2013, 129, 49–58. [Google Scholar] [CrossRef]
- Wiles, P.J.; Duren, L.A.; Häse, C.; Larsen, J.; Simpson, J.H. Stratification and mixing in the Limfjorden in relation to mussel culture. J. Mar. Syst. 2006, 60, 129–143. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadimitriou, C.A.; Savvidis, Y.G.; Galinou-Mitsoudi, S.; Moriki, A. The Role of Hydrodynamics on the Sustainable Mussels’ Culture Activity. The Case of the Chalastra Basin (NW Gulf of Thessaloniki). Hydrology 2021, 8, 105. https://doi.org/10.3390/hydrology8030105
Papadimitriou CA, Savvidis YG, Galinou-Mitsoudi S, Moriki A. The Role of Hydrodynamics on the Sustainable Mussels’ Culture Activity. The Case of the Chalastra Basin (NW Gulf of Thessaloniki). Hydrology. 2021; 8(3):105. https://doi.org/10.3390/hydrology8030105
Chicago/Turabian StylePapadimitriou, Chrysi A., Yiannis G. Savvidis, Sofia Galinou-Mitsoudi, and Amalia Moriki. 2021. "The Role of Hydrodynamics on the Sustainable Mussels’ Culture Activity. The Case of the Chalastra Basin (NW Gulf of Thessaloniki)" Hydrology 8, no. 3: 105. https://doi.org/10.3390/hydrology8030105
APA StylePapadimitriou, C. A., Savvidis, Y. G., Galinou-Mitsoudi, S., & Moriki, A. (2021). The Role of Hydrodynamics on the Sustainable Mussels’ Culture Activity. The Case of the Chalastra Basin (NW Gulf of Thessaloniki). Hydrology, 8(3), 105. https://doi.org/10.3390/hydrology8030105





