Sustainable Production of Reclaimed Water by Constructed Wetlands for Combined Irrigation and Microalgae Cultivation Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytical Methods
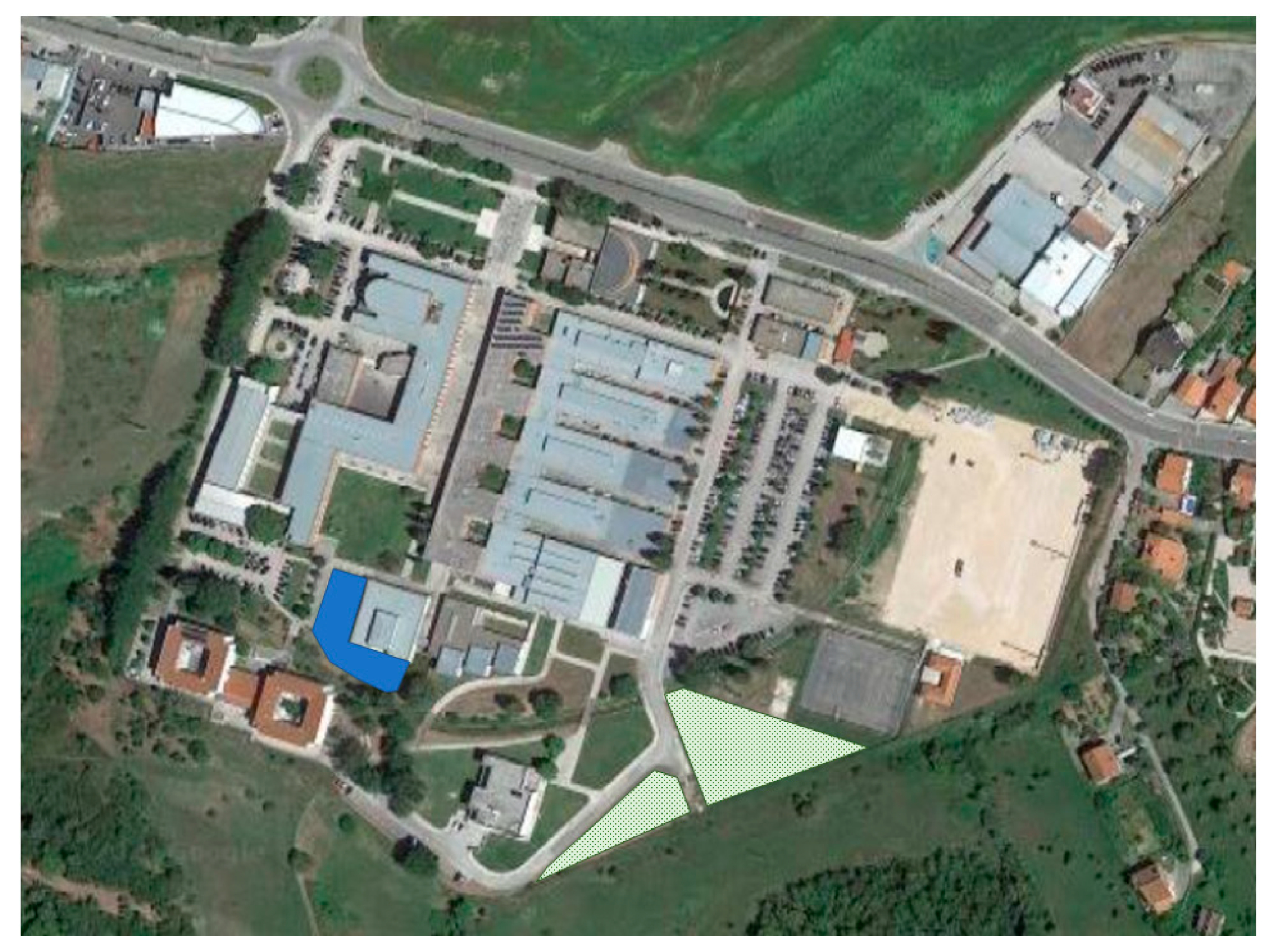
2.2. Wastewater Characterístics and Treatment Setup
2.3. Irrigation Experiments
2.4. Microalgae Cultivations
3. Results and Discussion
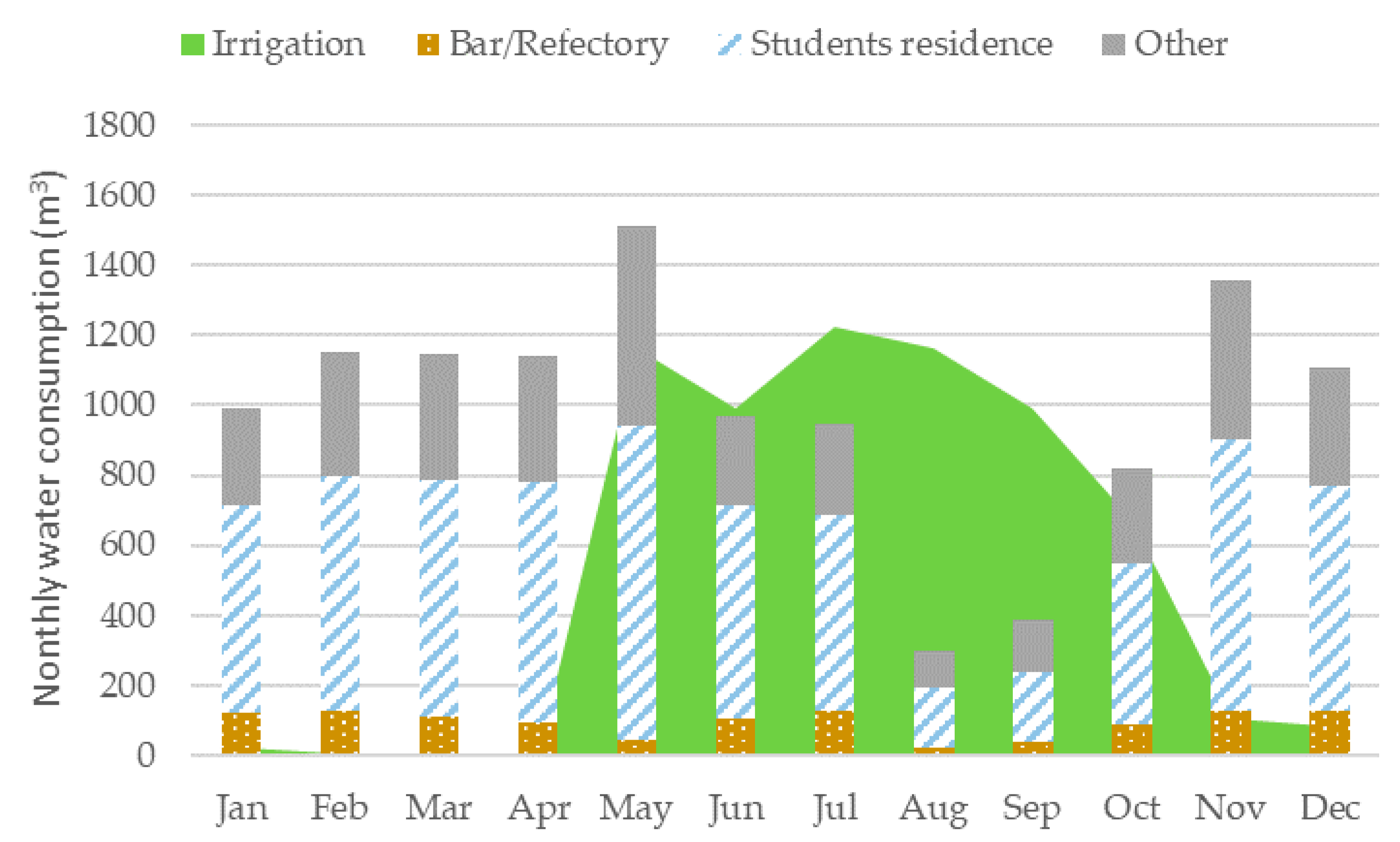
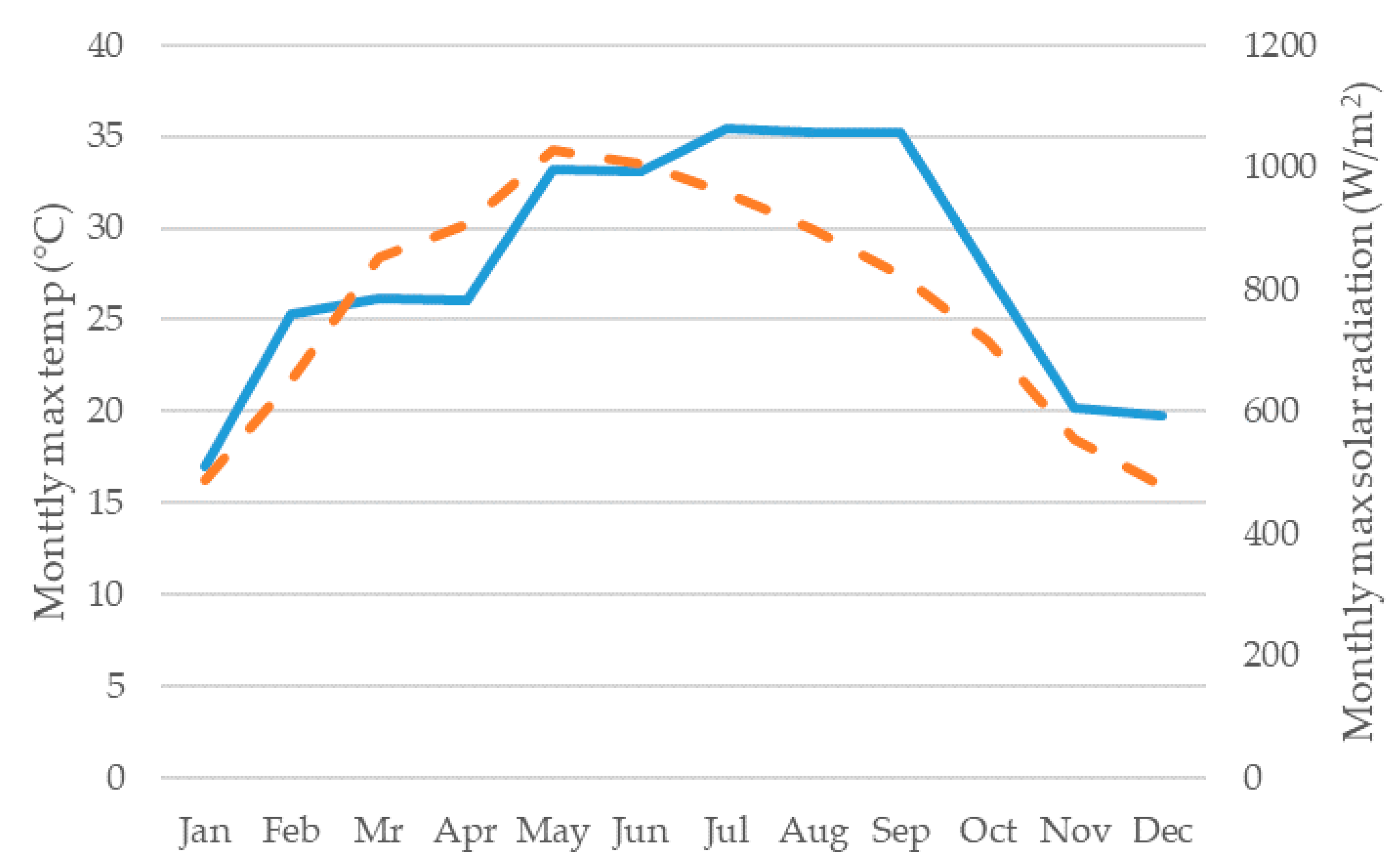
3.1. Irrigation Results
3.2. Microalgae Growth Results
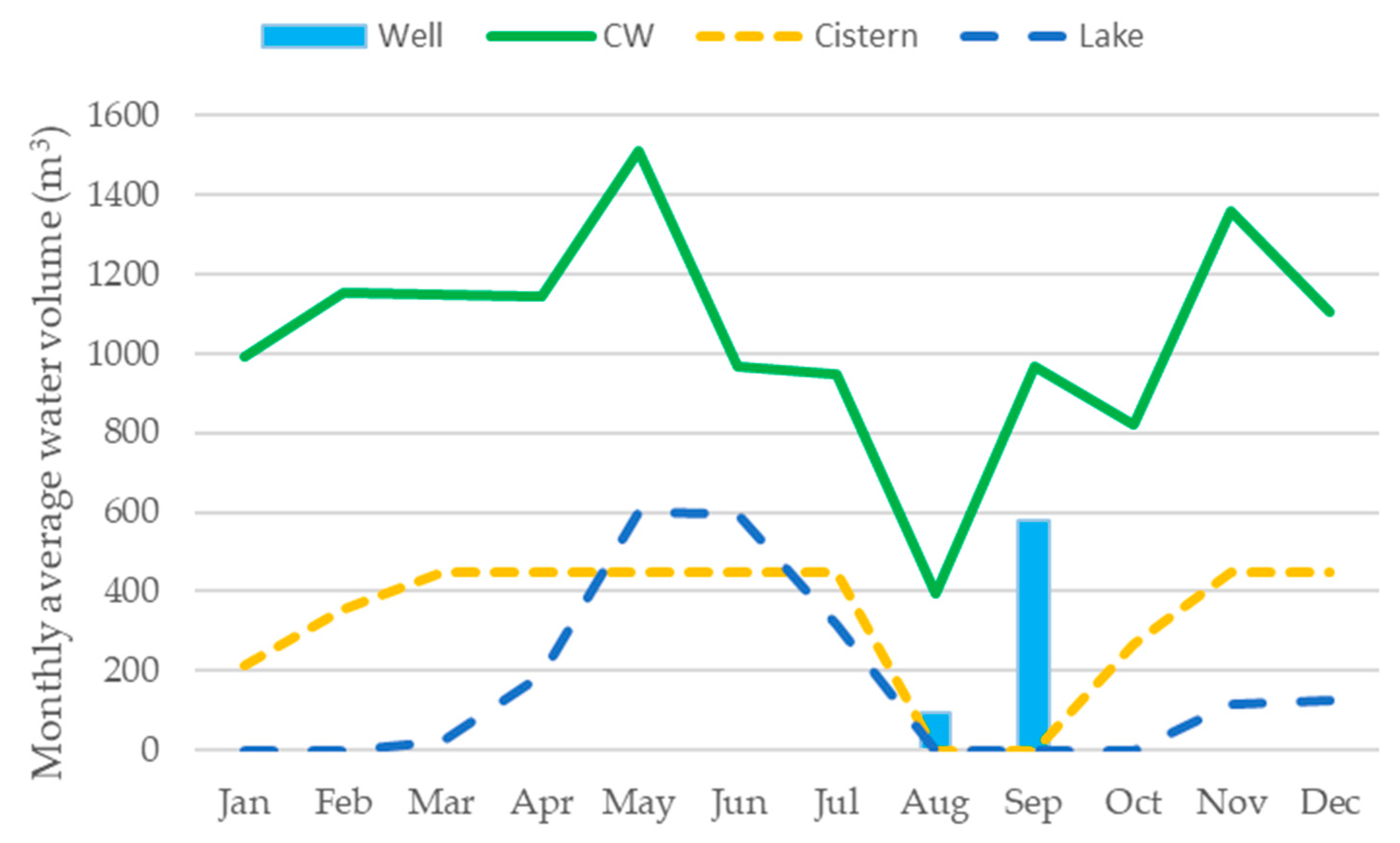
3.3. Simulation of Reclaimed Water Use for Green Space Irrigation and Microalgae Production
- (i).
- Estimation of investment and operational costs to set up the proposed framework, namely, the CW beds, the PBR plant, the compact WWTP, the disinfection units, the renewable energy station unit, and the additional tubing and pumps;
- (ii).
- Estimation of financial benefits from avoiding the use of commercial fertilizers and compost for treatment of the green spaces and from avoiding the consumption of nutrients for the microalgae cultivation;
- (iii).
- Experimental work to optimize microalgae cultivation in PBRs placed outdoors;
- (iv).
- Evaluation of potential uses of the microalgae biomass for valuable compounds under the biorefinery concept;
- (v).
- Scale-up of the proposed water recovery model for application to different size communities under the smart cities concept;
- (vi).
- Spread of an educational message and green behavior motivation for the protection of water as a fundamental resource.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN. Sustainable Development Goal 6 Synthesis Report on Water and Sanitation; United Nations: New York, NY, USA, 2018. [Google Scholar]
- Sun, C.; Zhou, X. Characterizing Hydrological Drought and Water Scarcity Changes in the Future: A Case Study in the Jinghe River Basin of China. Water 2020, 12, 1605. [Google Scholar] [CrossRef]
- Bao, C.; He, D. Scenario Modeling of Urbanization Development and Water Scarcity Based on System Dynamics: A Case Study of Beijing–Tianjin–Hebei Urban Agglomeration, China. Int. J. Environ. Res. Public Health 2019, 16, 3834. [Google Scholar] [CrossRef] [PubMed]
- UN. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Zhou, L.; Xu, K.; Cheng, X.; Xu, Y.; Jia, Q. Study on optimizing production scheduling for water-saving in textile dyeing industry. J. Clean. Prod. 2017, 141, 721–727. [Google Scholar] [CrossRef]
- AL-Washali, T.; Sharma, S.; Lupoja, R.; AL-Nozaily, F.; Haidera, M.; Kennedy, M. Assessment of water losses in distribution networks: Methods, applications, uncertainties, and implications in intermittent supply. Resour. Conserv. Recycl. 2020, 152, 104515. [Google Scholar] [CrossRef]
- Richter, B.D.; Benoit, K.; Dugan, J.; Getacho, G.; LaRoe, N.; Moro, B.; Rynne, T.; Tahamtani, M.; Townsend, A. Decoupling Urban Water Use and Growth in Response to Water Scarcity. Water 2020, 12, 2868. [Google Scholar] [CrossRef]
- Peng, Y.; Xiao, Y.; Fu, Z.; Dong, Y.; Zheng, Y.; Yan, H.; Li, X. Precision irrigation perspectives on the sustainable water-saving of field crop production in China: Water demand prediction and irrigation scheme optimization. J. Clean. Prod. 2019, 230, 365–377. [Google Scholar] [CrossRef]
- Rebelo, A.; Quadrado, M.; Franco, A.; Lacasta, N.; Machado, P. Water reuse in Portugal: New legislation trends to support the definition of water quality standards based on risk characterization. Water Cycle 2020, 1, 41–53. [Google Scholar] [CrossRef]
- EC. Guidelines on Integrating Water Reuse into Water Planning and Management in the Context of the WFD; European Commision Water Directors: Amsterdam, The Netherlands, 2016; Available online: https://ec.europa.eu/environment/water/pdf/Guidelines_on_water_reuse.pdf (accessed on 12 February 2021).
- Somoza-Tornos, A.; Rives-Jiménez, M.; Espuña, A.; Graells, M. A Circular Economy Approach to the Design of a Water Network Targeting the Use of Regenerated Water. Comp. Aided Chem. Eng. 2019, 47, 119–124. [Google Scholar] [CrossRef]
- Hagenvoort, J.; Ortega-Reig, M.; Botella, S.; García, C.; de Luis, A.; Palau-Salvador, G. Reusing Treated Waste-Water from a Circular Economy Perspective—The Case of the Real Acequia de Moncada in Valencia (Spain). Water 2019, 11, 1830. [Google Scholar] [CrossRef]
- Neczaj, E.; Grosser, A. Circular Economy in Wastewater Treatment Plant–Challenges and Barriers. Proceedings 2018, 2, 614. [Google Scholar] [CrossRef]
- Furumai, H. Rainwater and reclaimed wastewater for sustainable urban water use. Phys. Chem. Earth Parts A/B/C 2008, 33, 340–346. [Google Scholar] [CrossRef]
- Cherchi, C.; Kesaano, M.; Badruzzaman, M.; Schwab, K.; Jacangelo, J.G. Municipal reclaimed water for multi-purpose applications in the power sector: A review. J. Environ. Manag. 2019, 236, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Poustie, A.; Yang, Y.; Verburg, P.; Pagilla, K.; Hanigan, D. Reclaimed wastewater as a viable water source for agricultural irrigation: A review of food crop growth inhibition and promotion in the context of environmental change. Sci. Total Environ. 2020, 739, 139756. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, Y.; Chen, H. Graywater treatment technologies and reuse of reclaimed water for toilet flushing. Environ. Sci. Pollut. Res. 2020, 27, 34653–34663. [Google Scholar] [CrossRef] [PubMed]
- Digiano, F.A.; Weaver, C.C.; Okun Deceased, D.A. Benefits of shifting fire protection to reclaimed water. J. Am. Water Works Assoc. 2009, 101, 65–74. [Google Scholar] [CrossRef]
- Voulvoulis, N. Water reuse from a circular economy perspective and potential risks from an unregulated approach. Curr. Opin. Environ. Sci. Heal. 2018, 2, 32–45. [Google Scholar] [CrossRef]
- Fito, J.; Van Hulle, S.W.H. Wastewater reclamation and reuse potentials in agriculture: Towards environmental sustainability. Environ. Dev. Sustain. 2020, 1–24. [Google Scholar] [CrossRef]
- Yang, J.; Monnot, M.; Ercolei, L.; Moulin, P. Membrane-Based Processes Used in Municipal Wastewater Treatment for Water Reuse: State-Of-The-Art and Performance Analysis. Membranes (Basel) 2020, 10, 131. [Google Scholar] [CrossRef]
- Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M. Wetlands for wastewater treatment and subsequent recycling of treated effluent: A review. Environ. Sci. Pollut. Res. 2018, 25, 23595–23623. [Google Scholar] [CrossRef] [PubMed]
- Gorgoglione, A.; Torretta, V. Sustainable Management and Successful Application of Constructed Wetlands: A Critical Review. Sustainability 2018, 10, 3910. [Google Scholar] [CrossRef]
- Sandoval, L.; Zamora-Castro, S.; Vidal-Álvarez, M.; Marín-Muñiz, J. Role of Wetland Plants and Use of Ornamental Flowering Plants in Constructed Wetlands for Wastewater Treatment: A Review. Appl. Sci. 2019, 9, 685. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Y.; Xie, H.; Yang, Z. Constructed Wetlands: A Review on the Role of Radial Oxygen Loss in the Rhizosphere by Macrophytes. Water 2018, 10, 678. [Google Scholar] [CrossRef]
- Rajan, R.J.; Sudarsan, J.S.; Nithiyanantham, S. Microbial population dynamics in constructed wetlands: Review of recent advancements for wastewater treatment. Environ. Eng. Res. 2018, 24, 181–190. [Google Scholar] [CrossRef]
- Sanjrani, M.A.; Zhou, B.; Zhao, H.; Zheng, Y.P.; Wang, Y.; Xia, S.B. Treatment of Wastewater with Constructed Wetlands Systems and Plants used in this Technology–A Review. Appl. Ecol. Environ. Res. 2020, 18, 107–127. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Liu, R.; Morgan, D. Global development of various emerged substrates utilized in constructed wetlands. Bioresour. Technol. 2018, 261, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, N.; Vlăduț, V.; Voicu, G. Water Scarcity and Wastewater Reuse in Crop Irrigation. Sustainability 2020, 12, 9055. [Google Scholar] [CrossRef]
- Vergine, P.; Salerno, C.; Libutti, A.; Beneduce, L.; Gatta, G.; Berardi, G.; Pollice, A. Closing the water cycle in the agro-industrial sector by reusing treated wastewater for irrigation. J. Clean. Prod. 2017, 164, 587–596. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Priority-based multiple products from microalgae: Review on techniques and strategies. Crit. Rev. Biotechnol. 2020, 40, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Cinar, O.S.; Chong, Z.K.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic Production from Microalgae: A Review. Int. J. Environ. Res. Public Health 2020, 17, 3842. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Yew, G.Y.; Koyande, A.K.; Chew, K.W.; Vo, D.-V.N.; Show, P.L. Green technology for the industrial production of biofuels and bioproducts from microalgae: A review. Environ. Chem. Lett. 2020, 18, 1967–1985. [Google Scholar] [CrossRef]
- Han, W.; Clarke, W.; Pratt, S. Composting of waste algae: A review. Waste Manag. 2014, 34, 1148–1155. [Google Scholar] [CrossRef]
- Michalak, I.; Tuhy, Ł.; Chojnacka, K. Co-Composting of Algae and Effect of the Compost on Germination and Growth of Lepidium sativum. Polish J. Environ. Stud. 2016, 25, 1107–1115. [Google Scholar] [CrossRef]
- Ishizaki, R.; Noguchi, R.; Putra, A.S.; Ichikawa, S.; Ahamed, T.; Watanabe, M.M. Reduction in Energy Requirement and CO2 Emission for Microalgae Oil Production Using Wastewater. Energies 2020, 13, 1641. [Google Scholar] [CrossRef]
- Xiaogang, H.; Jalalah, M.; Jingyuan, W.; Zheng, Y.; Li, X.; Salama, E.-S. Microalgal growth coupled with wastewater treatment in open and closed systems for advanced biofuel generation. Biomass Convers. Biorefinery 2020, 1–20, in press. [Google Scholar] [CrossRef]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A Review of Algae-Based Produced Water Treatment for Biomass and Biofuel Production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Mateus, D.M.R.; Pinho, H.J.O.; Nogueira, I.M.D.P.; Rosa, M.A.N.H.; Cartaxo, M.A.M.; Nunes, V.M.B. Participation of students in the project Valorbio. Int. J. Sustain. High. Educ. 2020, 21, 244–263. [Google Scholar] [CrossRef]
- Pinho, H.; Alves, A.; Graça, N.; Mateus, D. Solid Waste Mixtures as Constructed Wetlands Filling: Effect of Hydraulic Loading Rate on Nutrient Removal From Wastewater. In Proceedings of the Water Resources and Wetlands. 4th International Conference; Gastescu, P., Bretcan, P., Eds.; Romanian Limnogeographical Association: Targoviste, Romania, 2018; pp. 108–113. Available online: http://www.limnology.ro/wrw2018/proceedings.html (accessed on 12 February 2021).
- Pinho, H.J.O.; Mateus, D.M.R. Projeto Valorbio–Tratamento de Águas Residuais por Zonas Húmidas Construídas Modulares: Uma Contribuição Para a Economia Circular; Instituto Politécnico de Tomar: Tomar, Portugal, 2019; ISBN 989-8840-27-1. [Google Scholar]
- Mateus, D.M.R.; Pinho, H.J.O. Screening of Solid Waste as Filler Material for Constructed Wetlands. IOP Conf. Ser. Earth Environ. Sci. 2018, 182, 012001. [Google Scholar] [CrossRef]
- Mateus, D.M.R.; Pinho, H.J.O. Evaluation of solid waste stratified mixtures as constructed wetland fillers under different operation modes. J. Clean. Prod. 2020, 253, 119986. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017; ISBN 9780875532875. [Google Scholar]
- Liu, J.; Bangert, K. Effect of nitrogen source in low-cost media on biomass and lipid productivity of Nannochloropsis salina for large-scale biodiesel production. Environ. Prog. Sustain. Energy 2015, 34, 297–303. [Google Scholar] [CrossRef]
- Yu, Z.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresour. Technol. 2018, 247, 904–914. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Mandel, M.; Cohen-Bazier, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Soeder, C.J.; Hegewald, E. Scenedesmus. In Micro-Algal Biotechnology; Borowitzka, M., Borowitzka, L., Eds.; Cambridge University Press: Cambridge, UK, 1988; pp. 59–84. ISBN 9780521323499. [Google Scholar]
- Apandi, N.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Gani, P.; Ibrahim, A.; Kassim, A.H.M. Scenedesmus Biomass Productivity and Nutrient Removal from Wet Market Wastewater, A Bio-kinetic Study. Waste Biomass Valorization 2019, 10, 2783–2800. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Cultivation of Chlorella vulgaris in a pilot-scale sequential-baffled column photobioreactor for biomass and biodiesel production. Energy Convers. Manag. 2014, 88, 399–410. [Google Scholar] [CrossRef]
- SNIRH-APA Sistema Nacional de Informação de Recursos Hídricos, Agência Portuguesa do Ambiente. Available online: https://snirh.apambiente.pt/ (accessed on 12 February 2021).
- Bitog, J.P.; Lee, I.-B.; Lee, C.-G.; Kim, K.-S.; Hwang, H.-S.; Hong, S.-W.; Seo, I.-H.; Kwon, K.-S.; Mostafa, E. Application of computational fluid dynamics for modeling and designing photobioreactors for microalgae production: A review. Comput. Electron. Agric. 2011, 76, 131–147. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q.; Horsman, M. Closed photobioreactors for production of microalgal biomasses. Biotechnol. Adv. 2012, 30, 904–912. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of Photobioreactors for Mass Cultivation of Photosynthetic Organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]


| Parameter 1 → | COD (mg/L) | BOD5 (mg/L) | TN (mg/L) | TP (mg/L) | TSS (mg/L) | NTU | pH |
|---|---|---|---|---|---|---|---|
| Result | 269 ± 8 | 130 ± 40 | 72.9 ± 0.6 | 1.5 ± 0.7 | 39.0 ± 0.3 | 11.4 ± 0.7 | 8.2 ± 0.1 |
| Parameter 1 → | COD (mg/L) | BOD5 (mg/L) | TN (mg/L) | TP (mg/L) | TSS (mg/L) | NTU | pH | Fluoride (mg/L) | E. coli3 |
|---|---|---|---|---|---|---|---|---|---|
| Result | 29 | 4.2 | 35 | 0.96 | 6.9 | 2.0 | 7.2 | 0.0 | 0 |
| NQAR 2 | n.r. | ≤10 | n.r. | n.r. | ≤10 | ≤5 | 6–9 | ≤2.0 | ≤10 |
| Parameter → (mg/L) | Al | B | Be | Co | Fe | Li | Mn | Mo | Se | V |
|---|---|---|---|---|---|---|---|---|---|---|
| Result | 0.0 | <0.01 | <0.01 | <0.02 | 0.25 | 0.0 | 0.19 | 0.0 | <0.02 | <0.02 |
| NQAR 1 | ≤5 | – 2 | ≤0.1 | ≤0.05 | ≤2.0 | ≤2.5 | ≤0.2 | ≤0.01 | ≤0.02 | ≤0.1 |
| Growth Indicator | Type of Irrigation Water | |
|---|---|---|
| Reclaimed | Fertilized | |
| Wet weight (g) | 4 ± 1 | 4 ± 2 |
| Plant height (cm) | 11 ± 1 | 12 ± 1 |
| Root length (cm) | 9 ± 3 | 9 ± 2 |
| Flowers per plant | 1.4 ± 0.7 | 1.3 ± 0.6 |
| Sprouts per plant | 0.8 ± 0.5 | 0.8 ± 0.6 |
| Chlorophyll a (μg/g) | 1210 ± 130 | 1476 ± 107 |
| Chlorophyll b (μg/g) | 412 ± 70 | 526 ± 73 |
| Productivity Indicator | Type of Microalgae and Reactor | |||
|---|---|---|---|---|
| Chlorella sp. | Scenedesmus sp. | |||
| SF | PBR | SF | PBR | |
| Specific growth rate (day−1) | 0.070 (a) | 0.378 (b) | 0.089 (a) | 0.470 (b) |
| Final biomass concentration (kg/m3) | 0.14 (a) | 0.37 (b) | 0.38 (b) | 0.64 (a) |
| Biomass productivity (mgdw/L/day) | 5.2 (a) | 33.1 (b) | 16.0 (c) | 61.2 (d) |
| Doubling time (day) | 9.96 (a) | 1.83 (b) | 7.77 (a) | 1.48 (b) |
| Total chlorophyll (mg/L) | 1.02 (a) | n.d. | 3.83 (b) | 4.31 (b) |
| Carotenoids (mg/L) | 0.33 (a) | n.d. | 1.26 (b) | 1.55 (c) |
| Lipid productivity (mg/L/day) | 0.70 (a) | n.d. | 1.43 (b) | 5.47 (c) |
| Storage Resources | Storage Capacity (m3) | Water Available for PBRs (m3/year) | Biomass Production (kgdw/year) | Water Extraction from the Well (m3/year) | Water Saving (%) |
|---|---|---|---|---|---|
| Cistern | 450 | 7380 | 2362 | 1276 | 80 |
| Cistern + Lake 50% | 750 | 6930 | 2218 | 976 | 85 |
| Cistern + Lake 100% | 1050 | 6480 | 2074 | 676 | 89 |
| Plus additional storage 1 | 1726 | 5467 | 1749 | 0 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinho, H.J.O.; Mateus, D.M.R. Sustainable Production of Reclaimed Water by Constructed Wetlands for Combined Irrigation and Microalgae Cultivation Applications. Hydrology 2021, 8, 30. https://doi.org/10.3390/hydrology8010030
Pinho HJO, Mateus DMR. Sustainable Production of Reclaimed Water by Constructed Wetlands for Combined Irrigation and Microalgae Cultivation Applications. Hydrology. 2021; 8(1):30. https://doi.org/10.3390/hydrology8010030
Chicago/Turabian StylePinho, Henrique J. O., and Dina M. R. Mateus. 2021. "Sustainable Production of Reclaimed Water by Constructed Wetlands for Combined Irrigation and Microalgae Cultivation Applications" Hydrology 8, no. 1: 30. https://doi.org/10.3390/hydrology8010030
APA StylePinho, H. J. O., & Mateus, D. M. R. (2021). Sustainable Production of Reclaimed Water by Constructed Wetlands for Combined Irrigation and Microalgae Cultivation Applications. Hydrology, 8(1), 30. https://doi.org/10.3390/hydrology8010030







