Groundwater Discharge in the Arctic: A Review of Studies and Implications for Biogeochemistry
Abstract
1. Introduction
2. Studies in the Arctic
2.1. Terrestrial Sites
2.2. Marine Sites
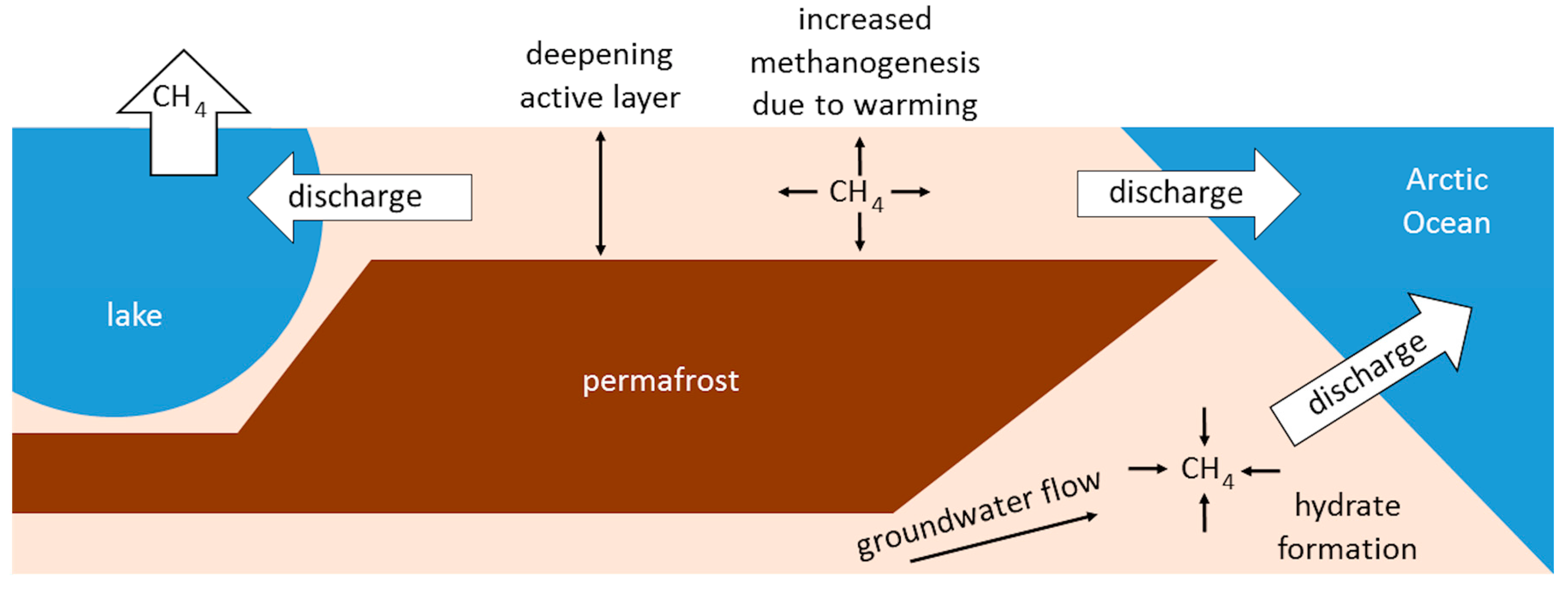
3. Implications for Biogeochemistry
3.1. Carbon
3.2. Nitrogen
3.3. Other Solutes
4. Research Priorities
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Taniguchi, M.; Burnett, W.C.; Cable, J.E.; Turner, J.V. Investigation of submarine groundwater discharge. Hydrol. Process. 2002, 16, 2115–2129. [Google Scholar] [CrossRef]
- Walvoord, M.A.; Striegl, R.G. Increased groundwater to stream discharge from permafrost thawing in the Yukon River basin: Potential impacts on lateral export of carbon and nitrogen. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Dimova, N.T.; Burnett, W.C. Evaluation of groundwater discharge into small lakes based on the temporal distribution of radon-222. Limnol. Oceanogr. 2011, 56, 486–494. [Google Scholar] [CrossRef]
- Knee, K.; Paytan, A. 4.08 Submarine groundwater discharge: A source of nutrients, metals, and pollutants to the coastal ocean. Treatise Estuar. Coast. Sci. 2011, 4, 205–234. [Google Scholar] [CrossRef]
- Sklash, M.G.; Farvolden, R.N. The role of groundwater in storm runoff. Dev. Water Sci. 1979, 12, 45–65. [Google Scholar] [CrossRef]
- Kishel, H.F.; Gerla, P.J. Characteristics of preferential flow and groundwater discharge to Shingobee Lake, Minnesota, USA. Hydrol. Process. 2002, 16, 1921–1934. [Google Scholar] [CrossRef]
- Raanan, H.; Vengosh, A.; Paytan, A.; Nishri, A.; Kabala, Z. Quantifying saline groundwater flow into a freshwater lake using the Ra isotope quartet: A case study from the Sea of Galilee (Lake Kinneret), Israel. Limnol. Oceanogr. 2009, 54, 119–131. [Google Scholar] [CrossRef]
- Moore, W.S. The subterranean estuary: A reaction zone of ground water and sea water. Mar. Chem. 1999, 65, 111–125. [Google Scholar] [CrossRef]
- Lecher, A.L.; Kessler, J.; Sparrow, K.; Garcia-Tigreros Kodovska, F.; Dimova, N.; Murray, J.; Tulaczyk, S.; Paytan, A. Methane transport through submarine groundwater discharge to the North Pacific and Arctic Ocean at two Alaskan sites. Limnol. Oceanogr. 2015, 61, S344–S355. [Google Scholar] [CrossRef]
- Frederick, J.M.; Buffett, B.A. Effects of submarine groundwater discharge on the present-day extent of relict submarine permafrost and gas hydrate stability on the Beaufort Sea continental shelf. J. Geophys. Res. Earth Surf. 2015, 120, 417–432. [Google Scholar] [CrossRef]
- Slomp, C.P.; Van Cappellen, P. Nutrient inputs to the coastal ocean through submarine groundwater discharge: Controls and potential impact. J. Hydrol. 2004, 295, 64–86. [Google Scholar] [CrossRef]
- Hosono, T.; Ono, M.; Burnett, W.C.; Tokunaga, T.; Taniguchi, M.; Akimichi, T. Spatial distribution of submarine groundwater discharge and associated nutrients within a local coastal area. Environ. Sci. Technol. 2012, 46, 5319–5326. [Google Scholar] [CrossRef] [PubMed]
- Lecher, A.L.; Mackey, K.R.M.; Kudela, R.; Ryan, J.; Fisher, A.; Murray, J.; Paytan, A. Nutrient loading through submarine groundwater discharge and phytoplankton growth in Monterey Bay, CA. Environ. Sci. Technol. 2015, 49, 6665–6673. [Google Scholar] [CrossRef] [PubMed]
- Knee, K.L.; Layton, B.A.; Street, J.H.; Boehm, A.B.; Paytan, A. Sources of nutrients and fecal indicator bacteria to nearshore waters on the north shore of Kaua`i (Hawai`i, USA). Estuaries Coasts 2008, 31, 607–622. [Google Scholar] [CrossRef]
- Knee, K.L.; Gossett, R.; Boehm, A.B.; Paytan, A. Caffeine and agricultural pesticide concentrations in surface water and groundwater on the north shore of Kauai (Hawaii, USA). Mar. Pollut. Bull. 2010, 60, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Black, F.J.; Paytan, A.; Knee, K.L.; De Sieyes, N.R.; Ganguli, P.M.; Gray, E.; Flegal, A.R. Submarine groundwater discharge of total mercury and monomethylmercury to central California coastal waters. Environ. Sci. Technol. 2009, 43, 5652–5659. [Google Scholar] [CrossRef] [PubMed]
- Bone, S.E.; Charette, M.A.; Lamborg, C.H.; Gonneea, M.E. Has submarine groundwater discharge been overlooked as a source of mercury to coastal waters? Environ. Sci. Technol. 2007, 41, 3090–3095. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.D.; White, E.S.; Gammons, C.H. Characterizing groundwater-lake interactions and its impact on lake water quality. J. Hydrol. 2013, 493, 69–78. [Google Scholar] [CrossRef]
- Bugna, G.C.; Chanton, J.P.; Cable, J.E.; Burnett, W.C.; Cable, P.H. The importance of groundwater discharge to the methane budgets of nearshore and continental shelf waters of the northeastern Gulf of Mexico. Geochim. Cosmochim. Acta 1996, 60, 4735–4746. [Google Scholar] [CrossRef]
- Paytan, A.; Lecher, A.L.; Dimova, N.; Sparrow, K.J.; Kodovska, F.G.-T.; Murray, J.; Tulaczyk, S.; Kessler, J.D. Methane transport from the active layer to lakes in the Arctic using Toolik Lake, Alaska, as a case study. Proc. Natl. Acad. Sci. USA 2015, 112, 3636–3640. [Google Scholar] [CrossRef] [PubMed]
- Burnett, W.C.; Aggarwal, P.K.; Aureli, A.; Bokuniewicz, H.; Cable, J.E.; Charette, M.A.; Kontar, E.; Krupa, S.; Kulkarni, K.M.; Loveless, A.; et al. Quantifying submarine groundwater discharge in the coastal zone via multiple methods. Sci. Total Environ. 2006, 367, 498–543. [Google Scholar] [CrossRef] [PubMed]
- Burnett, W.C.; Peterson, R.; Moore, W.S.; de Oliveira, J. Radon and radium isotopes as tracers of submarine groundwater discharge—Results from the Ubatuba, Brazil SGD assessment intercomparison. Estuar. Coast. Shelf Sci. 2008, 76, 501–511. [Google Scholar] [CrossRef]
- Moore, W.S.; Sarmiento, J.L.; Key, R.M. Submarine groundwater discharge revealed by 228Ra distribution in the upper Atlantic Ocean. Nat. Geosci. 2008, 1, 309–311. [Google Scholar] [CrossRef]
- Moore, W.S. Fifteen years experience in measuring 224Ra and 223Ra by delayed-coincidence counting. Mar. Chem. 2008, 109, 188–197. [Google Scholar] [CrossRef]
- Swarzenski, P.W.; Reich, C.; Kroeger, K.D.; Baskaran, M. Ra and Rn isotopes as natural tracers of submarine groundwater discharge in Tampa Bay, Florida. Mar. Chem. 2007, 104, 69–84. [Google Scholar] [CrossRef]
- Moore, W.S.; Krest, J. Distribution of 223Ra and 224Ra in the plumes of the Mississippi and Atchafalaya Rivers and the Gulf of Mexico. Mar. Chem. 2004, 86, 105–119. [Google Scholar] [CrossRef]
- Knee, K.L.; Garcia-solsona, E.; Garcia-orellana, J.; Boehm, A.B.; Paytan, A. Using radium isotopes to characterize water ages and coastal mixing rates: A sensitivity analysis. Limnol. Oceanogr. Methods 2011, 9, 380–395. [Google Scholar] [CrossRef]
- Shaw, R.D.; Prepas, E.E. Groundwater-lake interactions: I. Accuracy of seepage meter estimates of lake seepage. J. Hydrol. 1990, 119, 105–120. [Google Scholar] [CrossRef]
- Whalen, S.C.; Cornwell, J.C. Nitrogen, phosphorus, and organic carbon cycling in an Arctic lake. Can. J. Fish. Aquat. Sci. 1985, 42, 797–808. [Google Scholar] [CrossRef]
- Wilson, J.; Rocha, C. Regional scale assessment of submarine groundwater discharge in Ireland combining medium resolution satellite imagery and geochemical tracing techniques. Remote Sens. Environ. 2012, 119, 21–34. [Google Scholar] [CrossRef]
- Charette, M.A.; Sholkovitz, E.R. Trace element cycling in a subterranean estuary: Part 2. Geochemistry of the pore water. Geochim. Cosmochim. Acta 2006, 70, 811–826. [Google Scholar] [CrossRef]
- Charette, M.A.; Sholkovitz, E.R.; Hansel, C.M. Trace element cycling in a subterranean estuary: Part 1. Geochemistry of the permeable sediments. Geochim. Cosmochim. Acta 2005, 69, 2095–2109. [Google Scholar] [CrossRef]
- Michael, H.A.; Charette, M.A.; Harvey, C.F. Patterns and variability of groundwater flow and radium activity at the coast: A case study from Waquoit Bay, Massachusetts. Mar. Chem. 2011, 127, 100–114. [Google Scholar] [CrossRef]
- Kwon, E.; Kim, G.; Primeau, F.; Moore, W.; Cho, H.-M.; DeVries, T.; Sarmiento, J.; Charette, M.; Cho, Y.-K. Global estimate of submarine groundwater discharge based on an observationally constrained radium isotope model. Geophys. Res. Lett. 2014, 62–68. [Google Scholar] [CrossRef]
- Charette, M.A.; Morris, P.J.; Henderson, P.B.; Moore, W.S. Radium Isotope Distributions during the US GEOTRACES North Atlantic cruises. Mar. Chem. 2015, 177, 184–195. [Google Scholar] [CrossRef]
- Vonk, J.E.; Tank, S.E.; Bowden, W.B.; Laurion, I.; Vincent, W.F.; Alekseychik, P.; Amyot, M.; Billet, M.F.; Canrio, J.; Cory, R.M.; et al. Reviews and syntheses: Effects of permafrost thaw on Arctic aquatic ecosystems. Biogeosciences 2015, 12, 7129–7167. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Hinzman, L.D. Shrinking thermokarst ponds and groundwater dynamics in discontinuous permafrost near council, Alaska. Permafr. Periglac. Process. 2003, 14, 151–160. [Google Scholar] [CrossRef]
- Osterkamp, T.E. The recent warming of permafrost in Alaska. Glob. Planet. Change 2005, 49, 187–202. [Google Scholar] [CrossRef]
- Osterkamp, T.E. Characteristics of the recent warming of permafrost in Alaska. J. Geophys. Res. Surf. 2007, 112, F02S02. [Google Scholar] [CrossRef]
- Shiklomanov, I.A.; Shiklomanov, A.I. Climatic change and the dynamics of river runoff into the Arctic Ocean. Water Resour. 2003, 30, 593–601. [Google Scholar] [CrossRef]
- Dimova, N.T.; Paytan, A.; Kessler, J.D.; Sparrow, K.J.; Garcia-Tigreros Kodovska, F.; Lecher, A.L.; Murray, J.; Tulaczyk, S.M. Current magnitude and mechanisms of groundwater discharge in the Arctic: Case study from Alaska. Environ. Sci. Technol. 2015, 49, 12036–12043. [Google Scholar] [CrossRef] [PubMed]
- Lecher, A.L.; Chuang, P.; Singleton, M.; Paytan, A. Sources of methane to an Arctic lake in Alaska: An Isotopic Investigation. J. Geophys. Res. Biogeosci. 2017, 122, 753–766. [Google Scholar] [CrossRef]
- Dugan, H.A.; Gleeson, T.; Lamoureuz, S.F.; Novakowski, K. Tracing groundwater discharge in a High Arctic lake using radon-222. Environ. Earth Sci. 2012, 66, 1385–1392. [Google Scholar] [CrossRef]
- Kies, A.; Nawrot, A.; Tosheva, Z.; Jania, J. Natural radioactive isotopes in glacier meltwater studies. Geochem. J. 2011, 45, 423–429. [Google Scholar] [CrossRef]
- Linhoff, B.S.; Charette, M.A.; Nienow, P.W.; Wadham, J.L.; Tedstone, A.J.; Cowton, T. Utility of 222Rn as a passive tracers of subglacial distributed system drainage. Earth Planet. Sci. Lett. 2017, 462, 180–188. [Google Scholar] [CrossRef]
- Bhatia, M.P.; Das, S.B.; Kujawinski, E.B.; Henderson, P.; Burke, A.; Charette, M.A. Seasonal evolution of water contributions to discharge from a Greenland outlet glacier: Insight from a new isotope-mixing model. J. Glaciol. 2011, 57, 929–941. [Google Scholar] [CrossRef]
- Lecher, A.L.; Chien, C.; Paytan, A. Submarine groundwater discharge as a source of nutrients to the North Pacific and Arctic coastal ocean. Mar. Chem. 2016, 186, 167–177. [Google Scholar] [CrossRef]
- Charkin, A.N.; Rutgets van der Loeff, M.; Shakhova, N.E.; Gustafsson, O.; Dudarev, O.V.; Cherepnev, M.S.; Salyuk, A.N.; Koshurnikov, A.V.; Spivak, E.A.; Gunar, A.Y.; et al. Discovery and characterization of submarine groundwater discharge in the Aiberian Arctic seas: A case study of the Buor-Khaya Gulf, Laptev Sea. Cryosph. Discuss. 2017. in review. [Google Scholar] [CrossRef]
- Deming, D.; Sass, J.H.; Lachenbruch, A.H.; De Rito, R.F. Heat flow and subsurface temperature as evidence for basin-scale ground-water flow, North Slope of Alaska. Geol. Soc. Am. Bull. 1992, 104, 528–542. [Google Scholar] [CrossRef]
- Frederick, J.M.; Buffett, B.A. Submarine groundwater discharge as a possible formation mechanism for permafrost-associated gas hydrate on the circum-Arctic continental shelf. J. Geophys. Res. B Solid Earth 2016, 121, 1383–1404. [Google Scholar] [CrossRef]
- Hay, A.E. Remote acoustic imaging of the plume from a submarine spring in an Arctic Fjord. Science 1984, 225, 1154. [Google Scholar] [CrossRef] [PubMed]
- Bense, V.F.; Ferguson, G.; Kooi, H. Evolution of shallow groundwater flow systems in areas of degrading permafrost. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef]
- Toohey, R.C.; Herman-Mercer, N.M.; Schuster, P.F.; Mutter, E.A.; Koch, J.C. Multidecadal increases in the Yukon River Basin of chemical fluxes as indicators of changing flowpaths, groundwater, and permafrost. Geophys. Res. Lett. 2016, 43, 12120–12130. [Google Scholar] [CrossRef]
- Tranter, M.; Brown, G.; Raiswell, R.; Sharp, M.; Gurnell, A. A conceptual model of solute acquisition by Alpine glacial meltwaters. J. Glaciol. 1993, 39, 573–581. [Google Scholar] [CrossRef]
- Tranter, M.; Raiswell, R. The composition of the englacial and subglacial component in bulk meltwaters draining the Gornergletscher, Switzerland. J. Glaciol. 1991, 37, 59–66. [Google Scholar] [CrossRef]
- Raiswell, R. Chemical models of solute aquisition in glacial meltwaters. J. Glaciol. 1984, 30, 49–57. [Google Scholar] [CrossRef]
- Bartholomaus, T.C.; Anderson, R.S.; Anderson, S.P. Growth and collapse of the distributed subglacial hydrologic system of Kennicott Glacier, Alaska, USA and its effects on basal motion. J. Glaciol. 2011, 57, 985–1002. [Google Scholar] [CrossRef]
- Hawkings, J.R.; Wadham, J.L.; Tranter, M.; Raiswell, R.; Benning, L.G.; Statham, P.J.; Tedstone, A.; Nienow, P.; Lee, K.; Telling, J. Ice sheets as a significant source of highly reactive nanoparticulate iron to the oceans. Nat. Commun. 2014, 5, 3929. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.P.; Kujawinski, E.B.; Das, S.B.; Breier, C.F.; Henderson, P.B.; Charette, M.A. Greenland meltwater as a significant and potentially bioavailable source of iron to the ocean. Nat. Geosci. 2013, 6, 274–278. [Google Scholar] [CrossRef]
- Siegel, D.I. Evidence for dilution of deep, confined ground water by vertical recharge of isotopically heavy Pleistocene water. Geology 1991, 19, 433–436. [Google Scholar] [CrossRef]
- Garcia-Tigreros Kodovska, F.; Sparrow, K.J.; Yvon-Lewis, S.A.; Paytan, A.; Dimova, N.T.; Lecher, A.; Kessler, J.D. Dissolved methane and carbon dioxide fluxes in Subarctic and Arctic regions: Assessing measurement techniques and spatial gradients. Earth Planet. Sci. Lett. 2016, 436, 43–55. [Google Scholar] [CrossRef]
- Payette, S.; Delwaide, A.; Caccianiga, M.; Beauchemin, M. Accelerated thawing of subarctic peatland permafrost over the last 50 years. Geophys. Res. Lett. 2004, 31, L18208. [Google Scholar] [CrossRef]
- Smith, L.C.; Sheng, Y.; MacDonald, G.M.; Hinzman, L.D. Disappearing Arctic lakes. Science 2005, 308, 1429. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Birks, J.; Rover, J.; Guldager, N. Controls on recent Alaskan lake changes identified from water isotopes and remote sensing. Geophys. Res. Lett. 2013, 40, 3413–3418. [Google Scholar] [CrossRef]
- Blake, L.I.; Tveit, A.; Overeas, L.; Head, I.M.; Gray, N.D. Response of methanogens in Arctic sediments to temperature and methanogenic substrate availability. PLoS ONE 2015, 10, e0129733. [Google Scholar] [CrossRef] [PubMed]
- Hinzman, L.D.; Bettez, N.D.; Bolton, W.R.; Chapin, F.S.; Dyurgerov, M.B.; Fastie, C.L.; Griffith, B.; Hollister, R.D.; Hope, A.; Huntington, H.P.; et al. Evidence and implications of recent climate change in northern Alaska and other Arctic regions. Clim. Chang. 2005, 72, 251–298. [Google Scholar] [CrossRef]
- Clein, J.; McGuire, A.D.; Euskirchen, E.S.; Calef, M. The effects of different climate input datasets on simulated carbon dynamics in the Western Arctic. Earth Interact. 2007, 11, 1–24. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Aiken, G.R.; Swanson, D.K.; Panda, S.; Butler, K.D.; Baltensperger, A.P. Dissolved organic matter compostition of Arctic rivers: Linkingpermafrost and parent material to riverine carbon. Glob. Biogeochem. Cycles 2016, 30, 1811–1826. [Google Scholar] [CrossRef]
- Osterkamp, T.E.; Romanovsky, V.E. Evidence for warming and thawing of discontinuous permafrost in Alaska. Permafr. Periglac. Process. 1999, 10, 17–37. [Google Scholar] [CrossRef]
- Stieglitz, M.; Dery, S.J.; Romanovsky, V.E.; Osterkamp, T.E. The role of snow cover in the warming of arctic permafrost. Geophys. Res. Lett. 2003, 30, 1712. [Google Scholar] [CrossRef]
- McClelland, J.W.; Stieglitz, M.; Pan, F.; Holmes, R.M.; Peterson, B.J. Recent changes in nitrate and dissolved organic carbon export from the upper Kuparuk River, North Slope, Alaska. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef]
- Tremblay, J.É.; Simpson, K.; Martin, J.; Miller, L.; Gratton, Y.; Barber, D.; Price, N.M. Vertical stability and the annual dynamics of nutrients and chlorophyll fluorescence in the coastal, southeast Beaufort Sea. J. Geophys. Res. Oceans 2008, 113, 1–14. [Google Scholar] [CrossRef]
- Pabi, S.; van Dijken, G.L.; Arrigo, K.R. Primary production in the Arctic Ocean, 1998–2006. J. Geophys. Res. Oceans 2008, 113, 1998–2006. [Google Scholar] [CrossRef]
- Frey, K.E.; McClelland, J.W. Impacts of permafrost degradation on arctic river biogeochemistry. Hydrol. Process. 2009, 23, 169–182. [Google Scholar] [CrossRef]
- Hood, E.; Berner, L. Effects of changing glacial coverage on the physical and biogeochemical properties of coastal streams in southeastern Alaska. J. Geophys. Res. Biogeosci. 2009, 114. [Google Scholar] [CrossRef]
- Knee, K.L.; Crook, E.D.; Hench, J.L.; Leichter, J.J.; Paytan, A. Assessment of submarine groundwater discharge (SGD) as a source of dissolved radium and nutrients to Moorea (French Polynesia) coastal waters. Estuaries Coasts 2016, 39, 1651–1668. [Google Scholar] [CrossRef]
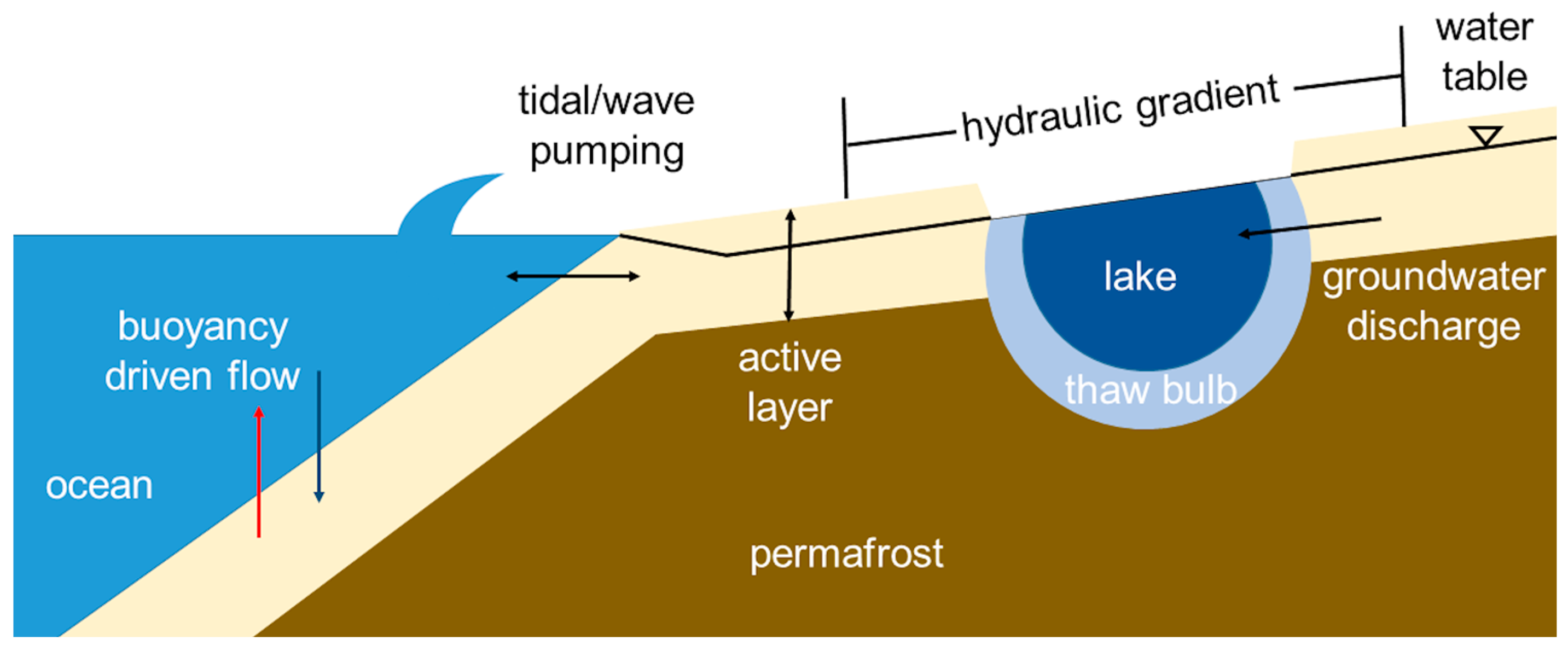
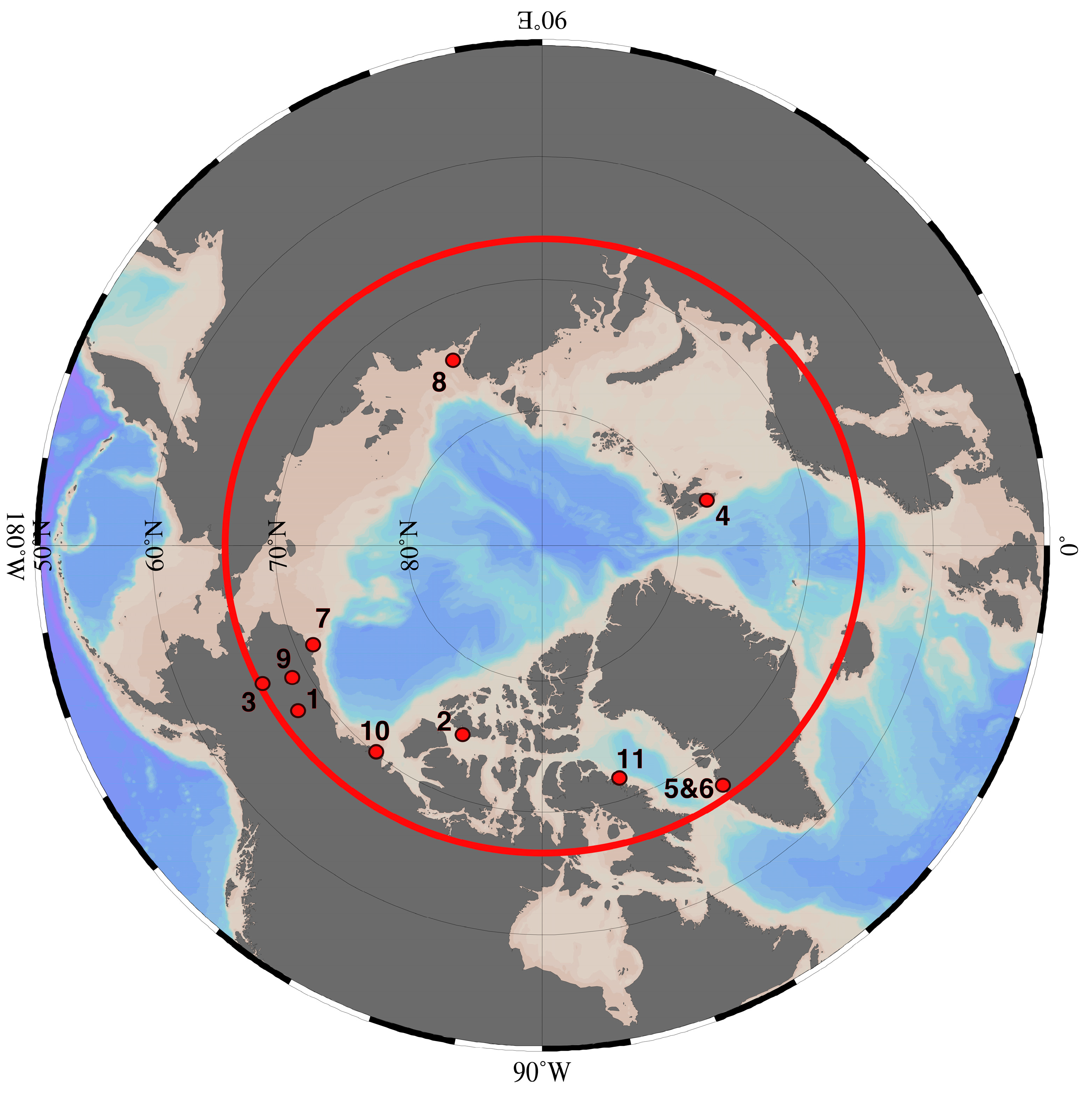
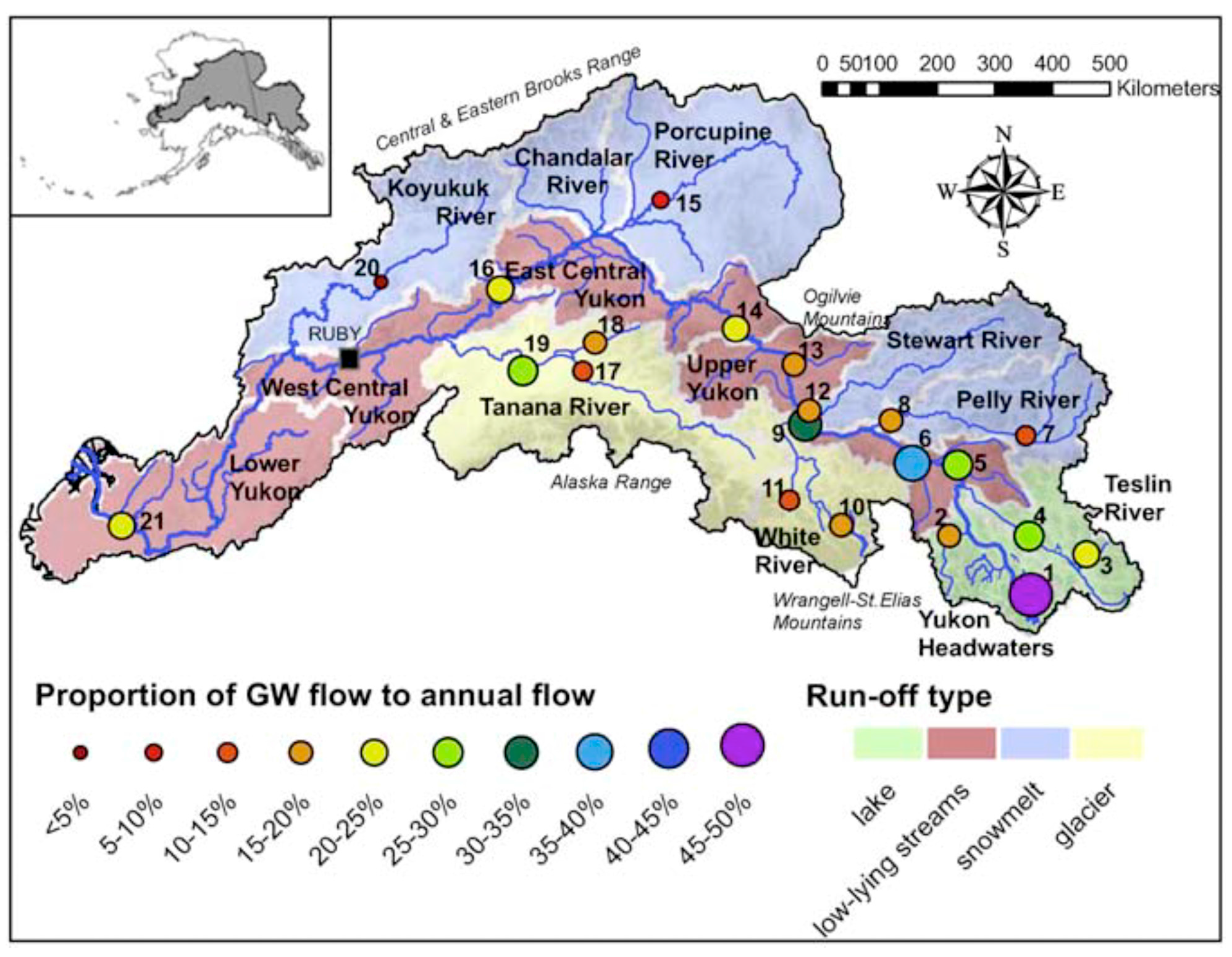
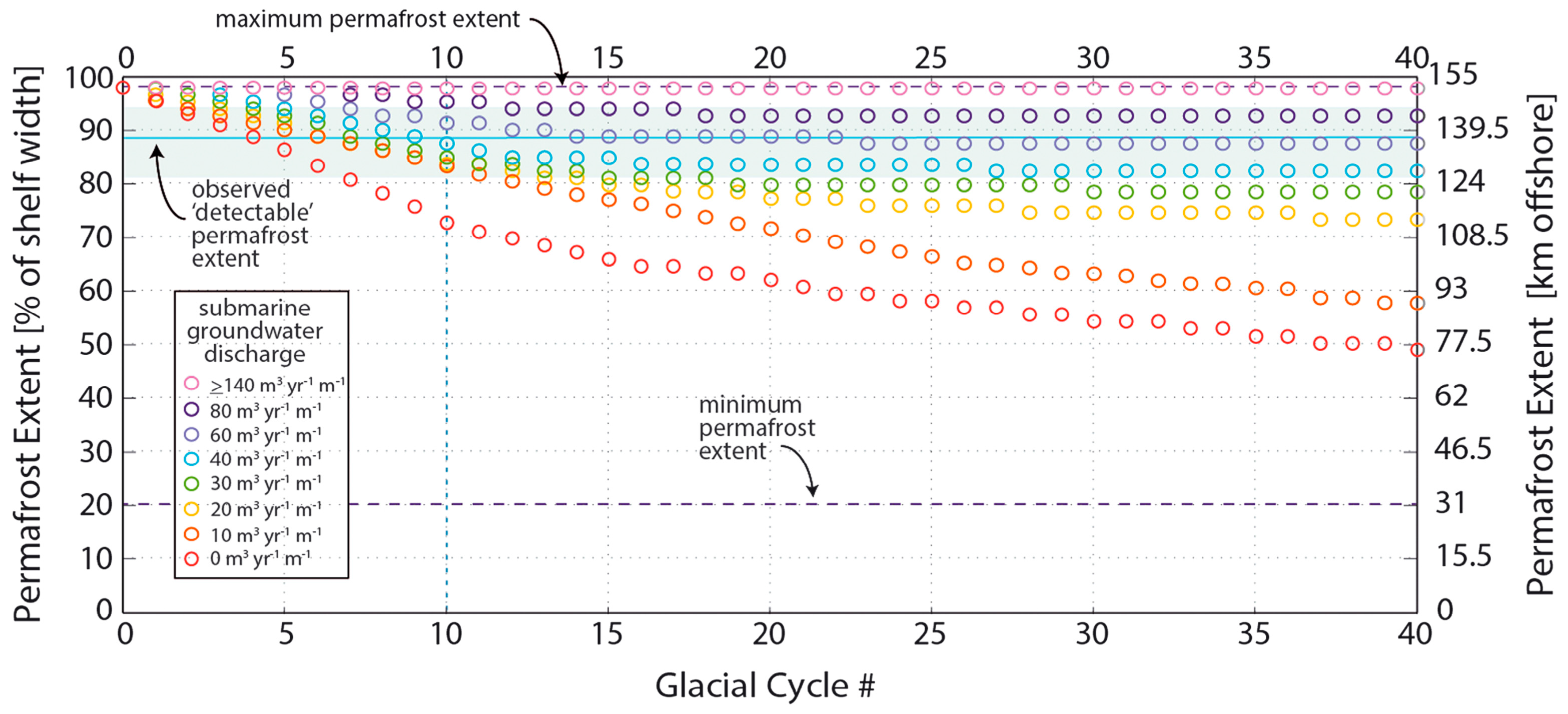
| Figure 2 Number | Location | Groundwater Discharge | Reference(s) |
|---|---|---|---|
| Terrestrial | |||
| 1 | Toolik Lake | 1.6–2.1 × 104 m3 day−1 1.25 ± 1.15 cm day−1 not calculated 3.64 × 104 m3 day−1 | Paytan et al. [20] Dimova et al. [41] Lecher et al. [42] Whalen and Cornwell [29] |
| 2 | Shellabear Lake | not calculated | Dugan et al. [43] |
| 3 | Yukon River Basin | up to 50% of river flow | Walvoord and Striegl [2] |
| 4 | Werenskiold Glacier | not calculated | Kies et al. [44] |
| 5 | Leverette Glacier | 1–5% of river flow | Linhoff et al. [45] |
| 6 | ‘N’ Glacier | 3.4–26% of outflow | Bhatia et al. [46] |
| Marine | |||
| 7 | Barrow | 13.0 ± 0.2 m3 day−1 m−1 1.0 ± 0.3 cm day−1 | Lecher et al. [9] Dimova et al. [41] Lecher et al. [47] |
| 8 | Laptev Sea | not calculated | Charkin et al. [48] |
| 9 | North Slope of Alaska | not calculated | Deming et al. [49] |
| 10 | Mackenzie River Mouth | 60–140 m3 yr−1 m−1 | Frederick and Buffett [10] Frederick and Buffett [50] |
| 11 | Cambridge Fjord | 0.14 m3 s−1 | Hay [51] |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecher, A.L. Groundwater Discharge in the Arctic: A Review of Studies and Implications for Biogeochemistry. Hydrology 2017, 4, 41. https://doi.org/10.3390/hydrology4030041
Lecher AL. Groundwater Discharge in the Arctic: A Review of Studies and Implications for Biogeochemistry. Hydrology. 2017; 4(3):41. https://doi.org/10.3390/hydrology4030041
Chicago/Turabian StyleLecher, Alanna L. 2017. "Groundwater Discharge in the Arctic: A Review of Studies and Implications for Biogeochemistry" Hydrology 4, no. 3: 41. https://doi.org/10.3390/hydrology4030041
APA StyleLecher, A. L. (2017). Groundwater Discharge in the Arctic: A Review of Studies and Implications for Biogeochemistry. Hydrology, 4(3), 41. https://doi.org/10.3390/hydrology4030041





