Lithological Identification and Underground Water Conditions in Jeddo Using Geophysical and Geochemical Methods
Abstract
:1. Introduction
2. Geology of Study Area
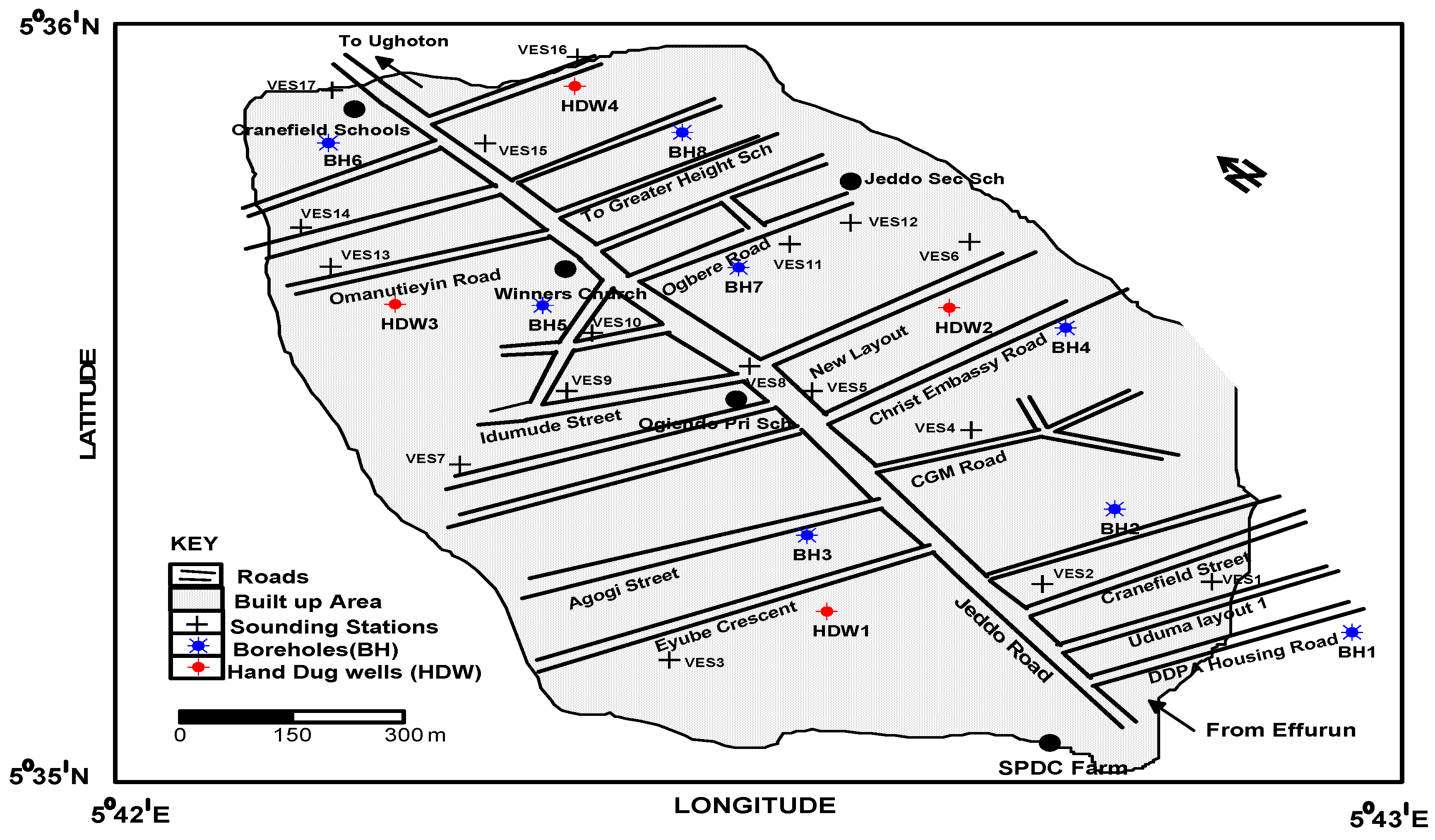
3. Methodology
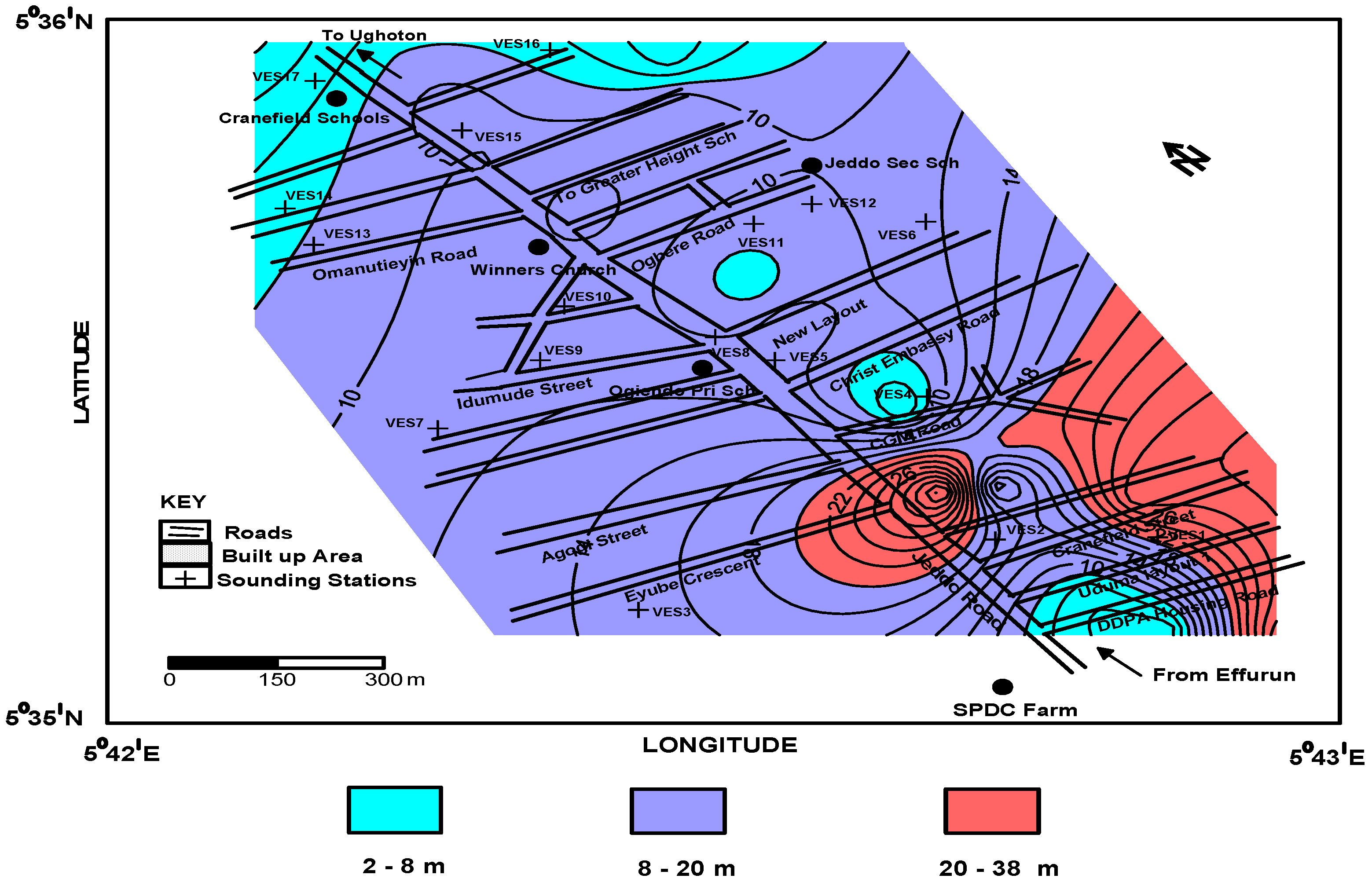
4. Results and Data Analysis
4.1. Geoelectric Model
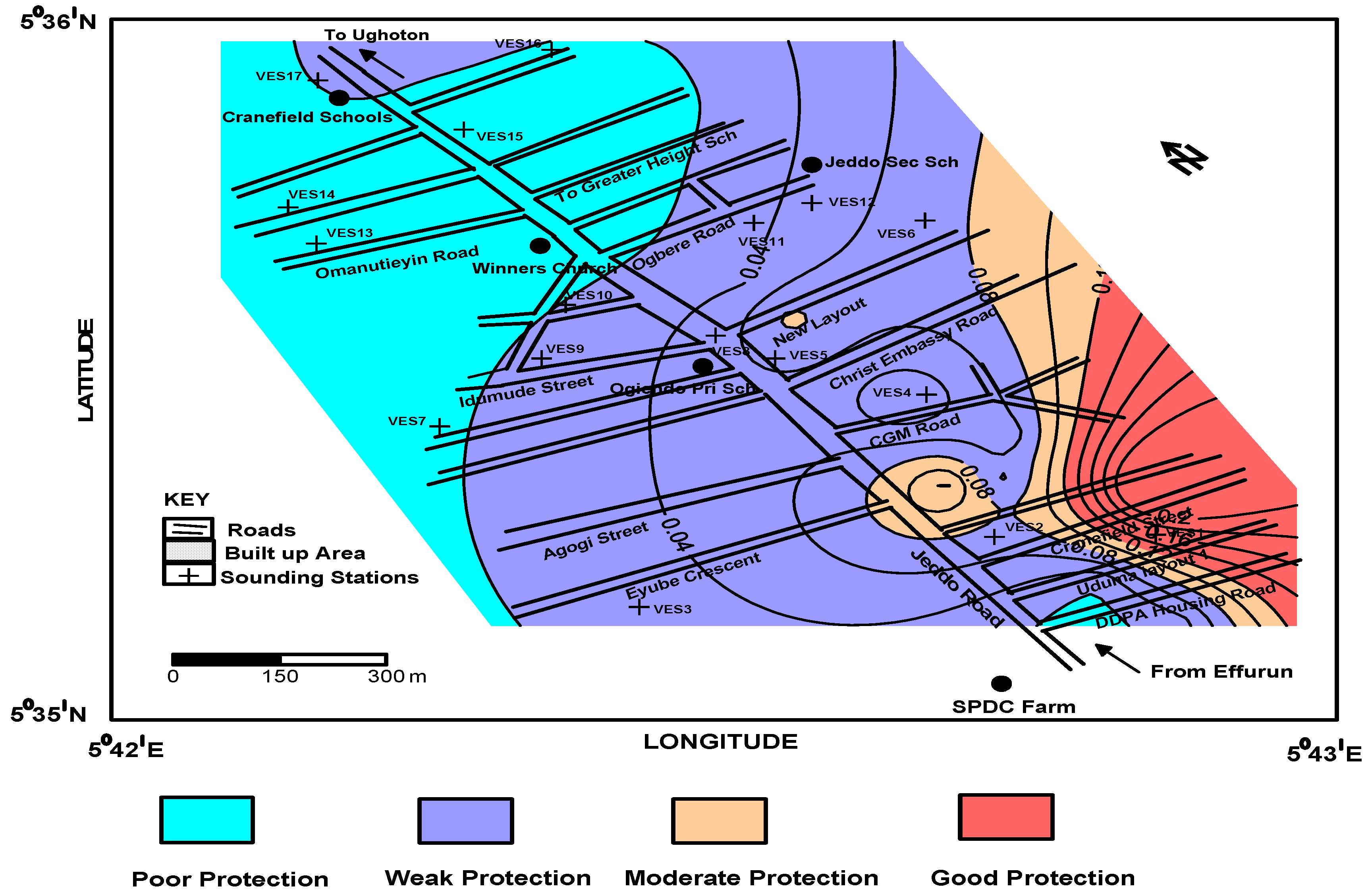
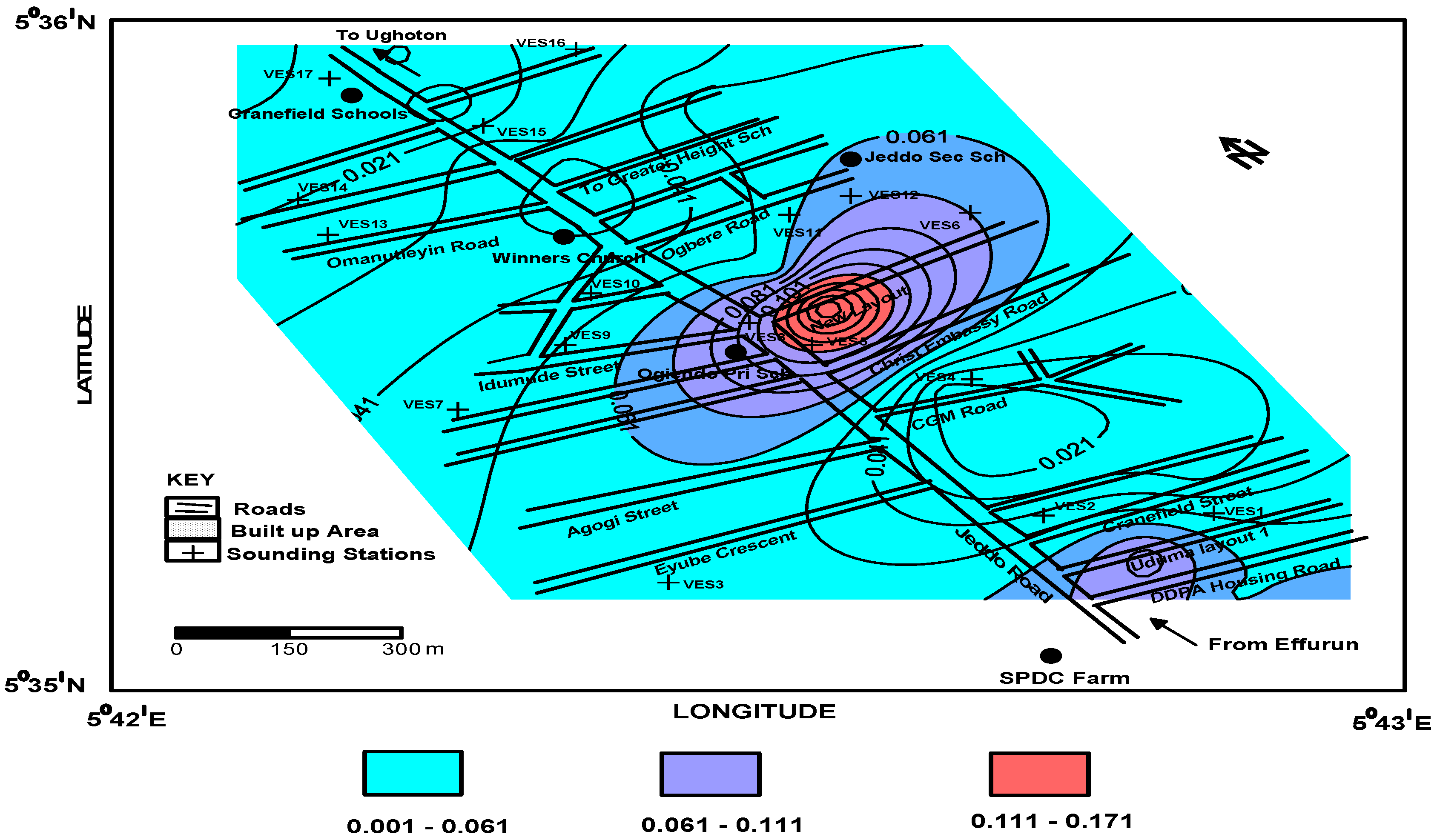
4.2. Aquifer Protective Capacity
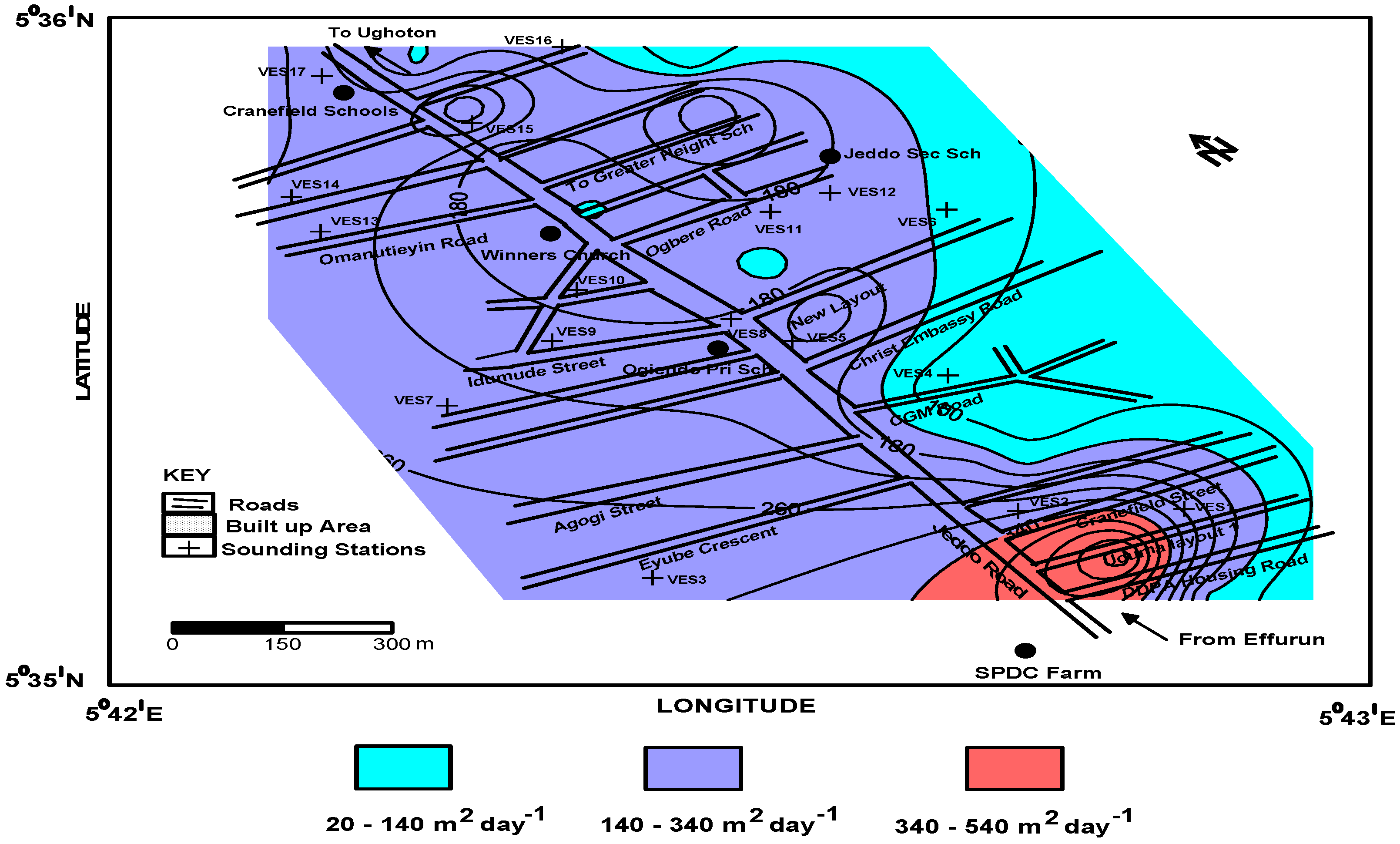
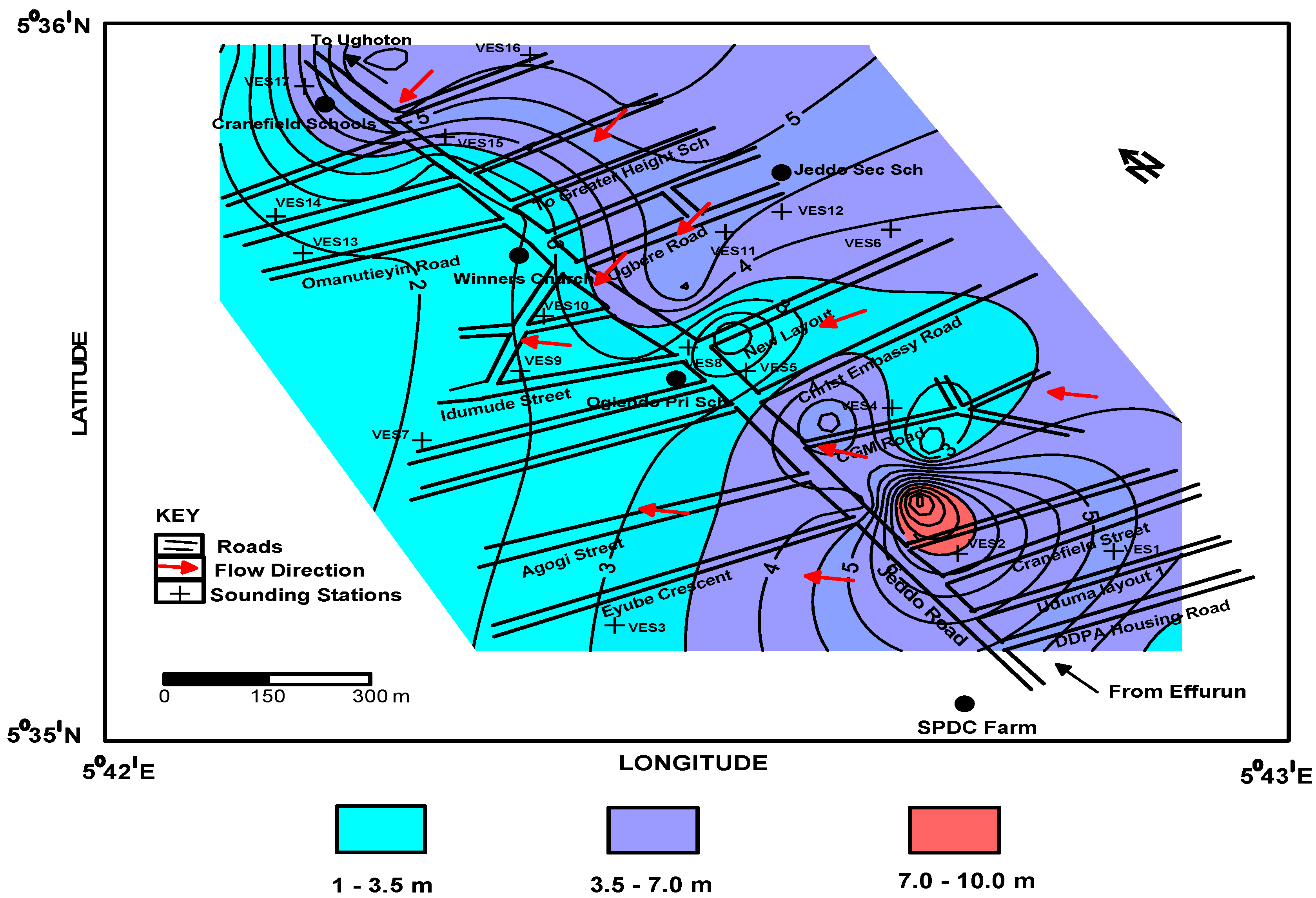
4.3. Aquifer Transmissivity
4.4. Hydrogeochemical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akpoborie, I.A.; Uriri, A.E.; Efobo, O. Ground water conditions and spatial distribution of lead and cadmium in the shallow aquifer at Effurun-Warri metropolis, Nigeria. Environ. Pollut. 2014, 3, 27–37. [Google Scholar] [CrossRef]
- Adejuwon, O.A. Rainfall seasonality in the Niger delta belt, Nigeria. J. Geogr. Reg. Plan. 2012, 5, 51–60. [Google Scholar]
- Ward, S.H. Resistivity and induced polarization methods. Geophysics 1990, 1, 147–189. [Google Scholar]
- William, L. Fundamentals of Geophysics; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Niwas, S.W.; Singhai, D.C. Estimation of aquifer transmissivity from Dar-Zarrouk parameters in porous media. J. Hydrol. 1981, 50, 393–399. [Google Scholar] [CrossRef]
- Aristedemou, E.; Thomas-Betts, A. DC Resistivity and induced polarization investigations at a waste disposal site and its environments. J. Appl. Geophys. 2000, 44, 275–302. [Google Scholar] [CrossRef]
- Hubbard, S.S.; Rubin, Y. Hydrogeophysics; Springer: Dordrecht, The Netherlands, 2005; Volume 50, p. 523. [Google Scholar]
- Butler, J.J., Jr. Hydrogeological Methods for Estimation of Spatial Variations in Hydraulic Conductivity; Springer: Dordrecht, The Netherlands, 2005; pp. 23–58. [Google Scholar]
- Karlik, G.; Kaya, A.M. Investigation of groundwater contamination using electric and electromagnetic methods at an open Waste-Disposal site: A case study from Isparta, Turkey. Environ. Geol. 2001, 40, 725–731. [Google Scholar] [CrossRef]
- Anomohanran, O. Determination of groundwater in Asaba, Delta State, Nigeria. Int. J. Phys. Sci. 2011, 6, 7651–7659. [Google Scholar]
- Jegede, S.I.; Ujuanbi, O.; Abdullahi, N.K.; Iserhien-Emekeme, R.E. Mapping and monitoring of leachate plume migration at an open waste disposal site using Non-Invasive methods. Res. J. Environ. Earth Sci. 2012, 4, 26–33. [Google Scholar]
- Udoinyang, I.E.; Igboekwu, M.U. A quifer transmissivity, Dar-Zarroukparameters and the direction of flow of suspended particulate matter in boreholes in MOUAU and the Kwa Ibo river, Umudike-Nigeria. Greener J. Phys. Sci. 2012, 2, 70–84. [Google Scholar]
- Imali, H.; Dharmagunwardhane, H.A. Use of Resistivity Sounding Results for Estimating Transmissivity of Aquifers: A Case Study from North Central Province, Sri Lanka. In Proceedings of the 29th Technical Sessions of Geophysical Society of Sri Lanka, Jinadasa Katupotha, 22 February 2013; pp. 21–23. [Google Scholar]
- Iserhien-Emekeme, R.E. Electrical resistivity survey for predicting aquifer at Onicha-Ugbo, Delta State, Nigeria. J. Appl. Math. Phys. 2014, 2, 520–527. [Google Scholar] [CrossRef]
- Anomohanran, O.; Iserhien-Emekeme, R.E. Estimation of aquifer parameters in Erho, Nigeria using the Cooper-Jacob evaluation method. Am. J. Environ. Sci. 2014, 10, 500–508. [Google Scholar] [CrossRef]
- Ofomola, M.O. Mapping of aquifer contamination using Geoelectric methods at a municipal solid waste disposal site in Warri, Southern Nigeria. J. Appl. Geol. Geophys. 2015, 3, 39–47. [Google Scholar]
- Short, K.C.; Stauble, A.J. Outline of the Niger delta. Bull. Am. Assoc. Pet. Geol. 1967, 54, 761–779. [Google Scholar]
- Murat, R.C. Stratigraphy and paleogeography of the cretaceous and lower tertiary in Southern Nigeria. In African Geology; University of Ibadan Press: Ibadan, Nigeria, 1970; pp. 635–648. [Google Scholar]
- Asseez, O.L. Review of the stratigraphy, sedimentation and structure of the Niger delta. In Geology of Nigeria; Kogbe, C.A., Ed.; Rock View (Nig.) Ltd.: Jos, Nigeria, 1989; pp. 311–324. [Google Scholar]
- Nwajide, C.S. A Guide for Geological Field Trips to Anambra and Related Sedimentary Basins in Southeastern Nigeria; Petroleum Development Trust Fund, University of Nigeria, Nsukka: Nsukka, Nigeria, 2006; p. 68. [Google Scholar]
- Tamunosiki, D.; Ming, G.H.; Uko, E.D.; Tamunobereton-ari, I.; Emudianughe, J.E. Porosity modeling of the South-East Niger delta basin, Nigeria. Int. J. Geol. Earth Environ. Sci. 2014, 4, 49–60. [Google Scholar]
- Henriet, J.P. Direct applications of the Dar Zarrouk Parameters in groundwater surveys. Geophys. Prospect. 1975, 24, 344–353. [Google Scholar] [CrossRef]
- Oladapo, M.I.; Mohammed, M.Z.; Adeoye, O.O.; Adetola, B.A. Geoelectrical investigation of the Ondo State Housing Corporation Estate, IjapoAkure Southwestern Nigeria. J. Min. Geol. 2004, 40, 41–48. [Google Scholar]
- Ofomola, M.O. Aquifer Characterization and groundwater quality studies in part of Niger Delta Area using geoelectric and hydrogeochemical methods. Niger. J. Phys. 2014, 25, 96–106. [Google Scholar]
- Mundel, J.A.; Lother, L.; Oliver, E.M.; Allen-long, S. Aquifer Vulnerability Analysis for Water Resource Production; Indian Department of Environmental Management: Indianapolis, IN, USA, 2003; Volume 25.
- Akpoborie, I.A.; Ekakite, O.A.; Adaikpoh, E.O. The quality of groundwater from dug wells in parts of the Western Niger Delta. Knowl. Rev. 2000, 2, 72–75. [Google Scholar]
- Gheorghe, A. Processing and Synthesis of Hydrological Data; Abacus Press: Tumbridge Wells, UK, 1978; pp. 122–136. [Google Scholar]
- Okiongbo, K.S.; Akpofure, E. Determination of aquifer properties and groundwater vulnerability mapping using geoelectric method in Yenagoa city and its environs in Bayelsa State, South Nigeria. J. Water Resour. Prot. 2012, 4, 354–362. [Google Scholar] [CrossRef]
- Uma, K.O. An appraisal of the groundwater resources of the Imo River Basin, Nigeria. J. Min. Geol. 1989, 25, 305–315. [Google Scholar]
- Buddermeier, R.W.; Schloss, J.A. Groundwater Storage and Flow. 2000. Available online: http://www.kgs.Ukans.edu/Height plains/atlas//apgengw.htm (accessed on 25 March 2017).
- Fetter, C.W. Applied Hydrology, 3rd ed.; Macmillan College Publishing Company, Inc.: New York, NY, USA, 1994; Volume 114. [Google Scholar]
- Amadi, A.N. Assessing the effects of aladimma dumpsite on soil and groundwater using water quality index and factor analysis. Aust. J. Basic Appl. Sci. 2011, 5, 763–770. [Google Scholar]
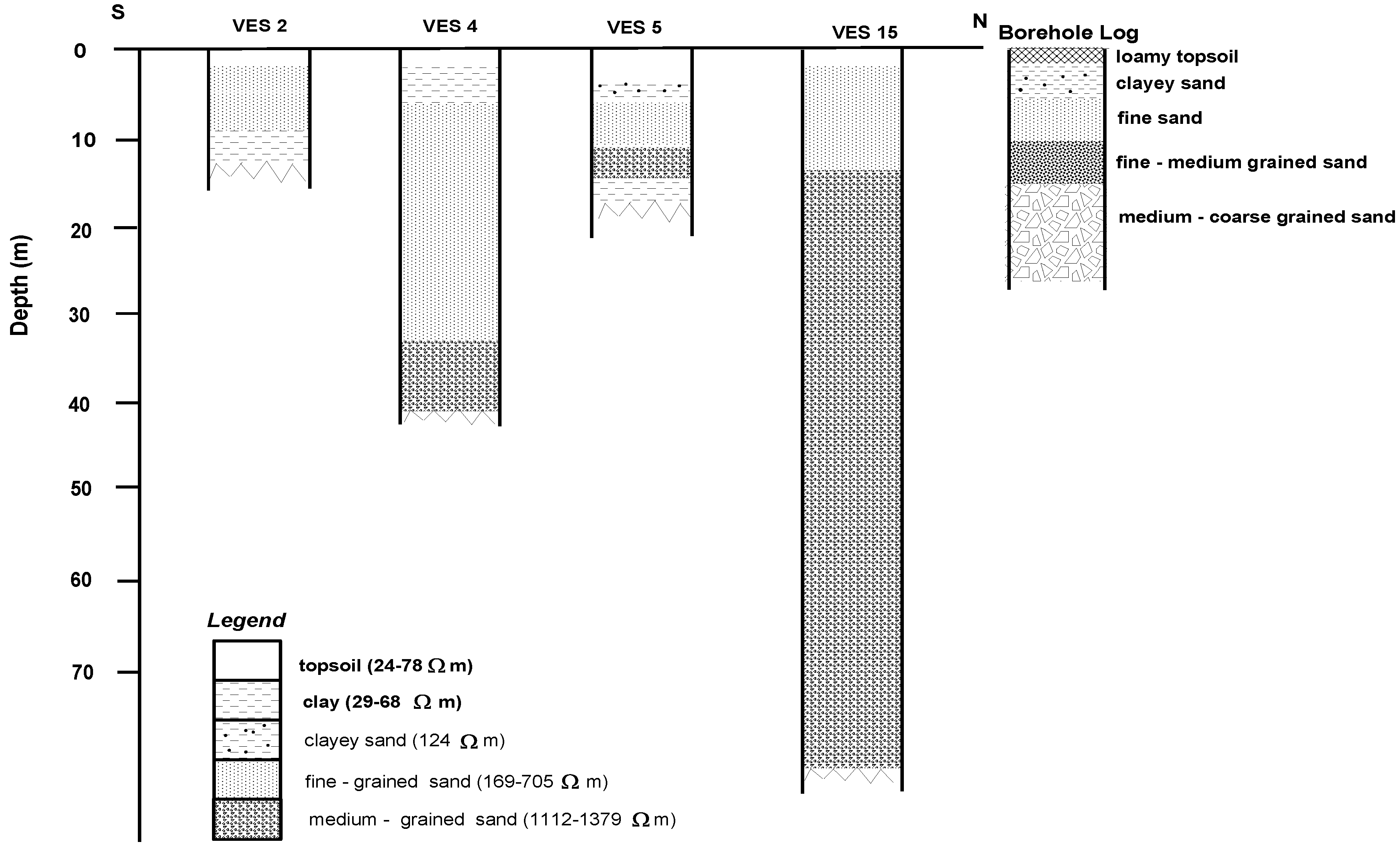
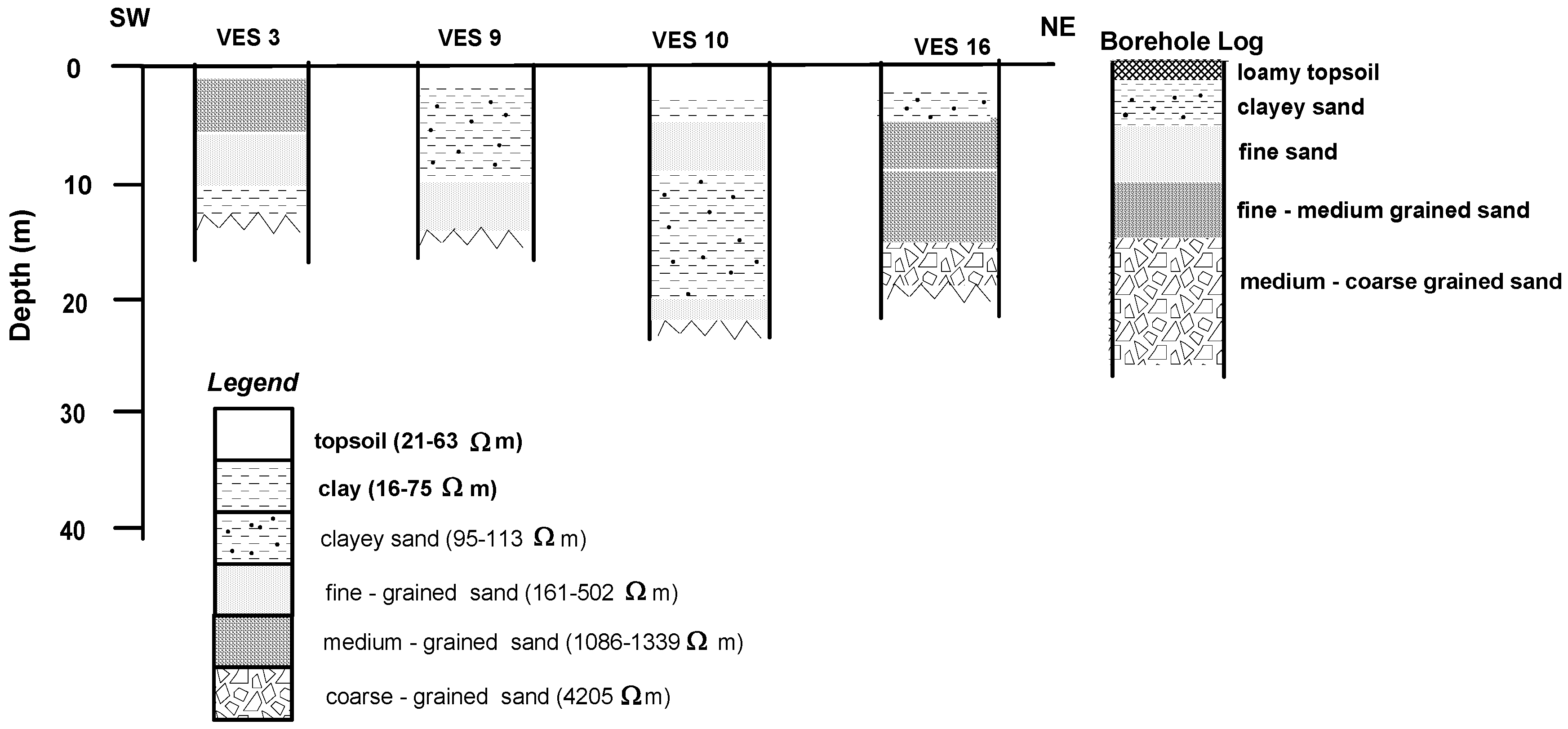
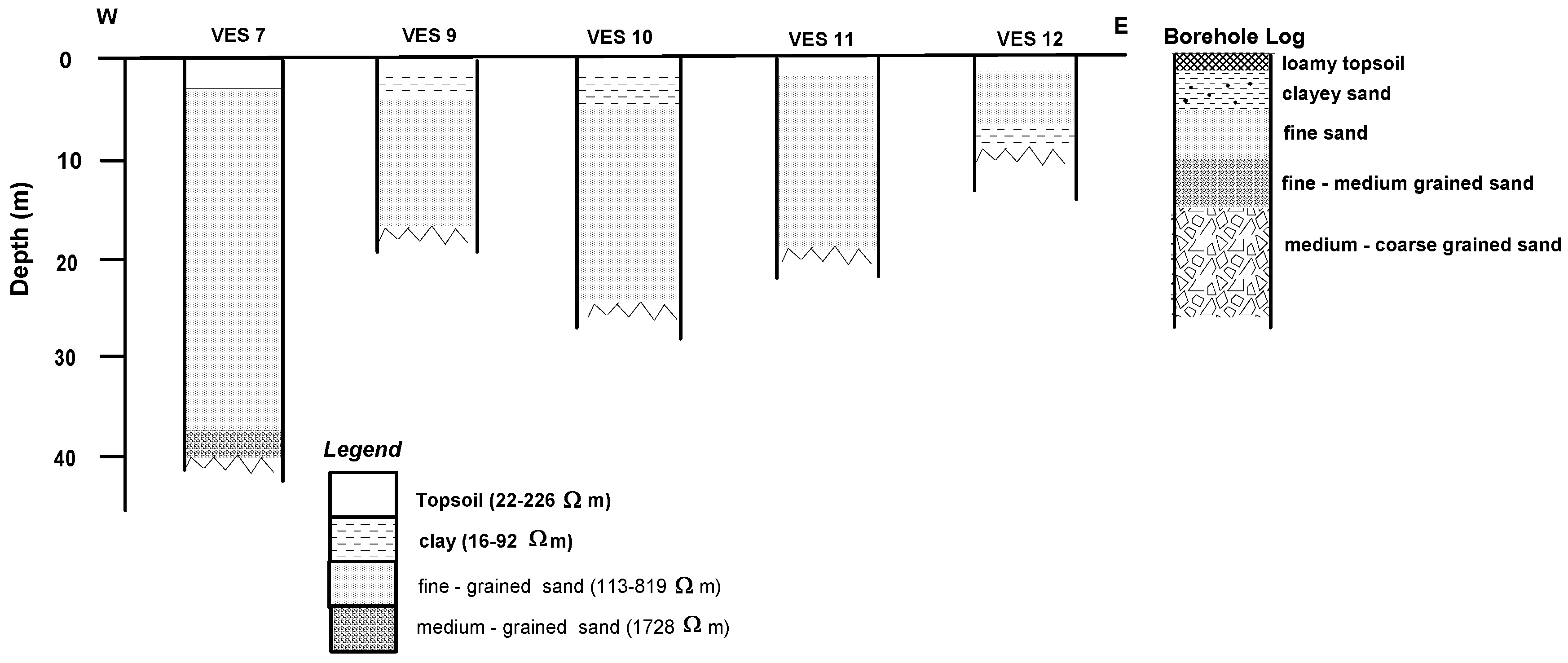

| Nature of Sediment | Lithology (Inferred) | Resistivity (Ωm) |
|---|---|---|
| Unsaturated sediment | Top soil | |
| Saturated sediments | Clay | |
| Clayey sand | ||
| Fine-grained sand | ||
| Medium-grained sand | ||
| Coarse sand | 4205 |
| VES STATION | Latitude | Longitude | Elevation (m) | Aquifer Resistivity (Ωm) | Depth to Aquifer (m) | Aquifer Thickness (m) | LongitudinalConductance (Ω−1) | TransverseResistance (Ωm2) | Conductivity, σ (Ω−1) | Kσ | Transmissivity, (m2 day−1) | Static Water Level | Aquifer Protective Capacity (Longitudinal Conductance) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.5881 | 5.7120 | 6.0 | 392 | 31.4 | 2.30 | 0.127 | 913 | 0.002550 | 0.0676 | 61.7 | 3.12 | Good |
| 2 | 5.5882 | 5.7104 | 6.3 | 402 | 2.5 | 5.80 | 0.035 | 2321 | 0.002490 | 0.0600 | 139.3 | 4.14 | Weak |
| 3 | 5.5890 | 5.7092 | 7.7 | 283 | 4.8 | 22.01 | 0.024 | 6231 | 0.003530 | 0.0865 | 334.0 | 5.49 | Weak |
| 4 | 5.5908 | 5.7099 | 8.1 | 1112 | 29.8 | 4.14 | 0.261 | 4603 | 0.000899 | 0.0258 | 188.8 | 5.59 | Good |
| 5 | 5.5910 | 5.7078 | 12.7 | 1185 | 8.3 | 5.70 | 0.057 | 6760 | 0.000844 | 0.0189 | 127.8 | 9.55 | Moderate |
| 6 | 5.5919 | 5.7080 | 5.3 | 1683 | 21.2 | 2.40 | 0.053 | 3970 | 0.000594 | 0.0178 | 70.7 | 2.06 | Moderate |
| 7 | 5.5909 | 5.7070 | 6.4 | 1728 | 37.5 | 6.80 | 0.126 | 11,732 | 0.000579 | 0.0166 | 194.8 | 3.88 | Good |
| 8 | 5.5925 | 5.7064 | 8.2 | 1397 | 3.6 | 3.30 | 0.020 | 4560 | 0.000716 | 0.0168 | 76.6 | 5.32 | Weak |
| 9 | 5.5942 | 5.7048 | 3.5 | 161 | 11.6 | 9.70 | 0.086 | 1567 | 0.006210 | 0.1645 | 257.8 | 1.40 | Moderate |
| 10 | 5.5950 | 5.7041 | 8.0 | 501 | 6.5 | 4.50 | 0.035 | 2270 | 0.001990 | 0.0545 | 123.7 | 5.10 | Weak |
| 11 | 5.5985 | 5.7031 | 8.6 | 517 | 11.9 | 10.50 | 0.015 | 5451 | 0.001930 | 0.0561 | 305.8 | 5.50 | Weak |
| 12 | 5.5994 | 5.7029 | 8.9 | 818 | 2.5 | 2.10 | 0.018 | 1729 | 0.001220 | 0.0366 | 63.3 | 5.70 | Weak |
| 13 | 5.5964 | 5.7016 | 4.6 | 995 | 12.8 | 6.10 | 0.008 | 6059 | 0.001000 | 0.0228 | 138.1 | 2.50 | Poor |
| 14 | 5.5974 | 5.7001 | 4.7 | 662 | 9.8 | 38.50 | 0.017 | 25,483 | 0.001510 | 0.0386 | 138.1 | 2.40 | Weak |
| 15 | 5.5984 | 5.6996 | 9.0 | 1379 | 11.3 | 75.90 | 0.010 | 104,674 | 0.000135 | 0.0033 | 245.2 | 5.60 | Weak |
| 16 | 5.5994 | 5.6989 | 9.9 | 1086 | 8.3 | 5.30 | 0.036 | 5810 | 0.000921 | 0.0225 | 130.7 | 6.00 | Weak |
| 17 | 5.5997 | 5.6967 | 3.4 | 1038 | 1.5 | 53.80 | 0.006 | 55,907 | 0.000182 | 0.0049 | 276.7 | 1.40 | Poor |
| Transmissivity Range | Transmissivity Potentials |
|---|---|
| Greater than 500 m2/day (5.79 × 10−3 m2/s) | High potential |
| Between 50 and 500 m2/day (5.58 × 10−3 and 7.39 × 10−3 m2/s) | Moderate potential |
| Between 5 and 50 m2/day (9.06 × 10−3 and 5.50 × 10−3 m2/s) | Low potential |
| Between 0.5 and 5 m2/day (5.01 × 10−3 and 5.58 × 10−3 m2/s) | Very low potential |
| Below 0.5 m2/day (5.01 × 10−3 m2/s) | Negligible flat |
| Water Quality Index Level | Water Quality Status |
|---|---|
| <50 | Excellent |
| 50–100 | Good |
| 100–200 | Poor |
| 200–300 | Very poor |
| >300 | Unsuitable for drinking |
| Parameters (mg/L) | Standard Values (Si) | Observed Values | Standard Deviation | Variance | Quality Rating (Qi) | Unit Weight (Wi) | QiWi | |||
|---|---|---|---|---|---|---|---|---|---|---|
| WHO | FEPA | Min | Max | Mean | ||||||
| Temperature (°C) | 35 | 27 | 28.00 | 28.20 | 28.10 | 0.100 | 0.1000 | 80.30 | 0.030 | 2.410 |
| TSS | 10 | 10 | 0.04 | 0.93 | 0.50 | 0.300 | 0.1000 | 5.00 | 0.100 | 0.500 |
| TDS | 500 | 500 | 0.83 | 18.66 | 8.00 | 7.900 | 62.1000 | 1.60 | 0.002 | 0.003 |
| Alkalinity | 250 | 250 | 0.13 | 2.93 | 1.50 | 1.000 | 1.0000 | 0.60 | 0.004 | 0.002 |
| Total hardness | 200 | 150 | 2.45 | 3.10 | 3.00 | 0.300 | 0.1000 | 1.55 | 0.005 | 0.008 |
| Color | 15 | 15 | 11.00 | 21.00 | 16.00 | 3.800 | 14.5000 | 106.70 | 0.067 | 7.149 |
| Carbonate | 51 | 50 | 23.16 | 61.73 | 49.20 | 15.200 | 230.7000 | 80.70 | 0.200 | 16.140 |
| Chloride | 250 | 250 | 0.15 | 0.74 | 0.40 | 0.300 | 0.1000 | 0.16 | 0.004 | 0.010 |
| Nitrate | 50 | 50 | 1.98 | 2.04 | 2.00 | 0.002 | 0.0006 | 1000 | 0.020 | 200 |
| Sulfate | 100 | 500 | 0.08 | 0.34 | 0.20 | 1.500 | 2.4000 | 0.20 | 0.010 | 0.002 |
| Lead | 0.01 | 0.01 | 0.01 | 0.04 | 0.02 | 0.001 | 0.0002 | 200 | 100 | 20,000 |
| Potassium | 100 | 100 | 1.24 | 2.38 | 1.70 | 0.700 | 0.5000 | 1.70 | 0.010 | 0.017 |
| Sodium | 200 | 200 | 0.04 | 0.08 | 0.08 | 0.003 | 0.0010 | 0.04 | 0.005 | 0.002 |
| Phosphate | 5 | 5 | 0.21 | 0.59 | 0.50 | 0.010 | 0.2000 | 10.00 | 0.200 | 2.000 |
| Calcium | 200 | 200 | 101.84 | 124.10 | 117.20 | 9.000 | 81.0000 | 58.60 | 0.005 | 0.293 |
| Magnesium | 150 | 100 | 10.84 | 9.46 | 10.90 | 1.100 | 1.1400 | 7.30 | 0.007 | 0.051 |
| Copper | 2 | 1 | 0.87 | 0.09 | 0.30 | 0.300 | 0.1000 | 1.50 | 0.500 | 7.500 |
| Iron | 0.30 | 0.30 | 0.20 | 0.40 | 0.30 | 0.095 | 0.0120 | 100 | 0.300 | 30.000 |
| Turbidity | 5 | 1 | 1.90 | 1.29 | 1.63 | 0.220 | 0.0670 | 32.60 | 0.200 | 6.520 |
| pH | 6.50–8.50 | 6–9 | 5.30 | 6.40 | 6.00 | 0.100 | 0.7000 | 92.30 | 0.010 | 0.923 |
| Counts (cfu/mL) | 10 | 10 | 2.08 | 2.64 | 2.30 | 0.800 | 0.1000 | 23.00 | 0.100 | 2.300 |
| Conductivity (µs/cm) | 1000 | 1000 | 92.10 | 93.10 | 92.80 | 0.400 | 0.2 | 0.04 | 0.001 | 0.00004 |
| ∑Wi = 101.78 | ∑QiWi=20,275.83 | |||||||||
| Parameters (mg/L) | Standard Values (Si) | Observed Values | Standard Deviation | Variance | Quality Rating (Qi) | Unit Weight (Wi) | QiWi | |||
|---|---|---|---|---|---|---|---|---|---|---|
| WHO | FEPA | Min | Max | Mean | ||||||
| Temperature (°C) | 35 | 27 | 28.00 | 28.40 | 28.100 | 0.10 | 0.0200 | 80.30 | 0.030 | 2.4090 |
| TSS | 10 | 10 | 0.17 | 1.56 | 0.800 | 0.50 | 0.2400 | 8.00 | 0.100 | 0.8000 |
| TDS | 500 | 500 | 3.38 | 22.17 | 13.800 | 7.20 | 5.2000 | 2.76 | 0.002 | 0.0060 |
| Alkalinity | 250 | 250 | 0.53 | 4.92 | 2.500 | 1.60 | 2.5000 | 1.00 | 0.004 | 0.0040 |
| Hardness | 200 | 150 | 3.02 | 3.21 | 3.100 | 0.07 | 0.0050 | 1.55 | 0.005 | 0.0080 |
| Color | 15 | 15 | 4.00 | 20.00 | 11.500 | 5.50 | 30.0000 | 76.70 | 0.067 | 5.1400 |
| Carbonate | 51 | 50 | 10.46 | 59.14 | 33.400 | 19.60 | 385.9000 | 58.00 | 0.020 | 1.1600 |
| Chloride | 250 | 250 | 0.09 | 0.53 | 0.300 | 0.20 | 0.0600 | 0.12 | 0.004 | 0.0050 |
| Nitrate | 50 | 50 | 1.93 | 2.00 | 2.000 | 0.03 | 0.0010 | 4.00 | 0.020 | 0.0800 |
| Sulfate | 100 | 500 | 0.01 | 0.16 | 0.100 | 0.07 | 0.0050 | 0.10 | 0.001 | 0.0030 |
| Lead | 0.01 | 0.01 | 0.01 | 0.02 | 0.002 | 0.01 | 0.0001 | 200.00 | 100.000 | 20,000 |
| Potassium | 100 | 100 | 1.48 | 2.84 | 2.000 | 0.40 | 0.2000 | 2.00 | 0.001 | 0.0020 |
| Sodium | 200 | 200 | 0.04 | 1.34 | 0.600 | 0.50 | 0.2600 | 0.30 | 0.050 | 0.1500 |
| Phosphate | 5 | 5 | 0.51 | 0.69 | 0.630 | 0.06 | 0.0040 | 1.26 | 2.000 | 0.2500 |
| Calcium | 200 | 200 | 119.30 | 128.43 | 123.000 | 4.40 | 19.800 | 61.50 | 0.005 | 3.1000 |
| Magnesium | 150 | 100 | 9.73 | 10.43 | 10.100 | 0.30 | 0.1000 | 6.73 | 0.070 | 0.4700 |
| Copper | 2.00 | 1.00 | 0.09 | 0.10 | 0.100 | 0.01 | 0.0001 | 5.00 | 0.500 | 2.5000 |
| Iron | 0.30 | 0.3 | 0.02 | 0.04 | 0.050 | 0.02 | 0.0003 | 166.70 | 0.200 | 33.3400 |
| Turbidity | 5 | 1 | 0.11 | 0.96 | 0.640 | 0.27 | 0.0730 | 128.00 | 0.300 | 38.4000 |
| pH | 6.5–8.5 | 6–9 | 4.10 | 6.60 | 5.000 | 0.90 | 0.8000 | 73.52 | 0.010 | 22.1000 |
| Coliform counts (cfu/mL) | 10 | 10 | 0.38 | 7.00 | 2.000 | 2.20 | 4.9000 | 20.00 | 0.100 | 2.0000 |
| Conductivity (µs/cm) | 1000 | 1000 | 93.50 | 92.30 | 9.3 | 1.40 | 2.0000 | 0.93 | 0.001 | 0.0009 |
| ∑Wi = 103.49 | ∑QiWi =20,111.93 | |||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iserhien-Emekeme, R.; Ofomola, M.O.; Bawallah, M.; Anomohanran, O. Lithological Identification and Underground Water Conditions in Jeddo Using Geophysical and Geochemical Methods. Hydrology 2017, 4, 42. https://doi.org/10.3390/hydrology4030042
Iserhien-Emekeme R, Ofomola MO, Bawallah M, Anomohanran O. Lithological Identification and Underground Water Conditions in Jeddo Using Geophysical and Geochemical Methods. Hydrology. 2017; 4(3):42. https://doi.org/10.3390/hydrology4030042
Chicago/Turabian StyleIserhien-Emekeme, Ruth, Merrious Oviri Ofomola, Musa Bawallah, and Ochuko Anomohanran. 2017. "Lithological Identification and Underground Water Conditions in Jeddo Using Geophysical and Geochemical Methods" Hydrology 4, no. 3: 42. https://doi.org/10.3390/hydrology4030042
APA StyleIserhien-Emekeme, R., Ofomola, M. O., Bawallah, M., & Anomohanran, O. (2017). Lithological Identification and Underground Water Conditions in Jeddo Using Geophysical and Geochemical Methods. Hydrology, 4(3), 42. https://doi.org/10.3390/hydrology4030042





