Differential Evolution in Hydrochemical Characteristics Amongst Porous, Fissured and Karst Aquifers in China
Abstract
1. Introduction
2. Materials and Methods
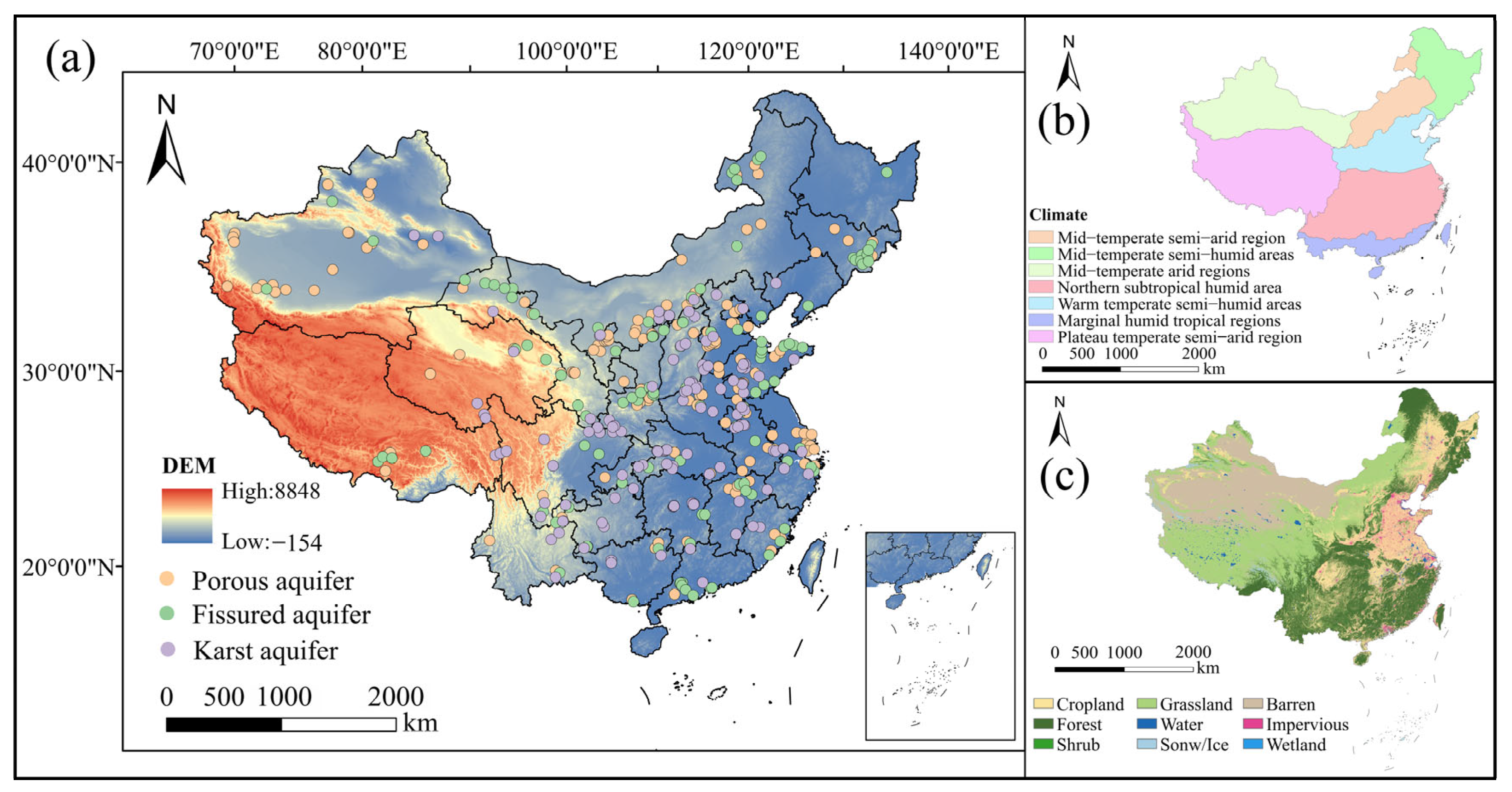
2.1. Study Area
2.1.1. Geographical Conditions and Climate
2.1.2. Hydrogeological Condition and Land-Use Types
2.1.3. Coastal Aquifers and River–Groundwater Interaction
2.2. Search Strategy
2.3. Hierarchical Cluster Analysis
2.4. Principal Component Analysis(PCA)
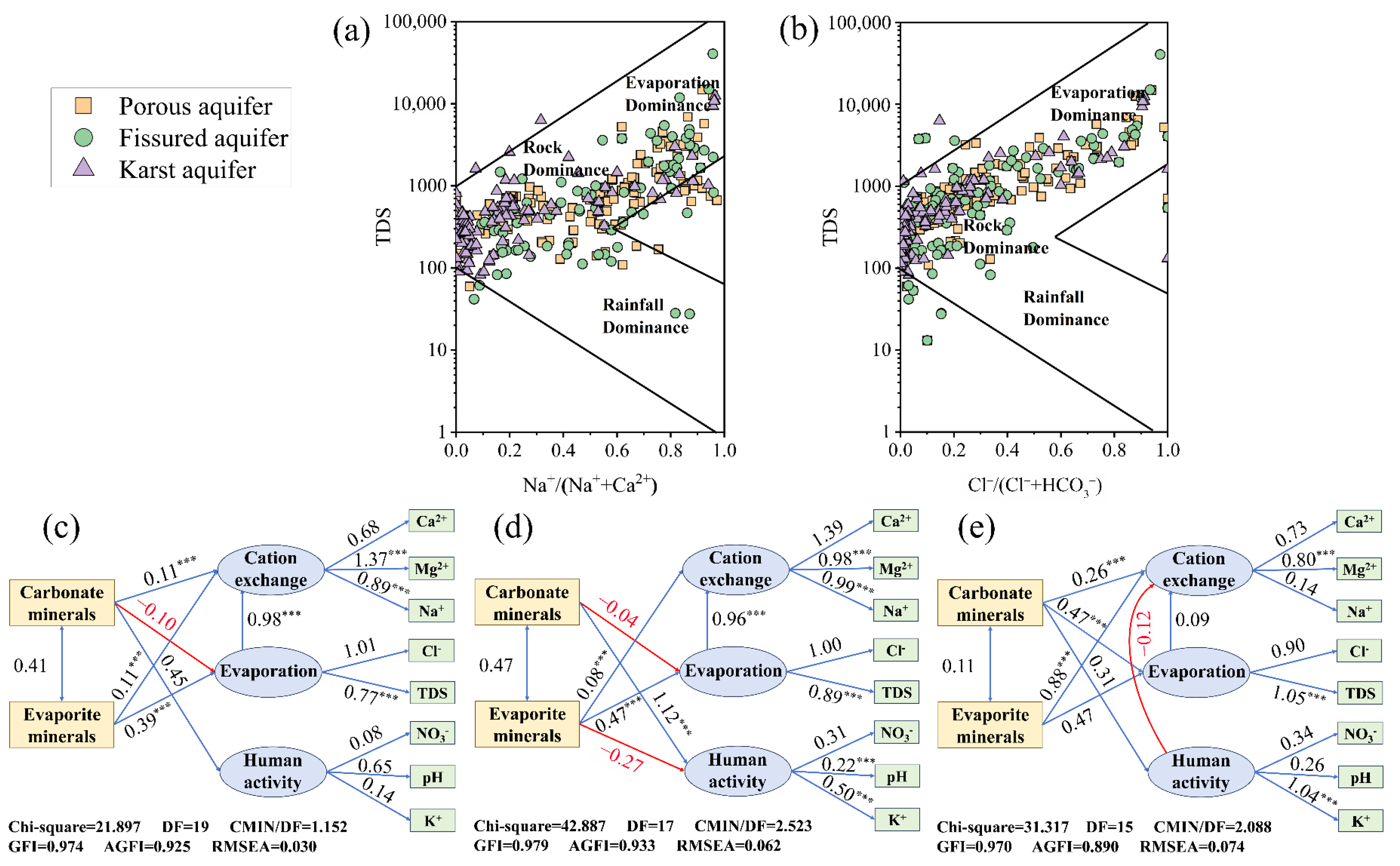
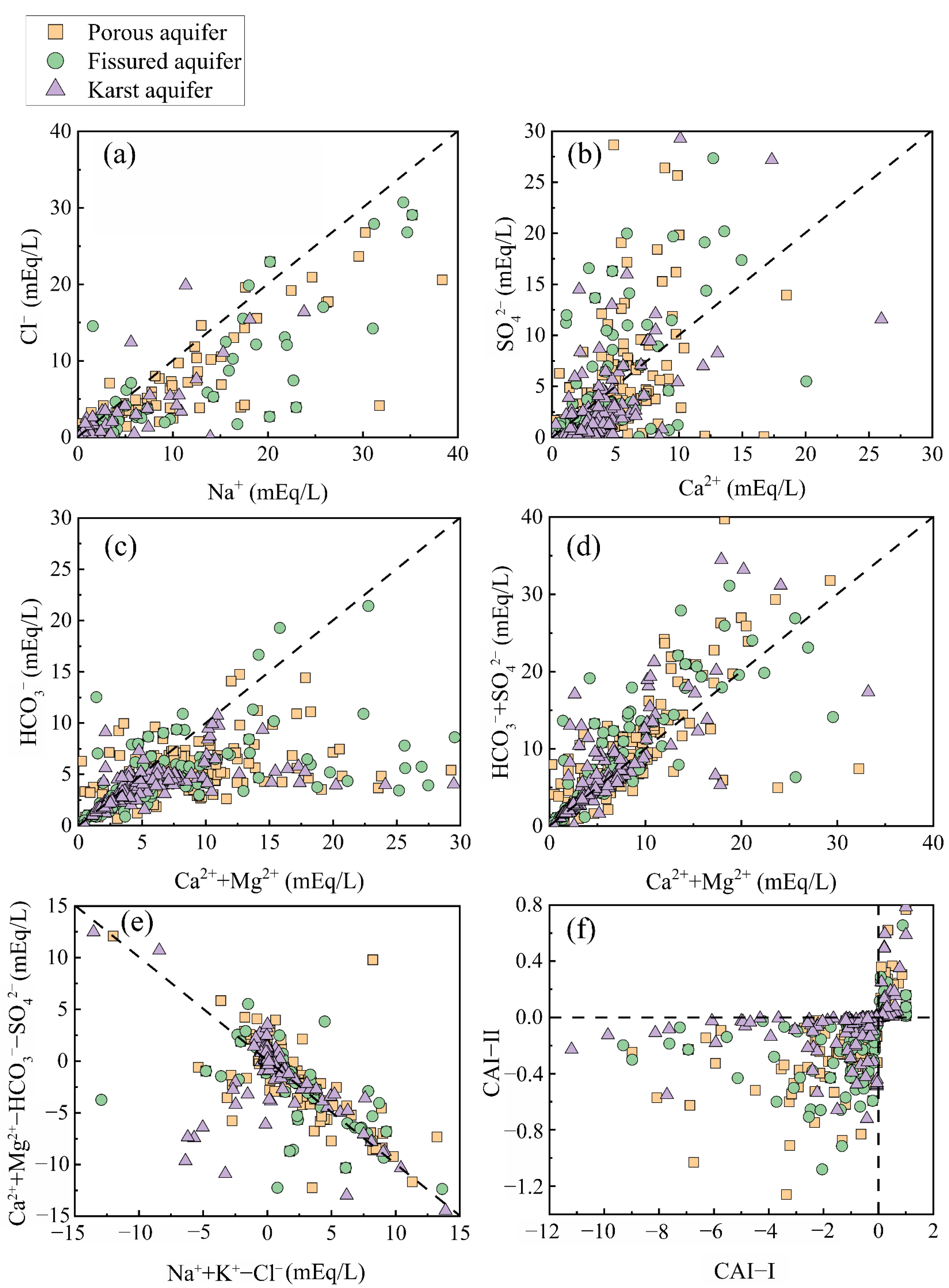
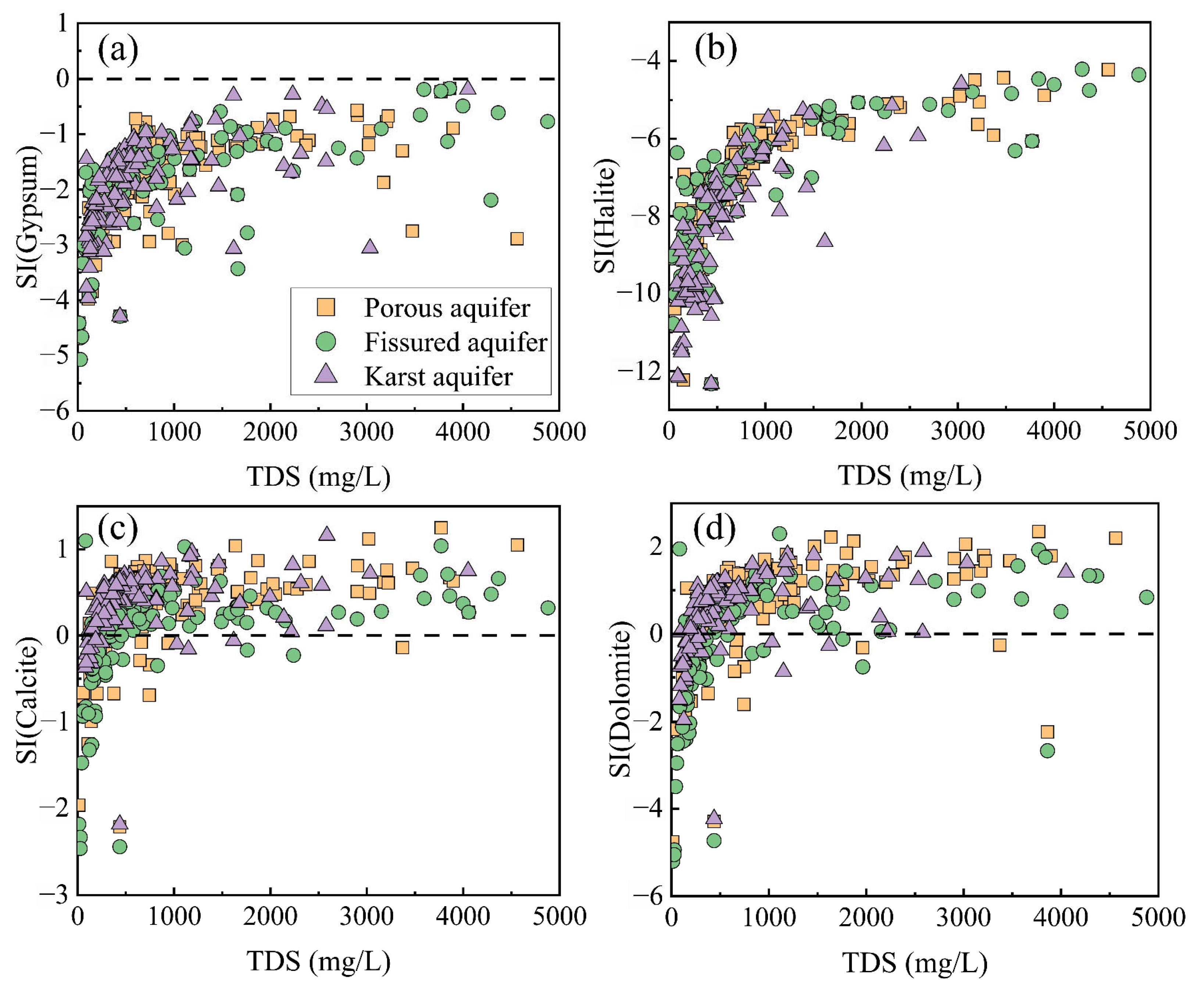
2.5. Saturation Index (SI) and Chloroalkaline Indices (CAI)
2.6. Structural Equation Modeling
3. Results
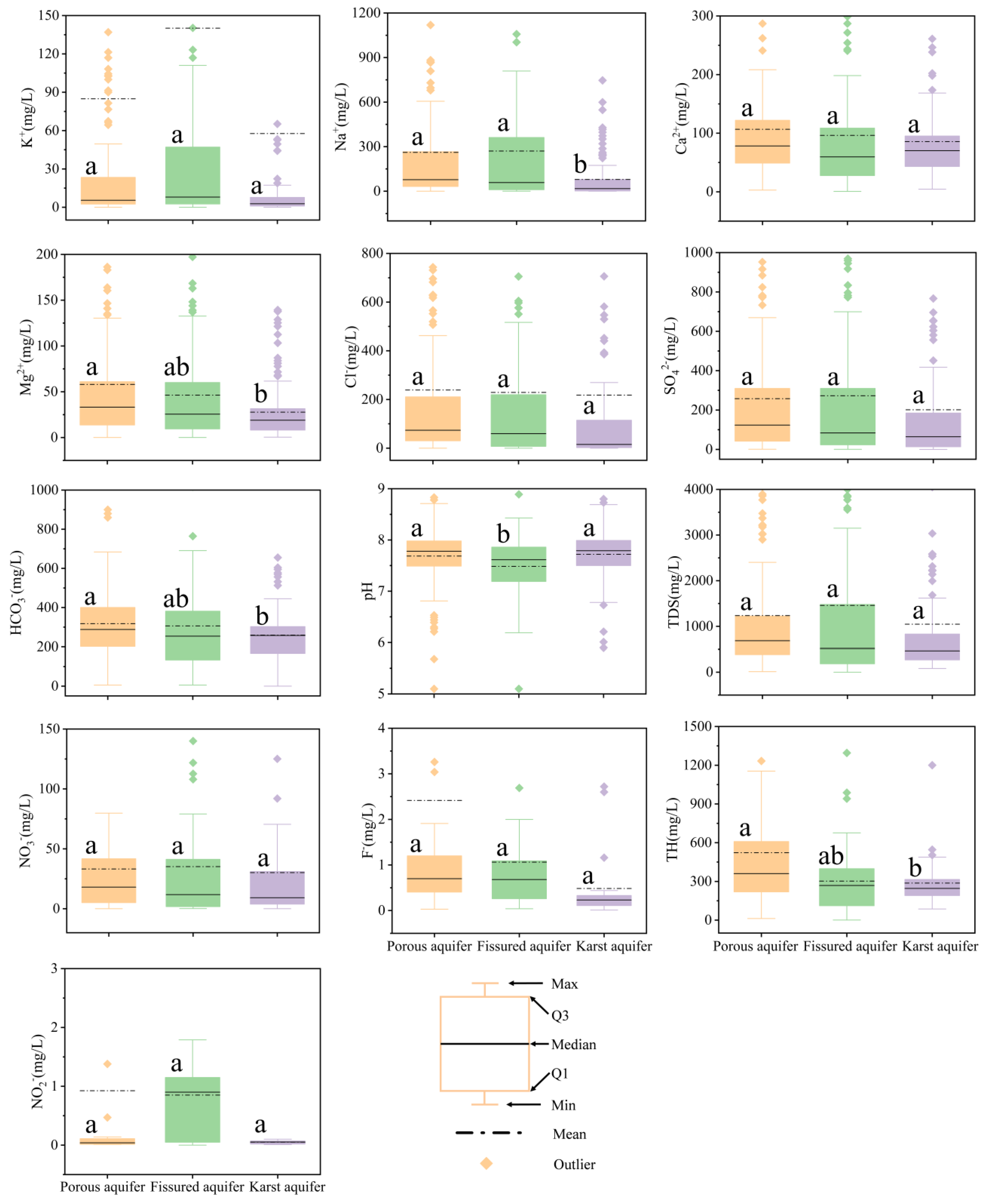
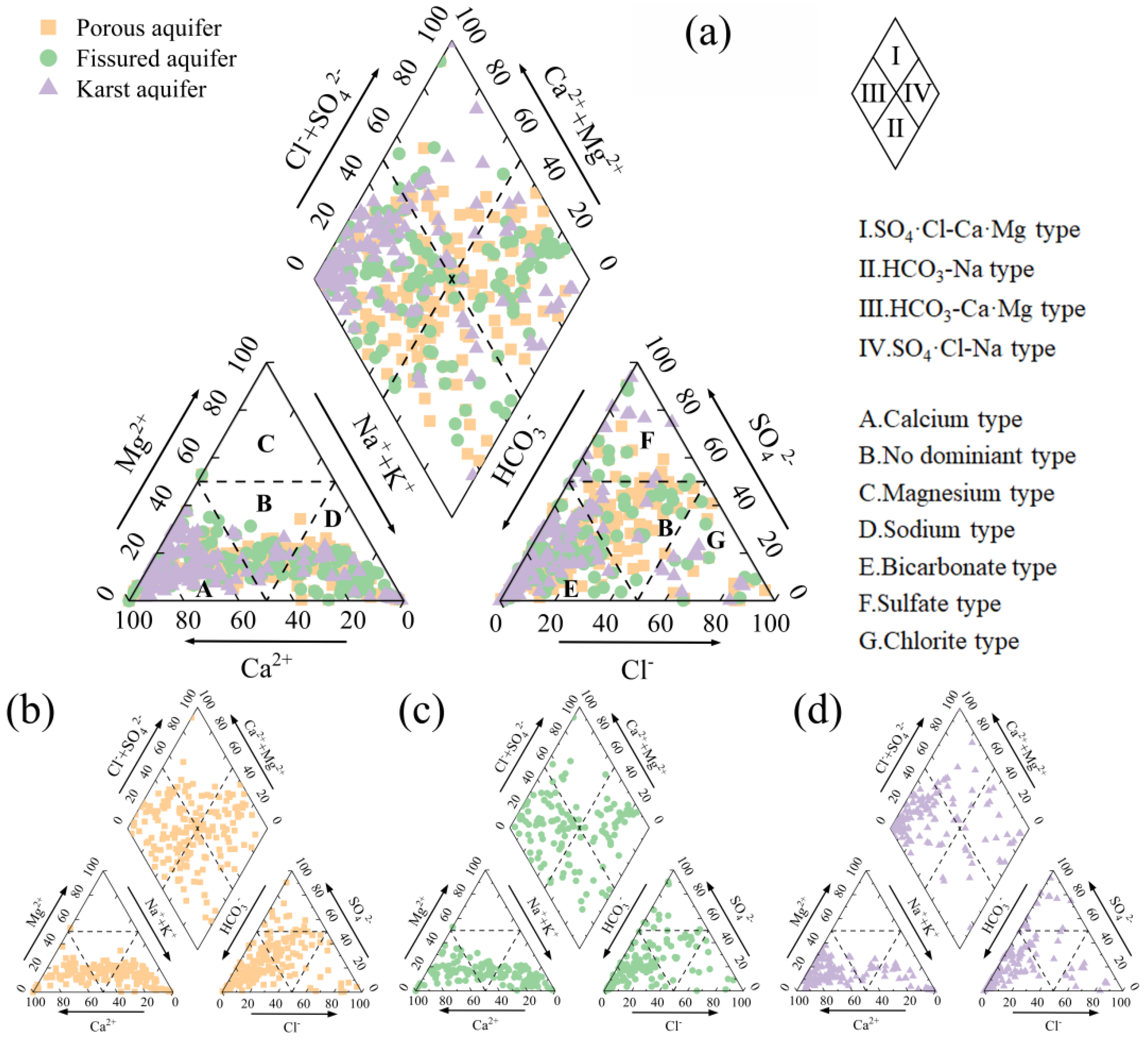
3.1. Hydrochemical Characteristics and Classification of Groundwater
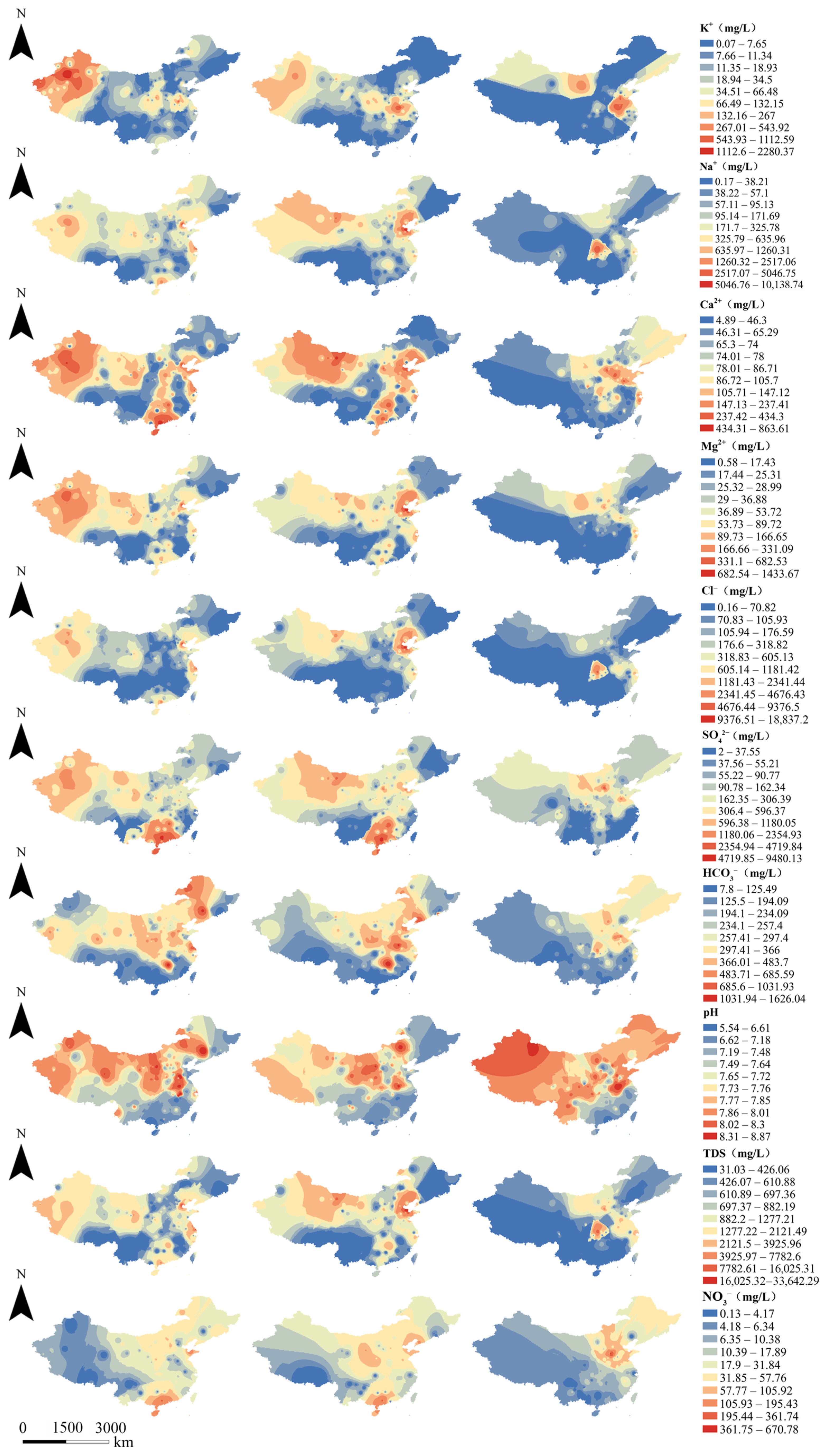
3.2. Spatial Distribution of Groundwater Chemistry in Different Types of Aquifers
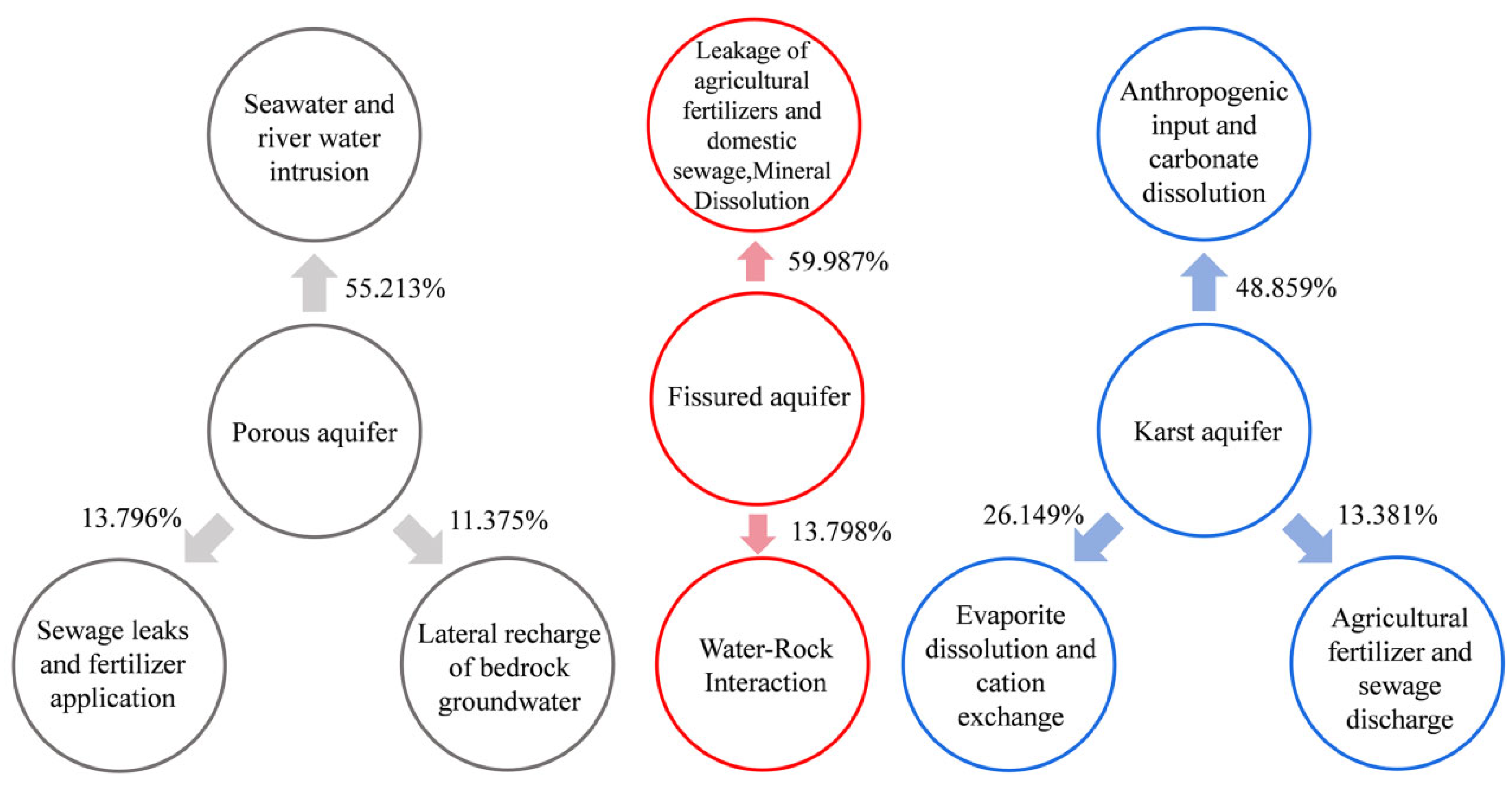
3.3. Source Allocation of Chemical Constituents of Groundwater from Different Types of Aquifers
3.4. Controlling Factors of Water Chemistry in Different Aquifers
4. Discussion
4.1. Hydrochemical Characteristics of Different Types of Aquifers
4.2. Main Sources of Groundwater Chemical Composition
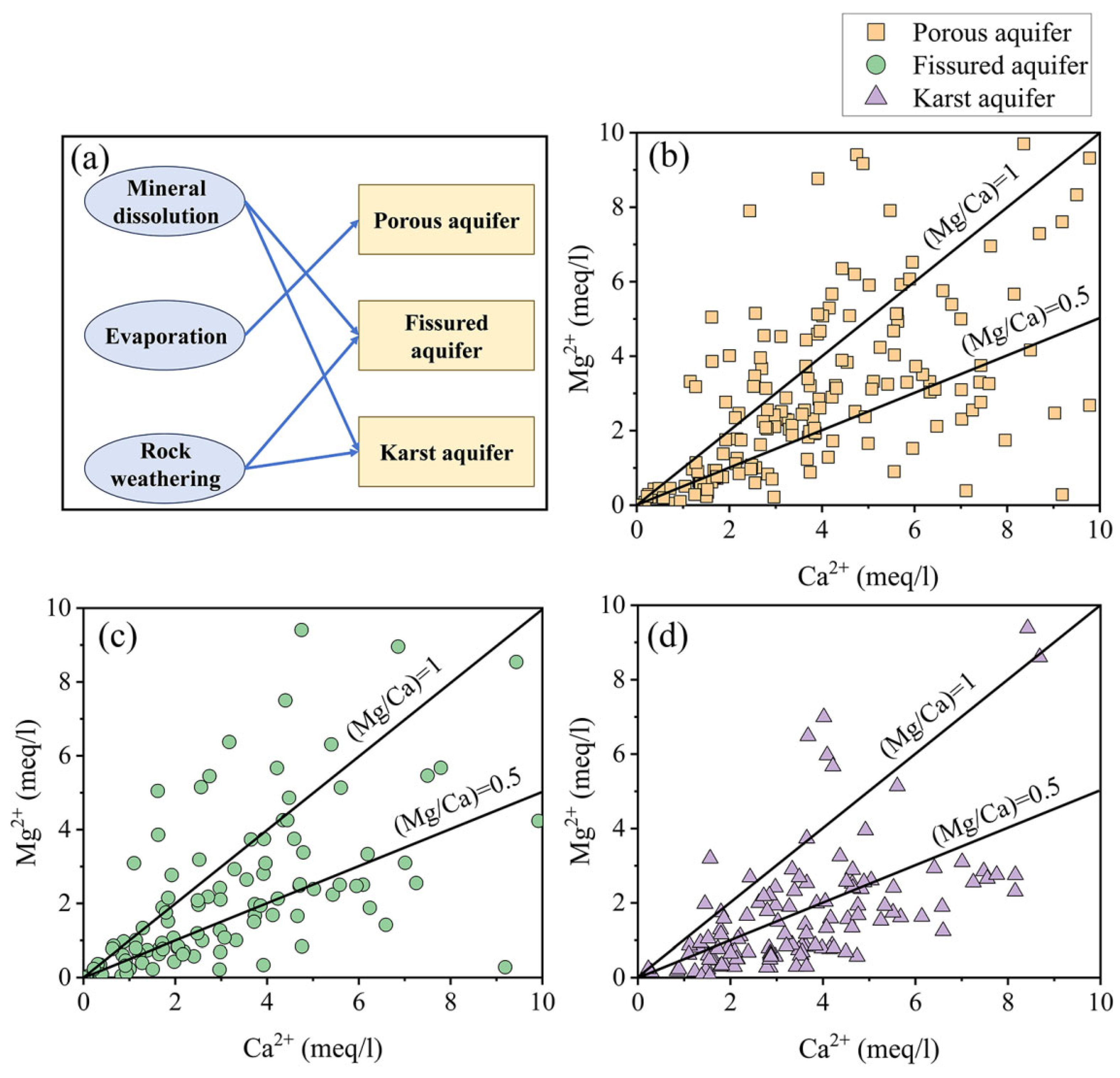
4.3. Hydrogeochemical Mechanisms of Groundwater Evolution in Different Aquifers
4.4. Influencing Factors Across Different Aquifer Types
4.5. Suggestions for Groundwater Protection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gleeson, T.; Befus, K.M.; Jasechko, S.; Luijendijk, E.; Cardenas, M.B. The global volume and distribution of modern groundwater. Nat. Geosci. 2015, 9, 161–167. [Google Scholar] [CrossRef]
- Barbosa, F.A.S.; Brait, L.A.S.; Coutinho, F.H.; Ferreira, C.M.; Moreira, E.F.; de Queiroz Salles, L.; Meirelles, P.M. Ecological landscape explains aquifers microbial structure. Sci. Total Environ. 2023, 862, 160822. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Pei, L.; Huang, G.; Han, D.; Song, J. Identification of Groundwater Contamination in a Rapidly Urbanized Area on a Regional Scale: A New Approach of Multi-Hydrochemical Evidences. Int. J. Environ. Res. Public Health 2021, 18, 12143. [Google Scholar] [CrossRef] [PubMed]
- Kazakis, N.; Oikonomidis, D.; Voudouris, K.S. Groundwater vulnerability and pollution risk assessment with disparate models in karstic, porous, and fissured rock aquifers using remote sensing techniques and GIS in Anthemountas basin, Greece. Environ. Earth Sci. 2015, 74, 6199–6209. [Google Scholar] [CrossRef]
- de León-Gómez, H.; Martin del Campo-Delgado, M.A.; Esteller-Alberich, M.V.; Velasco-Tapia, F.; Alva-Niño, E.; Cruz-López, A. Assessment of nitrate and heavy metal contamination of groundwater using the heavy metal pollution index: Case study of Linares, Mexico. Environ. Earth Sci. 2020, 79, 433. [Google Scholar] [CrossRef]
- Fandel, C.; Ferré, T.; Chen, Z.; Renard, P.; Goldscheider, N. A model ensemble generator to explore structural uncertainty in karst systems with unmapped conduits. Hydrogeol. J. 2020, 29, 229–248. [Google Scholar] [CrossRef]
- Ma, N.; Gao, L.; Ge, Z.; Li, M. Hydrochemical characteristics of groundwater in a plain river network region: Establishing linkages between source and water quality variables. Chemosphere 2023, 331, 138809. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Zhan, H.; Chen, X.; Liu, M.; Wang, M. Identification of hydrogeochemical processes and transport paths of a multi-aquifer system in closed mining regions. J. Hydrol. 2020, 589, 125344. [Google Scholar] [CrossRef]
- Nakhaei, M.; Dadgar, M.A.; Amiri, V. Geochemical processes analysis and evaluation of groundwater quality in Hamadan Province, Western Iran. Arab. J. Geosci. 2016, 9, 384. [Google Scholar] [CrossRef]
- Lane, A.D.; Kirk, M.F.; Whittemore, D.O.; Stotler, R.; Hildebrand, J.; Feril, O. Long-term (1970s–2016) changes in groundwater geochemistry in the High Plains aquifer in south-central Kansas, USA. Hydrogeol. J. 2019, 28, 491–501. [Google Scholar] [CrossRef]
- Tran, D.A.; Goeppert, N.; Goldscheider, N. Use of major ion chemistry and trace and rare earth elements to characterize hydraulic relations, mixing processes and water–rock interaction in the Dong Van karst aquifer system, Northern Vietnam. Hydrogeol. J. 2023, 31, 1735–1753. [Google Scholar] [CrossRef]
- Matengu, B.; Xu, Y.; Tordiffe, E. Hydrogeological characteristics of the Omaruru Delta Aquifer System in Namibia. Hydrogeol. J. 2019, 27, 857–883. [Google Scholar] [CrossRef]
- Tziritis, E.; Sachsamanoglou, E.; Güler, C. Evaluating spatiotemporal groundwater quality changes in the Rhodope coastal aquifer system (NE Greece) employing a GIS-assisted hybrid approach of multivariate statistics and inverse geochemical modeling. Sci. Total Environ. 2024, 947, 174676. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Liu, C.; Sun, J.; Zhang, M.; Jing, J.; Li, L. A regional scale investigation on factors controlling the groundwater chemistry of various aquifers in a rapidly urbanized area: A case study of the Pearl River Delta. Sci. Total Environ. 2018, 625, 510–518. [Google Scholar] [CrossRef]
- Spellman, P.; Pain, A.; Breithaupt, C.; Bremner, P.M. Using multivariate statistics to link major ion chemistry changes at karst springs to agriculture. Sci. Total Environ. 2024, 921, 170573. [Google Scholar] [CrossRef]
- Lamari, H.; Mesbah, M.; Cherchali, M.E.-H. Groundwater chemistry origin and quality assessment of the Sahel−Soummam aquifer, in northeast Algeria: A combined hydrochemical and isotopic approach. Groundw. Sustain. Dev. 2024, 26, 101294. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, P.; Wang, G.; Mao, H.; Feng, X.; Huang, H. Groundwater chemistry and isotope for interpreting the hydrogeological conditions and hydrochemical evolution of multilayer aquifer system of Donghai island, China. Appl. Geochem. 2023, 159, 105833. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, L.; Yao, D.; Hou, X.; Zhang, J.; Qin, H.; Ren, X.; Zheng, X. Hydrogeochemical Processes and Inverse Modeling for a Multilayer Aquifer System in the Yuaner Coal Mine, Huaibei Coalfield, China. Mine Water Environ. 2022, 41, 775–789. [Google Scholar] [CrossRef]
- Antonio Torres-Martínez, J.; Mahlknecht, J.; Mora, A.; Kaown, D.; Koh, D.-C.; Mayer, B.; Tetzlaff, D. Unveiling nitrate origins in semiarid aquifers: A comparative analysis of Bayesian isotope mixing models using nitrate and boron isotopes and a Positive Matrix Factorization model. J. Hydrol. 2024, 639, 131622. [Google Scholar] [CrossRef]
- Boumaiza, L.; Walter, J.; Chesnaux, R.; Zahi, F.; Huneau, F.; Garel, É.; Stotler, R.L.; Bordeleau, G.; Johannesson, K.H.; Vystavna, Y.; et al. Combined effects of seawater intrusion and nitrate contamination on groundwater in coastal agricultural areas: A case from the Plain of the El-Nil River (North-Eastern Algeria). Sci. Total Environ. 2022, 851, 158153. [Google Scholar] [CrossRef]
- Lone, S.A.; Jeelani, G.; Mukherjee, A. Hydrogeochemical controls on contrasting co-occurrence of geogenic Arsenic (As) and Fluoride (F−) in complex aquifer system of Upper Indus Basin, (UIB) western Himalaya. Environ. Res. 2024, 260, 119675. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zou, J.W.; Liu, J.R.; Zhang, J.K.; Zhen, P.N. Factors controlling groundwater chemical evolution with the impact of reduced exploitation. Catena 2022, 214, 106261. [Google Scholar] [CrossRef]
- Yu, D.; Yu, J.; Wu, D.; Han, Y.; Sun, B.; Zheng, L.; Chen, H.; Liu, R. Isotopic and Hydrochemical Characteristics of the Changqing-Xiaolipu Water Resource, Jinan, Eastern China: Implications for Water Resources in the Yellow River Basin. Sustainability 2023, 15, 2439. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, Z.; Pan, Z.; Zhang, S.; Li, X.; Ma, R. Influence of permafrost and hydrogeology on seasonal and spatial variations in water chemistry of an alpine river in the northeastern Qinghai-Tibet Plateau, China. Sci. Total Environ. 2022, 834, 155227. [Google Scholar] [CrossRef]
- Shishaye, H.A.; Tait, D.R.; Befus, K.M.; Maher, D.T.; Reading, M.J.; Jeffrey, L.; Tewolde, T.G.; Asfaw, A.T. Development of an improved hydrogeological and hydro-geochemical conceptualization of a complex aquifer system in Ethiopia. Hydrogeol. J. 2020, 28, 2727–2746. [Google Scholar] [CrossRef]
- Huang, G.; Han, D.; Song, J.; Li, L.; Pei, L. A sharp contrasting occurrence of iron-rich groundwater in the Pearl River Delta during the past dozen years (2006–2018): The genesis and mitigation effect. Sci. Total Environ. 2022, 829, 154676. [Google Scholar] [CrossRef]
- Huang, G.; Hou, Q.; Han, D.; Liu, R.; Song, J. Large scale occurrence of aluminium-rich shallow groundwater in the Pearl River Delta after the rapid urbanization: Co-effects of anthropogenic and geogenic factors. J. Contam. Hydrol. 2023, 254, 104130. [Google Scholar] [CrossRef]
- Subba Rao, N.; Das, R.; Sahoo, H.K.; Gugulothu, S. Hydrochemical characterization and water quality perspectives for groundwater management for urban development. Groundw. Sustain. Dev. 2024, 24, 101071. [Google Scholar] [CrossRef]
- Choudhary, S.; Subba Rao, N.; Chaudhary, M.; Das, R. Assessing sources of groundwater quality and health risks using graphical, multivariate, and index techniques from a part of Rajasthan, India. Groundw. Sustain. Dev. 2024, 27, 101356. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Zhang, Y.; Sun, Z.; Sun, T.; Fan, H.; Wu, B.; Li, M.; Qian, L. Hydrochemical evaluation of groundwater quality and human health risk assessment of nitrate in the largest peninsula of China based on high-density sampling: A case study of Weifang. J. Clean. Prod. 2021, 322, 129164. [Google Scholar] [CrossRef]
- Reischer, M.; Bichler, B.; Spötl, C.; Höfer-Öllinger, G.; Wyhlidal, S. Karst hydrogeology of the Untersberg massif and its interaction with the porous aquifer in the adjacent Salzburg Basin. Austrian J. Earth Sci. 2015, 108, 68–81. [Google Scholar] [CrossRef]
- Dantas, J.C.M.; Velásquez, L.N.M.; de Paula, R.S. Horizontal and vertical compartmentalization in the fissure and karstic aquifers of the Lagoa Santa Karst Environmental Protection Area and surroundings, Minas Gerais, Brazil. J. S. Am. Earth Sci. 2023, 123, 104219. [Google Scholar] [CrossRef]
- Jaunat, J.; Huneau, F.; Dupuy, A.; Celle-Jeanton, H.; Vergnaud-Ayraud, V.; Aquilina, L.; Labasque, T.; Le Coustumer, P. Hydrochemical data and groundwater dating to infer differential flowpaths through weathered profiles of a fractured aquifer. Appl. Geochem. 2012, 27, 2053–2067. [Google Scholar] [CrossRef]
- Musgrove, M.; Jurgens, B.C.; Opsahl, S.P. Karst Groundwater Vulnerability Determined by Modeled Age and Residence Time Tracers. Geophys. Res. Lett. 2023, 50, 102853. [Google Scholar] [CrossRef]
- Guo, X.; Luo, M.; Li, J.; Chen, Y.; Kuang, Y.; Jiang, C.; Zhou, H. Dual-tracer analysis of stable isotopes and thermal signals to quantify groundwater residence times in karst rhythmic spring systems. J. Hydrol. 2025, 661, 133493. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Y.; Zhang, Y.; Guo, L.; Wang, Z.; Zheng, Q. Impact of groundwater overexploitation on karst aquifer and delineation of the critical zones: Case study of Jinci spring in Shanxi, China. Carbonates Evaporites 2022, 37, 68. [Google Scholar] [CrossRef]
- Bi, P.; Huang, G.; Liu, C.; Li, L. Geochemical factors controlling natural background levels of phosphate in various groundwater units in a large-scale urbanized area. J. Hydrol. 2022, 608, 127594. [Google Scholar] [CrossRef]
- Abu-alnaeem, M.F.; Yusoff, I.; Ng, T.F.; Alias, Y.; Raksmey, M. Assessment of groundwater salinity and quality in Gaza coastal aquifer, Gaza Strip, Palestine: An integrated statistical, geostatistical and hydrogeochemical approaches study. Sci. Total Environ. 2018, 615, 972–989. [Google Scholar] [CrossRef]
- Liu, R.; Xie, X.; Hou, Q.; Han, D.; Song, J.; Huang, G. Spatial distribution, sources, and human health risk assessment of elevated nitrate levels in groundwater of an agriculture-dominant coastal area in Hainan Island, China. J. Hydrol. 2024, 634, 131088. [Google Scholar] [CrossRef]
- Huang, G.; Pei, L.; Li, L.; Liu, C. Natural background levels in groundwater in the Pearl River Delta after the rapid expansion of urbanization: A new pre-selection method. Sci. Total Environ. 2022, 813, 151890. [Google Scholar] [CrossRef]
- Ahmad, S.; Umar, R.; Ahmad, I. Assessment of groundwater quality using Entropy-Weighted Quality Index (EWQI) and multivariate statistical techniques in Central Ganga plain, India. Environ. Dev. Sustain. 2022, 26, 1615–1643. [Google Scholar] [CrossRef]
- Shuaibu, A.; Kalin, R.M.; Phoenix, V.; Lawal, I.M. Geochemical evolution and mechanisms controlling groundwater chemistry in the transboundary Komadugu–Yobe Basin, Lake Chad region: An integrated approach of chemometric analysis and geochemical modeling. J. Hydrol. Reg. Stud. 2025, 57, 102098. [Google Scholar] [CrossRef]
- Kanji, S.; Das, S.; Rajak, C. A comparative hydrochemical assessment of groundwater quality for drinking and irrigation purposes using different statistical and ML models in lower gangetic alluvial plain, eastern India. Chemosphere 2025, 372, 144074. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, X.; Liu, Y.; Jin, M.; Knappett, P.S.K.; Liu, Y. Hydrogeochemical Evolution Along Groundwater Flow Paths in the Manas River Basin, Northwest China. Groundwater 2018, 57, 575–589. [Google Scholar] [CrossRef]
- Arslan, H. Application of multivariate statistical techniques in the assessment of groundwater quality in seawater intrusion area in Bafra Plain, Turkey. Environ. Monit. Assess. 2012, 185, 2439–2452. [Google Scholar] [CrossRef]
- Ligavha-Mbelengwa, L.; Gomo, M. Investigation of factors influencing groundwater quality in a typical Karoo aquifer in Beaufort West town of South Africa. Environ. Earth Sci. 2020, 79, 196. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, F.; Sun, J.; Shan, Y.; Su, W.; Shang, W.; Li, Y. Distribution and source analysis of soil toxic organic compounds of coal-electricity production base in arid and semi-arid region of China. J. Hazard. Mater. 2024, 477, 135317. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, J.; Wang, S.; Zou, J.; Zhen, P. Multivariate statistical analysis of chemical and stable isotopic data as indicative of groundwater evolution with reduced exploitation. Geosci. Front. 2023, 14, 101476. [Google Scholar] [CrossRef]
- Su, H.; Wang, J.D.; Liu, J.T. Geochemical factors controlling the occurrence of high-fluoride groundwater in the western region of the Ordos basin, northwestern China. Environ. Pollut. 2019, 252, 1154–1162. [Google Scholar] [CrossRef]
- Elumalai, V.; Nwabisa, D.P.; Rajmohan, N. Evaluation of high fluoride contaminated fractured rock aquifer in South Africa—Geochemical and chemometric approaches. Chemosphere 2019, 235, 1–11. [Google Scholar] [CrossRef]
- Jampani, M.; Liedl, R.; Hülsmann, S.; Sonkamble, S.; Amerasinghe, P. Hydrogeochemical and mixing processes controlling groundwater chemistry in a wastewater irrigated agricultural system of India. Chemosphere 2020, 239, 124741. [Google Scholar] [CrossRef] [PubMed]
- Alfarrah, N.; Hweesh, A.; van Camp, M.; Walraevens, K. Groundwater flow and chemistry of the oases of Al Wahat, NE Libya. Environ. Earth Sci. 2016, 75, 985. [Google Scholar] [CrossRef]
- Elumalai, V.; Rajmohan, N.; Sithole, B.; Li, P.; Uthandi, S.; van Tol, J. Geochemical evolution and the processes controlling groundwater chemistry using ionic ratios, geochemical modelling and chemometric analysis in uMhlathuze catchment, KwaZulu-Natal, South Africa. Chemosphere 2023, 312, 137179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Venkatesh, A.S.; Singh, R.; Udayabhanu, G.; Saha, D. Geochemical signatures and isotopic systematics constraining dynamics of fluoride contamination in groundwater across Jamui district, Indo-Gangetic alluvial plains, India. Chemosphere 2018, 205, 493–505. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, W.; Guan, Q.; Sun, Y.; Du, Q.; Zhang, E.; Yan, Y.; Yang, X. The structural equation modeling constructed for runoff change attribution analysis outperforms traditional methods. J. Hydrol. 2024, 636, 131317. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, Y.; Lu, J. Structural equation modeling of the combined effect of urban population, gross regional product and area on air pollution in selected Chinese cities. J. Clean. Prod. 2024, 434, 140030. [Google Scholar] [CrossRef]
- Grace, J.B.; Keeley, J.E. A Structural Equation Model Analysis Of Postfire Plant Diversity In California Shrublands. Ecol. Appl. 2006, 16, 503–514. [Google Scholar] [CrossRef]
- Yinglan, A.; Wang, G.Q.; Liu, T.X.; Shrestha, S.; Xue, B.L.; Tan, Z.X. Vertical variations of soil water and its controlling factors based on the structural equation model in a semi-arid grassland. Sci. Total Environ. 2019, 691, 1016–1026. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Li, C.; Gao, Z.; Chen, S. An investigation into the hydrochemistry, quality and risk to human health of groundwater in the central region of Shandong Province, North China. J. Clean. Prod. 2021, 282, 125416. [Google Scholar] [CrossRef]
- Huang, G.; Sun, J.; Zhang, Y.; Chen, Z.; Liu, F. Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area, South China. Sci. Total Environ. 2013, 463–464, 209–221. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, J.J. Origin of groundwater salinity and hydrogeochemical processes in the confined Quaternary aquifer of the Pearl River Delta, China. J. Hydrol. 2012, 438–439, 112–124. [Google Scholar] [CrossRef]
- Fuentes-Rivas, R.M.; Santacruz-De Leon, G.; Ramos-Leal, J.A.; Alvarez-Bastida, C.; Moran-Ramirez, J. Hydrogeochemical processes, and health risk assessment of groundwater, in Santa María del rio aquifer: A case study of San Luis Potosí valley, Mexico. Groundw. Sustain. Dev. 2024, 26, 101268. [Google Scholar] [CrossRef]
- Patel, A.; Rai, S.P.; Puthiyottil, N.; Singh, A.K.; Noble, J.; Singh, R.; Hagare, D.; Saravana Kumar, U.D.; Rai, N.; Venyo Akpataku, K. Refining aquifer heterogeneity and understanding groundwater recharge sources in an intensively exploited agrarian dominated region of the Ganga Plain. Geosci. Front. 2024, 15, 101808. [Google Scholar] [CrossRef]
- Torres-Martínez, J.A.; Mora, A.; Knappett, P.S.K.; Ornelas-Soto, N.; Mahlknecht, J. Tracking nitrate and sulfate sources in groundwater of an urbanized valley using a multi-tracer approach combined with a Bayesian isotope mixing model. Water Res. 2020, 182, 115962. [Google Scholar] [CrossRef]
- Menció, A.; Mas-Pla, J.; Otero, N.; Regàs, O.; Boy-Roura, M.; Puig, R.; Bach, J.; Domènech, C.; Zamorano, M.; Brusi, D.; et al. Nitrate pollution of groundwater; all right…, but nothing else? Sci. Total Environ. 2016, 539, 241–251. [Google Scholar] [CrossRef]
- Ostad, H.; Mohammadi, Z.; Raeisi, E.; Azimi, M.H.; Liso, I.S.; Parise, M. An integrated approach for characterization of a fractured-rock carbonate aquifer in the Zagros Region of Iran. J. Hydrol. 2024, 640, 131681. [Google Scholar] [CrossRef]
- Satheeskumar, V.; Subramani, T.; Lakshumanan, C.; Roy, P.D.; Karunanidhi, D. Groundwater chemistry and demarcation of seawater intrusion zones in the Thamirabarani delta of south India based on geochemical signatures. Environ. Geochem. Health 2020, 43, 757–770. [Google Scholar] [CrossRef]
- Xiaojuan, Q.; Baoling, L.; Kai, L.; Xinyu, C.; Wenjin, Y. Hydrochemical characteristic and karst development in typical karst spring area, Northern China. Front. Environ. Sci. 2025, 12, 1494730. [Google Scholar] [CrossRef]
- Huang, G.; Song, J.; Han, D.; Liu, R.; Liu, C.; Hou, Q. Assessing natural background levels of geogenic contaminants in groundwater of an urbanized delta through removal of groundwaters impacted by anthropogenic inputs: New insights into driving factors. Sci. Total Environ. 2023, 857, 159527. [Google Scholar] [CrossRef]
- Liu, F.; Sun, J.; Wang, J.; Zhang, Y. Groundwater acidification in shallow aquifers in Pearl River Delta, China: Distribution, factors, and effects. Geochem. J. 2017, 51, 373–384. [Google Scholar] [CrossRef]
- Hou, Q.Y.; Yang, Z.F.; Ji, J.F.; Yu, T.; Yuan, J.X. Effects of Soil pH and Mineral Nutrients on Cadmium Uptake by Rice Grain in the Pearl River Delta, China. Bull. Environ. Contam. Toxicol. 2021, 106, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Song, X.; Yang, L.; Han, D.; Zhang, Y.; Ma, Y.; Bu, H. The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci. Total Environ. 2015, 538, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Li, P.; He, X.; Su, F.; Elumalai, V. Hydrogeochemical Processes Affecting Groundwater Chemistry in the Central Part of the Guanzhong Basin, China. Arch. Environ. Contam. Toxicol. 2020, 80, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Haghiri, M.; Mozafari, M. Characterization of karst aquifers of the Hashtgerd Basin (North Iran) using a combination of geological, hydrodynamical, hydrochemical and isotopic investigations. Hydrogeol. J. 2025, 33, 391–410. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.; Ma, B.; Liu, Y.; Jin, M.; Knappett, P.S.K.; Liu, Y. Using isotopes and hydrogeochemistry to characterize groundwater flow systems within intensively pumped aquifers in an arid inland basin, Northwest China. J. Hydrol. 2021, 595, 126048. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Zhang, Q.L.; Chen, W.F.; Shi, W.W.; Cui, Y.L.; Chen, L.L.; Shao, J.L. Hydrogeochemical analysis and groundwater pollution source identification based on self-organizing map at a contaminated site. J. Hydrol. 2023, 616, 128839. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, X.; Jiang, C.; Li, C.; Zhang, X.; Wang, W.; Duan, Y.; Luo, W.; Mao, Z.; Wang, Y. Multiple contamination sources, pathways and conceptual model of complex buried karst water system:constrained by hydrogeochemistry and δ2H, δ18O, δ34S, δ13C and 87Sr/86Sr isotopes. J. Hydrol. 2024, 639, 131614. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, R.; Han, P.-F.; Wang, J.; Wan, L. Evolution of Hydrogeochemistry in the Haolebaojinao Watershed of the Ordos Basin, China. Sustainability 2023, 15, 5091. [Google Scholar] [CrossRef]
- Nguyet, V.T.M.; Thanh, V.P.; Hai, V.D.; Roi, N.D.; Tra, D.T.T. Hydrogeochemical characterization and groundwater quality of the dong giao karst aquifer in Tam Diep, Ninh Binh, Vietnam. Acta Carsologica 2016, 45, 233–242. [Google Scholar] [CrossRef]
- Bloom, A.L. Water-formed structures: Geomorphology and hydrology of karst terrains. Science 1989, 243, 1618–1619. [Google Scholar] [CrossRef]
- Ji, H.; Chiogna, G.; Chang, W.; Richieri, B.; Chen, L.; Luo, M.; Guo, X.; Huang, K. Investigating the structure of a multiple karst aquifer system and its hydrological process response using high-resolution multi-tracer data. J. Hydrol. 2025, 657, 133152. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, Y.; Groves, C.; Yuan, D.; Kambesis, P. Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China. J. Contam. Hydrol. 2009, 109, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Abiriga, D.; Vestgarden, L.S.; Klempe, H. Groundwater contamination from a municipal landfill: Effect of age, landfill closure, and season on groundwater chemistry. Sci. Total Environ. 2020, 737, 140307. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Guo, H.; Zhang, L.; Wang, Z.; Sun, X. Silicate weathering contributed to arsenic enrichment in geotherm-affected groundwater in Pliocene aquifers of the Guide basin, China. J. Hydrol. 2022, 606, 127444. [Google Scholar] [CrossRef]
- Guo, X.; Chen, Q.; Huang, H.; Wang, Z.; Li, J.; Huang, K.; Zhou, H. Water source identification and circulation characteristics of intermittent karst spring based on hydrochemistry and stable isotope—An example from Southern China. Appl. Geochem. 2022, 141, 105309. [Google Scholar] [CrossRef]
- Chen, W.; Liu, P.; Luo, Y.; Li, B.; Peng, J.; Jin, X. Behavior of Sb and As in the hydrogeochemistry of adjacent karst underground river systems and the responses of such systems to mining activities. Sci. Total Environ. 2023, 857, 159411. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, C.; Satake, S.; Orimo, M.; Fukumoto, K.; Cao, Y. Heterogeneity of hydrological connectivity in coral limestone groundwater pool from vertical, spatial and temporal tracing of groundwater chemicals and isotopes. J. Hydrol. 2023, 623, 129636. [Google Scholar] [CrossRef]
- Said, I.; Abd-Elgawad, A.N.; Seleem, E.-M.M.; Zeid, S.A.M.; Salman, S.A. Multivariate statistics explaining groundwater chemistry, Asyut, Egypt. Environ. Monit. Assess. 2022, 194, 669. [Google Scholar] [CrossRef]
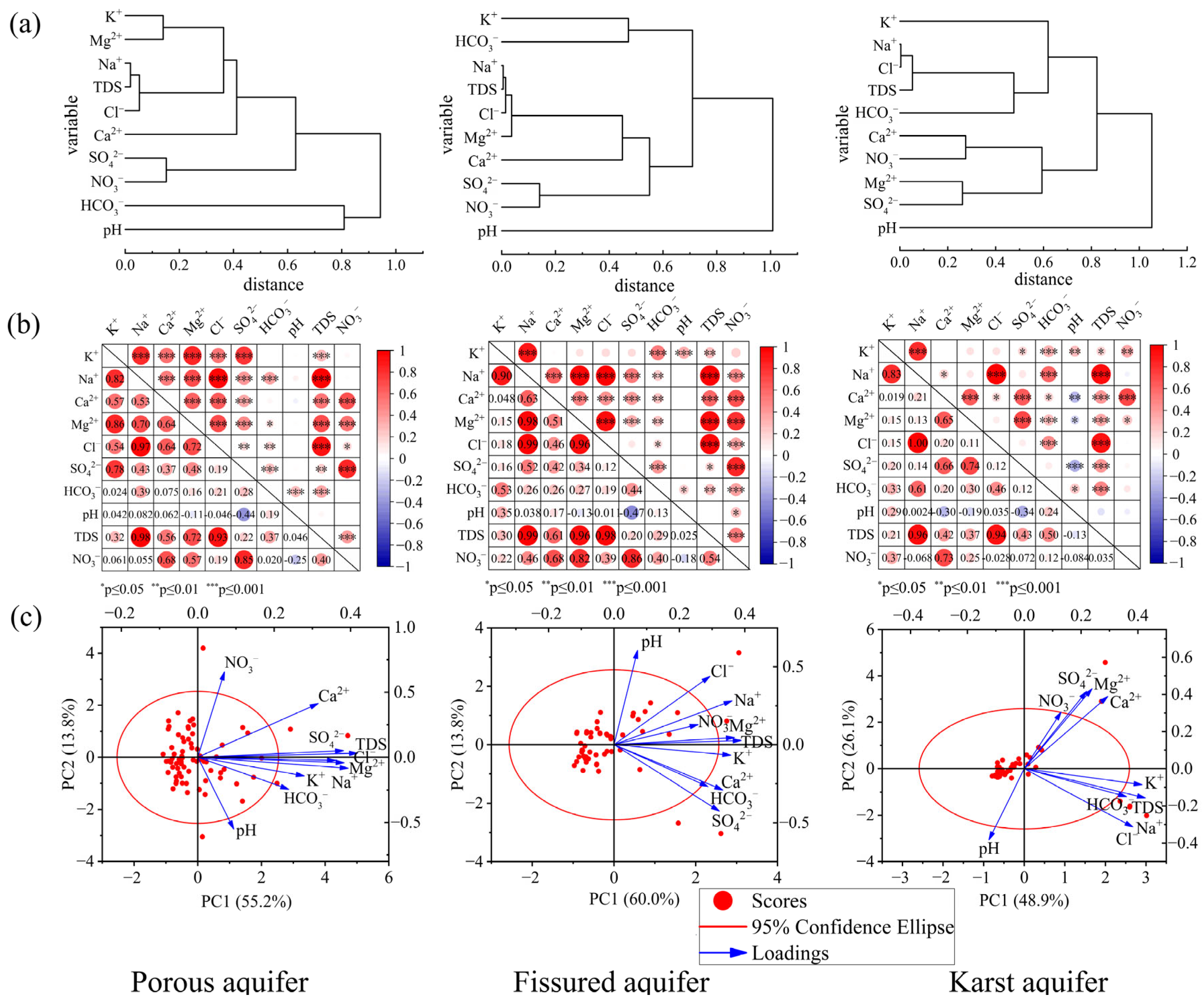
| Chemical Parameters | Porous Aquifer | Fissured Aquifer | Karst Aquifer | |||||
|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | PC1 | PC2 | PC3 | |
| K+ | 0.655 | −0.168 | −0.242 | 0.875 | −0.079 | 0.917 | −0.139 | 0.306 |
| Na+ | 0.925 | −0.100 | −0.103 | 0.885 | 0.331 | 0.846 | −0.506 | −0.105 |
| Ca2+ | 0.741 | 0.486 | 0.162 | 0.815 | −0.340 | 0.650 | 0.629 | 0.364 |
| Mg2+ | 0.900 | −0.050 | −0.129 | 0.902 | 0.054 | 0.527 | 0.697 | −0.265 |
| Cl− | 0.847 | −0.029 | −0.305 | 0.719 | 0.516 | 0.846 | −0.507 | −0.075 |
| SO42− | 0.897 | 0.060 | 0.049 | 0.789 | −0.499 | 0.485 | 0.674 | −0.495 |
| HCO3− | 0.560 | −0.288 | 0.604 | 0.705 | −0.316 | 0.795 | −0.241 | 0.040 |
| NO3− | 0.164 | 0.772 | 0.502 | 0.630 | 0.151 | 0.287 | 0.493 | 0.808 |
| pH | 0.221 | −0.647 | 0.554 | 0.176 | 0.712 | −0.275 | −0.618 | 0.310 |
| TDS | 0.983 | 0.037 | −0.081 | 0.953 | 0.031 | 0.942 | −0.253 | −0.173 |
| Eigenvalue | 5.521 | 1.380 | 1.138 | 5.999 | 1.380 | 4.886 | 2.615 | 1.338 |
| Explained variance (%) | 55.213 | 13.796 | 11.375 | 59.987 | 13.798 | 48.859 | 26.149 | 13.381 |
| Cumulative % of variance | 55.213 | 69.009 | 80.384 | 59.987 | 73.785 | 48.859 | 75.008 | 88.389 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Fang, J.; Feng, F.; Yao, T.; Shan, Y.; Su, W. Differential Evolution in Hydrochemical Characteristics Amongst Porous, Fissured and Karst Aquifers in China. Hydrology 2025, 12, 175. https://doi.org/10.3390/hydrology12070175
Li C, Fang J, Feng F, Yao T, Shan Y, Su W. Differential Evolution in Hydrochemical Characteristics Amongst Porous, Fissured and Karst Aquifers in China. Hydrology. 2025; 12(7):175. https://doi.org/10.3390/hydrology12070175
Chicago/Turabian StyleLi, Chengsong, Jie Fang, Feisheng Feng, Tingting Yao, Yongping Shan, and Wanli Su. 2025. "Differential Evolution in Hydrochemical Characteristics Amongst Porous, Fissured and Karst Aquifers in China" Hydrology 12, no. 7: 175. https://doi.org/10.3390/hydrology12070175
APA StyleLi, C., Fang, J., Feng, F., Yao, T., Shan, Y., & Su, W. (2025). Differential Evolution in Hydrochemical Characteristics Amongst Porous, Fissured and Karst Aquifers in China. Hydrology, 12(7), 175. https://doi.org/10.3390/hydrology12070175





