Activated Carbons for Bone Cell Growth: Structural Properties and Biological Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Activated Carbon Materials
2.2. Physicochemical Properties of ACs
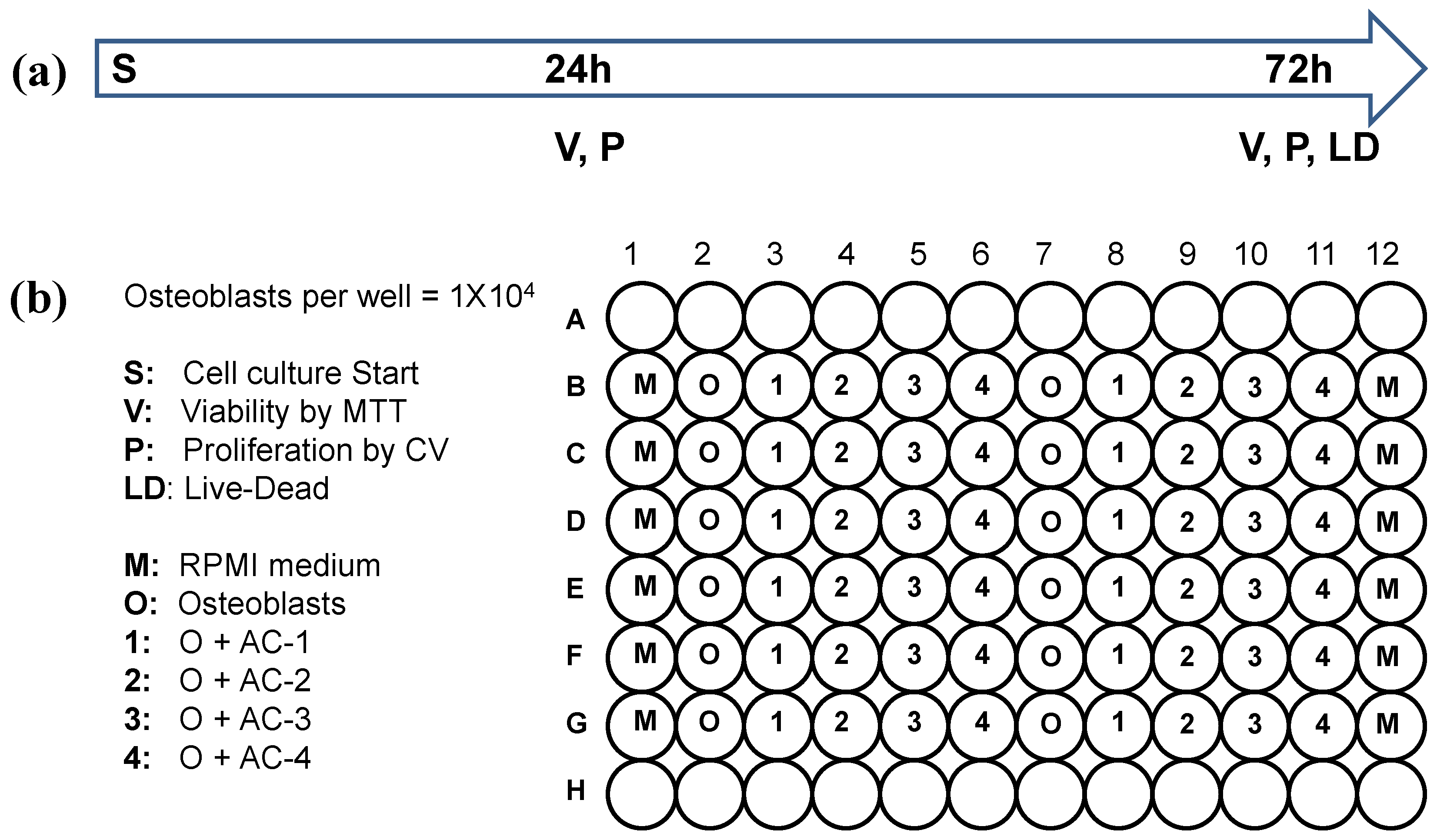
2.3. Osteoblast Culture in Carbon Material
3. Results and Discussion
3.1. Characterization of Activated Carbons
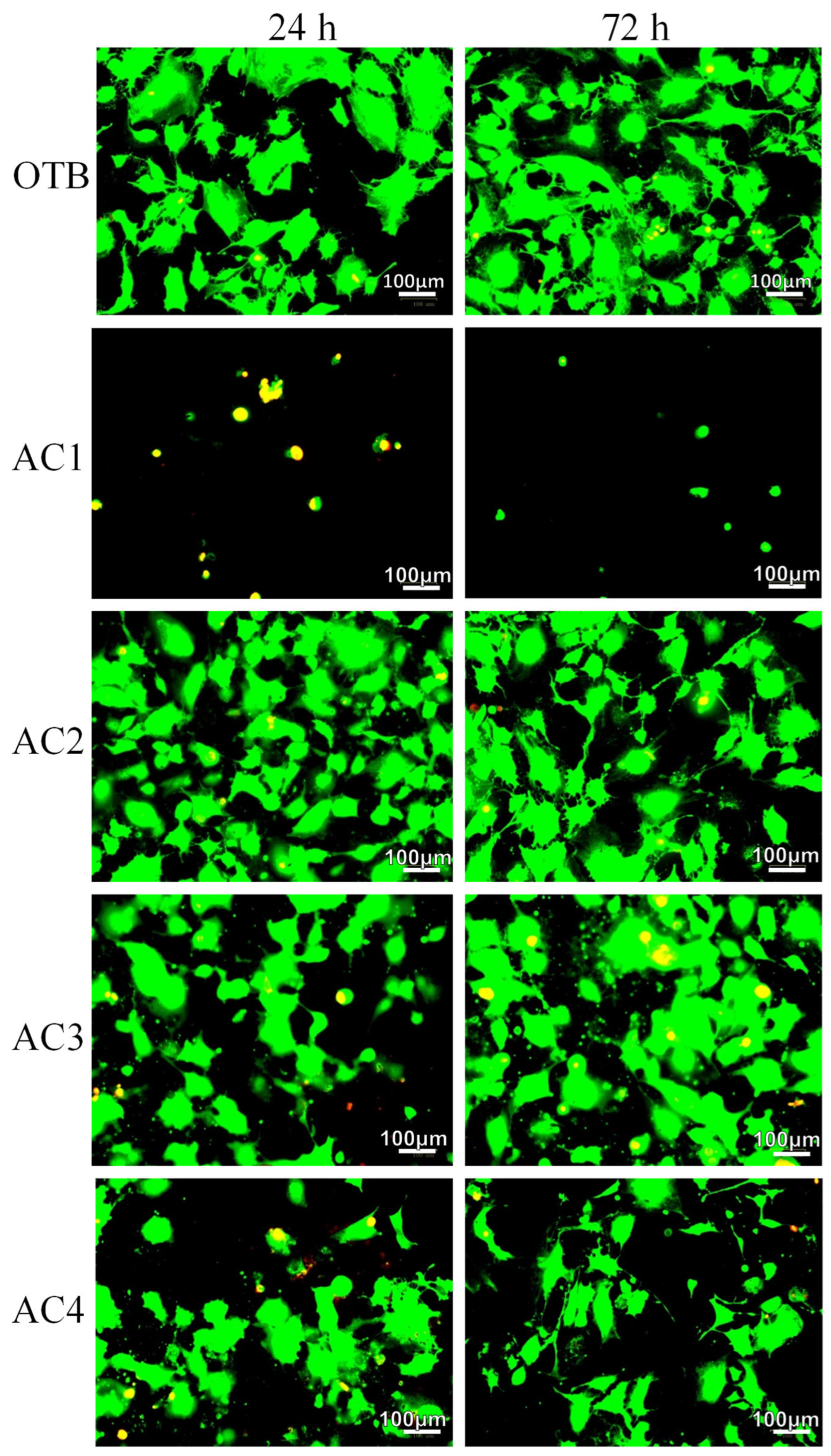
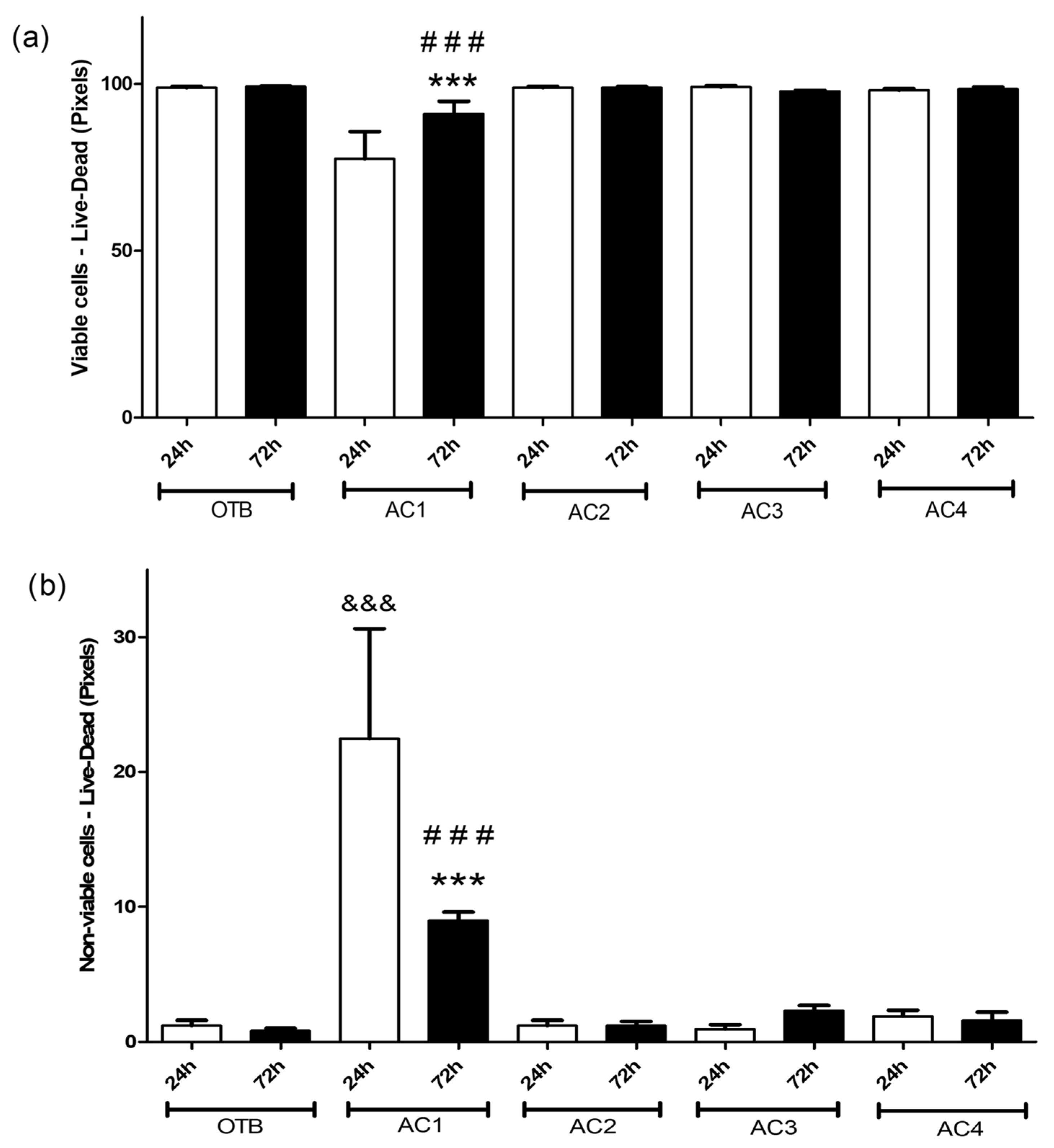
3.2. Osteoblast Culture in Activated Carbon Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Braghiroli, F.L.; Amaral-Labat, G. Chapter 18—Biochar Modification Methods: Property Engineering for Diverse Value-Added Applications. In Biochar Ecotechnology for Sustainable Agriculture and Environment; Kumar, A., Vara Prasad, M.N., Kumari, P., Solanki, M.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 523–559. ISBN 978-0-443-29855-4. [Google Scholar]
- Bratek, W.; Światkowski, A.; Pakuła, M.; Biniak, S.; Bystrzejewski, M.; Szmigielski, R. Characteristics of Activated Carbon Prepared from Waste PET by Carbon Dioxide Activation. J. Anal. Appl. Pyrolysis 2013, 100, 192–198. [Google Scholar] [CrossRef]
- Kundu, S.; Khandaker, T.; Anik, M.A.; Hasan, M.K.; Dhar, P.K.; Dutta, S.K.; Latif, M.A.; Hossain, M.S. A Comprehensive Review of Enhanced CO2 Capture Using Activated Carbon Derived from Biomass Feedstock. RSC Adv. 2024, 14, 29693–29736. [Google Scholar] [CrossRef]
- Quintas Salamba, M.; Melo, R.L.F.; da Silva Aires, F.I.; de Matos Filho, J.R.; Nascimento Dari, D.; Luz Lima, F.L.; Ferreira Alcântara Araújo, S.; da Costa Silva, L.; Fernandes da Silva, L.; Alexandre Chirindza, E.; et al. Porosity of Activated Carbon in Water Remediation: A Bibliometric Review and Overview of Research Perspectives. ACS EST Water 2025, 5, 2070–2086. [Google Scholar] [CrossRef]
- Zumra; Ahmed, S. Comprehensive Review on the Environmental Remediation of Pharmaceuticals in Water by Adsorption. Environ. Toxicol. Chem. 2025, 44, 1457–1476. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Jalil, A.A.; Nguyen, L.M.; Nguyen, D.H. A Comprehensive Review on the Adsorption of Dyes onto Activated Carbons Derived from Harmful Invasive Plants. Environ. Res. 2025, 279, 121807. [Google Scholar] [CrossRef]
- Jones, L.O. Poisoning. Anaesth. Intensive Care Med. 2006, 7, 132–134. [Google Scholar] [CrossRef]
- Bond, G.R. The Role of Activated Charcoal and Gastric Emptying in Gastrointestinal Decontamination: A State-of-the-Art Review. Ann. Emerg. Med. 2002, 39, 273–286. [Google Scholar] [CrossRef]
- Sampson, H.A.; Sicherer, S.H.; Birnbaum, A.H. AGA Technical Review on the Evaluation of Food Allergy in Gastrointestinal Disorders. Gastroenterology 2001, 120, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Publio, M.L.; Amaral-Labat, G.A.; Mattos, P.A.; Rodrigues, A.F.; Del Bel, M.L.S.; de Carvalho Pereira, D.; Mello, D.C.; De Souza, V.; Silva, G.F.; Fierro, V.; et al. Activated Carbon as a Bone Substitute: Enhancing Mechanical and Morphological Properties in a Rat Tibia Defect Model. J. Adv. Med. Med. Res. 2025, 37, 23–40. [Google Scholar] [CrossRef]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone Remodeling during Fracture Repair: The Cellular Picture. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 19, pp. 459–466. [Google Scholar]
- Murta, M.G.M.B.; Martucci, L.G.; Gomes Neto, L.F.; Ruinho, P.B.; de Souza Candido, S.; dos Santos, P.R.S.; da Fonseca Sarmento, J.P.; Santos, S.C.; da Silva, E.L.D.; Pacheco, T.S. Osteomyelitis within the SUS: Analysis of the Epidemiological Profile, Cost of Hospitalization, Average Length of Stay and Mortality in the Last 5 Years. Res. Soc. Dev. 2023, 12, e6612139291. [Google Scholar] [CrossRef]
- Hunter, S.; Alexander, Z.; Crawford, H.; Te Ao, B.; Selak, V.; Mutu-Grigg, J.; Lorgelly, P.; Grant, C. Hospitalisation Cost for Paediatric Osteomyelitis and Septic Arthritis in New Zealand. J. Paediatr. Child. Health 2025, 61, 54–59. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Einhorn, T.A. Developmental Aspects of Fracture Healing and the Use of Pharmacological Agents to Alter Healing. J. Musculoskelet. Neuronal Interact. 2003, 3, 297–303. [Google Scholar]
- Hadjiargyrou, M.; Lombardo, F.; Zhao, S.; Ahrens, W.; Joo, J.; Ahn, H.; Jurman, M.; White, D.W.; Rubin, C.T. Transcriptional Profiling of Bone Regeneration: Insight into the Molecular Complexity of Wound Repair* 210. J. Biol. Chem. 2002, 277, 30177–30182. [Google Scholar] [CrossRef] [PubMed]
- Miranda, E.S.; Cardoso, F.T.S.; Medeiros Filho, J.F.; Barreto, M.D.; Teixeira, R.M.; Wanderley, A.L.; Fernandes, K.E. Organic and Inorganic Bone Graft Use in Rabbits’ Radius Surgical Fractures Rapair: An Experimental and Comparative Study. Acta Ortop. Bras. 2005, 13, 245–248. [Google Scholar] [CrossRef]
- Oliveira, P.D.; Fernandes, K.R.; Sperandio, E.F.; Pastor, F.A.C.; Nonaka, K.O.; Parizotto, N.A.; Renno, A.C.M. Comparative Study of the Effects of Low-Level Laser and Low-Intensity Ultrasound Associated with Biosilicate® on the Process of Bone Repair in the Rat Tibia. Rev. Bras. Ortop. 2012, 47, 102–107. [Google Scholar] [CrossRef][Green Version]
- Flausse, A.; Henrionnet, C.; Dossot, M.; Dumas, D.; Hupont, S.; Pinzano, A.; Mainard, D.; Galois, L.; Magdalou, J.; Lopez, E. Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells in Hydrogel Containing Nacre Powder. J. Biomed. Mater. Res. A 2013, 101, 3211–3218. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Etinosa, P.O.; Osuchukwu, O.A.; Anisiji, E.O.; Lawal, M.Y.; Mohammed, S.A.; Ibitoye, O.I.; Oni, P.G.; Aderibigbe, V.D.; Aina, T.; Oyebode, D.; et al. In-Depth Review of Synthesis of Hydroxyapatite Biomaterials from Natural Resources and Chemical Regents for Biomedical Applications. Arab. J. Chem. 2024, 17, 106010. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon Based Nanomaterials for Tissue Engineering of Bone: Building New Bone on Small Black Scaffolds: A Review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef]
- Arambula-Maldonado, R.; Mequanint, K. Carbon-Based Electrically Conductive Materials for Bone Repair and Regeneration. Mater. Adv. 2022, 3, 5186–5206. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Heal. Healthc. Mater. 2020, 9, 1901495. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, D.; Saravanan, S.; Sudhakar, S.; Vimalraj, S. Graphene: A Multifaceted Carbon-Based Material for Bone Tissue Engineering Applications. ACS Omega 2024, 9, 67–80. [Google Scholar] [CrossRef]
- Okolie, J.; Rogachuk, B. Activated Carbon Utilization for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2025; pp. 225–234. ISBN 9780443138409. [Google Scholar]
- Bahrami, S.; Baheiraei, N.; Shahrezaee, M. Biomimetic Reduced Graphene Oxide Coated Collagen Scaffold for in Situ Bone Regeneration. Sci. Rep. 2021, 11, 16783. [Google Scholar] [CrossRef] [PubMed]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing Scaffold Pore Size for Tissue Engineering: Insights across Various Tissue Types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef]
- Islam, M.; Lantada, A.D.; Mager, D.; Korvink, J.G. Carbon-Based Materials for Articular Tissue Engineering: From Innovative Scaffolding Materials toward Engineered Living Carbon. Adv. Healthc. Mater. 2022, 11, 2101834. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-Based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Boss, A.F.N.; Munhoz, M.G.C.; Amaral-Labat, G.; Lima, R.G.A.; Medeiros, L.I.; Medeiros, N.C.F.L.; Fonseca, B.C.S.; Braghiroli, F.L.; Lenz e Silva, G.F.B. Why Sustainable Porous Carbon Should Be Further Explored as Radar-Absorbing Material? A Comparative Study with Different Nanostructured Carbons. J. Renew. Mater. 2024, 12, 1639–1659. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Cell Growth and Function on Calcium Phosphate Reinforced Chitosan Scaffolds. J. Mater. Sci. Mater. Med. 2004, 15, 255–260. [Google Scholar] [CrossRef]
- Bobe, K.; Willbold, E.; Morgenthal, I.; Andersen, O.; Studnitzky, T.; Nellesen, J.; Tillmann, W.; Vogt, C.; Vano, K.; Witte, F. In Vitro and in Vivo Evaluation of Biodegradable, Open-Porous Scaffolds Made of Sintered Magnesium W4 Short Fibres. Acta Biomater. 2013, 9, 8611–8623. [Google Scholar] [CrossRef]
- Burchacka, E.; Pstrowska, K.; Kułażyński, M.; Beran, E.; Fałtynowicz, H.; Chojnacka, K. Antibacterial Agents Adsorbed on Active Carbon: A New Approach for S. aureus and E. coli Pathogen Elimination. Pathogens 2021, 10, 1066. [Google Scholar] [CrossRef]
- Karsenty, G.; Wagner, E.F. Reaching a Genetic and Molecular Understanding of Skeletal Development. Dev. Cell 2002, 2, 389–406. [Google Scholar] [CrossRef]
- Zhao, W.; Fierro, V.; Fernández-Huerta, N.; Izquierdo, M.T.; Celzard, A. Impact of Synthesis Conditions of KOH Activated Carbons on Their Hydrogen Storage Capacities. Int. J. Hydrogen Energy 2012, 37, 14278–14284. [Google Scholar] [CrossRef]
- Ahmed, M.; Punshon, G.; Darbyshire, A.; Seifalian, A. Effects of Sterilization Treatments on Bulk and Surface Properties of Nanocomposite Biomaterials: Effects of Sterilization on the Properties of Nanocomposite Biomaterials. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1182–1190. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface Oxides on Carbon and Their Analysis: A Critical Assessment. Carbon. N. Y. 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Sponchiado, P.A.I.; Melo, M.T.; Cominal, J.G.; Martelli Tosi, M.; Ciancaglini, P.; Ramos, A.P.; Maniglia, B.C. Biomembranes Based on Potato Starch Modified by Dry Heating Treatment: One Sustainable Strategy to Amplify the Use of Starch as a Biomaterial. Biomacromolecules 2025, 26, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P. V Current Concepts of Molecular Aspects of Bone Healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Lim, J.; Park, Y.; Lee, J.-Y.; Yoon, J.; Choi, J.-W. Carbon-Based Nanocomposites for Biomedical Applications. RSC Adv. 2024, 14, 7142–7156. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. C J. Carbon Res. 2021, 7, 39. [Google Scholar] [CrossRef]
- Robin, M.; Mouloungui, E.; Castillo Dali, G.; Wang, Y.; Saffar, J.-L.; Pavon-Djavid, G.; Divoux, T.; Manneville, S.; Behr, L.; Cardi, D.; et al. Mineralized Collagen Plywood Contributes to Bone Autograft Performance. Nature 2024, 636, 100–107. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone Structure and Formation: A New Perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing Biomaterials of Human Origin for Tissue Engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Won, J.-E.; Knowles, J.C.; Kim, H.-W. Naturally and Synthetic Smart Composite Biomaterials for Tissue Regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef]
- Chiara, G.; Letizia, F.; Lorenzo, F.; Edoardo, S.; Diego, S.; Stefano, S.; Barbara, Z. Nanostructured Biomaterials for Tissue Engineered Bone Tissue Reconstruction. Int. J. Mol. Sci. 2012, 13, 737–757. [Google Scholar] [CrossRef]
- Li, H.; Jiang, F.; Ye, S.; Wu, Y.; Zhu, K.; Wang, D. Bioactive Apatite Incorporated Alginate Microspheres with Sustained Drug-Delivery for Bone Regeneration Application. Mater. Sci. Eng. C 2016, 62, 779–786. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Medeiros, N.C.F.L.; Amaral-Labat, G.; de Medeiros, L.I.; Boss, A.F.N.; Fonseca, B.C.d.S.; Munhoz, M.G.d.C.; Silva, G.F.B.L.e.; Baldan, M.R.; Braghiroli, F.L. Optimizing Activation Temperature of Sustainable Porous Materials Derived from Forestry Residues: Applications in Radar-Absorbing Technologies. J. Renew. Mater. 2025, 13, 1021. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-Generation Biomedical Materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Vincent, M.P.; Bobbala, S.; Karabin, N.B.; Frey, M.; Liu, Y.; Navidzadeh, J.O.; Stack, T.; Scott, E.A. Surface Chemistry-Mediated Modulation of Adsorbed Albumin Folding State Specifies Nanocarrier Clearance by Distinct Macrophage Subsets. Nat. Commun. 2021, 12, 648. [Google Scholar] [CrossRef]
- Stocco, T.D.; Zhang, T.; Dimitrov, E.; Ghosh, A.; da Silva, A.M.H.; Melo, W.C.M.A.; Tsumura, W.G.; Silva, A.D.R.; Sousa, G.F.; Viana, B.C.; et al. Carbon Nanomaterial-Based Hydrogels as Scaffolds in Tissue Engineering: A Comprehensive Review. Int. J. Nanomed. 2023, 18, 6153–6183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X. The Utilization of Carbon-Based Nanomaterials in Bone Tissue Regeneration and Engineering: Respective Featured Applications and Future Prospects. Med. Nov. Technol. Devices 2022, 16, 100168. [Google Scholar] [CrossRef]
- Teimouri, R.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Surface Modifications of Scaffolds for Bone Regeneration. J. Mater. Res. Technol. 2023, 24, 7938–7973. [Google Scholar] [CrossRef]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef]
- Park, K.-R.; Kwon, Y.-J.; Park, J.-E.; Yun, H.-M. 7-HYB, a Phenolic Compound Isolated from Myristica Fragrans Houtt Increases Cell Migration, Osteoblast Differentiation, and Mineralization through BMP2 and β-Catenin Signaling. Int. J. Mol. Sci. 2020, 21, 8059. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Abu Serea, E.S.; Salah-Eldin, R.E.; Al-Hafiry, S.A.; Ali, M.K.; Shalan, A.E.; Lanceros-Méndez, S. Recent Progress in Graphene-and Related Carbon-Nanomaterial-Based Electrochemical Biosensors for Early Disease Detection. ACS Biomater. Sci. Eng. 2022, 8, 964–1000. [Google Scholar] [CrossRef]
- Stoddart, M.J. Cell Viability Assays: Introduction. Methods Mol. Biol. 2011, 740, 1–6. [Google Scholar]
- Mojoudi, N.; Mirghaffari, N.; Soleimani, M.; Shariatmadari, H.; Belver, C.; Bedia, J. Phenol Adsorption on High Microporous Activated Carbons Prepared from Oily Sludge: Equilibrium, Kinetic and Thermodynamic Studies. Sci. Rep. 2019, 9, 19352. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tian, Y.; Ouyang, J.; Shen, Y.; Wang, X.; Luan, J. Carbon Nanomaterials for Drug Delivery and Tissue Engineering. Front. Chem. 2022, 10, 990362. [Google Scholar] [CrossRef]
- Amadou Kiari, M.N.; Konan, A.T.S.; Sanda Mamane, O.; Ouattara, L.Y.; Ibrahim Grema, M.H.; Siragi Dounounou Boukari, M.; Adamou Ibro, A.; Malam Alma, M.M.; Yao, K.B. Adsorption Kinetics, Thermodynamics, Modeling and Optimization of Bisphenol A on Activated Carbon Based on Hyphaene thebaica Shells. Case Stud. Chem. Environ. Eng. 2024, 10, 100903. [Google Scholar] [CrossRef]
- González-Rodríguez, L.; Yáñez, O.; Mena-Ulecia, K.; Hidalgo-Rosa, Y.; García-Carmona, X.; Ulloa-Tesser, C. Exploring the Adsorption of Five Emerging Pollutants on Activated Carbon: A Theoretical Approach. J. Environ. Chem. Eng. 2024, 12, 112911. [Google Scholar] [CrossRef]
- Pereira, R.G.; Veloso, C.M.; da Silva, N.M.; de Sousa, L.F.; Bonomo, R.C.F.; de Souza, A.O.; da Guarda Souza, M.O.; Fontan, R.d.C.I. Preparation of Activated Carbons from Cocoa Shells and Siriguela Seeds Using H3PO4 and ZnCL2 as Activating Agents for BSA and α-Lactalbumin Adsorption. Fuel Process. Technol. 2014, 126, 476–486. [Google Scholar] [CrossRef]
- Barnes, L.-M.; Phillips, G.J.; Davies, J.G.; Lloyd, A.W.; Cheek, E.; Tennison, S.R.; Rawlinson, A.P.; Kozynchenko, O.P.; Mikhalovsky, S. V The Cytotoxicity of Highly Porous Medical Carbon Adsorbents. Carbon N. Y. 2009, 47, 1887–1895. [Google Scholar] [CrossRef]
- Sarnatskaya, V.; Shlapa, Y.; Lykhova, A.; Brieieva, O.; Prokopenko, I.; Sidorenko, A.; Solopan, S.; Kolesnik, D.; Belous, A.; Nikolaev, V. Structure and Biological Activity of Particles Produced from Highly Activated Carbon Adsorbent. Heliyon 2022, 8, e09163. [Google Scholar] [CrossRef] [PubMed]
- Mashhadimoslem, H.; Safarzadeh Khosrowshahi, M.; Jafari, M.; Ghaemi, A.; Maleki, A. Adsorption Equilibrium, Thermodynamic, and Kinetic Study of O2/N2/CO2 on Functionalized Granular Activated Carbon. ACS Omega 2022, 7, 18409–18426. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, X.; Maimaitiniyazi, R.; Zhang, X.; Qu, Q. Discussion on the Thermodynamic Calculation and Adsorption Spontaneity Re Ofudje et al. (2023). Heliyon 2024, 10, e28188. [Google Scholar] [CrossRef] [PubMed]
- Mahtabian, S.; Mirhadi, S.M.; Tavangarian, F. From Rose Petal to Bone Scaffolds: Using Nature to Fabricate Osteon-like Scaffolds for Bone Tissue Engineering Applications. Ceram. Int. 2021, 47, 21633–21641. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 12 March 2025).
- Gillman, C.E.; Jayasuriya, A.C. FDA-Approved Bone Grafts and Bone Graft Substitute Devices in Bone Regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef]
- Matsumoto, M.A.; Caviquioli, G.; Biguetti, C.C.; de Andrade Holgado, L.; Saraiva, P.P.; Rennó, A.C.M.; Kawakami, R.Y. A Novel Bioactive Vitroceramic Presents Similar Biological Responses as Autogenous Bone Grafts. J. Mater. Sci. Mater. Med. 2012, 23, 1447–1456. [Google Scholar] [CrossRef]


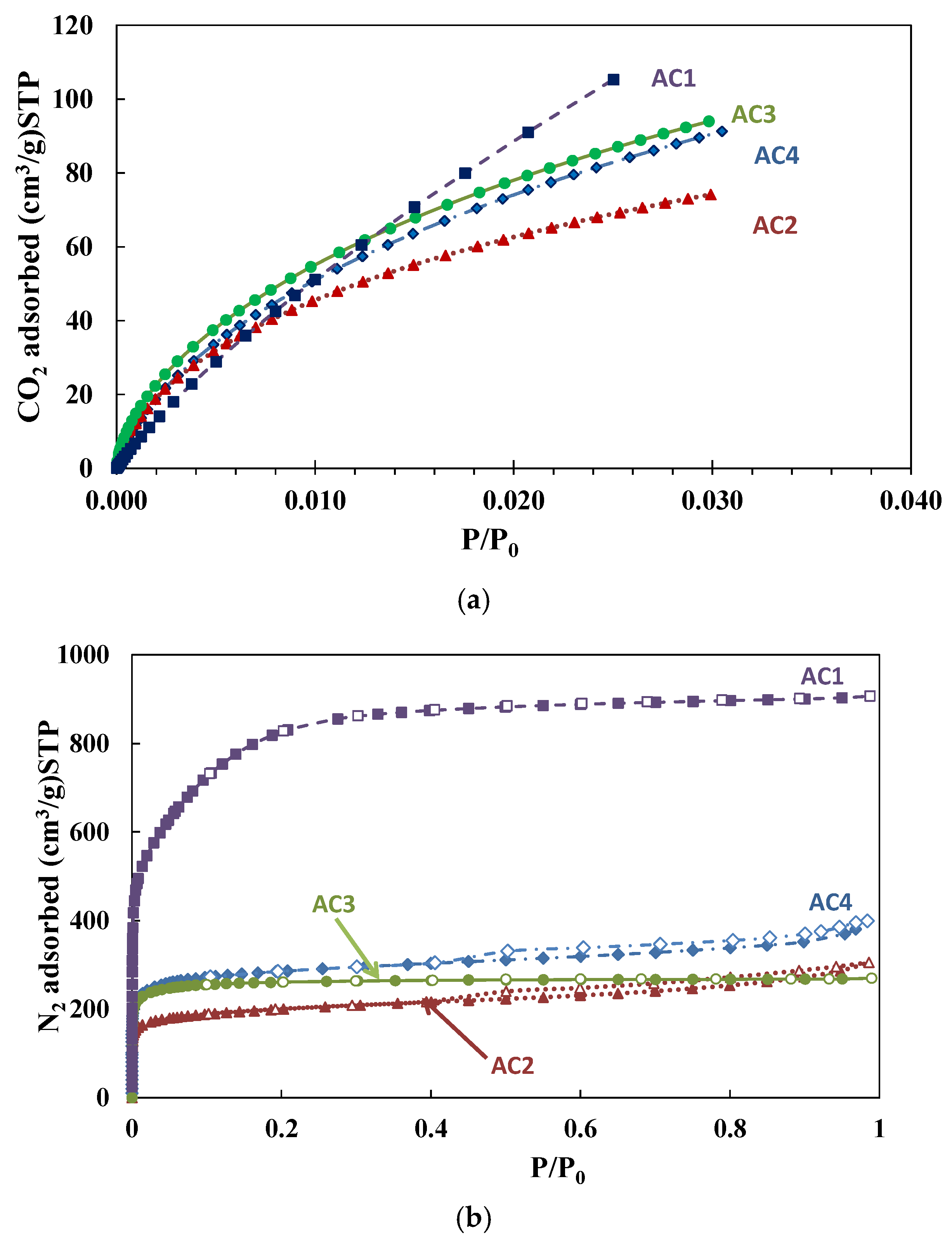



| CO2 Adsorption | N2 Adsorption | ||||||
|---|---|---|---|---|---|---|---|
| Samples | SBET (m2/g) | VDR (cm3/g) | SBET (m2/g) | VDR (cm3/g) | Vmeso (cm3/g) | VDR/V0.97 (%) | Vmeso/V0.97 (%) |
| AC1 | 1424 | 0.41 | 3072 | 0.97 | 0.43 | 69 | 31 |
| AC2 | 470 | 0.25 | 755 | 0.28 | 0.18 | 60 | 37 |
| AC3 | 632 | 0.31 | 1040 | 0.39 | 0.03 | 93 | 7 |
| AC4 | 635 | 0.33 | 1083 | 0.42 | 0.17 | 67 | 28 |
| Samples | Vmeso Hg (cm3/g) | Vmacro Hg (cm3/g) | Vmeso Hg/ Vtotal Hg | Vmacro Hg/ Vtotal Hg |
|---|---|---|---|---|
| AC1 | 0.59 | 4.04 | 13 | 87 |
| AC2 | 0.15 | 1.09 | 12 | 88 |
| AC3 | 0.08 | 0.18 | 31 | 69 |
| AC4 | 0.20 | 0.55 | 27 | 73 |
| Bio-Substitute Precursor | Composition | Code | Price ($) |
|---|---|---|---|
| Hydroxyapatite | Ca10(PO4)6(OH)2 | 900204-50G | $215.00/50 g |
| Bioactive glass powder | CaO:SiO2:P2O5 | 915084-25G | $273.00/25 g |
| Norit® PK 1-3 | Activated Carbon | 22874-250G | $71.50/250 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.d.C.; Souza, D.V.; Rodrigues, A.F.; Amaral-Labat, G.; Almeida-Mattos, P.; Lenz e Silva, G.F.B.; Braghiroli, F.L.; Oliveira, A.P.L.d.; Silva Júnior, J.A.; Zamuner, S.R.; et al. Activated Carbons for Bone Cell Growth: Structural Properties and Biological Interactions. ChemEngineering 2025, 9, 139. https://doi.org/10.3390/chemengineering9060139
Pereira DdC, Souza DV, Rodrigues AF, Amaral-Labat G, Almeida-Mattos P, Lenz e Silva GFB, Braghiroli FL, Oliveira APLd, Silva Júnior JA, Zamuner SR, et al. Activated Carbons for Bone Cell Growth: Structural Properties and Biological Interactions. ChemEngineering. 2025; 9(6):139. https://doi.org/10.3390/chemengineering9060139
Chicago/Turabian StylePereira, Damião de Carvalho, Drielli Viana Souza, Ayres Fernando Rodrigues, Gisele Amaral-Labat, Patrícia Almeida-Mattos, Guilherme Frederico Bernardo Lenz e Silva, Flavia Lega Braghiroli, Ana Paula Ligeiro de Oliveira, José Antônio Silva Júnior, Stella Regina Zamuner, and et al. 2025. "Activated Carbons for Bone Cell Growth: Structural Properties and Biological Interactions" ChemEngineering 9, no. 6: 139. https://doi.org/10.3390/chemengineering9060139
APA StylePereira, D. d. C., Souza, D. V., Rodrigues, A. F., Amaral-Labat, G., Almeida-Mattos, P., Lenz e Silva, G. F. B., Braghiroli, F. L., Oliveira, A. P. L. d., Silva Júnior, J. A., Zamuner, S. R., Fierro, V., Celzard, A., & Marcos, R. L. (2025). Activated Carbons for Bone Cell Growth: Structural Properties and Biological Interactions. ChemEngineering, 9(6), 139. https://doi.org/10.3390/chemengineering9060139












