Polyvinylidene Fluoride Membrane Modified by PEG Additive for Tofu Industrial Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Coagulation–Flocculation Process
2.5. Ultrafiltration Process
3. Results and Discussion
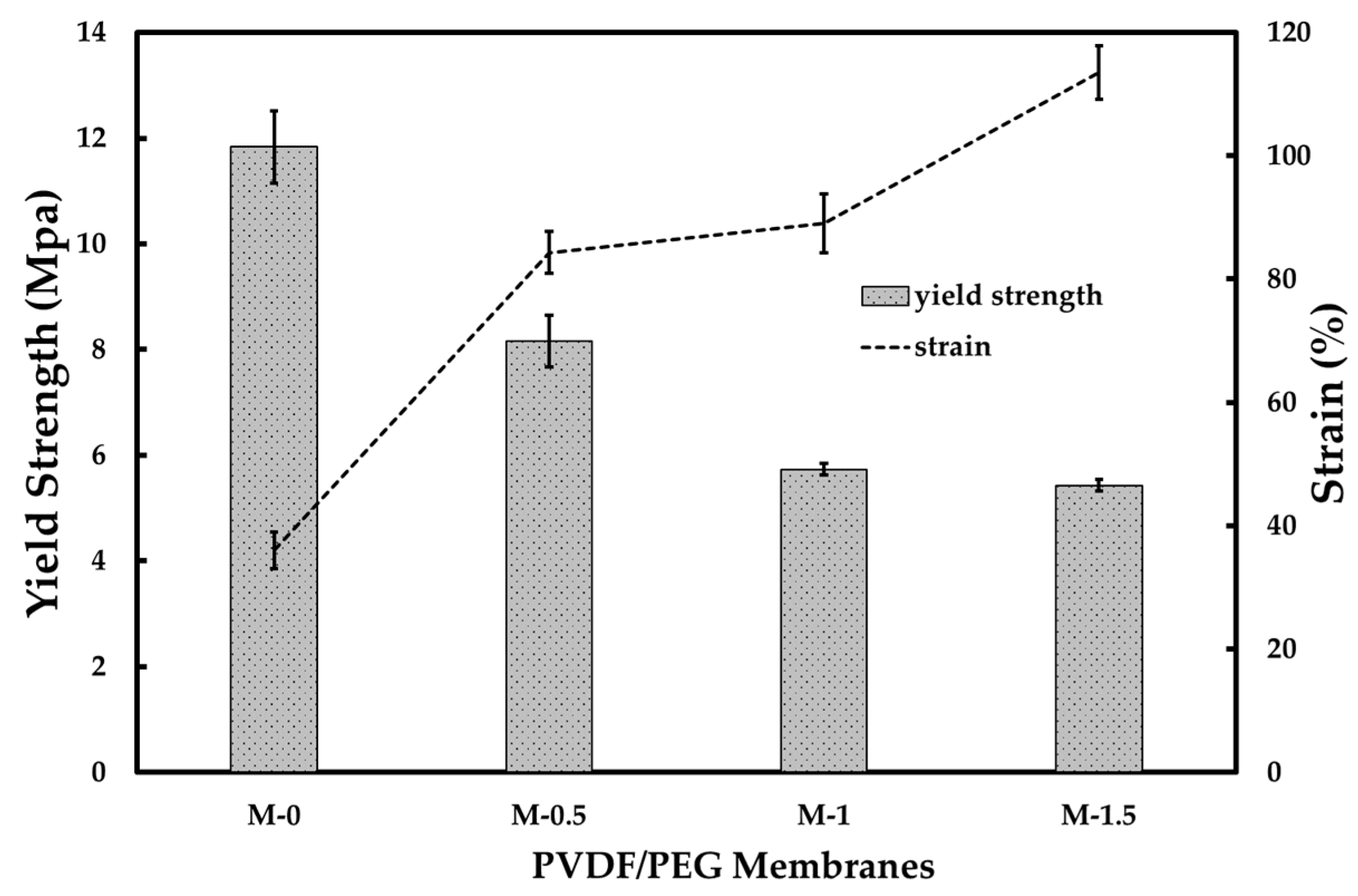
3.1. Membrane Characterization
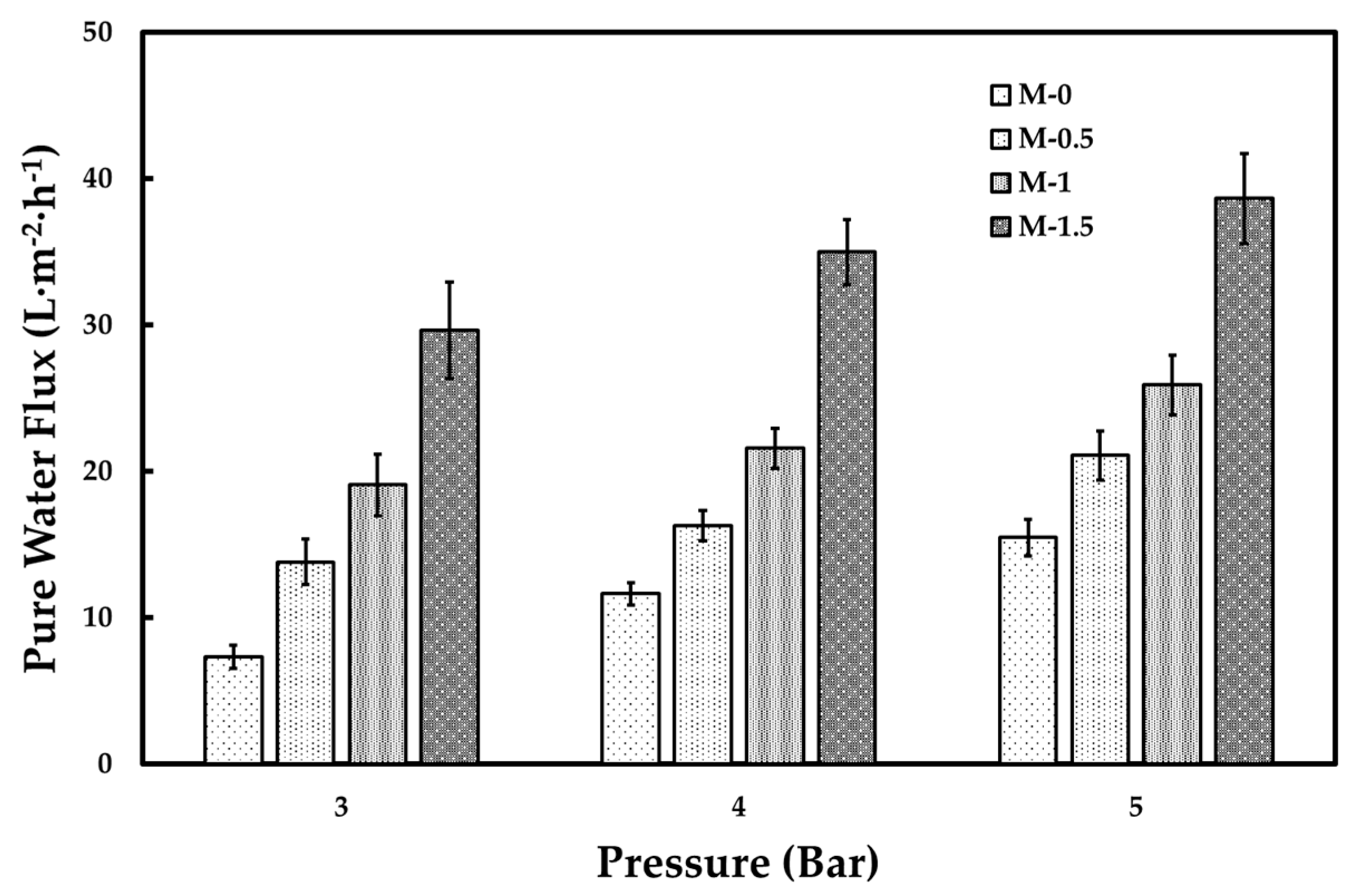
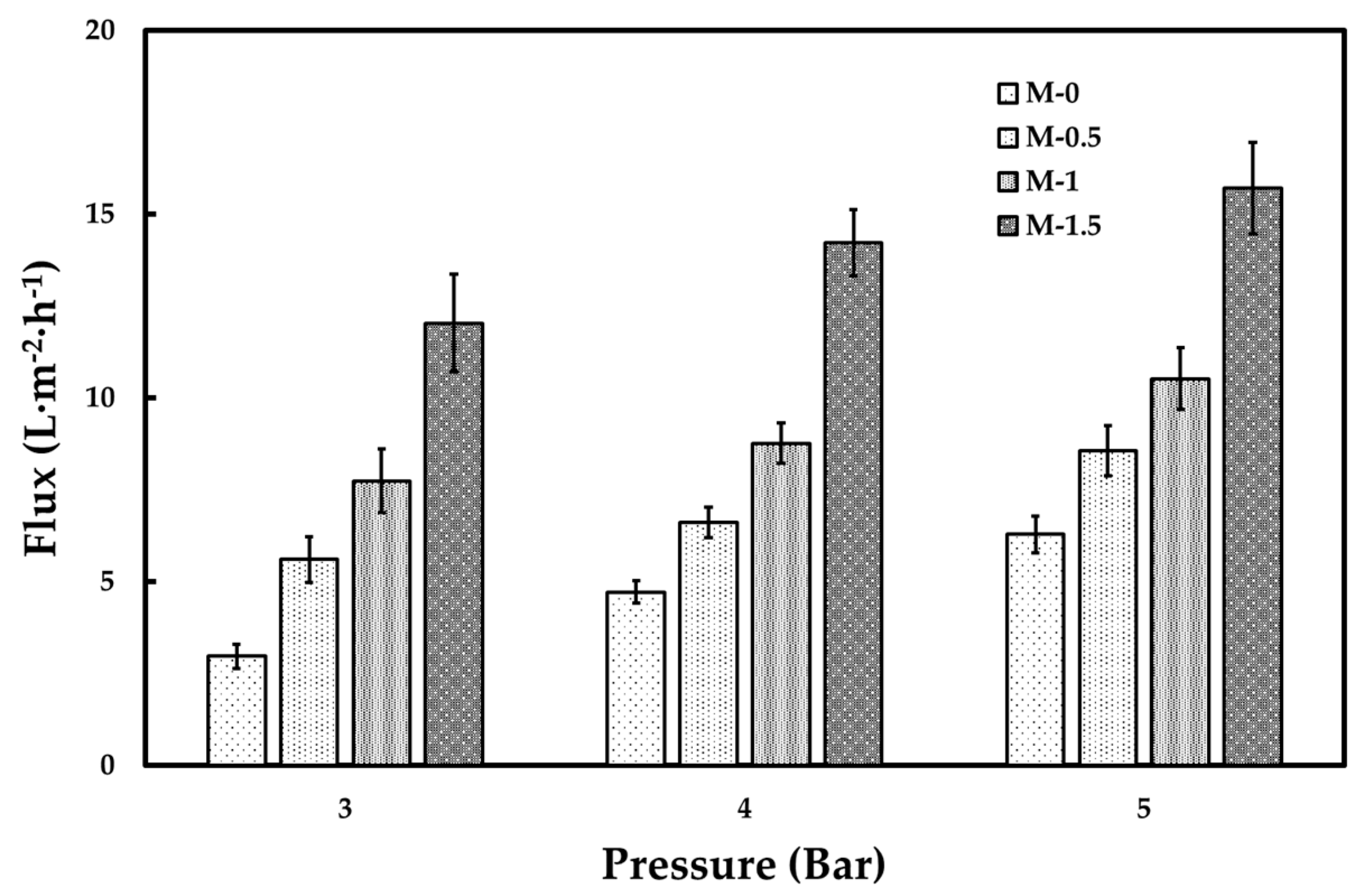
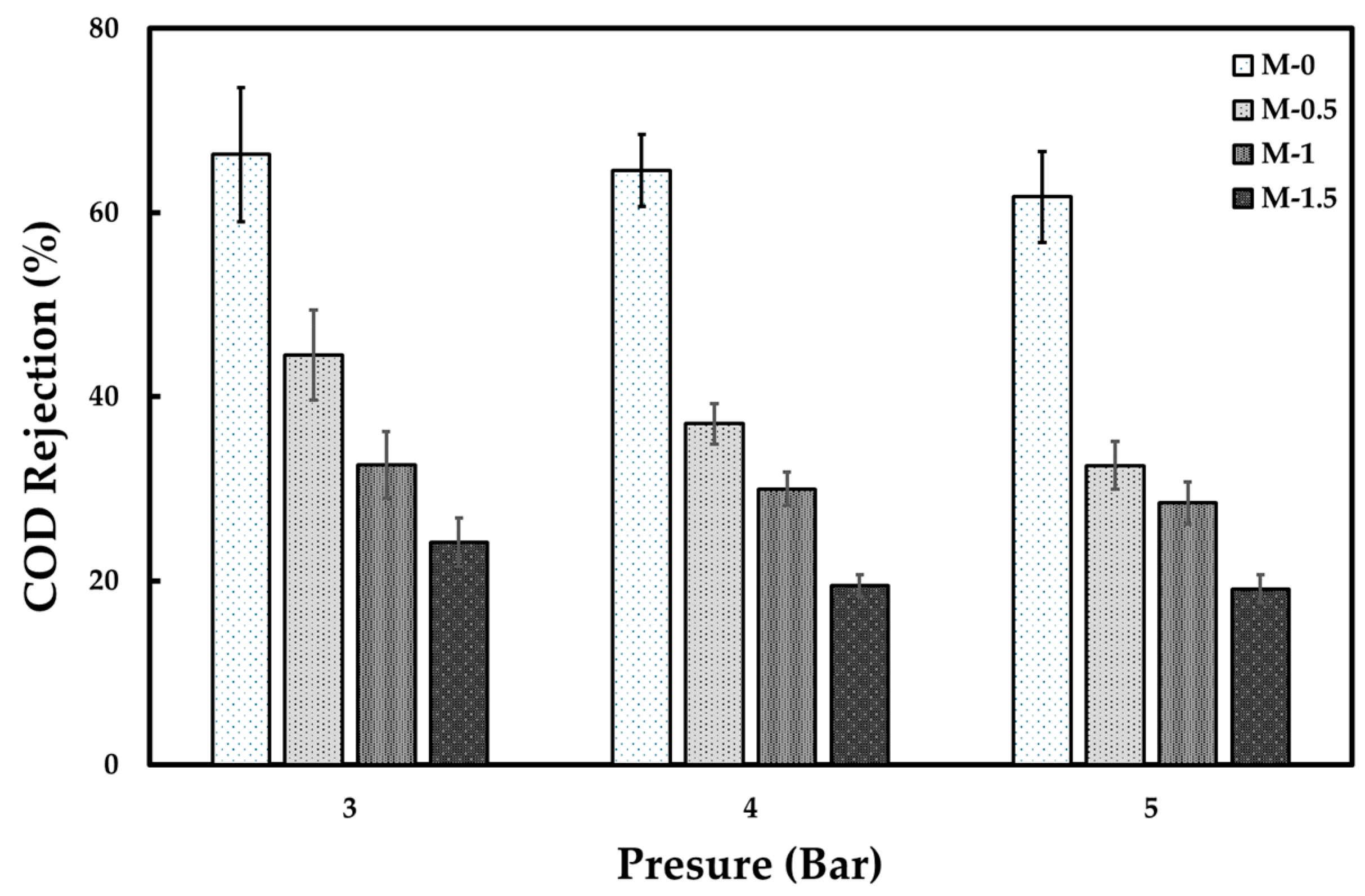
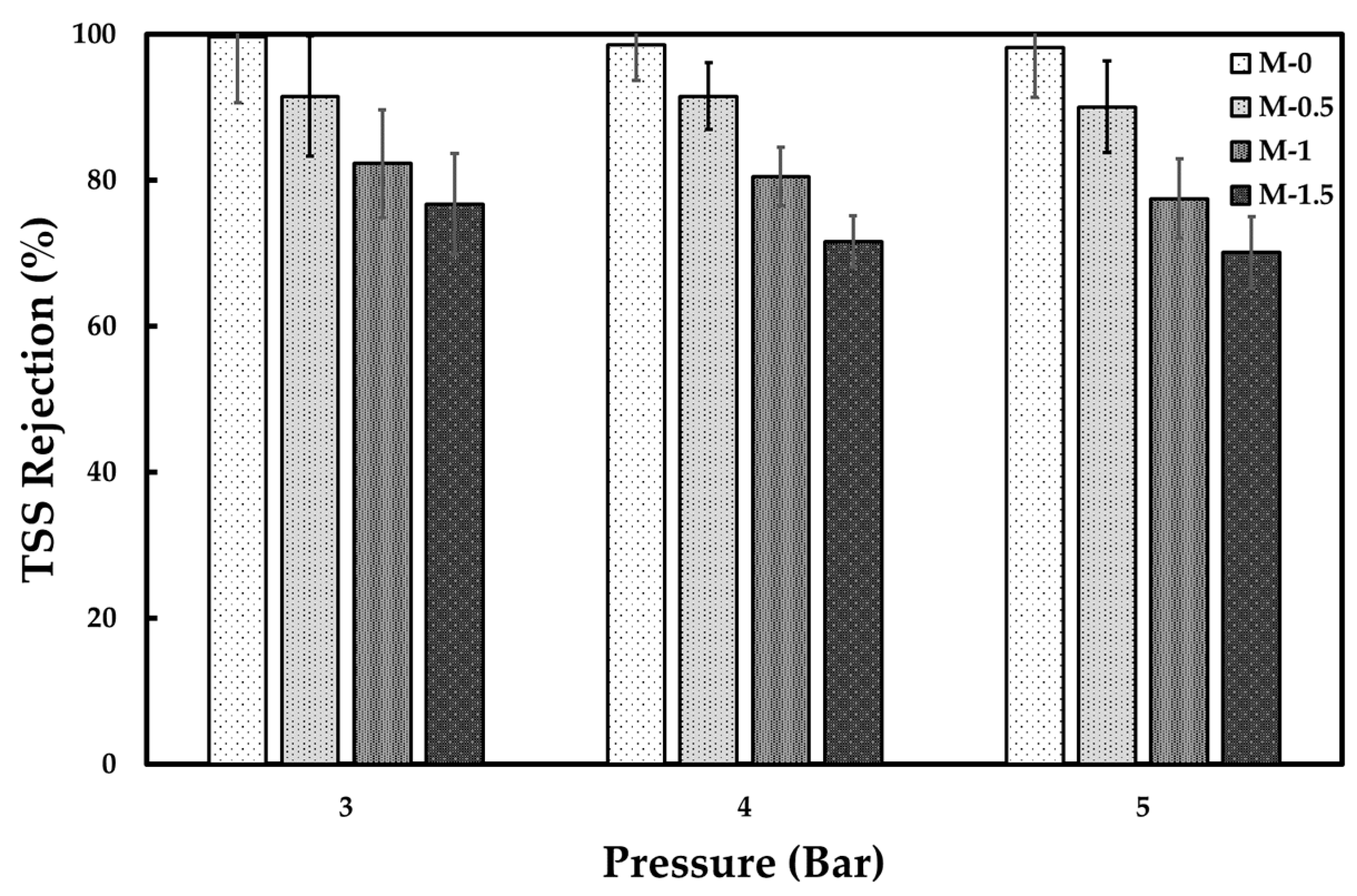
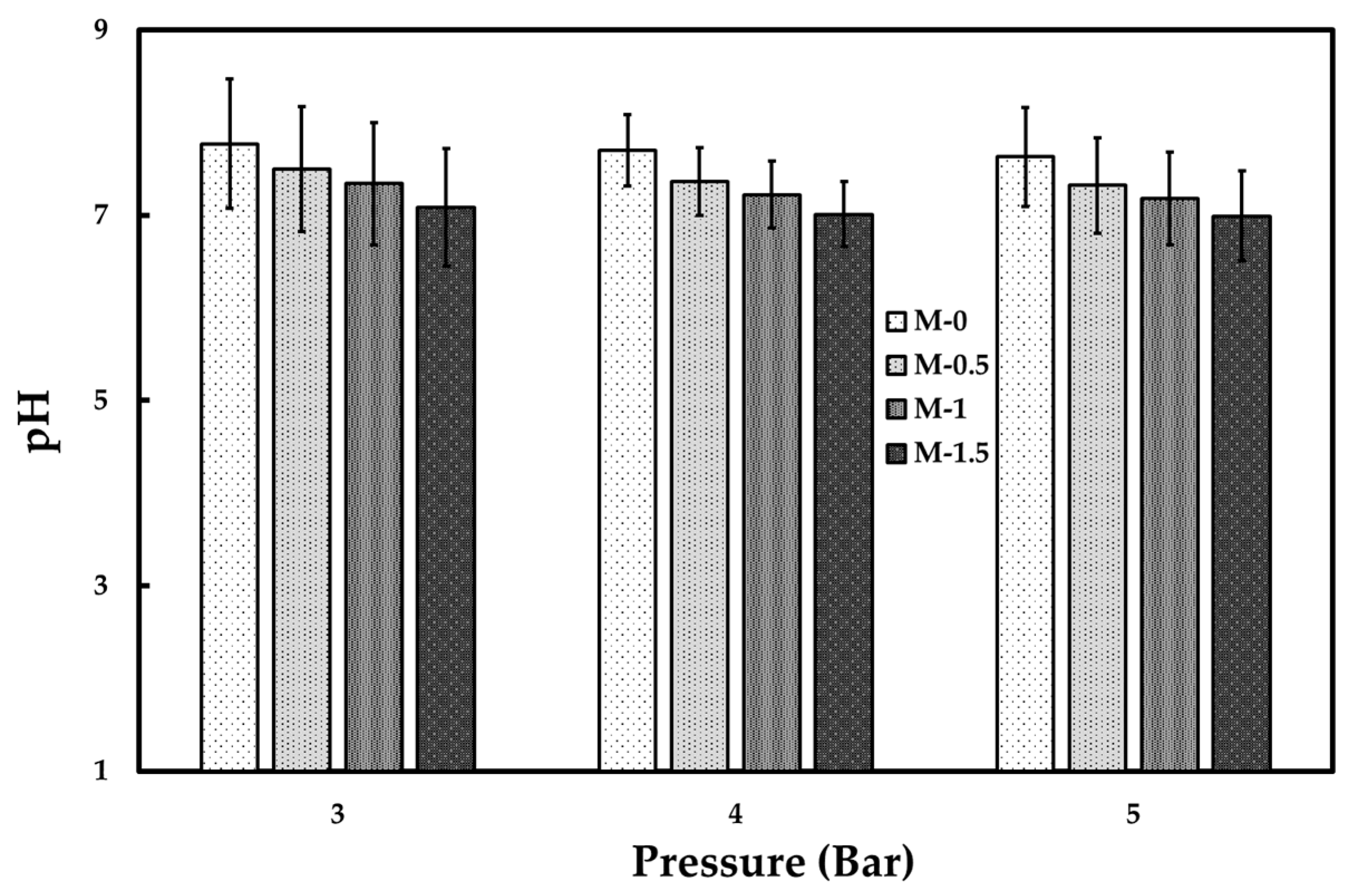
3.2. Tofu Wastewater Treatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Central Bureau of Statistics of Indonesia. Indonesian Statistics 2003; Central Bureau of Statistics of Indonesia: Jakarta, Indonesia, 2023. [Google Scholar]
- Faisal, M.; Gani, A.; Mulana, F.; Daimon, H. Treatment and utilization of industrial tofu waste in Indonesia. Asian J. Chem. 2016, 28, 501–507. [Google Scholar] [CrossRef]
- Oktariany, A.; Kartohardjono, S. Effect of Coagulant Dosage on Tofu Industry Wastewater Treatment in Combination with Ultrafiltration Process using Polysulfone Membrane. E3S Web Conf. 2018, 67, 04004. [Google Scholar] [CrossRef]
- Astuti, A.D.; Ayu, D.I. Treatment of tofu industry wastewater using bioreactor anaerobic-aerobic and bioball as media with variation of hidraulic retention time. Reaktor 2019, 19, 18–25. [Google Scholar] [CrossRef]
- Ministry of Environment of the Republic of Indonesia. Wastewater Quality Standards; Standard No. 5; Ministry of Environment of the Republic of Indonesia: Jakarta, Indonesia, 2014; Volume 2014. [Google Scholar]
- Purnawan, I.; Angputra, D.; Debora, S.C.; Karamah, E.F.; Febriasari, A.; Kartohardjono, S. Polyvinylidene fluoride membrane with a polyvinylpyrrolidone additive for tofu industrial wastewater treatment in combination with the coagulation–flocculation process. Membranes 2021, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Shukla, P. A comprehensive review on fundamental properties and applications of poly (vinylidene fluoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Barambu, N.U.; Bilad, M.R.; Bustam, M.A.; Kurnia, K.A.; Othman, M.H.D.; Nordin, N.A.H.M. Development of membrane material for oily wastewater treatment: A review. Ain Shams Eng. J. 2021, 12, 1361–1374. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, D.; Sun, X.; Jiang, S.; Liu, Y.; Zhang, D.; Zhang, L. Surface modified by green synthetic of Cu-MOF-74 to improve the anti-biofouling properties of PVDF membranes. Chem. Eng. J. 2021, 411, 128524. [Google Scholar] [CrossRef]
- Mortaheb, H.R.; Baghban Salehi, M.; Rajabzadeh, M. Optimized hybrid PVDF/graphene membranes for enhancing performance of AGMD process in water desalination. J. Ind. Eng. Chem. 2021, 99, 407–421. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef]
- Ren, G.; Wan, K.; Kong, H.; Guo, L.; Wang, Y.; Liu, X.; Wei, G. Recent advance in biomass membranes: Fabrication, functional regulation, and antimicrobial applications. Carbohydr. Polym. 2023, 305, 120537. [Google Scholar] [CrossRef]
- Sinha, M.; Purkait, M. Increase in hydrophilicity of polysulfone membrane using polyethylene glycol methyl ether. J. Membr. Sci. 2013, 437, 7–16. [Google Scholar] [CrossRef]
- Aktar, S.; Saha, M.; Mahbub, S.; Halim, M.A.; Rub, M.A.; Hoque, M.A.; Islam, D.S.; Kumar, D.; Alghamdi, Y.G.; Asiri, A.M. Influence of polyethylene glycol on the aggregation/clouding phenomena of cationic and non-ionic surfactants in attendance of electrolytes (NaCl & Na2SO4): An experimental and theoretical analysis. J. Mol. Liq. 2020, 306, 112880. [Google Scholar]
- Subagja, T.; Adhika, D.R.; Kurniawan, R.; Sumarlan, A.; Saraswaty, V. Nano-Encapsulated PEG 1000 and Paraffin Composite as Phase Change Material for Thermal Comfort Enhancement. Int. J. Technol. 2025, 16, 291–319. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Shao, X.-S.; Xu, Y.-Y.; Zhang, Q.-Q. Surface modification of PVDF porous membranes. Chin. J. Polym. Sci. 2013, 31, 994–1001. [Google Scholar] [CrossRef]
- Purnawan, I.; Febriasari, A.; Setyaputra, B.; Yolandini, T.T.; Windriyo, M.J.; Karamah, E.F.; Kartohardjono, S. Combined Process of Ozonation and Membrane Processes to treat Wastewater from Batik Industry. IOP Conf. Ser. Earth Environ. Sci. 2020, 442, 012003. [Google Scholar]
- Zhang, J.; Li, G.; Yuan, X.; Li, P.; Yu, Y.; Yang, W.; Zhao, S. Reduction of ultrafiltration membrane fouling by the pretreatment removal of emerging pollutants: A review. Membranes 2023, 13, 77. [Google Scholar] [CrossRef]
- Yunos, M.Z.; Harun, Z.; Basri, H.; Ismail, A.F. Studies on fouling by natural organic matter (NOM) on polysulfone membranes: Effect of polyethylene glycol (PEG). Desalination 2014, 333, 36–44. [Google Scholar] [CrossRef]
- Alamery, H.R.D.; Hatim, M.I.; Ahmad, M.S. A study of the effects of adding PEG on the properties and morphology of asymmetric membranes comprising PVDF-HFP co-polymer fabricated by phase inversion method. ARPN J. Eng. Appl. Sci. 2016, 11, 7130–7140. [Google Scholar]
- Lusiana, R.A.; Indra, A.; Prasetya, N.B.A.; Sasongko, N.A.; Siahaan, P.; Azmiyawati, C.; Wijayanti, N.; Wijaya, A.R.; Othman, M.H.D. The Effect of Temperature, Sulfonation, and PEG Addition on Physicochemical Characteristics of PVDF Membranes and Its Application on Hemodialysis Membrane. Indones. J. Chem. 2021, 21, 942–953. [Google Scholar] [CrossRef]
- Marmur, A.; Della Volpe, C.; Siboni, S.; Amirfazli, A.; Drelich, J.W. Contact angles and wettability: Towards common and accurate terminology. Surf. Innov. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Hakami, M.W.; Alkhudhiri, A.; Al-Batty, S.; Zacharof, M.-P.; Maddy, J.; Hilal, N. Ceramic microfiltration membranes in wastewater treatment: Filtration behavior, fouling and prevention. Membranes 2020, 10, 248. [Google Scholar] [CrossRef]
- Umam, K.; Sagita, F.; Pramono, E.; Ledyastuti, M.; Kadja, G.T.; Radiman, C.L. Polyvinylidenefluoride (PVDF)/surface functionalized-mordenite mixed matrix membrane for congo red dyes removal: Effect of types of organosilane. JCIS Open 2023, 11, 100093. [Google Scholar]
- Folgado, E.; Ladmiral, V.; Semsarilar, M. Towards permanent hydrophilic PVDF membranes. Amphiphilic PVDF-b-PEG-b-PVDF triblock copolymer as membrane additive. Eur. Polym. J. 2020, 131, 109708. [Google Scholar] [CrossRef]
- Isawi, H. Evaluating the performance of different nano-enhanced ultrafiltration membranes for the removal of organic pollutants from wastewater. J. Water Process Eng. 2019, 31, 100833. [Google Scholar] [CrossRef]
- Gittleman, C.S.; Coms, F.D.; Lai, Y.-H. Membrane durability: Physical and chemical degradation. In Polymer Electrolyte Fuel Cell Degradation; Springer: New York, NY, USA, 2011; Volume 15. [Google Scholar]
- Wang, L.-Y.; Yong, W.F.; Yu, L.E.; Chung, T.-S. Design of high efficiency PVDF-PEG hollow fibers for air filtration of ultrafine particles. J. Membr. Sci. 2017, 535, 342–349. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; He, Y.; Liu, B.; Zhong, X. Natural organic matter removal and flux decline with PEG–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Membr. Sci. 2012, 405–406, 48–56. [Google Scholar] [CrossRef]
- Fadaei, A.; Salimi, A.; Mirzataheri, M. Structural elucidation of morphology and performance of the PVDF/PEG membrane. J. Polym. Res. 2014, 21, 545. [Google Scholar] [CrossRef]
- Zirehpour, A.; Rahimpour, A. Membranes for wastewater treatment. Nanostruct. Polym. Membr. 2016, 2, 159–207. [Google Scholar]
- Katibi, K.K.; Yunos, K.F.; Che Man, H.; Aris, A.Z.; bin Mohd Nor, M.Z.; Binti Azis, R.S. Recent advances in the rejection of endocrine-disrupting compounds from water using membrane and membrane bioreactor technologies: A review. Polymers 2021, 13, 392. [Google Scholar] [CrossRef] [PubMed]
- Pervov, A.; Tikhonov, K. Control of ionic composition of wastewater treated by reverse osmosis membranes to increase product total dissolved solids and reduce concentrate utilization costs. Desalination Water Treat. 2023, 309, 49–57. [Google Scholar] [CrossRef]
- Wang, J.; Cahyadi, A.; Wu, B.; Pee, W.; Fane, A.G.; Chew, J.W. The roles of particles in enhancing membrane filtration: A review. J. Membr. Sci. 2020, 595, 117570. [Google Scholar] [CrossRef]
- Fan, H.; Duan, Y.; Elimelech, M. Compaction of Pressure-Driven Polymer Membranes: Measurements, Theory, and Mechanisms. Environ. Sci. Technol. 2025, 59, 14752–14763. [Google Scholar] [CrossRef]
- Alrasheedi, N.F.; Abdulazeez, I.; Baig, N.; Salhi, B.; Asmaly, H.A.; Haladu, S.A.; Elsharif, A.M. Antifouling macrocyclic-engineered PVDF membrane for the low-pressure separation of surfactant-stabilized oily wastewater. J. Environ. Chem. Eng. 2024, 12, 112850. [Google Scholar] [CrossRef]
- Sherhan, B.Y.; Abbas, A.D.; Alsalhy, Q.F.; Rashad, A.A.; Rashad, Z.W.; Shawkat, A.A.; Abbas, T.K.; Kareem, N.A.A. Produced water treatment using ultrafiltration and nanofiltration membranes. Al-Khwarizmi Eng. J. 2016, 12, 10–18. [Google Scholar]
- Adjovu, G.E.; Stephen, H.; James, D.; Ahmad, S. Measurement of total dissolved solids and total suspended solids in water systems: A review of the issues, conventional, and remote sensing techniques. Remote Sens. 2023, 15, 3534. [Google Scholar] [CrossRef]
- Abdullah, S.; Karmakar, S.; Mishra, S.; Pradhan, R.C. Ultrafiltration of cashew apple juice using hollow fibers for shelf life extension: Process optimization, flux modelling and storage study. J. Food Meas. Charact. 2023, 17, 2182–2192. [Google Scholar] [CrossRef]
- Chua, J.-Y.; Liu, S.-Q. Soy whey: More than just wastewater from tofu and soy protein isolate industry. Trends Food Sci. Technol. 2019, 91, 24–32. [Google Scholar] [CrossRef]
- Hardyanti, N.; Susanto, H.; Budihardjo, M.A. Removal of organic matter from tofu wastewater using a combination of adsorption, Fenton oxidation, and ultrafiltration membranes. Desalination Water Treat. 2024, 318, 100255. [Google Scholar] [CrossRef]





| Membrane | PVDF (g) | PEG (g) | DMAc (mL) |
|---|---|---|---|
| M-0 | 15.0 | 0.0 | 85 |
| M-0.5 | 14.5 | 0.5 | 85 |
| M-1.0 | 14.0 | 1.0 | 85 |
| M-1.5 | 13.5 | 1.5 | 85 |
| Membrane | Contact Angle (°) |
|---|---|
| M-0 | 65.3 ± 2.1 |
| M-0.5 | 62.3 ± 2.1 |
| M-1.0 | 57.3 ± 2.5 |
| M-1.5 | 53.3 ± 2.9 |
| Parameters | Initial Tofu Wastewater | After Coagulation- Flocculation Process | After UF Process | Government Regulation [5] |
|---|---|---|---|---|
| pH | 3.7 ± 0.2 | 6.9 ± 0.3 | 7.0–7.8 | 6–9 |
| COD (mg∙L−1) | 11,880 ± 255 | 4698 ± 199 | 1580–3800 | 150 |
| TDS (mg∙L−1) | 740 ± 14 | 1300 ± 32 | 759–1232 | 2000 |
| TSS (mg∙L−1) | 1640 ± 40 | 271 ± 18 | 1–81 | 50 |
| Turbidity (FAU) | 900 ± 54 | 346 ± 24 | 3–93 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartohardjono, S.; Owen, M.G.; Estella, S.; Purnawan, I.; Lau, W.J. Polyvinylidene Fluoride Membrane Modified by PEG Additive for Tofu Industrial Wastewater Treatment. ChemEngineering 2025, 9, 106. https://doi.org/10.3390/chemengineering9050106
Kartohardjono S, Owen MG, Estella S, Purnawan I, Lau WJ. Polyvinylidene Fluoride Membrane Modified by PEG Additive for Tofu Industrial Wastewater Treatment. ChemEngineering. 2025; 9(5):106. https://doi.org/10.3390/chemengineering9050106
Chicago/Turabian StyleKartohardjono, Sutrasno, Michael Gabriell Owen, Sherlyta Estella, Irfan Purnawan, and Woei Jye Lau. 2025. "Polyvinylidene Fluoride Membrane Modified by PEG Additive for Tofu Industrial Wastewater Treatment" ChemEngineering 9, no. 5: 106. https://doi.org/10.3390/chemengineering9050106
APA StyleKartohardjono, S., Owen, M. G., Estella, S., Purnawan, I., & Lau, W. J. (2025). Polyvinylidene Fluoride Membrane Modified by PEG Additive for Tofu Industrial Wastewater Treatment. ChemEngineering, 9(5), 106. https://doi.org/10.3390/chemengineering9050106








