The Cascade Transformation of Furfural to Cyclopentanone: A Critical Evaluation Concerning Feasible Process Development
Abstract
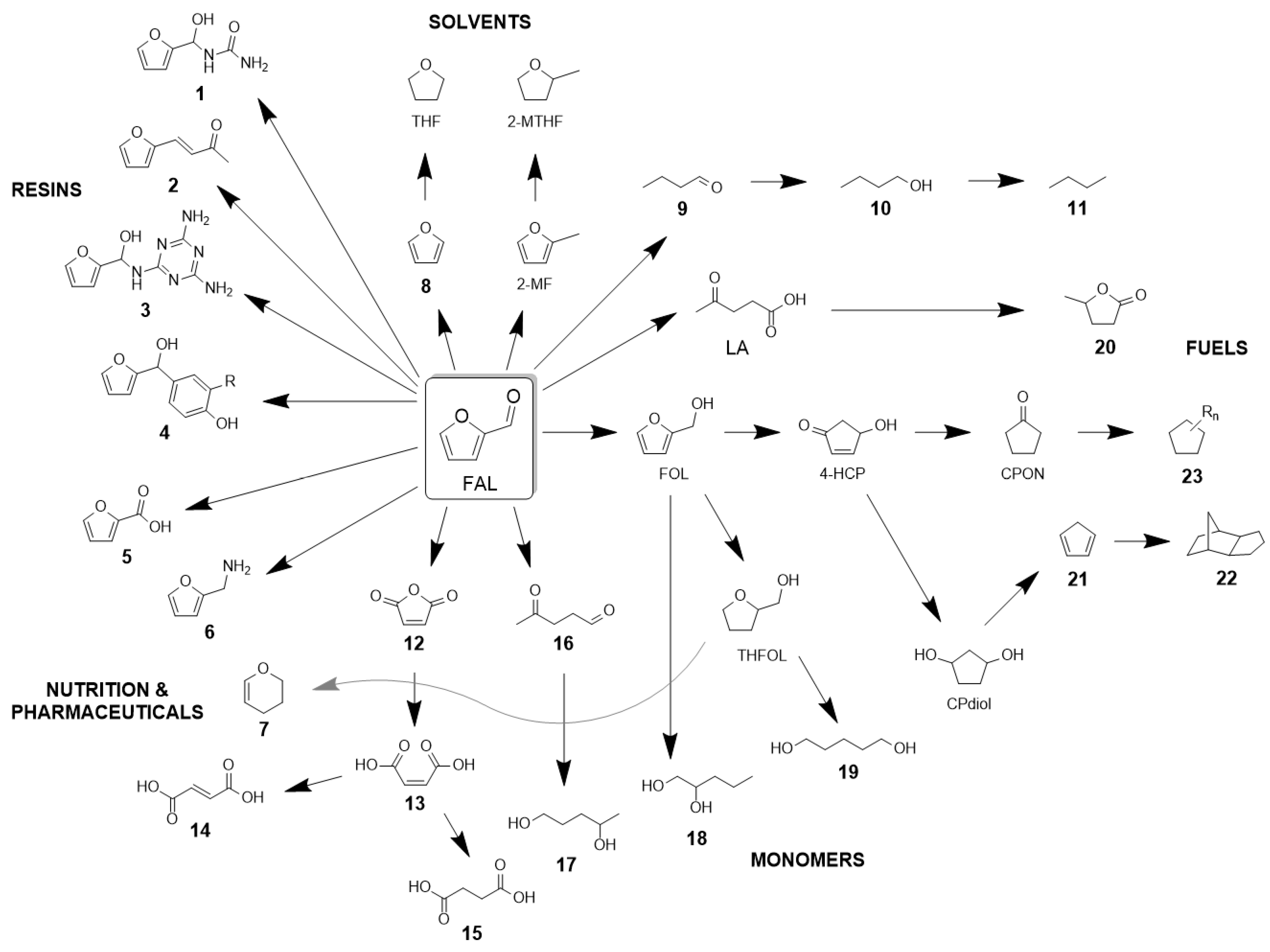
1. Introduction
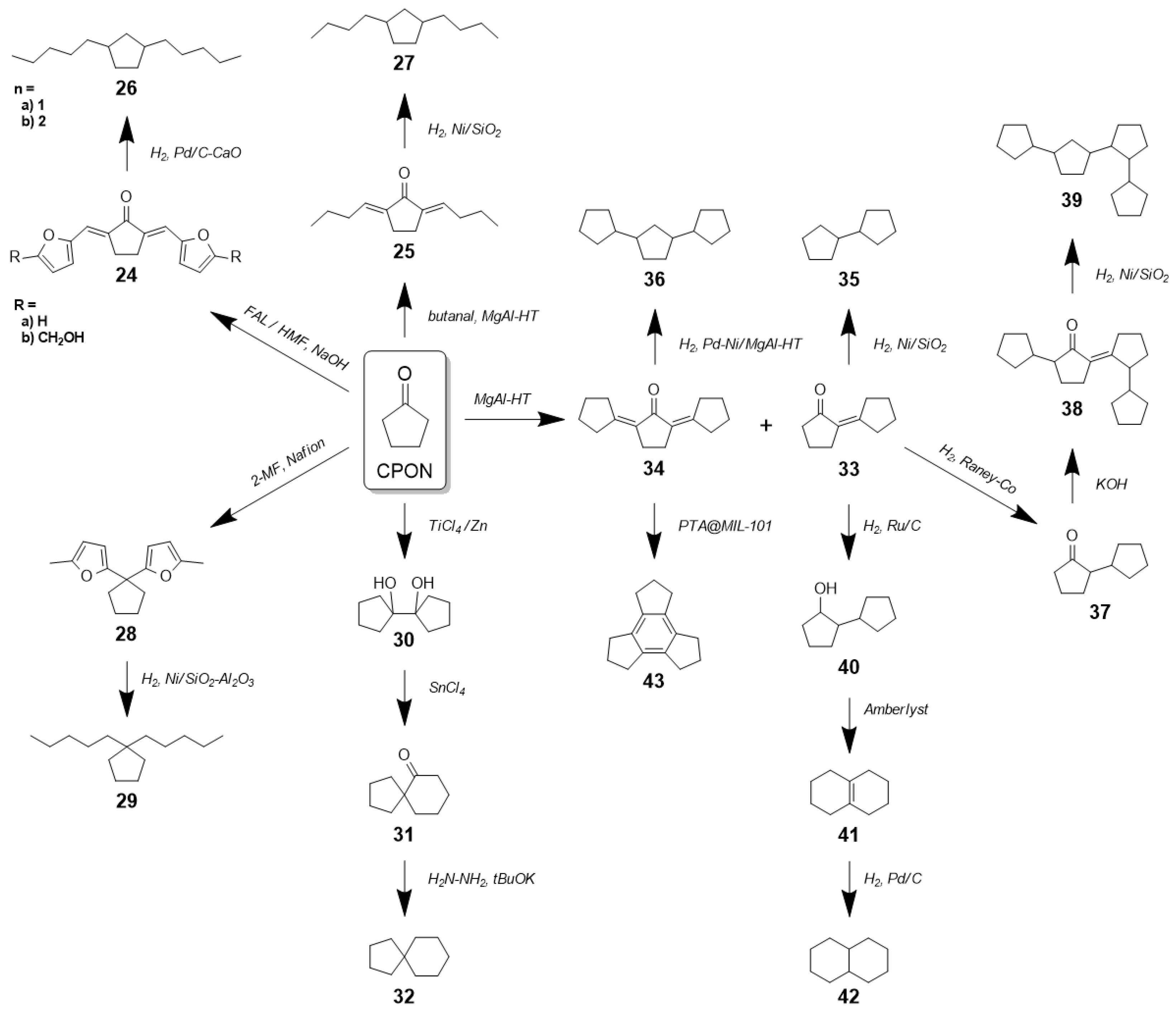
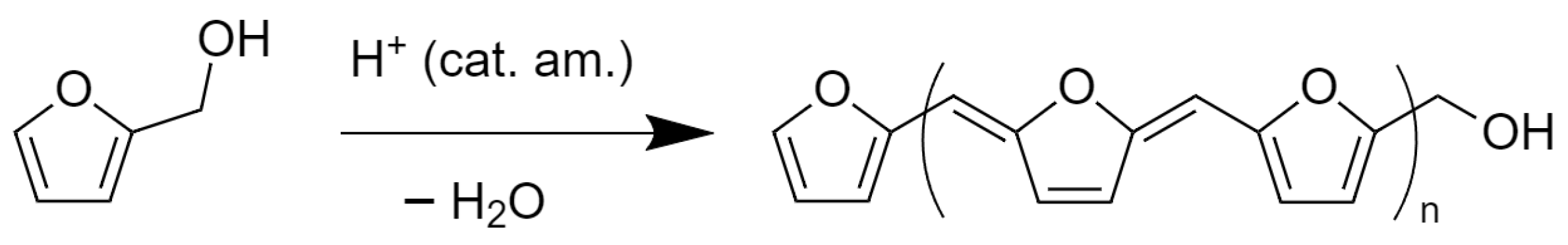
2. Technological Background
| Ref. | Author(s) | Catalyst System (Metal-to-Support wt%) | Cat. Load. (wt%) | T (°C) | PH2 (bar) | Time (h) | Sub. Conc. (v/v%) | Reactor Fill (v%) | CPON Yield (%) | CPOL Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| [82] | Hronec * | Pt/C (5%) | 5 | 160 | 80 | 0.5 | 5 | 20 | 76.5 | 5 |
| [115] | Yang, Xu * | Ni-Cu/SBA-15 (10%; 5%) | 2 | 160 | 40 | 4 | 5 | 16.7 | 62 | 3 |
| [116] | Guo, Guo * | CuZnAl | 41.6 | 150 | 40 | 6 | 3.3 | 36 | 60.3 | 2.5 |
| [181] | Liu, Xiao * | Ni/zeolite Y (20%) | 1.5 | 150 | 40 | 9 | 5 | N/D | 86.5 | 4.9 |
| [182] | Li, Fu * | Cu-Co3O4 (5%) | 26 | 170 | 10 | 1 | 2 | 50 | 67 | 10 |
| [183] | Zhu, Xiao * | Cu-Ni-Al/HT (ratio 1:14:5) | 25.9 | 140 | 40 | 8 | 5 | 50 | 95.8 | 3.0 |
| [117] | Fang, Li * | Ru/MIL-101 (3%) | 10 | 160 | 40 | 2.5 | 10 | 60 | 96 | 1 |
| [118] | Hronec * | Pd-Cu/C (5%; 10%) | 1 | 160 | 30 | 1 | 5 | 20 | 92.1 | 0.4 |
| [119] | Zhang, Cao * | Au/TiO2 (0.10%) | 10 | 160 | 40 | 1.2 | 5 | 20 | >99 | 0 |
| [120] | Wang, Xiao * | Cu-Ni@C (0.02%; 0.01%) | 2 | 130 | 50 | 5 | 5 | 50 | 96.9 | 1.1 |
| [121] | Liu, Wang * | Ru/CNT (6%) | 4.3 | 160 | 10 | 5 | 5 | 50 | 91 | 4 |
| [122] | Liu, Mu * | Pt/NC-BS-800 (5%) | 5 | 150 | 10 | 4 | 2 | N/D | 76 | 9 |
| [123] | Wang, Xiao * | Cu/MgO (20%) | 13.8 | 140 | 40 | 8 | 5 | 50 | 85 | 9 |
| [124] | Zhou, Jiang * | Cu-Zn/CNT (17%; 3%) | 2 | 140 | 40 | 10 | 5 | 50 | 85.3 | 5.8 |
| [125] | Li, Shi * | Ni-Co/TiO2 (10%; 10%) | 60 | 150 | 40 | 4 | 3.3 | 30 | 53.3 | 16.3 |
| [126] | Zhang, Li * | Cu/ZrO2 (39%) | 10.4 | 150 | 15 | 4 | 3.3 | N/D | 91.3 | 3.8 |
| [127] | Date, Rode * | Pd/f-SiO2 (4%) | 10 | 165 | 34.5 | 5 | 5 | 33 | 89 | 0 |
| [128] | Dohade, Dhepe * | Pt-Co/C (3.12%; 3.10%) | 22.3 | 180 | 10 | 5 | 5 | 33 | 75 | 2 |
| [129] | Shen, Ying * | Ru/C + Al11.6PO23.7 (0.5%) | 2.6 | 160 | 40 | 4 | 4 | 52 | 84 | 0 |
| [130] | Cherkasov, Rebrov * | Pd-Bi/SiO2 (4.87%; 1.36%) | 10.4 | 150 | 50 | 2.3 | 5 | 63 | 54.6 | N/D |
| [131] | Zhou, Huang * | Cu0.4Mg5.6Al2 | 66.6 | 180 | 2 | 5 | 5 | 4 | 98.1 | 0 |
| [132] | Li, Deng * | Pd/Fe-MIL-100 (5%) | 10 | 150 | 40 | 6 | 2.5 | 43 | 92 | N/D |
| [133] | Pan, Feng * | Cu-Fe3O4 (10%) | 50 | 160 | 30 | 4 | 1 | 40 | 91 | 6 |
| [134] | Deng, Zhang * | Pd/Cu-BTC (5%) | 1 | 150 | 40 | 6 | 2.5 | 43 | 93 | N/D |
| [135] | Mironenko * | Pd/CNT (1%) | 8.6 | 150 | 30 | 2 | 4 | N/D | 37 | N/D |
| [136] | Astuti, Mujiyanti * | Ni-Co/TiO2 (20%; 6.8%) | 47.3 | 170 | 30 | 6 | 5 | N/D | 27 | 41 |
| [137] | Wang, Zhang * | Pd-Co@UiO-66 (4.4%; 0.62%) | 17.3 | 120 | 30 | 12 | 1 | 20 | 96 | N/D |
| [138] | Ren, Li * | Cu4Zn/Al (film: 40 cm2) | N/D | 140 | 20 | 2 | 0.5 | 40 | 86.5 | 4 |
| [139] | Li, Deng * | Pd/FeZn-DMC (5%) | 10 | 150 | 40 | 6 | 2.5 | 41 | 87.5 | N/D |
| [140] | Deng, Deng * | Pd/pyrochlore (5%) | 10 | 150 | 40 | 6 | 2.5 | 50 | 92 | N/D |
| [141] | Lee, An * | Pd/CMK-3 (0.94%) | 1 | 160 | 30 | 5 | 5 | 21 | 41.9 | N/D |
| [142] | Liu, Li * | Ni2Cu1/Al2O3 (44%; 24%) | 4 | 140 | 10 | 1 | 3.3 | N/D | 89.5 | 7 |
| [143] | Zhu, He * | Cu0-Zn/(Al)(Zr)O-2 (11%) | 5 | 160 | 40 | 2.5 | 5 | N/D | 92 | 2 |
| [144] | Herrera, Escalona * | Ni/CNTox (10%) | 14 | 200 | 20 | 1 | 2.2 | N/D | 20 | 7 |
| [145] | Jia, Wang * | NiFe/SBA-15 (5.0%; 1.6%) | 20 | 160 | 34 | 5 | 6 | 53 | 90 | N/D |
| [146] | Mironenko * | Pd/CNT (1%) | 8.6 | 200 | 80 | 1 | 4 | N/D | 79 | N/D |
| [147] | Gao, Hu * | Ni-P/γ-Al2O 3 (15%; 10%) | 104 | 150 | 30 | 2 | 1 | 40 | 90.1 | 0 |
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of 4-Hydroxycyclopent-2-Enone (4-HCP)
3.3. Cascade Hydrogenation-Dehydration Reactions
3.4. Control Hydrogenation of 4-HCP at Room Temperature
4. Results and Discussion
4.1. Reproducing the Work of Hronec
4.2. Control Hydrogenations of 4-HCP
4.3. A Biphasic Water-Toluene Solvent System for Improved Down-Stream Processing
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-MF | 2-methylfuran |
| 2-MTHF | 2-methyltetrahydrofuran |
| 3-HCP | 3-hydroxycyclopentanone |
| 4-HCP | 4-hydroxycyclopent-2-enone |
| aq | aqueous |
| [c] | concentration |
| cat. am | catalytic amount |
| CPEON | cyclopent-2-enone |
| CPOL | cyclopentanol |
| CPON | cyclopentanone |
| DSP | downstream processing |
| FAL | furfural |
| FOL | furfuryl alcohol |
| FTIR | Fourier transform infrared spectroscopy |
| GC-FID | gas chromatography with flame ionization detection |
| HMF | 5-hydroxymethylfurfural |
| LA | levulinic acid |
| N/A | not applicable |
| N/D | not determined |
| NMR | nuclear magnetic resonance |
| PTFE | poly(tetrafluoro-ethylene) |
| SM | Supplementary Materials |
| STY | space-time yield |
| THF | tetrahydrofuran |
| THFOL | tetrahydrofurfuryl alcohol |
| Tol | toluene |
| TON | turnover number |
| v% | percentage by volume |
| wt% | percentage by weight |
References
- Meadows, D.H.; Meadows, D.L.; Randers, J.; Behrens, W.W., III. The Limits to Growth: A Report for THE CLUB OF ROME’S Project on the Predicament of Mankind; Universe Books: New York, NY, USA, 1972. [Google Scholar]
- World Commission on Environment and Development. Our Common Future; Oxford University Press: New York, NY, USA, 1987. [Google Scholar]
- Dale, B.E. ‘Greening’ the chemical industry: Research and development priorities for biobased industrial products. J. Chem. Technol. Biotechnol. 2003, 78, 1093–1103. [Google Scholar] [CrossRef]
- de Jong, E.; Higson, A.; Walsh, P.; Wellisch, M. Product developments in the bio-based chemicals arena. Biofuels Bioprod. Bioref. 2012, 6, 606–624. [Google Scholar] [CrossRef]
- Parajuli, R.; Dalgaard, T.; Jørgensen, U.; Adamsen, A.P.S.; Knudsen, A.P.S.; Birkved, M.; Gylling, M.; Schjørring, J.K. Biorefining in the prevailing energy and materials crisis: A review of sustainable pathways for biorefinery value chains and sustainability assessment methodologies. Renew. Sustain. Energ. Rev. 2015, 43, 244–263. [Google Scholar] [CrossRef]
- van den Oever, M.; Molenveld, K. Replacing fossil based plastic performance products by bio-based plastic products–Technical feasibility. New Biotechnol. 2017, 37 Pt A, 48–59. [Google Scholar] [CrossRef]
- Parida, V.; Sjödin, D.; Reim, W. Reviewing Literature on Digitalization, Business Model Innovation, and Sustainable Industry: Past Achievements and Future Promises. Sustainability 2019, 11, 391. [Google Scholar] [CrossRef]
- Brownlee, H.J.; Miner, C.S. Industrial Development of Furfural. Ind. Eng. Chem. 1948, 40, 201–204. [Google Scholar] [CrossRef]
- LaForge, F.B.; Mains, G.H. Furfural from Corncobs. Ind. Eng. Chem. 1923, 15, 823–829. [Google Scholar] [CrossRef]
- Brownlee, H.J. Furfural Manufacture from Oat Hulls: I–A Study of the Liquid-Solid Ratio. Ind. Eng. Chem. 1927, 19, 422–424. [Google Scholar]
- Peters, F.N., Jr. The Furans: Fifteen Years of Progress. Ind. Eng. Chem. 1936, 28, 755–759. [Google Scholar] [CrossRef]
- Buell, C.K.; Boatright, R.G. Furfural Extractive Distillation. Ind. Eng. Chem. 1947, 39, 695–705. [Google Scholar] [CrossRef]
- Market Value of Furfural Worldwide from 2015 to 2022, with a Forecast for 2023 to 2030. Available online: https://www.statista.com/statistics/1310467/furfural-market-value-worldwide/ (accessed on 24 April 2025).
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural–A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef]
- Khandarkar, K.M.; Ahmed, M.; Meshram, J.S. Indian Natural Zeolites Catalyzed Urea Furfural Green Polymerization. Int. J. Innov. Res. Technol. Sci. Eng. 2014, 3, 12079–12087. [Google Scholar]
- Ghafari, R.; DoostHosseini, K.; Abdulkhani, A.; Mirshokraie, S.A. Replacing formaldehyde by furfural in urea formaldehyde resin: Effect on formaldehyde emission and physical-mechanical properties of particleboards. Eur. J. Wood Wood Prod. 2016, 74, 609–616. [Google Scholar] [CrossRef]
- Barrett, C.J.; Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Single-reactor process for sequential aldol-condensation and hydrogenation of biomass-derived compounds in water. Appl. Catal. B Environ. 2006, 66, 111–118. [Google Scholar] [CrossRef]
- Desai, D.S.; Yadav, G.D. Green Synthesis of Furfural Acetone by Solvent-Free Aldol Condensation of Furfural with Acetone over La2O3-MgO Mixed Oxide Catalyst. Ind. Eng. Chem. Res. 2019, 58, 16096–16105. [Google Scholar] [CrossRef]
- Zhou, J.-B.; Li, P.-L.; Zhou, J.-F.; Liao, X.-P.; Shi, B. Preparation of Formaldehyde-free Melamine Resin using Furfural as Condensation Agent and its Retanning Performances Investigation. J. Am. Leather Chem. Assoc. 2018, 113, 198–206. [Google Scholar]
- Oliveira, F.B.; Gardrat, C.; Enjalbal, C.; Frollini, E.; Castellan, A. Phenol-Furfural Resins to Elaborate Composites Reinforced with Sisal Fibers–Molecular Analysis of Resin and Properties of Composites. J. Appl. Polym. Sci. 2008, 109, 2291–2303. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Chen, Z.; Ding, C.; Zheng, Q.; Xu, J.; Meng, Q. Synthesis of High-Water-Resistance Lignin-Phenol Resin Adhesive with Furfural as a Crosslinking Agent. Polymers 2020, 12, 2805. [Google Scholar] [CrossRef]
- Pérez, H.I.; Manjarrez, N.; Solís, A.; Luna, H.; Ramírez, M.A.; Cassani, J. Microbial biocatalytic preparation of 2-furoic acid by oxidation of 2-furfuryl alcohol and 2-furanaldehyde with Nocardia corallina. Afr. J. Biotechnol. 2009, 8, 2279–2282. [Google Scholar]
- Kikhtyanin, O.; Lesnik, E.; Kubička, D. The occurrence of Cannizzaro reaction over Mg-Al hydrotalcites. Appl. Catal. A Gen. 2016, 525, 215–225. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, B.; Zhou, X.; Kang, S.; Xu, Y.; Wei, J. Selective Synthesis of Furfurylamine by Reductive Amination of Furfural over Raney Cobalt. ChemCatChem 2019, 11, 5562–5569. [Google Scholar] [CrossRef]
- Sawyer, R.L.; Andrus, D.W. 2,3-Dihydropyran. Org. Synth. 1943, 23, 25. [Google Scholar] [CrossRef]
- Wuts, P.G.M.; Greene, T.W. Chapter 2: Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols. In Greene’s Protective Groups in Organic Synthesis; John Wiley & Sons, Inc.: New York, NY, USA, 2006. [Google Scholar]
- Copelin, H.B.; Garnett, D.I. Decarbonylation of Furfural. U.S. Patent US3007941 A, 7 November 1961. [Google Scholar]
- Singh, H.; Prasad, M.; Srivastava, R.D. Metal Support Interactions in the Palladium-Catalysed Decomposition of Furfural to Furan. J. Chem. Technol. 1980, 30, 293–296. [Google Scholar] [CrossRef]
- Lejemble, P.; Gaset, A.; Kalck, P. From Biomass to Furan through Decarbonylation of Furfural under Mild Conditions. Biomass 1984, 4, 263–274. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Niu, S.; Li, Y. A Study of furfural decarbonylation on K-doped Pd/Al2O3 catalysts. J. Mol. Catal. A Chem. 2011, 335, 71–81. [Google Scholar] [CrossRef]
- Sitthisa, S.; An, W.; Resasco, D.E. Selective conversion of furfural to methylfuran over silica-supported Ni–Fe bimetallic catalysts. J. Catal. 2011, 284, 90–101. [Google Scholar] [CrossRef]
- Srivastava, S.; Jadeja, G.C.; Parikh, J. Versatile bi-metallic Copper-Cobalt catalysts for liquid phase hydrogenation of furfural to 2-methylfuran. RSC Adv. 2016, 6, 1649–1658. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Lin, W.; Song, W.; Li, S. High efficient conversion of furfural to 2-methylfuran over Ni-Cu/Al2O3 catalyst with formic acid as a hydrogen donor. Appl. Catal. A Gen. 2017, 547, 248–255. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Moreno-Tost, R.; Maireles-Torres, P. Selective Production of 2-Methylfuran by Gas-Phase Hydrogenation of Furfural on Copper Incorporated by Complexation in Mesoporous Silica Catalysts. ChemSusChem 2017, 10, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Scholz, D.; Aellig, C.; Hermans, I. Catalytic Transfer Hydrogenation/Hydrogenolysis for Reductive Upgrading of Furfural and 5-(Hydroxymethylfurfural). ChemSusChem 2014, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Biradar, N.S.; Hengne, A.A.; Birajdar, S.N.; Swami, R.; Rode, C.V. Tailoring the Product Distribution with Batch and Continuous Process Options in Catalytic Hydrogenation of Furfural. Org. Process. Res. Dev. 2014, 18, 1434–1442. [Google Scholar] [CrossRef]
- García-Suárez, E.J.; Balu, A.M.; Tristany, M.; García, A.B.; Philippot, K.; Luque, R. Versatile dual hydrogenation–oxidation nanocatalysts for the aqueous transformation of biomass-derived platform molecules. Green Chem. 2012, 14, 1434–1439. [Google Scholar] [CrossRef]
- Nielsen, E.R. Vapor Phase Oxidation of Furfural. Ind. Eng. Chem. 1949, 41, 365–368. [Google Scholar] [CrossRef]
- Aldosari, O.F.; Iqbal, S.; Miedziak, P.J.; Brett, G.L.; Jones, D.R.; Liu, X.; Edwards, J.K.; Morgan, D.J.; Knight, D.K.; Hutchings, G.J. Pd-Ru/TiO2 catalyst–an active and selective catalyst for furfural hydrogenation. Catal. Sci. Technol. 2016, 6, 234–242. [Google Scholar] [CrossRef]
- Chang, X.; Liu, A.-F.; Cai, B.; Luo, J.-Y.; Pan, H.; Huang, Y.-B. Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran and 2-Methyltetrahydrofuran over Bimetallic Copper-Palladium Catalysts. ChemSusChem 2016, 9, 3330–3337. [Google Scholar] [CrossRef]
- Cukalovic, A.; Stevens, C.V. Feasibility of production methods for succinic acid derivatives: A marriage of renewable resources and chemical technology. Biofuel Bioprod. Biorefin. 2012, 6, 88–104. [Google Scholar] [CrossRef]
- Ni, Y.; Bi, Z.; Su, H.; Yan, L. Deep eutectic solvent (DES) as both solvent and catalyst for oxidation of furfural to maleic acid and fumaric acid. Green Chem. 2019, 5, 1075–1079. [Google Scholar] [CrossRef]
- Bechthold, I.; Bretz, K.; Kabasci, S.; Kopitzky, R.; Springer, A. Succinic Acid: A New Platform Chemical for Biobased Polymers from Renewable Resources. Chem. Eng. Technol. 2008, 31, 647–654. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Q.; Xu, J.; Li, L.; Cui, Y.-T.; Lang, R.; Li, L.; Su, Y.; Miao, S.; Sun, H.; et al. Catalytic cascade conversion of furfural to 1,4-pentanediol in a single reactor. Green Chem. 2018, 20, 1770–1776. [Google Scholar] [CrossRef]
- Liu, Q.; Qiao, B.; Liu, F.; Zhang, L.; Su, Y.; Wang, A.; Zhang, T. Catalytic production of 1,4-pentanediol from furfural in a fixed-bed system under mild conditions. Green Chem. 2020, 11, 3532–3538. [Google Scholar] [CrossRef]
- Stones, M.K.; Banz Chung, E.M.-J.; Tadeu da Cunha, I.; Sullivan, R.J.; Soltanipanah, P.; Magee, M.; Umphrey, G.J.; Moore, C.M.; Sutton, A.D.; Schlaf, M. Conversion of Furfural Derivatives to 1,4-Pentanediol and Cyclopentanol in Aqueous Medium Catalyzed by trans-[(2,9-Dipyridyl-1,10-phenanthroline)(CH3CN)2Ru](OTf)2. ACS Catal. 2020, 10, 2667–2683. [Google Scholar]
- Stadler, B.M.; Brandt, A.; Kux, A.; Beck, H.; de Vries, J.G. Properties of Novel Polyesters Made from Renewable 1,4-Pentanediol. ChemSusChem 2020, 13, 556–563. [Google Scholar] [CrossRef]
- Oh, M.-Y.; Jin, G.; Lee, B.; Kim, J.; Won, W. Co-production of 1,4-pentanediol and adipic acid from corn stover with biomass-derived co-solvent: Process synthesis and analysis. J. Clean. Prod. 2022, 359, 131920. [Google Scholar] [CrossRef]
- Merlo, A.B.; Vetere, V.; Ruggera, J.F.; Casella, M.L. Bimetallic PtSn catalyst for the selective hydrogenation of furfural to furfuryl alcohol in liquid-phase. Catal. Commun. 2009, 10, 1665–1669. [Google Scholar] [CrossRef]
- Fulajtarová, K.; Soták, T.; Hronec, M.; Vávra, I.; Dobročka, E.; Omastová, M. Aqueous phase hydrogenation of furfural to furfuryl alcohol over Pd-Cu catalysts. Appl. Catal. A Gen. 2015, 502, 78–85. [Google Scholar] [CrossRef]
- Tukacs, J.M.; Bohus, M.; Dibó, G.; Mika, L.T. Ruthenium-catalyzed solvent-free conversion of furfural to furfuryl alcohol. RSC Adv. 2017, 7, 3331–3335. [Google Scholar] [CrossRef]
- An, Z.; Li, J. Recent advances in the catalytic transfer hydrogenation of furfural to furfuryl alcohol over heterogeneous catalysts. Green Chem. 2022, 24, 1780–1808. [Google Scholar] [CrossRef]
- Merat, N.; Godawa, C.; Gaset, A. High selective production of tetrahydrofurfurylalcohol: Catalytic hydrogenation of furfural and furfuryl alcohol. J. Chem. Technol. Biotechnol. 1990, 48, 145–159. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakagawa, H.; Watanabe, H.; Tomishige, K. Total Hydrogenation of Furfural over a Silica-Supported Nickel Catalyst Prepared by the Reduction of a Nickel Nitrate Precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Biradar, N.S.; Hengne, A.M.; Birajdar, S.N.; Niphadkar, P.S.; Joshi, P.N.; Rode, C.V. Single-Pot Formation of THFAL via Catalytic Hydrogenation of FFR Over Pd/MFI Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 272–281. [Google Scholar] [CrossRef]
- Li, C.; Xu, G.; Liu, X.; Zhang, Y.; Fu, Y. Hydrogenation of Biomass-Derived Furfural to Tetrahydrofurfuryl Alcohol over Hydroxyapatite-Supported Pd Catalyst under Mild Conditions. Ind. Eng. Chem. Res. 2017, 56, 8843–8849. [Google Scholar] [CrossRef]
- Mizugaki, T.; Yamakawa, T.; Nagatsu, Y.; Maeno, Z.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Direct Transformation of Furfural to 1,2-Pentanediol Using a Hydrotalcite-Supported Platinum Nanoparticle Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 2243–2247. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Conversions of Furfural to Pentanediols. Catal. Surv. Asia 2015, 19, 249–256. [Google Scholar] [CrossRef]
- Ma, R.; Wu, X.-P.; Tong, T.; Shao, Z.-J.; Wang, Y.; Liu, X.; Xia, Q.; Gong, X.-Q. The Critical Role of Water in the Ring Opening of Furfural Alcohol to 1,2-Pentanediol. ACS Catal. 2017, 7, 333–337. [Google Scholar] [CrossRef]
- Pisal, D.S.; Yadav, G.D. Single-Step Hydrogenolysis of Furfural to 1,2-Pentanediol Using a Bifunctional Rh/OMS-2 Catalyst. ACS Omega 2019, 4, 1201–1214. [Google Scholar] [CrossRef]
- Liu, S.; Amada, Y.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Performance and characterization of rhenium-modified Rh-Ir alloy catalyst for one-pot conversion of furfural into 1,5-pentanediol. Catal. Sci. Technol. 2014, 4, 2535–2549. [Google Scholar] [CrossRef]
- Liu, S.; Amada, Y.; Tamura, M.; Nakagawa, Y.; Tomishige, K. One-pot selective conversion of furfural into 1,5-pentanediol over a Pd-added Ir-ReOx/SiO2 bifunctional catalyst. Green Chem. 2014, 16, 617–626. [Google Scholar] [CrossRef]
- Huang, K.; Brentzel, Z.J.; Barnett, K.J.; Dumesic, J.A.; Huber, G.W.; Maravelias, C.T. Conversion of Furfural to 1,5-Pentanediol: Process Synthesis and Analysis. ACS Sustain. Chem. Eng. 2017, 5, 4699–4706. [Google Scholar] [CrossRef]
- Al-Yusufi, M.; Steinfeldt, N.; Eckelt, R.; Atia, H.; Lund, H.; Bartling, S.; Rockstroh, N.; Köckritz, A. Efficient Base Nickel-Catalyzed Hydrogenolysis of Furfural-Derived Tetrahydrofurfuryl Alcohol to 1,5-Pentanediol. ACS Sustain. Chem. Eng. 2022, 10, 4954–4968. [Google Scholar] [CrossRef]
- Rackemann, D.W.; Doherty, W.O.S. The conversion of lignocellulosics to levulinic acid. Biofuel Bioprod. Biorefin. 2011, 5, 198–214. [Google Scholar] [CrossRef]
- González Maldonado, G.M.; Assary, R.S.; Dumesic, J.; Curtiss, L.A. Experimental and theoretical studies on the acid-catalyzed conversion of furfuryl alcohol to levulinic acid in aqueous solution. Energy Environ. Sci. 2012, 5, 6981–6989. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Gallo, J.M.R.; Alonso, D.M.; Dumesic, J.A. Selective Production of Levulinic Acid from Furfuryl Alcohol in THF Solvent Systems over H-ZSM-5. ACS Catal. 2015, 5, 3354–3359. [Google Scholar] [CrossRef]
- Wright, W.H.R.; Palkovits, R. Development of Heterogeneous Catalysts for the Conversion of Levulinic Acid to γ-Valero-lactone. ChemSusChem 2012, 5, 1657–1667. [Google Scholar] [CrossRef]
- Omoruyi, U.; Page, S.; Hallett, J.; Miller, P.W. Homogeneous Catalyzed Reactions of Levulinic Acid: To γ-Valerolactone and Beyond. ChemSusChem 2016, 9, 2037–2047. [Google Scholar] [CrossRef]
- Piancatelli, G.; Scretti, A.; Barbadoro, S. A useful preparation of 4-substituted 5-hydroxy-3-oxocyclopentene. Tetrahedron Lett. 1976, 39, 3555–3558. [Google Scholar] [CrossRef]
- Ulbrich, K.; Kreitmeier, P.; Reiser, O. Microwave- or microreactor-assisted conversion of furfuryl alcohols into 4-hydroxy-2-cyclopentenone. Synlett 2010, 28, 4719–4722. [Google Scholar]
- Kumaraguru, T.; Babita, P.; Sheelu, G.; Lavanya, K.; Fadnavis, N.W. Synthesis of Enantiomerically Pure 4-Hydroxy-2-cyclopentenones. Org. Process Res. Dev. 2013, 17, 1526–1530. [Google Scholar] [CrossRef]
- Piutti, C.; Quartieri, F. The Piancatelli Rearrangement: New Applications for an Intriguing Reaction. Molecules 2013, 18, 12290–12312. [Google Scholar] [CrossRef]
- Roche, S.P.; Aitken, D.J. Chemistry of 4-hydroxy-2-cyclopentenone derivatives. Eur. J. Org. Chem. 2010, 28, 5339–5358. [Google Scholar] [CrossRef]
- Verrier, C.; Moebs-Sanchez, S.; Queneau, Y.; Popowycz, F. The Piancatelli reaction and its variants: Recent applications of high added-value chemicals and biomass valorization. Org. Biomol. Chem. 2018, 16, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, N.; Zheng, M.; Li, S.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Industrially scalable and cost-effective synthesis of 1,3-cyclopentanediol with furfuryl alcohol from lignocellulose. Green Chem. 2016, 18, 3607–3613. [Google Scholar] [CrossRef]
- Li, G.; Hou, B.; Wang, A.; Xin, X.; Cong, Y.; Wang, X.; Li, N.; Zhang, T. Making JP-10 Superfuel Affordable with a Lignocellulosic Platform Compound. Angew. Chem. Int. Ed. 2019, 58, 12154–12158. [Google Scholar] [CrossRef] [PubMed]
- Hronec, M.; Fulajtárová, K. Selective transformation of furfural to cyclopentanone. Catal. Commun. 2012, 24, 100–104. [Google Scholar] [CrossRef]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance material review on cyclopentanone. Food Chem. Tox. 2012, 50, S608–S612. [Google Scholar] [CrossRef]
- Belsito, D.; Bickers, D.; Bruze, M.; Calow, P.; Dagli, M.L.; Dekant, W.; Fryer, A.D.; Greim, H.; Miyachi, Y.; Saurat, J.H.; et al. A toxicologic and dermatologic assessment of cyclopentanones and cyclopentenones when used as fragrance ingredients. Food Chem. Tox. 2012, 50, S517–S556. [Google Scholar] [CrossRef]
- Panten, J.; Surburg, H. Flavors and Fragrances, 2. Aliphatic Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Vichy, France, 2015. [Google Scholar]
- Duereh, A.; Guo, H.; Honma, T.; Hiraga, Y.; Sato, Y.; Smith, R.L., Jr.; Inomata, H. Solvent Polarity of Cyclic Ketone (Cyclopentanone, Cyclohexanone): Alcohol (Methanol, Ethanol) Renewable Mixed-Solvent Systems for Applications in Pharmaceutical and Chemical Processing. Ind. Eng. Chem. Res. 2018, 57, 7331–7344. [Google Scholar] [CrossRef]
- Siegel, H.; Eggersdorfer, M. Cyclopentanone. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Vichy, France, 2000. [Google Scholar]
- Durkó, G.; Jalsovszky, I. Solvent-induced, selective rearrangement of hydrogen cubane-1,4-dicarboxylate to hydrogen cuneane-2,6-dicarboxylate. Tetrahedron 2013, 69, 5160–5163. [Google Scholar] [CrossRef]
- Prentice, C.; Martin, A.E.; Morrison, J.; Smith, A.D.; Zysman-Colman, E. Benzophenone as a cheap and effective photosensitizer for the photocatalytic synthesis of dimethyl cubane-1,4-dicarboxylate. Org. Biomol. Chem. 2023, 21, 3307–3310. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, J.A.; Harvey, B.G. Bio-Based Cycloalkanes: The Missing Link to High-Performance Sustainable Jet Fuels. ChemSusChem 2020, 13, 5777–5807. [Google Scholar] [CrossRef] [PubMed]
- Hronec, M.; Fulajtárova, K.; Liptaj, T.; Štolcová, M.; Soták, T. Cyclopentanone: A raw material for production of C15 and C17 fuel precursors. Biomass Bioenerg. 2014, 63, 291–299. [Google Scholar] [CrossRef]
- Deng, Q.; Xu, J.; Han, P.; Pan, L.; Wang, L.; Zhang, X.; Zou, J.-J. Efficient synthesis of high-density aviation biofuel via solvent-free aldol condensation of cyclic ketones and furanic aldehydes. Fuel Process. Technol. 2016, 148, 361–366. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, C.; Shi, N.; Zhang, X.; Wang, C.; Ma, L. Production of renewable long-chained cycloalkanes from biomass-derived furfurals and cyclic ketones. RSC Adv. 2018, 8, 13686–13696. [Google Scholar] [CrossRef]
- Wang, W.; Sun, S.; Han, F.; Li, G.; Shao, X.; Li, N. Synthesis of Diesel and Jet Fuel Range Cycloalkanes with Cyclopentanone and Furfural. Catalysts 2019, 9, 886. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Li, G.; Wang, W.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of renewable high-density fuels using cyclopentanone derived from lignocellulose. Chem. Commun. 2014, 50, 2572–2574. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Li, N.; Wang, W.; Wang, A.; Zhang, T.; Cong, Y.; Wang, X.; Huber, G.W. Synthesis of Jet-Fuel Range Cycloalkanes from the Mixtures of Cyclopentane and Butanal. Ind. Eng. Chem. Res. 2015, 54, 11825–11837. [Google Scholar] [CrossRef]
- Sheng, X.; Li, G.; Wang, W.; Cong, Y.; Wang, X.; Huber, G.W.; Li, N.; Wang, A.; Zhang, T. Dual-bed catalyst system for the direct synthesis of high density aviation fuel with cyclopentanone from lignocellulose. React. Eng. Kinet. Catal. 2016, 62, 2754–2761. [Google Scholar] [CrossRef]
- Tang, H.; Chen, F.; Li, G.; Yang, X.; Hu, Y.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T.; Li, N. Synthesis of jet fuel additive with cyclopentanone. J. Energ. Chem. 2019, 28, 23–30. [Google Scholar] [CrossRef]
- Wang, W.; Ji, X.; Ge, H.; Li, Z.; Tian, G.; Shao, X.; Zhang, Q. Synthesis of C15 and C10 fuel precursors with cyclopentanone and furfural derived from hemicellulose. RSC Adv. 2017, 7, 16901–16907. [Google Scholar] [CrossRef]
- Ao, L.; Zhao, W.; Guan, Y.-S.; Wang, D.-K.; Liu, K.-S.; Guo, T.-T.; Fan, X.; Wei, X.-Y. Efficient synthesis of C15 fuel precursor by heterogeneously catalyzed aldol-condensation of furfural with cyclopentanone. RSC Adv. 2019, 9, 3661–3668. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Wang, X.; Sheng, X.; Li, S.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of Diesel or Jet Fuel Range Cycloalkanes with 2-Methylfuran and Cyclopentanone from Lignocellulose. Energy Fuels 2014, 28, 5112–5118. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Pan, L.; Nie, G.; Liu, Q.; Wang, P.; Li, Y.; Zou, J.-J. Renewable high-density spiro-fuels from lignocellulose-derived cyclic ketones. Chem. Commun. 2017, 53, 10303–10307. [Google Scholar] [CrossRef]
- Wang, R.; Li, G.; Tang, H.; Wang, A.; Xu, G.; Cong, Y.; Wang, X.; Zhang, T.; Li, N. Synthesis of Decaline-Type Thermal-Stable Jet Fuel Additives with Cycloketones. ACS Sustain. Chem. Eng. 2019, 7, 17354–17361. [Google Scholar] [CrossRef]
- Wang, W.; Li, N.; Li, G.; Li, S.; Wang, W.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of Renewable High-Density Fuel with Cyclopentanone Derived from Hemicellulose. ACS Sustain. Chem. Eng. 2017, 5, 1812–1817. [Google Scholar] [CrossRef]
- Deng, Q.; Nie, G.; Pan, L.; Zou, J.-J.; Zhang, X.; Wang, L. Highly selective self-condensation of cyclic ketones using MOF-encapsulating phosphotungstic acid for renewable high-density fuels. Green Chem. 2015, 17, 4473–4482. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Reduction of Biomass-Derived Furanic Compounds with Hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Supported Metal Catalysts for Total Hydrogenation of Furfural and 5-Hydroxy-methylfurfural. J. Jpn. Pet. Inst. 2017, 60, 1–9. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarová, K.; Liptaj, T. Effect of catalyst and solvent on the furan ring rearrangement of cyclopentanone. Appl. Catal. A Gen. 2012, 437–438, 104–111. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarová, K.; Mičušik, M. Influence of furanic polymers on selectivity of furfural rearrangement to cyclopentanone. Appl. Catal. A Gen. 2013, 468, 426–431. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárova, K.; Soták, T. Highly selective rearrangement of furfuryl alcohol to cyclopentanone. Appl. Catal. B Environ. 2014, 154–155, 294–300. [Google Scholar] [CrossRef]
- Li, D.; Tian, Z.; Cai, X.; Li, Z.; Zhang, C.; Zhang, W.; Song, Y.; Wang, H.; Li, C. Nature of polymeric condensates during furfural rearrangement to cyclopentanone and cyclopentanol over Cu-based catalysts. New. J. Chem. 2021, 45, 22767–22777. [Google Scholar] [CrossRef]
- Dutta, S.; Subray Bhat, N. Catalytic Transformation of Biomass-Derived Furfurals to Cyclopentanones and Their Derivatives: A Review. ACS Omega 2021, 6, 35145–35172. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Cheng, Y.; Hu, Z.; Wang, C.; Sui, D.; Yang, Y.; Lu, T. A Comprehensive Review on Metal Catalysts for the Production of Cyclopentanone Derivatives from Furfural and HMF. Molecules 2023, 28, 5397. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Likholobov, V.A. Aqueous-Phase Hydrogenation of Furfural in the Presence of Supported Metal Catalysts of Different Types. A Review. Dokl. Phys. Chem. 2023, 509, 33–50. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Z.; Huang, Y.; Lu, F.; Wang, F.; Gao, J.; Xu, J. Conversion of furfural into cyclopentanone over Ni-Cu bimetallic catalysts. Green Chem. 2013, 15, 1932–1940. [Google Scholar] [CrossRef]
- Guo, J.; Xu, G.; Han, Z.; Zhang, Y.; Fu, Y.; Guo, Q. Selective Conversion of Furfural to Cyclopentanone with CuZnAl Catalysts. ACS Sustain. Chem. Eng. 2014, 2, 2259–2266. [Google Scholar] [CrossRef]
- Fang, R.; Liu, H.; Luque, R.; Li, Y. Efficient and selective hydrogenation of biomass-derived furfural to cyclopentanone using Ru catalysts. Green Chem. 2015, 8, 4183–4188. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarová, K.; Vávra, I.; Soták, T.; Dobročka, E.; Mičušik, M. Carbon supported Pd-Cu catalysts for highly selective rearrangement of furfural to cyclopentanone. Appl. Catal. B Environ. 2016, 181, 210–219. [Google Scholar] [CrossRef]
- Zhang, G.-S.; Zhu, M.-M.; Zhang, Q.; Liu, Y.-M.; He, H.-Y.; Cao, Y. Towards quantitative and scalable transformation of furfural to cyclopentanone with supported gold catalysts. Green Chem. 2016, 18, 2155–2164. [Google Scholar] [CrossRef]
- Wang, Y.; Sang, S.; Zhu, W.; Gao, L.; Xiao, G. CuNi@C catalysts with high activity derived from metal-organic frameworks precursor for conversion of furfural to cyclopentanone. Chem. Eng. J. 2016, 299, 104–111. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Wang, X.; Liang, Y.; Yang, X.; Wang, Z. Highly Selective and Efficient Rearrangement of Biomass-Derived Furfural to Cyclopentanone over Interface-Active Ru/Carbon Nanotubes Catalyst in Water. ACS Sustain. Chem. Eng. 2017, 5, 744–751. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Fei, B.; Chen, X.; Zhang, J.; Mu, X. Tunable and selective hydrogenation of furfural to furfuryl alcohol and cyclopentanone over Pt supported on biomass-derived porous heteroatom doped carbon. Faraday Discuss. 2017, 202, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, W.; Sang, S.; Gao, L.; Xiao, G. Supported Cu catalysts for the hydrogenation of furfural in aqueous phase: Effect of support. Asia-Pac. J. Chem. Eng. 2017, 12, 422–431. [Google Scholar] [CrossRef]
- Zhou, M.; Li, J.; Wang, K.; Xia, H.; Xu, J.; Jiang, J. Selective conversion of furfural to cyclopentanone over CNT-supported Cu based catalysts: Model reaction for upgrading of bio-oil. Fuel 2017, 202, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Liu, D.; Mu, X.; Chen, X.; Shi, Y. Selective conversion of furfural to cyclopentanone or cyclopentanol using Co-Ni catalyst in water. Catalysts 2018, 8, 193. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, G.; Yang, L.; Li, F. Efficient conversion of furfural into cyclopentanone over high performing and stable Cu/ZrO2 catalysts. Appl. Catal. A Gen. 2018, 561, 117–126. [Google Scholar] [CrossRef]
- Date, N.S.; Kondawar, S.E.; Chikate, R.C.; Rode, C.V. Single-Pot Reductive Rearrangement of Furfural to Cyclopentanone over Silica-Supported Pd Catalysts. ACS Omega 2018, 3, 9860–9871. [Google Scholar] [CrossRef]
- Dohade, M.; Dhepe, P.L. Efficient method for the cyclopentanone synthesis from furfural: Understanding the role of solvents, solubilities and bimetallic catalytic system. Catal. Sci. Technol. 2018, 8, 5259–5269. [Google Scholar] [CrossRef]
- Shen, T.; Hu, R.; Zhu, C.; Li, M.; Zhuang, W.; Tang, C.; Ying, H. Production of cyclopentanone from furfural over Ru/C with Al11.6PO23.7 and application in the synthesis of diesel range alkanes. RSC Adv. 2018, 8, 37993–38001. [Google Scholar] [CrossRef]
- Cherkasov, N.; Expósito, A.J.; Aw, M.S.; Fernández-García, J.; Huband, S.; Sloan, J.; Paniwnyk, L.; Rebrov, E.V. Active site isolation in bismuth-poisoned Pd/SiO2 catalysts for selective hydrogenation of furfural. Appl. Catal. A Gen. 2019, 570, 183–191. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, Z.; Guo, W.; Liu, J.; Li, R.; Chen, R.; Huang, J. Hydrogenation and Hydrolysis of Furfural to Furfuryl Alcohol, Cyclopentanone, and Cyclopentanol with a Heterogeneous Copper Catalyst in Water. Ind. Eng. Chem. Res. 2019, 58, 3988–3993. [Google Scholar] [CrossRef]
- Li, X.; Deng, Q.; Zhang, L.; Wang, J.; Wang, R.; Zeng, Z.; Deng, S. Highly efficient hydrogenative ring-rearrangement of furanic aldehydes to cyclopentanone compounds catalyzed by noble metals/MIL-MOFs. Appl. Catal. A Gen. 2019, 575, 152–158. [Google Scholar] [CrossRef]
- Pan, P.; Xu, W.-Y.; Pu, T.-J.; Wang, X.-D.; Pei, X.-J.; Tang, F.; Feng, Y.-S. Selective Conversion of Furfural to Cyclopentanone and Cyclopentanol by Magnetic Cu-Fe3O4 NPs Catalyst. ChemistrySelect 2019, 4, 5845–5852. [Google Scholar] [CrossRef]
- Deng, Q.; Wen, X.; Zhang, P. Pd/Cu-MOF as a highly efficient catalyst for synthesis of cyclopentanone compounds from biomass-derived furanic aldehydes. Catal. Commun. 2019, 126, 5–9. [Google Scholar] [CrossRef]
- Jia, P.; Lan, X.; Li, X.; Wang, T. Highly selective hydrogenation of furfural to cyclopentanone over a NiFe bimetallic catalyst in a methanol/water solution with a solvent effect. ACS Sustain. Chem. 2019, 7, 15221–15229. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B. Effect of the conditions for the aqueous-phase hydrogenation of furfural over Pd/C catalysts on the reaction routes. AIP Conf. Proc. 2019, 2141, 020010. [Google Scholar]
- Astuti, M.D.; Kristina, D.; Rodiansono, R.; Mujiyanti, D.R. One-pot Selective Conversion of Biomass-derived Furfural into Cyclopentanone/Cyclopentanol over TiO2 Supported Bimetallic Ni-M (M = Co, Fe) Catalysts. Bull. Chem. React. Eng. Catal. 2020, 15, 231–241. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Zhang, X. One-Step Encapsulation of Bimetallic Pd-Co Nanoparticles Within UiO-66 for Selective Conversion of Furfural to Cyclopentanone. Catal. Lett. 2020, 150, 2158–2166. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, C.; Yang, L.; Fan, G.; Li, F. Robust structured Cu-based film catalysts with greatly enhanced catalytic hydrogenation property. Appl. Surf. Sci. 2020, 504, 114364. [Google Scholar] [CrossRef]
- Li, X.; Deng, Q.; Zhou, S.; Zou, J.; Wang, J.; Wang, R.; Zeng, Z.; Deng, S. Double-metal cyanide-supported Pd catalysts for highly efficient hydrogenative ring-rearrangement of biomass-derived furanic aldehydes to cyclopentanone compounds. J. Catal. 2019, 378, 201–208. [Google Scholar] [CrossRef]
- Deng, Q.; Gao, R.; Li, X.; Wang, J.; Zeng, Z.; Zou, J.-J.; Deng, S. Hydrogenative Ring-Rearrangement of Biobased Furanic Aldehydes to Cyclopentanone Compounds over Pd/Pyrochlore by Introducing Oxygen Vacancies. ACS Catal. 2020, 10, 7355–7366. [Google Scholar] [CrossRef]
- Lee, J.; Woo, J.; Nguyen-Huy, C.; Lee, M.S.; Joo, S.H.; An, K. Highly dispersed Pd catalysts supported on various carbons for furfural hydrogenation. Catal. Today 2020, 350, 71–79. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, L.; Fan, G.; Zheng, L.; Yang, L.; Li, F. NiCu Nanoparticles for Catalytic Hydrogenation of Biomass-Derived Carbonyl Compounds. ACS Appl. Nano Mater. 2020, 3, 9226–9237. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Ma, X.; An, Z.; Guo, S.; Shu, X.; Song, H.; Xiang, X.; He, J. A gradient reduction strategy to produce defects-rich nano-twin Cu particles for targeting activation of carbon-carbon- or carbon-oxygen in furfural conversions. J. Catal. 2020, 389, 78–86. [Google Scholar] [CrossRef]
- Herrera, C.; Fuentealba, D.; Ghampson, I.T.; Sepulveda, C.; García-Fierro, J.L.; Canales, R.I.; Escalona, N. Selective conversion of biomass-derived furfural to cyclopentanone over carbon nanotube-supported Ni catalysts in Pickering emulsions. Catal. Commun. 2020, 144, 106092. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Talsi, V.P.; Likholobov, V.A. Mechanism of Pd/C-catalyzed hydrogenation of furfural under hydrothermal conditions. J. Catal. 2020, 389, 721–734. [Google Scholar] [CrossRef]
- Gao, G.; Shao, Y.; Gao, Y.; Wei, T.; Gao, G.; Zhang, S.; Chen, Q.; Hu, X. Synergetic effects of hydrogenation and acidic sites in phosphorus-modified nickel catalysts for the selective conversion of furfural to cyclopentanone. Catal. Sci. Technol. 2021, 11, 575–593. [Google Scholar] [CrossRef]
- Zu, S.; Ma, H.; Sun, Y.; Liu, X.; Zhang, M.; Luo, Y.; Gao, J.; Xu, J. Selective tandem hydrogenation and rearrangement of furfural to cyclopentanone over CuNi bimetallic catalyst in water. Chin. J. Catal. 2021, 42, 2216–2224. [Google Scholar] [CrossRef]
- Tong, Z.; Gao, R.; Li, X.; Guo, L.; Wang, J.; Zeng, Z.; Deng, Q.; Deng, S. Highly Controllable Hydrogenative Ring Rearrangement and Complete Hydrogenation Of Biobased Furfurals over Pd/La2B2O7 (B=Ti, Zr, Ce). ChemCatChem 2021, 13, 4549–4556. [Google Scholar] [CrossRef]
- Kumar, A.; Shivhare, A.; Bal, R.; Srivastava, R. Metal and solvent-dependent activity of spinel-based catalysts for the selective hydrogenation and rearrangement of furfural. Sustain. Energy Fuels 2021, 5, 3191–3204. [Google Scholar] [CrossRef]
- Gao, R.; Li, X.; Guo, L.; Tong, Z.; Deng, Q.; Wang, J.; Zeng, Z.; Zou, J.-J.; Deng, S. Pyrochlore/Al2O3 composites supported Pd for the selective synthesis of cyclopentanone from biobased furfurals. Appl. Catal. A Gen. 2021, 612, 117985. [Google Scholar] [CrossRef]
- Tian, H.; Gao, G.; Xu, Q.; Gao, Z.; Zhang, S.; Hu, G.; Xu, L.; Hu, X. Facilitating selective conversion of furfural to cyclopentanone via reducing availability of metallic nickel sites. Mol. Catal. 2021, 510, 111697. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Z.; Yang, Y.; Sun, Z.; Wang, Y.; Shi, C.; Liu, Y.-Y.; Wang, A.; Leus, K.; Van Der Voort, P. Hydrogenative Ring-Rearrangement of Furfural to Cyclopentanone over Pd/UiO-66-NO2. Molecules 2021, 26, 5736. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tong, Z.; Zhu, S.; Deng, Q.; Chen, S.; Wang, J.; Zeng, Z.; Zhang, Y.; Zou, J.-J.; Deng, S. Water-mediated hydrogen spillover accelerates hydrogenative ring-rearrangement of furfurals to cyclic compounds. J. Catal. 2022, 405, 363–372. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, K.; Fan, M.; Gao, G.; Wang, J.; Zhang, L.; Zhang, S.; Hu, X. Synthesis of a Thermally and Hydrothermally Stable Copper-Based Catalyst via Alloying of Cu with Ni and Zn for Catalyzing Conversion of Furfural into Cyclopentanone. ACS Sustain. Chem. Eng. 2022, 10, 8763–8777. [Google Scholar] [CrossRef]
- Byun, M.Y.; Kim, Y.E.; Baek, J.H.; Jae, J.; Lee, M.S. Effect of surface properties of TiO2 on the performance of Pt/TiO2 catalysts for furfural hydrogenation. RSC Adv. 2022, 12, 860–868. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Cai, C.; Wang, H.; Huang, Y.; Wang, C.; Ma, L. Selectivity catalytic transfer hydrogenation of biomass-based furfural to cyclopentanone. Fuel 2023, 332, 126057. [Google Scholar] [CrossRef]
- Cheng, C.; Zhao, C.-S.; Zhao, D.; Ding, S.-M.; Chen, C. The importance of constructing Triple-functional Sr2P2O7/Ni2P catalysts for smoothing hydrogenation Ring-rearrangement of Biomass-derived Furfural compounds in water. J. Catal. 2023, 421, 117–133. [Google Scholar] [CrossRef]
- Balaga, R.; Balla, P.; Zhang, X.; Ramineni, K.; Du, H.; Lingalwar, S.; Perupogu, V.; Zhang, Z.C. Enhanced Cyclopentanone Yield from Furfural Hydrogenation: Promotional Effect of Surface Silanols on Ni-Cu/m-Silica Catalyst. Catalysts 2023, 13, 580. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, J.; Wu, D. Highly Efficient NiCu/SiO2 Catalyst Induced by Ni(Cu)-Silica Interaction for Aqueous-Phase Furfural Hydrogenation. Catal. Lett. 2023, 153, 1543–1555. [Google Scholar] [CrossRef]
- Rajpurohit, A.S.; Mohan, T.V.R.; Jaccob, M.; Ramswamy, K.K.; Viswanathan, B. Realizing Influence of Supports in Aqueous-Phase Hydrogenation of Furfural over Nickel Catalysts. ChemNanoMat 2023, 9, e202300158. [Google Scholar] [CrossRef]
- Long, W.; Huang, S.; Huang, Y. Selective catalytic hydrogenation of furfural to cyclopentanone over Ru-Co bimetallic catalyst. Sci. Asia 2023, 49, 116. [Google Scholar] [CrossRef]
- Orozco-Saumell, A.; Mariscal, R.; Vila, F.; López Granados, M.; Alonso, D.M. Hydrogenation of Furfural to Cyclopentanone in Tert-Butanol-Water Medium: A Study of the Reaction Intermediates Reactivity Using Cu/ZnO/Al2O3 as Catalyst. Catalysts 2023, 13, 1394. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, Y.; Ma, Z.; Sun, Z.; Liu, X.; Xu, C.C.; Nie, R. Synergy between Ni3Sn2 alloy and Lewis acidic ReOx enables selectivity control of furfural hydrogenation to cyclopentanone. Appl. Catal. B Environ. 2024, 340, 123191. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Wang, Y.; Zhou, F.; Xiang, Z.; Zhu, W.; Wang, H. P-induced electron transfer interaction for enhanced selective hydrogenation rearrangement of furfural to cyclopentanone. J. Energy Chem. 2024, 92, 43–51. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, H.; Liu, R.; Luo, H.; Lv, Y.; Liu, P. Highly efficient and selective hydrogenation of furfural to furfuryl alcohol and cyclopentanone over Cu-Ni bimetallic Catalysts: The crucial role of CuNi alloys and Cu+ species. J. Catal. 2024, 436, 115603. [Google Scholar] [CrossRef]
- Kittisabhorn, A.; Ahmed, I.; Pornputtapitak, W.; Ratchahat, S.; Chaiwat, W.; Koo-amornpattana, W.; Klysubun, W.; Limphirat, W.; Assabumrungrat, S.; Srifa, A. Constructing Ni-Pt Bimetallic Catalysts for Catalytic Hydrogenation and Rearrangement of Furfural into Cyclopentanone with Insight in H/D Exchange by D2O Labeling. ACS Omega 2024, 9, 28637–28647. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Zhou, F.; Wang, Y.; Xiang, Z.; Zhu, W.; Wang, H. Phytic acid-assisted phase-controlled synthesis of nickel phosphides for highly selective hydrogenation of biomass-derived furfural. Appl. Catal. B. Environ. Energy 2024, 359, 124413. [Google Scholar] [CrossRef]
- Baldenhofer, R.; Lange, J.-P.; Kersten, S.R.A.; Ruiz, M.P. Furfural to Cyclopentanone–a Search for Putative Oligomeric By-products. ChemSusChem 2024, 17, e2024000108. [Google Scholar] [CrossRef]
- Gao, G.; Hu, X.; Shao, Y.; Sun, K.; Li, C.; Chen, Q.; Zhang, S. Alloying nickel with phosphorus to switch the selective conversion of furfural from furans to cyclopentanones or 1,4-pentanediol. Fuel 2024, 371 Pt A, 131934. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Z.; Wu, D. Efficient CoCu/SiO2 Catalyst Derived from Co(Cu) Silicate for Aqueous-Phase Furfural Hydrogenation. Chem. Eng. Technol. 2024, 47, e202300265. [Google Scholar] [CrossRef]
- Patil, A.; Engelbert van Bevervoorde, M.J.S.; Neira d’Angelo, M.F. Intensifying Cyclopentanone Synthesis from Furfural Using Supported Copper Catalysts. ChemSusChem 2025, 18, e202401484. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Rosmini, C.; Kodyingal, A.K.; Simeonov, S.; Neira d’Angelo, M.F. Modulating activity and selectivity of aqueous phase furfuryl alcohol hydrogenation by tuning supported Pt on W-Zr Mixed oxides. Appl. Catal. B Environ. Energy 2025, 375, 125400. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, R.; Meng, C.; Zhang, Y.; Hu, W.; Gao, X.; Yang, D.; Wan, X.; Zhou, C.; Kustov, L.M.; et al. Biomass upgrading of furfural to cyclopentanone via selective aqueous-phase hydrogenative rearrangement over Pd/g-C3N4 catalysts. Chem. Eng. J. 2025, 505, 159752. [Google Scholar] [CrossRef]
- Soares, W.L.S.; Feitosa, L.F.; Moreira, C.R.; Bertella, F.; Lopes, C.W.; Duarte de Farias, A.M.; Fraga, M.A. Tailoring Cu-SiO2 Interaction through Nanocatalyst Architecture to Assemble Surface Sites for Furfural Aqueous-Phase Hydrogenation to Cycloketones. ACS Appl. Mater. Interfaces 2025, 17, 13146–13161. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, B.; He, Z.; Deng, Y.; Yang, Y.; Luo, Y.; Wang, H.; Song, X.; Du, X.; Wang, C.; et al. One-pot xylose-to-cyclopentanone conversion tuned by intermediate partitioning behavior and poisoning-resistant multifunctional Co/Nb2O5 catalysts. Appl. Catal. A Gen. 2025, 699, 120282. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, B.; Wu, H.; He, Z.; Du, X.; Ou, J.; Ren, T.; Wang, H.; Liao, Y.; Liu, Q.; et al. Correlation of the catalytic performance with Ruδ+ species on Ru/Nb2O5 in furfural aqueous reductive conversion. Catal. Sci. Technol. 2025, 15, 33–40. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Shi, J.; Wu, W.-P.; Zhu, R.; Li, X.-L.; Deng, J.; Fu, Y. Effect of Cp*Iridium(III) Complex and acid co-catalyst on conversion of furfural compounds to cyclopentanones or straight chain ketones. Appl. Catal. A Gen. 2017, 543, 266–273. [Google Scholar] [CrossRef]
- Bonacci, S.; Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Oliverio, M.; Procopio, A. Montmorillonite K10-Catalyzed Solvent-Free Conversion of Furfural into Cyclopentenones. Catalysts 2019, 9, 301. [Google Scholar] [CrossRef]
- Chabab, S.; Theveneau, P.; Coquelet, C.; Corvisier, J.; Paricaud, P. Measurements and predictive models of high-pressure H2 solubility in brine (H2O+NaCl) for Underground Hydrogen Storage application. Int. J. Hydrogen Energy 2020, 45, 32206–32220. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Wei, R.-P.; Geng, G.-L.; Zhou, M.-H.; Gao, L.-J.; Xiao, G.-M. Aqueous-phase catalytic hydrogenation of furfural over Ni-bearing hierarchical Y zeolite catalysts synthesized by a facile route. Fuel Process. Technol. 2015, 134, 168–174. [Google Scholar] [CrossRef]
- Li, X.-L.; Deng, J.; Shi, J.; Pan, T.; Yu, C.-G.; Xu, H.-J.; Fu, Y. Selective conversion of furfural to cyclopentanone or cyclopentanol using different preparation methods of Cu-Co catalysts. Green Chem. 2015, 17, 1038–1046. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, M.; Zeng, Z.; Xiao, G.; Xiao, R. Selective hydrogenation of furfural to cyclopentanone over Cu-Ni-Al hydrotalcite-based catalysts. Korean J. Chem. Eng. 2014, 31, 593–597. [Google Scholar]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Anet, F.A.L. The N.M.R. spectrum of cyclopentanone. Can. J. Chem. 1961, 39, 2316–2318. [Google Scholar] [CrossRef]
- Lambert, J.B.; Johnson, S.C.; Xue, L. Dynamics of Five-Membered Rings in the Solid State by NMR Spectroscopy. J. Am. Chem. Soc. 1994, 116, 6167–6174. [Google Scholar] [CrossRef]
- Abraham, R.J.; Koniotou, R.; Sancassan, F. Conformational analysis. Part 39. A theoretical and lanthanide induced shift (LIS) investigation of the conformations of cyclopentanol and cis- and trans-cyclopentane-1,2-diol. J. Chem. Soc. Perkin Trans. 2 2002, 12, 2025–2030. [Google Scholar] [CrossRef]
- Bruehwiler, A.; Semagina, N.; Grasemann, M.; Renken, A.; Kiwi-Minsker, L. Three-Phase Catalytic Hydrogenation of a Functionalized Alkyne: Mass Transfer and Kinetic Studies with in Situ Hydrogen Monitoring. Ind. Eng. Chem. Res. 2008, 47, 6862–6869. [Google Scholar] [CrossRef]
- Fulajtarová, K.; Hronec, M.; Liptaj, T.; Prónayová, N.; Soták, T. Catalytic hydrogenation of condensation product of furfural with cyclopentanone using molecular hydrogen and formic acid as hydrogen donor. J. Taiwan Inst. Chem. Eng. 2016, 66, 137–142. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, S.; Long, J.; Wang, C.; Chang, J.; Tan, J.; Liu, Q.; Ma, L.; Wang, T.; Zhang, Q. In situ hydrogenation of furfural with additives over a RANEY® Ni catalyst. RSC Adv. 2015, 111, 91190–91195. [Google Scholar] [CrossRef]
- Luo, J.; Monai, M.; Yun, H.; Arroyo-Ramírez, L.; Wang, C.; Murray, C.B.; Fornasiero, P.; Gorte, R.J. The H2 Pressure Dependence of Hydrodeoxygenation Selectivities for Furfural Over Pt/C Catalysts. Catal. Lett. 2016, 146, 711–717. [Google Scholar] [CrossRef]

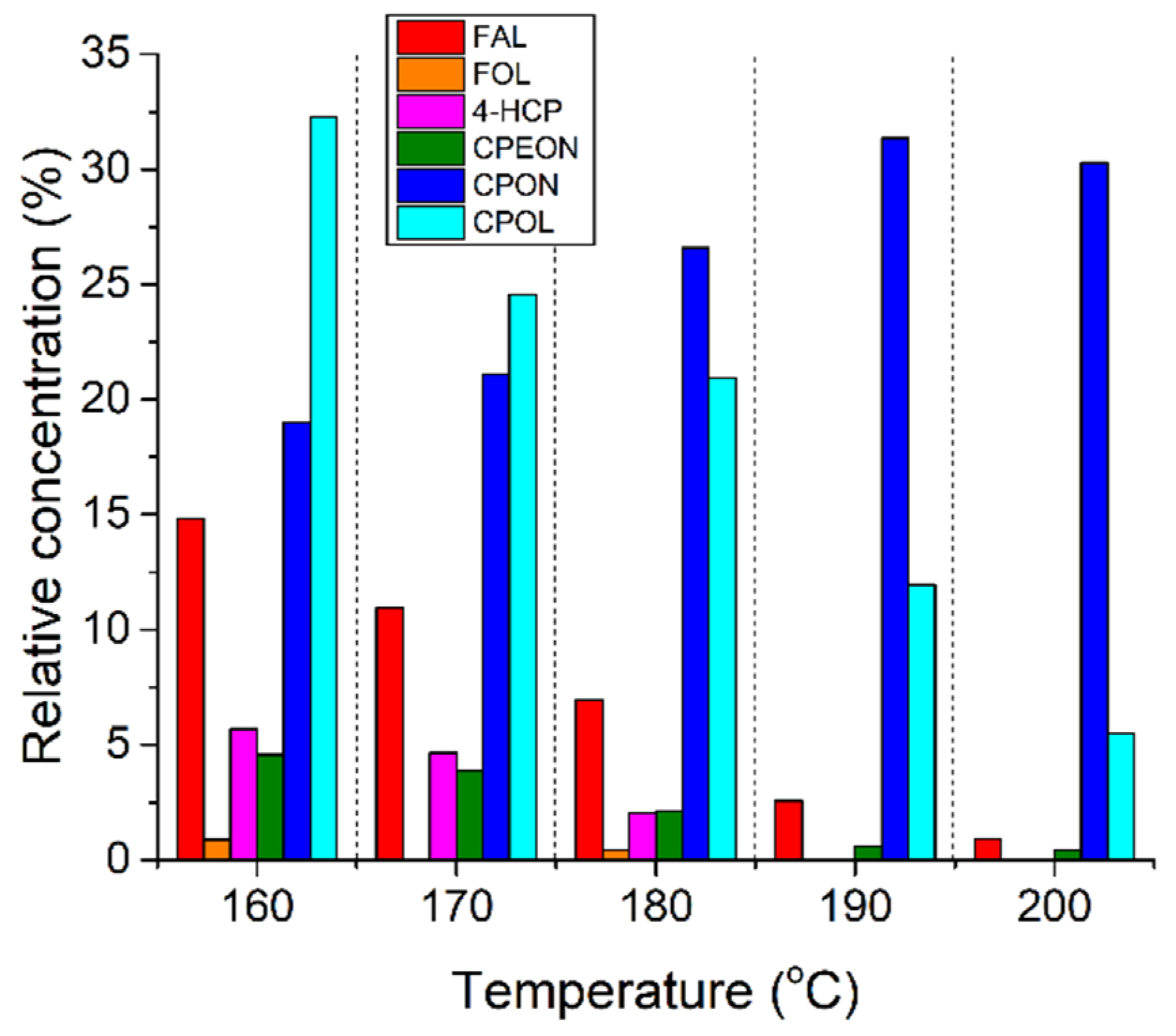

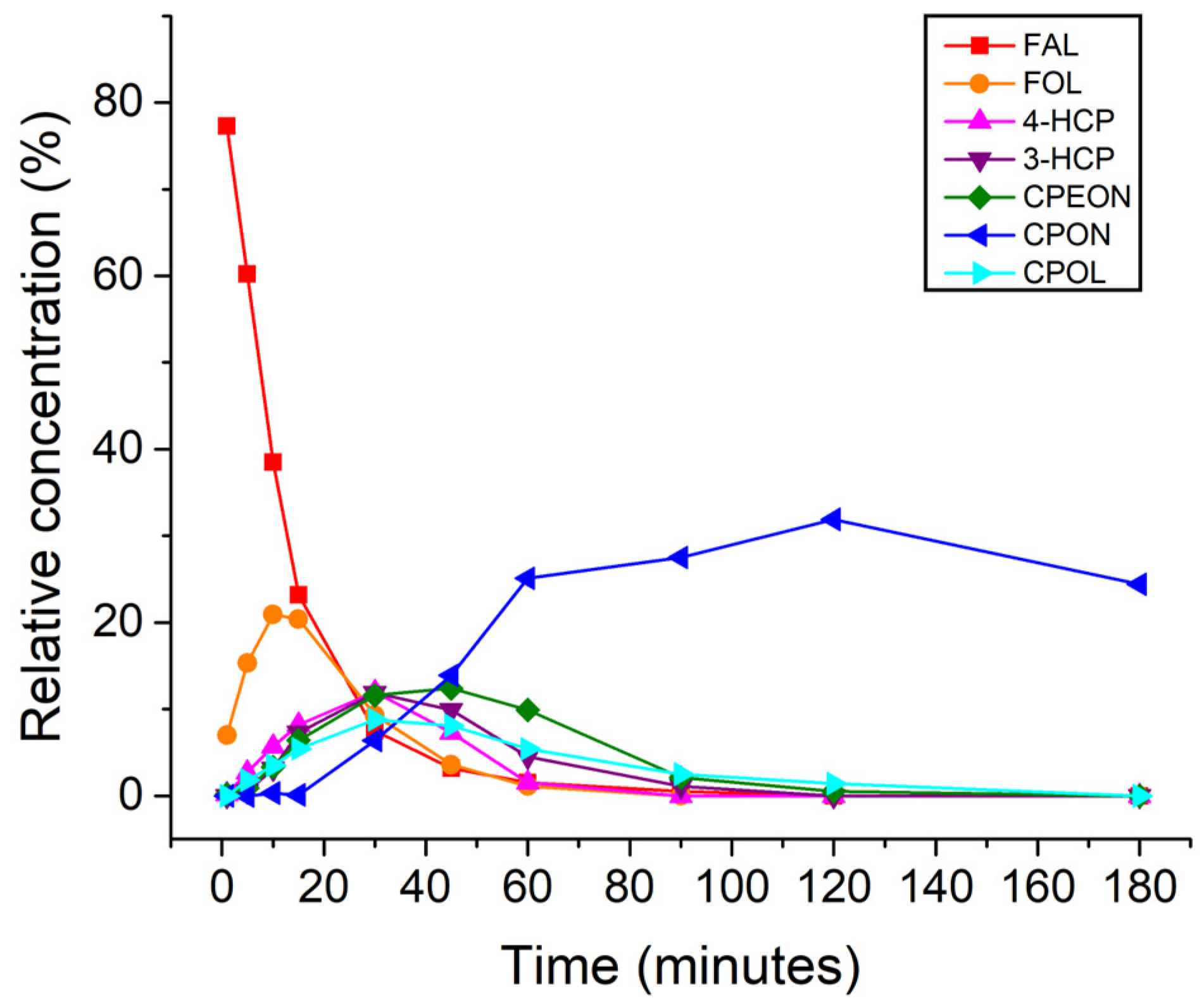
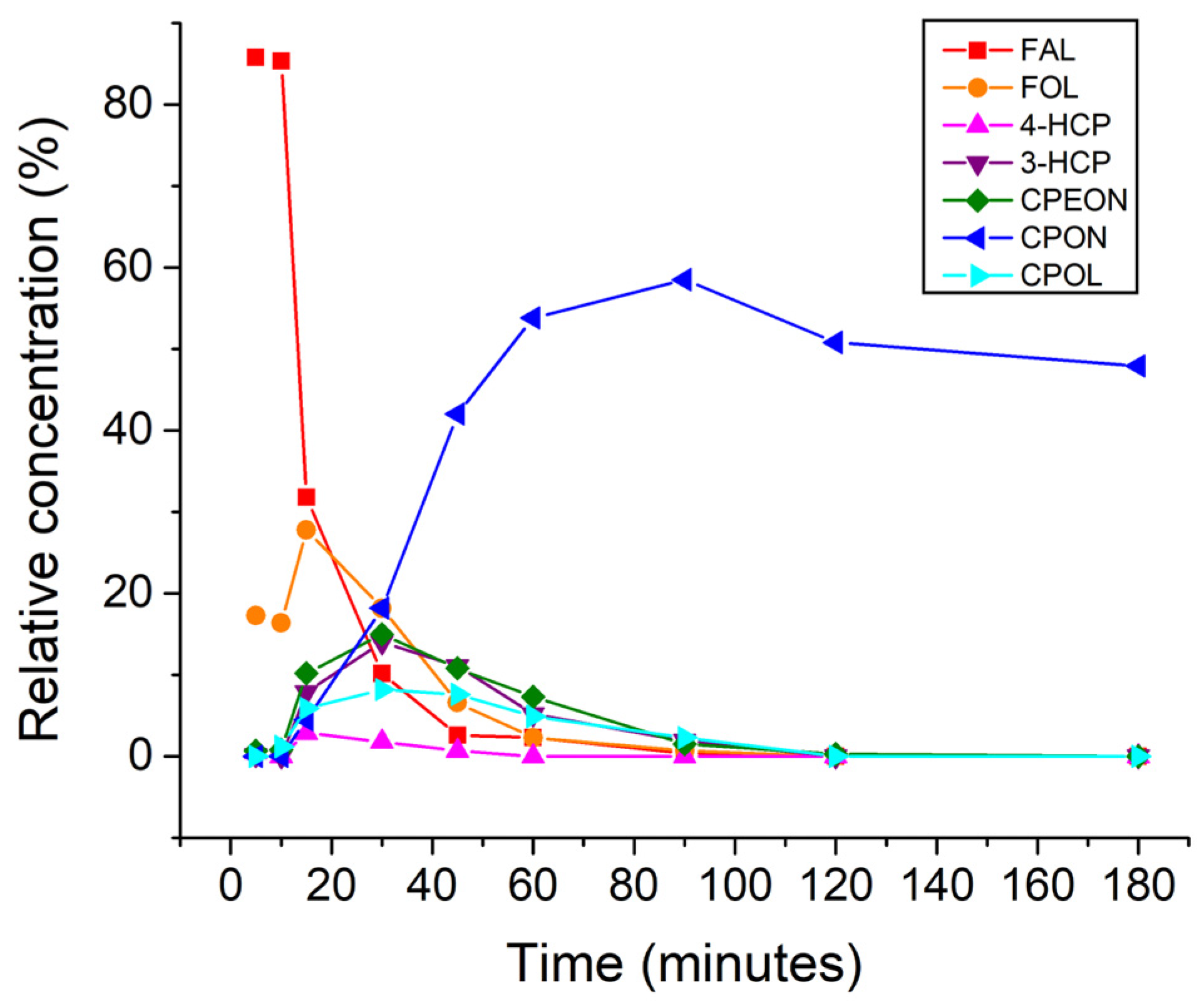


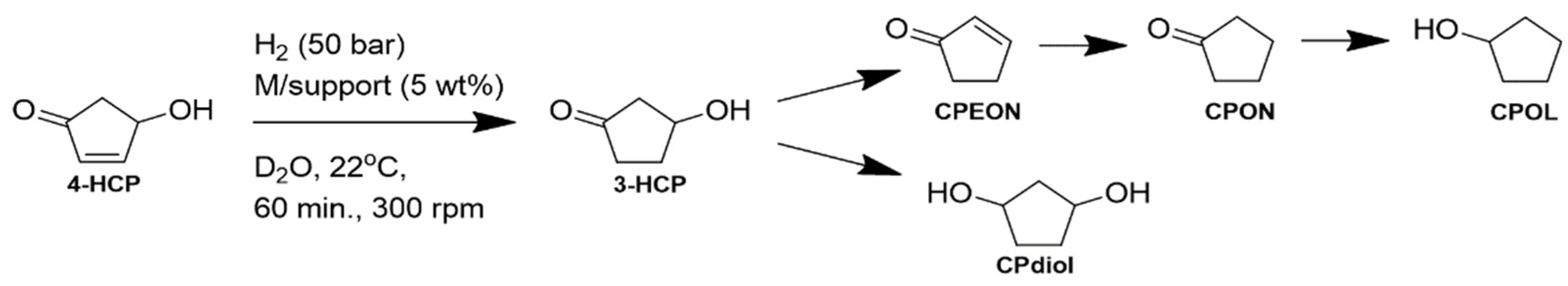

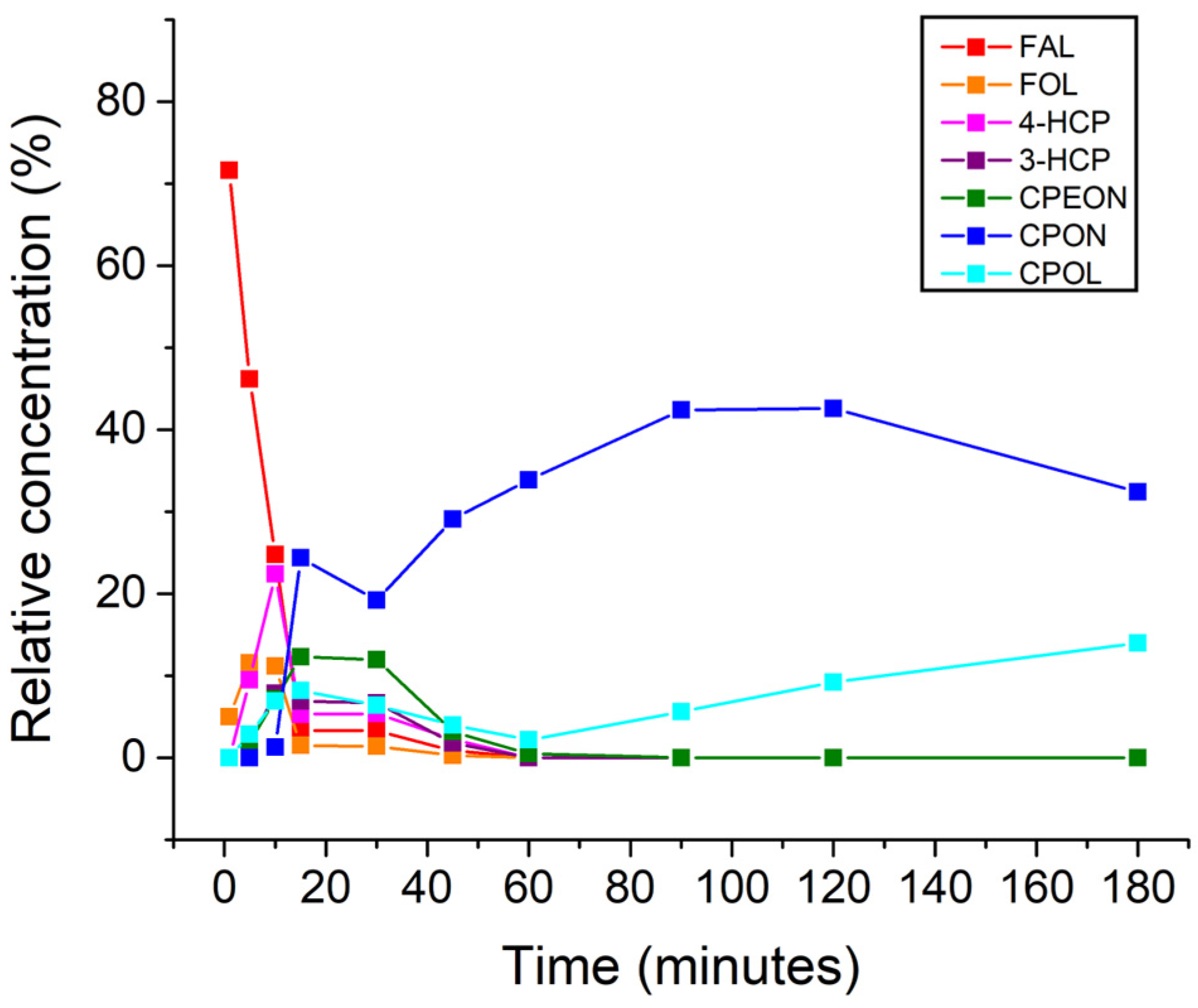


| Entry | Catalyst | T (°C) | FAL Conv. (%) b | Product Yields (%) b | Mass Balance (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FOL | THFOL | 4-HCP | CPEON | CPON | CPOL | |||||
| 1 | Pt/C | 25 | 3.5 | 2.3 | 0 | 0 | 0 | 0 | 0 | 98.8 |
| 2 | Pt/C | 160 | 85.2 | 0.9 | 0 | 5.7 | 4.6 | 19.0 | 32.3 | 77.2 |
| 3 | Pd/C | 25 | 100 | 0.9 | 64.3 | 0.2 | 0 | 0 | 0 | 65.4 |
| 4 | Pd/C | 160 | 100 | 0.9 | 46.8 | 0.6 | 0 | 0 | 11.8 | 60.1 |
| 5 | Ru/C | 25 | 16.9 | 8.0 | 1.5 | 0 | 0 | 0 | 0 | 92.7 |
| 6 | Ru/C c | 160 | 97.9 | 0 | 9.3 | 0 | 0 | 14.4 | 11.5 | 37.2 |
| 7 | Pt/C d | 160 | 59.1 | 0.8 | 0 | 10.1 | 4.3 | 7.9 | 23.4 | 59.1 |
| 8 | Pt/Al2O3 | 180 | 63.3 | 5.0 | 0 | 0.5 | 0 | 0.9 | 0 | 43.2 |
| 9 e | Pt/C | 160 | 100 | 0 | 0.4 | N/D | N/D | 76.5 | 4.8 | 85.2 |
| Entry | Catalyst | Conversion 4-HCP (%) b | Product Yields (%) b | Mass b | |||
|---|---|---|---|---|---|---|---|
| 3-HCP | CPdiol | CPON | CPOL | Balance (%) | |||
| 1 | Ru/C | 95.3 | 49.9 | 24.8 | 6.3 | 15.2 | 100.9 |
| 2 | Pd/C | 95.6 | 66.7 | 0.5 | 8.9 | 19.6 | 100.1 |
| 3 | Rh/C | 75.1 | 45.9 | 7.6 | 7.3 | 15.2 | 100.9 |
| 4 | Pt/C | 91.6 | 55.9 | 8.6 | 8.5 | 18.7 | 100.1 |
| 5 c | Pd/C | 95.5 | 77.2 | 0.3 | 6.1 | 11.8 | 99.9 |
| 6 | Pd/Al2O3 | 68.5 | 53.1 | 0.2 | 5.4 | 9.9 | 100.1 |
| 7 | Pd/CaCO3 | 34.6 | 18.6 | 0 | 5.5 | 10.5 | 100.0 |
| 8 | Pd/BaSO4 | 41.8 | 26.2 | 0 | 5.3 | 10.3 | 100.0 |
| Water–Toluene Volume Ratio | [caq/ctol] Per Reaction Component | |||||
|---|---|---|---|---|---|---|
| FAL | FOL | 4-HCP | CPEON | CPON | CPOL | |
| 75:25 | 0.63 | N/A | 2.99 | 2.56 | 0.58 | 10.51 |
| 50:50 | 0.23 | 0.97 | N/A | 0.86 | 0.20 | 3.15 |
| 25:75 | 0.07 | N/A | 0.22 | 0.25 | 0.10 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Slagmaat, C.A.M.R. The Cascade Transformation of Furfural to Cyclopentanone: A Critical Evaluation Concerning Feasible Process Development. ChemEngineering 2025, 9, 74. https://doi.org/10.3390/chemengineering9040074
van Slagmaat CAMR. The Cascade Transformation of Furfural to Cyclopentanone: A Critical Evaluation Concerning Feasible Process Development. ChemEngineering. 2025; 9(4):74. https://doi.org/10.3390/chemengineering9040074
Chicago/Turabian Stylevan Slagmaat, Christian A. M. R. 2025. "The Cascade Transformation of Furfural to Cyclopentanone: A Critical Evaluation Concerning Feasible Process Development" ChemEngineering 9, no. 4: 74. https://doi.org/10.3390/chemengineering9040074
APA Stylevan Slagmaat, C. A. M. R. (2025). The Cascade Transformation of Furfural to Cyclopentanone: A Critical Evaluation Concerning Feasible Process Development. ChemEngineering, 9(4), 74. https://doi.org/10.3390/chemengineering9040074






