Abstract
In this study, a novel anode-supported solid oxide fuel cell (SOFC) comprising a Bi2O3-doped NiO-ScSZ anode and an ScSZ electrolyte was successfully fabricated via a low-temperature co-sintering process at 1300 °C. The incorporation of 3 wt% Bi2O3 effectively promoted the sintering of both the anode support and electrolyte layer, resulting in a dense, gas-tight electrolyte and a mechanically robust porous anode support. X-ray diffraction (XRD) and scanning electron microscopy (SEM) analyses confirmed the formation of phase-pure, highly crystalline ScSZ with an optimized microstructure. Electrochemical performance measurements demonstrated that the fabricated cells achieved excellent power density, reaching a peak value of 0.861 W cm−2 at 800 °C under humidified hydrogen fuel conditions. The cells maintained stable performance under dry methane operation, with a maximum power density of 0.624 W cm−2 at 800 °C, indicating resistance to carbon deposition. Gas chromatographic analyses further revealed that the Bi2O3-doped NiO-ScSZ anode facilitated earlier and more stable electrochemical oxidation of methane-derived species compared with the conventional NiO-YSZ system, even under conditions of an elevated methane partial pressure. These findings demonstrate that Bi2O3 co-doping, combined with low-temperature co-sintering, provides an effective approach for fabricating high-performance intermediate-temperature SOFCs with enhanced structural integrity and electrochemical stability. The developed methodology presents a promising pathway toward achieving cost-effective and durable SOFC technologies.
1. Introduction
Solid oxide fuel cells (SOFCs) are recognized as one of the most promising electrochemical energy conversion technologies owing to their high efficiency, broad fuel flexibility, and environmental compatibility [1,2]. Unlike polymer electrolyte fuel cells (PEFCs) or molten carbonate fuel cells (MCFCs), SOFCs operate at elevated temperatures (typically 600–1000 °C), enabling the direct utilization of a wide variety of fuels, including hydrogen, natural gas, and hydrocarbons, without extensive external reforming [1,3,4,5,6]. The high operating temperature also permits the use of cost-effective, non-precious metal catalysts, offering significant economic advantages [7]. However, these high temperatures impose stringent requirements on material stability and mechanical robustness, which have motivated intensive efforts to reduce the operating temperature into the intermediate range (600–800 °C) [8]. Achieving this reduction necessitates the careful optimization of the electrolyte and electrode materials regarding composition, microstructure, and interfacial properties [2,9,10,11].
Among candidate electrolyte materials for intermediate-temperature SOFCs, scandia-stabilized zirconia (ScSZ) has attracted considerable attention due to its superior oxygen ion conductivity, which surpasses the conventional yttria-stabilized zirconia (YSZ) under comparable conditions [12,13]. This enhanced conductivity is attributed to the stabilized cubic fluorite structure and the high mobility of oxygen vacancies introduced by scandia doping [14]. Nevertheless, ScSZ faces several critical challenges that limit its practical application. First, ScSZ typically requires high sintering temperatures (above 1400 °C) to achieve full densification, leading to increased fabrication costs and higher energy consumption [13]. Second, ScSZ may suffer from phase instability at intermediate temperatures, where undesirable tetragonal or rhombohedral phases can emerge and degrade long-term conductivity [15]. Furthermore, mismatches in thermal expansion coefficients and shrinkage behaviors between the electrolyte and anode materials, such as NiO-ScSZ composites, often cause interfacial compatibility issues during co-sintering [16].
To address these challenges, several strategies have been explored, including co-doping with additional oxides and the incorporation of sintering aids [17]. In addition, emerging fabrication technologies, such as 3D printing, have shown potential in enabling low-temperature and structurally controlled ceramic layers [18]. Among them, bismuth oxide (Bi2O3) has received significant attention owing to its exceptionally high ionic conductivity (~0.1 S/cm at 750 °C) and low melting point (~825 °C) [9,14]. Particularly in its δ-phase, Bi2O3 acts as an effective sintering promoter, substantially lowering the densification temperature of ceramic electrolytes. Studies have demonstrated that even small amounts of Bi2O3 doping can stabilize the desired cubic phase of ScSZ, promote grain growth, and enhance microstructural densification, enabling low-temperature processing without sacrificing electrochemical performance. Moreover, the simultaneous Bi2O3 doping of both electrolyte and anode materials has been shown to improve interfacial adhesion, reduce porosity, and facilitate enhanced ionic transport across the electrode/electrolyte interface. However, careful control of the Bi2O3 content is critical, as excessive doping may lead to phase segregation or unwanted electronic conduction pathways, adversely affecting cell performance [9,13].
Previous research has shown that the addition of Bi2O3 to Sc-stabilized zirconia (ScSZ) electrolytes can promote cubic phase stabilization and enhance sintering at lower temperatures, thereby improving ionic conductivity and reducing the required sintering temperature [19]. In this study, Bi2O3 was introduced into both the anode and electrolyte layers at a concentration of 3 wt% to facilitate low-temperature co-sintering. For the electrolyte, a comparative evaluation of 1 wt%, 3 wt%, and 5 wt% Bi2O3 was conducted. While 1 wt% offered limited densification, and 5 wt% caused abnormal grain growth and possible secondary phases, 3 wt% resulted in a dense and uniform microstructure without structural degradation. For the anode support, Bi2O3 levels above 3 wt% led to excessive sintering and mechanical collapse during co-firing. Therefore, 3 wt% was chosen as the optimal concentration for both layers to ensure structural integrity, enhanced sintering, and high electrochemical performance. We successfully achieved co-sintering at a relatively low temperature of 1300 °C by introducing 3 wt% Bi2O3 into both the anode and electrolyte layers, effectively overcoming the limitations associated with conventional high-temperature sintering processes [13,20]. The resulting half-cells demonstrated excellent microstructural integrity and high electrochemical performance under both hydrogen and methane atmospheres, achieving stable operation and notable power densities [21,22]. These results highlight the potential of Bi2O3 co-doping as an effective strategy for enabling cost-effective, high-performance intermediate-temperature SOFCs, offering a viable pathway toward the commercial realization of SOFC technologies.
2. Materials and Methods
2.1. Cell Fabrication
The Bi2O3 powder used in this study was synthesized via the thermal decomposition of bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, ≥99.9%, Aladdin, Riverside, CA, USA). The precursor was dissolved in deionized water, dried at 120 °C, and then calcined at 600 °C for 4 h in air to obtain phase-pure yellow Bi2O3.
Building upon our previous methodology [23], we adapted the process to fabricate Bi2O3-doped NiO-ScSZ substrates. Figure 1 illustrates the fabrication process of the anode-supported SOFC, including the preparation of the NiO-ScSZ-Bi2O3 anode, spray-coating the Bi2O3-modified ScSZ electrolyte, and the deposition of the LSM-based cathode. The anode support was fabricated via a dry-pressing method using a ternary mixture of NiO, ScSZ, and Bi2O3, with a mass ratio of 56:44:3. Commercial NiO (99.9%, Kojundo Chemical Lab, Nerima, Japan), 11 mol% Sc2O3-stabilized ZrO2 (ScSZ, Japan Fine Ceramics Co., Ltd., Sendai, Japan), and Bi2O3 powders were weighed accordingly and thoroughly mixed with 10 wt% starch as the pore-forming agent in ethanol. The mixture was transferred into a ball mill jar and subjected to 24 h of planetary ball milling to ensure uniform dispersion of the powders. After milling, the slurry was dried at 80 °C, sieved through a 100-mesh screen, and granulated. Subsequently, 4 wt% of polyvinyl butyral (PVB) dissolved in ethanol was added as a temporary binder, and the mixture was further hand-ground until the ethanol fully evaporated. The resulting granulated powder was pressed into 24 mm diameter green disks under a uniaxial pressure of 220 MPa. The green bodies were then pre-sintered at 1000 °C for 2 h in air to obtain mechanically robust and porous NiO–ScSZ–Bi2O3 substrates [24].
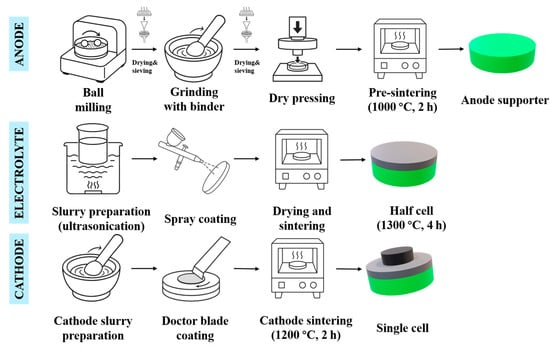
Figure 1.
Schematic of the fabrication process of the anode-supported SOFC.
The ScSZ–Bi2O3 composite electrolyte was deposited directly onto the pre-sintered anode substrates using a spray-coating technique [17,25]. The electrolyte slurry was formulated by dispersing ScSZ powder and 3 wt% Bi2O3 in anhydrous ethanol with 3 wt% PVB and 1 wt% ethyl cellulose as binders. The initial dispersion was carried out in a beaker using magnetic stirring for 30 min, followed by ultrasonic treatment for another 30 min to improve the suspension stability and minimize particle agglomeration. The optimized slurry exhibited good flowability and homogeneity, which are essential for uniform spraying. Prior to spraying, the surface of the anode substrate was cleaned with ethanol and fixed in position. The slurry was loaded into a pneumatic spray gun operated at 0.2 MPa, and multiple thin coats were applied, with drying at 80 °C between cycles to build up sufficient thickness. After the final coating layer was dried, the electrolyte-coated anode support was co-sintered at 1300 °C for 4 h in air to achieve a dense and gas-tight electrolyte membrane. The diameter of the half-cell after sintering was 20 mm.
For the cathode material, lanthanum strontium manganite (LSM-20, 99%, Sigma-Aldrich®, St. Louis, MO, USA) was selected. The LSM powder was manually ground using an agate mortar and thoroughly mixed with a predetermined ratio of pore-forming agents and organic binders to prepare a printable slurry. The resulting cathode slurry was uniformly deposited onto the sintered electrolyte surface using the doctor blade technique. The sample was then dried at 80 °C and subsequently sintered in air at 1200 °C for 2 h to complete the cell fabrication. The effective cathode area was defined as 0.78 cm2.
2.2. Cell Testing and Characterization
The phase composition of the prepared SOFC half-cells was analyzed using X-ray diffraction (XRD, Rigaku SmartLab 9 kW, Akishima, Japan) with Cu Kα radiation. Scans were conducted in the 2θ range of 20–80°, with a step size of 0.01°. The microstructural and morphological features of the SOFC single cells were observed using a field-emission scanning electron microscope (FE-SEM, JSM-7000F, JEOL Ltd., Akishima, Japan), focusing on the cross-section of the electrolyte layer and the porosity distribution in the anode. In addition, energy-dispersive X-ray spectroscopy (EDS) was employed to analyze the materials’ elemental compositions.
The porosity of the NiO-ScSZ-Bi2O3 anode was evaluated using the Archimedes method to assess structural variations before and after reduction. Three types of samples were tested: (i) the pre-sintered anode without an electrolyte coating, (ii) the sample sintered in air at 1300 °C for 4 h, and (iii) the reduced Ni-ScSZ-Bi2O3 support obtained by reduction in H2 at 800 °C for 2 h. Each sample was weighed under three conditions: dry weight (Wd), suspended in water (Ws), and immersed weight after boiling in deionized water (Wi) to eliminate trapped air. The porosity (P) was calculated using the following equation:
All measurements were conducted at room temperature using deionized water, and each test condition was repeated at least three times to ensure reproducibility and accuracy.
As illustrated in Figure 2, mass flow controllers (MFCs) were used to regulate the gas flow rates to both the anode and cathode sides of the SOFC. During periods when fuel gases were not introduced, argon was supplied as a protective gas to prevent the oxidation or contamination of the cell components. The anode exhaust gases could either be directly vented or directed to a gas chromatograph (GC) for compositional analysis.
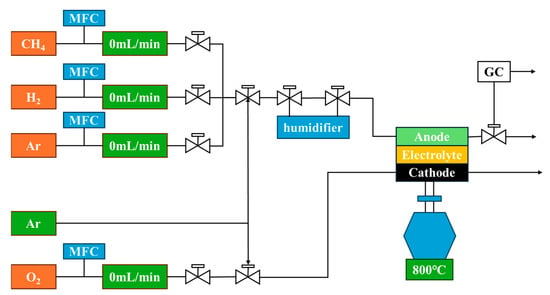
Figure 2.
Schematic diagram of SOFC performance testing system.
For cell assembly, a single cell was sealed onto an alumina tube using glass rings and a ceramic sealant. Platinum mesh (1 × 1 cm) was employed as the current collector on both the anode and cathode sides to ensure good electrical contact. The mesh was carefully placed over the central region of the SOFC to cover the active reaction area fully. The cell was heated at a rate of 5 °C/min to 800 °C and held for 1 h to reach thermal equilibrium. The electrochemical performance was tested under both humidified hydrogen (~3 vol% H2O; flow rate: 50 mL/min) and dry methane atmospheres. Ambient air was supplied to the cathode. Current–voltage (I-V) curves and power density outputs were recorded using a programmable direct current (DC) electronic load. The open-circuit voltage (OCV) was monitored throughout the heating process and gas switching.
Dry methane (CH4, 99.999%) was directly introduced at three concentrations (3.85%, 5.6%, and 10.6%) to investigate the anode’s carbon deposition tolerance, with the total gas flow rate fixed at 50 mL/min. These concentrations were achieved by adjusting the dilution ratio with inert gas to simulate different hydrocarbon fuel conditions. The anode tail gas was analyzed using an online gas chromatograph (Agilent GC 7890A, Santa Clara, CA, USA). Calibration was performed using two certified reference gases, and the total measurement error was controlled within ±2%. The carbon deposition rate was calculated using the carbon balance method, while the water production was estimated based on hydrogen and oxygen consumption.
3. Results and Discussion
3.1. Material Characterizations
XRD analysis was conducted to investigate the phase composition and crystallinity of the electrolyte and anode support layers, as shown in Figure 3. The diffraction patterns of the electrolyte (Sample A) and the anode support (Sample B) are compared alongside the standard reference patterns for NiO (PDF#04-011-2340), Bi2O3 (PDF#97-017-3834), and ScSZ (PDF#00-051-1604 and PDF#97-009-2133).
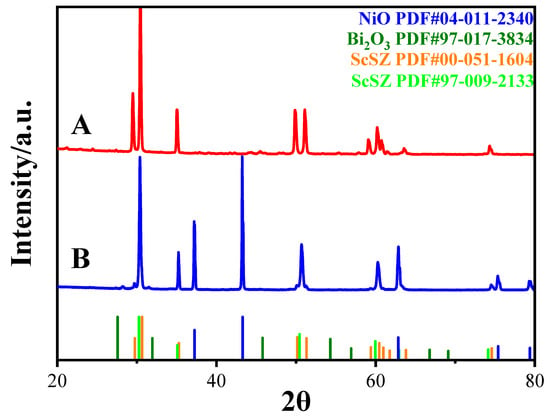
Figure 3.
XRD patterns of ScSZ (A) and NiO-ScSZ (B).
Sample A primarily exhibited an ScSZ structure, as evidenced by major diffraction peaks located at approximately 30.2°, 35.1°, 50.4°, and 59.9°, which corresponded to the standard reflections of cubic ScSZ (PDF#04-008-1973). Additionally, a slight peak shift and minor broadening compared with the ideal cubic positions indicated a rhombohedral distortion, which is consistent with the structural model of ScSZ described in PDF#00-051-1604. This indicates that the sample retained a highly symmetric structure, intermediate between cubic and rhombohedral phases, characteristic of ScSZ with a high scandia content. Although no distinct diffraction peaks corresponding to δ-Bi2O3 were observed, a slight intensity perturbation near 27.5° indicated the possible presence of a minor amount of a segregated Bi2O3 phase. It can be inferred that while most of the Bi2O3 was incorporated into the ScSZ lattice, a small fraction may have existed as isolated nanocrystalline or amorphous domains below the detection limit. The sharpness and symmetry of the major peaks, combined with a low background intensity, confirm the excellent crystallinity. Full-width at half-maximum (FWHM) analysis of the (111) peak at ~30.2° yielded an estimated crystallite size of approximately 45–55 nm, indicating high crystallinity.
Sample B showed dominant crystalline phases identified as NiO and ScSZ. The diffraction peaks at approximately 37.2°, 43.3°, and 62.8° corresponded to the 101, 012, and 110/104 reflections from the NiO phase, while additional peaks near 30.2°, 35.1°, and 50.4° matched those of cubic ScSZ. The possibility of minor rhombohedral distortion, similar to that observed in the electrolyte, cannot be completely excluded. No distinct Bi2O3-related peaks were detected in Sample B, although the potential existence of an amorphous Bi2O3 domain remains plausible. FWHM analysis of the major NiO peak at ~43.3° indicated an estimated crystallite size of approximately 40–50 nm. The clear and sharp diffraction peaks, coupled with a low and stable background, further demonstrated the high crystallinity and low defect density of the anode support.
The rhombohedral distortion in the anode layer appeared less pronounced compared with the electrolyte layer, as no evident peak shift or broadening beyond the cubic ScSZ references was observed. This may be attributed to the presence of NiO and the different local chemical environment in the anode layer, which may have inhibited the degree of rhombohedral distortion in the ScSZ phase.
While the XRD results confirm high crystallinity and phase purity, microstructural features such as porosity, grain connectivity, and interfacial adhesion cannot be inferred from XRD and are evaluated using SEM analysis.
ScSZ samples with 0, 1, 3, and 5 wt% Bi2O3 were prepared and sintered at high temperatures to obtain dense structures to investigate the phase evolution induced by Bi2O3 doping. The X-ray diffraction (Cu-Kα; 2θ = 20–80°) results are shown in Figure 4. All samples exhibited major reflections at 2θ ≈ 29.6°, 30.6°, 35.2°, 50.1°, and 51.3°, corresponding to the hexagonal phase reflections of ScSZ (PDF#97-008-6704), including the 006, 152, 154, 158, and 740 planes.
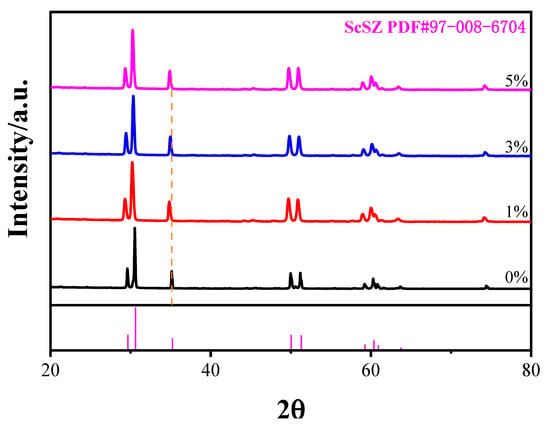
Figure 4.
XRD patterns of ScSZ samples with varying Bi2O3 doping contents (0 wt%, 1 wt%, 3 wt%, and 5 wt%).
No tetragonal (t-ZrO2; 2θ ≈ 30.3°) or cubic (δ-Bi2O3; 2θ ≈ 27.9°) peaks were observed, indicating that up to 5 wt% Bi2O3 was fully incorporated into the ScSZ lattice without forming detectable secondary phases. This suggests that Bi3+ ions were successfully doped into the ScSZ crystal structure, stabilized by the cooperative substitution of Sc3+ and Bi3+, which suppressed phase separation.
As shown by comparison with the orange dashed line, a slight peak shift toward lower angles and a broader full-width at half-maximum (FWHM) was observed with an increasing Bi2O3 content, implying lattice expansion due to Bi3+ substitution. The expansion was attributed to the significantly larger ionic radius of Bi3+ (1.17 Å) compared with Zr4+ (0.84 Å), leading to local strain and crystal swelling.
Two major effects may arise from the observed lattice expansion:
- (1)
- Enhanced ionic transport, as enlarged oxygen pathways may facilitate oxygen ion migration and thereby improve conductivity;
- (2)
- Compromised structural stability since excessive expansion could induce internal stress and increase the risk of microcracking during thermal cycling.
3.2. SOFC Microstructure
The influence of the Bi2O3 doping content on the sintering behavior of ScSZ electrolytes was examined using cross-sectional SEM images (Figure 5). These images illustrate the microstructural evolution of samples sintered at 1300 °C for 4 h under varying doping conditions.
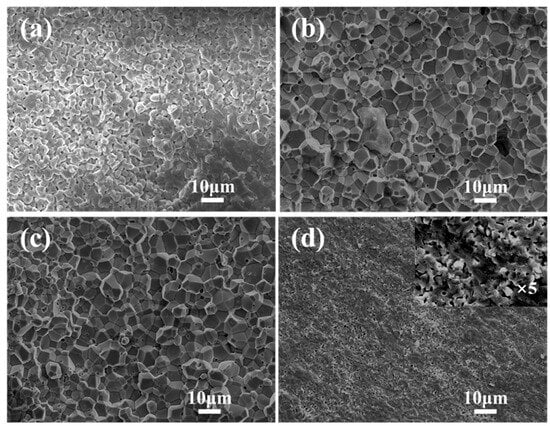
Figure 5.
SEM images of ScSZ electrolyte sections sintered at 1300 °C for 4 h under different Bi2O3 doping levels. (a) Undoped ScSZ; (b) ScSZ with 1 wt% Bi2O3; (c) ScSZ with 3 wt% Bi2O3; and (d) ScSZ with 5 wt% Bi2O3.
The undoped sample (Figure 5a) exhibited insufficient densification, evidenced by a high density of interconnected pores across the cross-section. Nonetheless, distinct grain boundaries were still visible. Upon the addition of 1 wt% Bi2O3 (Figure 5b), the grain boundaries became more well-defined, and the overall porosity was reduced, although a small number of isolated pores remained.
At a doping level of 3 wt% Bi2O3 (Figure 5c), the sample exhibited a well-balanced microstructure characterized by homogeneous grain growth and significantly reduced porosity, with no large pores detected. This suggests that 3 wt% Bi2O3 facilitated optimal densification and enhanced the electrolyte’s structural integrity.
In contrast, when the Bi2O3 content was increased to 5 wt% (Figure 5d), abnormal grain growth and heterogeneous particle distribution became apparent. The high-magnification image (×5000) reveals many residual pores dispersed throughout the cross-section, indicating that excessive Bi2O3 hindered effective densification.
This deterioration was likely due to the low melting point of Bi2O3, which, when present in excess, may lead to uneven liquid-phase formation and the entrapment of volatile species during sintering. The presence of Bi-rich glassy phases along grain boundaries, as reported in a previous study [26], supports the interpretation that Bi2O3 can promote liquid-phase sintering. However, excessive Bi2O3 may result in microstructural instability, residual porosity, or pore clustering, ultimately compromising densification and uniformity.
EDS mapping and spot analysis confirmed the presence of Bi in all doped electrolyte samples. However, the Bi signals were marked with asterisks (*), indicating that the detected concentrations were close to the instrument’s detection limit and subject to quantification uncertainty. While these results verify the presence of Bi, the exact chemical state and influence of residual Bi-containing species on ionic conductivity remain to be clarified through further investigation.
Figure 6 presents cross-sectional SEM images of the single cell incorporating 3 wt% Bi2O3-doped ScSZ electrolyte. In the annotated Figure 6a, the layered structure of the anode-supported SOFC is clearly distinguished, including the porous cathode, dense electrolyte, and porous anode support. The interfaces between layers appear well bonded, with no visible delamination or interfacial defects, indicating good structural integration.
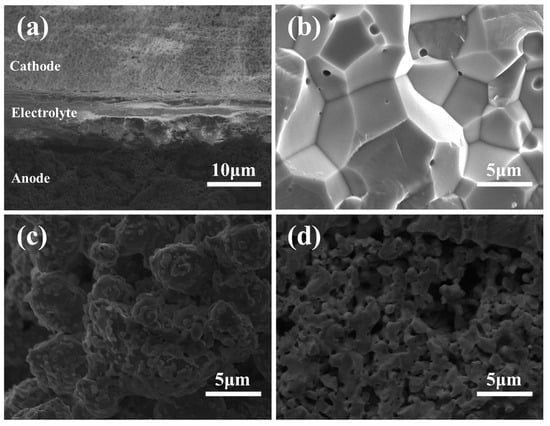
Figure 6.
Cross-sectional SEM images of an SOFC with 3 wt% Bi2O3-doped ScSZ electrolyte: (a) single cell; (b) dense electrolyte; (c) anode; and (d) cathode.
Figure 6b presents a high-magnification SEM image of the electrolyte layer doped with 3 wt% Bi2O3. The microstructure exhibits high densification, with well-defined grain boundaries and the absence of interconnected pores. These features confirm the enhanced sintering behavior promoted by Bi2O3 addition. Such a dense and structurally uniform layer is critical for preventing gas crossover and achieving a high open-circuit voltage (OCV) in SOFC operation.
Figure 6c presents the porous microstructure of the anode layer doped with Bi2O3. A well-developed network of interconnected pores and aggregated particles is observed, indicating that the introduction of Bi2O3 did not disrupt the original anode architecture. This preserved porosity facilitated gas diffusion and provided a high surface area, which was favorable for the formation of triple-phase boundaries (TPBs) during fuel cell operation.
Figure 6d depicts the cathode layer, which was fabricated without Bi2O3 doping. The cathode maintained a loose, highly porous microstructure with small, uniformly distributed grains. This morphology offered a sufficient surface area and gas pathways to facilitate the oxygen reduction reaction (ORR), demonstrating that the undoped cathode retained excellent catalytic performance. These results confirm that selective doping of Bi2O3 into the electrolyte layer improves sintering and densification without compromising the porous structure and functionality of the electrodes.
The porosity was quantified using the Archimedes method to provide further insights into the anode microstructure. Unlike previous studies, in which pre-sintered NiO–ScSZ anodes without Bi2O3 doping tended to fracture due to insufficient mechanical integrity, the present sample doped with 3 wt% Bi2O3 remained intact, enabling porosity assessment at the pre-sintering stage. This improvement highlights the role of Bi2O3 in enhancing low-temperature sintering and mechanical robustness. The pre-sintered NiO-ScSZ support exhibited a porosity of approximately 58.0%, indicating a well-connected porous structure. Subsequent sintering at 1300 °C resulted in a reduced porosity of 44.7%, suggesting significant densification. Upon reduction at 800 °C in hydrogen for 2 h, the porosity increased to approximately 50.0%, attributed to the volumetric shrinkage during the NiO-to-Ni transformation. These results are consistent with the SEM observations in Figure 6c and confirm the effectiveness of the adopted fabrication strategy. The preservation and partial recovery of porosity upon reduction is beneficial for fuel diffusion and TPB formation, which are both critical for high electrochemical performance.
3.3. SOFC Electrochemical Performance
Figure 7 presents the current–voltage (I–V) and current density–power density (I–P) characteristics of the fabricated anode-supported SOFC at various operating temperatures under a humidified hydrogen (~3 vol% H2O) atmosphere. Before electrochemical measurements, the sealing integrity of the single cell was assessed using a soap bubble flow meter. The gas flow rates at the anode and cathode outlets were nearly identical, confirming excellent sealing performance and electrolyte gas tightness. During the operation, humidified hydrogen and air were continuously supplied to the anode and cathode sides, respectively, with the flow rates kept constant throughout the experiment, ensuring the reliability of the electrochemical data.
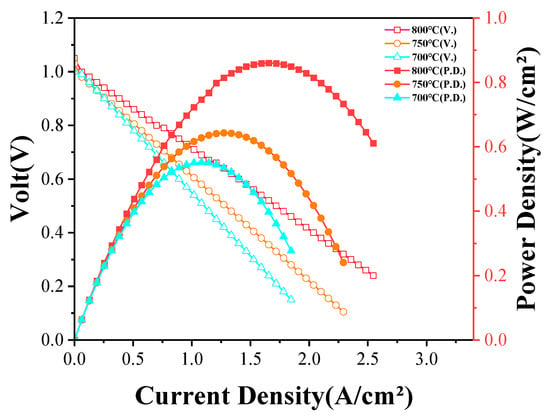
Figure 7.
Electrochemical performances of SOFC powered by hydrogen at different temperatures.
The measured OCVs were approximately 1.05 V at 800 °C, 1.03 V at 750 °C, and 1.00 V at 700 °C, indicating the sufficient densification and impermeability of the electrolyte layer. The measured OCV at 800 °C was slightly lower than the theoretical value of ~1.10 V for a system with 3 vol% H2O. This deviation can be attributed to hydrogen dilution by water vapor. As the operating temperature increased, both the current density and peak power density significantly improved. The SOFC achieved peak power densities of 0.861 W/cm2 at 800 °C, 0.643 W/cm2 at 750 °C, and 0.552 W/cm2 at 700 °C.
The performance enhancement at elevated temperatures can be mainly attributed to the increased ionic conductivity of the YSZ electrolyte, reduced ohmic resistance, lower activation overpotential at the electrode interfaces, and accelerated electrochemical reaction kinetics. The I–V profiles exhibited a typical monotonic voltage decrease with increasing current density, reflecting the combined effects of activation, ohmic, and concentration polarizations. At 700 °C, a steeper voltage drop was observed, suggesting that activation polarization was likely the dominant factor under low-temperature operation due to the limited electrocatalytic activity of the LSM cathode. In contrast, the voltage decrease at 800 °C was much more gradual, indicating improved charge transfer characteristics and enhanced electrode reaction kinetics.
This observation underscores the critical importance of maintaining a high operating temperature to minimize polarization loss and optimize cell performance.
Electrochemical impedance spectroscopy (EIS) measurements were not included in this study due to persistent technical issues during frequency sweeps. Future work will incorporate EIS analysis to further distinguish the contributions of ohmic resistance and electrode polarization.
The electrochemical performance of the SOFC under a dry methane atmosphere (CH4 concentration: 16.7 vol%) was further evaluated, as shown in Figure 8. The initial OCVs under methane were recorded at approximately 1.22 V at 800 °C, 1.20 V at 750 °C, and 1.17 at 700 °C, which were slightly higher than those obtained under a hydrogen atmosphere, consistent with the higher theoretical potential of methane oxidation. Moreover, the stability of the OCVs during the initial open-circuit operation confirmed the gas tightness of the electrolyte, even under hydrocarbon conditions.
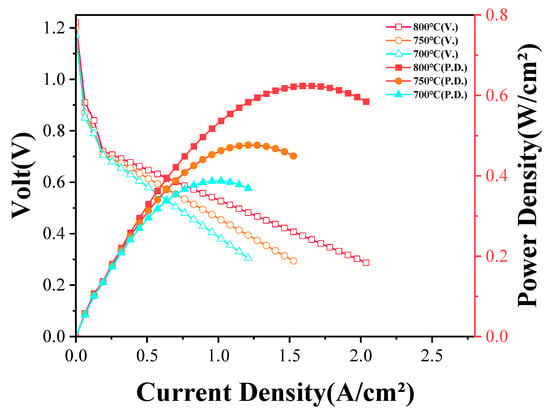
Figure 8.
Electrochemical performances of SOFC powered by methane at different temperatures.
Despite the high initial OCVs, a more pronounced voltage decline with increasing current density was observed during methane operation, particularly at lower temperatures. This behavior was primarily attributed to the sluggish electrochemical oxidation kinetics of methane compared with hydrogen, as well as the increased anode polarization resistance associated with limited fuel oxidation rates. The maximum power densities achieved under a methane atmosphere were 0.624 W/cm2 at 800 °C, 0.477 W/cm2 at 750 °C, and 0.386 W/cm2 at 700 °C, all lower than those measured under hydrogen operation. The performance degradation was likely due to the combined effects of slower methane oxidation, local fuel depletion at high current densities, and potential carbon deposition on the anode surface, which could block active sites and increase polarization losses.
Overall, the fabricated SOFC demonstrated a strong temperature-dependent performance under both humidified hydrogen and dry methane atmospheres, with a significantly enhanced output at elevated temperatures. Nevertheless, even at 800 °C, the power output under methane remained inferior to that under hydrogen, highlighting the necessity for further optimization of the anode microstructure, catalytic activity, and carbon tolerance to achieve efficient and stable direct hydrocarbon-fueled SOFC operation.
3.4. Gas Analysis
Methane undergoes thermal decomposition at elevated temperatures, including at the TPB on the anode side. The decomposition products—primarily carbon and hydrogen—can subsequently react with oxygen ions (O2−) transported from the cathode. In addition, methane may adsorb onto the surface of the anode catalyst and directly participate in electrochemical oxidation reactions with O2− ions, thereby contributing to the overall fuel oxidation process.
The gas analysis at the anode outlet revealed the presence of six major components: CH4, CO, CO2, H2, H2O, and deposited carbon. These species were involved in a network of electrochemical and thermochemical reactions that collectively defined the local reaction environment and fuel conversion behavior.
The reaction mechanisms of methane reforming and oxidation were previously detailed in [23] and are briefly summarized in Table 1.

Table 1.
Summary of electrochemical and chemical reactions at the anode.
The gas evolution behaviors of NiO-ScSZ/ScSZ/LSM anode-supported SOFCs were systematically investigated under varying methane concentrations (3.85%, 5.6%, and 10.6%) at 800 °C to assist in evaluating the anode performance, particularly its oxidation activity and carbon tolerance. The results were compared with those of a previously reported NiO–YSZ-based SOFC to clarify the effect of anode composition on electrochemical reaction initiation, methane-reforming behavior, and carbon deposition suppression.
In the NiO-YSZ/YSZ/LSM system under 3.85% methane, gas chromatographic analysis showed that at low current densities (<0.192 A·cm−2), the anode exhaust predominantly contained CH4, CO, and H2, with no detectable CO2 or H2O. This indicates that methane decomposition was the dominant process in this regime, with negligible electrochemical oxidation. As the current density increased to approximately 0.192 A·cm−2, H2O appeared, signaling the onset of hydrogen electrochemical oxidation. CO2 formation was only observed above 0.256 A·cm−2, reflecting the delayed initiation of CO and carbon oxidation. This late activation of oxidation pathways implies insufficient early oxygen ion transport, which can lead to the accumulation of solid carbon and subsequent anode degradation under direct hydrocarbon operation [23,27,28].
In contrast, the Bi2O3-doped NiO-ScSZ/ScSZ/LSM cell operating under 3.85% methane exhibited significantly improved oxidation behavior, as shown in Figure 9. CO2 formation initiated at approximately 0.128 A·cm−2, accompanied by earlier H2O generation and quicker CH4 consumption with increasing current density. This earlier activation of oxidation reactions suggests that the improved oxide ion conductivity of the ScSZ electrolyte, together with the optimized anode microstructure, enhanced the oxygen ion flux to the anode, thus enabling the prompt oxidation of reforming intermediates even under mild operating conditions. The ability to rapidly initiate CO and carbon oxidation is critical for minimizing the risk of carbon deposition, ensuring stable anode operation during hydrocarbon fuel utilization.
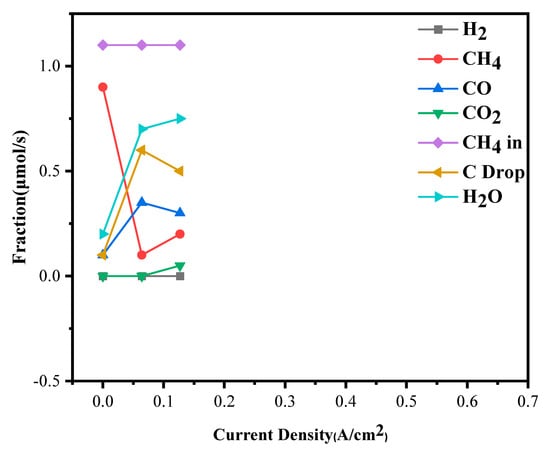
Figure 9.
Anode exhaust gas compositions under 3.85% dry methane.
At a 5.6% methane concentration, as shown in Figure 10, the gas evolution behavior exhibited noticeable changes. The actual onset of CO2 formation was delayed to approximately 0.256 A·cm−2. Although minor CO2 signals were detected at lower current densities, these were considered instrumental artifacts. The elevated methane partial pressure increased the concentrations of H2 and CO in the anode environment, intensifying the local reducing atmosphere and thereby suppressing the early electrochemical oxidation of intermediate species. Consequently, a higher external current load was required to supply sufficient oxygen ions to overcome the local chemical environment and drive CO and carbon oxidation reactions. Nevertheless, even under these conditions, the NiO-ScSZ/ScSZ/LSM cell maintained stable CH4 consumption and showed gradual CO2 and H2O generation as the current increased, confirming the robust anode reaction kinetics.
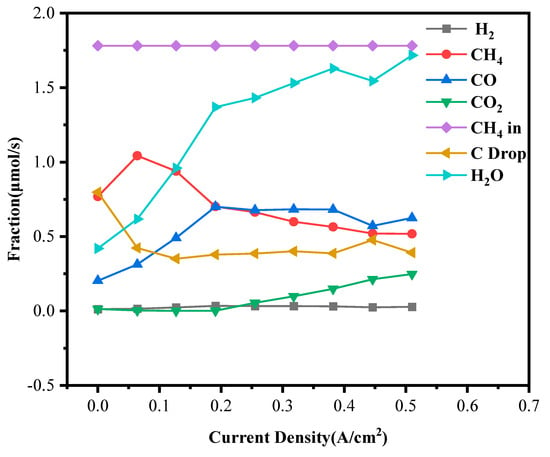
Figure 10.
Anode exhaust gas compositions under 5.6% dry methane.
At a 10.6% methane concentration (Figure 11), the suppression of early oxidation reactions became even more pronounced. The onset of substantial CO2 production shifted further to approximately 0.445 A·cm−2. Under such high methane partial pressure, the strongly reducing environment significantly inhibited the early stages of oxygen-ion-involved oxidation reactions. Despite these harsher conditions, the Bi2O3-doped NiO-ScSZ/ScSZ/LSM cell exhibited steady CH4 conversion and continued the generation of CO and H2, indicating effective reforming activity. Moreover, the emergence of H2O at relatively low current densities suggested that hydrogen oxidation could proceed even before the full activation of CO or carbon oxidation pathways, reflecting the high electrochemical activity supported by the enhanced oxide ion transport properties of ScSZ.
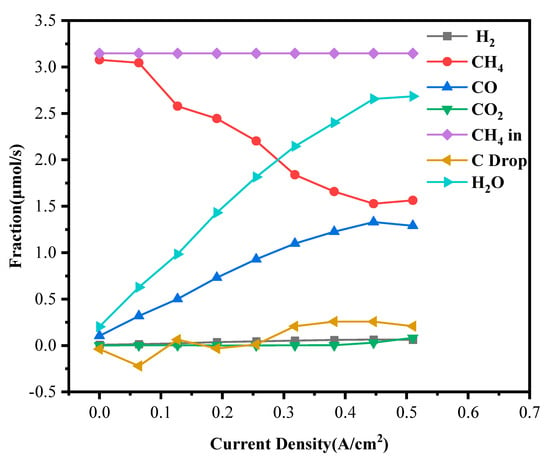
Figure 11.
Anode exhaust gas composition under 10.6% dry methane.
Overall, the gas analysis results demonstrate that the Bi2O3-doped NiO–ScSZ/ScSZ/LSM anode, combined with the ScSZ electrolyte, effectively promoted earlier and more stable electrochemical oxidation of methane-derived species compared with the conventional NiO-YSZ anode structure. Even as the methane concentration increased and the local anode environment became increasingly reducing, the ScSZ-based system retained strong oxidation activity and maintained fuel conversion without signs of serious carbon accumulation. These characteristics confirm the superior stability and carbon tolerance of the developed anode, supporting its excellent performance after low-temperature co-sintering. The gas evolution behavior under dry methane atmospheres thus provides important auxiliary evidence for the enhanced anode microstructural and electrochemical properties achieved in this work.
4. Conclusions
In this study, a novel anode-supported SOFC configuration employing Bi2O3-doped NiO-ScSZ as the anode and ScSZ as the electrolyte was successfully fabricated via a low-temperature co-sintering process at 1300 °C. The introduction of 3 wt% Bi2O3 effectively enhanced the sintering ability of both the anode and electrolyte layers, enabling the formation of dense and gas-tight ScSZ electrolytes and mechanically robust porous anode supports without compromising structural integrity.
Comprehensive material characterizations confirmed that the electrolyte had a highly dense microstructure with well-preserved cubic/rhombohedral ScSZ phases, while the anode support maintained a favorable porous framework suitable for gas transport and electrochemical activity. The optimized microstructure contributed to excellent electrochemical performance, achieving peak power densities of 0.861 W cm−2 at 800 °C under hydrogen operation and maintaining open-circuit voltages close to the theoretical values, indicating superior electrolyte densification and gas-sealing quality.
Under dry methane operation, the cells demonstrated stable output with maximum power densities reaching 0.624 W cm−2 at 800 °C. Although the performance under methane was lower than that under hydrogen, the cell exhibited good tolerance to carbon deposition, as further evidenced by systematic gas analysis. The Bi2O3-doped NiO-ScSZ/ScSZ/LSM cell promoted earlier and more stable electrochemical oxidation of methane-derived species compared with conventional NiO-YSZ systems, even under increasingly reducing environments at higher methane concentrations. This behavior reflects the enhanced oxygen ion transport capability and improved anode structural stability enabled by Bi2O3 addition.
Overall, the results highlight that Bi2O3 co-doping combined with optimized low-temperature co-sintering is an effective strategy to achieve high-performance anode-supported SOFCs with ScSZ electrolytes. The proposed fabrication approach reduces the sintering temperature and processing cost while also improving cell performance and stability, providing valuable insights into the development of cost-effective and durable intermediate-temperature SOFC technologies.
Author Contributions
S.P.: Data curation, formal analysis, investigation, and writing—original draft. Z.L., P.X., T.F. and J.W.: Investigation. G.G. and A.A.: Conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, grant number 22K04732. The APC was funded by Hirosaki University.
Data Availability Statement
The data will be made available upon request.
Acknowledgments
The authors acknowledge the Shared Facility Center for Science and Technology, Hirosaki University (SFCST), for SEM and XRD analyses. Liu Zhao, Pairuzha Xiaokaiti, Fang Tiancheng, Wang Jiwei and Guan Guoqing gratefully acknowledge the scholarship from the Kyoritsu International Foundation of Japan.
Conflicts of Interest
Author Pairuzha Xiaokaiti was employed by the company Frontier Laboratories Ltd. The remaining authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Nomenclature and Abbreviations
| SOFC | Solid oxide fuel cell |
| ScSZ | Scandia-stabilized zirconia |
| YSZ | Yttria-stabilized zirconia |
| PEFC | Polymer electrolyte fuel cell |
| MCFC | Molten carbonate fuel cell |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| PVB | Polyvinyl butyral |
| OCV | Open-circuit voltage |
| TPB | Triple-phase boundary |
| DC | Direct current |
| MFCs | Mass flow controllers |
| GC | Gas chromatograph |
| EIS | Electrochemical impedance spectroscopy |
References
- Singh, M.; Zappa, D.; Comini, E. Solid oxide fuel cell: Decade of progress, future perspectives and challenges. Int. J. Hydrogen Energy 2021, 46, 27643–27674. [Google Scholar] [CrossRef]
- Corigliano, O.; Pagnotta, L.; Fragiacomo, P. On the technology of solid oxide fuel cell (sofc) energy systems for stationary power generation: A review. Sustainability 2022, 14, 15276. [Google Scholar] [CrossRef]
- Tu, B.; Qi, H.; Yin, Y.; Zhang, T.; Liu, D. Effects of methane processing strategy on fuel composition, electrical and thermal efficiency of solid oxide fuel cell. Int. J. Hydrogen Energy 2021, 46, 26537–26549. [Google Scholar] [CrossRef]
- Quach, T.-Q.; Giap, V.-T.; Lee, D.K.; Pineda, I.T.; Ahn, K.Y. Parametric study of a high-performance ammonia-fed SOFC standalone system. J. Mech. Sci. Technol. 2022, 36, 3193–3201. [Google Scholar] [CrossRef]
- Fu, Q.; Freundt, P.; Bomhard, J.; Hauler, F. SOFC Stacks Operating under Direct Internal Steam Reforming of Methane. Fuel Cells 2017, 17, 151–156. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, Z.; Xia, L.; He, Q.; Li, Z. A comprehensive review of solid oxide fuel cells operating on various promising alternative fuels. Energy Convers. Manag. 2022, 253, 115175. [Google Scholar] [CrossRef]
- Golkhatmi, S.Z.; Asghar, M.I.; Lund, P.D. A review on solid oxide fuel cell durability: Latest progress, mechanisms, and study tools. Renew. Sustain. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Dokmaingam, P.; Irvine, J.T.S.; Assabumrungrat, S.; Charojrochkul, S.; Laosiripojana, N. Modeling of IT-SOFC with indirect internal reforming operation fueled by methane: Effect of oxygen adding as autothermal reforming. Int. J. Hydrogen Energy 2010, 35, 13271–13279. [Google Scholar] [CrossRef]
- Arifin, N.A.; Afifi, A.A.; Samreen, A.; Hafriz, R.S.R.M.; Muchtar, A. Characteristic and challenges of scandia stabilized zirconia as solid oxide fuel cell material—In depth review. Solid State Ion. 2023, 399, 116302. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, S.P.; Wang, W.; Zhang, Y. NiO/YSZ, anode-supported, thin-electrolyte, solid oxide fuel cells fabricated by gel casting. J. Power Sources 2007, 170, 55–60. [Google Scholar] [CrossRef]
- Hussain, S.; Li, Y. Review of solid oxide fuel cell materials: Cathode, anode, and electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Vinchhi, P.; Khandla, M.; Chaudhary, K.; Pati, R. Recent advances on electrolyte materials for SOFC: A review. Inorg. Chem. Commun. 2023, 152, 110724. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Tian, Y.; Sun, J.; Huang, P. Preparation of Bi2O3–YSZ and YSB–YSZ composite powders by a microemulsion method and their performance as electrolytes in a solid oxide fuel cell. Materials 2023, 16, 4673. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Z.; Tian, H.; Li, J. Effect of bismuth oxide on the microstructure and electrical conductivity of yttria stabilized zirconia. Sensors 2016, 16, 369. [Google Scholar] [CrossRef] [PubMed]
- Curi, M.; Silva, E.; Furtado, J.; Ferraz, H.; Secchi, A. Anodes for SOFC: Review of material selection, interface and electrochemical phenomena. Quím. Nova 2020, 44, 86–97. [Google Scholar] [CrossRef]
- Koh, J.-H.; Yoo, Y.-S.; Park, J.-W.; Lim, H.C. Carbon deposition and cell performance of Ni-YSZ anode support sofc with methane fuel. Solid State Ion. 2002, 149, 157–166. [Google Scholar] [CrossRef]
- Yusupandi, F.; Ilham, M.; Yafi, I.A.; Widiatmoko, P.; Nurdin, I. Improvement of an anode-supported intermediate temperature solid oxide fuel cell with spray-coated calcia-stabilized zirconia electrolytes. Int. J. Technol. 2024, 15, 1971. [Google Scholar] [CrossRef]
- Sun, D.; Lu, Y.; Karaki, T. Review of the applications of 3D printing technology in the field of piezoelectric ceramics. Resour. Chem. Mater. 2023, 2, 128–142. [Google Scholar] [CrossRef]
- Hirano, M.; Oda, T.; Ukai, K.; Mizutani, Y. Effect of Bi2O3 additives in Sc stabilized zirconia electrolyte on a stability of crystal phase and electrolyte properties. Solid State Ion. 2003, 158, 215–223. [Google Scholar] [CrossRef]
- Abarzua, G.; Udayabhaskar, R.; Mangalaraj, R.V.; Durango-Petro, J.; Usuba, J. A feasible strategy for tailoring stable spray-coated electrolyte layer in micro-tubular solid oxide fuel cells. Int. J. Appl. Ceram. Technol. 2022, 19, 1389–1396. [Google Scholar] [CrossRef]
- Sumi, H.; Puengjinda, P.; Muroyama, H.; Matsui, T.; Eguchi, K. Effects of crystal Structure of yttria- and scandia-stabilized zirconia in nickel-based SOFC anodes on carbon deposition and oxidation behavior. J. Power Sources 2011, 196, 6048–6054. [Google Scholar] [CrossRef]
- Vafaeenezhad, S.; Hanifi, A.R.; Laguna-Bercero, M.A.; Etsell, T.H.; Sarkar, P. Microstructure and long-term stability of Ni–YSZ anode supported fuel cells: A review. Mater. Futures 2022, 1, 042101. [Google Scholar] [CrossRef]
- Peng, S.; Liu, Z.; Xiaokaiti, P.; Fang, T.; Wang, J.; Guan, G.; Abudula, A. Low-cost fabrication of high-performance anode-supported sofcs with anti-carbon deposition capability. Resour. Chem. Mater. 2025, 4, 100117. [Google Scholar] [CrossRef]
- Chourashiya, M.G.; Jadhav, L.D. Synthesis and characterization of 10%Gd doped ceria (GDC) deposited on NiO-GDC anode-grade-ceramic substrate as half cell for IT-SOFC. Int. J. Hydrogen Energy 2011, 36, 14984–14995. [Google Scholar] [CrossRef]
- Berges, C.; Wain, A.; Andújar, R.; Naranjo, J.A.; Gallego, A. Fused filament fabrication for anode supported SOFC development: Towards advanced, scalable and cost-competitive energetic systems. Int. J. Hydrogen Energy 2021, 46, 26174–26184. [Google Scholar] [CrossRef]
- Mori, M.; Liu, Y.; Ma, S.; Hashimoto, S.; Yasumoto, K. Effects of Bi Addition on Sintering and Electrical Properties of Scandia Stabilized Zirocnia as Intermediate-Temperature SOFC Electrolyte. Electrochemistry 2009, 77, 184–189. [Google Scholar] [CrossRef]
- You, H.; Abudula, A.; Ding, X.; Zhou, Y. Reactions of low and middle concentration dry methane over Ni/YSZ anode of solid oxide fuel cell. J. Power Sources 2007, 165, 722–727. [Google Scholar] [CrossRef]
- You, H.; Gao, H.; Chen, G.; Abudula, A.; Ding, X. The conversion among reactions at Ni-based anodes in solid oxide fuel cells with low concentrations of dry methane. J. Power Sources 2011, 196, 2779–2784. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).