1. Introduction
The deoxygenation of water is a vital process in the oil and gas industry [
1]. The presence of oxygen in water can cause several problems in the oil industry, including the corrosion of equipment and pipes, which can be costly and potentially dangerous. To reduce or control these problems, deoxygenation techniques are used to remove or reduce the oxygen content of water used in drilling operations [
2].
Membrane deoxygenation is a valuable method for removing dissolved oxygen from water used in various industrial applications [
3].
Polyargansiloxanes are a type of silicone-based polymer known as siloxane. The properties of polyarganosiloxanes can be affected by attaching different organic groups to silicon atoms. Some common organic groups include methyl, phenyl, and fluorine-containing alkyl groups. These changes in chemical structure vary the properties of polyarganosiloxanes such as flexibility, heat stability, and chemical resistance [
4].
Many industries use silicon in their fields due to its unique properties. It is used in coatings, oil and gas separation processes, adhesives, lubricants, and many other products because of its resistance to heat, cold, moisture, and chemicals. For example, silicone polymers are used in coatings at low ratios to help substrates wet easily, resulting in a smooth, uniform appearance after drying [
5]. In oil and gas separation processes, siloxane compounds are employed in membranes and filters to improve the efficiency of separating hydrocarbons from water; it is also used to remove oxygen from water in petroleum processes [
6]. In the adhesives and sealants industry, silicon’s flexibility and chemical resistance make it ideal for applications that require long-lasting bonds, such as in construction materials [
7].
The most common methods to prepare polyarganosiloxanes are condensation polymerization and addition polymerization. The properties of the synthesized siloxane can be affected by the choice of synthesis method [
8]. The main type of polymer in the group of polyorganosiloxanes is polydimethylsiloxane (PDMS). PDMS is composed of repeating dimethylsiloxane units, as illustrated in
Figure 1 [
9].
In PDMS, each silicon atom is bonded to two oxygen atoms and two methyl groups (CH3), resulting in a flexible and relatively inert polymer chain. The methyl groups in PDMS have low polarity and contribute to a low surface tension, meaning that PDMS tends not to wet surfaces easily. This property makes PDMS suitable for applications where low adhesion to surfaces is desired, such as in mold release agents or coatings for water-repellent surfaces. Another property of PDMS is that it is permeable to gasses due to its flexible polymeric chains and the large space between methyl groups; this high permeability to gasses makes PDMS a valuable membrane in applications such as oil–gas separation [
9].
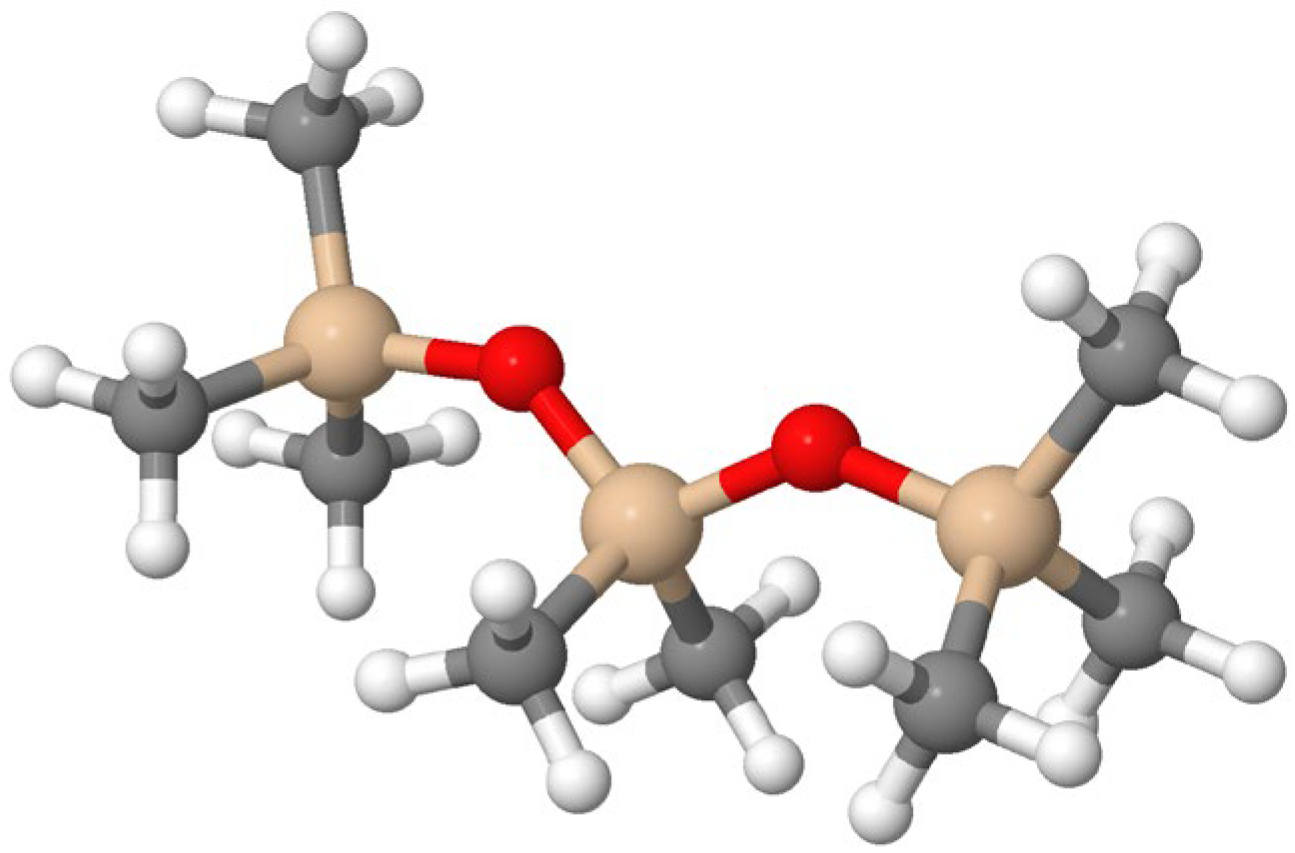
The methyl group in PDMS can be replaced by other functional groups to synthesize polymers for a wide range of other exciting applications; for example, fluorine-containing alkyl groups as shown in
Figure 2 lead to a high resistance to hydrocarbon solubility while maintaining low surface tension. This property is important for its application in gas–oil separation and is of direct importance for applications in the oil industry [
9,
10].
Many fundamental questions related the relationship between the backbone structure and the functional groups attached to the backbones can be addressed using molecular simulations, with carefully chosen interaction potentials and target boundary conditions with correct sampling procedures.
The beneficial properties of PDMS motivated us to study and better understand the surface behavior of siloxane, aiming to enhance oxygen removal from water in petroleum industry applications to reduce corrosion.
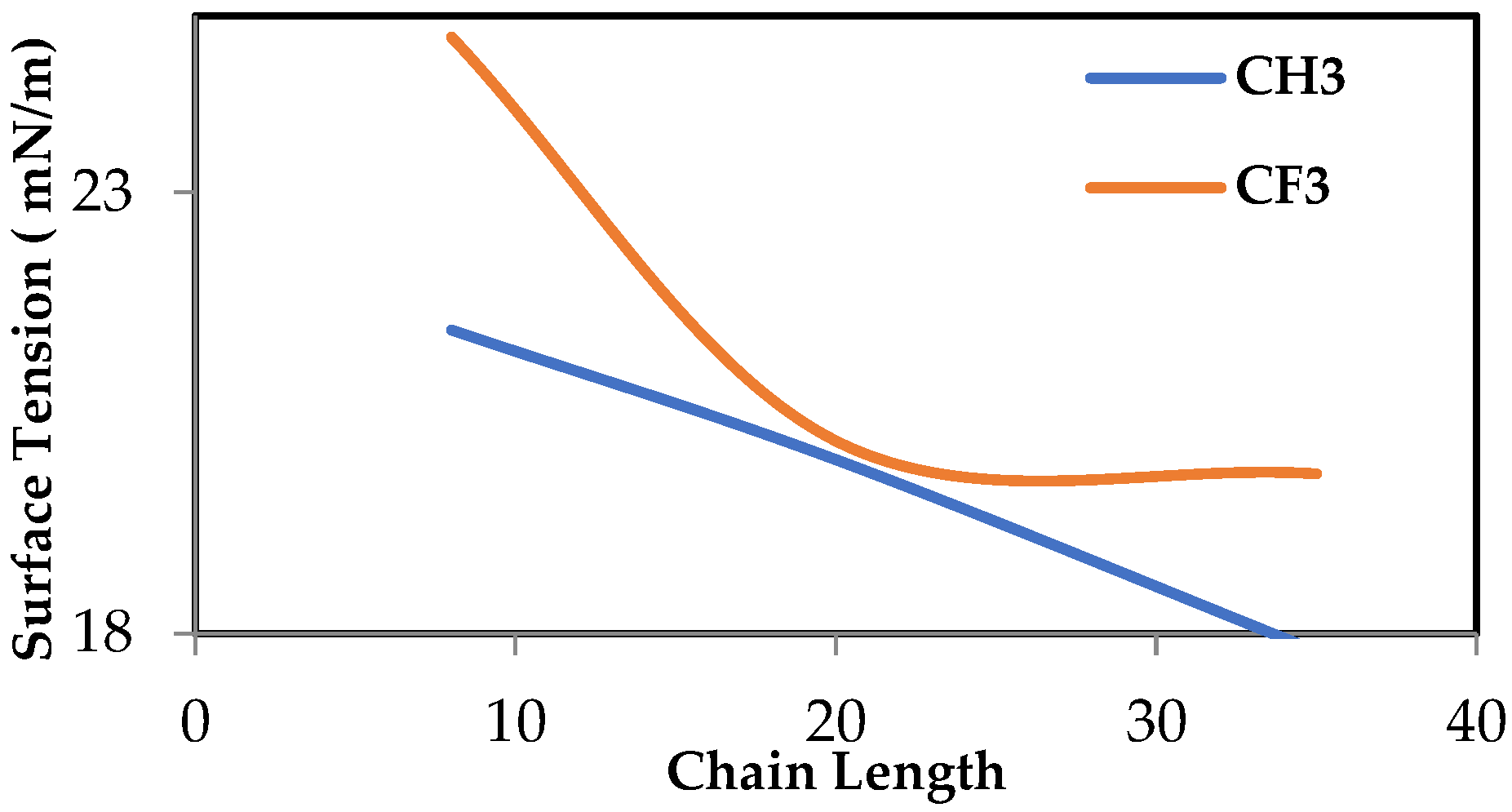
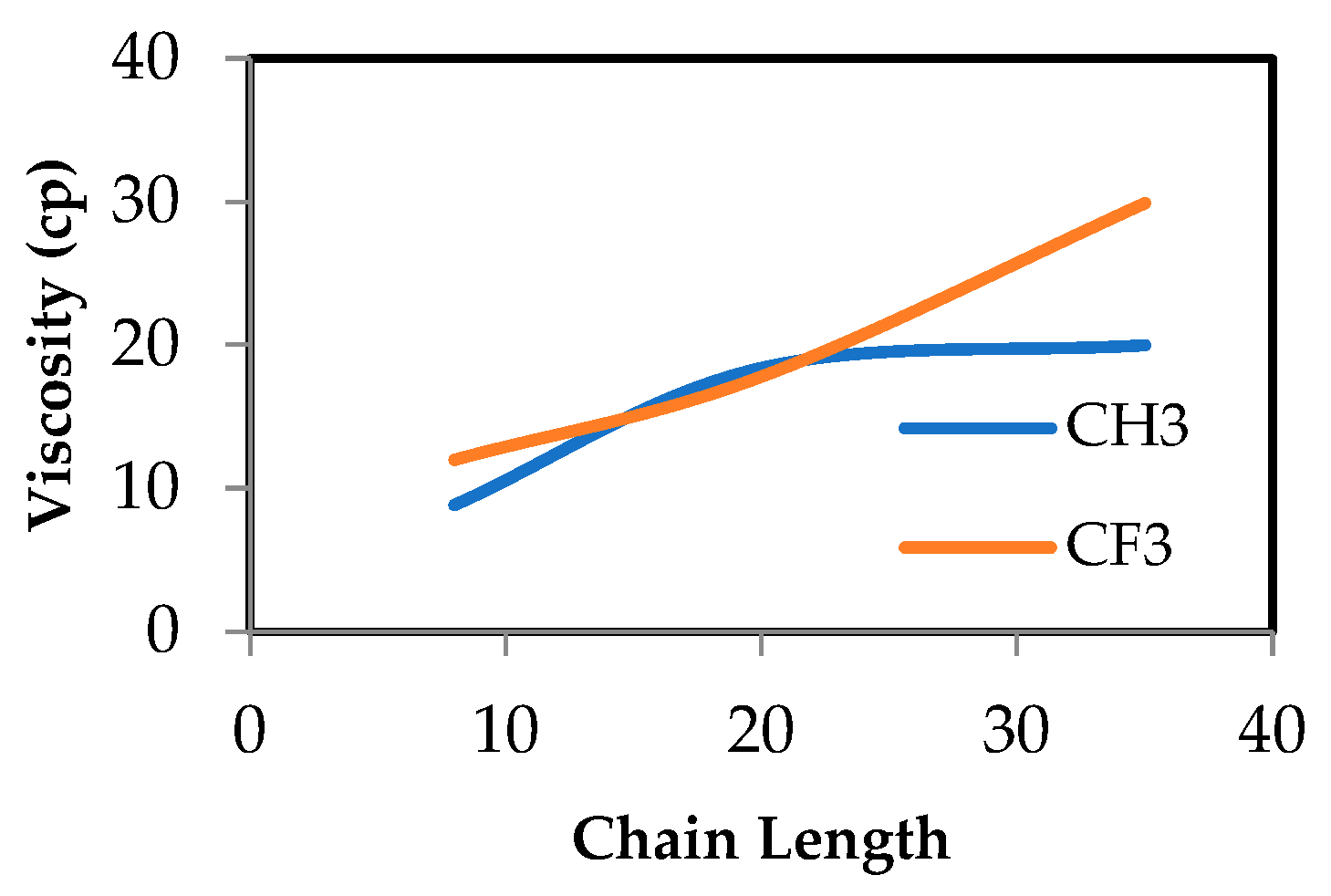
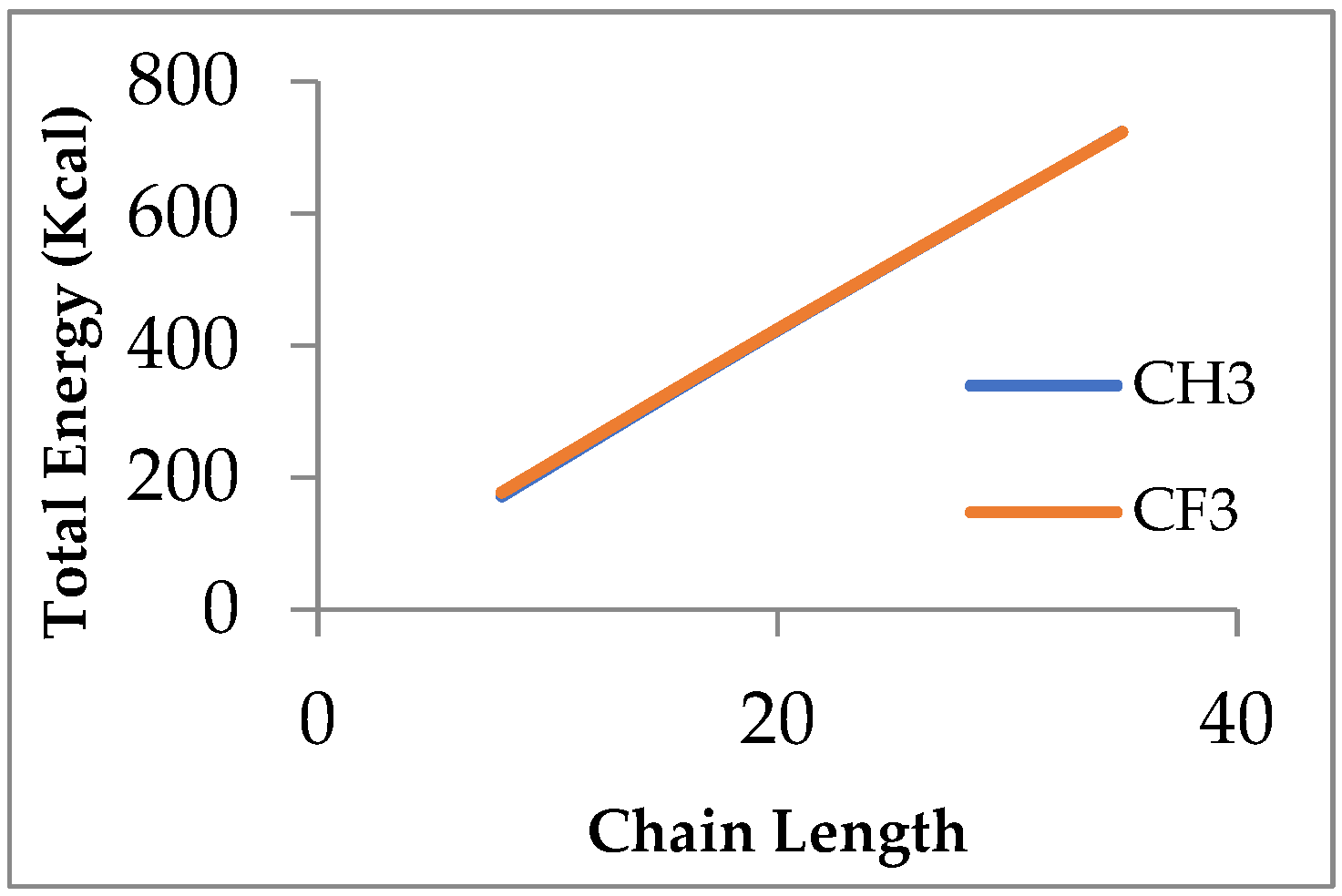
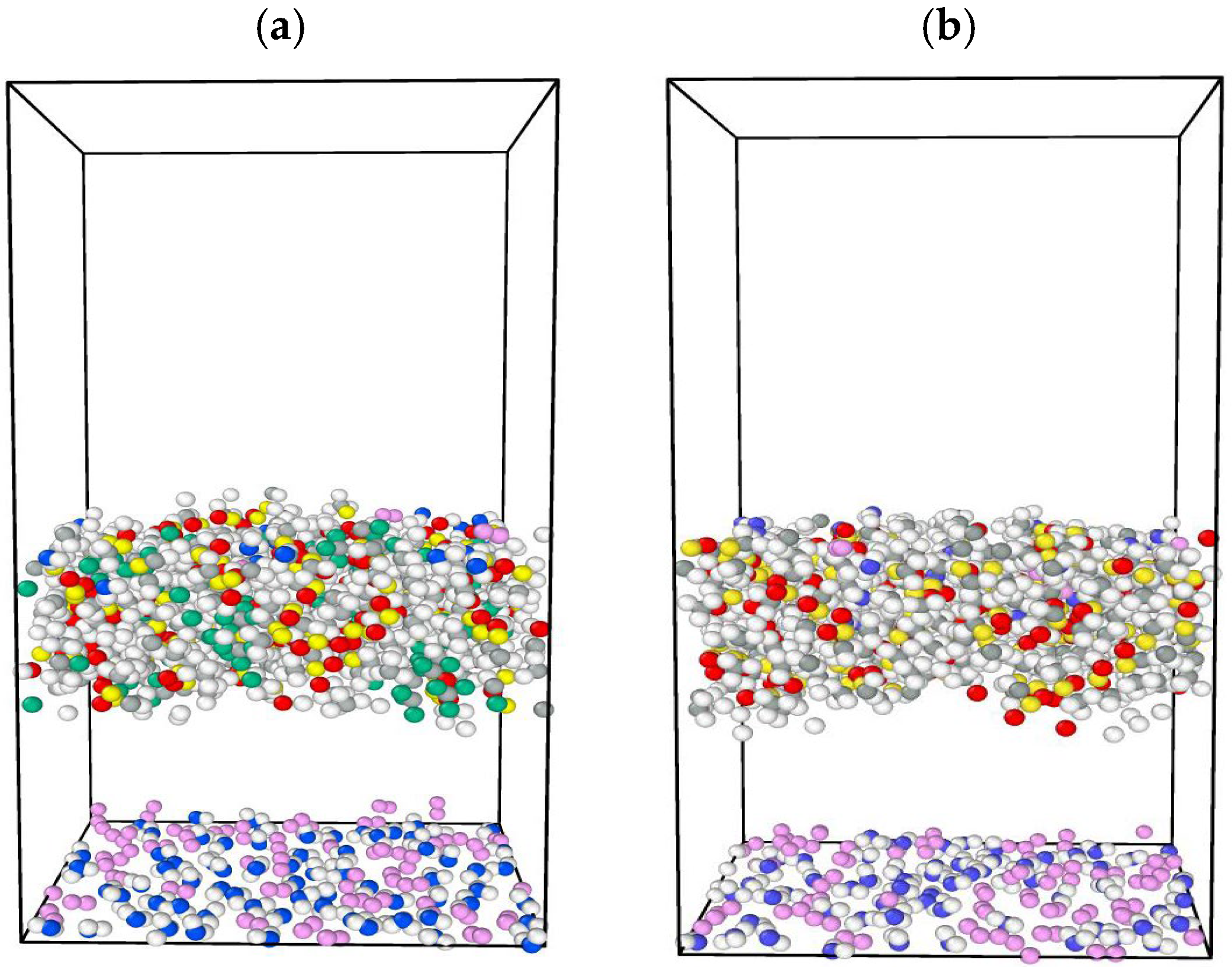
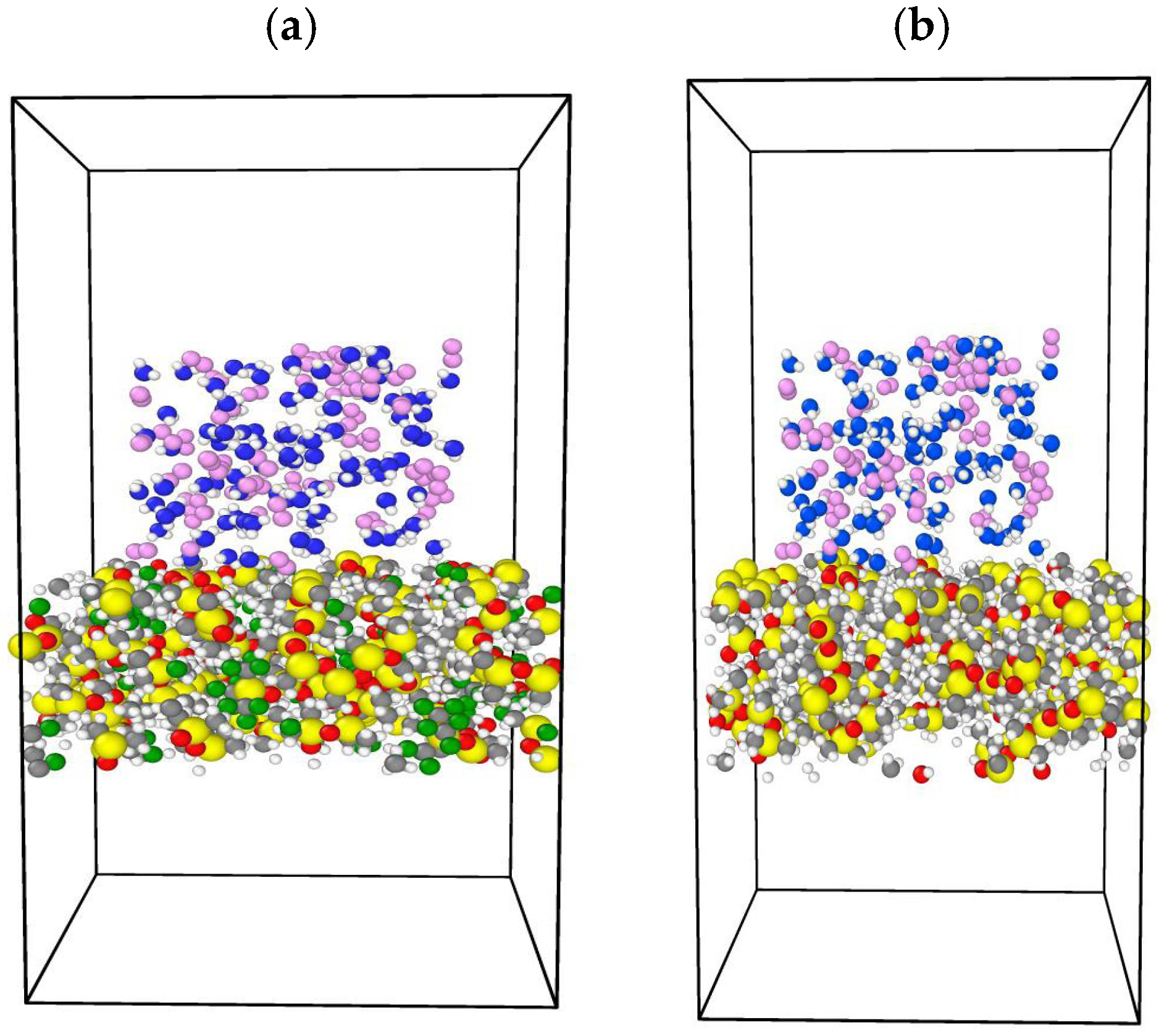
In this study, MD simulations by LAMMPS were performed to evaluate the surface properties of siloxane with two different functional groups attached to the end of the chain, which are the methyl group and fluorinated alkyl, represented by trifluoromethyl. Three types of PDMS chain lengths were chosen, namely (8, 20, 35) monomers per chain. By modifying the chain length, the surface tension, wettability, and adhesion characteristics of PDMS can be adjusted to meet particular requirements. For example, the accurate surface tension estimation of oil and gas systems is important in gas well stimulation where minimizing surface tension is required to minimize capillary forces that trap the aqueous phase in the formation [
11]. Surface tension, viscosity, and total energy were examined for both cases. Subsequently, we replicated the 20 monomer per chain for both end functional groups and used this to evaluate the efficiency of the deoxygenation of water.
4. Conclusions
In this paper, the effect of functional groups and chain length on the properties and performance of siloxane-based membranes in separation processes was investigated. Our study revealed that CF3 functional groups, due to their high electronegativity and polarity, slightly increase the surface tension compared to CH3 groups. However, the overall surface tension remains low, making these membranes suitable for gas–oil separation applications. As well as this, we concluded that increasing the chain length reduces the surface tension of both functional groups; understanding this behavior is important for optimizing the chain length to enhance membrane performance.
In terms of studying the effect of chain length on the total energy, we concluded that the increasing chain length in both functional groups will increase the total energy within the siloxane structure, while replacing the CH3 functional group with the CF3 functional group has a relatively small effect on the stability of the total energy. This result suggests that siloxanes containing CF3 and CH3 functional groups can provide proportionate energy profiles, which is an advantage for industrial applications that require stable membrane performance under variable conditions.
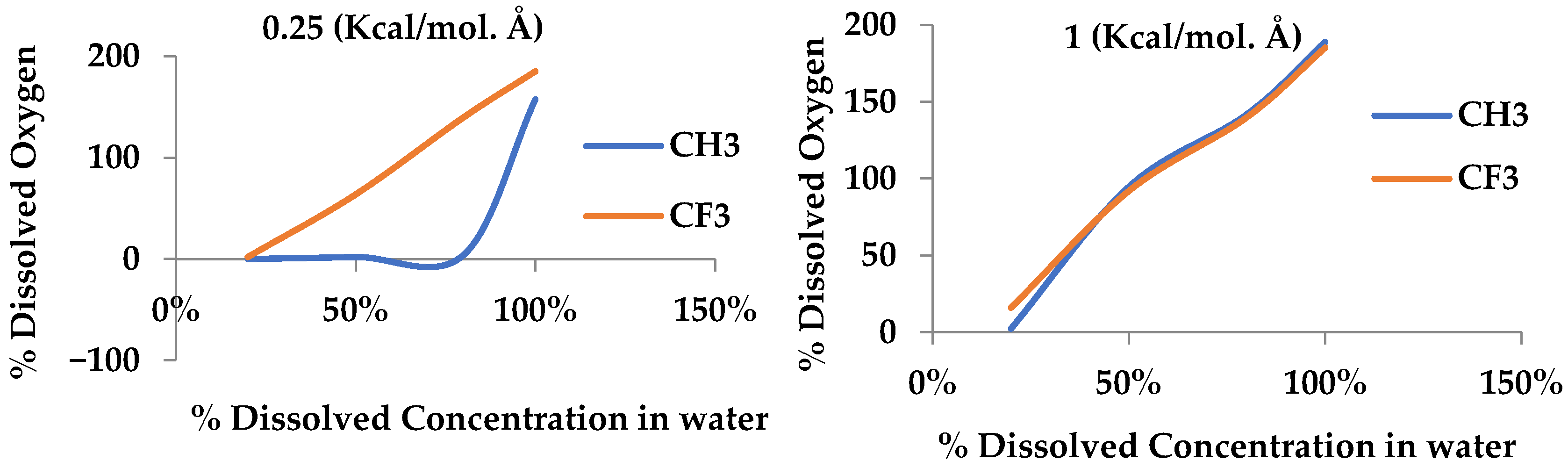
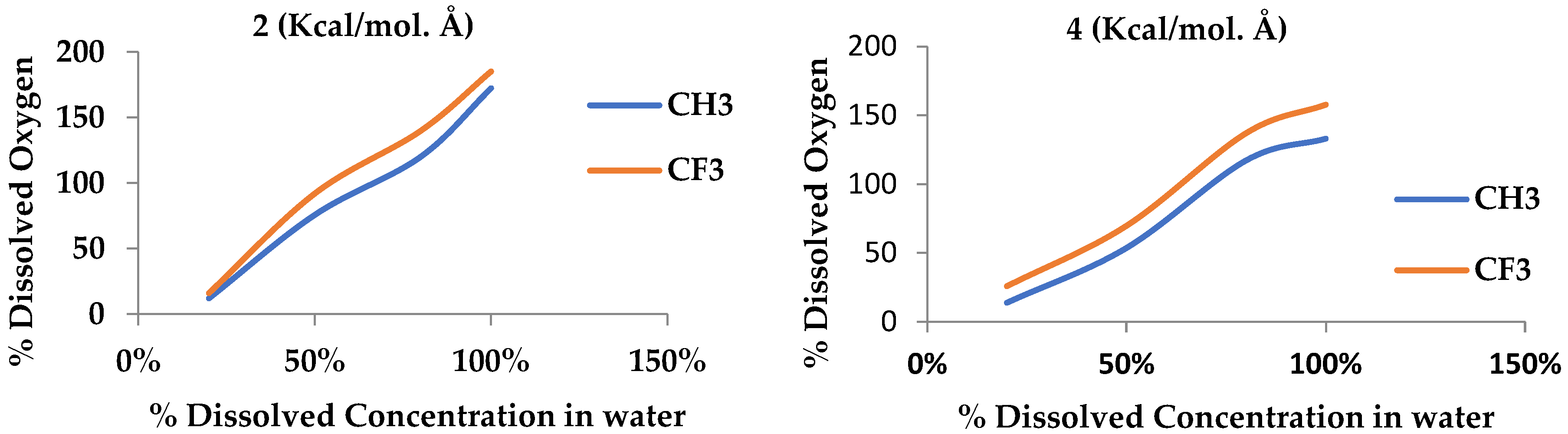
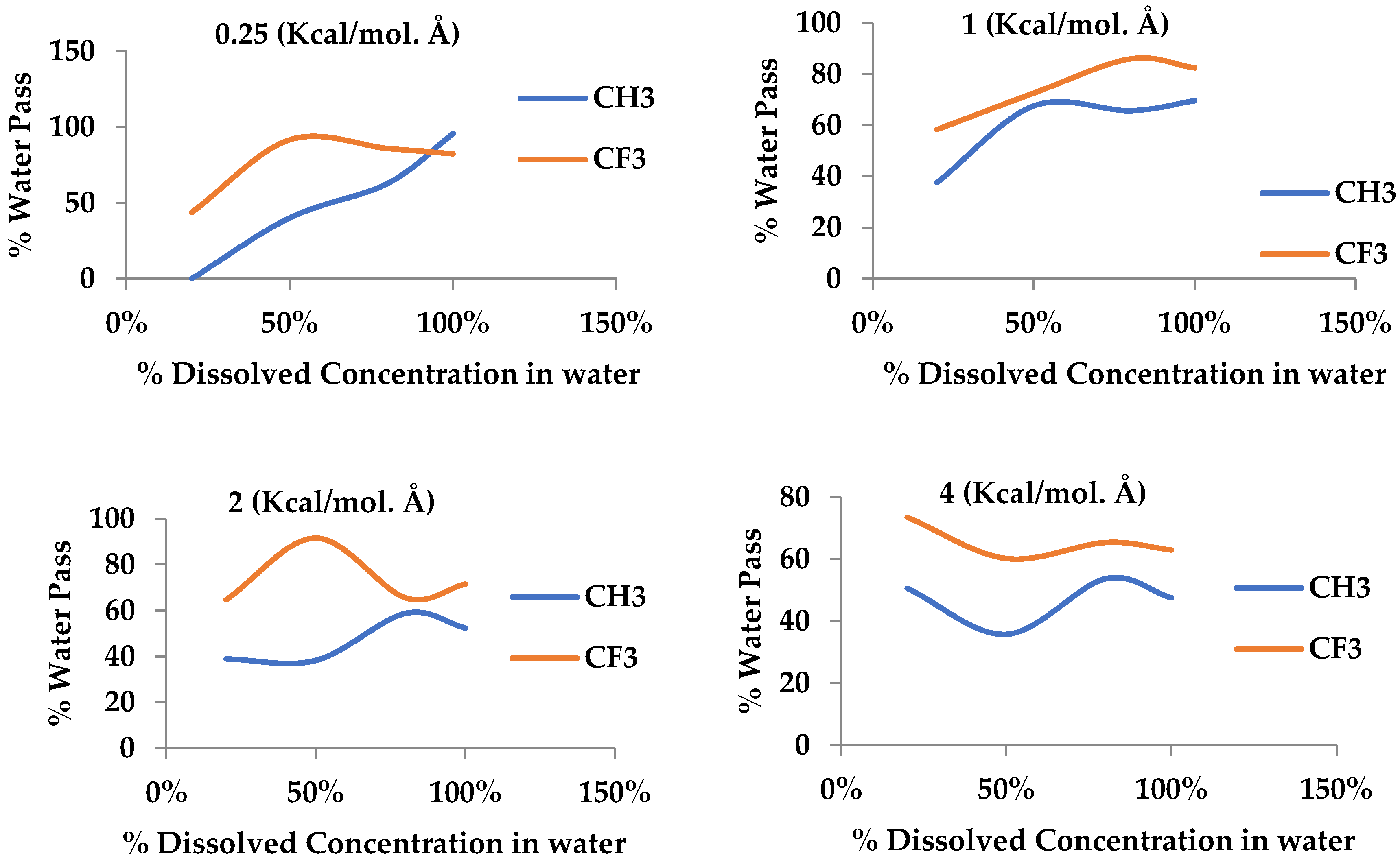
Additionally, we concluded that the efficiency of removing dissolved oxygen from water for membranes with the CF3 functional group is higher than for the CH3 functional group due to the stronger dipole–dipole interactions between CF3 groups and oxygen molecules. Despite this, a higher percentage of water is allowed to pass through. Conversely, membranes with CH3 functional groups are less efficient in removing dissolved oxygen than membranes with CF3 functional groups but with less water permeability, making them more suitable for applications where water retention is required.
These results emphasize the importance of tailoring membrane design to the specific needs of the application. For processes that prioritize maximum oxygen removal, membranes containing the CF3 group are more effective. Conversely, in cases where minimizing water passage is essential, membranes containing the CH3 group offer a more balanced solution. This precise understanding of the interaction between functional groups and membrane performance provides valuable insights for optimizing siloxane membranes in various industrial applications, including gas–oil separation and water treatment.