Abstract
Microplastic contamination in terrestrial environments has risen significantly, far exceeding levels in marine environments. This shift underscores the concerning prevalence of microplastics (MPs) in sewage sludge and soil, raising environmental apprehensions. Microplastics from various sources accumulate in sewage systems, consequently, sewage sludge and soil have transformed into primary reservoirs of microplastic pollutants, capable of infiltrating aquatic ecosystems. While using sludge to enrich soil provides nutrients, it simultaneously introduces substantial microplastic content, posing environmental hazards. These microplastics can accumulate in the soil, altering its properties and potentially polluting deeper soil layers and groundwater, compounding environmental risks. This review scrutinizes the abundance, types, and shapes of microplastics in sewage sludge and soil, evaluating their impacts and suggesting future research directions. Statistical analysis reveals higher microplastic concentrations in sludge (271 Particles/kg dry weight) than in soil (34.6 Particles/kg). Strong correlations between microplastic concentrations in soil and sludge (R2 = 0.95) underscore the significant influence of sludge application on soil ecosystems. The p-value of 0.0001 indicates a significant correlation between MP amounts in soil and sludge, while the p-value of 0.47 suggests no significant association between MP concentrations in wastewater and sludge. Research confirms that microplastics influence sludge properties, microbial communities, and soil characteristics, contingent on microplastic attributes and soil conditions. Predominantly, microplastic shapes found in sludge and soil are fibers and fragments, often linked to agricultural fertilizer use. Microplastics detrimentally affect soil bulk density and aggregate stability, impairing soil structure and surface. Furthermore, their presence alters pollutant transport behavior in soil, emphasizing the imperative to investigate microplastics’ effects and transport mechanisms for mitigating environmental and health risks.
1. Introduction
The annual production of plastics has been steadily rising and now stands at over 330 million tons, making it considered an emerging contaminant [1,2]. When plastic debris is released into the environment, it can break down into various sizes and forms, including microplastic (MPs) particles in the 1–5000 µm size range [3]. Due to their hydrophobicity and high molecular weight, these materials are highly non-biodegradable and accumulate in various environments [4]. MPs are divided into two main types: primary MPs and secondary MPs. Primary MPs are purposefully produced in tiny sizes for use in various industries, including sandblasting, textiles, paints, adhesives, detergents, and cosmetic products in the form of pellets [5,6,7]. These primary MPs have entered the environment through wastewater discharge and floods. It has been established that 35% of the major primary MPs in water matrices originate from washing clothes [6]. In contrast, secondary MPs are produced when plastic waste disintegrates in an environment due to exposure to weathering, solar UV radiation, and tidal waves [8].
Previous investigations on MPs in wastewater treatment plants (WWTPs) found that wastewater treatment technologies do not completely remove MPs from wastewater [9]. Wastewater treatment plants have been identified as a significant source and sink of MPs [10]. Around 90% of MPs may be trapped in sewage sludge during wastewater treatment [11]. Treatment using mechanical, chemical, and biological methods removed almost 99% of the MPs entering a WWTP [12]. After treatment, the removed MPs were mostly transferred to the sludge phase [13]. Reusing sewage sludge for land application is common in various nations [14]. Due to the extensive utilization of sewage sludge in agriculture, directly or indirectly, it can significantly increase the entrance of MP into croplands [15].
Numerous fibrous and fragmented MPs have been detected in soils worldwide [16,17,18,19]. For instance, fragmented MPs were widespread in the soil of agricultural areas, where the application of sewage sludge promotes the accumulation of MPs in agricultural soils [20]. MPs pose a chronic threat to soil animals, microbes, and plants [21]. They are passed into the terrestrial environment and can be present for many years [22]. Once MPs accumulate in the soil, plants can uptake them and transfer them along the food chain [23]. Nevertheless, the origin and potential pathways of MPs getting into the soil are diverse, including the application of sewage sludge and compost for various purposes, fertilization, irrigation, plastic mulching, littering, and atmospheric deposition [24].
Numerous review articles have examined methods for investigating MPs in water and soil [25,26,27]. However, a thorough examination of MP pollution in sludge-based fertilizers used in agriculture is lacking in the research. To understand the distribution, properties, and impacts of MPs in wastewater sludges and soils and support environmental management, a comprehensive examination of the subject is required. Buoyant plastic polymers sink when larger particles are covered in calcareous microorganisms, a phenomenon known as biofouling [28]. Biofouling impacts the buoyancy and hydrophobicity of plastic [29]. It is still unclear how low-density MP is transported and deposited to reach the sediments. Therefore, it is essential to provide a critical overview of the features of MPs in soil. Moreover, it is necessary to clarify the interlinkage and source pathways discovered in soil and other environmental matrices. Accordingly, the objectives of this paper are to provide a comprehensive review of the quantity, polymer size, and morphology of MPs in the sludge and soils; to analyze the efficiency of applied techniques for the removal of MPs from the soil; and finally, to describe the impact of biofouling on MPs.
2. Microplastics in Wastewater
The recognition of relevant available literature under the objectives of this study is carried out through a comprehensive examination of several studies conducted until 2023. The literature search was carried out in an online scientific database called ‘Scopus’, using the keywords “microplastics”, “sludge”, and “soil”. The MP concentration and characteristics are presented in Table 1, Tables S1 and S2. Various studies across different nations, including China [30,31,32,33], Finland [34], Italy [35], Spain [36,37,38], and the United Kingdom [39] have investigated the presence of MPs in WWTPs. These studies reveal significant differences in MP concentrations between influent and effluent streams, ranging from 1.2 to 2102.1 Particles/L in influent and from 2 × 10−3 to 13,200 × 10−3 Particles/L in effluent. It has been noted that most studies on MPs in WWTPs, both municipal and industrial WWTP examinations, have been conducted in China and Europe.
For instance, Tadsuwan et al. measured MPs in the influent and effluent of the WWTP with an average of 77 Particles/L and 2.33 Particles/L [40], whereas Yahyanezhad et al. measured MPs with an average of 206 Particles/L and 94 Particles/L [41]. Several factors might be responsible for a significant variation in the effluent quality, such as the type of treatments used in these investigations. Additionally, the removal of MPs from a variety of wastewater sources, including urban wastewater (UW), urban wastewater that contains a significant amount of industrial wastewater (mixed wastewater (MW)), and industrial wastewater (IW), has been examined. The concentration of MPs in WWTPs from various sources varies greatly. For example, in the study by Wang et al., the concentration of MPs in urban wastewater was 26 Particles/L, which is higher than the concentration of MPs in industrial wastewater, 12 Particles/L [42]. It is worth noting that the population serviced, the type of wastewater sources (municipal or industrial), the local economy, and lifestyles are just a few of the numerous factors that may be responsible for the variations in the abundance of MPs [30]. The sampling and detection techniques had an impact on the number of MPs as well [43]. Small wastewater sample amounts raised the ambiguity surrounding the amounts of MPs and the accuracy of the analysis [44]. Therefore, conducting studies on the fate of MPs in WWTPs has become more difficult due to these issues.
Studies have identified microplastics (MPs) across a wide range of sizes, indicating a variety of potential sources, including runoff and direct waste disposal through sewage systems [42]. According to several studies, the concentrations of MPs in sewage sludge are typically lower than those in influent and effluent streams [45,46]. MP concentration in the released effluent is reduced because of treatment procedures used during WW treatment, including sedimentation, filtering, and biological degradation, which seek to separate and remove solid particles [34,46]. However, a study by Magni et al. indicates that the concentration of MPs in sludge may be higher than that of the effluent, this may be because MPs are buoyant and lightweight, which means they frequently stay suspended in water or are filtered out of wastewater rather than settling in sludge. Furthermore, some might not stick to the organic material in the sludge and remain in the aqueous phase [35].

Table 1.
Microplastic concentrations in influent and effluents of wastewater treatment plants.
Table 1.
Microplastic concentrations in influent and effluents of wastewater treatment plants.
| Location | WW Type | MPs in Influent (Particles/L) | MPs in Effluent (Particles/L) | MP Size (µm) | MP Polymer Type | MPs in Sludge (Particle/kg) | SS Treatment Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| China | UW | 8.72 | 6908 | 500–5000 | PE, PET, PP, PAN | N/A | Dewatering | [33] |
| China | UW & IW | 6.55 | 0. 59 | 43–5000 | PE, PP, PS, PP-PE | 7.0 | N/A | [30] |
| China | UW | 12.03 | 0. 59 | 0–5000 | PA, PE, PES, PET, PP, PI, PVC, PAC, PU, others | N/A | N/A | [31] |
| China | UW | 1.75 | 2190.4 | N/A | PE, PET, PP, PAN | N/A | Dewatering | [30] |
| China | 1.2 | 893 | 500–5000 | PA, PE, PET, PVAC, PP, PS, PES, EP, EPM, CN, Acrylic | 1.0 | N/A | [45] | |
| China | MW | 44.07 | 1.97 | 0–5000 | PET, PE, PP, PA | N/A | Dewatering | [47] |
| China | UW | 16 | 8.7 | 500–3500 | PA, PET, PE, PP, PS, PC, PU, PVC | 2.9 | Dewatering | [46] |
| China | UW & IW | 18–890, 8–23 | 6–26, 6–12 | 50–400 | PE, PET, PA, PP, PS, PVC | N/A | N/A | [42] |
| China | N/A | N/A | N/A | 100–5000 | PE, PB, PP | 16.5–38.5 | Anaerobic digestion | [48] |
| Denmark | N/A | N/A | N/A | 10–25, >300 | PA, PP, PE, PU, PS, PES, Acrylic | 13 | N/A | [49] |
| Finland | UMW | 61 | 27 | 20–300 | PE, PET, PP, PMMA, PS, POM | 0.8 | Anaerobic digestion | [34] |
| France | UMW | 244 | 16130 | 20–500 | PS, PE, PP, PET, PA | 2.84 | N/A | [50] |
| Italy | UMW | 2.5 | 113 | 10–5000 | PA, PE, PTU, PP, PS, PU, others | 0.4 | N/A | [35] |
| Iran | MW | 206 | 94 | 1–5000 | N/A | 183 | N/A | [41] |
| Korea | UMW | 10.16–23.75 | 13,200 | 106–300 | N/A | 7.34–13.27 | Thickening, Anaerobic digestion, Dewatering | [51] |
| Spain | UW | 11.1 | 112,000 | 150–5000 | PE, PP | 2.8 | N/A | [38] |
| Spain | UM & IW | 264, 1567 | 39, 131 | 100–5000 | PP, PS, PE, PVC, HDPE, PEMMA | N/A | N/A | [36] |
| Spain | UMW | 236 | 165,000 | 25–5000 | PE, PP, PU, PCL, PET, PS, PMMA | 26 | Dewatering | [37] |
| Thailand | UW | 77 | 10.6 | N/A | PE, PET | N/A | N/A | [40] |
| Thailand | UM | 4–50 | 2–30 | 50–5000 | PA, PE, PET, PVAC, PP, PS, PES, EP, Acrylic, others | 42–214 | N/A | [52] |
| Turkey | 72.6 | 2934 | 250–2000 | N/A | 8.2 | N/A | [53] | |
| UK | UW | 2102.1 | 129.1 | 2–1000 | PA, PE, PET, PS, PVC, PP | 1974 | Anaerobic digestion | [39] |
3. Microplastics in Sludge
The movement of MP from the liquid phase to the solid phase or sludge in WWTPs depends on the treatment methods. There were no substantial variations in the WWTP configuration in either the wastewater or the sludge in the aforementioned situations [40]. Recycled activated sludge is a possible concern for MPs [35]. The initial concentration of MPs in untreated wastewater is one of the key factors affecting the concentration of MPs in sewage sludge, and sludge is frequently utilized in agriculture as fertilizer or as a raw material for concrete [54].
This review looked at 30 papers that discussed the size, shape, color, and type of polymers of MPs in sewage sludge and their abundance. Their relative unique characteristics are reported in Table S1 and Figure 1. The presence of MPs in sewage sludge has also been researched in ten European nations, including 15 treatment facilities: Denmark, Finland, France, Germany, Netherlands, Italy, Ireland, Spain, Norway, and Sweden [55,56,57,58,59,60,61,62,63,64,65,66]. It shows that, despite differences between the plants, WWTPs are among the major contributors to the annual release of MPs into the environment, which ranges from 10.6 to 165 Particles/L. Referring to the studies cited in Table S1, MP concentrations in sludge vary significantly depending on the country or location, ranging from 510 to 495,000 Particles/kg dry weight of sludge. Numerous elements, such as the diversity of urbanization, population density, plastic usage, seasonality, and treatment procedures at the investigated WWTP, as well as the inconsistent sampling and analytical methodologies, might be attributed to the substantial range in the results [60,67,68]. As a result, it becomes imperative to evaluate the MP concentration in wastewater and sludge annually while considering seasonal fluctuations and relating the results to the WWTP procedures. In other words, the overall destiny of MPs in the WWTP, including sludge, should be assessed. It also emphasizes the reported MP discharge rates through sludge and has been noted that sludge is responsible for the emission of up to billions of MP particles into the environment each year [69]. Given these polymers’ resistance to breakdown, it is simple to imagine how MPs would quickly accumulate in environmental compartments [70]. The properties of the MPs in soil and sludge are thoroughly reviewed in this work.
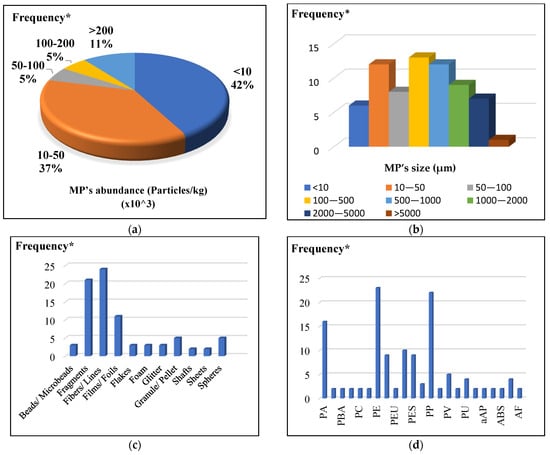
Figure 1.
MP’s abundance and characteristics in sludge: (a) abundance (Particles/kg); (b) size (µm); (c) shape; (d) composition type (* Frequency is referred to the MPs points reported by the previous papers).
3.1. Abundance of Microplastics in Sludge
The available data reveal that the pollution of MPs occurs in sludge. As a result, this review examines the MP concentration in sludge investigations. Italy and Germany had the greatest MP concentration in sewage sludge, ranging from 120 to 465 Particles/kg [59,60]. China, Australia, and Morocco came in second and third, respectively, with 40.5–74 and (0.1–36) × 103 Particles/kg of MP, respectively [71,72,73]. In Denmark, sludge MP concentration was examined by Chand et al., who discovered that the abundance was 30.2 Particles/kg [55]. According to Menéndez-Manjón et al., the MP content in the sludge in Spain ranged from 0.59 to 1.58 Particles/kg [74] and based on the Yang et al. study, the average MP concentration in China was (0.1–220) × 103 Particles/kg [68,75,76]. In Australia, MPs concentrations in sludge samples varied from 37.8 × 103 to 46.1 × 103 Particles/kg [73]. In conclusion, the range of MPs in sludge varied considerably, which makes it challenging to compare data from different sites since there is currently no standardized procedure for collecting, pretreating, separating, and describing MPs in sludge.
The population size, plastics use laws, the type of connected sanitation networks, and industrial activities can all impact the variance in MP abundance between nations. For example, sewage sludge from a WWTP in a small city with a small population (Sweden) exhibited lower MP concentrations (6.07 × 103 Particles/kg) than in a larger country with a greater population [68], as a result, a higher volume of produced wastewater (China with the abundance of 220 × 103 Particles/kg) [46]. Additionally, seasonality significantly impacts the quantity of MP in sludges. In the influents, Bayo et al. discovered that the abundance of MPs varied seasonally and was higher in warm than cold seasons, consistent with higher temperatures and rainfall [77]. Mason et al. also discovered that the amount and percentage of rainfall in the sewer system would affect the concentration of MPs in the sewage treatment system [22].
On the other hand, the amounts of MP in sludges depend on the circumstances surrounding the WWTP, such as its daily average capacity and the wastewater source. The quantity of MP in sludges varies depending on treatment capacity, servicing population, and wastewater treatment techniques [78]. Mason et al. performed statistical research on WWTPs in the United States, and the findings revealed a positive relationship between the serviced population and the total MPs in wastewater [22]. A comprehensive investigation into the behavior of MPs within sewage sludge was conducted by Jiang et al. According to these authors, there were 30.6 Particles/L of effluent and 126 Particles/L of influent, moving nearly 75% of the MPs to the sludge [76]. In their analysis, the dewatered sludge and filter cake contained 36.3 and 46.3 Particles/g of MPs, respectively. These numbers correspond to the MPs accumulated in the sludges [76].
Figure 1 demonstrates an in-depth analysis of the data gathered in Table S1 about the MP characteristics in sewage sludge. As shown in Figure 1a, most studies (80%) reported an average concentration of less than 50 × 103 Particles/kg, while 14% of all studies reported an MP concentration of greater than 100 × 103 Particles/kg, and 6% reported values between 50 × 103 and 100 × 103 Particles/kg. Most MPs taken out of the wastewater were kept in the sludge. It was discovered that there were much more MPs in the sludge than in the wastewater. According to a study by Ziajahromi et al. in Australia, MPs abundance in WWTP effluent ranged from 0.18 × 103 to 0.96 × 103 Particles/kg while it was between 37.8 × 103 and 46.1 × 103 Particles/kg in secondary sludge [73]. According to research, 20% of the MPs in the secondary sludge would flow back into the wastewater, which is worth mentioning [79]. As a result, sludge usage has drawn much interest recently. A thorough investigation is required to accurately assess the presence of MPs in sludges because sludges originate from various treatment plants, which causes complexity and diversity.
3.2. Size and Shape Distribution of Microplastics in Sludge
In previous research, the recorded size fractions of the MPs had the smallest MP size of 1.5 µm and the largest of 5000 µm. In 80% of the investigations, a MP with a size of less than 500 µm was the most prevalent [35,56]. Figure 1b illustrates the size distribution of MPs in sludges, with 58.92% of MPs 0.5 mm and a fall to 39.3% of MPs 500–5000 µm. Only 1.78% of the MPs in these sludges were bigger than 5000 µm. The findings show that the majority of microparticles matched the minimum measured proportion. Ustun et al. reported that more MPs with a size of 300–500 µm than 500–1000 µm were detected in the filter cake, sludge thickener, and return sludge, respectively [80]. Additionally, Magni et al. reported that treatment plant sludge from Italy contained up to 90% MPs, smaller than 1.0 mm [35]. Vardar et al. also discovered that the predominant size range in wastewater samples was 500–1000 µm, which was 1000–2000 µm, followed by 500–1000 µm, in sludge samples [53].
According to a study that looked at a WWTP’s effluent and sludge treatment lines, the MPs in the effluent ranged from 34.0% to 49.7% and from 29.0% to 48.5%, respectively, and the MPs in the sludge ranged from 4.0–78.0% and (2–66%) to 0.02–0.1 and 3.0–5.0 mm, respectively [76]. Similarly, Raju et al. found that MPs in WAS were greater in size than those in wastewater (>1 mm (9.04%), 250 µm–1 mm (12.65%), 125–250 µm (39.76%), 38–125 µm (21.08%), and 1.5–38 µm (9.68%) [72]. In contrast, some research has found that sludge contains a higher proportion of smaller-sized MPs than larger MPs [16]. It might be explained by the fact that smaller MPs are removed more quickly via adsorption on sludge during sedimentation [60]. According to research by Murphy et al., Hou et al., and Yang et al., larger MPs (e.g., 5000 µm) decrease along wastewater treatment units while growing in sludge samples, whereas smaller MPs continue to migrate along the WWTP [81,82,83]. The mechanical forces of large sizes of MPs reduce the formation of sludge flocs and impact sludge dewatering [84]. The complexity of MP sources in sludge causes a wide variation in particle size in different WWTPs. Therefore, it is vital to research MPs’ size and concentration in activated sludge.
Various research has recorded the morphology of MPs in sludge, commonly categorized into pellet/granule, spherule, fragment, sheet, foam, fiber/line, and film/foils and each of these kinds has unique characteristics [85,86]. To illustrate, spheres are characterized by their spherical shape and a wide variety of sizes, while granules exhibit irregular shapes and a wide size range from minuscule particles to larger fragments. In contrast, pellets are uniform in size, consistently measuring between 3 to 5 mm in diameter [87]. Additionally, fibers are characterized by their thin, elongated structure, spanning sizes from a few micrometers to several millimeters in length. In contrast, lines are thicker and more structured than fibers, typically larger in both diameter and length, ranging from a few millimeters to several centimeters [87,88]. To clarify the distinction between foils and films, it is important to note that films are generally thinner and more flexible than foils [87].
Figure 1c displays the shape distribution of MPs in sludge. In the sludge tests, fibers were the most prevalent shape (26.83%), whereas foils and beads had the lowest form (1.22%). Fragments, films, and spheres were three more shapes that appeared more frequently in the studies—25.6%, 12.2%, and 6.1%, respectively. The remaining reported shapes (28.05%) were made up of shafts, sheets, microbeads, flakes, foam, glitter, granules, lines, and glitter. From 30.5% to 78.5% of all the particles in sewage sludge were in the shape of fiber, which was also the most prevalent shape [35,56]. In Spain’s WWTP sludge samples, Hernández-Arenas et al. discovered that 89.0–97.0% of MPs were fibers [65]. Another study discovered that sludge samples from Chinese treatment plants primarily comprised fibers (43–59%) [76,89]. According to Hou et al., fibers in the wastewater treatment process could easily become entangled with flocs and be retained in sludges instead of fragments [82]. Washing processes used in homes and companies may release many fibers into the wastewater [90]. Depending on the population lifestyle in a country or region, the percentage of MP forms varies by country and city. For instance, Italian sewage sludge from the city of Sicily had the highest concentration of fragments (66–68%), while Thailand had the highest concentration of fibers (78%) [40,91].
It is important to note that the sampling season may affect the entry of various types of MPs into WWTPs [92]. For instance, Browne et al. hypothesized that as individuals wear more clothing in the winter than in summer, more microfibers would be anticipated to enter WWTPs during the winter [93]. However, variations in sample processing can impact the number of MPs [94]. As a result, various factors influence how MPs sludge sample shapes. Given that various sample techniques and mesh sizes for sieves and filters can be used, the form of collected MPs can vary significantly.
3.3. Composition Distribution of Microplastics in Sludge
Polyethylene (PE), polypropylene (PP), polyamide (PA), polystyrene (PS), polyvinyl chloride (PVC), and polyester (PET) are the most prevalent forms of polymers found in sludge [74,95]. Variation in the composition of MPs in the sludge depends on the source of wastewater; for example [96], PE is used in personal care products, shampoo bottles, and pipes [97]; PP is used in food packaging, snack wrappers, and auto parts [98]; PA is used in synthetic clothes [99]; PES is used in synthetic clothes [99]; PET is used for water and other beverage bottles [100]; and PS is used in foam food containers, eyeglasses, and building insulation [101]. According to Figure 1d, the most common MP forms in sewage sludge were PE, PP, and PA, followed by polyethylene terephthalate, polyester, and polystyrene. Polycarbonates (PC), Polyvinyl chloride (PVC), and Polyesterurethane (PEU) were infrequently found. This may be because polyethylene and polypropylene are the most widely used and produced polymers and because washing synthetic clothing releases polyamide and polyester [35,102].
3.4. Surface Morphology of Microplastics in Sludge
Regardless of the wastewater source, MP surfaces in the sludge exhibit varying degrees of deterioration [11]. The MPs in sludge are not as smooth on the surface as the original MPs and instead feature folds and aggregated structures [103]. In addition to demonstrating that MPs’ surfaces were heavily weathered and energy dispersive, Keerthika et al. suggested that the presence of inorganic components connected with the surface of MPs might be caused by environmental factors or plastic additives [104]. In a different study, Taghizadeh R.A. et al. looked at the surface morphology of MPs and showed how erosion alters their surface properties, causing them to be effectively degraded into smaller MPs and even NPs [105]. The erosion of several MP types found in the investigation is depicted in Figure 2. Physical effects, such as physical abrasion from interaction with ambient materials like sand and other MPs [106], as well as chemical and biological effects [107,108], such as photooxidation and bacterial activity, can all cause erosion on MP surfaces. MPs break down into smaller particles as a result of all these factors together [109].

Figure 2.
Microplastic surface morphology (Adopted from studies by Taghizadeh R.A. et al. [105,110]).
Additionally, a variety of morphologies were discovered in a study on MP imaging [110]. The observed morphologies on the MP surface were protrusion, indentation, shallow cracks, deep grooves, pits, breakage, and a scaly appearance. Figure 2 shows a schematic of these conditions. The biological or non-biological particles that were adherent or deposited were attributed to physical entrapment within surface cracks and electrostatic interactions. Further surface characteristics such as protrusion, indentation, shallow cracks, and deep grooves were caused by mechanical stress that was brought on by wave action, currents, or physical contact. It was observed that the MP surface had pits that were the result of localized erosion or dissolution brought on by chemical reactions with ambient factors such salts, acids, or biological substances. There have also been reports of breakage and the appearance of scaly looks, which have been attributed to physical impacts and environmental elements like UV radiation, oxidation, or biological activities, respectively [110].
Previous findings demonstrated that the kind and shape of the MPs impacted the degree and type of surface erosion [111]. It is important to note that the surfaces of the pieces have shown the most deformation, whereas fibers and beads have shown modest erosion, which suggests that they are durable in the environment and degrade slowly [110].
The photos of MP surfaces obtained through analysis in a study by Tajwar et al. [112] revealed distinctive cracks. In a wrapped state, fiber displayed relatively straighter lines, whereas film had a severely deformed margin and a rough surface with a translucent, thin, flexible strip along the edge. In contrast, smaller fragments had more broken edges, representing small pieces detached or broken from something distinct. Additionally, beads displayed morphological variance compared to other varieties of MP, displaying an overall rough surface and extremely obvious edges [112]. Therefore, their physical characteristics can influence the reactivity and behavior of plastic microparticles in the environment. In order to comprehend the behavior of MPs, it is crucial to investigate the interaction between physical properties (such as surface roughness) and the sorption behavior of various polymers.
4. Microplastics in Soil
Wastewater discharges and sewage sludge, the main by-products of waste-water treatment plants (WWTPs), have been discovered to be an important contributor to MP contamination in soil, groundwater, and surface waters [113]. MPs have been found in agricultural soils, notably some points with sludge compost utilization [20,114]; soils in urbanized areas [16]; coastal soils [19]; farmland soils [32]; landfill trash; and leachates [115]. In addition to the previously mentioned ways, MPs also reach soil ecosystems through the irrigation of wastewater and agricultural mulching layers [116]. Additional routes include dumping grounds, beach trash, and runoff from agricultural, industrial, and urban areas [64]. The alteration of the biological community is significantly influenced by the movement of MPs through the food chain as evidenced by numerous studies [117,118,119]. Moreover, MPs’ particle sizes get smaller, increasing their accumulation and transmission in food chains until they eventually reach people [120].
Most nations allow MPs to be contained in sludge that will be used to amend the soil. In Norway (82%), Ireland (63%), the US (55%), China (45%), Sweden (36%), the Netherlands (99%), Korea (55%) and Canada (47%), the sludge from WWTPs was mostly used for agricultural soil applications, woodland, and paddy-soil, as well in Finland (89%) and Scotland (40%) it was used as soil compost [121]. Sludge could be a significant source of MP pollution in farming, yet it is frequently disregarded. The amount of MPs that enter agricultural soil each year through sewage soil amendment is estimated by Nizzetto et al. to be 6.3 × 107–4.3 × 108 Particles/year in Europe and 4.4 × 107–3 × 108 Particles/year in North America, exceeding the projected global marine annual input of MPs (9.3 × 107–2.36 × 108 Particles/kg) [122]. Most MPs in soil are mostly found as micro-aggregates and are intimately associated with soil aggregates [32]. Compared to other media, where MP degradation is primarily influenced by UV radiation and physical wear, MP breakdown in the soil is more constrained and slower [123]. UV light and rising temperatures damage the MPs in the topsoil [124]. Although the photooxidation route cannot effectively break down the MPs buried in the soil, it can be inferred that MPs will build up in the soil [125]. After interacting with the soil, MPs’ migration ability is mostly influenced by their size and texture. The ability of MPs to migrate in fine-grained homogenous soils is not immediately apparent [126]. Usual agricultural production, biological properties, and scabbing will amplify MPs’ migration behavior. Additionally, MPs’ mobility will increase with low Fe/Al oxide content and high pH in the soil, which could increase their impact on the soil and groundwater at high water levels [127].
Based on the data, the research on MPs in soil is less than that of sewage sludge between 2019 and 2023 because studies on MPs in biosolids and soil are less prevalent than those on MPs in water and wastewater (Table S2). Only 14% of the publications analyzed focused on the occurrence of MPs in agricultural soils amended with biosolids. In comparison, 60% of the publications reviewed discussed the prevalence of MPs in sludge samples from WWTPs, 26% on MPs in agricultural soils with no obvious signs of biosolid application, and 26% on agricultural soils. China accounted for 36% of all studies, with Germany accounting for 8% of publications, followed by Morocco and Mauritius.
It seems that most of the studies in this area that have been published have investigated SS application to agricultural soils. According to estimates, between 2800 and 300,000 t of MPs are released each year through SS application in agricultural areas in Australia, Europe, and North America, respectively [122]. The majority of MPs eventually migrate from the soil into receiving water bodies, influencing the MPs pollution in water bodies, according to the claim that only 16–38% of MPs entering the soil through agricultural soil amendment will remain in the soil [122].
According to Corradini et al. [114], different biosolids treatment rates resulted in different MP concentrations in agricultural soils. In soils that had received one and five applications of biosolids, respectively, the median estimated mass of MPs increased, rising from 1.37 to 4.38 mg/kg, and 97% of these MPs were classified as fibers [114]. The prevalent shape of MPs in agricultural soils in north-western and south-western China was likewise discovered to be fibers, which accounted for 49% and 92% of all MPs, respectively. The need for a precise and proper dentition of MP fibers is further emphasized by Corradini et al., who noted that MP morphologies were classified at the operator’s choice. However, applying biosolids made from sewage sludge offers a significant route for transferring MPs, particularly fibers, to agricultural soils, according to robust evidence provided by Corradini et al. [114].
4.1. Abundance of Microplastics in Soils
The findings indicate that the application of sludge in the soil, which exacerbates soil MP pollution, results in a higher abundance of MPs in the soil than in the sludge and aquatic environments [24,128]. Recent research includes 19 studies investigating MPs due to the potential contribution of land-spread municipal sewage sludge as a source of MPs in soil. According to the reported data in Table S2, it is discovered that the MP abundance in sewage sludge ranges between 13 × 105 and 6.9 × 105 Particles/kg of dry soil [19,129,130]. The data only displays the mean concentrations regardless of the type, size, or morphotype.
A detailed evaluation of MP characteristics in soil provided in Table S2 is illustrated in Figure 3. According to Figure 3a, most MP concentrations in soil were less than 50 × 103 Particles/kg, with 31% of studies showing values higher than 100 × 103 Particles/kg. The following sections will further detail these factors (polymer type, morphotype, and size). The discrepancy may result from different MP testing procedures/methods and WWTP factors, such as wastewater sources [19,131]. Research revealed that applying SS to the ground could enhance the amount of MP in the soil [20,114].
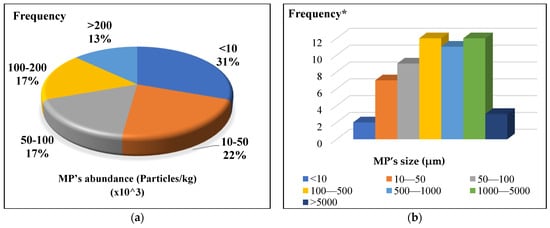
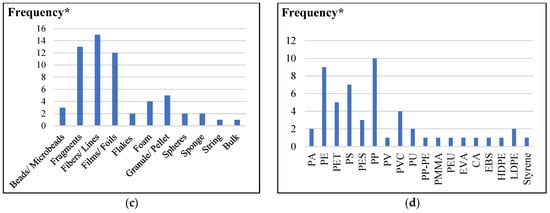
Figure 3.
MP characteristics in soil: (a) abundance (Particles/kg); (b) size (µm); (c) shape; (d) composition type (* Frequency is referred to the MP points reported by the previous papers).
Application of sludge on the agricultural fields can significantly raise the MPs level in the soil. According to Schell et al., the sludge-added agricultural plot gained roughly 16,000 MPs. It compared the MP concentration in the five years which had concentrations of 226–412 and 177–235 Particles/kg at the start and end of the experiment, respectively. Similarly, Corradini et al. [114] discovered that MPs accumulated in soils with subsequent sludge application after studying MPs in two soil layers (0–10 and 10–20 cm).
Considering MP abundances in soil for four different land-use types, such as grassland, dry land, paddy field, and greenhouse, ranging between 2693 Particles/kg and 2351 Particles/kg [132]. The abundance of MPs on dry land had the maximum quantity in the surface layer (4225 Particles/kg), whereas the abundance of MPs in paddy fields had the highest value (2675 Particles/kg). In a greenhouse, the surface and deep layers of the MP abundance showed the lowest values, 1813 Particles/kg and 1875 Particles/kg, respectively. On dry ground, however, there was a significant difference in the abundance of MPs between the two soil layers, but for soil samples taken from a greenhouse, the abundance of MPs in the surface layer was comparable to that in the deep layer. These findings demonstrate that the number of MPs in soil varied by location and based on their origin, migration attempts, and deterioration [132]. Hence, a low-intensity agricultural activity that led to a reduced abundance of MPs in the soil may be the main explanation for a smaller abundance of MPs in the soil in the sources.
Furthermore, another study found that without the addition of sewage sludge, soils typically contained 930 to 740 light-density Particles/kg and 1100 to 570 heavy-density Particles/kg [20]. There were, on average, 3060–1680 heavy-density Particles/kg and 2130–950 light-density Particles/kg in soils treated with sewage sludge. For both plastic densities, there was a statistically significant difference in MP concentration between soils that had previously received sewage sludge and those without sewage sludge application. A total average of 3.78 × 108 light-density Particles/ha and 6.74 × 108 heavy-density Particles/ha would infiltrate agricultural fields per application due to the MP concentration of sewage sludge [20]. Thus, these results emphasize that increased densities of MPs resulting from MP concentrations in sludge-amended soil may have negative environmental effects that may influence human health through different pathways such as the food chain.
According to Zhou et al. [133], MP abundance was significantly higher in woodland (9.6 × 104–6.9 × 105 Particles/kg) than in vegetable plots (4.3 × 104–6.2 × 105 Particles/kg) and vacant ground (2.2 × 104–2 × 105 Particles/kg). This outcome could be explained by factors like the various sources of pollution present at the sampling locations, deposition, accumulation due to rough terrain, reduced disturbance, vegetation trapping, soil differences, runoff effects, presence of wildlife, and historical land-use practices [133,134]. The woodlands behave as sinks for MPs carried by wind or water runoff, while dense vegetation and less intensive human activity contribute to their accumulation [133].
According to Ziajahromi et al. [73], sludge/biosolids had a substantially greater concentration of MPs (43.8 Particles/g) than virgin soils (0.21 Particles/g) and soils supplemented with biosolids (1.58 Particles/g). According to research from China, the concentrations of MPs in virgin soil and agricultural soil treated with biosolids were higher than normal, at 18.7 and 4.49 Particles/g, respectively [135,136]. As a result, sludge and biosolids have considerably higher concentrations of MPs than virgin soils and soils that have been enhanced with biosolids.
It should be noted that the kind of treatment procedure (e.g., primary, secondary, tertiary/advanced) can affect the concentration of MPs in sludge/biosolid in a WWTP [137]. A plausible reason is regional differences in population and economic development. WWTP capacity has a bigger impact on the concentrations of MPs retained in the sludge/biosolid as well as the level of MPs at the WWTP [56,138]. Similarly, variations in MP concentration observed in agricultural soils among studies are probably due to regional characteristics, the biosolid application pace, the soil depth, and site conditions [139].
Studies have revealed that the application of sewage sludge and agricultural methods affect the concentrations of MPs in soils. In samples utilizing sludge compost, Zhang et al. found 545.9 Particles/kg, which was significantly greater than those with 87.6 and 5.0 Particles/kg [140]. Due to sewage sludge dumping, Corradini et al. discovered an average of 3500 Particles/kg in agricultural soils [141], but soils from intensive agriculture, which included the application of sewage sludge and plastic greenhouses, had up to 18,760 Particles/kg [32]. On the other hand, Lv et al. discovered a mere 10.3 Particles/kg in soils utilized for co-cultivating rice and fish in the absence of compost products [142]. This suggests that the incorporation of sludge compost considerably elevates MP levels in soils.
The physicochemical characteristics of the soil (such as redox, pH, bulk density, porosity, and organic matter content), the climate (wind, rainfall, and temperature), the physics and biochemistry of the soil (such as the soil biota), and other environmental factors (such as mechanical disturbance) may all be thought of as potential causes of the variation in MP abundance between different agricultural practice types [143]. Also, the interaction between soil particles and sunlight can lead to the formation of an electrical charge on the surface of MPs [126]. Agronomic techniques, soil biota, and the spatial arrangement of MPs in the soil, both vertically and horizontally can have an impact. Surface runoff and wind erosion aid MP migration in the horizontal direction. The frequency of wetting-drying (w-d) cycles is observed to greatly increase the migratory depth of MPs [126].
Moreover, soil microbes significantly impact how MPs migrate and change. Soil organisms like earthworms and fungi can transport MPs from the soil surface to deeper soil horizons, causing changes in their distribution [144]. Earthworms can transport MPs from the soil’s surface into its pores horizontally and vertically; the easier the migration, the smaller the MP particle size [145]. Three soil arthropods were the subject of Zhu et al.‘s investigation into how they affected soil MP movement and transformation. The movement of MPs within soil alters the duration of their presence, enhances the potential for MPs to reach groundwater, and increases the likelihood of interactions between MPs and other microorganisms [144]. According to some research, the earthworm gut can break down MPs in soil [146], yet the precise mechanism still has to be further investigated through tests.
The quantity of MP found in the soil is determined by the origin of the sludge, as indicated by previous studies [24,147,148]. These findings demonstrate that the number of MPs in soil varied by location and based on their origin, migration activities, and deterioration [149]. Hence, low-intensity agricultural activity that led to a lower abundance of MPs in the soil could be the main reason for a lower abundance of MPs in the soil, as mentioned earlier. In this regard, Zhang and Liu [32] showed that compared to other soil types, the MPs in the plastic-greenhouse vegetable system were greater (7100–42,960 Particles/kg). Another explanation for a higher abundance value could be the absence of analytical standard operating procedures [16,149]. The abundance and characteristics of MPs in farmland soils can be significantly influenced by the specific agricultural practices and conditions, highlighting the importance of considering field circumstances in both the introduction and removal of MPs despite their known sources.
4.2. Size and Shape Distribution of Microplastics in Soils
The findings showed that MPs were present in soil samples regardless of whether they had been exposed to sludge, with MPs being divided into various categories based on size and shape. According to the size distribution of MPs in soils (Figure 3b), 47% of MPs were 0.5 mm, dropping to 52% for MPs between 500 and 5000 µm. There are significant discrepancies in the size range of MP monitored across various studies and sample types, even though the general trend indicated above was visible when examining mean concentrations of total MPs in different sample types. This variation in size can lead to variations in the reported concentrations, making it challenging to compare these findings meaningfully [150]. Although there is agreement that MPs should not be larger than 1 µm, no investigations on soil have revealed any particles between 1 and 10 µm (Table S2). This is most likely due to the limits of present methodologies and the difficulties in precisely detecting and quantifying the smaller particle size ranges in such intricate matrices.
The lowest size limit of MPs discovered was explicitly disclosed in half of the articles in Table S2. It should be noted that the filter size used to segregate MPs, and the instrumental size detection limit often define the lowest detectable size of MPs. However, MP size classes were absent from some research, and the size differs significantly between sample types and geographical regions, making it challenging to offer a straightforward quantitative summary of MP size classes. For instance, although Liu et al. observed MPs in a larger size classification of 30–1000, 1000–3000, and 3000–5000 µm, van den Berg et al. recorded MPs within size ranges of 50–150, 150–250, 250–500, 500–1000, and 1000–5000 µm [16,20]. Because of this lack of harmonization, it is more challenging to precisely quantify MPs in the environment, which makes evaluating the dangers related to various size ranges of MPs in the environment more challenging. MPs with a size of 1000 µm or smaller are the most common in soil [17,19,129,148]. Only 10% of the MPs in these soil samples are larger than 5000 mm, with the size distribution of MPs in soil being thoroughly below 0.5 mm [19,42,142,151].
The majority of MP in soil layers, according to Liu et al., were smaller than 1 mm; they accounted for about 69.8% and 97.4% of the 0–10 cm layer and 62.5% and 98.5% of the 10–20 cm layer [152]. Further evidence of limited downward migration of larger MP was found in the higher fraction of the smallest MP size category (0–0.3 mm) in deeper soil layers as compared to surface layers [153,154]. To be more precise, there was a notable fluctuation in the percentage of MPs larger than 1 mm across the 0–10 cm and 10–20 cm layers, ranging from 8.1% to 2.9% in greenhouse soils and 22.9% to 20.6% in paddy field soils [153]. Notably, the distribution of MP may vary depending on land use and environmental factors, as seen by the significant concentrations of smaller MP in deeper soil layers as opposed to top levels, especially in grassland and greenhouse environments [153].
Research conducted by Ragoobur et al. revealed that whereas MPs greater than 1 mm (58%) were detected in shallow soil, MPs between 3 and 5 mm are prevalent in deep soils [155]. Similarly, Li et al. (2019) found that most MPs in paddy soils at 0–10 cm depth were between 3 and 1 mm [156]. In addition, Liu et al. showed that deep soils had 96% more MPs smaller than 1 mm than shallow soils, demonstrating the variation in MP size distribution over soil profiles [16]. These results demonstrated that larger MPs typically remain in shallow soil layers. This could also be related to the finding that soil erosion, runoff, and wind all carry smaller MPs more easily [157]. Additionally, soil plowing increases the migration of MPs between shallow and deep soil layers, which in turn leads to their fragmentation and accumulation in deeper soil layers. Furthermore, year-round exposure to UV light in Mauritius accelerates the photodegradation of larger plastics in shallow soils, resulting in a greater concentration of smaller MPs in deeper strata [155]. These results demonstrate how reported MP concentrations across different sample types are greatly impacted by smaller size fractions, highlighting the necessity of advanced methods to precisely identify and measure MPs in sludge, biosolids, and soil textures.
The effectiveness of MP separation techniques is greatly influenced by the morphology of MPs and the filter design [158]. Ziajahromi et al. [12] used a 25 µm stainless steel filter to show how fibers of 100–200 µm ended up there, highlighting the unusual sizes found on smaller filters. MPs are categorized as fiber, fragment, film, and flakes in most research; however, other morphologies such as foam, line, filament, granular, and bulk are also occasionally used, indicating the diversity of descriptors employed in various investigations [23]. As indicated by the research in Table S2, the most prevalent morphotypes of MPs throughout all investigations were found in 85.7% of all MPs reported in soils, fibers, and fragments. Furthermore, fibers were the most prevalent form (25%) while string and bulk were the least common (1.67%), according to Figure 3c on the distribution of MP morphologies in soils. Three more typologies that were frequently seen in the study were foam, films, and fragments. The two most common MPs across all sampling sites, according to Liu et al.‘s (evaluation of the MP morphologies in various types of soil samples, were fragment and fiber, which accounted for at least 65.1% and 65.6% of the deep (10–20 cm) and surface (0–10 cm) soil layers, respectively [16]. In addition to fragment and fiber, the most common morphologies among those MPs in soil were film and microbead.
There are significant differences in the distribution of MP types in soil between different land uses and geographical areas [159]. According to Zhou et al., fragments account for 51.7% of MP types, whereas fibers make up the remaining 15.2% [133]. Different environments, including vegetable plots, forests, and bare ground, exhibit different compositions, with greater proportions of fragments being seen [160]. On the other hand, when compared to wooded regions and vacant land, the proportion of fibers in vegetable patches was much higher. These results highlight the impact of geography and land use variables on the distribution and prevalence of MP morphologies in soil. This pattern is further supported by Yang et al.’s study, which shows that fibers consistently account for a considerable majority (66.7–82.5%) of MP types across various treatments, highlighting the fibers’ persistent majority in soil MP contamination [83].
A variety of human activities, including washing synthetic clothing, treating wastewater, and agricultural practices, are linked to the presence of MPs in soil [161]. Synthetic fibers point to a major route for MP contamination in soils and are frequently connected to the application of sewage sludge [54]. Films were more common in soils that had been mulched over an extended period, while microspheres were regularly found in soil samples that had been modified with sewage sludge, indicating a connection to wastewater treatment plants [147]. The presence of microspheres in soil following extended sludge application was further validated by Corradini et al.‘s investigation in Chile, supporting the idea that sewage treatment facilities are a source of these particles [141]. These results highlight different pathways and sources of MPs in agricultural soils, and based on the aforementioned previous studies in this section, synthetic fibers are used as indicators of sludge application [128].
4.3. Composition Distribution of Microplastics in Soils
Various MP compositions were discovered in the soil employing diverse application methods. As per in Figure 3d, Polyvinyl chloride (PVC), polyethylene terephthalate (PET), polyester (PES), polyamide (PA), polyester urethane (PEU), and others were sporadically identified. Polypropylene (PP), polyethylene (PE), and polystyrene (PS) were the most prevalent MP forms found in soils. There were differences between the polymer types of MPs reported across different sample types [162]. Virgin soils contained significantly more PE and PP than sludge/biosolids did, and PE was significantly more common in the latter [162]. The abundance of MPs with similar polymer characteristics in both sludge/biosolids and soils modified with biosolids is probably due to the transfer of MPs from biosolids to soils [163].
According to Ragoobur et al., PP, PA, PE, PS, and EVA had the maximum proportion of MPs in the soil [155]. Additionally, Liu et al. determined that of all the plastic particles, PE (polyethylene) accounted for the largest fraction (22.9%), followed by PA and PS, with the remaining materials being PP, PB, and PVC [152]. Furthermore, PE, PP, PS, PVC, and PA were frequently discovered at each test site, according to Zhou et al. [133]. PE was the most common polymer form at all test locations, including bare soil, wooded areas, and vegetable plots. According to the study by Schell et al., polyester (22–53%) and acrylic (22–37%) were the most prevalent polymer types in the soil samples [128]. Yang et al. reported that following long-term sludge application, eight distinct MP compositions were found in the soil [83]. The majority was made up of PP and PES. PE was found in tests of soil. PA and a trace quantity of PS were found in treated soil samples. Nearly all sludge treatment procedures included the discovery of PAN and poly (styrene: acrylate) (PS-AC). A large number of MPs, primarily PE, found in sludges are derived from pharmaceutical and personal care products (PCCPs) [164]. The use of plastic mulch films on agricultural soils may be the cause of the higher level of PE and PP in virgin soils. PE and PP have been used frequently as plastic mulch on agricultural grounds to improve soil quality [165].
According to the aforementioned findings, prolonged application of sludge compost products containing MPs can result in their presence and accumulation in soils, where earthworms can then take them up and transmit them deeper into the soil profile. Due to soil biota’s ability to decompose and break large polymers, they can become small detritus and even partially digest after being ingested by earthworms [166]. According to a recent study, PE could enter the trophic chains from soil to earthworms and then to chickens, potentially leading to the passage of MPs into the human food chain [167]. This transfer poses a partial risk to human health. Therefore, further research on the ecological risks of MPs in soil ecosystems is needed.
4.4. Surface Morphology of Microplastics in Soils
The investigations showed that several factors influencing degradation are related to the morphology of MPs in soils [168]. According to Yang et al.’s study results, the MPs in soil surfaces displayed varying degrees of disintegration regardless of the source of sludge applied [83]. On fragment surfaces, fractures and numerous cracks were seen as opposed to fiber surfaces, which displayed more roughness and irregular protrusions. MPs age in agricultural soils with a comparable regularity to manure-treated soils and sediments [83]. The primary elements affecting deterioration were UV light, temperature, mechanical action, and microbiological activities [19,165]. Compared to PES fibers, PA fibers are more susceptible to deterioration [169]. The environment or the type of polymer may be associated with some tearing cracks in fragments seen in soil supplemented with municipal sewage.
5. Statistical Analysis of Microplastic Distribution
The results of a descriptive statistical analysis on the MPs’ concentration (Conc.) and size in WW, sludges, and soils are represented in Table 2 conducted by Statistical Package for Social Sciences (SPSS) based on the data recorded in Table 1, Tables S1 and S2, encompassing 72 studies. Furthermore, a histogram analysis of the mentioned parameters is illustrated in Figure 4, demonstrating each parameter’s recorded values and frequencies.

Table 2.
Descriptive statistical analysis of MPs’ concentration (Conc.) and Size in Wastewater (WW), Sludges, and Soils.
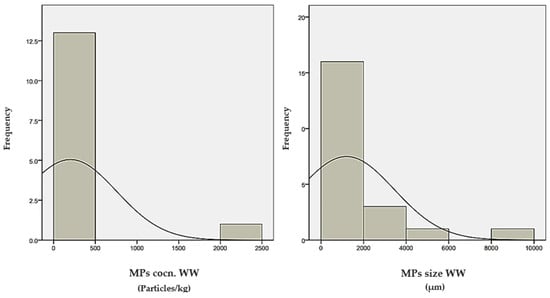
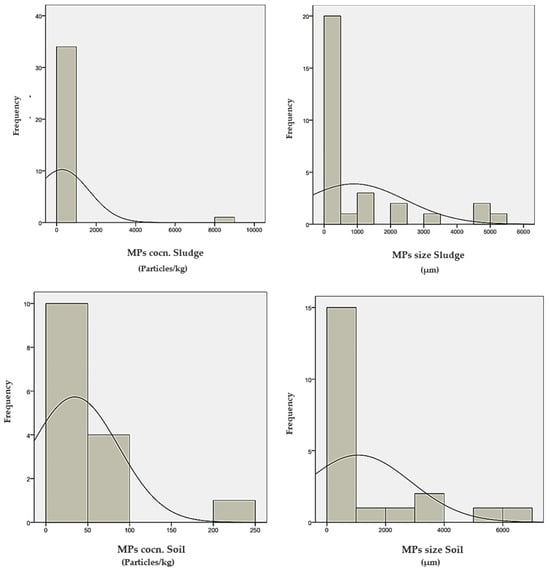
Figure 4.
Histogram distribution of data for MPs’ concentration and size in WW, sludges, and soils.
According to this table, the mean values of MPs’ Conc. in WW, sludges, and soils are 201, 271, and 34.6 Particles/kg, respectively. Moreover, the mean values of MPs’ size in WW, sludges, and soils are 1199, 899, and 1072 µm, respectively. Furthermore, the median values of these variables are reported as 14, 10, and 18 Particles/kg, 150, 66, and 125 µm, correspondingly. The considerable disparity between these parameters’ means and median values can confirm the existence of wrong data records in the sample collection. According to Figure 4, the histogram evaluations reveal that the observed parameters of MPs’ Conc. in WW, sludges, and soils, and MPs’ size in WW, sludges, and soils are mainly concentrated in the range of 0–500, 0–1000 and 0–100, 0–2000, 0–1000, and 0–100 µm, respectively.
Regarding the MP’s concentration in wastewater, sludge, and soil gathered in Table 1, Tables S1 and S2, the correlation between MP concentrations in wastewater and sludge and sludge and soil is evaluated using a linear regression analysis using SPSS software (version 21) (Figure 5). The slope values of 0.95 and 0.72 indicate a direct relationship between the MP concentration in sludge and soil and the MP concentration in wastewater and sludge. For every unit increase in MP concentration in sludge, there is an expected increase of 0.95 units in MP concentration in soil, while for every unit increase in MP concentration in wastewater, there is an expected increase of 0.72 units in MP concentration in sludge. These results suggest that as the concentrations of MPs increase in both sludge and wastewater, there is a proportional increase in their concentrations throughout the system. With a linear regression coefficient (R2) of 0.95, the analysis showed a significant positive correlation between the concentration of MPs in sludge and soil. The p-value of 0.0001 (‘p < 0.05’) in MP concentration in soil and sludge shows the observed correlation is statistically significant, meaning there is a significant correlation between these concentrations. The strong link shows that sludge is a substantial source of MPs in the soil environment, which may negatively affect soil quality and environmental concerns.
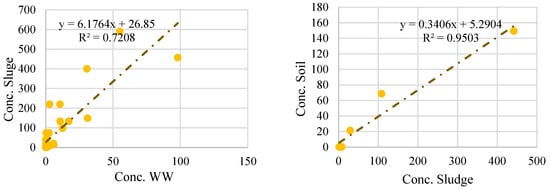
Figure 5.
Correlation plot of MPs’ concentration in WW (Particles/L), sludges, and soil (Particles/kg).
In contrast, an R2 value of 0.72 indicated a weaker association between MP concentrations in wastewater and sludge. The correlation between these two matrices is still positive but not as strong as between sludge and soil. These figures suggest that changes in MP concentration in soil have a larger impact on the resulting MP concentration in sludge than changes in MP concentration in wastewater. The p-value of 0.47 (‘p > 0.05’) in MP concentration in WW and sludge suggests that this correlation is not statistically significant Therefore, there is no statistically significant evidence to suggest that MP concentration in wastewater has an effect on MP concentration in sludge. Furthermore, it shows that factors other than the MP concentration in wastewater affect the MP load in sludge. Additional sources, such as industrial or agricultural operations, and changes in the treatment procedures may impact the MP concentrations in sludge. In order to properly manage and minimize MP pollution, additional research is required to pinpoint additional sources and enhance treatment procedures. Understanding the relationships between MP concentrations in various environmental compartments will help formulate focused strategies for this expanding environmental problem.
6. Effects of Microplastics on Media’s Characteristics
MPs remained in sludge as a result of sedimentation in WWTPs [11]. Specifically, particles with a higher density than water are more likely to be trapped in sewage sludge, like polyvinyl chloride [170]. One of the most economically sensible sewage sludge disposal methods is reusing sludge for terrestrial use, and applying sewage sludge for agricultural property could have both advantages and disadvantages. It has been understood that the application of sludge contributes to soil pollution, as evidenced by enhanced brominated diphenyl ether concentrations in the soil [171], influenced biological soil features [172], and exacerbated metal contamination [173]. Consequently, MPs can affect the environment and the operations of WWTPs.
6.1. Effect of Microplastics on Sludge Properties
The MPs’ composition and particle size affect the sewage sludge, which may impact the sludge’s floc properties and microbial community. The summary of the MPs’ effects on sludge characteristics is shown in Figure 6.
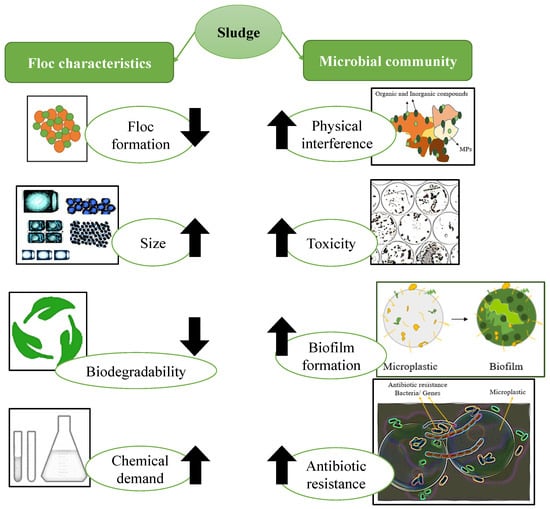
Figure 6.
Microplastics’ effects on sludge’s features.
Microplastics’ impact on floc characteristics
MPs can alter the floc properties in several ways following their entry into the sludge, as detailed below.
- Disrupting Floc Formation: MPs might hinder the development of flocs by decreasing the efficacy of coagulants and flocculants used in wastewater treatment [174]. In numerous ways, this can cause the sludge’s settling and dewatering properties to be weak [175]; (a) surface charge: it is one factor that may prevent MPs from forming flocs since it differs from the surface charge of natural sludge particles. (b) Size: Because MPs are much smaller than natural sludge particles, they settle more slowly and are more likely to stay suspended in the liquid phase, where they can help create flocs. (c) Hydrophobicity: MPs tend to cluster together rather than adhere to other sludge particles because they are hydrophobic, which means they repel water. (d) Adsorption: Organic substances and other pollutants in sludge can be absorbed by MPs, which can obstruct the chemical reactions resulting in floc formation. They can bind to the surface of chemicals that help produce flocs, like calcium hydroxide and aluminum sulfate, which lessens the effectiveness of those substances. Due to the decreased ability of these compounds to form strong connections between particles, weaker flocs that are more challenging to remove from the water are produced. MPs can also prevent floc formation as a physical barrier between particles [174,176]. To illustrate, MPs may become entangled in flocs and be unable to settle out of the water adequately. This process causes treated wastewater to be more turbid and decreases the effectiveness of removing suspended solids. According to studies, including MPs in sludge can interfere with floc formation, which can greatly impact how the sludge settles, is dewatered, and is treated [177]. The researchers discovered that the inclusion of MPs decreased the sludge’s settling velocity, indicating that the flocs were less compact and less able to settle. It can be because the MPs interfered with floc formation by giving bacteria a surface to adhere to, preventing the production of larger, more stable flocs. The researchers found that the capillary suction time decreased by approximately 17% for smaller MPs, indicating that a low concentration of MPs can improve sludge dewatering to a degree [84]. Similarly, Qian et al. investigated how MPs affected the dewatering capabilities of employed activated sludge. As the MPs interfered with floc formation, the researchers observed that the presence of MPs hindered the sludge’s ability to be dewatered [178]. Researchers discovered that the MPs prevented extracellular polymeric substances (EPS) from adhering to the sludge flocs, reducing the flocs’ strength and stability and making dewatering more complicated.
- Altering Floc Size and Structure: MPs can change the shape and size of flocs, which affects how dense, porous, and compressible they are, which may impact the effectiveness of sludge dewatering and disposal. Through various methods, MPs can change the size and density of flocs in sludge [179]. (a) Adsorption: MPs can adhere to the surface of flocs, changing their surface characteristics and leading particles to aggregate or disperse, resulting in changes in the floc size and density. (b) Interference with flocculation agents: by striving for binding sites on the floc surface, MPs can inhibit the activity of flocculation agents such as polymers or coagulants. This may result in smaller or less thick flocs and lessen the efficacy of these agents. (c) Physical obstruction: MPs can physically prevent flocs from arising by occupying the space in the sludge matrix. As a result, smaller, less dense flocs may result from the prevention of particles from aggregating to create bigger aggregates [180]. In one such study, Zhang et al. discovered that adding MPs to sludge caused the floc size to expand significantly. The study employed polystyrene MPs and found that they had up to 25% larger average floc sizes. The researchers credited the ability of the MPs to build a scaffold-like structure within the sludge, which improved the size and stability of the flocs, as the cause of this increase [181]. Xu et al.‘s investigation evaluated the MPs’ impact on sludge floc structure. According to the study, the structure of the flocs significantly changed when MPs were added to sludge. The scientists noticed that the MPs gathered within the flocs, forming a more compact and regular structure. The sludge’s settling and dewatering characteristics may vary due to this structural change. It is revealed that the size of sludge flocs decreased by 9.8% and 30.8% when the concentration of MPs was 100 and 300 mg/L, respectively. This suggests that MPs may interfere with forming larger particles in the sludge flocs [84]. Additionally, Zhang et al. concluded that adding MPs to sludge greatly decreased the flocs’ settling velocity. The ability of the MPs to expand and stabilize the flocs, making them more difficult to settle, was identified by the researchers as the cause of this decline [182].
- Reducing Biodegradability: MPs cannot degrade and may end up in sludge over time. As a result, sludge’s biodegradability is lowered, making treatment and disposal more challenging [183]. To begin with, by interfering with the formation and stability of flocs, MPs can decrease the dewaterability of flocs in sludge [84]. Therefore, smaller and weaker flocs that are more challenging to dewater can be the outcome [184]. MPs may potentially have a detrimental effect on the cellular mechanisms involved in floc production [184]. Microorganisms produce EPS, which aids in binding sludge particles together, and these substances are critical for the creation and stability of flocs [185]. However, the adsorption of EPS and other organic molecules by MPs, which decreases their availability for floc formation, can interfere with these activities [185]. The surface area of the sludge particles can also be increased, which can result in increased water absorption and decreased dewaterability [84]. Smaller particles are inclined to absorb water and swelling because they have a higher surface area to volume ratio [186]. Overall, by interfering with both physical and biological processes involved in floc formation and stability, the presence of MPs in sludge can have considerable detrimental effects on its dewaterability.
Additionally, the presence of MPs in the sludge drastically decreased its capacity to biodegrade [84]. The scientists attributed this reduction to the MPs’ capacity to lower microbial activity by blocking the access of microorganisms to the organic materials in the sludge [177]. It is demonstrated that the presence of 100 mg/L MPs resulted in a substantial decrease of 44.8% in Adenosine triphosphate levels, indicating that the high concentration of MPs had a noticeable inhibitory effect on sludge activity [84]. Additionally, the findings demonstrated that 100 mg/L MPs significantly reduced the proportion of viable cells within the sludge [84]. According to the study, Zhang et al. investigated the impact of MPs on the microbial community and the biodegradability of sludge. The study discovered that the abundance and diversity of microbial communities in the sludge significantly decreased after the addition of MPs. The researchers noticed that the sludge’s biodegradability decreased due to this decrease in microbial activity [187].
- Increasing chemical demand: MPs have the potential to raise the chemical demand essential to efficient treatment. To illustrate, by adsorbing organic and inorganic contaminants, MPs can increase the chemical demand in sludge, producing more complex and stable particles. Because of the increased chemical demand, it may be more challenging to treat the sludge using traditional methods because more chemicals must be added to the treatment process. Furthermore, MPs could operate as a source of nutrients for the microorganisms in the sludge, increasing their activity and demand for chemicals [188]. Overall, the presence of MPs in sludge can make treatment more difficult and raise the price of chemical application. The impact of MPs on sludge’s chemical oxygen demand (COD) was studied by Wei et al. According to this study, the COD of sludge increased by 12% when MPs were added. The ability of the MPs to adsorb organic contaminants, which might raise the COD of the sludge, was acknowledged by the researchers as the cause of this increase [189]. Another study by Shi et al. investigated the impact of MPs on the heavy metal content of sludge. According to the research, adding MPs to sludge caused a noticeable rise in the leaching level of heavy metals. The ability of the MPs to adsorb heavy metals, which might add to the overall chemical demand of the sludge, was identified by the researchers as the reason for this rise [190].
As a result, MPs have a detrimental effect on the sludge’s physical and chemical characteristics, which may hinder the effectiveness of its treatment and disposal alternatives. To lessen MP’s effect on sludge quality, it is crucial to reduce the amount of plastic waste that enters wastewater treatment plants.
Microplastics’ impact on microbial community in sludge
The microbial community may be significantly impacted by MPs in sludge. According to studies, MPs can influence both the diversity and composition of microbial communities in sludge as well as their metabolic activity, which can alter nutrient cycling and other ecological processes [191]. Additionally, MPs can serve as a substrate for bacterial development and biofilm formation, which may result in the agglomeration of pathogens and dangerous microorganisms [191]. MPs can have a variety of effects on the microbial population in sludge in different ways.
- Physical interference: The development and function of microorganisms in sludge can be physically hampered by MPs [186]. They have the potential to block pores and decrease the supply of nutrients, oxygen, and other vital elements needed by microbes [192]. Multiple mechanisms exist for MPs to physically affect the sludge’s microbiological community: MPs can absorb organic and inorganic contaminants, which can change the chemical structure of the sludge and impact the microbial population [192]. They can also combine with other particles in the sludge to produce larger particles, which can settle out of suspension. As a result, microbes may have less access to nutrients and oxygen, which may inhibit their growth and alter their rate of metabolism [193]. In one such investigation, Wei et al., (2020) discovered that adding MPs to sludge significantly increased the viscosity and yield stress of the sludge. Their examination of the pore structure indicated that the presence of MPs resulted in a 12% reduction in porosity. This suggests that the aggregation of MPs may block the cavities in sludge, hindering the formation of granular sludge and potentially impeding the accumulation and movement of MPs into deeper sludge layers. The ability of the MPs to create a network-like structure inside the sludge enhanced its overall viscosity and yield stress, which the researchers attributed to this rise [194].
- Toxicity: MPs have the potential to leak hazardous substances such as plasticizers, flame retardants, antioxidants, light stabilizers, and other compounds utilized in their production [195]. In addition to altering the growth and metabolism of the microbial population in sludge, these chemicals can also cause soil pollution as a result of the sludge amendment [196,197]. It is found that additives generated by MPs can directly damage microbial cells, which can impact microbial activities [198]. According to Wei et al., the main inhibitory mechanism that promotes the breakdown of anaerobic digesting bacteria cell walls is bisphenol A produced by PVC [189]. PVC plastic products used in the medical industry have antibacterial qualities because they contain plasticizers that are selective to types of microbes like Gram-negative bacteria and sulfate-reducing bacteria and are resistant to nitrifying bacteria.
- Biofilm formation: MPs may also promote the development of biofilm, a protective bacterial layer that develops on surfaces. As a result, dangerous bacteria may build up in the sludge, further upsetting the microbial population [199]. To be precise, a procedure known as microbial adhesion allows MPs to create biofilms in the sludge’s microbial community. Bacteria and fungi in the sludge can adhere to the MPs’ surface and create a slimy covering known as a biofilm [200]. The microorganisms can live and develop on the surface of the MP owing to this biofilm’s protective habitat. The development of biofilms on MPs may also be aided by the sludge’s organic matter content. Organic substances give the microorganisms access to nutrition, which can promote their development and adherence to the MP’s surface. A biofilm that has developed on an MP can entice additional bacteria to join the community. As a result, diverse microbial communities may develop on the surface of the MPs, which may impact the MPs’ transport and fate in the environment [201].
According to one study by Chen et al., adding MPs to wastewater caused an increase in biofilm production on the MPs’ surface. According to the researchers, the MPs may have offered a surface for the adhesion and proliferation of bacteria, which in turn aided in the development of biofilms. They have shown that with increasing MPS concentrations, biofilm formation in the sludge has experienced an increasing trend of 60–90% [202]. Further study discovered that the MPs changed the wastewater’s microbial population, which impacted biofilm development [9]. According to a different study by He et al., adding MPs to activated sludge increased biofilm production on the MPs’ surfaces. According to the researchers, these might have served as a surface for the adhesion and expansion of bacteria, which in turn aided in the development of biofilms [115].
- Antibiotic resistance: Antibiotic-resistant bacteria can accumulate in MPs and pass their resistance genes to other microbes in sludge [203]. MPs in the sludge can offer bacteria a surface to adhere to and build biofilms. Bacteria can exchange genetic material within these biofilms, including genes that confer antibiotic resistance. As a result, MPs in sludge may help the microbial community develop increased antibiotic resistance [204]. According to one study by Wang et al., adding MPs to sludge led to a noticeable effect of antibiotic resistance genes (ARGs) in the sludge. The researchers hypothesized that the MPs might have served as a means for the distribution of ARGs, which could have significant consequences on the emergence of antibiotic resistance in the environment [205]. According to a different study by Wang et al., the presence of MPs in sludge significantly increased the amount of mobile genetic elements (MGEs) in the sludge. MGEs, which include ARGs, are genetic elements capable of transferring genetic material between bacteria. According to the researchers, the MPs may have served as a surface for the attachment and transfer of MGEs. This possibility has substantial consequences for the spread of antibiotic resistance in the environment [206].
Overall, MPs can potentially upset the microbial community’s delicate equilibrium in sludge, which would limit treatment effectiveness. MPs have complex and varied effects on the microbial community in sludge. Therefore, more investigation is required to completely comprehend how MP pollution will affect these ecosystems in the near future.
6.2. Effect of Microplastics on Soil Ecosystems
MPs can interact with a range of soil parameters, so they can potentially alter soil properties in addition to their direct effects on sludge, soil animals, and crops. The functional and structural diversity of the soil microbial community is directly impacted by changes to the physical and chemical features of the soil environment, which could result in more severe soil environmental issues [9]. Figure 7 shows the outline of the MPs’ effects on soil features.
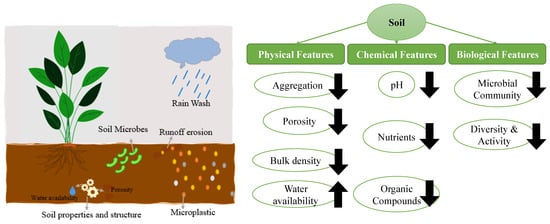
Figure 7.
Microplastics’ effects on Soil properties.
Microplastics’ impact on Physical properties of soils
By limiting water infiltration and changing soil structure, MPs can change the physical characteristics of soil ecosystems [207]. It can result in less water being available for plants and other organisms because they can build physical barriers that stop water from accessing the soil [208]. As a result, the soil’s capacity to hold water and nutrients is diminished. They can also alter the soil’s porosity. Along with altered soil microbial populations and nutrient cycling, this can result in lower plant growth and production [208]. The behavior and survival of creatures that live in the soil can also be affected by MPs, which has an even greater effect on the ecosystem’s physical characteristics [209]. MPs offer a variety of approaches by which they might impact soil’s physical characteristics.
- Aggregation: By decreasing soil stability and increasing soil erosion, MPs can have an adverse impact on the soil’s ability to aggregate. Aggregates in the soil are crucial for preserving soil stability and structure but can be disrupted by MPs [210]. A rise in soil erosion and topsoil loss could result from this, which would be detrimental to the health and productivity of plants. Additionally, they may have a negative impact on soil aggregation by reducing the activity of soil microbes that are crucial for preserving soil stability and structure [211]. It is worth noting that the presence of MPs in soils may decline soil aggregation by about 15–30% [212]. Chen et al.‘s research revealed that soil aggregation significantly decreased when MPs were added to the soil. The researchers credited this to MPs’ potential to obstruct the natural mechanisms that cause soil aggregation. Particularly, the MPs can physically displace soil aggregates, which lowers their stability and leads to their disintegration. According to the study, the MPs also changed the microbial community in the soil, which impacted soil aggregation [213]. A study by Liu et al. showed that the presence of MPs in soil decreased soil organic carbon and increased bulk density, both of which are signs of soil degradation. Researchers hypothesized that the MPs may have impeded the natural processes that cause soil aggregation, which in turn decreased the stability of soil aggregates and affected soil structure and water retention [214].
- Porosity: The porosity characteristics of soils may be negatively impacted by MPs [210]. They may remain in soil pores, decreasing the soil’s capacity to retain air and water. Reduced soil fertility and plant growth may result from this. MPs may also change the microbial communities in the soil, which may have an additional effect on the soil’s porosity and capacity for nutrient cycling [215]. According to one study by Yu et al., adding MPs to soil caused a noticeable reduction in soil porosity. The scientists explained this by stating that they can block soil pores, limiting the quantity of air and water the soil can hold. The investigation also discovered that MPs changed the soil’s microbial population, which affected soil porosity [216]. The addition of low-density polyethylene (LDPE) MPs to soil led to a reduction in soil porosity by around 3–5% and an increase in soil compaction, according to Qi et al.‘s findings in a related investigation. The researchers hypothesized that MPs may have impeded the natural processes that result in soil aggregation, reducing the stability of soil aggregates and affecting soil structure and water retention [217].
- Bulk Density: By creating more soil compaction, MPs can change the bulk density characteristics of soils [218]. This is because MPs are denser than the majority of naturally occurring soil particles and can fill up the soil’s pore spaces, decreasing the soil’s capacity to store water and air. As a result, the soil has a larger bulk density and is denser. Porosity loss can have a negative effect on plant development and microbial activity by reducing air and water infiltration into the soil [219,220]. According to a study by Qi et al., soils with LDPE-MP concentrations showed a loss of 14% in bulk density [217]. The researchers hypothesized that MPs may have impeded the natural processes that result in soil aggregation, reducing the stability of soil aggregates and affecting soil structure and water retention. Similar findings were obtained by Chai et al. in another investigation. According to the study, soils with MP concentrations had noticeably affected bulk densities [221]. The reason for this, according to the researchers, is that by reducing soil pore space, they may also decrease the amount of air and water that the soil can hold. According to de Souza Machado et al., MPs have a stronger influence on soil parameters than MPs that are only slightly different from the shape of the soil particles. MPs will decrease the bulk density by 2–6% and enhance evapotranspiration of the soil. Plant development and agricultural production may be negatively impacted by the increase in bulk density brought on by the presence of MPs. Compacted soils are less able to store water and have poor aeration, which can cause waterlogging and lessened plant nutrient availability [211].
- Water availability: MPs can also alter the water availability characteristics of soils in other ways. (a) Reduced water holding capacity: MPs can lower the soil’s ability to hold water by clogging pore pores that would otherwise be filled with air or water. Due to this, plant growth may be hindered, and runoff may be exacerbated [218]. (b) Enhanced soil erosion: MPs can exacerbate soil erosion by lowering the stability of soil aggregates. This may result in more sediment being deposited in adjacent water bodies, which may have an even greater effect on water availability. (c) Greater runoff: MPs can potentially produce greater runoff by lowering infiltration rates. As a result, more water evaporates off the soil’s top rather than seeping in. (d) Changed soil structure: MPs can change soil structure by aggregating with other particles. This may impact how water moves through the soil and make it more challenging for plant roots to get to water. Some research revealed that polyester, polyacrylic, and polyethylene have decreased soil water retention capacity (~5%) and decreased bulk density [211,219]. A change in the physical characteristics of the soil may accelerate root growth and exudation since MPs are becoming more widely recognized as a significant emerging contaminant. Accordingly, MP’s presence in soils can reduce the soil’s ability to retain water, and it can be concluded that this might be brought on by modifications in soil structure brought on by the presence of MPs.
Microplastics’ impact on chemical properties of soils
MPs can impact the chemical composition of soil in many ways [222]. First, they might change the soil’s pH to make it more acidic or alkaline. Plants and other organisms that depend on a particular pH level in the soil may not develop or survive as a result of this. Second, they can influence soil nitrogen levels. They can, therefore, bind to nutrients like nitrogen and phosphorus, reducing their availability to plants and other living things. Reduced plant growth and nutritional deficits may result from this. Thirdly, they may affect the soil’s capacity to hold onto water and may lessen its porosity, which makes it more difficult for plants to absorb water. Plants and other soil-dwelling species may experience water stress [217]. Overall, the presence of MPs in the soil can significantly modify its chemical characteristics, thus likely impacting the health and productivity of ecosystems that depend on healthy soil.
- Soil pH: The kind, size, and quantity of MPs in the soil, as well as the type of soil, are all variables that affect how MPs affect soil pH. Studies have revealed that MPs can modify the chemical structure of the soil, changing the pH. They have numerous potential effects on the pH of the soils. Firstly, they can discharge poisons and compounds that change the chemical composition of soil. Many types of plastic contain substances like phthalates and bisphenol A (BPA) that can leak out of the material and enter the soil. By making the soil more or less acidic, these compounds can change the pH of the soil. Second, MPs can absorb and hold water, which may result in changes in moisture levels and ultimately impact pH. They may also lessen the oxygen accessible to soil bacteria, which may affect their capacity to control the pH of the soil [219]. Gharahi et al. investigated the impact of polyethylene on soil pH. The research revealed that polyethylene affects the pH of the soil, which varied between 7.03–7.53 compared to the pH = 7.87 of the control sample, leading to a rise in adsorption of heavy metal ions and this can be attributed to the higher negative charge of the MPs surface in higher pH values, which generates an electrostatic attraction to the metal cation [223]. The effects of different MPs on soil pH are studied by Liu et al. and represent a 2% loss in soil pH when adding MPs [224]. Additionally, Yang et al. studied the impact of MPs on soil pH, and according to the study, MPs raised the soil’s alkalinity by raising its pH. The researchers attributed this impact to the interaction of MPs with soil organic matter, minerals, and various organic and inorganic contaminants [225].
- Nutrients: According to studies, MPs can alter soil pH, decrease microbial activity, and interfere with nutrient cycle mechanisms, which all impact the nutritional characteristics of soils [226]. They can also adsorb minerals, which lessens their availability for plant uptake and has a variety of other effects on soil nutrients; (a) MPs can decrease soil fertility by obstructing the passage of water and air through the soil. This may result in lower plant nutrient uptake and decreased agricultural production. (b) Microbial community alteration: MPs can potentially change the soil microbial community diversity and activity. This may impact nutrient cycle procedures like nitrogen fixation and mineralization. (c) Toxic chemical contamination of soil: MPs can absorb and store harmful substances like pesticides and heavy metals from the environment [227]. These MPs can pollute soil when they enter it with these hazardous compounds, which can harm plant development and health. The circulation system for nutritional components such as nitrogen is powered by microorganisms. The primary processes that link nitrogen transformation and nitrogen cycle are ammunition, nitrogen fixation, nitrification, and denitrification. The correspondence microorganisms must be present for each process [228].
According to [229], the quantity, activity, and flora structure of the microorganisms participating in nitrogen cycles determine the process and outcomes of Nitrogen (N) cycles. Nutrient mineralization and fixation are two processes that are controlled by these factors. According to a recent study by Rong et al., the presence of MPs in soil impacted nutritional competition and the relationship between soil bacteria, which changed the nitrogen cycling carried out by soil microorganisms [230]. Moreover, Awet et al. (2018) found that PS can inhibit the activity of extracellular enzymes related to Carbon (C), N, and Phosphorus (P) cycling and reduce microbial biomass [231].
According to Qian et al., residual plastic film in agricultural soil might decrease the amount of inorganic nitrogen in the soil, as well as the activity of functional enzymes and the expression of functional genes involved in nitrogen cycling. Urease is crucial in the soil’s nitrogen cycle and is typically found in bacteria. These bacteria experienced a reduction of 20% in urease expression [232]. In 2021, Huang et al. found that MPs also impact the health and activity of algae in sediments. PE in sediments has been shown to increase the growth of anaerobic ammonia-oxidizing bacteria and denitrifying bacteria by 45–60% [233]. The functional bacteria involved in nitrogen cycling can be influenced by environmental factors such as polymer type, particle size, and concentration of MPs, which may be related to the ability of MPs to act as organic substrates and release additives.
Therefore, it is evident that the impacts of MPs on N cycling are likely, and this calls for further additional tests applying a variety of MP materials. While some MPs, like PA and PAN, directly increase the accessibility of biogeochemically active N [211], sometimes their derivatives, including phthalate esters, might harm soil, which may interfere with nutrient cycling by limiting key soil enzyme activity and they showed that biomass nitrogen declined by 38.2–76.2% [228]. Additionally, these substances can combine to create complexes with substances released during the breakdown of plastic and the breakdown of organic materials, which may ultimately alter the decomposition or ammonification of those substances. The impact of MPs on the nutritional properties of soils has been examined in several studies. Zhang et al.‘s study looked at the impact of MPs on soil N availability. The study discovered that MPs decreased the amount of soil N available, which may have detrimental effects on plant growth and ecosystem health [234]. The impact of MPs on soil P availability was examined in a study by [235]. According to the study, MPs reduced the amount of phosphorus in the soil, which could limit plant development and production.
Overall, these studies imply that MPs negatively impact soil nutrient properties. MPs can potentially reduce the amount of N and P in the soil, which may restrict plant development and production. Additionally, MPs can change the processes that control how soil C and N are cycled, which can harm ecosystem health and soil fertility. Therefore, more study is required to comprehend the long-term consequences of MPs on soil nutrient properties and to create mitigation measures for their detrimental effects on soil functions.
- Organic compounds: Organic compounds in soils can be impacted by MPs in several ways [236]. Some ways that MPs can affect organic compounds in soils include (a) Changing the soil structure: MPs can build up in soil pores and alter the soil’s structure, impacting the amount of nutrients and water plants can access. Changes in microbial activity and decomposition rates may result from this, which may affect the organic molecules found in soil. (b) Adsorption of organic compounds: MPs can absorb organic pollutants like pesticides and herbicides due to their large surface area. As a result, these compounds may be less readily available for microbial or plant uptake. (c) Leaching of organic compounds: MPs can also help organic molecules in soils leach into groundwater or bodies of surface water. Aquatic ecosystems may be impacted, and water resources may become contaminated. (d) Modifying microbial communities: The diversity and composition of the microorganisms inhabiting the soil can be altered by MPs. These microorganisms are crucial in nutrient cycling and decomposition processes [237]. It is worth noting that MPs interact with organic compounds in soils in various complex ways, depending on the type of soil, the size and concentration of the MP, the length of exposure, and the environmental circumstances. Therefore, changes in microbial communities can have implications for plant nutrition and soil health.
High MP concentrations have been shown in certain studies to enhance soil concentrations of humus, fulvic acid, soluble organic carbon, organic nitrogen, and organic phosphorus, as well as to boost the activity of soil enzymes [214]. Numerous research studies have examined how MPs affect the organic molecules in the soil structure. Shi et al. looked at the impact of MPs on soil organic matter (SOM). According to the study, MPs changed the composition and lowered the concentration of SOM. The research also revealed that MPs impacted the microbial ecosystem that is in charge of decomposing SOM [238]. Chen et al. investigated the impact of MPs on soil dissolved organic matter (DOM) in a different study. The study discovered that MPs boosted DOM concentration and changed its diversity. The study also discovered that MPs impacted the microbial population in charge of DOM [239].
Overall, these investigations indicate that MPs are harmful to the organic molecules that make up soil. MPs change the content and concentration of SOM, DOM, and humic compounds, which are crucial for soil activities like nutrient cycling and water retention. MPs also impact the microbial population in charge of degrading organic compounds, which has an additional impact on soil activities.
Microplastics’ impact on biological properties of soils
MPs may affect the biological characteristics of the soil [222]. The presence of MPs can significantly impact soil’s biological characteristics, which may, therefore, impact the health and productivity of ecosystems that depend on healthy soil [240]. Soil harbors a wide range of organisms, including fauna, nematodes, collembolans, various soil-dwelling animals, and microorganisms.
Microbial and invertebrate community: MPs have several potential effects on soil microbial community. The community of the plastisphere, is defined as the microbial communities found on plastic waste, and its environs differ dramatically [241,242]. According to Zettler et al., whereas community homogeneity was higher, the average plastisphere abundance was lower than that of the nearby microorganisms [241]. The same conclusion was later reached by Tender et al., who found that the bacterial community on the plastic substrate clearly differs from that in the surroundings. For instance, some bacterial groups, such as Vibrionaceae or Pseudoalteromonadaceae, are frequently found in plastic trash but very infrequently in the environment [243]. Accordingly, the MPs’ surface plastisphere makes a considerable contribution to the degrading process, and it is presented that both bacteria and fungi may contribute to the deterioration of plastics [244].
For resident organisms, the soil system is a crucial habitat. It is simple to envision how many animals, after ingesting MPs unknowingly, would be unable to digest the plastic particles, preventing food from passing through the gut [245]. However, by breaking the delicate plastic pieces, soil organisms, particularly earthworms, can consume MPs [246]. According to some studies, earthworms can consume huge amounts of MPs, which could injure their intestinal tract and lower their survival rate. According to Rillig and Bonkowski, the consumption of MPs may be especially damaging to natural filter feeders, such as ciliates and flagellates [247]. In another study, Lei et al. observed that polystyrene (PS) can specifically cause evident harm to cholinergic and GABAergic neurons as well as oxidative stress kinase [248]. Moreover, MPs may potentially have unintended consequences on the food chain [249]. Higher trophic levels, such as birds or mammals may eat species that live in the soil and are harmed by MPs [250]. As a result, these organisms may accumulate MPs, negatively affecting their survival and general health.
Gaylor et al. discovered that the combination of polybrominated diphenyl ether (PBDE) and polyurethane foam had synergistic effects on the earthworm species Eisenia fetida, leading to the accumulation of chemicals in the earthworms, which could then be transferred to predators or cause earthworms to relocate from their habitat [251]. Earthworm Lumbricus terrestrosin was treated with low-density polyethylene (LDPE) for 60 days by Lwanga et al. Based on studies of mortality, growth, and tunnel formation, they discovered that MPs could cause toxicity to earthworms. Additionally, earthworms were found to selectively consume MPs, accumulate them in their bodies, and transfer them within the soil ecosystem. Higher concentrations of MPs led to increased mortality, while lower concentrations required more time to cause harm(e.g., for L. terrestris) [146]. Similarly, Rodriguez-Seijo et al. also reported that polyethylene (PE) exposure caused histopathological damage and immune system defects in Eisenia andrei but did not significantly affect survival, juvenile numbers, or adult weight [252].
MPs can influence the variety of soil microbial communities in several ways [253]. In the beginning, MPs can offer a surface for microbial development, which can result in microhabitats that are distinct from those found in organic soils. Changes in the diversity and abundance of various microbial groups may result from this, which may affect the cycling of nutrients and the activity of soil microbes. Second, MPs can have hazardous effects on species that live in soil, including bacteria. This may also affect the diversity and abundance of certain microbial groups, which may affect their activity. For instance, several studies have revealed that MPs can lower the diversity and abundance of soil fungi, which are essential for plant growth and the cycling of nutrients. Thirdly, MPs can change the physical characteristics of soil, including its porosity and ability to retain water [246]. The diversity of microbial communities may be impacted by these changes, which may also affect the distribution and activity of soil microorganisms. MPs can also have an impact on the diversity of soil fauna. Numerous species, including earthworms, mites, and springtails, are part of the soil fauna. Due to their contributions to the nutrient cycle and soil structure, these organisms are essential for maintaining healthy soil. According to studies, MPs can negatively impact the development and survival of soil fauna by 60–90% with the increment in MPs concentration, which ultimately results in a decrease in the diversity of that fauna. MPs affect soil diversity in ways other than just soil fauna and microbes. The diversity of plant species that flourish in contaminated soils can also be impacted by MPs [254]. Overall, the diversity, activity, and functionality of soil microbial communities can be significantly impacted by the presence of MPs in the soil.
Microorganisms play a major role in the environment’s movement of materials and energy. These organisms are directly linked to the composition of communities and metabolic activity. The structure of the microbial population and the activity of the enzymes can both be greatly impacted by the deposition of MPs [255]. The impact of MPs on bacterial community structure can be mitigated by altering the physical, chemical, or nutritional circumstances. According to a study by Fei et al., the presence of both PE and PVC in acid-cropped soil significantly inhibited the activity of fluorescein diacetate hydrolase, stimulated the activity of acid phosphorus and urease and decreased the diversity and richness of the microbial community. The effects of PE on soil were more severe than those of PVC [227]. According to Huang et al., adding LDPE can change the urease activity and composition of the microbial community in soil, disrupting the hydrolysis of organic nitrogen. They also noted that MPs in soil can serve as a special habitat, potentially changing the ecological function of the soil ecosystem [256]. It was observed that MPs in soil can reduce microbial activity by 9–14% compared to the control sample [219]. Ren et al.‘s study discovered that MPs decreased the diversity and richness of soil microbial communities [257]. MPs can alter the composition of soil microbial communities and diminish soil diversity. In this regard, Wei et al. showed that soil enzymes experienced a 6% loss in the presence of MPs in the soil [189].
Additionally, Yi et al. discovered that MPs decreased soil microbial biomass and activity by 31–41%. The processes of nitrogen mineralization and soil respiration, which are crucial for preserving the health and productivity of the soil, were found to be declining [258]. In another study, Awet et al. (2018) examined how MPs affected soil microbial activity and biomass as well as the diversity of functional enzymes, and the results revealed that the presence of NPs significantly reduced N- (leucine aminopeptidase) activity and constrained the soil’s potential ability to use nitrogen. Since carbon is the primary constituent of MPs, it may impact how carbon is converted and stored in the environment [231]. Overall, MPs cannot only have a detrimental effect on the diversity and abundance of soil bacteria but also change the bacterial communities in the soil and decrease soil fungal diversity, which results in a loss in the carbon and nitrogen cycling in the soils.
7. How Biofouling Influences Microplastics Accumulation and Transport in the Soil and Sludge
Considering that the accumulation of organisms on MPs may have significant ecological and environmental consequences, it is imperative to understand this phenomenon. MPs can facilitate the growth of microbes, algae, and other organisms. By accumulating organisms on the surface of MPs, a process known as biofouling, it is possible to change these objects’ physical and chemical characteristics [259]. The size, shape, and surface qualities of MPs, as well as the type of organisms engaged in the biofouling process, may impact biofouling. These effects are complex and dependent on some variables. Research has looked into how MPs affect sludge biofouling in some experiments [29,260]. It is known that MPs can accumulate and remain in the soil for a long time due to their resistance to biodegradation, even though there is little research on the effects of MPs on soil biofouling.
It is worth noting that MPs might have some general effects on biofouling (Figure 8). The community structure of the organisms engaged in biofouling may change in the presence of MPs [259]. For instance, research has revealed that MPs can promote some bacterial species’ growth while hindering others’ growth. Additionally, the accumulation of organisms on MPs’ surfaces can change certain aspects of their physical characteristics, including their buoyancy and settling velocity [115]. Due to this, MPs may be transported to the water surface or the coastline depending on their fate and location in the ecosystem. By modifying the qualities and structure of the sludge, MPs can also affect how they are transported in sludge, which may affect transport and fate. According to a study by Sun et al. MPs in wastewater treatment plants may accumulate in the activated sludge and promote the development of biofilm, which can clog the system and lessen the treatment process’ effectiveness. Decreasing the flow rate and prolonging the sludge’s presence in the treatment system can have an impact on the transport of MPs in sludge [78]. Additionally, MPs can affect their transportation in soil by altering soil properties and structure, which can impact their movement and fate. Increased sedimentation and erosion may impact the movement and fate of MPs in soil [261]. The impact of biofouling might be more considerable on films due to their larger, uniform surfaces and greater potential for biofilm formation. This uniform surface allows for more extensive and cohesive biofilm development compared to the more irregular surfaces of fragments [262]. Furthermore, the surfaces of films are frequently smoother than those of fibers, which may help bacteria attach to their surface. More niches for colonization may be available on rougher surfaces, but in general, films with bigger flat areas can accumulate more biomass [211].
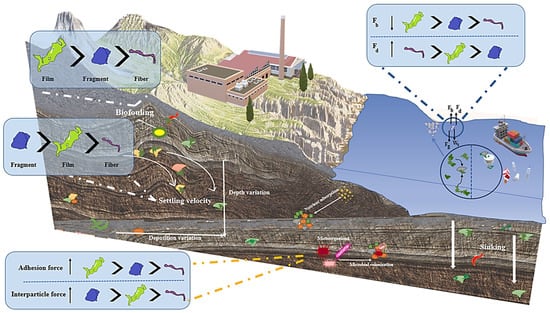
Figure 8.
Schematic diagram of biofouling effect on MPs.
MPs can also impact settling and sinking behavior in sludge and soil. MPs may interact with other sludge constituents and soil particles, such as organic matter and minerals when incorporated into sludge and soils [263]. This interaction may affect the mobility and retention of the MPs and alter the soil’s structure and characteristics. This may change the sludge’s and the soil’s chemical and physical properties, impacting the settling behavior [264].
MPs’ settling behavior may also be influenced by their size and form. Smaller MPs could settle more slowly due to their larger surface area to volume ratio, which increases their buoyancy and makes them more resistant to settling. Additionally, the structure of the MPs can affect how they settle, with flat or disc-shaped MPs potentially settling more slowly than spherical MPs [265]. Additionally, the settling behavior of other sludge or soil components may be impacted by the presence of MPs in soils and sludge. This is because they can attach to other particles and aggregate, changing the structure of sludge and the characteristics of the soil. It has been demonstrated that aggregation considerably boosted settling rates [266]. Their size and shape can also impact MPs’ sludge sinking rate. In a study by Koelmans et al., larger and non-spherical MPs tend to sink in water more quickly than smaller and spherical ones. This may also be true of MPs found in sludge; larger and more asymmetrical MPs may sink more quickly than spherical and smaller MPs [267]. The impact of biofouling on the fate of MPs was examined by Kooi et al. and they revealed that all particles can settle as a result of biofouling, with larger particles beginning to settle last. Additionally, larger particles (10 mm and 1 mm) fluctuate rapidly and reappear with each oscillation, resulting in a size-selective removal of smaller particles from the surface [268]. The higher density and more compact structure of fragments allows them to sink more quickly, making it possible to more effectively overcome soil resistance [29,260]. Compared to fragments, films and fibers settle more slowly because of their greater surface area and flexibility, which causes them to become entangled with roots, soil particles, and organic substances [269].
According to earlier research, fouling causes plastic debris’ buoyancy to decrease to the point where it sinks, and fouling has also been proposed as a potential explanation for the lack of MPs determined through surface net sampling techniques [270,271]. It is found that debris with greater relative surface areas had higher fouling rates, but the rates at which buoyancy was lost for different-sized fragments of the same material have not been particularly investigated [272]. Ryan examined how the size and characteristics of floating debris vary with distance from a pollution source and discovered scientific evidence that an object’s size, shape, and buoyancy affect the duration it remains on the surface. This study predicted that size becomes increasingly significant for tiny litter items while shape is most relevant for large ones [271]. According to a study by Quik et al., larger and non-spherical MPs tend to sink in water more quickly than smaller and spherical ones. This may also be relevant to MPs found in sludge; larger and more irregular MPs may sink more quickly than spherical and smaller MPs [273]. As in a study by Horton et al., larger and non-spherical MPs tend to settle in water more quickly than smaller and spherical ones. This may also be true when transmitted through soil MPs, where larger and irregularly shaped particles may sink more quickly than spherical and smaller particles [274]. Khatmullina et al.‘s research emphasizes the impact of particle shape on the sinking behavior of MPs and makes the case for the need for studies using actual MPs of various shapes [275]. Recent experimental studies by Waldschläger et al. and Kaiser et al. help better understand MPs’ sinking behavior by including particles of various shapes. Films, a common and distinctive plastic shape anticipated to impact sinking behavior significantly, were excluded from these studies. With a market share of less than 40%, the packaging industry dominates the plastics sector, which suggests that film particles play a significant role in MP pollution [276,277]. In a 2020 study by Michiel van Melkebeke, the sinking behavior of typical MPs derived from actual plastic waste samples, including films, is examined. The significance of particle shape was validated, and suitable shape descriptors were found to quantitatively characterize the MP shapes that occur most frequently. They discovered that, on average, the terminal sinking velocity of ordinary MPs is 3 to 4 times lower than expected by the reference law for spheres, and up to 7 times lower for fibrous MPs in particular [260]. Overall, fragments settle more quickly and face a greater decrease in buoyancy because of biofouling because of their irregular form, higher initial density, and compact structure [28]. In contrast, films have a smoother surface, are more flexible, and have a lower initial density, which causes a less noticeable decrease in buoyancy despite having a greater surface area for biofilm growth [28].
By modifying the sludge’s physical and chemical properties, MPs can change the velocity at which it sinks. According to a study by Kowalski et al., the presence of MPs in sludge can make it denser and more viscous, slowing down the MPs’ sinking rate. This is due to the possibility that the increased density and viscosity will hamper the mobility of MPs inside the sludge [278]. It has been discovered that biofouling increases the hydrophilicity of plastic surfaces, making it more challenging to separate them from sediment mixtures based on polarity. Moreover, It is shown that marine biofouling may play a role in the settling motion of low-density MPs, particularly film MPs, which naturally float in saltwater [260].
Additionally, other sludge components, including organic matter and minerals, may impact the sinking velocity of MPs. MPs in the soil have been shown to increase soil bulk density and decrease soil porosity, which can reduce water infiltration and increase surface runoff [279]. Modifying the flow of water and sediment, which may vary the speed at which MPs sink, may impact the transport of MPs in soil. Furthermore, other soil constituents, including organic matter and minerals, may impact the velocity at which MPs sink. Soil MPs can bind to organic matter and mineral surfaces, which decreases their mobility and increases their retention in soil. It is represented that biofouling served as an adhesive agent to absorb inorganic minerals and raise MP density. As a result, all MPs treated with water sunk in the seawater due to the adherence of biofouling and inorganic debris [127]. It is concluded that biofouling contributes to enhancing the sinking of MPs. In this regard, Kaiser et al. demonstrated that biofouling enhances the deposition of MPs to marine sediment [29]. The growth of a biofilm accelerates the rate at which negatively buoyant MP particles sink. Therefore, biofouling significantly influences the sinking of MPs by gradually lessening the impact of density differences between particles and fluids, and the specific density of buoyant MP particles does not substantially increase by the formation of a tiny biofilm to result in their sinking. As a result, fouling microorganisms are required to move buoyant MP through the water [280]. Additionally, van Melkebeke et al. revealed that sinking rate assessments and estimates of films had a far higher likelihood than other plastic shapes of developing a biofilm, which is strong enough to sink buoyant polymers [260]. It is also expressed that thin films were the objects most likely to sink quickly as a result of biofouling [281].
A drag force that affects the behavior and mobility of particles is another element that experiences the biofouling effect. Because biofouling increases surface roughness and modifies the hydrodynamic characteristics of MPs, it affects the drag force that these particles encounter in soil settings. In soil conditions, the bio-fouled MPs settle more quickly due to their decreased buoyancy and increased density. Research has shown that biofouling affects the way MPs sink in estuary waters, a change that can also be detected in soil habitats [29]. Due to entanglement and biofouling with soil and sludge particles, fibers undergo a considerable increase in drag force because of their elongated shape and high surface area [260]. While films are more flexible than fibers, they also expose a greater amount of their effective surface area to the medium, which increases drag force. However, because of their dense and irregular structure, fragments show less surface area, which results in a significantly smaller increase in drag force. Compared to more flexible MPs, their rigidity and compactness cause a lesser rise in drag force [260].
8. Conclusions
MPs can enter the environment through direct human use of products or indirectly through sludge from wastewater treatment facilities. The concentration of MPs in sludge and soil is influenced by factors such as the wastewater source, treatment technology, and sampling technique. MP concentrations in sludge can range from 510 to 495,000 Particles/kg of dry weight (dw), highlighting the necessity of analyzing MP concentrations in sludge and wastewater. On average, MP concentrations in wastewater, sludge, and soil are 201, 271, and 34.6 Particles/kg, respectively. The concentration of MPs in soils treated with sludge is roughly 2–3 times higher than in untreated soils, with levels ranging from 300 to 50,000 Particles/kg.
The correlation analysis of MP concentrations shows that there is a direct relationship between the MP concentration in sludge and soil and the MP concentration in wastewater and sludge. The results of the linear regression coefficients suggest that as the concentrations of MPs increase in both sludge and wastewater, there is a proportional increase in their concentrations throughout the system. The correlation between WW and sludge matrices is not as strong as between sludge and soil. This suggests that changes in MP concentration in soil have a larger impact on the resulting MP concentration in sludge than changes in MP concentration in wastewater. There is a statistically significant correlation between the amounts of MP in soil and sludge, as indicated by The p-value of 0.0001 (‘p < 0.05’) in these samples. The correlation between MP concentrations in wastewater (WW) and sludge has a p-value of 0.47, which is greater than the significance level of 0.05. This indicates that there is no statistically significant association between the two variables. Therefore, we cannot statistically conclude that MP concentration in wastewater influences MP concentration in sludge.
It can be concluded that the MPs may have smaller sizes in sludge than in soils which may be attributed to the soil’s dynamic contribution in sink and source from various sources and the histogram analysis represents that their highest size ranges between 100 and 2000 µm. Moreover, the abundance and morphology of MPs can vary significantly depending on the type of polymer and other environmental factors. The abundance of MPs differs between sludge and soil due to factors such as the amount of sewage sludge used and its history of applications. Using sludge compost products can increase the number of MPs in soils. Fibers are the most common type of MP found in both sludge and soil. However, other factors like the type of wastewater treatment plant, sampling strategy, sample season, and land use activities can impact the diversity of MP shapes in sludge and soil. Polyethylene (PE) and polypropylene (PP) are the most common types of MPs found in both sludge and soil due to their wide uses. The morphology of MPs varies with characteristics such as deposited or adherent biological or non-biological particles, surface cracks, grooves, pits, breakages, and scaly appearances. It is important to note that the type and geometry of MPs affect their surface erosion.
The presence of MPs in sewage sludge can have both positive and negative effects when used as fertilizer on agricultural land. MPs can alter the properties of both the sludge and the soil, leading to changes in floc size, chemical demand, physical interference, toxicity risk, biofilm production, and accumulation of antibiotic-resistant bacteria. The addition of MPs can decrease floc formation and biodegradability. Furthermore, while MPs in soils can increase available water and soil pH, they may decrease soil aggregation ability, porosity, bulk density, available nutrients, organic compounds, and microbial community diversity and activity. In addition, understanding the biofouling process, where organisms deposit on MPs, is crucial due to its ecological and environmental consequences. Biofouling can make MPs denser and less buoyant, causing them to settle. The impact of MPs on biofouling depends on their size, shape, surface characteristics, and the types of organisms involved.
9. Recommendations on Future Work
The study of characteristics of MPs in sludge is imperative due to their significant impact on soil quality and, subsequently, the potential contamination of soils by MPs. This contamination poses serious threats to both the environment and human health. Consequently, some potential areas of investigation are recommended to be conducted by researchers in the future:
- study the effect of MPs on wastewater treatment methods;
- develop standardized sampling and analysis methods to evaluate the fate of MPs in WWTPs or other environmental media;
- evaluate the pollution risks to humans from MPs in sludge-amended soils;
- evaluate the possibility of uptake of MPs by plants;
- assess the effects of MPs on trophic transfer along the food chain;
- analyze the potential risks of degradation byproducts released from MPs;
- study the plastic weathering and transport processes within the soil profile.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemengineering8050086/s1, two tables are published online alongside the manuscript titled; Table S1 Microplastic characteristics in sewage sludge from various wastewater treatment plants, Table S2 Occurrence of microplastics in soil. References [282,283,284,285,286,287,288,289] are cited in Supplementary Materials.
Author Contributions
M.A. Conceptualization, methodology, software, writing-original draft, and writing-review and editing. J.Y. Supervision, conceptualization, and writing-review and editing. B.N. Methodology, the software, review of the manuscript, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
| aAP | Aromatic polyamide |
| ABS | Acrylonitrile butadiene styrene |
| AF | Acrylic fibers |
| CA | Cellulose acetate |
| CN | Cellulose nitrate |
| EE | Ethylene ethyl |
| EP | Epoxy resins |
| EPM | Ethylene-propylene polymer |
| EVA | Ethylene-vinyl acetate |
| HDPE | High-density polyethylene |
| IW | Industrial wastewater |
| LDPE | Low-density polyethylene |
| MW | Mixed wastewater |
| SBR | Styrene butadiene rubber |
| SS | Sewage Sludge |
| PA | Polyamide |
| PAC | Polyacrylochloride |
| PAN | Polyacrylonitrile |
| PB | Polybutylene |
| PBM | Polybutylene |
| PC | Polycarbonate |
| PCL | Poly ε-caprolactone |
| PDS | Polydimethyl Siloxane |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PES | Polyester |
| PI | Polyimide |
| PMMA | Polymethyl methacrylate |
| PO | Polyolefin |
| POM | Polyoxymethylene |
| PP | Polypropylene |
| PP-PE | Polypropylene- polyethylene |
| PS | Polystyrene |
| PTU | Polytereurethane |
| PU | Polyurethane |
| PVAC | Polyvinyl acetate |
| PVC | Polyvinylchloride |
| WW | Wastewater |
| WWTP | Wastewater Treatment Plant |
| UW | Urban Wastewater |
References
- Jiang, C.; Yin, L.; Li, Z.; Wen, X.; Luo, X.; Hu, S.; Yang, H.; Long, Y.; Deng, B.; Huang, L. Microplastic pollution in the rivers of the Tibet Plateau. Environ. Pollut. 2019, 249, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Nayebi, B.; Ghalebizade, M.; Niavol, K.P. Removal of Acid Red 131 by peroxi-coagulation using stainless steel and aluminum electrodes: A comparative study. Water Conserv. Sci. Eng. 2021, 6, 201–211. [Google Scholar] [CrossRef]
- Frias, J.P.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, P.; Manna, C.; Jain, M. Abundance, interaction, ingestion, ecological concerns, and mitigation policies of microplastic pollution in riverine ecosystem: A review. Sci. Total Environ. 2021, 782, 146695. [Google Scholar] [CrossRef]
- Arab, M.; Danesh, S. A sustainable system for decontamination of cephalexin antibiotic using electrocoagulation technology and response surface methodology. Remediat. J. 2023, 33, 365–378. [Google Scholar] [CrossRef]
- Duan, J.; Bolan, N.; Li, Y.; Ding, S.; Atugoda, T.; Vithanage, M.; Sarkar, B.; Tsang, D.C.; Kirkham, M. Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Res. 2021, 196, 117011. [Google Scholar] [CrossRef]
- Acarer, S. Microplastics in wastewater treatment plants: Sources, properties, removal efficiency, removal mechanisms, and interactions with pollutants. Water Sci. Technol. 2023, 87, 685–710. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, D.; Li, Z.; Zhu, C. Advancement and challenges of microplastic pollution in the aquatic environment: A review. Water Air Soil Pollut. 2018, 229, 140. [Google Scholar] [CrossRef]
- Xu, Z.; Bai, X.; Ye, Z. Removal and generation of microplastics in wastewater treatment plants: A review. J. Clean. Prod. 2021, 291, 125982. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef] [PubMed]
- Lofty, J.; Muhawenimana, V.; Wilson, C.; Ouro, P. Microplastics removal from a primary settler tank in a wastewater treatment plant and estimations of contamination onto European agricultural land via sewage sludge recycling. Environ. Pollut. 2022, 304, 119198. [Google Scholar] [CrossRef]
- Milojevic, N.; Cydzik-Kwiatkowska, A. Agricultural use of sewage sludge as a threat of microplastic (Mp) spread in the environment and the role of governance. Energies 2021, 14, 6293. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef]
- Hossain, M.N.; Rahman, M.M.; Afrin, S.; Akbor, M.A.; Siddique, M.A.B.; Malafaia, G. Identification and quantification of microplastics in agricultural farmland soil and textile sludge in Bangladesh. Sci. Total Environ. 2023, 858, 160118. [Google Scholar] [CrossRef]
- Piehl, S.; Leibner, A.; Löder, M.G.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro-and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Fu, C.; Zhou, Y.; Dai, Z.; Li, Y.; Tu, C.; Luo, Y. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- van den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef]
- Bostan, N.; Ilyas, N.; Akhtar, N.; Mehmood, S.; Saman, R.U.; Sayyed, R.; Shatid, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Pandiaraj, S. Toxicity assessment of microplastic (MPs); a threat to the ecosystem. Environ. Res. 2023, 234, 116523. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the environment: Intake through the food web, human exposure and toxicological effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef]
- Büks, F.; Kaupenjohann, M. Global concentrations of microplastic in soils, a review. Soil Discuss. 2020, 2020, 649–662. [Google Scholar] [CrossRef]
- Nayebi, B.; Khurana, P.; Pulicharla, R.; Karimpour, S.; Brar, S.K. Preservation, storage, and sample preparation methods for freshwater microplastics—A comprehensive review. Environ. Sci. Adv. 2023, 2, 1060–1081. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.; Sin, A.; Kim, G.; Khan, S.; Nadagouda, M.N.; Sahle-Demessie, E.; Han, C. Pretreatment methods for monitoring microplastics in soil and freshwater sediment samples: A comprehensive review. Sci. Total Environ. 2023, 871, 161718. [Google Scholar] [CrossRef] [PubMed]
- Stock, F.; Kochleus, C.; Bänsch-Baltruschat, B.; Brennholt, N.; Reifferscheid, G. Sampling techniques and preparation methods for microplastic analyses in the aquatic environment—A review. TrAC Trends Anal. Chem. 2019, 113, 84–92. [Google Scholar] [CrossRef]
- Fazey, F.M.; Ryan, P.G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. Pollut. 2016, 210, 354–360. [Google Scholar] [CrossRef]
- Kaiser, D.; Kowalski, N.; Waniek, J.J. Effects of biofouling on the sinking behavior of microplastics. Environ. Res. Lett. 2017, 12, 124003. [Google Scholar] [CrossRef]
- Long, Z.; Pan, Z.; Wang, W.; Ren, J.; Yu, X.; Lin, L.; Lin, H.; Chen, H.; Jin, X. Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res. 2019, 155, 255–265. [Google Scholar] [CrossRef]
- Yang, L.; Li, K.; Cui, S.; Kang, Y.; An, L.; Lei, K. Removal of microplastics in municipal sewage from China’s largest water reclamation plant. Water Res. 2019, 155, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Y. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Xie, Y.; Zhong, S.; Gao, P. Occurrence and removal of microplastics from wastewater treatment plants in a typical tourist city in China. J. Clean. Prod. 2021, 291, 125968. [Google Scholar] [CrossRef]
- Salmi, P.; Ryymin, K.; Karjalainen, A.K.; Mikola, A.; Uurasjärvi, E.; Talvitie, J. Particle balance and return loops for microplastics in a tertiary-level wastewater treatment plant. Water Sci. Technol. 2021, 84, 89–100. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Franco, A.; Arellano, J.; Albendín, G.; Rodríguez-Barroso, R.; Zahedi, S.; Quiroga, J.M.; Coello, M.D. Mapping microplastics in Cadiz (Spain): Occurrence of microplastics in municipal and industrial wastewaters. J. Water Process Eng. 2020, 38, 101596. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef]
- Cunsolo, S.; Williams, J.; Hale, M.; Read, D.S.; Couceiro, F. Optimising sample preparation for FTIR-based microplastic analysis in wastewater and sludge samples: Multiple digestions. Anal. Bioanal. Chem. 2021, 413, 3789–3799. [Google Scholar] [CrossRef]
- Tadsuwan, K.; Babel, S. Microplastic abundance and removal via an ultrafiltration system coupled to a conventional municipal wastewater treatment plant in Thailand. J. Environ. Chem. Eng. 2022, 10, 107142. [Google Scholar] [CrossRef]
- Yahyanezhad, N.; Bardi, M.J.; Aminirad, H. An evaluation of microplastics fate in the wastewater treatment plants: Frequency and removal of microplastics by microfiltration membrane. Water Pract. Technol. 2021, 16, 782–792. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Duan, L.; Zhang, Y.; Zhou, Y.; Sui, Q.; Xu, D.; Qu, H.; Yu, G. Occurrence and distribution of microplastics in domestic, industrial, agricultural and aquacultural wastewater sources: A case study in Changzhou, China. Water Res. 2020, 182, 115956. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Horton, A.A.; Cross, R.K.; Read, D.S.; Jürgens, M.D.; Ball, H.L.; Svendsen, C.; Vollertsen, J.; Johnson, A.C. Semi-automated analysis of microplastics in complex wastewater samples. Environ. Pollut. 2021, 268, 115841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Su, Y.; Zhu, J.; Shi, J.; Huang, H.; Xie, B. Distribution and removal characteristics of microplastics in different processes of the leachate treatment system. Waste Manag. 2021, 120, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Dou, M.; Wang, C.; Li, G.; Jia, R. Abundance and removal characteristics of microplastics at a wastewater treatment plant in Zhengzhou. Environ. Sci. Pollut. Res. 2020, 27, 36295–36305. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Xu, J.; Su, X.; Lu, M.; Wang, Z.; Zhang, Y. Occurrence and characteristics of microplastics in a wastewater treatment plant. Bull. Environ. Contam. Toxicol. 2021, 107, 677–683. [Google Scholar] [CrossRef]
- Kong, W.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A.; Almadiy, A.A.; Thakur, N.; Salama, E.-S. Occurrence, characteristics, and microbial community of microplastics in anaerobic sludge of wastewater treatment plants. Environ. Pollut. 2024, 344, 123370. [Google Scholar] [CrossRef]
- Klemmensen, N.D.; Chand, R.; Blanco, M.S.; Vollertsen, J. Microplastic abundance in sludge-treated fields: Variance and estimated half-life. Sci. Total Environ. 2024, 922, 171394. [Google Scholar] [CrossRef]
- Kazour, M.; Terki, S.; Rabhi, K.; Jemaa, S.; Khalaf, G.; Amara, R. Sources of microplastics pollution in the marine environment: Importance of wastewater treatment plant and coastal landfill. Mar. Pollut. Bull. 2019, 146, 608–618. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y. Treatment characteristics of microplastics at biological sewage treatment facilities in Korea. Mar. Pollut. Bull. 2018, 137, 1–8. [Google Scholar] [CrossRef]
- Maw, M.M.; Boontanon, N.; Aung, H.K.Z.Z.; Jindal, R.; Fujii, S.; Visvanathan, C.; Boontanon, S.K. Microplastics in wastewater and sludge from centralized and decentralized wastewater treatment plants: Effects of treatment systems and microplastic characteristics. Chemosphere 2024, 361, 142536. [Google Scholar] [CrossRef] [PubMed]
- Vardar, S.; Onay, T.T.; Demirel, B.; Kideys, A.E. Evaluation of microplastics removal efficiency at a wastewater treatment plant discharging to the Sea of Marmara. Environ. Pollut. 2021, 289, 117862. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.; Lee, J.-Y.; Cha, J. Microplastics in soil and freshwater: Understanding sources, distribution, potential impacts, and regulations for management. Sci. Prog. 2022, 105, 00368504221126676. [Google Scholar] [CrossRef] [PubMed]
- Chand, R.; Kohansal, K.; Toor, S.; Pedersen, T.H.; Vollertsen, J. Microplastics degradation through hydrothermal liquefaction of wastewater treatment sludge. J. Clean. Prod. 2022, 335, 130383. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Xing, R.; Xie, S.; Yang, X.; Cui, P.; Lü, J.; Liao, H.; Yu, Z.; Wang, S. Enhanced in situ biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology. J. Hazard. Mater. 2020, 384, 121271. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- El Hayany, B.; El Fels, L.; Quénéa, K.; Dignac, M.-F.; Rumpel, C.; Gupta, V.K.; Hafidi, M. Microplastics from lagooning sludge to composts as revealed by fluorescent staining-image analysis, Raman spectroscopy and pyrolysis-GC/MS. J. Environ. Manag. 2020, 275, 111249. [Google Scholar] [CrossRef]
- Sujathan, S.; Kniggendorf, A.-K.; Kumar, A.; Roth, B.; Rosenwinkel, K.-H.; Nogueira, R. Heat and bleach: A cost-efficient method for extracting microplastics from return activated sludge. Arch. Environ. Contam. Toxicol. 2017, 73, 641–648. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Di, M.; Li, Z.; Wang, J. Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. Chem. Eng. J. 2019, 362, 176–182. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in sewage sludge: Effects of treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Pittura, L.; Foglia, A.; Akyol, Ç.; Cipolletta, G.; Benedetti, M.; Regoli, F.; Eusebi, A.L.; Sabbatini, S.; Tseng, L.Y.; Katsou, E. Microplastics in real wastewater treatment schemes: Comparative assessment and relevant inhibition effects on anaerobic processes. Chemosphere 2021, 262, 128415. [Google Scholar] [CrossRef]
- Leslie, H.; Brandsma, S.; Van Velzen, M.; Vethaak, A. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hurley, R.; Vogelsang, C.; Nizzetto, L.; Olsen, M. Mapping Microplastics in Sludge; Norwegian Institute for Water Research: Oslo, Norway, 2017. [Google Scholar]
- Hernández-Arenas, R.; Beltrán-Sanahuja, A.; Navarro-Quirant, P.; Sanz-Lazaro, C. The effect of sewage sludge containing microplastics on growth and fruit development of tomato plants. Environ. Pollut. 2021, 268, 115779. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.A.; Iordachescu, L.; Tumlin, S.; Vollertsen, J. A complete mass balance for plastics in a wastewater treatment plant-macroplastics contributes more than microplastics. Water Res. 2021, 201, 117307. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, E.A.; Habibi, M.; Haddad, E.; Hasanin, M.; Angel, D.L.; Booth, A.M.; Sabbah, I. Microplastic distributions in a domestic wastewater treatment plant: Removal efficiency, seasonal variation and influence of sampling technique. Sci. Total Environ. 2021, 752, 141880. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Petroody, S.S.A.; Hashemi, S.H.; van Gestel, C.A. Transport and accumulation of microplastics through wastewater treatment sludge processes. Chemosphere 2021, 278, 130471. [Google Scholar] [CrossRef]
- Vockenberg, T.; Wichard, T.; Ueberschaar, N.; Franke, M.; Stelter, M.; Braeutigam, P. The sorption behaviour of amine micropollutants on polyethylene microplastics–impact of aging and interactions with green seaweed. Environ. Sci. Process. Impacts 2020, 22, 1678–1687. [Google Scholar] [CrossRef]
- El Hayany, B.; El Fels, L.; Ouhdouch, Y.; Hafidi, M. Fate of pathogenic microorganisms during lagooning sludge composting and exploration of bacteriophages as indicator of hygienization. Environ. Technol. Innov. 2021, 21, 101268. [Google Scholar] [CrossRef]
- Raju, S.; Carbery, M.; Kuttykattil, A.; Senthirajah, K.; Lundmark, A.; Rogers, Z.; Suresh, S.; Evans, G.; Palanisami, T. Improved methodology to determine the fate and transport of microplastics in a secondary wastewater treatment plant. Water Res. 2020, 173, 115549. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Neale, P.A.; Silveira, I.T.; Chua, A.; Leusch, F.D. An audit of microplastic abundance throughout three Australian wastewater treatment plants. Chemosphere 2021, 263, 128294. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Manjón, A.; Martínez-Díez, R.; Sol, D.; Laca, A.; Laca, A.; Rancaño, A.; Díaz, M. Long-term occurrence and fate of microplastics in WWTPs: A case study in southwest Europe. Appl. Sci. 2022, 12, 2133. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Ma, J.; An, Y.; Liu, Q.; Yang, S.; Qu, Y.; Chen, H.; Zhao, W.; Tian, Y. Microplastics pollution in the soil mulched by dust-proof nets: A case study in Beijing, China. Environ. Pollut. 2021, 275, 116600. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Ren, H.; Cao, G.; Xie, G.; Xing, D.; Liu, B. Investigation and fate of microplastics in wastewater and sludge filter cake from a wastewater treatment plant in China. Sci. Total Environ. 2020, 746, 141378. [Google Scholar] [CrossRef] [PubMed]
- Bayo, J.; López-Castellanos, J.; Olmos, S. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar. Pollut. Bull. 2020, 156, 111211. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; Van Loosdrecht, M.C.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef]
- Üstün, G.E.; Bozdaş, K.; Can, T. Abundance and characteristics of microplastics in an urban wastewater treatment plant in Turkey. Environ. Pollut. 2022, 310, 119890. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, L.; Li, R.; Xu, L.; Shen, Y.; Li, S.; Tu, C.; Wu, L.; Christie, P.; Luo, Y. Microplastics in an agricultural soil following repeated application of three types of sewage sludge: A field study. Environ. Pollut. 2021, 289, 117943. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Zhang, Z.; Yan, Z.; Zhang, Y. Effects of chronic exposure to different sizes and polymers of microplastics on the characteristics of activated sludge. Sci. Total Environ. 2021, 783, 146954. [Google Scholar] [CrossRef]
- Yu, J.T.; Diamond, M.L.; Helm, P.A. A fit-for-purpose categorization scheme for microplastic morphologies. Integr. Environ. Assess. Manag. 2023, 19, 422–435. [Google Scholar] [CrossRef]
- Panno, S.V.; Kelly, W.R.; Scott, J.; Zheng, W.; McNeish, R.E.; Holm, N.; Hoellein, T.J.; Baranski, E.L. Microplastic contamination in karst groundwater systems. Groundwater 2019, 57, 189–196. [Google Scholar] [CrossRef]
- Rosal, R. Morphological description of microplastic particles for environmental fate studies. Mar. Pollut. Bull. 2021, 171, 112716. [Google Scholar] [CrossRef] [PubMed]
- Gesamp, G. Guidelines for the monitoring and assessment of plastic litter in the ocean. GESAMP Rep. Stud. 2019, 99, 130. [Google Scholar]
- Yang, Z.; Li, S.; Ma, S.; Liu, P.; Peng, D.; Ouyang, Z.; Guo, X. Characteristics and removal efficiency of microplastics in sewage treatment plant of Xi’an City, northwest China. Sci. Total Environ. 2021, 771, 145377. [Google Scholar] [CrossRef]
- Jiang, L.; Yin, M.; Tang, Y.; Dai, R.; Mo, L.; Yang, W.; Liang, Y.; Huang, K. Microfibers shed from synthetic textiles during laundry: Flow to wastewater treatment plants or release to receiving waters through storm drains? Process Saf. Environ. Prot. 2022, 168, 689–697. [Google Scholar] [CrossRef]
- Di Bella, G.; Corsino, S.F.; De Marines, F.; Lopresti, F.; La Carrubba, V.; Torregrossa, M.; Viviani, G. Occurrence of Microplastics in Waste Sludge of Wastewater Treatment Plants: Comparison between Membrane Bioreactor (MBR) and Conventional Activated Sludge (CAS) Technologies. Membranes 2022, 12, 371. [Google Scholar] [CrossRef]
- Kukkola, A.; Schneidewind, U.; Haverson, L.; Kelleher, L.; Drummond, J.D.; Sambrook Smith, G.; Lynch, I.; Krause, S. Snapshot Sampling May not Be Enough to Obtain Robust Estimates for Riverine Microplastic Loads. ACS EsT Water 2024, 4, 2309–2319. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Azari, A.; Vanoirbeek, J.A.; Van Belleghem, F.; Vleeschouwers, B.; Hoet, P.H.; Ghosh, M. Sampling strategies and analytical techniques for assessment of airborne micro and nano plastics. Environ. Int. 2023, 174, 107885. [Google Scholar] [CrossRef]
- Harley-Nyang, D.; Memon, F.A.; Jones, N.; Galloway, T. Investigation and analysis of microplastics in sewage sludge and biosolids: A case study from one wastewater treatment works in the UK. Sci. Total Environ. 2022, 823, 153735. [Google Scholar] [CrossRef]
- Talukdar, A.; Kundu, P.; Bhattacharya, S.; Dutta, N. Microplastic contamination in wastewater: Sources, distribution, detection and remediation through physical and chemical-biological methods. Sci. Total Environ. 2024, 916, 170254. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D. PE-RT: A New Class of Polyethylene for Industrial Pipes. In Proceedings of the International Conference on Offshore Mechanics and Arctic Engineering, Hamburg, Germany, 4–9 June 2006; pp. 513–521. [Google Scholar]
- Paiva, R.; Wrona, M.; Nerín, C.; Veroneze, I.B.; Gavril, G.-L.; Cruz, S.A. Importance of profile of volatile and off-odors compounds from different recycled polypropylene used for food applications. Food Chem. 2021, 350, 129250. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.-X.; Tang, R.-C.; Zhai, A.-D. Hydrophilic and antibacterial surface functionalization of polyamide fabric by coating with polylysine biomolecule. Prog. Org. Coat. 2020, 142, 105571. [Google Scholar] [CrossRef]
- Tsironi, T.N.; Chatzidakis, S.M.; Stoforos, N.G. The future of polyethylene terephthalate bottles: Challenges and sustainability. Packag. Technol. Sci. 2022, 35, 317–325. [Google Scholar] [CrossRef]
- Turner, A. Foamed polystyrene in the marine environment: Sources, additives, transport, behavior, and impacts. Environ. Sci. Technol. 2020, 54, 10411–10420. [Google Scholar] [CrossRef]
- Prata, J.C. Microplastics in wastewater: State of the knowledge on sources, fate and solutions. Mar. Pollut. Bull. 2018, 129, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mei, Q.; Chen, L.; Zhang, H.; Dong, B.; Dai, X.; He, C.; Zhou, J. Enhancement in adsorption potential of microplastics in sewage sludge for metal pollutants after the wastewater treatment process. Water Res. 2019, 157, 228–237. [Google Scholar] [CrossRef]
- Keerthika, K.; Padmavathy, P.; Rani, V.; Jeyashakila, R.; Aanand, S.; Kutty, R. Contamination of microplastics, surface morphology and risk assessment in beaches along the Thoothukudi coast, Gulf of Mannar region. Environ. Sci. Pollut. Res. 2022, 29, 75525–75538. [Google Scholar] [CrossRef]
- Abadi, Z.T.R.; Abtahi, B.; Fathi, M.; Mashhadi, N.; Grossart, H.-P. Size, shape, and elemental composition as predictors of microplastic surface erosion. J. Hazard. Mater. 2024, 476, 134961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, P.L.; Biesinger, M.C.; Grifi, M. Plastics and beaches: A degrading relationship. Mar. Pollut. Bull. 2009, 58, 80–84. [Google Scholar] [CrossRef]
- Veerasingam, S.; Saha, M.; Suneel, V.; Vethamony, P.; Rodrigues, A.C.; Bhattacharyya, S.; Naik, B. Characteristics, seasonal distribution and surface degradation features of microplastic pellets along the Goa coast, India. Chemosphere 2016, 159, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ou, Q.; van der Hoek, J.P.; Liu, G.; Lompe, K.M. Photo-oxidation of Micro-and Nanoplastics: Physical, Chemical, and Biological Effects in Environments. Environ. Sci. Technol. 2024, 58, 991–1009. [Google Scholar] [CrossRef]
- Abadi, Z.T.R.; Abtahi, B.; Grossart, H.-P.; Khodabandeh, S. Microplastic content of Kutum fish, Rutilus frisii kutum in the southern Caspian Sea. Sci. Total Environ. 2021, 752, 141542. [Google Scholar] [CrossRef]
- Bullard, J.E.; Zhou, Z.; Davis, S.; Fowler, S. Breakdown and modification of microplastic beads by aeolian abrasion. Environ. Sci. Technol. 2022, 57, 76–84. [Google Scholar] [CrossRef]
- Tajwar, M.; Yousuf Gazi, M.; Saha, S.K. Characterization and spatial abundance of microplastics in the coastal regions of Cox’s Bazar, Bangladesh: An integration of field, laboratory, and GIS techniques. Soil Sediment Contam. Int. J. 2022, 31, 57–80. [Google Scholar] [CrossRef]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/microplastics in water and wastewater treatment processes–origin, impact and potential solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- He, S.; Tong, J.; Xiong, W.; Xiang, Y.; Peng, H.; Wang, W.; Yang, Y.; Ye, Y.; Hu, M.; Yang, Z. Microplastics influence the fate of antibiotics in freshwater environments: Biofilm formation and its effect on adsorption behavior. J. Hazard. Mater. 2023, 442, 130078. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater treatment plants as a source of microplastics to an urban estuary: Removal efficiencies and loading per capita over one year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef]
- Roy, T.; Dey, T.K.; Jamal, M. Microplastic/nanoplastic toxicity in plants: An imminent concern. Environ. Monit. Assess. 2023, 195, 27. [Google Scholar] [CrossRef]
- Rose, P.K.; Yadav, S.; Kataria, N.; Khoo, K.S. Microplastics and nanoplastics in the terrestrial food chain: Uptake, translocation, trophic transfer, ecotoxicology, and human health risk. TrAC Trends Anal. Chem. 2023, 167, 117249. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C. Fate and Impacts of Microplastics in the Environment: Hydrosphere, Pedosphere, and Atmosphere. Environments 2023, 10, 70. [Google Scholar] [CrossRef]
- Eze, C.G.; Nwankwo, C.E.; Dey, S.; Sundaramurthy, S.; Okeke, E.S. Food chain microplastics contamination and impact on human health: A review. Environ. Chem. Lett. 2024, 22, 1889–1927. [Google Scholar] [CrossRef]
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health 2020, 14, 16–22. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.; Andrady, A.L.; Bornman, J.F.; Aucamp, P.J.; Bais, A.F.; Banaszak, A.T.; Barnes, P.W.; Bernhard, G.H.; Bruckman, L.S.; Busquets, R. Plastics in the environment in the context of UV radiation, climate change and the Montreal Protocol: UNEP Environmental Effects Assessment Panel, Update 2023. Photochem. Photobiol. Sci. 2024, 23, 629–650. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, Y.; Li, W.; Wang, J.; Zhang, X.; He, J.; Li, J.; Ma, Y.; Niu, Z. Co-effects of biofouling and inorganic matters increased the density of environmental microplastics in the sediments of Bohai Bay coast. Sci. Total Environ. 2020, 717, 134431. [Google Scholar] [CrossRef] [PubMed]
- Schell, T.; Hurley, R.; Buenaventura, N.T.; Mauri, P.V.; Nizzetto, L.; Rico, A.; Vighi, M. Fate of microplastics in agricultural soils amended with sewage sludge: Is surface water runoff a relevant environmental pathway? Environ. Pollut. 2022, 293, 118520. [Google Scholar] [CrossRef]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss floodplain soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Pérez-Reverón, R.; González-Sálamo, J.; Hernández-Sánchez, C.; González-Pleiter, M.; Hernández-Borges, J.; Díaz-Peña, F.J. Recycled wastewater as a potential source of microplastics in irrigated soils from an arid-insular territory (Fuerteventura, Spain). Sci. Total Environ. 2022, 817, 152830. [Google Scholar] [CrossRef]
- Reddy, A.S.; Nair, A.T. The fate of microplastics in wastewater treatment plants: An overview of source and remediation technologies. Environ. Technol. Innov. 2022, 28, 102815. [Google Scholar] [CrossRef]
- Gautam, K.; Sadasivam, A. Recent trends in analytical measures of microplastic in soil and toxicopathological risk assessment in earthworms. TrAC Trends Anal. Chem. 2023, 168, 117292. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Characterization of microplastics and the association of heavy metals with microplastics in suburban soil of central China. Sci. Total Environ. 2019, 694, 133798. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of plastic pollution on ecosystem services, sustainable development goals, and need to focus on circular economy and policy interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018, 616, 1056–1065. [Google Scholar] [CrossRef]
- Franco, A.; Martín-García, A.; Egea-Corbacho, A.; Arellano, J.; Albendín, G.; Rodríguez-Barroso, R.; Quiroga, J.; Coello, M. Assessment and accumulation of microplastics in sewage sludge at wastewater treatment plants located in Cádiz, Spain. Environ. Pollut. 2023, 317, 120689. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tang, W.; Wu, S.; Liu, H.; Yang, C. Fate and effects of microplastics in wastewater treatment processes. Sci. Total Environ. 2021, 757, 143902. [Google Scholar] [CrossRef]
- Koutnik, V.S.; Leonard, J.; Alkidim, S.; DePrima, F.J.; Ravi, S.; Hoek, E.M.; Mohanty, S.K. Distribution of microplastics in soil and freshwater environments: Global analysis and framework for transport modeling. Environ. Pollut. 2021, 274, 116552. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Allen, S.; Luo, X.; Allen, D. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics. Sci. Total Environ. 2021, 758, 143634. [Google Scholar] [CrossRef]
- Corradini, F.; Casado, F.; Leiva, V.; Huerta-Lwanga, E.; Geissen, V. Microplastics occurrence and frequency in soils under different land uses on a regional scale. Sci. Total Environ. 2021, 752, 141917. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, W.; Lu, S.; Huang, W.; Yuan, Q.; Tian, M.; Lv, W.; He, D. Microplastic pollution in rice-fish co-culture system: A report of three farmland stations in Shanghai, China. Sci. Total Environ. 2019, 652, 1209–1218. [Google Scholar] [CrossRef]
- Chen, S.; Ai, X.; Dong, T.; Li, B.; Luo, R.; Ai, Y.; Chen, Z.; Li, C. The physico-chemical properties and structural characteristics of artificial soil for cut slope restoration in Southwestern China. Sci. Rep. 2016, 6, 20565. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chen, Q.-L.; An, X.-L.; Yang, X.-R.; Christie, P.; Ke, X.; Wu, L.-H.; Zhu, Y.-G. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol. Biochem. 2018, 116, 302–310. [Google Scholar] [CrossRef]
- Rillig, M.C.; de Souza Machado, A.A.; Lehmann, A.; Klümper, U. Evolutionary implications of microplastics for soil biota. Environ. Chem. 2018, 16, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Lwanga, E.H.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the terrestrial ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.K.A.; Raju, S.; Singh, A.; Senathirajah, K.; Bhagwat-Russell, G.; Daggubati, L.; Kandaiah, R.; Palanisami, T. Occurrence and distribution of microplastics in long-term biosolid-applied rehabilitation land: An overlooked pathway for microplastic entry into terrestrial ecosystems in Australia. Environ. Pollut. 2023, 336, 122464. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and identification of microplastics from soil and sewage sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef]
- Fuller, S.G.; Gautam, A. A procedure for measuring microplastics using pressurized fluid extraction. Environ. Sci. Technol. 2016, 50, 5774–5780. [Google Scholar] [CrossRef]
- Leusch, F.D.; Lu, H.-C.; Perera, K.; Neale, P.A.; Ziajahromi, S. Analysis of the literature shows a remarkably consistent relationship between size and abundance of microplastics across different environmental matrices. Environ. Pollut. 2023, 319, 120984. [Google Scholar] [CrossRef]
- Long, Z.; Pan, Z.; Jin, X.; Zou, Q.; He, J.; Li, W.; Waters, C.N.; Turner, S.D.; do Sul, J.A.I.; Yu, X. Anthropocene microplastic stratigraphy of Xiamen Bay, China: A history of plastic production and waste management. Water Res. 2022, 226, 119215. [Google Scholar] [CrossRef]
- Liu, X.; Tang, N.; Yang, W.; Chang, J. Microplastics pollution in the soils of various land-use types along Sheshui River basin of Central China. Sci. Total Environ. 2022, 806, 150620. [Google Scholar] [CrossRef]
- O’Connor, D.; Pan, S.; Shen, Z.; Song, Y.; Jin, Y.; Wu, W.-M.; Hou, D. Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 2019, 249, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, Y.; Li, Y.; Hu, Q.; Wang, C.; Hu, J.; Zhang, W.; Wang, L.; Zhang, C.; Zhang, H. New insights into the vertical distribution and microbial degradation of microplastics in urban river sediments. Water Res. 2021, 188, 116449. [Google Scholar] [CrossRef] [PubMed]
- Ragoobur, D.; Huerta-Lwanga, E.; Somaroo, G.D. Microplastics in agricultural soils, wastewater effluents and sewage sludge in Mauritius. Sci. Total Environ. 2021, 798, 149326. [Google Scholar] [CrossRef]
- Li, N.-Y.; Qu, J.-H.; Yang, J.-Y. Microplastics distribution and microbial community characteristics of farmland soil under different mulch methods. J. Hazard. Mater. 2023, 445, 130408. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef] [PubMed]
- Golgoli, M.; Khiadani, M.; Shafieian, A.; Sen, T.K.; Hartanto, Y.; Johns, M.; Zargar, M. Microplastics fouling and interaction with polymeric membranes: A review. Chemosphere 2021, 283, 131185. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, S.; Qin, W.; Zhang, C.; Lv, C.; Zou, M. Effects of land use on the distribution of soil microplastics in the Lihe River watershed, China. Chemosphere 2023, 324, 138292. [Google Scholar] [CrossRef]
- Sarkar, S.; Diab, H.; Thompson, J. Microplastic pollution: Chemical characterization and impact on wildlife. Int. J. Environ. Res. Public Health 2023, 20, 1745. [Google Scholar] [CrossRef]
- Gayathri, N.; Prasad, G.; Prabhakaran, V.; Priya, V. Understanding the impact of microplastic contamination on soil quality and eco-toxicological risks in horticulture: A comprehensive review. Case Stud. Chem. Environ. Eng. 2024, 9, 100633. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Leusch, F.D. Systematic assessment of data quality and quality assurance/quality control (QA/QC) of current research on microplastics in biosolids and agricultural soils. Environ. Pollut. 2022, 294, 118629. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Slynkova, N.; Dwyer, J.; Griffith, M.; Fernandes, M.; Jaeger, J.E.; Leusch, F.D. Comprehensive assessment of microplastics in Australian biosolids: Abundance, seasonal variation and potential transport to agroecosystems. Water Res. 2024, 250, 121071. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hernandez-Milian, G. Microplastic extraction from marine vertebrate digestive tracts, regurgitates and scats: A protocol for researchers from all experience levels. Bio-Protoc. 2018, 8, e3087. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.R.; Kim, Y.-N.; Yoon, J.-H.; Dickinson, N.; Kim, K.-H. Plastic contamination of forest, urban, and agricultural soils: A case study of Yeoju City in the Republic of Korea. J. Soils Sediments 2021, 21, 1962–1973. [Google Scholar] [CrossRef]
- Khaldoon, S.; Lalung, J.; Maheer, U.; Kamaruddin, M.A.; Yhaya, M.F.; Alsolami, E.S.; Alorfi, H.S.; Hussein, M.A.; Rafatullah, M. A Review on the Role of Earthworms in Plastics Degradation: Issues and Challenges. Polymers 2022, 14, 4770. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic transport in soil by earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, G.; Feng, F.; Ye, J.; Liao, C.-H.; Wu, C.-H.; Chen, S.-C. Microplastics in the soil environment: Focusing on the sources, its transformation and change in morphology. Sci. Total Environ. 2023, 896, 165291. [Google Scholar] [CrossRef]
- Sørensen, L.; Groven, A.S.; Hovsbakken, I.A.; Del Puerto, O.; Krause, D.F.; Sarno, A.; Booth, A.M. UV degradation of natural and synthetic microfibers causes fragmentation and release of polymer degradation products and chemical additives. Sci. Total Environ. 2021, 755, 143170. [Google Scholar] [CrossRef]
- Kabir, M.S.; Wang, H.; Luster-Teasley, S.; Zhang, L.; Zhao, R. Microplastics in landfill leachate: Sources, detection, occurrence, and removal. Environ. Sci. Ecotechnol. 2023, 16, 100256. [Google Scholar] [CrossRef]
- Sellström, U.; de Wit, C.A.; Lundgren, N.; Tysklind, M. Effect of sewage-sludge application on concentrations of higher-brominated diphenyl ethers in soils and earthworms. Environ. Sci. Technol. 2005, 39, 9064–9070. [Google Scholar] [CrossRef]
- Banerjee, M.; Burton, D.; Depoe, S. Impact of sewage sludge application on soil biological characteristics. Agric. Ecosyst. Environ. 1997, 66, 241–249. [Google Scholar] [CrossRef]
- Pradas del Real, A.E.; Castillo-Michel, H.; Kaegi, R.; Sinnet, B.; Magnin, V.; Findling, N.; Villanova, J.; Carrière, M.; Santaella, C.; Fernández-Martínez, A. Fate of Ag-NPs in sewage sludge after application on agricultural soils. Environ. Sci. Technol. 2016, 50, 1759–1768. [Google Scholar] [CrossRef]
- Koul, B.; Bhat, N.; Abubakar, M.; Mishra, M.; Arukha, A.P.; Yadav, D. Application of Natural Coagulants in Water Treatment: A Sustainable Alternative to Chemicals. Water 2022, 14, 3751. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- Timilsina, A.; Adhikari, K.; Yadav, A.K.; Joshi, P.; Ramena, G.; Bohara, K. Effects of microplastics and nanoplastics in shrimp: Mechanisms of plastic particle and contaminant distribution and subsequent effects after uptake. Sci. Total Environ. 2023, 894, 164999. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef]
- Qian, J.; He, X.; Wang, P.; Xu, B.; Li, K.; Lu, B.; Jin, W.; Tang, S. Effects of polystyrene nanoplastics on extracellular polymeric substance composition of activated sludge: The role of surface functional groups. Environ. Pollut. 2021, 279, 116904. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, Q.; Li, J.; Li, Q.; Xu, H.; Ye, Q.; Wang, Y.; Shu, S.; Zhang, J. Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: Performance and mechanism. Sci. Total Environ. 2021, 752, 141837. [Google Scholar] [CrossRef] [PubMed]
- Shahi, N.K.; Maeng, M.; Kim, D.; Dockko, S. Removal behavior of microplastics using alum coagulant and its enhancement using polyamine-coated sand. Process. Saf. Environ. Prot. 2020, 141, 9–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Yue, J.; Xing, X.; Yang, Z.; Wang, X.; Wang, Q.; Zhang, J. Enhanced removal of polyethylene terephthalate microplastics through polyaluminum chloride coagulation with three typical coagulant aids. Sci. Total Environ. 2021, 800, 149589. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Li, J. The removal of microplastics in the wastewater treatment process and their potential impact on anaerobic digestion due to pollutants association. Chemosphere 2020, 251, 126360. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Zhang, X.; Yang, X.; Niu, S.; Zheng, Z.; Dong, B.; Hur, J.; Dai, X. Aging and mitigation of microplastics during sewage sludge treatments: An overview. Sci. Total Environ. 2024, 922, 171338. [Google Scholar] [CrossRef] [PubMed]
- Zita, A.; Hermansson, M. Effects of ionic strength on bacterial adhesion and stability of flocs in a wastewater activated sludge system. Appl. Environ. Microbiol. 1994, 60, 3041–3048. [Google Scholar] [CrossRef]
- Huang, L.; Jin, Y.; Zhou, D.; Liu, L.; Huang, S.; Zhao, Y.; Chen, Y. A review of the role of extracellular polymeric substances (EPS) in wastewater treatment systems. Int. J. Environ. Res. Public Health 2022, 19, 12191. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Milojevic, N.; Jachimowicz, P. The fate of microplastic in sludge management systems. Sci. Total Environ. 2022, 848, 157466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-T.; Wei, W.; Huang, Q.-S.; Wang, C.; Wang, Y.; Ni, B.-J. Insights into the microbial response of anaerobic granular sludge during long-term exposure to polyethylene terephthalate microplastics. Water Res. 2020, 179, 115898. [Google Scholar] [CrossRef]
- Zöhre, K.; James, A. Effectiveness of microplastics removal in wastewater treatment plants: A critical analysis of wastewater treatment processes. J. Environ. Chem. Eng. 2022, 10, 107831. [Google Scholar]
- Wei, W.; Huang, Q.-S.; Sun, J.; Wang, J.-Y.; Wu, S.-L.; Ni, B.-J. Polyvinyl chloride microplastics affect methane production from the anaerobic digestion of waste activated sludge through leaching toxic bisphenol-A. Environ. Sci. Technol. 2019, 53, 2509–2517. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Rong, H.; Li, J.; Zhao, Y.; Ren, L.; Chen, S.; Liu, J.; Mei, M.; Xue, Y.; Wang, T. Mechanistic insights into effect of in-situ microplastics on heavy metals leaching behavior from its dyeing sludge incineration bottom ash. J. Environ. Chem. Eng. 2023, 11, 110089. [Google Scholar] [CrossRef]
- Ma, J.; Gong, Z.; Wang, Z.; Liu, H.; Chen, G.; Guo, G. Elucidating degradation properties, microbial community, and mechanism of microplastics in sewage sludge under different terminal electron acceptors conditions. Bioresour. Technol. 2022, 346, 126624. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Mahmud, A.; Wasif, M.M.; Roy, H.; Mehnaz, F.; Ahmed, T.; Pervez, M.; Naddeo, V.; Islam, M. Aquatic Microplastic Pollution Control Strategies: Sustainable Degradation Techniques, Resource Recovery, and Recommendations for Bangladesh. Water 2022, 14, 3968. [Google Scholar] [CrossRef]
- Wei, W.; Hao, Q.; Chen, Z.; Bao, T.; Ni, B.-J. Polystyrene nanoplastics reshape the anaerobic granular sludge for recovering methane from wastewater. Water Res. 2020, 182, 116041. [Google Scholar] [CrossRef]
- European Commission. Review of Reach with Regard to the Registration Requirements on Polymers 070307/2011/602175/SER/D3 Final Report Part A: Polymers; European Commission DG Environment: Brussels, Belgium, 2012. [Google Scholar]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, H.; Hua, X.; Dang, Y.; Han, Y.; Yu, Z.; Chen, X.; Ding, P.; Li, H. Polystyrene microplastics (PS-MPs) toxicity induced oxidative stress and intestinal injury in nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 726, 138679. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.M.M.; Hai, F.I.; Lu, W.; Al-Mamun, A.; Dhar, B.R. A review of mechanisms underlying the impacts of (nano) microplastics on anaerobic digestion. Bioresour. Technol. 2021, 329, 124894. [Google Scholar]
- Cholewińska, P.; Moniuszko, H.; Wojnarowski, K.; Pokorny, P.; Szeligowska, N.; Dobicki, W.; Polechoński, R.; Górniak, W. The occurrence of microplastics and the formation of biofilms by pathogenic and opportunistic bacteria as threats in aquaculture. Int. J. Environ. Res. Public Health 2022, 19, 8137. [Google Scholar] [CrossRef]
- Glaser, J. The Importance of Biofilms to the Fate and Effects of Microplastics. In Bacterial Biofilms; IntechOpen: London, UK, 2020. [Google Scholar]
- Yang, Y.; Liu, W.; Zhang, Z.; Grossart, H.-P.; Gadd, G.M. Microplastics provide new microbial niches in aquatic environments. Appl. Microbiol. Biotechnol. 2020, 104, 6501–6511. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Wang, X.; Cheng, T.; Fu, K.; Qin, Z.; Feng, K. Biofilm structural and functional features on microplastic surfaces in greenhouse agricultural soil. Sustainability 2022, 14, 7024. [Google Scholar] [CrossRef]
- Perveen, S.; Pablos, C.; Reynolds, K.; Stanley, S.; Marugán, J. Growth and prevalence of antibiotic-resistant bacteria in microplastic biofilm from wastewater treatment plant effluents. Sci. Total Environ. 2023, 856, 159024. [Google Scholar] [CrossRef]
- Shi, J.; Wang, B.; Li, X.; Su, Y.; Wu, D.; Xie, B. Distinguishing removal and regrowth potential of antibiotic resistance genes and antibiotic resistant bacteria on microplastics and in leachate after chlorination or Fenton oxidation. J. Hazard. Mater. 2022, 430, 128432. [Google Scholar] [CrossRef]
- Wang, Z.; Su, Y.; Zhu, J.; Wu, D.; Xie, B. Size-dependent effects of microplastics on antibiotic resistance genes fate in wastewater treatment systems: The role of changed surface property and microbial assemblages in a continuous exposure mode. Sci. Total Environ. 2022, 851, 158264. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, J.; Dai, H.; Zhao, Y.; Li, D.; Duan, W.; Guo, Y. Microplastics affect the ammonia oxidation performance of aerobic granular sludge and enrich the intracellular and extracellular antibiotic resistance genes. J. Hazard. Mater. 2021, 409, 124981. [Google Scholar] [CrossRef]
- Yang, X.; Bento, C.P.; Chen, H.; Zhang, H.; Xue, S.; Lwanga, E.H.; Zomer, P.; Ritsema, C.J.; Geissen, V. Influence of microplastic addition on glyphosate decay and soil microbial activities in Chinese loess soil. Environ. Pollut. 2018, 242, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Chukwuemeka, I.S.; Li, G.; Mo, Y.; Jacques, K.J. Impacts of microplastics and urbanization on soil health: An urgent concern for sustainable development. Green Anal. Chem. 2024, 8, 100095. [Google Scholar]
- Zhai, Y.; Bai, J.; Chang, P.; Liu, Z.; Wang, Y.; Liu, G.; Cui, B.; Peijnenburg, W.; Vijver, M.G. Microplastics in terrestrial ecosystem: Exploring the menace to the soil-plant-microbe interactions. TrAC Trends Anal. Chem. 2024, 174, 117667. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Liang, X.; Lu, S.; Ren, J.; Zhang, Y.; Han, Z.; Gao, B.; Sun, K. Effects of microplastics on soil carbon pool and terrestrial plant performance. Carbon Res. 2024, 3, 37. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Lehmann, A.; Leifheit, E.F.; Gerdawischke, M.; Rillig, M.C. Microplastics have shape-and polymer-dependent effects on soil aggregation and organic matter loss—An experimental and meta-analytical approach. Microplast. Nanoplast. 2021, 1, 7. [Google Scholar] [CrossRef]
- Chen, L.; Han, L.; Feng, Y.; He, J.; Xing, B. Soil structures and immobilization of typical contaminants in soils in response to diverse microplastics. J. Hazard. Mater. 2022, 438, 129555. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, F.; Li, X. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, J.; Liu, Y.; Chen, L.; Tao, S.; Liu, W. Distribution characteristics of microplastics in agricultural soils from the largest vegetable production base in China. Sci. Total Environ. 2021, 756, 143860. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Beriot, N.; Gort, G.; Lwanga, E.H.; Gooren, H.; Yang, X.; Geissen, V. Impact of plastic mulch film debris on soil physicochemical and hydrological properties. Environ. Pollut. 2020, 266, 115097. [Google Scholar] [CrossRef]
- Yu, Y.; Battu, A.K.; Varga, T.; Denny, A.C.; Zahid, T.M.; Chowdhury, I.; Flury, M. Minimal impacts of microplastics on soil physical properties under environmentally relevant concentrations. Environ. Sci. Technol. 2023, 57, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef]
- Kim, S.W.; An, Y.-J. Soil microplastics inhibit the movement of springtail species. Environ. Int. 2019, 126, 699–706. [Google Scholar] [CrossRef]
- Chai, M.; Li, R.; Li, B.; Wu, H.; Yu, L. Responses of mangrove (Kandelia obovata) growth, photosynthesis, and rhizosphere soil properties to microplastic pollution. Mar. Pollut. Bull. 2023, 189, 114827. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, W.; Duan, C.; Zhu, X.; Wu, H.; Zhang, X.; Fang, L. Microplastics pollution from different plastic mulching years accentuate soil microbial nutrient limitations. Gondwana Res. 2022, 108, 91–101. [Google Scholar] [CrossRef]
- Gharahi, N.; Zamani-Ahmadmahmoodi, R. Effect of plastic pollution in soil properties and growth of grass species in semi-arid regions: A laboratory experiment. Environ. Sci. Pollut. Res. 2022, 29, 59118–59126. [Google Scholar] [CrossRef]
- Liu, S.; Huang, K.; Yuan, G.; Yang, C. Effects of Polyethylene Microplastics and Phenanthrene on Soil Properties, Enzyme Activities and Bacterial Communities. Processes 2022, 10, 2128. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, P.; Adams, C.A.; Zhang, S.; Sun, Y.; Yu, H.; Wang, F. Effects of microplastics on plant growth and arbuscular mycorrhizal fungal communities in a soil spiked with ZnO nanoparticles. Soil Biol. Biochem. 2021, 155, 108179. [Google Scholar] [CrossRef]
- You, J.; Liu, X.; Zhang, B.; Xie, Z.; Hou, Z.; Yang, Z. Seasonal changes in soil acidity and related properties in ginseng artificial bed soils under a plastic shade. J. Ginseng Res. 2015, 39, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, S.; Zhang, M.; Chen, G.; Zhu, T.; Zhang, S.; Teng, Y.; Christie, P.; Luo, Y. Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere 2016, 151, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Liu, Q.; Li, J.; Wang, C.; Li, Y.; Zhang, C. Microplastic pollution in marine environment: Occurrence, fate, and effects (with a specific focus on biogeochemical carbon and nitrogen cycles). Microplast. Pollut. 2021, 105–126. [Google Scholar]
- Rong, L.; Zhao, L.; Zhao, L.; Cheng, Z.; Yao, Y.; Yuan, C.; Wang, L.; Sun, H. LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 2021, 773, 145640. [Google Scholar] [CrossRef]
- Awet, T.; Kohl, Y.; Meier, F.; Straskraba, S.; Grün, A.-L.; Ruf, T.; Jost, C.; Drexel, R.; Tunc, E.; Emmerling, C. Effects of polystyrene nanoparticles on the microbiota and functional diversity of enzymes in soil. Environ. Sci. Eur. 2018, 30, 11. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, M.; Liu, G.; Lu, T.; Qu, Q.; Du, B.; Pan, X. Effects of soil residual plastic film on soil microbial community structure and fertility. Water Air Soil Pollut. 2018, 229, 261. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Gao, J.; Wang, F.; Yang, W.; Han, L.; Lin, D.; Min, B.; Zhi, Y.; Grieger, K. Effect of microplastics on ecosystem functioning: Microbial nitrogen removal mediated by benthic invertebrates. Sci. Total Environ. 2021, 754, 142133. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, L.; Zhao, Y.; Shan, J.; Zheng, X.; Xu, G.; Sun, Y.; Wang, F. Effects of microplastics and nitrogen deposition on soil multifunctionality, particularly C and N cycling. J. Hazard. Mater. 2023, 451, 131152. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, Y.; Cui, W.; Sun, Y.; Zhang, S.; Wang, F. Effects of microplastics and carbon nanotubes on soil geochemical properties and bacterial communities. J. Hazard. Mater. 2022, 433, 128826. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.; Angon, P.B.; Roy, A. Effects of microplastics on soil physical, chemical and biological properties. Nat. Hazards Res. 2024. [Google Scholar] [CrossRef]
- Hüffer, T.; Metzelder, F.; Sigmund, G.; Slawek, S.; Schmidt, T.C.; Hofmann, T. Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total Environ. 2019, 657, 242–247. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Lv, J.; Wang, Z.; Peng, Y.; Shang, J.; Wang, X. Microplastic additions alter soil organic matter stability and bacterial community under varying temperature in two contrasting soils. Sci. Total Environ. 2022, 838, 156471. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, X.; Wu, D.; Peng, L.; Fan, C.; Zhang, W.; Li, Q.; Ge, C. Addition of biodegradable microplastics alters the quantity and chemodiversity of dissolved organic matter in latosol. Sci. Total Environ. 2022, 816, 151960. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, E.; Bodor, A.; Kovács, E.; Papp, S.; Kovács, K.; Perei, K.; Feigl, G. Impacts of plastics on plant development: Recent advances and future research directions. Plants 2023, 12, 3282. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Osborn, A.M.; Duhaime, M.B. Microbes on a bottle: Substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS ONE 2016, 11, e0159289. [Google Scholar] [CrossRef]
- De Tender, C.A.; Devriese, L.I.; Haegeman, A.; Maes, S.; Ruttink, T.; Dawyndt, P. Bacterial community profiling of plastic litter in the Belgian part of the North Sea. Environ. Sci. Technol. 2015, 49, 9629–9638. [Google Scholar] [CrossRef]
- Carson, H.S.; Nerheim, M.S.; Carroll, K.A.; Eriksen, M. The plastic-associated microorganisms of the North Pacific Gyre. Mar. Pollut. Bull. 2013, 75, 126–132. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Mendoza Vega, J.; Ku Quej, V.; Chi, J.d.l.A.; Sanchez del Cid, L.; Chi, C.; Escalona Segura, G.; Gertsen, H.; Salánki, T.; van der Ploeg, M. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic in Terrestrial Ecosystems and the Soil? ACS Publications: Washington, WA, USA, 2012. [Google Scholar]
- Rillig, M.C.; Bonkowski, M. Microplastic and soil protists: A call for research. Environ. Pollut. 2018, 241, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, Z.; Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Effect of polyvinyl chloride microplastics on bacterial community and nutrient status in two agricultural soils. Bull. Environ. Contam. Toxicol. 2021, 107, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Xu, X.; Yu, H.; Xi, B.; Tan, W. Comparing the long-term responses of soil microbial structures and diversities to polyethylene microplastics in different aggregate fractions. Environ. Int. 2021, 149, 106398. [Google Scholar] [CrossRef] [PubMed]
- Gaylor, M.O.; Harvey, E.; Hale, R.C. Polybrominated diphenyl ether (PBDE) accumulation by earthworms (Eisenia fetida) exposed to biosolids-, polyurethane foam microparticle-, and penta-BDE-amended soils. Environ. Sci. Technol. 2013, 47, 13831–13839. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Seijo, A.; Lourenço, J.; Rocha-Santos, T.; Da Costa, J.; Duarte, A.; Vala, H.; Pereira, R. Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environ. Pollut. 2017, 220, 495–503. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and Arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- Wan, L.; Cheng, H.; Liu, Y.; Shen, Y.; Liu, G.; Su, X. Global meta-analysis reveals differential effects of microplastics on soil ecosystem. Sci. Total Environ. 2023, 867, 161403. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Sun, X.; Peng, Y.; Xiao, L. Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere 2020, 243, 125271. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Tang, J.; Liu, X.; Liu, Q. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ. Pollut. 2020, 256, 113347. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zhou, S.; Zhang, L.; Ding, S. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res. 2021, 93, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Moyal, J.; Dave, P.H.; Wu, M.; Karimpour, S.; Brar, S.K.; Zhong, H.; Kwong, R.W. Impacts of biofilm formation on the physicochemical properties and toxicity of microplastics: A concise review. Rev. Environ. Contam. Toxicol. 2023, 261, 8. [Google Scholar] [CrossRef]
- Van Melkebeke, M.; Janssen, C.; De Meester, S. Characteristics and sinking behavior of typical microplastics including the potential effect of biofouling: Implications for remediation. Environ. Sci. Technol. 2020, 54, 8668–8680. [Google Scholar] [CrossRef]
- Enfrin, M.; Lee, J.; Le-Clech, P.; Dumee, L.F. Kinetic and mechanistic aspects of ultrafiltration membrane fouling by nano-and microplastics. J. Membr. Sci. 2020, 601, 117890. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ingraffia, R.; de Souza Machado, A.A. Microplastic incorporation into soil in agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Chen, L.; Chao, J.; Teng, J.; Wang, Q. Microplastics in soils: A review of possible sources, analytical methods and ecological impacts. J. Chem. Technol. Biotechnol. 2020, 95, 2052–2068. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; Lu, S.; Qiu, R.; Hu, J.; Li, X.; Bigalke, M.; Shi, H.; He, D. A method for extracting soil microplastics through circulation of sodium bromide solutions. Sci. Total Environ. 2019, 691, 341–347. [Google Scholar] [CrossRef]
- Komar, P.D.; Reimers, C. Grain shape effects on settling rates. J. Geol. 1978, 86, 193–209. [Google Scholar] [CrossRef]
- Fiett, P.P. The Effects of Biofouling on Distribution of Microplastics in the Aquatic Environment. Master’s Thesis, Roskilde University, Roskilde, Denmark, 2017. [Google Scholar]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Kooi, M.; Nes, E.H.V.; Scheffer, M.; Koelmans, A.A. Ups and downs in the ocean: Effects of biofouling on vertical transport of microplastics. Environ. Sci. Technol. 2017, 51, 7963–7971. [Google Scholar] [CrossRef]
- Jalón-Rojas, I.; Romero-Ramírez, A.; Fauquembergue, K.; Rossignol, L.; Cachot, J.; Sous, D.; Morin, B. Effects of biofilms and particle physical properties on the rising and settling velocities of microplastic fibers and sheets. Environ. Sci. Technol. 2022, 56, 8114–8123. [Google Scholar] [CrossRef]
- Lobelle, D.; Cunliffe, M. Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 2011, 62, 197–200. [Google Scholar] [CrossRef]
- Ryan, P.G. A brief history of marine litter research. Mar. Anthropog. Litter 2015, 1–25. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Quik, J.T.; de Klein, J.J.; Koelmans, A.A. Spatially explicit fate modelling of nanomaterials in natural waters. Water Res. 2015, 80, 200–208. [Google Scholar] [CrossRef]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef]
- Khatmullina, L.; Isachenko, I. Settling velocity of microplastic particles of regular shapes. Mar. Pollut. Bull. 2017, 114, 871–880. [Google Scholar] [CrossRef]
- Waldschläger, K.; Schüttrumpf, H. Effects of particle properties on the settling and rise velocities of microplastics in freshwater under laboratory conditions. Environ. Sci. Technol. 2019, 53, 1958–1966. [Google Scholar] [CrossRef]
- Kaiser, D.; Estelmann, A.; Kowalski, N.; Glockzin, M.; Waniek, J.J. Sinking velocity of sub-millimeter microplastic. Mar. Pollut. Bull. 2019, 139, 214–220. [Google Scholar] [CrossRef]
- Kowalski, N.; Reichardt, A.M.; Waniek, J.J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 2016, 109, 310–319. [Google Scholar] [CrossRef]
- Guo, Z.; Li, P.; Yang, X.; Wang, Z.; Lu, B.; Chen, W.; Wu, Y.; Li, G.; Zhao, Z.; Liu, G. Soil texture is an important factor determining how microplastics affect soil hydraulic characteristics. Environ. Int. 2022, 165, 107293. [Google Scholar] [CrossRef]
- Fazey, F.M.; Ryan, P.G. Debris size and buoyancy influence the dispersal distance of stranded litter. Mar. Pollut. Bull. 2016, 110, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Leiser, R.; Wu, G.-M.; Neu, T.R.; Wendt-Potthoff, K. Biofouling, metal sorption and aggregation are related to sinking of microplastics in a stratified reservoir. Water Res. 2020, 176, 115748. [Google Scholar] [CrossRef] [PubMed]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, Y.; Xu, L.; Shi, W.; Wang, F.; Leblanc, G.A.; Cui, S.; An, L.; Lei, K. Investigation of the microplastics pro fi le in sludge from China’s largest Water reclamation plant using a feasible. J. Hazard. Mater. 2020, 388, 122067. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.; Nor’en, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant; Swedish Environmental Research Institute: Stockholm, Sweden, 2014. [Google Scholar]
- Hongprasith, N.; Kittimethawong, C.; Lertluksanaporn, R.; Eamchotchawalit, T.; Kittipongvises, S.; Lohwacharin, J. IR microspectroscopic identification of microplastics in municipal wastewater treatment plants. Environ. Sci. Pollut. Res. 2020, 27, 18557–18564. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Brandes, E.; Henseler, M.; Kreins, P. Identifying hot-spots for microplastic contamination in agricultural soils—A spatial modelling approach for Germany. Environ. Res. Lett. 2021, 16, 104041. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Kim, B.-H.; Kim, K.-H. Distribution of microplastics in soil by types of land use in metropolitan area of Seoul. Appl. Biol. Chem. 2024, 67, 15. [Google Scholar] [CrossRef]
- Wang, K.; Min, W.; Flury, M.; Gunina, A.; Lv, J.; Li, Q.; Jiang, R. Impact of long-term conventional and biodegradable film mulching on microplastic abundance, soil structure and organic carbon in a cotton field. Environ. Pollut. 2024, 356, 124367. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).