Enhanced Removal of Chlorpyrifos, Cu(II), Pb(II), and Iodine from Aqueous Solutions Using Ficus Nitida and Date Palm Biochars
Abstract
1. Introduction
2. Materials and Methods
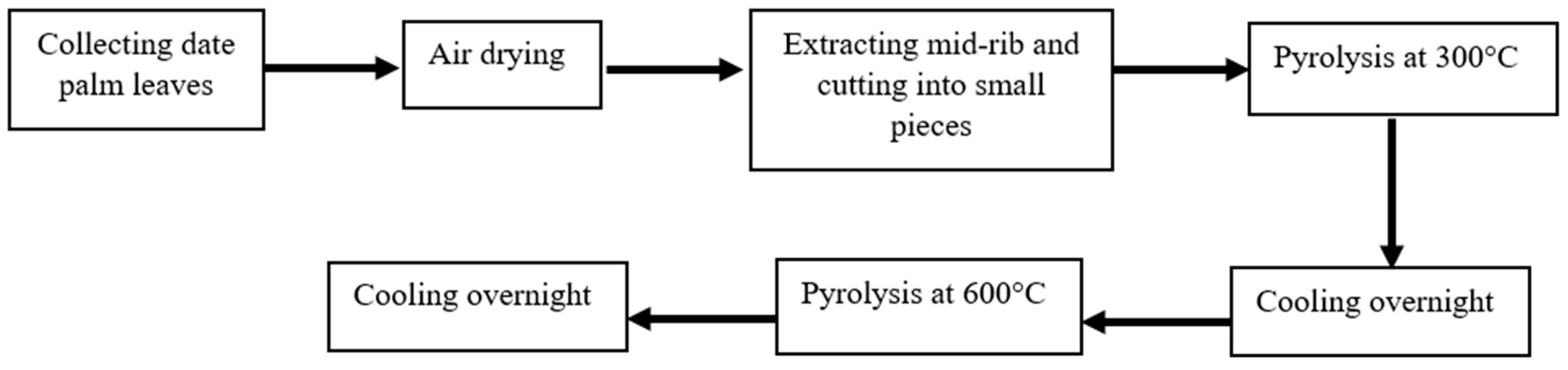
2.1. Biochar Preparation
2.2. Biochar Characterization
2.3. Adsorption of Heavy Metals by Biochar
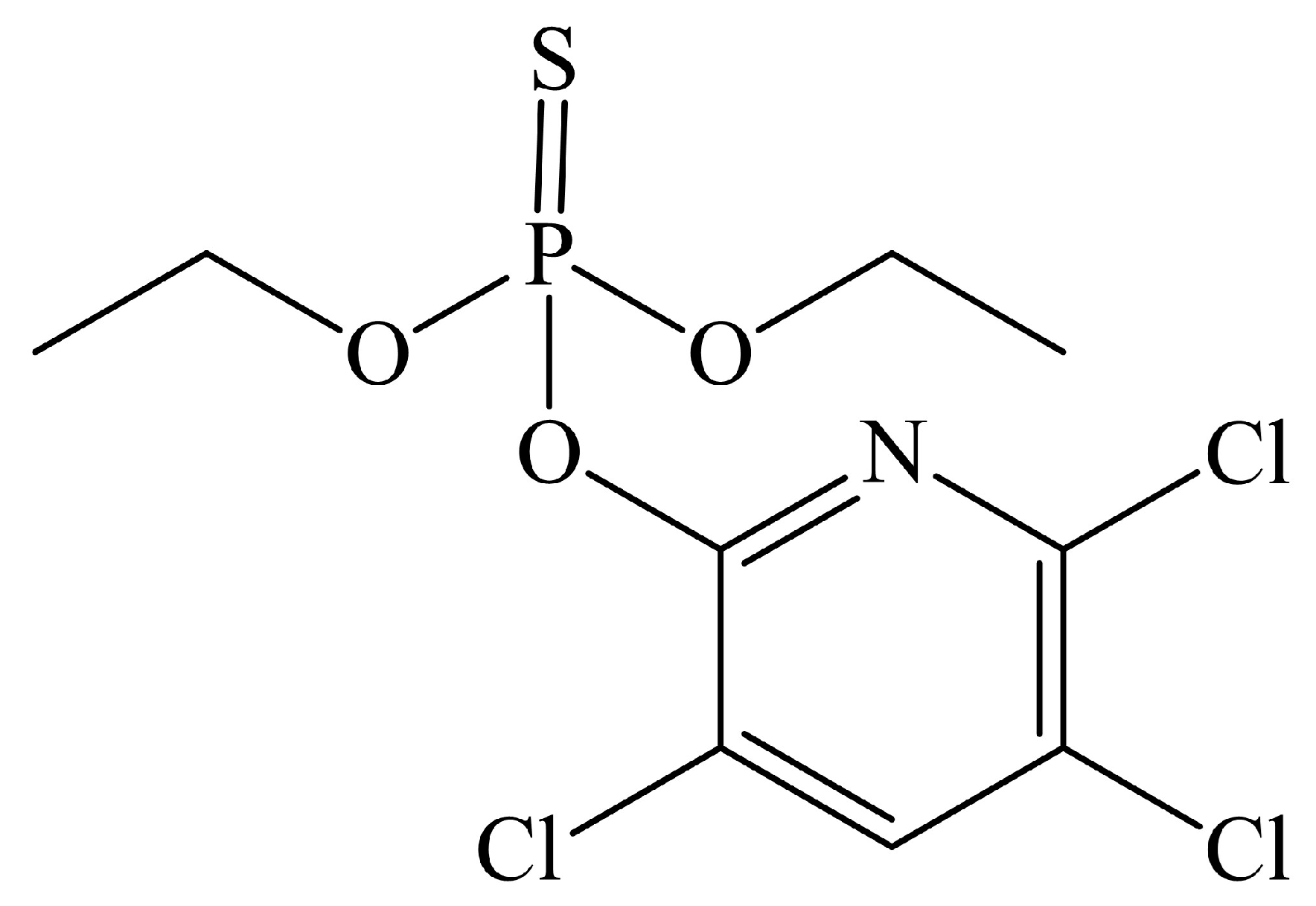
2.4. Adsorption of Chlorpyrifos by Biochar
3. Results and Discussion
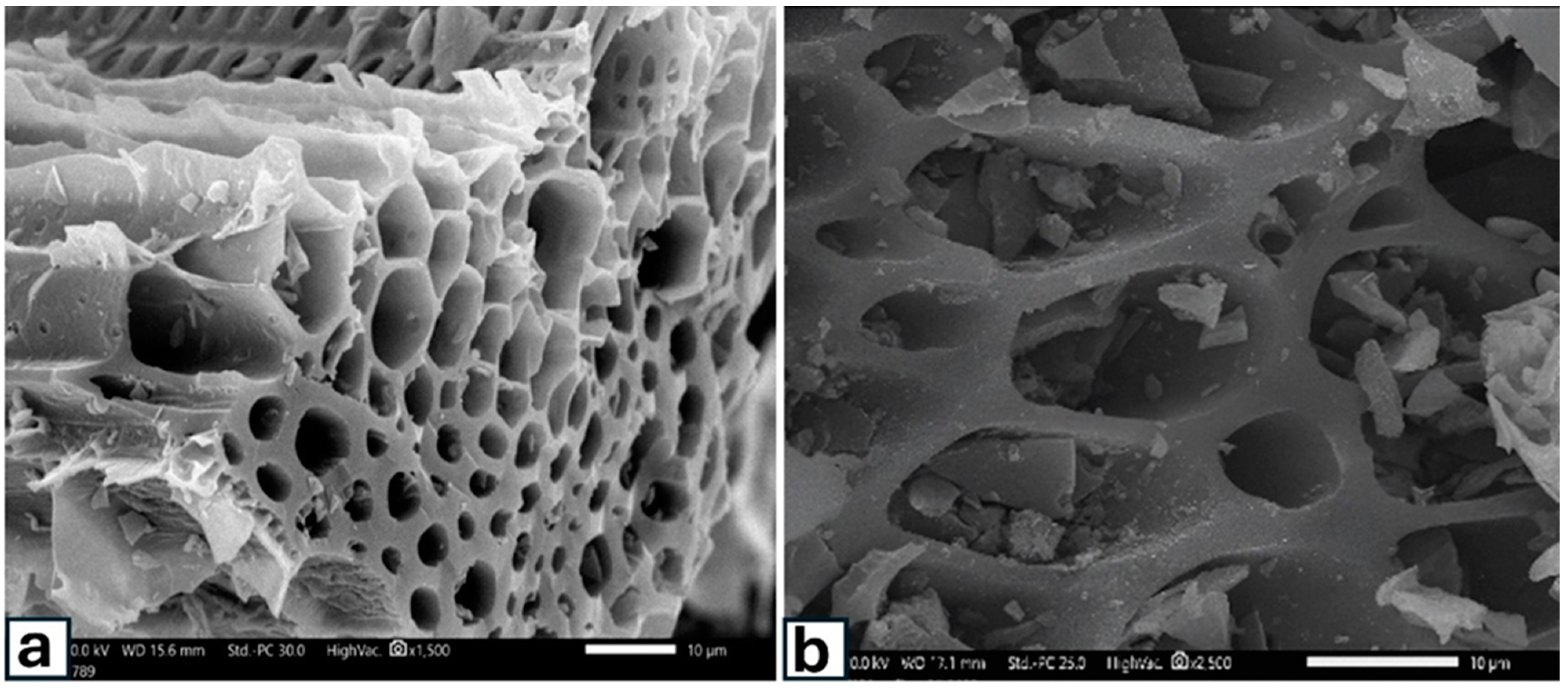
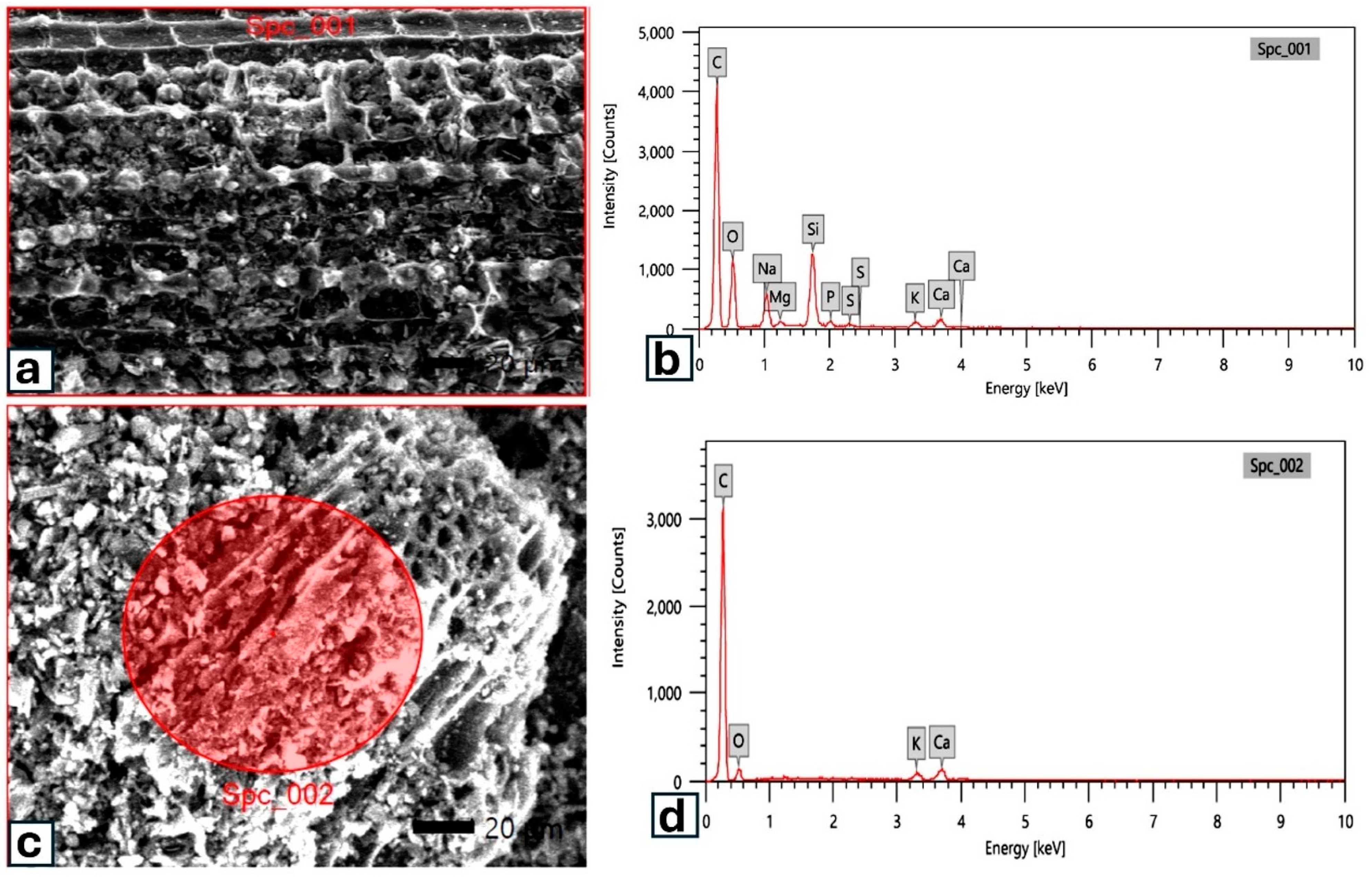
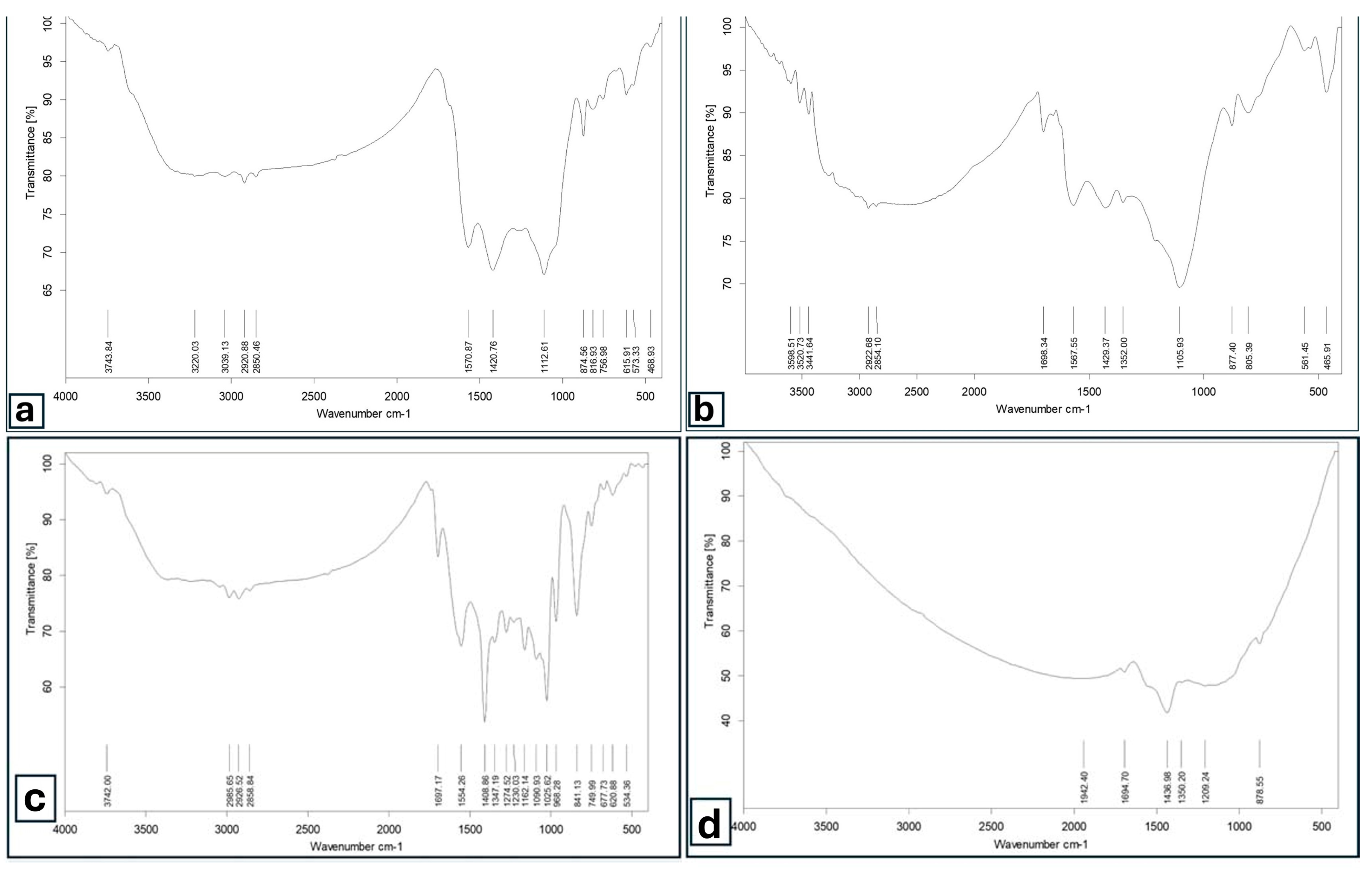
3.1. Main Features and Functional Groups of Biochar Surfaces
3.2. Adsorption of Cu(II) from Aqueous Solution on Different Amounts of Biochar
3.3. Adsorption of Pb(II) from Aqueous Solution on Different Amounts of Biochar
3.4. Adsorption of Iodine on Different Amounts of Biochar
3.5. Adsorption of Chlorpyrifos on Biochar
3.6. Regeneration of Biochar for Cu(II) Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi, A. A critical review of advanced nanotechnology and hybrid membrane-based water recycling, reuse, and wastewater treatment processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.; De la Rosa, J.M. Assessing the Effects of Biochar on the Immobilization of Trace Elements and Plant Development in a Naturally Contaminated Soil. Sustainability 2020, 12, 6025. [Google Scholar] [CrossRef]
- Lewoyehu, M. Comprehensive review on synthesis and application of activated carbon from agricultural residues for the remediation of venomous pollutants in wastewater. J. Anal. Appl. Pyrolysis 2021, 159, 105279. [Google Scholar] [CrossRef]
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E.; et al. Toxicity of Heavy Metals and Recent Advances in Their Removal: A Review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef]
- Hashem, A.; Fletcher, A.; Younis, H.; Mauof, H.; Abou-Okeil, A. Adsorption of Pb (II) ions from contaminated water by 1,2,3,4-butanetetracarboxylic acid-modified microcrystalline cellulose: Isotherms, kinetics, and thermodynamic studies. Int. J. Biol. Macromol. 2020, 164, 3193–3203. [Google Scholar] [CrossRef]
- Dai, K.; Liu, G.; Xu, W.; Deng, Z.; Wu, Y.; Zhao, C.; Zhang, Z. Judicious fabrication of bifunctionalized graphene oxide/MnFe2O4 magnetic nanohybrids for enhanced removal of Pb (II) from water. J. Colloid Interface Sci. 2020, 579, 815–822. [Google Scholar] [CrossRef]
- Abidli, A.; Huang, Y.; Rejeb, Z.B.; Zaoui, A.; Park, C.B. Sustainable and efficient technologies for removal and recovery of toxic and valuable metals from wastewater: Recent progress, challenges, and future perspectives. Chemosphere 2022, 292, 133102. [Google Scholar] [CrossRef]
- Hikmat Hama Aziz, K.H.; Kareem, R. Recent advances in water remediation from toxic heavy metals using biochar as a green and efficient adsorbent: A review. Case Stud. Chem. Environ. Eng. 2023, 8, 100495. [Google Scholar] [CrossRef]
- Abdelmoaty, Y.H.; Tessema, T.D.; Choudhury, F.A.; El-Kadri, O.M.; El-Kaderi, H.M. Nitrogen rich porous polymers for carbon dioxide and iodine sequestration for environmental remediation. ACS Appl. Mater. Interfaces 2018, 10, 16049. [Google Scholar] [CrossRef]
- Zhang, T.; Yue, X.; Gao, L.; Qiu, F.; Xu, J.; Rong, J.; Pan, J. Hierarchically porous bismuth oxide/layered double hydroxide composites: Preparation, characterization, and iodine adsorption. J. Clean. Prod. 2017, 144, 220. [Google Scholar] [CrossRef]
- Luo, Y.; Kawashima, A.; Ishido, Y.; Yoshihara, A.; Oda, K.; Hiroi, N.; Ito, T.; Ishii, N.; Suzuki, K. Iodine excess as an environmental risk factor for autoimmune thyroid disease. Int. J. Mol. Sci. 2014, 15, 12895. [Google Scholar] [CrossRef] [PubMed]
- Nallapaneni, A.; Pope, C.N. Chlorpyrifos. In Encyclopedia of Toxicology, 2nd ed.; Wexler, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 583–585. [Google Scholar]
- Huang, L.; Wu, B.; Wu, Y.; Yang, Z.; Yuan, T.; Alhassan, S.I.; Yang, W.; Wang, H.; Zhang, L. Porous and flexible membrane derived from ZIF-8-decorated hyphae for outstanding adsorption of Pb2+ ion. J. Colloid Interface Sci. 2020, 565, 465–473. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Teng, F.; Zhou, C. Surfactant changes lead adsorption behaviors and mechanisms on microplastics. Chem. Eng. J. 2020, 405, 126989. [Google Scholar] [CrossRef]
- Mahmoud, E.R.I.; Aly, H.M.; Hassan, N.A.; Abdulrahman Aljabri, A.; Khan, A.L.; El-Labban, H.F. Biochar from Date Palm Waste via Two-Step Pyrolysis: A Modified Approach for Cu (II) Removal from Aqueous Solutions. Processes 2024, 12, 1189. [Google Scholar] [CrossRef]
- Akhil, D.; Lakshmi, D.; Kartik, A.; Vo, D.V.N.; Arun, J.; Gopinath, K.P. Production, characterization, activation, and environmental applications of engineered biochar: A review. Environ. Chem. Lett. 2021, 19, 2261–2297. [Google Scholar] [CrossRef]
- Rani, L.; Kaushal, J.; Lal Srivastav, A. Biochar as sustainable adsorbents for chromium ion removal from aqueous environment: A review. Biomass Convers. Bioref. 2024, 14, 6083–6096. [Google Scholar] [CrossRef]
- Ghanim, A.N. Utilization of date pits derived bio-adsorbent for heavy metals in wastewater treatment: Review. Al-Qadisiyah J. Eng. Sci. 2023, 16, 058–069. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Alasmary, Z. Efficient Remediation of Cadmium- and Lead-Contaminated Water by Using Fe-Modified Date Palm Waste Biochar-Based Adsorbents. Int. J. Environ. Res. Public Health 2023, 20, 802. [Google Scholar] [CrossRef]
- Thabeta, W.M.; Ahmedb, S.B.; Abdelwahaba, O.; Soliman, N.F. Enhancement Adsorption of Lead and Cadmium Ions from Waste Solutions Using Chemically Modified Palm fibers. Egypt. J. Chem. 2020, 63, 14917–14927. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Removal of lead(II) from aqueous solution using date seed-derived biochar: Batch and column studies. Appl. Water Sci. 2018, 8, 181. [Google Scholar] [CrossRef]
- Al-Fulaiti, B.; El-Shafey, E.S.I.; Al Kindi, S.H.S.; Abdel-Jalil, R.J. Adsorption of Iodine from Aqueous Solution on Modified Silica Gel with Cyclodextrin Derivatives. Pol. J. Environ. Stud. 2022, 31, 5571–5582. [Google Scholar] [CrossRef] [PubMed]
- Barman, B.K.; Barman, S.; Roy, M.N. Inclusion complexation between tetrabutylphosphonium methanesulfonate as guest and α- and β-cyclodextrin as hosts investigated by physicochemical methodology. J. Mol. Liq. 2018, 264, 80. [Google Scholar] [CrossRef]
- Mahmoud, E.R.I.; Aly, H.M.; Hassan, N.A.; Abdulrahman Aljabri, A.; Khan, A.L.; El-Labban, H.F. Utilizing Date Palm Leaf Biochar for Simultaneous Adsorption of Pb(II) and Iodine from Aqueous Solutions. Processes 2024, 12, 1370. [Google Scholar] [CrossRef]
- Hirota, M.; Higaki, S.; Ito, S.; Ishida, Y.; Terao, K. Effects of 2-hydroxypropyl α-cyclodextrin on the radioactive iodine sorption on activated carbon. J. Radioanal. Nucl. Chem. 2021, 328, 659. [Google Scholar] [CrossRef]
- Windiastuti, E.; Indrasti, N.S.; Hasanudin, U.; Suprihatin, Y.B. The Influence of Pretreatment and Post Treatment with Alkaline Activators on the Adsorption Ability of Biochar from Palm Oil Empty Fruit. J. Ecol. Eng. 2023, 24, 242–251. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrol. 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Cheng, Y.; Jiang, P. Online analysis method for pyrolysis products with large volatility difference at high temperature and pressure: Pyrolysis kinetics of supercritical pressure n-decane. Fuel 2023, 346, 128245. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Pasieczna-Patkowska, S.; Madej, J. Comparison of photoacoustic, diffuse reflectance attenuated total reflectance and transmission infrared spectroscopy for the study of biochar. Pol. J. Chem. Technol. 2018, 20, 75–83. [Google Scholar] [CrossRef]
- Abdulrazzaq, H.; Jol, H.; Husni, A.; Abu-Bakr, R. Characterization and stabilisation of biochars obtained from empty fruit bunch, wood, and rice husk. BioResources 2014, 9, 2888–2898. [Google Scholar] [CrossRef]
- Alcazar-Ruiz, A.; Dorado, F.; Sanchez-Silva, L. Bio-phenolic compounds production through fast pyrolysis: Demineralizing olive pomace pretreatments. Food Bioprod. Process. 2023, 137, 200–213. [Google Scholar] [CrossRef]
- Yu, J.; Sun, J.; Sun, M.; Li, W.; Qi, D.; Zhang, Y.; Han, C. Protective mechanism of Coprinus comatus polysaccharide on acute alcoholic liver injury in mice, the metabolomics and gut microbiota investigation. Food Sci. Hum. Wellness 2024, 13, 401–413. [Google Scholar] [CrossRef]
- Cavaglia, J.; Garcia, S.M.; Roger, J.M.; Mestres, M.; Boqué, R. Detection of bacterial spoilage during wine alcoholic fermentation using ATR-MIR and MCR-ALS. Food Control 2022, 142, 109269. [Google Scholar] [CrossRef]
- Xue, T.; Wang, R.-Q.; Zhang, M.M.; Dai, J.-L. Adsorption, and desorption of mercury (II) in three forest soils in Shandong Province, China. Pedosphere 2013, 23, 265–272. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, Y.; Xu, Y.; Zhang, Q.; Shi, X.; Li, D.; Tian, D.; Jiang, D. Improved charge transfer in polymeric carbon nitride synergistically induced by the aromatic rings modification and Schottky junctions for efficient photocatalytic CO2 reduction. Chem. Eng. J. 2023, 463, 142395. [Google Scholar] [CrossRef]
- Yan, J.; Li, Z.; Zhang, Y.; Liu, R.; Zhou, L.; Fu, P. Hydrodeoxygenation of lignin phenolic derivatives to aromatics: A review of catalyst functionalization for targeted deoxygenation and active site modification strategies. Fuel Process. Technol. 2023, 250, 107914. [Google Scholar] [CrossRef]
- Ban, Y.; Jin, L.; Li, Y.; Yang, H.; Hu, H. Pyrolysis behaviors of model compounds with representative oxygen-containing functional groups in carbonaceous feedstock over calcium. Fuel 2023, 335, 127137. [Google Scholar] [CrossRef]
- Sizirici, B.; Fseha, Y.H.; Yildiz, I.; Delclos, T.; Khaleel, A. The effect of pyrolysis temperature and feedstock on date palm waste derived biochar to remove single and multi-metals in aqueous solutions. Sustain. Environ. Res. 2021, 31, 9. [Google Scholar] [CrossRef]
- Chen, H.; Xie, A.; You, S. A Review: Advances on Absorption of Heavy Metals in the Wastewater by Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2018, 301, 012160. [Google Scholar] [CrossRef]
- Tong, Y.; Mcnamara, P.J.; Mayer, B.K. Adsorption of organic micropollutants onto biochar: A review of relevant kinetics, mechanisms and equilibrium. Environ. Sci. Water Res. Technol. 2019, 5, 821–838. [Google Scholar] [CrossRef]
- Chen, X.C.; Chen, G.C.; Chen, L.G.; Chen, Y.X.; Lehmann, J.; McBride, M.B.; Hay, A.G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Goodman, B.A. Utilization of waste straw and husks from rice production: A review. J. Bioresour. Bioprod. 2020, 5, 143–162. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Li, N.; Tao, J.; Yan, B.; Cui, X.; Chen, G. Adsorption of Lead from Aqueous Solution by Biochar: A Review. Clean Technol. 2022, 4, 629–652. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, H.; Cheng, H.; Yan, Y.; Chang, M.; Cao, Y.; Huang, F.; Zhang, G.; Yan, M. Spent Ganoderma lucidum substrate derived biochar as a new bio-adsorbent for Pb2+/Cd2+ removal in water. Chemosphere 2020, 241, 125121. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Wu, J.; Miao, L. The effect of carbonization temperature on the capacity and mechanisms of Pb (II) adsorption by microalgae residue-derived biochar. Ecotoxicol. Environ. Saf. 2021, 225, 112750. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Zhang, M.; Feng, C.; Qin, B.; Zhang, W.; Zhao, N.; Li, Y.; Ni, Z.; Xu, Z.; et al. Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies. Sci. Total Environ. 2020, 717, 136894. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Zhao, T.; Yao, Y.; Li, D.; Wu, F.; Zhang, C.; Gao, B. Facile low-temperature one-step synthesis of pomelo peel biochar under air atmosphere and its adsorption behaviors for Ag (I) and Pb (II). Sci. Total Environ. 2018, 640–641, 73–79. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, T.; Li, Q.; Yang, H.; Yan, Y.; Sarkar, B.; Lam, S.S.; Bolan, N. Influence of pyrolysis temperature on the characteristics and lead (II) adsorption capacity of phosphorus-engineered poplar sawdust biochar. J. Anal. Appl. Pyrolysis 2020, 154, 105010. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Deng, L.; Wang, Y.; Kang, D.; Li, C.; Chen, H. Enhanced adsorption of Pb (II) by nitrogen and phosphorus co-doped biochar derived from Camellia oleifera shells. Environ. Res. 2020, 191, 110030. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Singh, P.; Sarswat, A.; Steele, P.H.; Pittman, C.U., Jr. Lead sorptive removal using magnetic and nonmagnetic fast pyrolysis energy cane biochars. J. Colloid Interface Sci. 2015, 448, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fan, X.; Tsang, D.C.; Wang, F.; Shen, Z.; Hou, D.; Alessi, D.S. Removal of lead by rice husk biochars produced at different temperatures and implications for their environmental utilizations. Chemosphere 2019, 235, 825–831. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, Y.; Xing, B.; Qin, X.; Zhang, C.; Xia, H. Lead and cadmium clean removal from wastewater by sustainable biochar derived from poplar saw dust. J. Clean. Prod. 2021, 314, 128074. [Google Scholar] [CrossRef]
- Gao, R.; Xiang, L.; Hu, H.; Fu, Q.; Zhu, J.; Liu, Y.; Huang, G. High-efficiency removal capacities and quantitative sorption mechanisms of Pb by oxidized rape straw biochars. Sci. Total Environ. 2019, 699, 134262. [Google Scholar] [CrossRef]
- Balraju, W.; Upadhyay, K.K.; Tripathi, S.K. Remediation of Toxic Metals by Forest Trees: Concepts and Strategies. Environ. Ecol. 2022, 40, 1798–1810. [Google Scholar]
- El-Khatib, A.A.; Barakat, N.A.; Youssef, N.A.; Samir, N.A. Bio accumulation of heavy metals air pollutants by urban trees. Int. J. Phytoremed. 2020, 22, 210–222. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wang, S.; Fang, J.; Xue, Y.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from Aqueous Solutions by Biochar Derived from KMnO4 Treated Hickory Wood. 2015. Available online: http://www.elsevier.com/open-access/userlicense/1.0/ (accessed on 15 September 2024).
- Yenisoy-Karakas, S.; Aygün, A.; Günes, M.; Tahtasakal, E. Physical and chemical characteristics of polymerbased spherical activated carbon and its ability to adsorb organics. Carbon 2004, 42, 477–484. [Google Scholar] [CrossRef]
- Hernandez-Maglinao, J.; Capareda, S.C. Improving the Surface Areas and Pore Volumes of Biochar Produced from Pyrolysis of Cotton Gin Trash via Steam Activation Process. Int. J. Eng. Sci. 2019, 3, 15–18. [Google Scholar]
- Saleh, M.E.; Mahmoud, A.H.; Rashad, M. Biochar usage as a cost-effective bio-sorbent for removing NH4-N from wastewater. In Proceedings of the Global Climate Change, Biodiversity and Sustainability: An International Conference Focused on the Arab Mena Region and Euromed, Alexandria, Egypt, 15–19 April 2013; pp. 15–18. [Google Scholar]
- Castiglioni, M.; Rivoira, L.; Ingrando, I.; Del Bubba, M.; Bruzzoniti, M.C. Characterization Techniques as Supporting Tools for the Interpretation of Biochar Adsorption Efficiency in Water Treatment: A Critical Review. Molecules 2021, 26, 5063. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Bahrudin, N.N.; Hum, N.N.M.F.; Surip, S.N.; Syed-Hassan, S.S.A.; Yousif, E.; Sabar, S. Microporous activated carbon developed from KOH activated biomass waste: Surface mechanistic study of methylene blue dye adsorption. Water Sci. Technol. 2021, 84, 1858–1872. [Google Scholar] [CrossRef] [PubMed]
- Tamrin, K.F.; Zahrim, A.Y. Determination of optimum polymeric coagulant in palm oil mill effluent coagulation using multiple-objective optimisation on the basis of ratio analysis (MOORA). Environ. Sci. Pollut. Res. 2017, 24, 15863–15869. [Google Scholar] [CrossRef] [PubMed]
- Schroder, A.; Kluppel, M.; Schuster, R.H.; Heidberg, J. Energetic surface heterogeneity of carbon black. Kautsch. Gummi Kunststoffe. 2001, 54, 260–266. Available online: http://www.plastverarbeiter.de/ai/resources/36a867629d7.pdf (accessed on 15 September 2024).
- Wampler, W.A.; Nikiel, L.; Evans, E.N. Carbon black. In Rubber Compounding: Chemistry and Applications, 2nd ed.; Rodgers, B., Ed.; CRC Press: Boca Raton, FL, USA, 2016; Chapter 6; pp. 209–250. [Google Scholar]
- Hess, W.M.; Herd, C.R. Microstructure, morphology, and general physical properties. In Carbon Black: Science and Technology, 2nd ed.; Donnet, J.-B., Bansal, R.C., Wang, M.J., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1993; Chapter 3; pp. 89–174. [Google Scholar]
- Joshi, V.; Jindal, M.K.; Sar, S.K. Approaching a discussion on the detachment of chlorpyrifos in contaminated water using different leaves and peels as bio adsorbents. Sci. Rep. 2023, 13, 11186. [Google Scholar] [CrossRef]
- Okoya, A.A.; Adegbaju, O.S.; Akinola, O.E.; Akinyele, A.B.; Amuda, O.S. Comparative Assessment of the Efficiency of Rice Husk Biochar and Conventional Water Treatment Method to Remove Chlorpyrifos from Pesticide Polluted Water. CJAST 2020, 39, CJAST.54205. [Google Scholar] [CrossRef]
- Wahba, T.F.; Hassan, N.A.; Aly, H.M. Biochar prepared from Ficus nitida as a carrier for frankincense essential oil (Boswellia sacra) to control some stored product insects. Pol. J. Entomol. 2022, 91, 94–108. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, L.; Hu, B.; Li, T.; Tian, H. Adsorption Behavior and Residue Degradation of Triazine Herbicides in Soil Amended with Rice Straw Biochar. Agriculture 2023, 13, 1282. [Google Scholar] [CrossRef]
- Klasson, K.T. Biochar characterization and a method for estimating biochar quality from proximate analysis results. Biomass Bioenergy 2017, 96, 50–58. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Sunita Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, 00570. [Google Scholar] [CrossRef]
- Johnson, E. Utilization of Biochar Produced from Agricultural Residues for the Removal of Pesticide and Pharmaceutical Micropollutants in Surface Water Biofiltration. Ph.D. Thesis, School of Engineering, Newcastle University, Newcastle upon Tyne, UK, March 2022. [Google Scholar]

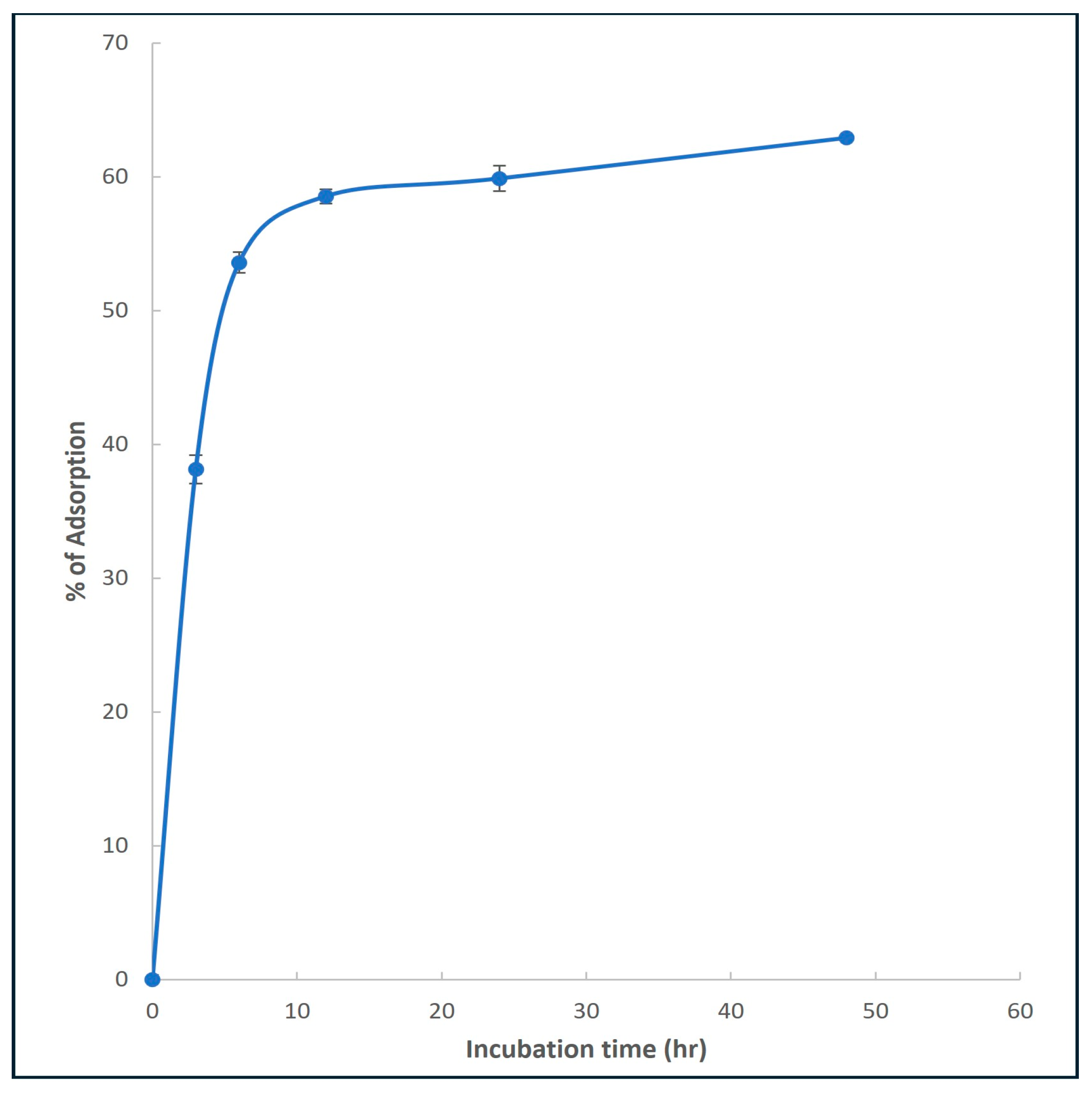
 ) and Ficus nitida biochar (
) and Ficus nitida biochar ( ) after different incubation times.
) after different incubation times.
 ) and Ficus nitida biochar (
) and Ficus nitida biochar ( ) after different incubation times.
) after different incubation times.

| Element | Biochar Type | |
|---|---|---|
| Date palm | Ficus nitida | |
| Mass % ± SD | Mass % ± SD | |
| C | 62.06 ± 0.21 | 72.93 ± 0.39 |
| O | 27.89 ± 0.31 | 23.80 ± 0.71 |
| Mg | 0.28 ± 0.02 | -- |
| Na | 3.32 ± 0.07 | -- |
| Si | 4.37 ± 0.06 | -- |
| P | 0.46 ± 0.02 | -- |
| S | 0.17 ± 0.01 | -- |
| Ca | 0.54 ± 0.03 | 1.11 ± 0.09 |
| K | 0.91 ± 0.03 | 2.16 ± 0.11 |
| O/C ratio | 0.45 | 0.33 |
| Wave Number (cm−1) | Assignments |
|---|---|
| 3000–3700 | -OH group |
| 2800–3000 | C-H (methyl) |
| ≈2000 | C-C |
| 1600–1800 | C=O/COOH |
| 1560 | C=C |
| 1420–1450 | C-H asymmetric |
| 1317–1375 | C-O asymmetric of aromatic |
| 1000–1260 | C-O |
| 700–900 | C-H aromatic (out of plan) |
| 400–600 | In organic matter |
| Amount of Biochar (g/10 mL) | Ficus nitida Biochar | Date palm Biochar | ||
|---|---|---|---|---|
| The Mean Concentration of Cu(II) (mg/L) | % of Adsorption | The Mean Concentration of Cu(II) (mg/L) | % of Adsorption | |
| 0.02 | 125.6 | 37 | 42 | 79 |
| 0.04 | 56.8 | 72 | 1.78 | 99.11 |
| 0.06 | 35 | 83 | 0.6 | 99.7 |
| 0.08 | 23.8 | 88 | 0.2 | 99.9 |
| Amount of Biochar (g/10 mL) | Date palm Biochar | Ficus nitida Biochar | ||
|---|---|---|---|---|
| The Mean Concentration of Pb(II) (mg/L) | % of Adsorption | The Mean Concentration of Pb(II) (mg/L) | % of Adsorption | |
| 0.02 | 0.15 | 99.9 | 24.6 | 75 |
| 0.04 | 0.11 | 99.9 | 19. 4 | 80 |
| 0.06 | 0 | 100 | 15.1 | 85 |
| 0.08 | 0 | 100 | 8.1 | 92 |
| Amount of Biochar (g/50 mL) | Biochar Type | |
|---|---|---|
| Date palm | Ficus nitida | |
| % of Adsorption | % of Adsorption | |
| 0.1 | 24 | 18 |
| 0.2 | 27 | 31 |
| 0.3 | 33 | 46.8 |
| 0.4 | 39 | 68 |
| Amount of Biochar (g/10 mL) | Biochar Type | |
|---|---|---|
| Date palm | Ficus nitida | |
| % of Adsorption | % of Adsorption | |
| 0.02 | 14 | 18 |
| 0.04 | 26 | 50 |
| 0.06 | 29 | 62 |
| 0.08 | 50 | 87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, E.R.I.; Aly, H.M.; Hassan, N.A.; Aljabri, A.; Khan, A.L.; F. El-Labban, H. Enhanced Removal of Chlorpyrifos, Cu(II), Pb(II), and Iodine from Aqueous Solutions Using Ficus Nitida and Date Palm Biochars. ChemEngineering 2024, 8, 105. https://doi.org/10.3390/chemengineering8050105
Mahmoud ERI, Aly HM, Hassan NA, Aljabri A, Khan AL, F. El-Labban H. Enhanced Removal of Chlorpyrifos, Cu(II), Pb(II), and Iodine from Aqueous Solutions Using Ficus Nitida and Date Palm Biochars. ChemEngineering. 2024; 8(5):105. https://doi.org/10.3390/chemengineering8050105
Chicago/Turabian StyleMahmoud, Essam R. I., Hesham M. Aly, Noura A. Hassan, Abdulrahman Aljabri, Asim Laeeq Khan, and Hashem F. El-Labban. 2024. "Enhanced Removal of Chlorpyrifos, Cu(II), Pb(II), and Iodine from Aqueous Solutions Using Ficus Nitida and Date Palm Biochars" ChemEngineering 8, no. 5: 105. https://doi.org/10.3390/chemengineering8050105
APA StyleMahmoud, E. R. I., Aly, H. M., Hassan, N. A., Aljabri, A., Khan, A. L., & F. El-Labban, H. (2024). Enhanced Removal of Chlorpyrifos, Cu(II), Pb(II), and Iodine from Aqueous Solutions Using Ficus Nitida and Date Palm Biochars. ChemEngineering, 8(5), 105. https://doi.org/10.3390/chemengineering8050105








