Abstract
Continuous distillation (CD) by steam is a patented emerging technology that allows us to obtain essential-oil fractions from citrus juices. It presents benefits such as reducing steam consumption by 50%, lowering environmental impact, and, by its design, obtaining fractions enriched in terpenic and oxygenated compounds that can be further processed. The CD of essential oils from Mexican lime juice (Citrus aurantifolia) was studied and the results were compared with conventional steam distillation (batch) in terms of steam consumption, extraction yield, chemical composition, and quality of the essential oils. Different steam flows were used: distillation without a packed column (sc); with packed column (cc); and steam flows of 10, 15, and 20 mL/min with a reflux ratio of 0.5, 1, and 2, respectively. CD was superior in terms of composition, extraction energy savings (0.63 kg steam/kg juice with 1.39 kg steam/kg juice in the conventional), and the extraction yield recovery efficiency was >90%. Gas chromatography-mass spectrometry analysis of the extracted essential oils indicated that the use of CD with a column increases the fractionation of volatile compounds. The result of this study demonstrates that CD can be used as an alternative method to extract the essential oil from lime or any citrus fruit, obtaining differentiated fractions in aroma and composition.
1. Introduction
Mexico ranks second worldwide in the production of Mexican lime, with an annual production of 3.1 million tons and a commercial value of USD 758 million. The main producing states are Michoacan, Veracruz, and Colima according to the Agrifood and Fisheries Information Service [1].
Citrus processing generates a large amount of by-products, such as citrus peel, which represents almost half of the weight of the fruit (40–50%). Dry citrus-peel waste mainly contains sugar, cellulose, pectin, and hemicellulose flavonoids, as well as up to 4% essential oils [2].
Lime is the most important citric fruit for essential-oil extraction [3]. Essential oils have a consolidated international market with estimated sales of USD 500 billion [4].
Essential oils have multiple applications in the food, pharmaceutical, and cosmetic industries for their aroma and numerous biological activities as follows: antioxidant, antidiabetic, insecticidal, antifungal, and antibacterial [5,6,7]. Citrus essential oils are a complex mixture of approximately 400 compounds, comprising 85–90% volatile and 1–15% nonvolatile components [8]. Its most abundant volatile components are terpenes, and those that give it an aroma are oxygenated compounds [5,7]. The volatile portion is mainly composed of limonene (40–60%), γ-terpinene (10–20%), β-pinene (10–16%), α-pinene (1–3%), neral (1–5%), and geranial (1–6%) (w/w) [9].
Traditional methods commonly used to obtain essential oils are steam distillation, solvent extraction, and mechanical expression [2,4]. The disadvantages of these traditional methods are high-energy consumption, low yield, prolonged time consumption, high operating temperature, and environmental pollution caused by the use of toxic solvents [2].
Currently, there are green or emerging technologies that allow for reductions in solvent use and energy consumption. Among these are supercritical fluid extraction, microwave-assisted extraction, ultrasound-assisted extraction [10], and, recently, horizontal continuous distillation.
Horizontal continuous distillation is a new patented technology for distilling citrus juices that reduces energy consumption by 50%, and it provides distilled fractions of essential oils with different compositions [11].
Continuous distillation refers to the actions of lime juice being fed, and effluent juice being released continuously, unlike batch processes where the juice is loaded, processed, and discharged.
The horizontal continuous distillation process consists of a multi-stage successive distillation (Figure 1). Firstly, the room-temperature juice feed (F) passes through a preheating system via heat exchange, where it is preheated with the hot residual juice that comes out of the last stage. This system allows the recovery of sensible heat from the effluent juice. Once preheated, the fed juice enters the first stage at a temperature close to its boiling point (70 °C). In the first stage, the fed juice is brought to its boiling point and partially evaporated utilizing a heat exchanger or heating coil fed with steam from the boiler. The generated vapors rise and are conducted through a packed column that concentrates the most volatile components in the vapor phase and are subsequently condensed by a condenser to generate the distillate of the first stage. Then, using a decanting pear, this distillate is separated into oil and water by density difference. In a similar way, the fed juice passes to each of the stages consecutively to be evaporated, and it then fractionated by the packed column and condensed, thus obtaining the distilled fractions of essential oils [11].
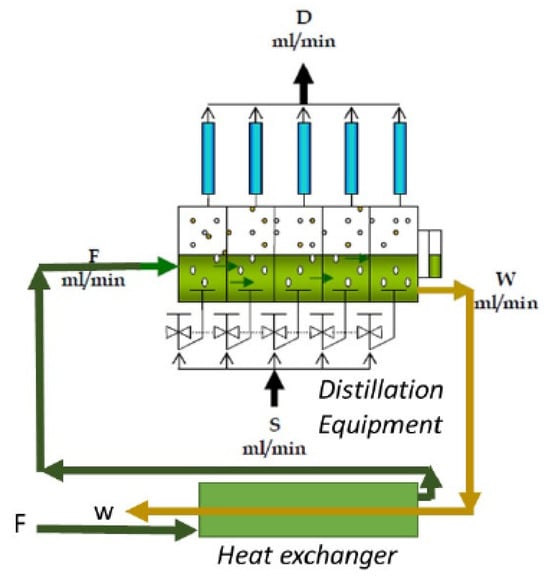
Figure 1.
Diagram of the continuous distillation process, taken from Padilla et al., 2021 [11].
In one of the previous studies reported by Padilla et al. [11], this technology was applied to Persian Lime juice and was operated in a simpler configuration, without the use of packed columns, achieving distilled fractions that were differentiated in the minor volatile compounds.
Therefore, the objective was to study the continuous-steam distillation process conditions using a new modality, packed columns, to evaluate the effect on its volatile composition and its comparison with conventional distillation.
2. Materials and Methods
2.1. Raw Material
The Mexican lime (Citrus aurantifolia) was obtained from the supply market in the city of Guadalajara, Jalisco. It was in good condition and free of visible bruises, damage, and fungal growth.
Treatment of the Raw Material
The lime was weighed and washed with distilled water to prevent inert materials such as soil, dust, insects, or microorganisms from interfering in subsequent operations. Once the lime was washed, the pressing process followed, using a helical press to obtain the concentrated oil in the lime juice.
2.2. Extraction Process
Once the appropriate particle size was obtained with the help of the helical press and the lime juice was weighed, it was deposited in the continuous-distillation extractor tank (the equipment was maintained at a constant feed flow of 250 mL/min for 4 h with 60 L of lime juice). In the packed column, 43 spherical packings with a diameter of 1.5 cm were used for each of the columns.
The extraction process began by passing the steam at different pressures (distillation without column at a steam flow rate of 30, 20, and 10 mL/min; distillation with column a reflux ratio 0.5, 1, and 2, respectively, and these were measured inside the extraction tank and referred to the steam force exerted on an area of raw material). Five fractions were obtained for each treatment. Condensation and separation of the water/oil mixture was then carried out in a phase separator or Florentine glass, and the yield was calculated using the following Equation (1):
A mass balance was performed to estimate steam consumption as follows:
where
F + S = D + W,
F = milliliters per minute of feed entering the distillation equipment.
S = milliliters per minute of water vapor entering the distillation equipment required for entrainment.
D = milliliters per minute of water and oil leaving the distillation equipment.
W = milliliters per minute of residue leaving the distillation equipment.
For the batch-steam distillation, 4 L of lime juice were deposited in the tank. Then, the tank was hermetically closed (to avoid possible leaks), and the gooseneck that conducts the vapor mixture to the condenser was placed there. At the outlet of the condenser, there was a Florentine glass, its function being to receive water/oil. The duration of the process was 4 h, with a vapor pressure of 0.6 kg/cm2 and a distillate flow of 30 mL/min.
The steam consumption of the traditional-batch process was 1.2 kg of steam/1 kg of lime juice [11].
For the economic and availability reasons related to the raw material, the tests were carried out in duplicate under different operating conditions.
2.3. Physical Characterization
2.3.1. Determination of Density
The relative density was determined according to French Standard NF T 75-111 [12].
A Density2Go portable digital density meter was used to determine the density; the equipment calculates the data and displays the reading on the instrument. Before starting the measurements, the equipment was calibrated with distilled water at 20 °C, and the measuring tube of the densimeter was introduced directly into the sample, taking 5 mL of the essential oil from each fraction obtained from the continuous distillation and from the batch-distillation samples. The readings were taken in triplicate from each of the essential-oil samples.
2.3.2. Determination of Refractive Index
The determination of the refractive index was carried out according to French Standard NF T 75-112 [13].
A Leica ABBE refractometer, model Mark II plus, whose principle is the ratio of air to substance measured at 20 °C, was used. The refractometer works by using an illumination prism and a refraction prism. When the prism and, consequently, the sample, is illuminated, the light passes through the internal mechanism of the equipment showing the field of vision, in which the critical angle is sought, and the instrument calculates the ratio of the angles by reflecting the measurement. The readings were made in triplicate for each of the samples of the essential oils obtained from both distillations.
2.3.3. Determination of Optical Rotation
For the determination of the optical rotation, it was carried out according to French Standard NF V 75-113 [14].
The determination was made by taking 10 mL of the essential oil from each sample obtained. The oil was placed in a 100 mm observation tube inside the POLAX-2L polarimeter of the ATAGO brand, which has a LED lamp that acts as a light source and a filter that ensures a wavelength of 589 nm and is equivalent to the D line of sodium with a measurement accuracy of 0.10°. The light beam passes through the cell with the sample inside showing in the field of view, and the light deviation and the digital analyzer allows one to obtain the reading of such a deviation. The readings were taken in triplicate for each of the essential-oil samples.
2.4. Gas Chromatography
The volatile composition of the essential oil or fractions was determined according to the norm AFNOR [15].
To evaluate the volatile components of interest, the samples were injected through an autoinjector into an Agilent Technologies 7890B gas chromatograph coupled to a 5977A mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The components were separated on an HP-5MS capillary column (30 m × 250 μm ID × 0.25 μm ft). The oven was initially programmed at 75 °C, increasing to 195 °C at a rate of 3 °C/min and then to 270 °C at a rate of 10 °C/min, and this was maintained for 7.5 min. The flow rate of the carrier gas (He) was 0.8 mL/min. The injection volume was 0.4 μL in split mode at a temperature of 250 °C. The temperatures in the transfer line, MS quad, and MS source were 270 °C, 150 °C, and 230 °C, respectively. The detector was operated at 70 eV (EI) and in scan acquisition mode (29–450 amu). The identification of the compounds was based on a comparison of the spectra of the peaks in the samples with those of the NIST14L library and retention indices from the literature. Samples were analyzed using identical conditions on the same chromatographic system equipped with an FID. The concentration of the compounds of interest was reported based on peak area (area units).
3. Results and Discussions
3.1. Steam Expenditure
Evaluation of the steam flow rate in the different continuous distillation flows with and without a column was conducted to evaluate the steam consumption during the distillation process. Different steam flows were used in the continuous distillation without a column (30, 20, and 10 mL/min), and, in the distillations with a column, they were carried out with reflux ratio of 0.5. 1 and 2 when compared to conventional distillation (Figure 2).
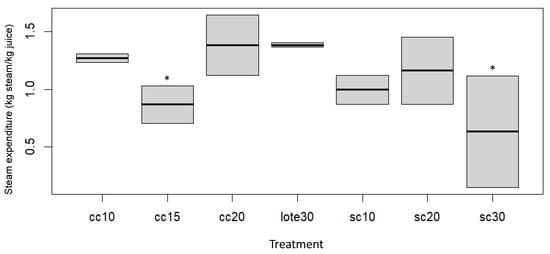
Figure 2.
Steam expenditure in the continuous-steam distillation by steam entrainment compared to conventional distillation (batch30) for an extraction period of 4 h. Column distillation with a steam flow of 10 mL/min (cc10). Column distillation with a steam flow of 15 mL/min (cc15). Distillation with a column with a steam flow of 20 mL/min (cc20). Distillation without column with a steam flow of 10 mL/min (sc10). Distillation without a column with a steam flow of 20 mL/min (sc20). Distillation without a column with a steam flow of 30 mL/min (sc30). An asterisk in superscript expresses a statistically significant difference in the columns of the medians of the treatments by Dunn’s post hoc test, adjusted by Bonferroni’s method (α = 0.05).
For the column-distillation treatments, using a steam flow of 15 mL/min showed a lower steam consumption of 0.87 ± 0.23 kg steam/kg of juice followed by a steam flow of 10 mL/min (1.27 ± 0.05 kg steam/kg of juice) when compared to conventional distillation, where the steam consumption was 1.39 ± 0.03 kg steam/kg of juice. In the case of distillation without a column, the steam flows of 30 and 10 mL/min (0.63 ± 0.69 and 1.00 ± 0.18 kg of steam/kg of juice) were the ones that presented lower steam consumption in comparison to batch distillation.
The reduction in steam consumption of the technology was possible for two reasons: one, that it was carried out on multi-stage separations, which allowed the steam consumption to be used more efficiently; and, secondly, it underwent a heat recovery that preheats the juice fed with the effluent juice.
These results suggest that continuous distillation with and without a column are similar in terms of steam consumption to conventional distillation. However, Masango studied different steam flow rates and found that using a low-steam-flow rate favors a higher oil yield and substantial energy savings [16].
3.2. Effect of the Steam Flow Rate on Performance
Evaluations of the steam flows when using a column (sc30, sc20, and sc10) and when not using a column (cc20, cc15, and cc10) were carried out to evaluate the effect that steam pressure has on lime essential-oil yield. Figure 3 shows the data obtained.
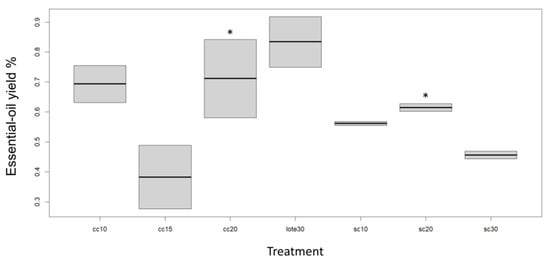
Figure 3.
Extraction yield of the lime essential oil in relation to continuous-steam distillation compared to conventional distillation (batch30) for an extraction period of 4 h. Column distillation with a steam flow of 10 mL/min (cc10). Column distillation with a steam flow of 15 mL/min (cc15). Distillation with a column and a steam flow of 20 mL/min (cc20). Distillation without a column and a steam flow of 10 mL/min (sc10). Distillation without a column and a steam flow of 20 mL/min (sc20). Distillation without a column and a steam flow of 30 mL/min (sc30). An asterisk in superscript expresses statistically significant differences in the columns of the medians of the treatments by Dunn’s post hoc test, adjusted by Bonferroni’s method (α = 0.05).
It is noteworthy that the highest yield was obtained from conventional distillation at 0.83 ± 0.12%. In the column-distillation treatments with a steam flow of 20 mL/min (cc20), the extraction yield increased drastically. It could be inferred that the steam flow favorably influenced the mass-transfer coefficient from the raw material to the vapor phase. For steam flow rates of 15 and 10 mL/min, the yield was 0.38 ± 0.15% and 0.69 ± 0.09%, respectively.
In the case of distillation without a column, the 20 mL/min steam flow was favored for the highest essential-oil yield of 0.61 ± 0.02%, followed by the 10 mL/min steam flow yield of 0.56 ± 0.01%, and the lowest was for the 30 mL/min steam flow with a yield of 0.46 ± 0.02%.
In the sc30 condition, the greater amount of steam generated a great deal of turbulence in the process, making it more inefficient.
These results show that the 20 mL/min steam flow with and without a column is effective for essential-oil yield. As Mistretta also reported, the yield increased when using a low steam flow, and they stated that the steam in distillation has two uses: to provide thermal energy and as a transport medium for distillate [17].
3.3. Physical Analysis of Mexican Lime Essential Oil (Citrus aurantifolia)
The physical characteristics of the lime essential oil obtained from continuous distillation with and without a column were density, the refractive index, and optical rotation. As already mentioned, five fractions were obtained from the continuous distillation with and without a column, and their physical characteristics were determined.
3.3.1. Density
Figure 4 shows the results obtained from determining the density in the five fractions of the essential oil obtained from the steam flows used in the continuous distillation with and without a column.
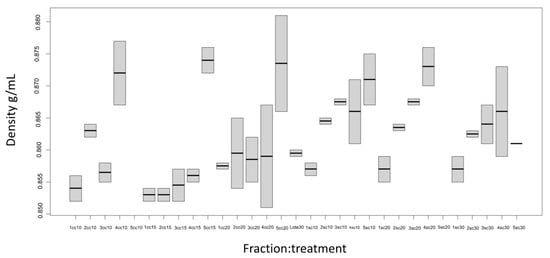
Figure 4.
Density of the essential oil obtained from Mexican lime juice by continuous steam distillation with a column and without a column. Sc, without column; Cc, with column; F1, Fraction 1; F2, Fraction 2; F3 Fraction 3; F4, Fraction 4; and F5, Fraction 5.
In the treatments with and without a column, as the number of fractions increased, the density of the essential oil increased. For the treatments with a column and without a column in the different steam flows, the Fraction 5 (F5) density was 0.871 ± 0.006 g/mL higher than in the other four fractions, which were 0.854 ± 0.003 g/mL. Compared with the density of the oil obtained from batch distillation, which was 0.86 ± 0.00 g/mL, having a higher density in Fraction 5 could be due to its composition and the extraction method used. During the first hours of distillation process, the highest amount of terpenes (limonene, β-pinene, α-pinene, β-phellandrene, and p-cymene) were concentrated [18]. In addition, in the first fractions (F1, F2, and F3), the presence of oxygenated compounds prevailed (linalool, geranial, nerol, citronellol, citral, and citronellal).
The density results obtained coincide with those reported by Njoku and Evbuomwan [19], who obtained a value of 0.85 mg/mL for lime with the Clevenger extraction method.
3.3.2. Refractive Index
The refractive index varies with the wavelength, corresponding to the D line 589.3 nm of sodium light.
There was a pattern in regard to density, where the refractive index increased as the fraction also increased (Figure 5). In Fraction 1 (F1), Fraction 2 (F2), and Fraction 3 (F3) in the treatments with a column and without a column of the different vapor flows, the refractive index was 1.475 ± 0.000 (like the one from the essential oil obtained from the conventional distillation of 1.47 ± 0.00). For Fraction 4 (F4) and Fraction 5 (F5), the refractive index of 1.487 ± 0.001 was higher in comparison to the three fractions and the oil obtained from the conventional distillation.
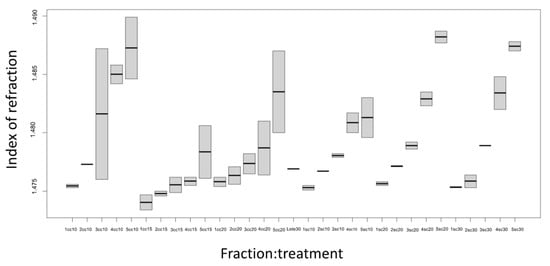
Figure 5.
Refractive index of the essential oil obtained from Mexican lime juice by continuous steam distillation with a column and without a column. Sc, without column; Cc, with column; F1, Fraction 1; F2, Fraction 2; F3, Fraction 3; F4, Fraction 4; and F5, Fraction 5.
Gamarra et al. [18] mentioned that the relationship between the distillation time and the refractive index is direct as the distillation time increases as the refractive index increases. The compounds of the essential oil are modified during distillation, and the compounds distilled in the first few minutes can make the essential oil more opaque in color, which would explain the difference between the fractions with increased indexes and the higher fractions of the others.
3.3.3. Optical Rotation
The optical rotation decreased as the fraction increased in the treatments with a column and those without a column with the different steam flows (Figure 6). In Fraction 1 (F1), Fraction 2 (F2), Fraction 3 (F3), and Fraction 4 (F4), the rotational power of 47.20 ± 0.21° was higher for the treatments with and without a column in the different steam flows. Fraction 5 (F5) produced a low rotational power of 15.93 ± 0.43° when compared to the rotational power of the essential oil obtained from conventional distillation, which yielded 38.50 ± 0.14°, and the four fractions. However, the essential oil obtained from the column treatments showed higher rotational power compared to the non-column treatment.
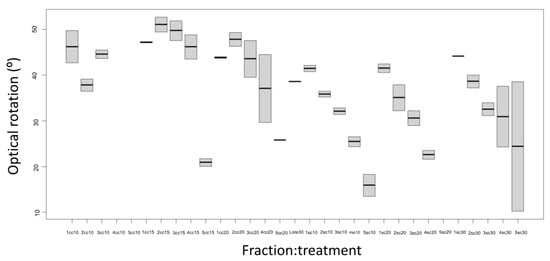
Figure 6.
Optical rotation of the essential oil obtained from Mexican lime juice by continuous steam distillation with a column and without a column Sc, without column; Cc, with column; F1, Fraction 1; F2, Fraction 2; F3, Fraction 3; F4, Fraction 4; and F5, Fraction 5.
The decrease in rotational power as the fraction increased may be due to the fact that the main compound (limonene) was depleted as the distillation time passed. The obtained results of the rotary power were lower than those reported by Albaladejo [20] in the lime essential oil with an optical rotation of +62.98°. The differences in optical rotation values between the aromatic samples were attributed to the presence of major components (limonene and β-pinene).
3.4. Chemical Composition of the Obtained Lime Essential Oil
After performing continuous distillation, a higher yield of essential oil at 0.61 ± 0.02% was obtained for the treatment without a packed column at 20 mL/min of steam flow, and a yield of 0.71 ± 0.19% was obtained for the treatment with a packed column at 20 mL/min of steam flow. Table 1 and Table 2 present the relative composition of the five fractions obtained from the continuous distillation of the sc20 and cc20 treatment compared to the composition of the essential oil from the conventional distillation (batch).
The results obtained agree with what was reported by Bourgou et al. [21], which is that citrus essential oil is mainly constituted of non-oxygenated aliphatic hydrocarbons, i.e., limonene, γ-terpinene, α-pinene, β-pinene, and α-terpinolene, as well as the oxygenated hydrocarbons found in higher concentrations than linalool, α-terpineol, neral, and geranial.



Table 1.
Volatile composition of the fractions in continuous distillation at sc20 mL/min vs. batch distillation.
Table 1.
Volatile composition of the fractions in continuous distillation at sc20 mL/min vs. batch distillation.
| a Treatment sc20 (Area Units ×107) | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | b RIlit | Component | c F1 | c F2 | c F3 | c F4 | c F5 | Batch |
| 1 | 932 | α-Pinene | 4.595 | 3.310 | 1.913 | 0.904 | 0.311 | 4.479 |
| 2 | 974 | β-Pinene | 28.551 | 8.828 | 1.805 | 0.317 | 0.061 | 23.327 |
| 3 | 1024 | D-Limonene | 110.160 | 105.697 | 87.626 | 65.33 | 38.03 | 114.698 |
| 4 | 1054 | γ-Terpinene | 15.245 | 15.660 | 14.009 | 11.407 | 7.233 | 18.131 |
| 5 | 1086 | α-Terpinolene | 3.593 | 7.004 | 8.397 | 8.954 | 7.892 | 5.066 |
| 6 | 1186 | α-Terpineol | 9.196 | 19.769 | 18.826 | 12.002 | 5.821 | 13.419 |
| 7 | 1235 | Neral | 1.41 | 1.02 | 0.51 | 0.22 | 0.11 | 0.988 |
| 8 | 1264 | Geranial | 2.836 | 2.297 | 1.139 | 0.476 | 0.177 | 1.992 |
a sc20 treatment, steam flow of 20 mL/min. b Literature retention index according to Adams [22]. c F1, Fraction 1; F2, Fraction 2; F3, Fraction 3; F4, Fraction 4; and F5, Fraction 5.

Table 2.
Volatile composition of the fractions in continuous distillation at cc20 mL/min vs. batch distillation.
Table 2.
Volatile composition of the fractions in continuous distillation at cc20 mL/min vs. batch distillation.
| a Treatment cc20 (Area units ×107) | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | b RIlit | Component | c F1 | c F2 | c F3 | c F4 | c F5 | Batch |
| 1 | 932 | α-Pinene | 4.688 | 3.363 | 2.116 | 0.841 | 0.246 | 4.479 |
| 2 | 974 | β-Pinene | 27.136 | 10.819 | 3.014 | 1.025 | 0.150 | 23.327 |
| 3 | 1024 | D-Limonene | 114.884 | 112.542 | 103.658 | 88.44 | 36.60 | 114.698 |
| 4 | 1054 | γ-Terpinene | 14.505 | 15.155 | 14.339 | 13.543 | 7.132 | 18.131 |
| 5 | 1086 | α-Terpinolene | 3.509 | 6.433 | 9.084 | 10.838 | 7.621 | 5.066 |
| 6 | 1186 | α-Terpineol | 9.259 | 15.225 | 13.653 | 12.354 | 13.945 | 13.419 |
| 7 | 1235 | Neral | 1.32 | 0.76 | 0.33 | 0.19 | 0.16 | 0.988 |
| 8 | 1264 | Geranial | 2.653 | 1.654 | 0.628 | 0.236 | 0.279 | 1.992 |
a cc20 treatment, steam flow of 20 mL/min. b Literature retention index according to Adams [20]. c F1, Fraction 1; F2, Fraction 2; F3, Fraction 3; F4, Fraction 4; and F5, Fraction 5.
Differences related to the composition of the oil, D-limonene, and the presence of other minority components can be explained by variations in the ecological conditions (climate, soil type, season of the year, and geographical location) and extraction conditions (extraction method, time, and raw material conditions) in which the plant grows. These can produce qualitative and quantitative changes in the oil [23].
4. Conclusions
Continuous distillation was superior in terms of composition, extraction energy savings (0.63 kg steam/kg juice with 1.39 kg steam/kg juice in the conventional), and recovery efficiency (>90%).
The gas chromatography-mass spectrometry analysis of the extracted essential oils indicated that the use of horizontal continuous distillation by steam with a column increases the fractionation of volatile compounds, facilitating desterpenation. Its composition was mainly of monoterpenes and oxygenated compounds, with D-limonene being the major component.
Tests are about to be carried out in an industrial setting to scale up from this pilot study to one at an industrial level.
5. Patents
Padilla, J.D.; Rodríguez, E.; Alba, A.; Vega, H. Sistema multifuncional de destilación, evaporación y extracción de moléculas orgánicas derivadas de productos naturales. Patent 330996. México 2015. Available online: https://siga.impi.gob.mx/newSIGA/content/common/principal.jsf (accessed on 9 January 2024).
Author Contributions
Conceptualization, J.D.P.-d.l.R., S.L.A.-L., M.E.-E., E.C.-T. and E.M.M.-S.; methodology, T.P.-A., M.E.-E., S.L.A.-L., E.C.-T., J.D.P.-d.l.R. and E.M.M.-S.; validation, M.E.-E., T.P.-A. and E.C.-T.; formal analysis, T.P.-A., E.C.-T., S.L.A.-L. and J.D.P.-d.l.R.; investigation, T.P.-A., J.D.P.-d.l.R. and M.E.-E.; data curation, T.P.-A., E.C.-T. and J.D.P.-d.l.R.; writing—original draft preparation, T.P.-A., S.L.A.-L. and J.D.P.-d.l.R.; writing—review and editing, S.L.A.-L., J.D.P.-d.l.R. and E.M.M.-S.; visualization, T.P.-A. and E.C.-T.; supervision, S.L.A.-L., J.D.P.-d.l.R. and M.E.-E.; project administration, J.D.P.-d.l.R.; funding acquisition, J.D.P.-d.l.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by COECYTJAL (grant number 8850-2020).
Data Availability Statement
The data are contained within the article.
Acknowledgments
To Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT) for the fellowship granted. Thanks is given to CIATEJ for use of their facilities and their support during the development of the present work. Thanks are also given to Abiel Alba, Jaime Gómez, and Ernesto Rodríguez for their technical support in the pilot plant.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Servicio de Información Agroalimentaria y Pesquera. Limón. 2024. Available online: https://www.gob.mx/siap/acciones-y-programas/panorama-agroalimentario-258035 (accessed on 9 January 2024).
- Song, Z.; Wei, X.; Xie, M.; Zhao, X.; Sun, J.; Mao, Y.; Wang, X.; Wang, W. Study on the microwave extraction process and product distribution of essential oils from citrus peel. Chem. Eng. Process.-Process Intensif. 2022, 171, 108726. [Google Scholar] [CrossRef]
- Sánchez Aldana, D.; Andrade-Ochoa, S.; Aguilar, C.N.; Contreras-Esquivel, J.C.; Nevárez-Moorillón, G.V. Antibacterial activity of pectic-based edible films incorporated with Mexican lime essential oil. Food Control 2015, 50, 907–912. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Yadav, M.P. Insights into the chemical composition and bioactivities of citrus peel essential oils. Food Res. Int. 2021, 143, 110231. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Saleem, M.; Siddique, S.; Ahmed, R.; Khanum, R.; Perveen, Z. Volatile components, antioxidant and antimicrobial activity of Citrus acida var. sour lime peel oil. J. Saudi Chem. Soc. 2009, 13, 195–198. [Google Scholar] [CrossRef]
- Al-Aamri, M.S.; Al-Abousi, N.M.; Al-Jabri, S.S.; Alam, T.; Khan, S.A. Chemical composition and in-vitro antioxidant and antimicrobial activity of the essential oil of Citrus aurantifolia L. leaves grown in Eastern Oman. J. Taibah Univ. Med. Sci. 2018, 13, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhao, L.; Cheng, H.; Qi, Z. Enhancing fractionation of terpenoids and terpenes in citrus essential oils by a biphasic extraction system. Chem. Eng. Sci. 2024, 299, 120476. [Google Scholar] [CrossRef]
- Shaw, D.; Tripathi, A.D.; Paul, V.; Agarwal, A.; Mishra, P.K.; Kumar, M. Valorization of essential oils from citrus peel powder using hydro-distillation. Sustain. Chem. Pharm. 2023, 32, 101036. [Google Scholar] [CrossRef]
- Gonçalves, D.; Paludetti, M.F.; Gonçalves, C.B.; Rodrigues, C.E.C. Extraction of oxygenated compounds from crude citrus latifolia peel oil using ethanol/water mixtures as solvents: Phase equilibrium and continuous equipment operation. Sep. Purif. Technol. 2018, 199, 271–281. [Google Scholar] [CrossRef]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Padilla-de la Rosa, J.D.; Manzano-Alfaro, M.D.; Gómez-Huerta, J.R.; Arriola-Guevara, E.; Guatemala-Morales, G.; Cardador-Martínez, A.; Estarrón-Espinosa, M. Innovation in a Continuous System of Distillation by Steam to Obtain Essential Oil from Persian Lime Juice (Citrus latifolia Tanaka). Molecules 2021, 26, 4172. [Google Scholar] [CrossRef] [PubMed]
- AFNOR. Dètermination de la densité relative à 20 °C. NF T 75-111. In Reçueil de Normes Françaises: Huiles Essentielles, 3rd ed.; La Bayeusainc Graphique: Paris, France, 1989. [Google Scholar]
- AFNOR. Dètermination de l´ indice de réfraction. NF T 75-112. In Reçueil de Normes Françaises: Huiles Essentielles, 3rd ed.; La Bayeusainc Graphique: Paris, France, 1989. [Google Scholar]
- AFNOR. Dètermination du pouvoir rotatoire. NF T 75-113. In Reçueil de Normes Françaises: Huiles Essentielles, 3rd ed.; La Bayeusainc Graphique: Paris, France, 1989. [Google Scholar]
- AFNOR. Dètermination de la teneur en huiles essentielles. NF V 03-409. In Reçueil de Normes Françaises: Huiles Essentielles, 3rd ed.; La Bayeusainc Graphique: Paris, France, 1989. [Google Scholar]
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Mistretta, A.M. Diseño de una Columna de Destilación para Recuperación de una Sustancia Termosensible. Ph.D. Thesis, Pontificia Universidad Católica de Valparaiso, Valparaíso, Chile, 2012. [Google Scholar]
- Gamarra, F.M.C.; Sakanaka, L.S.; Tambourgi, E.B.; Cabral, F.A. Influence on the quality of essential lemon (Citrus aurantifolia) oil by distillation process. Braz. J. Chem. Eng. 2006, 23, 147–151. [Google Scholar] [CrossRef]
- Njoku, V.I.; Evbuomwan, B.O. Analysis and Comparative Study of Essential Oil Extracted from Nigerian Orange, Lemon and Lime Peels. Greener J. Chem. Sci. Technol. 2014, 1, 6–14. [Google Scholar] [CrossRef]
- Albaladejo Meroño, Q. El Aceite Esencial de Limón Producido en España. Contribución a su Evaluación por Organismos Internacionales. 1999. Available online: https://www.tesisenred.net/handle/10803/11059;jsessionid=F2D3F901AB262FE9DD87CA6BCCA22076#documents (accessed on 24 January 2024).
- Bourgou, S.; Rahali, F.Z.; Ourghemmi, I.; Saïdani Tounsi, M. Changes of peel essential oil composition of four Tunisian citrus during fruit maturation. Sci. World J. 2012, 2012, 528593. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publ.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Sánchez, Y.; Pino, O.; Correa, T.M.; Naranjo, E.; Iglesia, A. Estudio químico y microbiológico del aceite esencial de Piper auritum Kunth (caisimón de anís). Rev. Prot. Veg. 2009, 24, 39–46. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).