Abstract
This study aimed to evaluate the extraction of Corymbia citriodora (Hook.) K.D.Hill and L.A.S.Johnson essential oil by steam distillation under reduced pressure. Yield and composition of the essential oils obtained at different system pressures were analyzed. System pressure had a significant influence on essential oil yield, resulting in a reduction of 78.6% when the pressure was reduced from 690 Torr to 240 Torr. There were also changes in essential oil composition, with an increase in citronellol content (oxygenated monoterpene). However, the major compound (citronellal) remained at a high content in all tests. Regarding the extracted mass of the major compounds (citronellal, citronellol), there was a significant reduction for all when the system pressure was reduced. Although the reduction in the pressure of the system caused a reduction in oil yield, it was possible to carry out the steps of extraction and purification of the major compound simultaneously. Reduced pressure extraction may decrease process time, increasing its efficiency and reducing costs in the extraction of essential oils.
1. Introduction
Today, the use of substances from natural sources in the development and production of cosmetics, foods, cleaning products, perfumery, and agricultural and medical applications is increasing. This trend follows the concerns regarding the effect of synthetic chemicals on the environment and human and animal health. Among these substances, essential oils highlight themselves as one of the natural products with the most potential and interest in the replacement of synthetic molecules [1,2,3].
Most of the essential oils are complex mixtures of terpenes, extracted from aromatic, medicinal, spice, and forest species. Several terpenes have demonstrated bioactive properties, with a potential for use in many areas, such as cosmetics, perfumery, medicine (antioxidant and antimicrobial activity, uses in aromatherapy), industrial uses (eco-friendly solvents), and agricultural applications (antimicrobial, antifungal, insecticidal/repellent activities), among other uses [4,5].
However, for effective use of these compounds, in several situations, the terpenes must be purified and/or separated from the mixture. In the industry, this process is carried out by vacuum fractional distillation, although the extraction and fractionation using supercritical carbon dioxide has been widely used in recent years [6,7].
Relative to the industrial methods used in the separation/purification of essential oil components, vacuum fractional distillation has the main drawbacks of high energy costs required to generate a high vacuum and the exposition of the essential oil to high temperatures (<120 °C), which may cause the degradation of thermolabile compounds, reducing the quality, or even rendering the product unusable [6,8,9]. On the other hand, the extraction or fractionation using supercritical carbon dioxide, while occurring at mild temperatures (approx. 31 °C), operates at pressures above 7.3 MPa (approx. 73 atm), which requires the design and construction of robust equipment and the strict control of operational parameters, increasing the costs of this process [10,11].
In this sense, the use of a process that simultaneously carries out the extraction and fractionation in a single stage is interesting because it reduces total process time; removes the exposure time of essential components to heat, maintaining product quality; and removes the need for post-treatment steps, reducing additional costs with separation/purification [6,12].
The reduction of the absolute pressure of the system may help in the separation/purification of essential oil components through the modulation of steam temperature in contact with the plant material, altering the volatilization rates of the several terpenes present in the feedstock [4,13].
Cusin et al. [14] carried out the extraction of the petitgrain essential oil of Citrus deliciosa Tenore by steam distillation at different absolute pressures. The author reported a reduction in essential oil yield as the pressure was reduced; however, there was an increase in the content of the major compound. On the other hand, Wu et al. [15], studying the reduced pressure extraction of Origanum vulgare L., reported an increase in essential oil yield when the absolute pressure of the system was reduced.
Corymbia citriodora (Hook.) K.D.Hill and L.A.S.Johnson is a large-sized aromatic species, related to eucalyptus, and is distributed worldwide. The name ‘Eucalyptus citriodora Hook.’ is synonym of this species. Native to Eastern Australia [16], C. citriodora has a high content of essential oil in its leaves (2.8–6.9% v/w), whose chemotype reported in the literature is the citronellal one [17,18].
The essential oil of C. citriodora has several uses: it can be used in cosmetics, perfumery, pharmaceuticals, foods, and other fields, such as hygiene and cleaning products [5,19,20]. The literature also has reports on other possible applications due to the antifungal, antibacterial, and herbicidal properties of C. citriodora leaf essential oil [20,21]. However, there are few reports addressing the use of pressure-reduced distillation on the yield and composition of essential oils, and even less on those of C. citriodora.
The aim of this work was to assess the extraction of the leaf essential oil of Corymbia citriodora (Hook.) K.D.Hill and L.A.S.Johnson by steam distillation at different absolute pressures, verifying the influence of system pressure on the yield and composition of the obtained essential oil.
2. Experimental
2.1. Collection and Preparation of Plant Material
The plant materials used in the experiments were Corymbia citriodora (Hook.) leaves. The material was collected from an adult plant with an age of approximately 17 years, located in a rural property in the municipality of Estrela, South Brazil, at the geographical coordinates of 29°28′43″ S and 51°54′34″ W, and an altitude of 39 m above sea level [22]. The collection was carried out early in the morning, and about 5 kg of leaves were collected on 4 April 2021. The plant species was identified by Dr. Felipe Gonzatti, the head of the Herbarium of University of Caxias do Sul (HUCS).
The plant material was dried using a kiln with forced air circulation at room temperature (20 ± 5 °C) for 96 h. After drying, the leaves were manually chopped using a scissor to render a particle size smaller than 2.0 mm, aiming to homogenize the samples. In each extraction, 50 g of chopped leaves were weighed in a semi-analytical balance, and then transferred to the extraction chamber with a volume of 1.0 L, rendering a plant material density of 0.05 g·mL−1 in all extractions [14]. The heating power of the mantle was kept at 415 W and the condenser temperature was kept at 10 ± 2 °C in all experiments.
2.2. Essential Oil Extraction
Firstly, the essential oil was extracted for 4 h at atmospheric pressure (since the location where the study was carried out was 817 m above sea level, the local atmospheric pressure was 690 Torr) to verify the yield and chemical composition of the essential oil obtained at ‘standard’ conditions.
Afterwards, extractions were carried out for 1 h, at different absolute pressures, to verify the influence of system pressure on the yield and chemical composition of the essential oil. The extraction period of 1 h was chosen to optimize extraction time since industrial extractions are generally conducted for 1 h. The absolute pressures of 690, 540, 390, and 240 Torr were tested. A scheme of the extraction system used is presented in Figure 1.
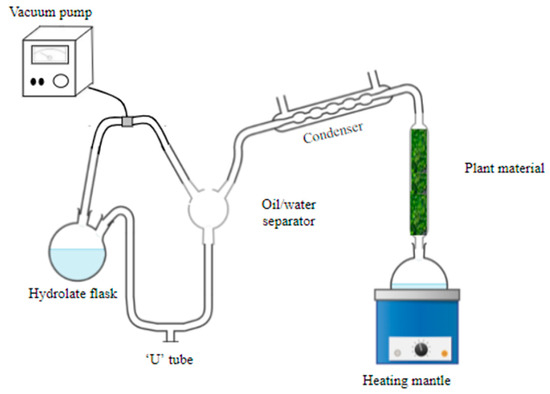
Figure 1.
Simplified scheme of the system used in the extractions at different absolute pressures. Source: adapted from Cusin et al. [14].
Essential oil yield was calculated using the measured volume of essential oil obtained and the mass of plant material used in the extraction, according to Equation (1).
where ‘Y’ is the essential oil yield, ‘V’ is the volume of essential oil obtained (mL), and ‘M’ is the mass of plant material used in the extraction (g). The extracted mass of the major compounds of the essential oil was also determined, at the four pressures analyzed, using Equation (2).
where ‘mc’ is the extracted mass of each component (mg), ‘Y’ is the essential oil yield (% v/w), ‘ρ’ is the specific mass of the essential oil (g·mL−1), and ‘C’ is the content of the compound in the essential oil (wt.%).
The obtained essential oils were stored in amber glass flasks and kept in a cold chamber (4 ± 2 °C) until they were ready for chromatographic analysis.
2.3. Chromatographic Analysis
The essential oils were analyzed by GC/MS (qualitative analysis) and GC-FID (quantitative analysis), following the procedures described by Silvestre et al. [23] and Vicenço et al. [24].
Qualitative analysis (GC/MS) was carried out using an HP gas chromatograph, model 6890, coupled to a MSD5973 mass spectrometer equipped with the HP Chemstation software and the Wiley 275 spectra library. An HP-5 MS fused silica column (30 m × 250 μm and 0.50 μm film thickness) was used (HP, Palo Alto, CA, USA). Temperature programming was 60 °C for 8 min, heating to 180 °C at 3 °C · min−1, and heating to 230 °C at 20 °C · min−1. The injector temperature was 200 °C and interface temperature was 250 °C, with a split ratio of 1:100, using helium as a carrier gas at 56 kPa and flow rate of 1.0 mL · min−1, with ionization energy of 70 eV.
Quantitative analysis (GC-FID) was carried out using an HP gas chromatograph, model 6890, coupled to a flame ionization detector, and the HP Chemstation software. An HP-5 MS fused silica column (30 m × 250 μm and 0.50 μm film thickness) was used (HP, Palo Alto, CA, USA), following the same temperature programming of GC/MS analysis. The detector temperature was 250 °C, with a split ratio of 1:100, using hydrogen as a carrier gas at 34 kPa and flow rate of 1.0 mL · min−1, and a sample injection volume of 1 μL. Compound quantification was performed using 1-octanol as an internal standard, injecting 25 μL of a 30.22 g · L−1 of a 1-octanol solution in hexane (755 μg of 1-octanol injected in each analysis).
The identification of the essential oil components was carried out by comparing their respective mass spectra to the ones in the Wiley 275 spectral library, which were then selected by the equipment through match percentage; we then compared the calculated linear retention indexes (LRI) to the ones reported by Adams [25]. A C7-C30 normal alkane solution (Sigma Aldrich, San Luis, MO, USA) was used, and 1 μL of a 20 g∙L−1 solution of alkanes diluted in hexane was injected.
Only the compounds whose LRI values and mass spectra were concordant were regarded as identified; otherwise, they were reported as ‘not identified’. The determination of the contents of each component of the essential oils was carried out based on the procedures described by Rebelo et al. [26], with the determination of response factors injecting curves of compounds representative of the chemical classes present in the essential oils.
2.4. Experimental Design and Statistical Analysis
The experimental design was completely randomized, with one factor (system absolute pressure). All extractions were carried out three times for each treatment, totaling 12 extractions for the experiment. The obtained results underwent Levene’s test (homoscedasticity) and Shapiro–Wilk’s test (homogeneity of residuals), followed by analysis of variance (ANOVA) and Tukey’s multiple range test at 5% probability. The statistical analysis was carried out using the Statistica 12 software (Statsoft, Tulsa, OK, USA).
3. Results and Discussion
3.1. Determination of Corymbia citriodora (Hook.) Leaf Essential Oil Yield
In the experiment in which the essential oil was extracted for 4 h at atmospheric pressure (690 Torr), the average essential oil yield was 7.32% v/w, which is considered the maximum yield for the tested sample of plant material tested in this study.
Tolba et al. [18], also carrying out the extraction of the essential oil of C. citriodora by steam distillation using only the leaves (without stalks) and dried at room temperature, reported a yield of 2.26 wt.% (approx. 2.86% v/w). In another study, Benchaa et al. [21] observed an essential oil yield of 3.40% v/w for an extraction time of 2 h, whereas Silou et al. [17], testing the same extraction time, reported an essential oil yield in the range of 6.10–6.90 wt.%.
As observed by Pauletti et al. [27] and Pansera et al. [28], the essential oil yield is quite variable, being influenced by environmental conditions and plant genetics, which may explain the wide variation of results reported in the literature, as well as the maximum essential oil yield, observed for C. citriodora in this study.
3.2. Effect of the Absolute Pressure of the System on Essential Oil Yield
The data on essential oil yield as a function of the different absolute pressures of the extraction system, for an extraction time of 1 h, are presented in Table 1.

Table 1.
Average essential oil yield as a function of the absolute pressure of the system, after an extraction time of 1 h.
With the data shown in Table 1, it is possible to observe that, in terms of yield, the extraction at atmospheric pressure (690 Torr) was the most efficient, i.e., had the highest essential oil yield. It was also possible to verify that the absolute pressure of the system had a significant effect on essential oil yield, resulting in a reduction of 78.6% in the yield when the system pressure was reduced from 690 Torr to 240 Torr. The essential oil yield extracted for 1 h at atmospheric pressure was 6.53% v/w. Pino et al. [29] reported a yield of 2.25% v/w for C. citriodora leaves extracted for 1 h at atmospheric pressure.
The terpenes present in the plant material have lower saturation pressures when the temperature of the steam used in distillation is lower. This ends up lowering the volatilization rates [13], which may explain the reduction in essential oil yield as the absolute pressure is reduced, considering a fixed extraction time.
Cusin et al. [14] used an apparatus similar to the one used in this study to evaluate the influence of system pressure on the extraction of the petitgrain essential oil of Citrus deliciosa Tenore. In this study, when the pressure was reduced from 690 to 310 Torr, there was a reduction in essential oil yield from 0.6% v/w at 690 Torr to 0.3% v/w at 310 Torr.
However, in the study carried out by Wu et al. [15], who tested the effect of pressure on the hydrodistillation of Origanum vulgare L. with a Clevenger apparatus at atmospheric and reduced pressure, there was an increase in essential oil yield at 500 mbar (approx. 375 Torr) relative to the extraction at 760 Torr. According to the authors, this behavior may be related to material texture and changes in the diffusion and volatilization rates of the terpenes that composed the essential oil during extraction.
Ha et al. [30] performed the steam distillation at reduced pressure (95 Torr) of the roots of Angelica tenuissima Nakai, and the leaves of Mentha arvensis L. and Acorus gramineus Rhizoma, and needles of Pinus sylvestris L., reporting essential oil yields of 3.16 wt.%, 3.48 wt.%, 4.41 wt.%, and 2.51 wt.%, respectively. According to literature data, the extraction at reduced pressure facilitated the extraction of the essential oil of Pinus sylvestris L. cones (essential oil content of 0.13% v/w), probably due to the smaller content of oxygenated monoterpenes and sesquiterpenes [31]. Relative to the species Mentha arvensis L., it was observed that the extraction at reduced pressure caused no important differences in the obtained essential oil relative to the data reported in the literature (2.7–5.1 wt.%) due to the higher amounts of hydrocarbon monoterpenes and oxygenated monoterpenes, which are more volatile [32].
Given the studies present in the literature, it is possible to notice that extraction efficiency is related to the kind of feedstock used (terpenes present in the sample) and the occurrence of variations due to the method employed. Thus, it is important to consider the joint effect, i.e., the kind of material and the extraction method used to obtain the essential oil and its real impact on its extraction efficiency [4].
3.3. Effect of the Absolute Pressure of the System on Essential Oil Composition
Aiming to evaluate the influence of the absolute pressure of the system on the essential oil, the chemical composition of the obtained oils at each operating pressure was determined, whose results are compiled in Table 2.

Table 2.
Chemical composition of the essential oils obtained by steam distillation at different absolute pressures for 1 h.
The GC/MS chromatograms of the essential oils obtained by reduced pressure extraction at different absolute pressures are presented in Figure 2.
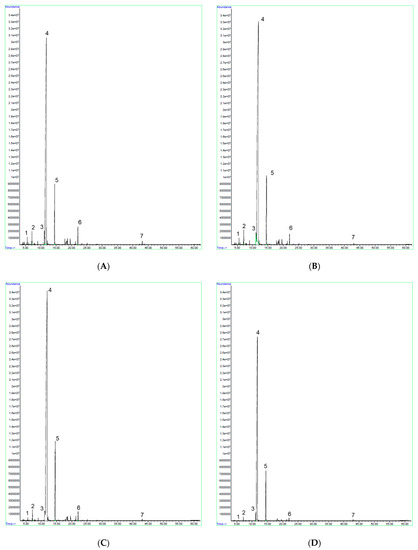
Figure 2.
GC/MS chromatograms of Corymbia citriodora essential oil, obtained by reduced pressure extraction at 690 Torr (A), 540 Torr (B), 390 Torr (C), and 240 Torr (D). 1—β-pinene; 2—eucalyptol (1,8-cineole); 3—isopulegol; 4—citronellal; 5—citronellol; 6—PMD (p-Menthane-3,8-diol), 7—(E)-caryophyllene.
Tolba et al. [18], extracting the leaf essential oil of Corymbia citriodora at atmospheric pressure, reported the following chemical composition: 0.43 wt.% β-pinene, 4.66 wt.% isopulegol, 69.77 wt.% citronellal (major compound), 10.63 wt.% citronellol, 2.76 wt.% PMD, and 1.34 wt.% caryophyllene. In a similar study, Benchaa et al. [21] reported 0.80 wt.% β-pinene, traces of isopulegol (content below 0.05 wt.%), 64.70 wt.% citronellal, 10.90 wt.% citronellol, and 0.50 wt.% caryophyllene.
When comparing the composition with the ones reported in Table 2, for the pressure of 690 Torr, it is possible to observe the existence of differences between them since the major compound content was considerably high, consequently interfering with the contents of the other compounds.
With the obtained results, it was possible to verify that by reducing the absolute pressure from 690 Torr to 240 Torr, there was a fractionation of the essential oil during the extraction. A reduction in PMD and (E)-caryophyllene contents was noticed, which increased the amounts of citronellol (the extraction at reduced pressures favored an increase in the contents of this compound in the obtained essential oil). Citronellal remained at high contents (about 92 wt.%) in all tested pressures. The contents of β-pinene, eucalyptol, and isopulegol did not differ statistically, probably due to the small amounts in the essential oil (<0.40 wt.%).
For citronellal, it was possible to verify that there was no statistical difference between the pressure of 240 Torr and the others; the pressure of 690 Torr differed from the pressures of 540 and 390 Torr. This indicates that the operating pressure of the system had little influence on the concentration of this compound, very probably due to its high content in the essential oil (>90 wt.%).
For citronellol, we observed a trend of increase in its content with the reduction of the operating pressure of the system; the pressures of 690 and 540 Torr were statistically similar. At 240 Torr, the citronellol content was 6.55 wt.%, an increase of 34% relative to the contents obtained at the pressure of 690 Torr (4.89 wt.%).
For (E)-caryophyllene (only hydrocarbon sesquiterpene identified), we observed a trend of reduction in its contents as the system pressure was reduced; the pressures of 390 and 240 Torr had similar performances, and the others were statistically different. At 240 Torr, the content of this compound reduced by 61% relative to the extraction at atmospheric pressure (0.70 wt.% at 690 Torr and 0.27 wt.% at 240 Torr).
For PMD, no clear trend could be observed, with no statistical difference between the pressure of 540 Torr and the others. The test at 690 Torr differed from 390 Torr and 240 Torr.
Kim and Lee [34] performed the steam distillation at reduced pressure (100 Torr) of Lavandula angustifolia leaves. The authors observed that the essential oil obtained in these conditions did not present more volatile monoterpene hydrocarbons, nor oxygenated sesquiterpenes in its composition, a different composition than the one observed by solid-phase extraction and simultaneous distillation-extraction (SDE) at atmospheric pressure, which were also evaluated.
Tamura et al. [35] extracted the essential oil of Ulva pertusa by steam distillation at the absolute pressure of 20–30 Torr, reporting important changes in the aroma of the obtained product due to alterations in its chemical composition.
As noted by Smith [36], although the increase in system pressure may help accelerate the extraction of volatile compounds by speeding up the kinetics, the increase in pressure hinders the volatilization of the less volatile compounds. In this sense, it would be preferable to operate the system at lower pressures, facilitating the volatilization of the compounds and exposing them to lower temperatures.
The relationships between the content and the absolute pressures for the compounds citronellal, citronellol, and (E)-caryophyllene are presented in Figure 3.
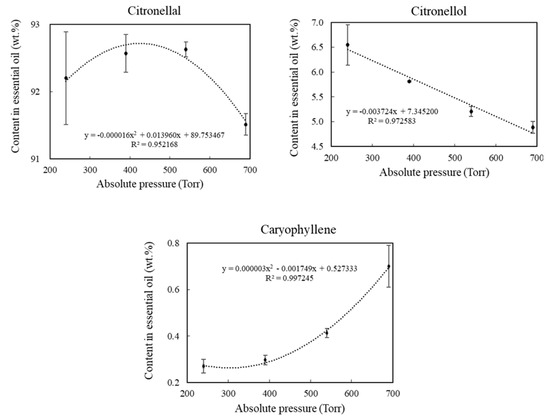
Figure 3.
Contents of the compounds found in highest amounts in the essential oil as a function of the absolute pressure of the extraction system.
According to the obtained data, it can be seen that citronellal remained at high contents in all pressures tested, showing that the extraction at reduced pressure had no important impact on this compound due to its large amounts in the essential oil.
On the other hand, two distinct behaviors for citronellol and (E)-caryophyllene can be observed, in which the former had its content increased as the system pressure was reduced, and the latter had its content reduced as the pressure decreased.
These different behaviors between citronellol and (E)-caryophyllene were probably the result of the different volatilities of these compounds, considering that citronellol is an oxygenated monoterpene and (E)-caryophyllene is a sesquiterpene. The lower inherent volatility of (E)-caryophyllene, associated with a lower steam temperature caused by the reduction of the system pressure, ended up reducing the extraction efficiency of this compound, increasing the citronellol contents. As citronellal, which is the major compound of the essential oil, citronellol is also an oxygenated monoterpene; they volatilized more easily, being extracted, while most of the (E)-caryophyllene remained in plant material due to the lower volatilization rate.
Similar to the results of this study, Kubota et al. [37] carried out the extraction of the volatile compounds of Alpinia galanga Willd by steam distillation at reduced pressure (20 Torr), reporting an increase in the amounts of oxygenated monoterpenes to the detriment of the most volatile (hydrocarbon monoterpenes) and less volatile (sesquiterpenes) fractions. Moreover, the same authors also cited that there was an increase in the aroma of the obtained material compared to the extraction at atmospheric pressure.
3.4. Effect of the Absolute Pressure of the System on the Extracted Mass of the Major Compounds
The effect of the absolute pressure of the system on the extracted mass of the major compounds was also analyzed, whose results are presented in Table 3.

Table 3.
Influence of the absolute pressure of the system on the extracted mass of the major compounds of Corymbia citriodora leaf essential oil.
According to the results compiled in Table 3, it is possible to observe that, when reducing the absolute pressure of the system from 690 to 240 Torr, there was a significant reduction of the extracted mass for all three compounds. This reduction was 78.4% for citronellal, 71.5% for citronellol, and 90.5% for (E)-caryophyllene.
As noted by Cavalcanti et al. [38], Lei et al. [39], and Cusin et al. [14], the extraction kinetics of terpenes are highly dependent on process conditions, such as the extraction time and absolute pressure of the system, which is directly related to steam temperature as a function of saturation pressure.
Thus, considering the same extraction time for all four pressures tested, the extractions at lower pressures, due to the lower temperature of the extracting fluid (steam), had slower extraction kinetics and smaller volatilization rates [14]. This reduced the extraction efficiency of the compounds, especially the less volatile ones, such as (E)-caryophyllene (hydrocarbon sesquiterpene).
With a mechanism similar to the process of vacuum fractional distillation, the extraction at reduced pressure promoted differential volatilization of the compounds, reducing their extraction efficiency, and concentrating the oxygenated monoterpenes (citronellal, citronellol) to the detriment of hydrocarbon sesquiterpenes ((E)-caryophyllene).
4. Conclusions
It was possible to observe a significant reduction in the essential oil yield with the reduction of the absolute pressure of the system. Regarding the contents of individual components, the content of the major compound (citronellal) was not influenced by the operating pressure of the system. However, all compounds had their extraction efficiency reduced with the reduction of the pressure. Steam distillation under reduced pressure may help in the purification of essential oil components by reducing the extraction efficiency of the less volatile compounds and concentrating on the more volatile ones. Thus, while the extraction efficiency is smaller, reduced pressure extraction may decrease process time and reduce costs in the extraction of essential oils.
Author Contributions
Conceptualization, J.d.A., W.P.S. and L.A.R.M.; Formal analysis, W.P.S.; Investigation, J.d.A.; Methodology, J.d.A., W.P.S. and G.F.P.; Resources, G.F.P.; Supervision, L.A.R.M.; Validation, L.A.R.M.; Writing—original draft, J.d.A. and W.P.S.; Writing—review and editing, G.F.P. and L.A.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data used in the study is reported in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A step forward on sustainability in the cosmetics industry: A review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Sahraoui, A.L.-H. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Simões, C.M.O.; Schenkel, E.P.; Mello, J.C.P.; Mentz, L.A.; Petrovick, P.R. Farmacognosia: Do Produto Natural ao Medicamento; Artmed: Porto Alegre, Brazil, 2016. [Google Scholar]
- Almeida, R.N.; Soares, R.D.P.; Cassel, E. Fractionation process of essential oils by batch distillation. Braz. J. Chem. Eng. 2018, 35, 1129–1140. [Google Scholar] [CrossRef]
- Silvestre, W.; Livinalli, N.; Baldasso, C.; Tessaro, I. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- de Souza, E.L.; Stamford, T.L.M.; Lima, E.d.O.; Filho, J.M.B.; Marques, M.O.M. Interference of heating on the antimicrobial activity and chemical composition of Origanum vulgare L. (Lamiaceae) essential oil. Food Sci. Technol. 2008, 28, 418–422. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Soares, V.B.; Coelho, G.L.V. Safety study of an experimental apparatus for extraction with supercritical CO2. Braz. J. Chem. Eng. 2012, 29, 677–682. [Google Scholar] [CrossRef]
- Iovine, A.; Molino, A.; Casella, P.; Marino, T.; Chianese, S.; Musmarra, D. Risk Analysis of a Supercritical Fluid Extraction Plant Through the Phast Safeti Software with Extraction Vessel Rupture as Scenario. Chem. Eng. Trans. 2021, 86, 253–258. [Google Scholar] [CrossRef]
- Silvestre, W.; Agostini, F.; Muniz, L.; Pauletti, G. Fractionating of green mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. J. Food Eng. 2016, 178, 90–94. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C.; Abbott, M.M. Introdução à Termodinâmica da Engenharia Química; LTC: Rio de Janeiro, Brazil, 2007. [Google Scholar]
- Cusin, Y.T.; Silvestre, W.P.; Pauletti, G.F.; Muniz, L.A.R. Extraction of Citrus deliciosa Tenore petitgrain (leaf) essential oil by steam distillation under different operating pressures. Indian Chem. Eng. 2022, 1–11. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, L.; Li, Y.; Wang, Y.; Wang, X.; Wan, N.; Huang, X.; Zhang, X.; Yang, M. A novel application of the vacuum distillation technology in extracting Origanum vulgare L. essential oils. Ind. Crops Prod. 2019, 139, 111516. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, S.; Luo, J.; Liu, X.; Lu, W.; Wang, C.; Arnold, R.J. Landrace origins and phenotypic diversity through seedling morphology in Corymbia citriodora subsp. citriodora. Aust. For. 2017, 80, 43–56. [Google Scholar] [CrossRef]
- Silou, T.; Loumouamou, A.N.; Loukakou, E.; Chalchat, J.-C.; Figuérédo, G. Intra and Interspecific Variations of Yield and Chemical Composition of Essential Oils From Five Eucalyptus Species Growing in the Congo-Brazzaville. Corymbia Subgenus. J. Essent. Oil Res. 2009, 21, 203–211. [Google Scholar] [CrossRef]
- Tolba, H.; Moghrani, H.; Benelmouffok, A.; Kellou, D.; Maachi, R. Essential oil of Algerian Eucalyptus citriodora: Chemical composition, antifungal activity. J. Med. Mycol. 2015, 25, e128–e133. [Google Scholar] [CrossRef]
- Ayinde, B.A. Eucalyptus (Eucalyptus citriodora Hook., Myrtaceae) oils. In Essential Oils in Food Preservation, Flavor, and Safety, 1st ed.; Preedy, V.R., Ed.; Academic Press: London, UK, 2016; pp. 413–418. [Google Scholar]
- Miguel, M.G.; Gago, C.; Antunes, M.D.; Lagoas, S.; Faleiro, M.L.; Megías, C.; Cortés-Giraldo, I.; Vioque, J.; Figueiredo, A.C. Antibacterial, Antioxidant, and Antiproliferative Activities of Corymbia citriodora and the Essential Oils of Eight Eucalyptus Species. Medicines 2018, 5, 61. [Google Scholar] [CrossRef]
- Benchaa, S.; Hazzit, M.; Abdelkrim, H. Allelopathic Effect of Eucalyptus citriodora Essential Oil and Its Potential Use as Bioherbicide. Chem. Biodivers. 2018, 15, e1800202. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Estrela. 2021. Available online: https://cidades.ibge.gov.br/brasil/rs/estrela/historico (accessed on 3 April 2022).
- Silvestre, W.P.; Medeiros, F.R.; Agostini, F.; Toss, D.; Pauletti, G.F. Fractionation of rosemary (Rosmarinus officinalis L.) essential oil using vacuum fractional distillation. J. Food Sci. Technol. 2019, 56, 5422–5434. [Google Scholar] [CrossRef] [PubMed]
- Vicenço, C.B.; Silvestre, W.P.; Da Silva, V.T.; Menegol, I.V.; Hahn, R.C.; Lima, T.S.; Agostini, F.; Pauletti, G.F. Bioactivity of Schinus molle L. and Schinus terebinthifolia Raddi. Essential Oils on Anticarsia gemmatalis (Hübner 1818). Braz. Arch. Biol. Technol. 2020, 63, e20200111. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing: Waco, TX, USA, 2017. [Google Scholar]
- Rebelo, R.A.; Tizziani, T.; Begnini, I.M.; Crestani, I.; Dognini, J.; de Gasper, A.L.; de Cordova, C.M.M.; dos Santos, L.; Hochheim, S.; Baldovini, N. Essential oils from leaves of Vernonanthura montevidensis (Spreng.) H. Rob.: Chemical profile and antimollicute potential. Nat. Prod. Res. 2020, 36, 2393–2398. [Google Scholar] [CrossRef]
- Pauletti, G.F.; Silvestre, W.P.; Rota, L.D.; Echeverrigaray, S.; De Barros, I.B.I. Poejo (Cunila galioides Benth.) Production in Five Agroecological Regions of Rio Grande do Sul. Braz. Arch. Biol. Technol. 2020, 63, e20190481. [Google Scholar] [CrossRef]
- Pansera, M.R.; Silvestre, W.P.; Gonzatti, F.; Pauletti, G.F.; Sartori, V.C. Chemical composition and antifungal activity of the essential oils from native species of the ‘Campos de Cima da Serra’ region, South Brazil. J. Essent. Oil Res. 2021, 33, 488–501. [Google Scholar] [CrossRef]
- Pino, J.A.; Quert, R.; Hernández, I.; Rodeiro, I.; Fernández, M.D.; Cuellar, C.; Pérez, J.C. Chemical composition and antioxidant activity of the essential oil from leaves of Corymbia citriodora Hook grown in western Cuba. Am. J. Essent. Oils Nat. Prod. 2020, 8, 18–22. [Google Scholar]
- Ka, M.-H.; Choi, E.H.; Chun, H.-S.; Lee, K.-G. Antioxidative Activity of Volatile Extracts Isolated from Angelica tenuissimae Roots, Peppermint Leaves, Pine Needles, and Sweet Flag Leaves. J. Agric. Food Chem. 2005, 53, 4124–4129. [Google Scholar] [CrossRef]
- Tumen, I.; Hafizoglu, H.; Kilic, A.; Dönmez, I.E.; Sivrikaya, H.; Reunanen, M. Yields and Constituents of Essential Oil from Cones of Pinaceae spp. Natively Grown in Turkey. Molecules 2010, 15, 5806. [Google Scholar] [CrossRef]
- Soltanbeigi, A.; Özgüven, M.; Hassanpouraghdam, M.B. Planting-date and cutting-time affect the growth and essential oil composition of Mentha × piperita and Mentha arvensis. Ind. Crops Prod. 2021, 170, 113790. [Google Scholar] [CrossRef]
- Yadegarinia, D.; Gachkar, L.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry 2006, 67, 1249–1255. [Google Scholar] [CrossRef]
- Kim, N.S.; Lee, D.S. Comparison of different extraction methods for the analysis of fragrances from Lavandula species by gas chromatography-mass spectrometry. J. Chromatogr. A 2002, 982, 31–47. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamoto, H.; Yang, R.-H.; Sugisawa, H. Characteristic Aroma Compounds in Green Algae (Ulva pertusa) Volatiles. J. Jpn. Soc. Food Sci. Technol. 1995, 42, 887–891. [Google Scholar] [CrossRef]
- Smith, R.M. Extractions with superheated water. J. Chromatogr. A 2002, 975, 31–46. [Google Scholar] [CrossRef]
- Kubota, K.; Someya, S.; Kurobayashi, Y.; Kobayashi, A. Flavor characteristics and stereochemistry of the volatile constituents of greater galangal (Alpinia galanga Willd.). In Flavor Chemistry of Ethnic Foods; Shahidi, F., Ho, C.T., Eds.; Springer Science + Business Media: New York, NY, USA, 1999; pp. 97–104. [Google Scholar]
- Cavalcanti, A.D.S.; Alves, M.D.S.; da Silva, L.C.P.; Patrocínio, D.D.S.; Sanches, M.N.; Chaves, D.S.D.A.; de Souza, M.A.A. Volatiles composition and extraction kinetics from Schinus terebinthifolius and Schinus molle leaves and fruit. Braz. J. Pharmacogn. 2015, 25, 356–362. [Google Scholar] [CrossRef]
- Lei, G.; Song, C.; Shi, H.; Xing, Y.; Song, X.; Liang, G. Parameter Effects and Kinetics of Ultrasound Assisted Ionic Liquid Mediated Hydro-distillation and Essential Oil Composition of Flowers of Paeonia suffruticosa Andr‘Jitsugetsu Nishiki’ from Central China. J. Essent. Oil Bear. Plants 2019, 22, 762–773. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).