Abstract
Cosmetics have always been in demand across the globe among people of all age groups. In the modern cosmetic world, nanostructured materials have proven hugely advantageous in producing cosmeceuticals or ‘nano-cosmeceuticals’ and various beauty products. The application of nanostructured materials in cosmetic products possesses some challenges in terms of short- and long-term safety and environmental issues, despite their growing popularity. The nanostructured particles in cosmeceuticals provide a targeted route of administration due to their high penetrability, site selectivity, high effectiveness, prolonged activity, and drug encapsulation potential. However, standard methods for toxicity evaluation may not be relevant for cosmeceuticals, leading to the need for an alternative methodology. This review article compiles detailed descriptions of all significant aspects of nanostructured materials in the cosmetics industry, which include the synthesis and characterization of relevant nanostructured materials for cosmeceuticals, state-of-the-art practices, mechanisms for the synthesis of advanced materials, toxicological concerns in terms of health risks in humans, and environmental concerns. Also, a proposal for new approaches in terms of regulatory measures to mitigate these problems has been suggested. The primary focus of this article is to provide a comprehensive outlook on this subject area and contribute to the exploration of new prospects and emerging roles of nanostructured materials in the cosmetics industry.
1. Introduction
The word ‘nanotechnology’ is made up of two words; the first word is the Greek word ‘nano’, which means tiny (e.g., of the nanometer length scale of ~1 to 100 nm), and the second word is ‘technology’ [1]. Nanotechnology possesses immense potential to improve a wide range of materials in research and applications and is therefore capable of gaining considerable attention in medical and pharmaceutical domains [2]. In recent years, the cosmetics industry has gained huge advantages through nanotechnology [3,4]. Cosmetics are designed to be applied on skin, sprayed, powdered, sprinkled or penetrated. These products are useful for enhancing a person’s attractiveness or modifying one’s appearance, and include skin moisturizers, fragrances, lipsticks, nail paints, eye and face makeup products, cleaning shampoos, permanent waves products, hair colors and deodorants, etc., as along with any material intended as a component of a cosmetic [5]. Nanostructured materials are useful in certain cosmetics, such as sun protection lotions, long-lasting cosmetics, and skin creams, as well as in pigment manufacturing [6]. These materials are helpful in creating enhanced properties such as improved fixation to the skin, penetration into the deeper layers of the skin, and the imparting of a consistent effect. Cosmeceuticals are cosmetic products which impart medicinal or druglike effects and benefits. The term ‘cosmeceuticals’ is not recognized by the United States FD&C Act (Federal Food, Drug and Cosmetic Act). However, this term is used by cosmetics industries and manufacturers to combine these two classes of products, i.e., cosmetics and pharmaceuticals, to create a new class of products, i.e., medicinal cosmetics. Cosmetics temporarily treat the skin, and the effect is immediate and fast, while cosmeceuticals are designed to treat the root cause of skin problems and create long-lasting results. For example, anti-aging and photoprotective nano-cosmeceuticals can penetrate deep into the skin’s layers and establish dynamic aftereffects [7]. A variety of nano-cosmeceuticals containing cubosomes, liposomes, nano-emulsions, nanocrystals, hydrogels, and other nanostructured materials are extensively utilized in branded cosmetic products and have proven advantageous in terms of the dynamic stability of molecules, particularly in the inner skin layers [8], to obtain enhanced beauty effects [9].
The nanostructured materials in cosmetics have an edge over their conventional bulk counterparts such as improved activity, distinctive smoothness, clarity, reduced degradation, and less irritation to skin [10]. Oxides of zinc and titanium, which are white and opaque in their physical appearance at the bulk scale, create transparent formulations at the nanoscale and are employed in moisturizers and foundations [11]. Aluminum oxide nanoparticles are also useful to impart a calming effect in foundations, face powders, and high-end concealer sticks to reduce visible flaws and expression lines [12]. This review article is an endeavor to compile the different aspects of nanostructured materials employed in cosmetics, viz., synthesis, characterization, and applications. A comprehensive discussion on the range of products in commercial production employing various nanomaterials along with the toxicological aspects, health risks, environmental concerns, regulatory requirements, and future directions are also presented in this article.
1.1. Significance of Nanomaterials
Nanotechnology has been utilized since ancient times by the Indians, Romans, Greeks, and Egyptians in the form of hair dyes and pigments. Indian data for cosmetic use span back many centuries (to more than 4000 BC), much like those from other regions of the world. Indians continue to utilize ritual-based as well as tradition-related items today in everyday life, even if some of them blend with modern cosmetics, such as carbon black (kajal) hair dyes and pigments. Historically, cosmetic goods were either made from natural ingredients or from natural ingredients modified in some way and are classified as herbal, e.g., sandalwood (Santalum album), red-vermillion (Bixa orellana), turmeric (Curcuma longa), amla or gooseberries (Phyllanthus emblica), reetha (Sapindus mukorossi), chickpea flour (Cicer reticulatum), henna (Lawsonia inermis), etc.), mineral (fuller’s earth, black earth, and red earth), or animal (milk, wax, honey, and curd); as well, some cosmetics are made from black soot and mineral powders [13]. ‘Kajal’ is similar to kohl, or carbon black; it is technically made from carbon nanoparticles and has been used for eye-liner makeup since ancient times by the people in the Indian subcontinent.
For over four decades, modern nanotechnology has been employed in the areas of processing skin repair and health-care products [14,15].
The term ‘cosmetics’ was coined by Raymond Reed in 1961, a founding member of the Cosmetic Chemists US Society. Cosmeceuticals have skin care solutions that enhance the skin tone and texture, and improve cleanliness and skin complexion [14]. These are physiologically and therapeutically active substances utilized in beauty products in order to add to their therapeutic values, e.g., healing of hyperpigmentation, uneven complexion, dark spots, skin irritation, photo-aging, wrinkles, hair damage, etc.; furthermore, they provide several additional benefits, e.g., drug-releasing capability is one of the most promising benefits of nano-cosmeceuticals which relies on parameters such as production process, the ratio of additives and drugs in the composition, and chemical or physical interactions between different components [16]. Nanostructured materials are tiny and can easily penetrate the deeper layers of the skin; therefore, they actively transfer the active components [17]. Most nanostructured-material-based cosmeceuticals can deliver both hydrophilic and lipophilic drugs [18]. Better distribution of cosmetic bioactive compounds into the skin is one probable explanation cited by several academicians and researchers for the significant interest raised regarding the usage of these materials in cosmetics [19]. However, the outcomes of several research studies do not tend to support such a conclusion. This might be attributed to the non-reproducibility of data from various groups of researchers [4]. The key purpose of nano-cosmeceuticals is to attain, enhance, and sustain the desired properties in the target part of the body by releasing the correct amount of nanomaterials. Therefore, nanostructured particles are used in perfumes to help the aroma sustain for longer durations, in skin-whitening treatments, moisturizers, and antiwrinkle creams to improve the attractiveness of the skin, and in sunscreens and other skin care products to boost sunscreen effectiveness by increasing UV protection [20,21,22]. Although, nanostructured materials in cosmetics and cosmeceuticals have advantages as discussed above, there are also several adverse effects that have raised serious concerns for cosmetics and health scientists as well as environmentalists. Ratio and types of nanostructured materials utilized in the manufacturing of personal care products and in cosmetic applications are shown in Figure 1a,b. The relative pros and cons of nanostructured materials in cosmetics are shown in Figure 2.
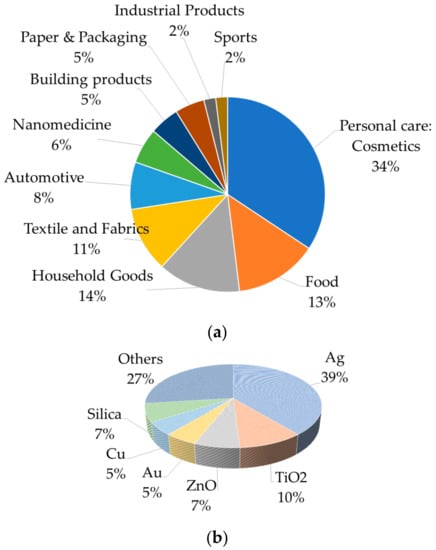
Figure 1.
Nanostructured materials in cosmetics and cosmeceuticals (a) and types of nanostructured materials in cosmetic applications (b).
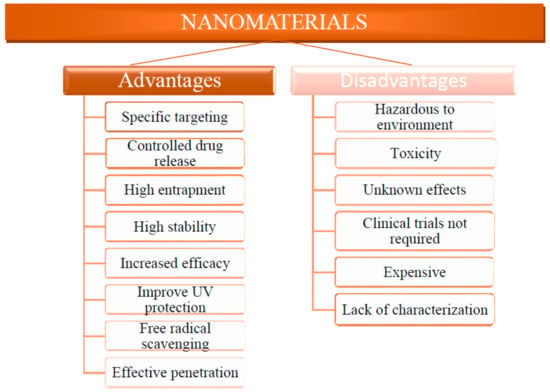
Figure 2.
Relative merits of nanostructured materials in cosmetics and cosmeceuticals.
1.2. Worldwide Scenario of Cosmetics Production
The global cosmetic sector was valued at ~USD 380.2 billion in the year 2019. It is predicted to grow up to USD 463.5 billion by 2027, with a compound annual growth rate (CAGR) of 5.3 percent in the years 2021 to 2027. In this, the share of nano-cosmetic pigments in the market size has been estimated to be ~USD 1.1 billion by the year 2026 after increasing at a CAGR of 6% during the years 2021 to 2026. It has been observed that consumers for commercial cosmetics and cosmeceuticals come from every corner of the globe, irrespective of age, gender, culture, ethnicity, and economic status, probably because people are becoming alarmingly mindful of their physical appearance in addition to skills and intelligence. The market for skin care products has five major categories: moisturizers, face creams, body lotions, sun protection creams, and anti-aging creams. Hair care, skin care, bath and shower, oral care, men’s grooming, deodorants, and antiperspirants are the major segments of the beauty and wellness product market. The cosmetic products market also includes the sections of lips, nails, eyes, and facials [23].
1.3. Market Analysis of Nano-Cosmetic Pigments on the Basis of Type, Application, and Geography
The demand for nanopigment-bearing cosmetics for coloring materials has gained significant attention and demand due to their efficiency of easy customization of makeup products to match unique choices and requirements to enhance the attractiveness of skin, hair, and overall appearances [24]. The share in the nano-cosmetics pigments market is dominated by inorganic nanoparticles and shows a growing rate, at a CAGR of 6.45%, during the years 2021–2026. Since inorganic metallic nanoparticles including TiO2, ZnO, SiO2, and Al2O3 play a significant role in the cosmetics industry for enhancing color, coating capability, and achieving a smooth texture, these have therefore been termed ‘nano-cosmetic pigments.’ These pigments can combine with organic materials to improve the overall quality and effectiveness of cosmetics [25]. Nontoxic in nature and showing hydrophilic, and biocompatible behavior as well as high stability are some of the features which are offered by these pigments over organic nanoparticles. Mostly, ZnO and TiO2 nanostructured particles are employed in sunscreens and similar skin-care products. These show appreciable results and enhance cosmetic effects because of better dispersion as UV filters; they begin at the particle size of 20 nm. TiO2 particles makes sunscreens more effective due to a higher sun protection factor (SPF) at the nanoscale. Therefore, inorganic nanostructured particles are expected to increase the demand for the development of future nano-cosmetic pigments in many regions. The facial makeup segment dominates the nano-cosmetic pigments market, which includes titanium dioxide, zinc oxide, carbon black, and iron oxide as key materials. This segment is expected to grow at the CAGR of 7.3% from the year 2021 to 2026. Titanium oxide is widely used in foundations, blushers, and powders to enhance the skin’s clarity and appearance. Sun protection is a significant feature of face powders, which act by scattering light by means of nanoparticles like ZnO. The size and distribution of the nanoparticles also play an important role in these products by regulating their appearance, stability, and protection against sunrays. Therefore, the demand for these pigments increased to 41% in European industries in the year 2020. One of the largest markets in Europe is in France, worth EUR 720 million (according to the Centre for the Promotion of Imports from developing countries (CBI) and the Ministry of Foreign Affairs). In the year 2017, the cosmetics industry of Italy had the turnover of USD 12.3 million (according to the International Trade Administration (ITA)). It is anticipated that this is due to increase in market value, and demand for nano-cosmetic pigments will also rise in the coming years. The different global cosmetics/cosmeceuticals market statistics are shown in shown in the Figure 3.
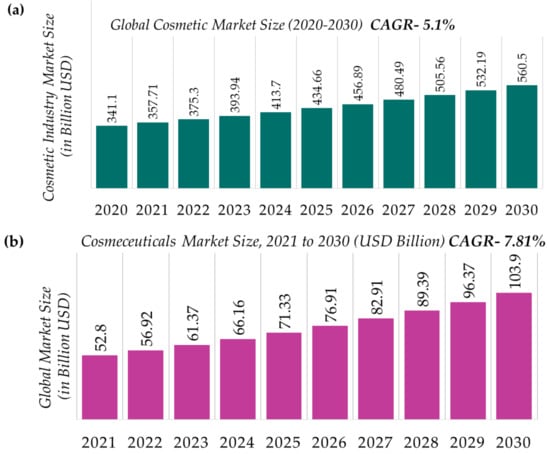
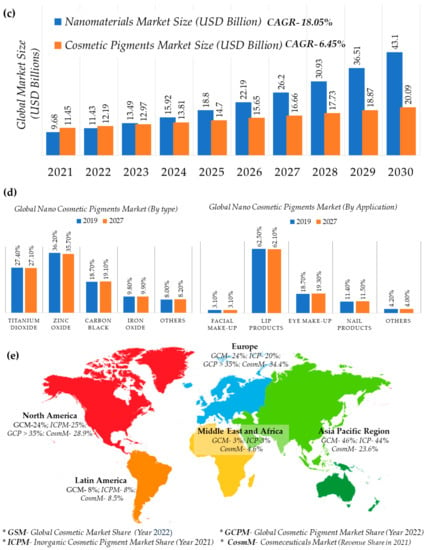
Figure 3.
(a) Global cosmetic market (2020–2030) (b) cosmeceutical market size (c) cosmeceuticals, and nano-cosmetic pigments market by revenue, (d) type andapplication, (e) geographic region [26,27,28,29,30,31,32]. * Others: hair color products; special effect and special purpose products; and miscellaneous products.
2. Synthesis of Nanostructured Materials
Nanostructured materials can be created using several methods, viz., top-down and bottom-up methods, in addition to conventional chemical synthesis, physical, and biological techniques.
2.1. Phytogenic and Microbial Biosynthesis of Metallic Nanoparticles
Nanotization has been reported as one of the microbial defense mechanisms against metallic-ion-induced stress. This process exploits various biological reducing agents in an eco-friendly condition [33]. Some specific biomolecules present in the microbial biomass can prevent agglomeration of the particles and inhibit steric effects by covering the metallic nanostructured particles (meta-NPs) and are called capping agents. These biomolecules can alter biological activity and surface chemistry of meta-NPs as well as prepare a stable medium for biosynthesis [34]. Meta-NPs can exert an influence on microbial cells by protein channels, efflux pump, or feasible penetration through the cell membrane [35].
Bacteria, algae, fungi, yeasts, and plants have been used as biofactories of meta-NPs that encourage the development of green chemistry. Also, vigorous viruses have the potential to engage in transferring meta-NPs. Therefore, these are identified to be potent carriers for meta-NPs, and are well-established for encapsulation [36]. Some biological reducing agents, such as enzymes, electron shuttle quinones, peptides, proteins, and polysaccharides, have been known to play an essential role in transforming metallic salt/ions to meta-NPs including silver, gold, platinum, palladium, copper, cadmium, titanium oxide, zinc oxide, and cadmium nanoparticles [37].
Bacterial biosynthesis of meta-NPs occurs in the presence of endogenous enzymes, proteins, and pigments. This technique requires a low consumption of energy and therefore creates an eco-friendly method. Fungi secrete considerable amounts of reducing agents, such as enzymes and proteins, which can provide a befitting media for the extracellular generation of meta-NPs [38]. The fungal cell wall is composed of functional molecules suitable for the intracellular biomineralization of Ag-NPs. Thus, the application of fungi in the biosynthesis of silver nanostructured particles has been elaborately explored [39]. Cytochrome b5 reductase enzyme, which is extracted from the fungus Mucor racemosus, is known to be a potent generator of meta-NPs. It can produce well-dispersed and stable meta-NPs within the size range of 70–180 nm [40]. The development of fungi-oriented systems for microbial biosynthesis of meta-NPs has emerged as an advancing, key branch of myco-nanotechnology. Also, fungi have appeared to be an efficient candidate for the generation of stable, polydispersed, and appropriately sized meta-NPs [41]. In addition, a variety of meta-NPs such as Ag-NP, Au-NP, Se-NP, CdS-NP, Fe-NP, Pa-NP, and ZnS-NP have been synthesized using edible mushrooms [42]). The generation of meta-NPs using yeast extract is an efficient process, due to amino acid components which tend to be used as a capping agent for covering meta-NPs. Amino acids can induce a negative net charge on the molecular surface of meta-NPs and maximize electrostatic repulsion in alkaline solutions [43].
Certain classes of algae, such as cynophyceae, chlorophyceae, phaeophycea, and rhodophyceae, have been reported to couple with the synthesis of meta-NPs both intra- and extracellularly. This is due to the presence of bioactive compounds, such as algal phytopigments and antioxidants, which exhibit reductase activity with the highest biocompatibility [44].
Moreover, the use of plants or plant extracts in biosynthesis of NPs is known as a phytogenic biosynthesis of meta-NPs, which can be categorized as an environmentally friendly, bottom-up strategy. Figure 4 illustrates the different sources of phytogenic biosynthesis as well as microbial biosynthesis of meta-NPs.

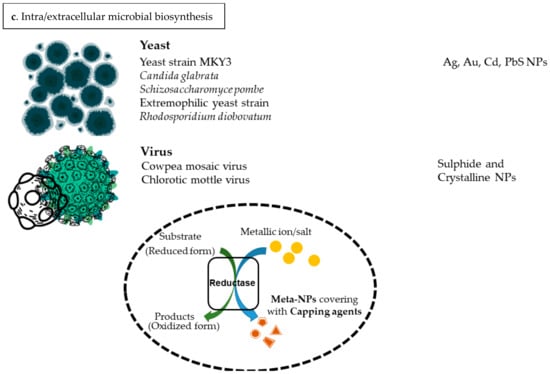
Figure 4.
Biosynthesis of meta-NPs. (a) Phytogenic biosynthesis of meta-NPs via exploiting plants and algae. (b) Microbial biosynthesis of meta-NPs via employing bacteria, fungi, yeasts, and viruses. (c) Intra/extracellular microbial biosynthesis of meta-NPs. This nanotization process is conducted by employing reductase, capping agents, and metallic ions. (The figures were created using the website Canva.com (accessed on 1 May 2023)).
2.2. Chemical and Physical Methods of Synthesis
The two main approaches to synthesizing nanomaterials, viz., top-down and bottom-up techniques, are illustrated in Figure 5.
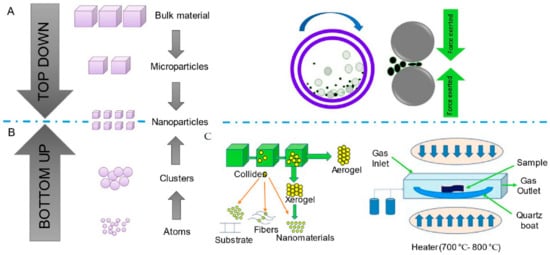
Figure 5.
Synthesis of nanostructured particles: (A) top-down and bottom-up approaches; (B) ball-milling method of a top-down approach; and (C) sol-gel and hydrothermal methods of a bottom-up approach.
Chemical synthesis is a bottom-up method to synthesize nanostructured materials from molecules, compounds, or complexes. On the other hand, cutting, grinding, and engraving methods, employed in the top-down strategy, necessitate the size reduction of the particles down to nanosized dimensions [45]. Chemical reduction, micro-emulsion formation, electrochemical synthesis, sol-gel synthesis, microwave assistance synthesis, and hydrothermal synthesis are a few examples of chemical processes employed to create nanomaterials. The physical processes include mechanical milling, vacuum vapor deposition, and laser ablation methods [46]. Physically manufactured nanostructured materials often possess a lower quality than those created by chemical methods. In addition, the finished product of the physical procedures requires expensive equipment, e.g., a vacuum system. On the other hand, the procedures involved in the bottom-up approach are easier to control and are more efficient compared to the top-down approach; therefore, these are frequently employed in industries [47]. The different methods of synthesis of NPs have been summarized in Table 1.

Table 1.
Methods and mechanism of NPs synthesis.
3. Organic Nanostructured Materials in Cosmetics Industry
Organic NPs are variable in size, biodegradable, nontoxic, and sensitive to thermal and electrical radiation. These are used in different pharmaceutical applications, such as skin care and transdermal drug delivery [82]. Nanotubes, carbon nanofibers, carbon nano-fullerenes, graphene, and nanosized soot and activated carbon are examples of carbon-only NPs [83]. Nano-emulsions, nanostructured crystals, vesicular delivery methods for nano-encapsulation, micelles, polymeric nanostructured capsules, solid lipid nanostructured particles (SLN), nanostructured lipid carriers (NLCs), liposomes, niosomes, and dendrimers are some examples of organic NPs [84]. Various organic NPs used in cosmetics industries are shown in Figure 6.
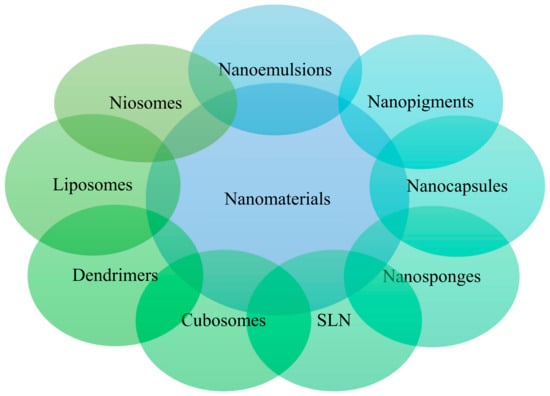
Figure 6.
Organic nanostructured materials used in cosmetics industries.
3.1. Liposomes
The term ‘liposome’ is derived from the terms ‘lipo’ and ‘soma’, which refer to fat bodies. The structure of a liposome includes hydrophobic phospholipid bilayers containing an aqueous medium within spherical vesicles [85].
Liposomes form from cholesterol and natural phospholipids in an aqueous environment with proper lipid-to-water ratio [86]. The lipid bilayer is made up of phospholipids and considered as a safe or low-risk component for skin [87]. Liposomes can improve skin smoothness, minimize acne rashes, and diminish wrinkles in clinical tests. These have been also designed to distribute vitamins, phytochemicals, and perfumes in waterless products like lipsticks, deodorants, body sprays, and antiperspirants [88]. Phospholipids are nontoxic and may be unsaturated or saturated. For example, unsaturated phosphatidylcholine originates from a natural source that is less stable but more permeable. Long acyl chains produce a hard, impermeable bilayer structure in the saturated ones, such as dipalmitoyl phosphatidylcholine [89].
3.2. Nano-Emulsions
Nano-emulsions, closely related to micro-emulsions, are liquid droplets that are spread in another liquid on the nanoscale. These are flexible, and their morphologies may be altered by manipulating the parameters during the production process [90]. Nano-emulsions can be made from a variety of components that are typically harmless. They possess better stability and are efficient in delivering active chemicals in cosmetics due to their size [91]. Molecules are encapsulated or stabilized as nano-emulsions for the manufacturing of skin creams to increase skin penetration [92] and are widely utilized in cosmeceuticals, e.g., lotions, shampoos, nail polishes, and hair conditioners, as a medium for the regulated release of biologically active chemicals [93]. Nano-emulsions more effectively deliver lipophilic chemicals compared to liposomes. Nanopigments can be also incorporated, in addition to nano-emulsions, to obtain a product with enhanced properties [94].
3.3. Niosomes
These are nanosized vesicles generated in an aqueous media using non-ionic surfactants and cholesterol [95]. In the 1970s, a popular cosmetic company developed the first niosomes from synthetic liposomes and patented their product in the year 1987 [96]. Cholesterol and non-ionic surfactants, such as alkyl amines, alkyl ethers, spans, polyoxyethylene, sorbitan esters, tweens, and bridges; steroid-bound surfactants; and crown esters are all important elements in the synthesis of niosomes [97]. Niosomes can be utilized to carry drug/medications that are not easily absorbed or entrapped. Niosomes have also been used as a carrier of iobitridol, which is a diagnostic agent used for X-ray imaging [98]. Several noisome-based cosmeceutical products are available in the market, including conditioner, shampoo, moisturizer, skin-lightening creams, and antiwrinkle creams [99].
3.4. Nanocapsules
Nanocapsules, either solid- or liquid-core, contain cosmetically active components in a cavity surrounded by a synthetic or natural polymer membrane. These are utilized in cosmetics to safeguard essential active ingredients [100]. The various components are encapsulated by polymeric nanocapsule solutions and can be directly applied to the skin. The properties of a nanocapsule can be altered by employing various polymers and detergents [101]. These nanocapsules are employed in a variety of deodorants due to their biocompatibility [102]. Polymeric nanocapsules are used to deliver retinol to the deeper layers of the skin in some cosmetics [103]. Nanocapsules also shows to restrict the penetration of UV-filtered octyl methoxycinnamate in faux leather compared to non-nano-size capsules [104].
3.5. Solid Lipid Nanostructured Particles (SLN)
Solid lipid NPs are solid nanostructured components at normal room temperature. The structure contains stabilizers and lipid droplets which crystallize to form SLN and are known to protect the skin’s surface [105]. Active compounds in SLN-based formulations enter the deeper layers of the skin more easily [106]. SLN can also increase skin moisture compared to saline. Other benefits include improved hydration, bioavailability, stability, skin coverage, etc. [107]. These are also employed in the production of fragrances and perfumes to ensure the perfume’s long-lasting aroma. Due to UV-resistant characteristic, they are utilized in sunscreen creams to guard against deterioration of the skin [108].
3.6. Cubosomes
Cubosomes are self-assembled fluid–crystalline structures which include specified amphiphilic lipids in their arrangement and are organized into three-dimensional ordered bilayers resembling a honeycomb structure [109]. When water is combined with a surfactant system and aqueous lipids, the microstructure of cubosomes is generated in a defined ratio [110]. The cubic phases have a unique geometry, capable of releasing hydrolyzed active substances, such as medicines and proteins, as well as cosmetic active compounds, in a regulated manner, and they are physiologically friendly [111]. Due to their geometry, their surfaces are quite large, and due to low density, these can be readily prepared from very dilute solutions. Due to the high moisture ratio, cubosomes exhibit special features. They have benefits from both polar and nonpolar groups and possess good thermal stability [112].
3.7. Dendrimers
The word ‘dendrimer’ is formed from two Greek words, viz., ‘dendron’, which means tree, and ‘meros’, which means portion [113]. Dendrimers are monodispersed, monomolecular, hyper branched, treelike structures having a diameter of roughly 20 nm [114]. Dendrimers can have monomolecular, monodispersion, or micellar structures. Different functional groups attached are responsible for their functionality [115]. These functional groups are associated with different applications like drug transport, catalysis, photo-activity, modulation of molecular weight, size measurement, and rheology [116]. Multiple permutations of these components can be employed to create a variety of forms and sizes of product with protected inner cores which are excellent for biological and materials science applications [117]. An individual nanostructured material has its specific function and application(s) in different cosmetics.
Basic structures of organic nanostructured particles used in cosmetics industries are displayed in Figure 7. Some of the nanostructured materials, their properties, and specific applications are given below in Table 2.
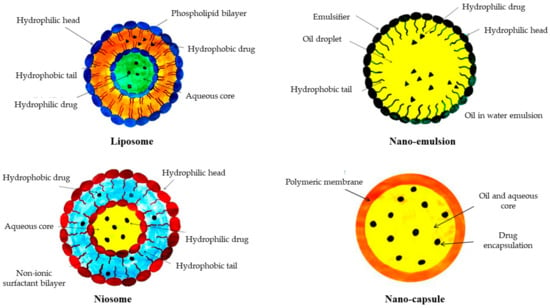
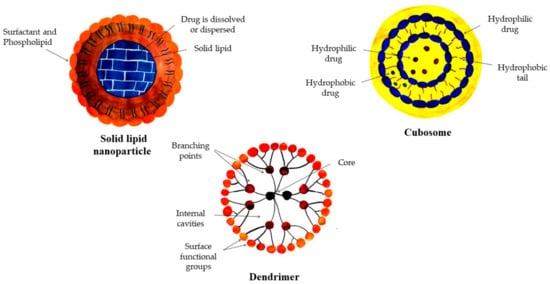
Figure 7.
Basic structures of different organic nanostructured materials used in cosmetics industries.

Table 2.
Organic nanostructured materials in various cosmetics, structure properties, and their applications in different cosmetic products.
4. Inorganic Nanostructured Materials in Cosmetics Industries
Inorganic NPs are made up of metal/metal oxides. The different types of inorganic NPs employed in cosmetics industries are shown in Figure 8. Some of the inorganic nanostructured materials and their characteristic properties are discussed here.
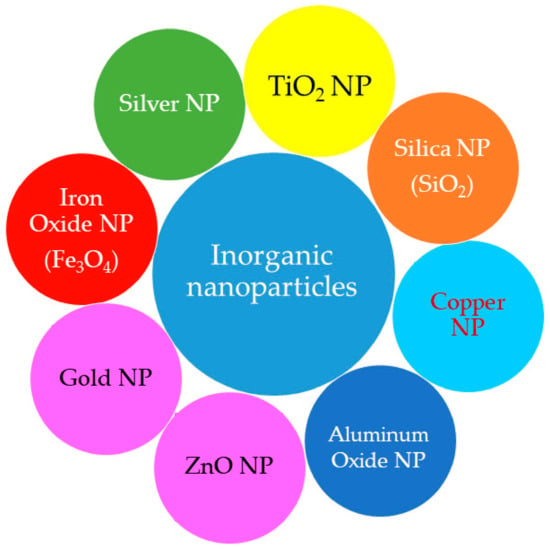
Figure 8.
Types of inorganic nanostructured particles used in cosmetics.
4.1. Nanostructured Particles of Silver and Gold
Silver and gold NPs are well-known to possess antimicrobial properties. Deodorants, face creams, anti-aging creams, and moisturizers include Ag and Au NPs. Some cosmetics industries report that Ag NPs employed in cosmetics give day-long protection from microorganisms. Ag NPs are employed at an amount of 12% in the composition in most of the nano-cosmetics formulations across the world [122]. Similarly, Au NPs possess unique capabilities of drug/medication delivery and discharge, ease of synthesis and functionalization, and are one of the most popular ingredients employed in cosmetics. The functionalization of Au NPs are usually carried out via thiol linkages, which enable easier conjugation with cosmetic components, resulting in enhanced product quality. Au NPs have been reported to be included in toothpaste formulations to provide efficient mouth cleansing [123]. Ag NPs’ antimicrobial activity has been attributed to the release of silver ions, and analogous research on nanostructured silver particle based products have been undertaken extensively to harness their unique antibacterial and antifungal capabilities. Similar studies have also been conducted on Au nanostructured particles as well. A TEM image of skin toner containing silver nanostructured particles is shown in Figure 9 [124].
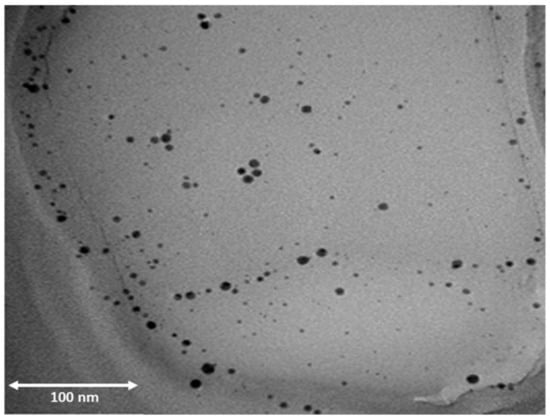
Figure 9.
TEM image of Ag nanoparticles from TEM in a skin toner [124].
4.2. Silica Nanostructured Particles
Several cosmetic sectors have paid close attention to the addition of silica NPs to cosmetic products. Silica NPs have attracted huge attention due to their unique qualities, such as their pleasant touch and ability to encapsulate and entrap lipophilic and hydrophilic molecules to their site of action [125]. Furthermore, silane chemistry, adaptability for surface functionalization, low-cost manufacturing, and ease of large-scale synthesis are additional important characteristics. Silica NPs are commonly employed in toothpaste, cosmetics, hair products, deodorants, and skin care. These provide emulsifying, emollient, and water-barrier activities, with the unique potential of improving protection against sunburn. Silica NPs make sunscreen formulations easier to distribute and reduce phototoxicity or deterioration [126]. Silica NPs with diameters ranging in size size of 5 and 100 nm are stabilized as nanodispersions and characterized by different microscopic techniques, viz., SEM (Figure 10a–c) and TEM (Figure 10d).

Figure 10.
SEM-based characterization of synthetic silica particles (a–c) [101]; (d) TEM-based characterization of synthetic silica particles [126].
4.3. Titanium Oxide and Zinc Oxide Nanostructured Particles
UV filters made from titanium oxide (TiO2) and zinc oxide (ZnO) NPs have been effectively integrated into a variety of cosmetic items. ZnO NPs are likely to reflect UVA radiation, whereas TiO2 NPs are accountable for reflecting UVB rays. When these two oxides are employed together, it enhances effectiveness in terms of protection against sunburn and other desirable properties, viz., transparency, spreadability, and superior texture, without causing skin inflammation [127]. Since TiO2 and ZnO nanostructured particles accumulate on the outer surface of stratum corneum, the UV protection offered by these systems is frequently utilized. Due to its antibacterial characteristics, ZnO has also proven to be a desirable solution in the pharmaceutical and cosmetic sectors. The antibacterial effect of these nanostructured particles is attributed to the generation of ROS and subsequent release of Zn2+ ions, which are poisonous to bacteria and cause instability of bacterial cell walls [128]. However, the chemicals employed in topical formulations have a significant impact on the efficiency of ZnO NPs since they can mask the surface of the particle and reduce the ZnO antibacterial activity [129]. ZnO and TiO2 NPs observed under SEM and TEM are shown in Figure 11 and Figure 12.
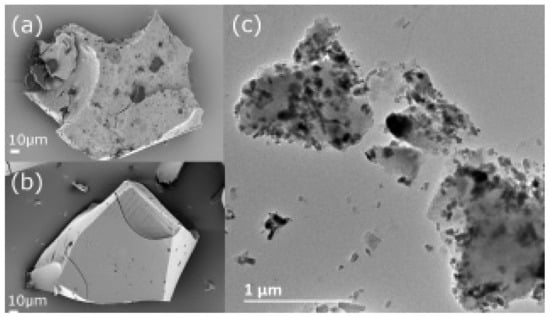
Figure 11.
SEM images of (a) MMC-TiO2-ZnO and (b) MMC; and a TEM image of (c) MMC-TiO2-ZnO [127].
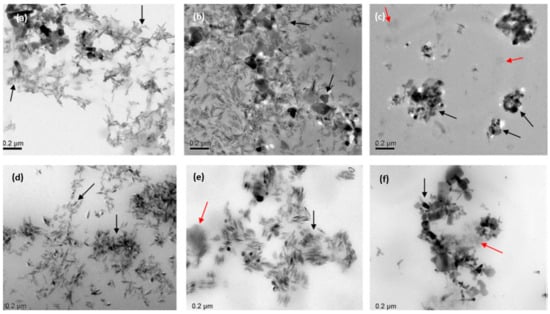
Figure 12.
Analysis of marketed sunscreens with inorganic nanoparticles (TiO2 and ZnO) using transmission electron microscopy (TEM). (a–f). Images taken at a magnification of 10,000–20,000 with an incident light of 200 kV [129] (Black and red arrows indicated metal oxide NPs and the formulation matrix, respectively).
Each inorganic nanostructured material has unique properties and function in various kinds of cosmetics. Some of the inorganic nanomaterials with their respective functions and uses in cosmetics are listed below in Table 3.

Table 3.
Functions of inorganic nanostructured materials in various cosmetics.
5. Major Cosmetic Products Containing NPs
Cosmetics are not limited to a particular kind; they have various subbranches, and these branches consist of different types of products specifically formulated and manufactured for specific skin/body parts. Similarly, nano-cosmeceuticals are used in various personal-care products; their subcategories are shown in Figure 13.
Figure 13.
Categories of nano-cosmeceuticals.
5.1. Skin Care
Skin-care products enhance the function and quality of the skin by promoting collagen formation and combating damage caused by free radicals. These improve the texture of the skin by maintaining the moisture level and protecting skin’s outer layer from various dust particles present in the environment. The most popular cosmetic products that contain nanostructured particles are moisturizers. The water in skin-care products evaporates quickly, creating cracks and dryness. This problem can be solved using nanostructured particles, which generate a thin moisture-retention layer to prevent water from evaporating too quickly [132]. As a result, the moisture content in the skin is preserved, resulting in a more youthful appearance. The frequently utilized nanostructured particles in moisturizing formulations are liposomes, nano-emulsions, and solid lipid nanostructured particles. These can also be used to treat skin diseases like dermatitis, psoriasis, and itching [133]. The use of sunscreen to protect our skin from the sun’s damaging UV radiation is widespread. Besides nanostructured particles, microstructured particles of metal oxides, such as insoluble blooms of ZnO and TiO2, are also used in several sunscreen actives. Sunscreen formulations containing TiO2 nanostructured particles are less oily and are clear, odorless, and visually attractive, and the nanostructured particles do not leave a chalky white deposit on the skin like other larger particles do [134].
5.2. Lip Care
Lipsticks and lip glosses designed to nourish and beautify consumers are now infused with NPs to impart bioactivity which softens and soothes lips by limiting water loss from the surface and keeping them hydrated. Nanocapsules of vitamin E reduce the bleeding caused by wrinkles and cracking. Lip volumizers with liposomes enhance volume effects of lips, fill in wrinkles, shape lips, and hydrate them. Silica NPs in the formulation ensure even distribution of colors throughout the composition. Pigment transfer into the fine creases of the lips is avoided, resulting in a much-improved elegant quality [135].
5.3. Nail Care
Nano-cosmetic nail-care products have a significant advantage over traditional nail-care treatments. Nanotechnology-based nail polishes include characteristics such as longevity, rapid drying, enhanced durability, scratch resistance, and ease of application due to their elastic nature [135]. NPs can protect the nails from infections that enter through skin tissues. Along with this, these also promote the keratin production for healthy nails.
5.4. Hair Care
Conditioners, shampoos, hair-growth accelerators, styling treatments, and coloring agents are all examples of nano-cosmetic hair products. Unique NP sizes and intrinsic features enable targeting of the shaft or hair follicles, and release an increased number of active substances [136]. NPs in shampoos lock in moisture in the cuticles and extend the time of contact with hair follicles and scalp by forming protective coatings. Unlike typical hair products, nano-emulsions used in hair cosmetics do not break the hair cuticle. NPs used in conditioners provide shine, softness, stickiness, and an increase in hair detangling [137]. A summary of NPs used in different cosmetics and their effects is provided in Figure 14. Functions of nanostructured materials in various marketed cosmetics are given in Table 4.

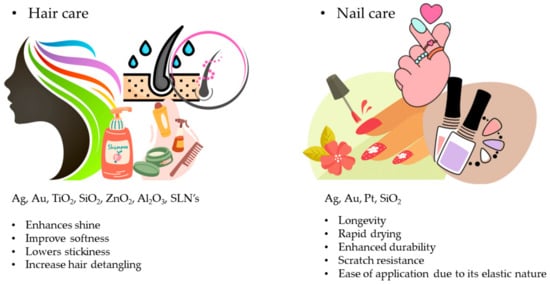
Figure 14.
Summary of NPs used in different cosmetics and their effects.

Table 4.
Functions of nanostructured materials in various marketed cosmetics.
6. Toxicity Factors in Cosmetic Products
Due to the advanced properties and extensive use of nanomaterials in cosmetic systems, safety concerns are important to address. Therefore, there is a necessity to evaluate and identify certain parameters to ensure safe cosmetic formulation. If nanostructured particles invade the hepatic circulation, they can cause hepatotoxicity [149,150]. Nanostructured particles are easily absorbed in the gastrointestinal system (upon accidental ingestion), and might enter the bloodstream through the vessel wall and spread to other bodily organs. Oral intake of copper nanostructured particles has been proven to cause serious damage to the liver, spleen, and kidneys of rats [151]. Metal-based NPs, such as TiO2 NPs, have been shown to cause the production of cytokines, which can lead to inflammatory processes and potential demise. TiO2 has been classified as hazardous for the respiratory system. Several investigations have been carried out to figure out the extent of damage caused by nanostructured particles of various metals to their micro- and large-scale equivalents. The observations suggest that the nanostructured materials cause more harm to organs [152]. The different ways of exposure of NPs to the interior body organs and soft tissues are summarized in Figure 15.
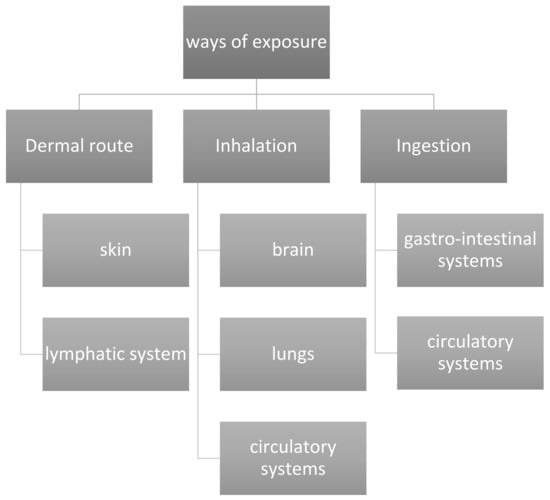
Figure 15.
Ways of exposure and organs affected by nanoparticles.
When preliminary tests on gold nanostructured particles were carried out, it was discovered that they are safe and possess low toxicity. Nanostructured particles with a diameter of less than 10 nm can easily permeate the epidermal layer and nuclear membrane, posing a risk of genotoxicity. Therefore, toxicity-triggering characteristics attributable to the shape, structure, elemental composition, and concentration of the nanostructured particles should be considered when manufacturing nanostructured particles for cosmetic goods [153]. Manufactured nanomaterials are used in a wide range of products, including electronics, cosmetics, paints, and even drugs, but little is known about how they affect the environment. In a recent laboratory investigation, researchers discovered that saltwater mussels and oysters accumulate and retain considerable amounts of produced nanoparticles from seawater in the form of so-called marine snow clumps [154]. Guidelines on the safety of nanostructured materials in cosmetics have been published by the Scientific Committee on Consumer Safety (SCCS) under the title “Guidance on the Safety Assessment of Nanomaterials in Cosmetics” [53,155] and followed by industries in the European Union (EU) [10,84,89]. Concerns regarding the NMs’ inherent negative impacts on the environment and human health have grown as their use is in both consumer and industrial items is growing at an exponential rate. Nanomaterials are mostly sunk in aquatic environments, where they enter the marine ecosystem through a wide range of direct and indirect routes. For the removal of contaminants from aquatic environments, a variety of nanomaterials have been utilized, including nanoscale zero-valent copper, nanocomposites made of Fe2O3 and biochar, and nanocomposites of magnesium oxide. These membranes made of nanomaterials can be created with characteristics like antifouling, antibacterial, photodegradation, and good permeability, creating new possibilities for highly selective, quick water-purification systems. As a result, toxicological issues may arise from the presence of nanoparticles in many environmental contexts. A new area of toxicology called nanotoxicology examines the hazards and risks associated with the toxicological characteristics of nanoparticles and their impacts on the environment and living things. Nanomaterials’ properties differ from those of their bulk counterparts in that they depend on their size, shape, and structure. The toxicity of nanomaterials in different organisms is influenced by these material characteristics, especially concentration. These characteristics affect how quickly nanomaterials are absorbed by cells. Humans and mammals can be exposed by oral, cutaneous, inhalation, paints and coatings, food, dietary supplements, and medical applications that use NMs [156]. Biosafety research on nanostructured materials is a challenge for 21st-century frontier science and technology, as well as raising serious scientific concerns for the scientific community in several nations [157]. Nanotechnology’s rapid development involves the implementation of additional safety evaluation approaches and procedures. Biodegradability, biocompatibility, and biodistribution of nanostructured materials hence become a prime concern regarding their safety and effectiveness in clinical and domestic usages. Nanostructured materials with poor biocompatibility, biodistribution, and biodegradability can harm the body by causing oxidative stress, DNA damage, and inflammation. However, as technology progresses, it is extremely likely that these problems will be rectified in the future [158]. Human health can be severely affected by the toxicity of nanostructured particles. NPs have been observed to cause higher toxicity per unit mass of blood passing through the blood vessels. The toxicity spreads because of their size, structure, and surface properties. The NPs tend to accumulate, agglomerate, and adhere to the skin surface of the workers involved in the manufacturing process. The adverse effects that may arise in skin and body organs via chemical and biological activities are listed in Table 5.

Table 5.
Safe concentration of some metallic nanoparticles and their target organs.
7. Health and Environmental Aspects
The two main categories of nanostructured materials used in cosmetics are soluble/biodegradable particles and insoluble/non-biodegradable particles. The organic nanostructured particles function as nanocarriers to improve product stability and active ingredient absorption while reducing harmful effects of irritants. Applications in beauty and personal-care products must anticipate their fundamental interactions with human tissues and resulting physico-chemical effects [160]. Compared to reactive carrier-mediated transport, passive diffusion is a popular transport technique for delivering active substances through the skin. The skin pores and hair follicle openings are typically the only places where nanostructured particles can enter the body. Nanostructured materials in cosmetic goods are exposed to humans through the skin [161]. Figure 16 illustrates the structure of human skin and penetrating nanostructured particles at the bases of the hair follicles.
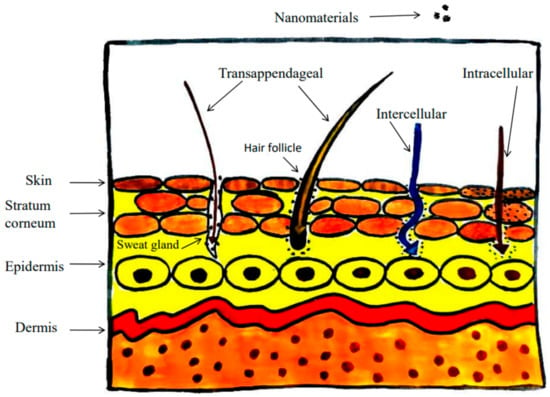
Figure 16.
Penetration of nanostructured particles in skin.
The epidermis, the top layer of skin; the dermis, which houses sweat glands, hair follicles, and tough connective tissues; and the subcutaneous layer are the three layers of the skin. Due to its hydrophilic nature and high cell cohesion, the stratum corneum, the epidermis’s topmost layer, serves as a barrier [162]. Nanostructured particles of a size less than 10 nm can diffuse through the stratum corneum and enter the dermis via intercellular or intracellular pathways; however, particles larger than 20 nm can only be ingested by transappendageal pathways like the hair follicle or sweat glands. Smaller nanostructured particles (1–100 nm) easily interact with cellular components and pass through cell membranes [163].
It is possible to perceive the special qualities of nanostructured particles employed in cosmetics as a two-edged sword. On the one hand, they provide alluring advantages with the added benefits of aesthetics, and on the other, they have been observed to cause unfavorable effects on the body’s systems [164]. This raises safety issues and health risks, especially given that when particles are shrunk down to the nanoscale, their known characteristics may quickly change. Additionally, it is yet unclear how hugely harmful the nanostructured particles are and what the extent of the damage is [165]. Nanostructured particles are being produced and utilized in various applications frequently in our everyday lives. Nanostructured particles made of metals and metal oxides have been employed to clean up air and water pollution. Since these are being used extensively, this increases the probability of their occurrence in waste discharges and causes environmental problems. This has led to their entry into water systems through the dumping of toxic wastewater, municipal sewage treatment plants, surface runoff to agricultural lands, underground water tables, and oceans [166]. Production, transportation, usage, and disposal all result in their discharge to the environment. Silver nanostructured particles physically interface with bacteria, triggering the creation of pits that enhance membrane permeability and eventually lead to cell death by allowing critical components to seep out. Nanostructured particles with such bactericidal characteristics will significantly impede wastewater treatment, especially when microbial communities are used to remove organic pollutants [167].
Consumers are likely to be at risk from the distinctive negative effects of any specific nanostructured material arising from the usage of cosmetic products [168]. Considering this, a general assessment of the safety of all nanostructured materials is required, including tests addressing the characteristic properties of nanostructures. The mechanism of exposure of nanostructured materials to the human skin and interior vital organs of the body is one of the most crucial factors to consider. The stratum corneum, the very first layer of the epidermis, is exposed directly. Exposure of tissues in the deeper layers of the skin to nanostructured materials via penetration through the skin barrier to influence functional activities has been under active research in recent years [169]. When nanostructured particles are absorbed into the skin, damage to the skin, lungs, brain, and other organs (through the blood) may occur [170]. Inorganic nanostructured materials like SiO2, TiO2, and ZnO produce significant toxicity to microorganisms by generating reactive oxygen species and cause oxidative stress effects. Certain nanostructured particles can have dangerous impacts on the brain. ZnO and TiO2 nanostructured particles are extensively utilized as UV blockers in cosmetic products. These are also recognized for producing ROS following photo-excitation. It has been reported that the existence of ROS in water bodies is detrimental to phytoplankton, seriously harming the aquatic ecology. An experiment for 21 days involving extended exposure of zebra fish to TiO2 nanostructured particles was observed to impair their reproduction significantly by lowering the fraction of viable embryos. Additionally, the genetic complement of an individual, which offers the biochemical toolbox through which it may adapt or combat harmful compounds, determines the toxic effects of almost any nanostructured particle on an organism. Inhaled nanoparticles have been linked to lung cancer, emphysema, asthma, bronchitis, and neurological illnesses. Also, colon cancer and Crohn’s disease can be caused by the presence of nanostructured particles in the digestive tract [171]. Furthermore, the growth of arteriosclerosis, blood clots, arrhythmia, heart disorders, and eventually cardiac mortality have been reported to be caused by the presence of nanostructured particles in the circulatory system. The development of autoimmune illnesses such as chronic erythematosus, scleroderma, and rheumatoid arthritis as well as diseases of multiple organs, such as the liver, spleen, and so on, are related to exposure to certain nanostructured particles [172]. For nanostructured materials to be safe for living beings, the organisms must be capable of modifying, accumulating, and degrading them, thus eliminating from circulating further. The current understanding on nanostructured particle safety is limited. Therefore, research that focuses on synthesis techniques, properties, safety evaluations in both in vitro and in vivo methods, and dose calculations is required [173]. Figure 17 provides a schematic of the human body showing the impacted organs, diseased areas, and pathways of nanostructured particle exposure. Researchers stress that not all nanostructured particles have harmful effects on human health; rather, the toxicity arises from several characteristics, such as size, accumulation, composition, crystalline nature, surface functionalization, etc. [174].
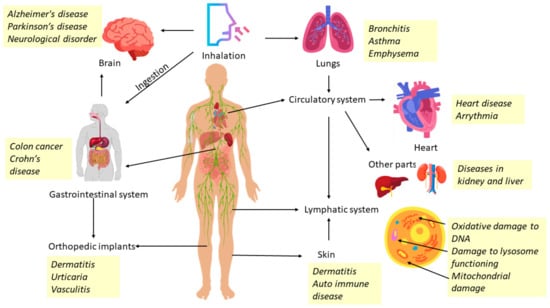
Figure 17.
Schematic of the human body showing the impacted organs, diseased areas, and pathways of nanostructured particle exposure from epidemiological, in vivo, and in vitro research.
7.1. Toxicity of Inorganic Nanoparticles on Skin
Nanoparticles (NPs) and nanostructured materials in pharma/cosmeceutical industry has emerged. They have been designed to create sustainable and target-oriented delivery of drugs and active ingredients in a controlled manner [175]. The capability of NPs and nanostructured materials to distribute into human organs and cross every cell membrane can cause cell injury and nanotoxicity [173]. Here, the biological routes of NPs and nanostructured material in cosmetics have on the skin is elaborated in Figure 18 [176].
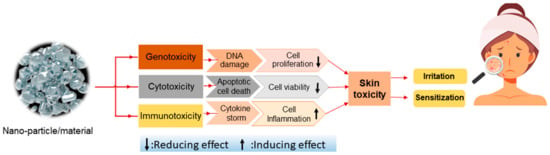
Figure 18.
Biological routes of nanoparticle and nanomaterial toxicity on skin.
7.1.1. Copper Oxide Nanoparticles (CuO-NP)
In-vitro treatment of copper oxide nanoparticles on intact epidermis has shown a significant decrease in cell vitality. Furthermore, it can stimulate an immunogenic response through the induction of pro-inflammatory cytokines, such as IL-1α, IL-6, and TNF-α. The histological study revealed the presence of atypical organelles, including degraded mitochondria, disappeared chromatin texture, and increased lysosomal vacuoles, which are indicative of oxidative stress caused by the topical application of copper oxide nanoparticles [177].
7.1.2. Iron Oxide Nanoparticles (FeO-NP)
Iron oxide nanoparticles in the form of a colloidal suspension were applied to human keratinocytes (HaCat cell line) at concentrations of 5, 10, and 25 μg/mL. The results showed no significant reduction in cell proliferation, indicating their nontoxic nature. In addition, an in vivo study of iron oxide nanoparticles revealed minor changes in physiological skin parameters but no significant decrease in skin barrier function. Therefore, iron oxide nanoparticles can be suggested as a safe but not highly efficient agent for transdermal delivery in topical applications [178].
7.1.3. Zinc Oxide Nanoparticles (ZnO-NP)
Zinc oxide nanoparticles were applied to epidermal samples excised from human skin, and no significant transmission into cells was observed. Therefore, the nanoparticles accumulated on the surface of the epidermis (stratum corneum), leaving the cytoplasm unaffected by toxic action. This may be the most crucial factor that makes it a commonly used nanoparticle in broad-spectrum sunscreen formulations. In addition, the toxicity of agglomerated zinc oxide nanoparticles was evaluated in volunteers, and no local toxicity was observed on their skin [179]. Recent in vitro experiments were conducted on human-derived keratinocytes (HaCat) and reconstructed human epidermis (RHE), which revealed a dose-dependent decrease in cell proliferation and an increase in damaged DNA [180]. Photo-corrosion can result in the degradation of chemical ingredients and cause poor stability. This can lead to of genotoxicity, which is manifested by DNA fragmentation [131,147].
7.1.4. Silver Nanoparticles (Ag-NP)
The topical application of silver nanoparticles in gel-based formulations has shown a safe toxic profile and can serve as an alternative agent for use in topical antibacterial formulas. In vivo studies have demonstrated that silver nanoparticles can induce oxidative-stress-induced cell death. However, they can also stimulate the cell’s antioxidant defense system, including glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT), which allows cells to overcome oxidative damage and apoptotic cell death [181]. Given the ever-growing cosmetics industry, silver nanoparticles have been incorporated into nail polish to exert antifungal activity. Additionally, they can be used as nanopigments in colored cosmetics such as lipstick and eye shadow. However, the mechanism of action and toxic effects of silver nanoparticles as nanopigments in cosmetics have not been explored widely [182]. Only mild irritation of eyes and nose has been shown [125].
7.1.5. Titanium Dioxide Nanoparticles (TiO2-NP)
Titanium dioxide is a widely used nanoparticle in various industries, with cosmeceuticals being one of the most well-established ones. Due to its UV-filtering capacity, it is commonly used as a sun protection factor (SPF) in sunscreen formulations. Furthermore, its use in day creams and lip balms. In vivo studies and clinical trials have been conducted using different concentrations of the nanoparticle, demonstrating its safety up to 25% in formulation. The only known route of its toxic effects on human biology is through inhalation [183,184]. It is known as carcinogen that can disturb the immune system and trigger oxidative-stress-induced organs’ irritation and cancer [84,149].
7.1.6. Silica Nanoparticles (Si-NP)
The accumulation of intracellular reactive oxygen species (ROS)-induced oxidative stress has been demonstrated as the mechanism of toxicity for silica nanoparticles on keratinocytes and skin fibroblasts, resulting in a reduction in cell viability. The cytotoxicity of silica nanoparticles appears to be size-dependent, with particles of 20 nm showing greater toxicity than larger particles of 100 nm. These findings are consistent with cutaneous irritation and skin sensitization as signs of skin toxification, as reported in previous studies [185]. In an in vivo study, the toxic effects of colloidal silica were investigated by topically applying it to rat skin for 90 days. The results showed no significant toxicity or visible alterations in internal organs, even at a dose of 2000 mg/kg [186]. Overall, these findings suggest that while silica nanoparticles may be toxic to skin cells in vitro, the systemic toxicity of colloidal silica appears to be low and only causes cellular inflammation in the body’s organs [133]. However, further studies are needed to fully understand the potential health risks associated with exposure to silica nanoparticles.
7.1.7. Gold Nanoparticles (Au-NP)
Gold nanoparticles are known for their anti-aging properties, as they scavenge free radicals and exhibit antioxidant activity. To evaluate the toxicity of gold nanoparticles, normal human dermal fibroblasts (NHDF) were incubated with neutral red, a dye used to assess cutaneous irritation and skin sensitization. Chronic light exposure was also applied to NHDF to evaluate morphological changes in dermal fibroblasts. Our results indicated that gold nanoparticles did not exhibit phototoxicity up to a concentration of 1000 μg/mL, and skin sensitization tests conducted in vitro showed a new gene expression pattern associated with the skin sensitization process. However, we observed that gold nanoparticles did not induce nuclear factor erythroid 2-related factor 2 (Nrf2), which initiates an antioxidative signaling pathway and helps maintain redox homeostasis [187]. This non-sensitive manner towards the cellular antioxidative signaling pathway causes skin rashes, jaundice, as well as certain systematic pathological conditions such as bone marrow depression, stomach and intestinal bleeding, vomiting, and headaches [124,156].
7.1.8. Aluminum Oxide Nanoparticles (Al2O3-NP)
Aluminum oxide nanoparticles have not been widely used in topical applications, but they are abundantly produced and applied in various industries. Instead of relying solely on dermatological toxicity tests such as skin irritation and sensitization, it may be beneficial to consider the toxic effects of these nanoparticles on different cell lines. Studies have shown that the toxicity of aluminum oxide nanoparticles is concentration-dependent and cell-specific. Specifically, reduced cell viability and blood–brain-barrier-induced neural toxicity can be attributed to the adverse effects of oxidative stress and inflammatory responses [188].
7.2. Toxicity of Organic Nanostructured Materials Carrier on the Skin
7.2.1. Nano-emulsion
The nano-emulsion is a nanostructured material commonly used in cosmeceuticals due to its feasible permeability into the skin layers. However, its toxicity can induce local skin irritation, which is closely related to the physicochemical properties of its constituents. To evaluate the toxicity of nano-emulsions, MTT and LDH assays have been used to determine the viability of skin cells. Surfactants, the main constituent of nano-emulsion, have been identified as crucial cytotoxic agents. Ionic and highly water-soluble surfactants induce the most skin irritation, while ionic crystalline surfactants with moderate levels of water solubility cause less skin irritation. Non-ionic surfactants’ toxicity is linked to the chemical linkage bond between the polar head and alkyl chain, and PEG-E esters have been found to cause less skin irritation than PEG-E ethers. This information can guide the selection of safe and effective surfactants for cosmeceutical formulations [189,190].
7.2.2. Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLN) have been reported to be Generally Recognized As Safe (GRAS). However, similar to nano-emulsions, the physicochemical properties of their constituents, particularly the surfactant, can have a significant impact on the toxicity-induced skin irritancy of SLN. To reduce skin irritation, the implementation of non-ionic and PEG-free surfactants has been shown to be effective in constructing SLN [191,192]. Furthermore, SLN have been used in topical applications and cosmetics to mitigate the toxic effects of bioactive compounds by reinforcing the lipid film of the stratum corneum and repairing damaged skin [193]. Considering the physicochemical properties of the constituents of SLN can help ensure the safe and effective use of this nanostructured material in cosmeceuticals.
7.2.3. Liposome
Poly 3-hydroxy butyrate (PHB) incorporation with phospholipids has resulted in the creation of a liposome structure that exhibits high permeability through the skin layers. This makes it an efficient nanostructured material for use in topical applications and cosmetics. However, its safety on human biology needs to be thoroughly investigated. To this end, an in vitro study was designed to evaluate its toxicity on two specific human keratinocyte cell lines, HaCat and HEK. The results of the MTT assay showed no significant reduction in cell viability, suggesting that liposomes have a safe toxic profile and can be used in skin-care products and cosmetics [194]. This information can guide the safe and effective use of PHB-incorporated liposomes in cosmeceutical formulations.
7.2.4. Nanocapsule
The nanocapsule tends to exhibit site-specific manner. For example, a combination of polylactide (PLA) and poly (ethylene glycol)—block-poly (lactide) for nanocapsulation is known as an efficient carrier for topical delivery of the anti-aging agent retinol to the target cells embedded with oxidative stress. The toxicity of the nanocapsule has been evaluated and showed no significant decrease in cell viability of human keratinocyte cell line (HaCat). So, it can safely be use in skin-care products and cosmetics without any dermal toxicity [195].
7.2.5. Nanosponge
The nanosponge has been designed to overcome poor solubility and stability of some volatile bioactive compounds. This formula has been carried out for improving physicochemical properties of Bachi oil loaded with cyclodextrin onto a nanosponge. Then, its toxic effect was evaluated by MTT assay on keratinocyte cells (HaCat cell line). The result shows that Bachi-oil-loaded nanosponges can increase cell viability as well as an inhibitory concentration of that compound (IC50 value). Due to this, it attenuates the toxic effect of Bachi oil on the keratinocyte cells [196].
7.2.6. Dendrimer
The dendrimer has been reported to be proper for use in topical applications. Dermal toxicity of different concentrations of cationic polyamidoamine (PAMAM) dendrimer has been evaluated by skin irritation assays. In this regard, histopathological, immunohistochemical, and visual examinations have been conducted. Also, a microscopic study has shown the dose-dependent manner of dendrimers and proves their safety up to a dose of 6 mg/mL. Higher doses of dendrimers show morphological changes in keratinocytes at the basal and spinous layer. To elaborate on it, keratinocytes have induced vacuolization as a consequence of dendrimers’ cytotoxic effect. Also, dendrimers can induce hyperplasia of connective tissue fibers that leads to leukocytes infiltration. Necrosis can also occur and causes visible granulocyte infiltration in the upper dermis and sockets [197].
7.2.7. Cubosome
Cubosome cytoxicity has been determined by fluorescence-activated cell sorting (FACS) in fibroblast cells. The result proves that its toxic effect is attributed to the lipid component rather than internal bioactive compounds. In this regard, it has been observed that cubosomes composed with a lipid concentration in the range of 1–100 mg/mL can exhibit a cytotoxic effect. Also, Alamar Blue for assaying cell viability proves that this type of lipid component has an essential role in cytotoxicity, e.g., monoolein-based cubosomes can induce a more toxic effect on different cell lines by interfering with nuclear and mitochondrial membranes [198].
7.2.8. Niosome
The toxicity of niosomes in cosmetic and topical applications has not been investigated. However, incorporation of its main constituent, non-ionic surfactant, in liquid soap and washing products has shown niosomes’ protecting and safe effect on skin [199]. In vitro toxicity assays have been conducted by incubation of niosomes with various cell lines. Result shows that the niosome exhibited a higher cytotoxic effect on cancer cells compare to a normal cell line (hGF), which were obtained from gingiva tissue [200]. This can suggest niosomes as an absorbing nanostructured material for cosmeceutical and dermatology researchers for use in topical applications.
The accumulation of nanoparticles in cytoplasm and cell organelles causes oxidative damage, which typically appears in a form of degraded mitochondria, disappeared chromatin, and vacuolization. They can induce NRF2 signaling pathways, resulting in rodex homeostasis by activation of antioxidative enzymes including GSH, SOD, and CAT, which reduces intracellular ROS. Also, the NRF2 signaling pathway [201,202] eads to an anti-inflammatory response by proteasomal degradation of NF-κB and thus, reduced generation of pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α [203,204]. That results in increasing the risk to the skin cells towards nanotoxicity-induced apoptosis and necrosis. Nanoparticle-induced oxidative damage can lead to atypical changes in organelles, such as mitochondrial degradation, chromatin disappearance, and lysosomal vacuolization [205,206,207]. The biological routes of nanoparticles into the skin, nanomaterial toxicity, and effects of nanoparticle accumulation in cell cytoplasm and cell organelles are shown in Figure 18 and Figure 19. Different techniques and conventional cytotoxicity assays commonly used to evaluate the cytotoxic effects of nanomaterials are shown in Figure 20. Different studies on the toxic effects of nanostructured particles from published scientific reports are listed in Table 6.
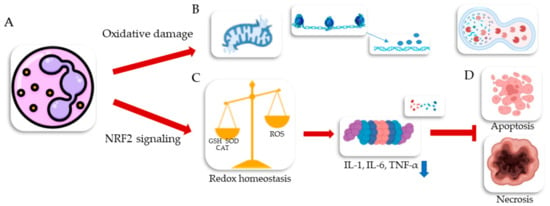
Figure 19.
The effects of nanoparticle accumulation in a living cell cytoplasm and cell organelles (A); can lead to oxidative damage, typically manifested as degraded mitochondria, disappeared chromatin, and vacuolization (B). NPs can also activate the NRF2 signaling pathway, resulting in redox homeostasis by activating antioxidant enzymes, such as GSH, SOD, and CAT, thereby reducing intracellular ROS levels (C). The NRF2 signaling pathway can also lead to an anti-inflammatory response by proteasomal degradation of NF-κB, resulting in reduced generation of pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α TNF (D). These effects can prevent skin cells from undergoing nanotoxicity-induced apoptosis and necrosis [203,204,206,207].
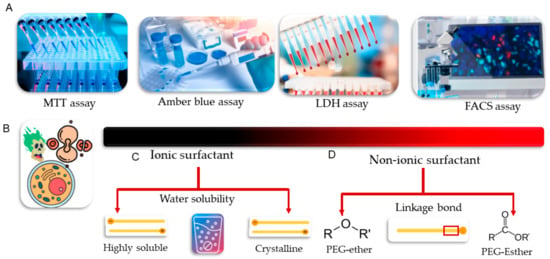
Figure 20.
MTT, amber blue, LDH, and FACS techniques are commonly used conventional cytotoxicity assays to evaluate the cytotoxic effects of nanomaterials (A). The red color gradient represents the range of cytotoxicity for different categories of surfactants, the main constituents of nanomaterials (B). There is a direct correlation between the cytotoxicity of ionic surfactants and their water solubility. As a result, highly soluble surfactants exhibit greater toxicity compared to moderately water-soluble crystalline surfactants (C). Non-ionic surfactants have lower cytotoxicity ranges compared to their ionic counterparts. The cytotoxicity of non-ionic surfactants is also dependent on the chemical linkage bond between the polar head and alkyl group of the surfactants. For instance, the PEG-ether surfactant has been shown to have greater cytotoxicity than the PEG-esther surfactant (D).

Table 6.
Toxic effects of various nanostructured particles.
8. Regulatory Affairs Involved for Safety Concerns
Although nanostructured particles have numerous advantages, there are also significant environmental risks and toxicity issues. Specific legal laws have been implemented to control them and offer validated limitations. The low concentration of these materials, as well as the absence of comparative data and toxicity thresholds for specific types of nanoparticles, is the main cause of the problems in the detection of nanostructured materials in cosmetics, food, trash, soil, water, etc. [219]. The most significant challenges in reducing the risk posed by nanostructured particles are associated with investigation and control of various parameters, e.g., concentration, chemical composition, source material, and synthesis/manufacturing methods; and technology for detecting these materials, especially portable equipment for quick diagnosis [220].
Concerned nations have recently started to monitor and assess the working of nanostructured materials in various spheres of human beings and the environment. The Danish Legislature has decided to compile a list of mixtures and goods that release nanostructured materials. According to the European Parliament’s resolution on ‘Regulatory Aspects of Nanomaterials’, the public should have access to nanostructured materials while maintaining safety. Because, as the dimensions of these materials become smaller, their toxicity becomes worse [221]. The EC Regulation 1223/2009 serves as primary regulatory framework for cosmetics in the EU. Based on this paradigm, customers should have access to a list including all nanostructured materials used in cosmetics [222]. In the EU, the person responsible is required to electronically communicate all cosmetic products to the EC and through the Cosmetic Products Notification Portal (CPNP) before they are placed on the market. This is performed for the specific purpose of algorithmic trading and for immediate and appropriate medical treatment. According to Regulation (EC) No. 1223/2009, if a cosmetic product contains any nanostructured materials, it must be disclosed [222,223]. The EU regulation’s fundamental premise is to increase consumer protection for EU citizens and transparency of cosmetic items. The commission and the relevant national authorities now have more authority. The FDA in the U.S.A. keeps a check on nanostructured material’s usage in cosmetics. The FDA provided an evaluation of scientific and legal factors relevant to the safety and efficacy of goods in the FDA Nanotechnology Inquiry Report of 2007. The task force suggested that a recommendation be released outlining safety concerns for cosmetic goods. Based on it, producers ought to think about assuring the safety of nanostructured-material-based cosmetic goods [224]. The FDA released the ‘Final Guideline for Industry—Safety of Nanomaterials in Cosmeceutical’ as industry guidance in 2014. It evaluates safety concerns and offers advice to the cosmetics industry [225]. Organizations can utilize the FDA’s two points to consider assessing whether their products include nanotechnology:
- If a substance or finished product is designed to have at least one intrinsic or surface structure, or at least one exterior dimension, in the nanoscale range (about 1–100 nm) [225].
- Whether a material or finished product has been designed to display characteristics or phenomena, such as physical or chemical characteristics or biological consequences, which are due to its parameters, even if those dimensions are beyond the nanoscale range up to 1000 nm [225].
Physical and chemical properties, particle agglomeration and size distribution, contaminants, exposure pathways, and in vitro and in vivo emerging pollutants on nanomaterial components are some of the safety issues to consider. The FDA’s biggest issue will be conducting thorough risk assessments. Data gathering seems to be the FDA’s top priority going ahead so that it can more effectively oversee the safety of nanoparticles used in cosmetics and other items with nanotechnology [226].
9. Future Perspective and Conclusions
By extracting and using advanced, efficient, and secure natural components, most of the customers’ ongoing demand for creative cosmetics may be satisfied. These chemicals need to be nanostructured and coupled with carriers formed from nano-emulsions with small amounts of emulsifiers and preservatives, as initially suggested in order to increase their efficiency [20]. Nano-cosmeceuticals are becoming more and more well-liked worldwide. Nanotechnology and cosmetics have been shown to be effective in treating a range of skin conditions [224]. To boost the use of nanotechnology in the cosmetics sector, these avenues could be pursued: An extensive toxicity evaluation of TiO2 and ZnO nanostructures could be performed before their widespread acceptance in the cosmetics industry, because earlier studies had shown conflicting results [25]. Although they do not wipe off and maintain the stability of the skin’s lipid bilayers, liposomes and nano-emulsions may be employed due to their stability [225]. For more secure and cost-effective products for consumers, nano-cosmeceuticals, a combination of cosmetics and medications, is best recommended.
Nanotechnology is a broad area that allows for the systematic production of nanoparticles and their numerous uses in various fields for a variety of reasons. There is a major lack of information regarding how nanotechnology will impact humans and the environment because it is still in development. It is challenging to make judgments about the impacts of the global development of nanotechnology due to a lack of study on nanosystems. These types of characteristics, such as transparency, solubility strength, and metal color, as well as physical and chemical reactivity, are all included in the definition of nanoparticles in this context, which are crucial for growth in the field of personal care and beauty, and by promoting the cosmetic’s diffusion into the skin’s surface, they may effectively assist skin absorption. It could be interesting to modify some of the actual cosmetic rules underlining that both cosmetics and cosmeceuticals penetrate the skin layers until the viable skin is without the range of the blood circulation. As a result, nanotechnology has a better future in the cosmetics industry since it can deliver drugs more effectively and improve the texture of cosmetics. A comprehensive overview of the application of nanotechnology in various cosmeceuticals is provided in this article, which highlights the general and advanced trends. Consideration is given to the creation of cosmetics and the increase in their use on a global scale. The potential for harmful consequences, such as skin inflammation, skin cancer, and genotoxicity, must be carefully investigated. Research in this aspect can investigate the interaction behavior of nanostructured materials with skin. A comprehensive study is required to understand the harmful effects of nanostructured materials, since the side-effect-free nature of personal-care products is a critical factor. This review would be advantageous to understand the connection between biosafety and nanomaterials along with its applications for sustainable future growth.
Author Contributions
Conceptualization, P.A.; methodology, P.A. and N.M.; writing—original draft preparation, A.S. and Z.S.; writing—review and editing, P.A. and N.M.; supervision, P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barik, T.K.; Maity, G.C.; Gupta, P.; Mohan, L.; Santra, T.S. Nanomaterials: An Introduction. Nanomater. Their Biomed. Appl. 2021, 16, 1–27. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, C. Emerging trends of nanotechnology in beauty solutions: A review. Mater. Today Proc. 2021, 81, 1052–1059. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef] [PubMed]
- Dhapte-Pawar, V.; Kadam, S.; Saptarsi, S.; Kenjale, P.P. Nanocosmeceuticals: Facets and aspects. Futur. Sci. OA 2020, 6, FSO613. [Google Scholar] [CrossRef]
- Manikanika; Kumar, J.; Jaswal, S. Role of nanotechnology in the world of cosmetology: A review. Mater. Today Proc. 2021, 45, 3302–3306. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- Nath, R.; Chakraborty, R.; Roy, R. Nanotechnology Based Cosmeceut icals. Int. J. Sci. Res. Sci. Technol. 2021, 8, 94–106. [Google Scholar]
- Shende, P.; Patel, D.; Takke, A. Nanomaterial-based cosmeceuticals. In Handbook of Functionalized Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 775–791. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. New Insights on Unique Features and Role of Nanostructured Materials in Cosmetics. Cosmetics 2020, 7, 24. [Google Scholar] [CrossRef]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.D.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the development of new cosmetic formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-Based Cosmeceuticals. Int. Sch. Res. Not. 2014, 2014, 843687. [Google Scholar] [CrossRef]
- Hameed, A.; Fatima, G.R.; Malik, K.; Muqadas, A. Scope of Nanotechnology in Cosmetics: Dermatology and Skin Care Products. J. Med. Chem. Sci. 2019, 2019, 9–16. [Google Scholar]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Ekpa Effiong, D.; Uwah, T.O.; Udofa Jumbo, E.; Akpabio, A.E. Nanotechnology in Cosmetics: Basics, Current Trends and Safety Concerns—A Review. Adv. Nanopart. 2020, 9, 1–22. [Google Scholar]
- Singh, T.G.; Sharma, N. Nanobiomaterials in cosmetics: Current status and future prospects. In Nanobiomaterials in Galenic Formulations and Cosmetics: Applications of Nanobiomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 149–174. [Google Scholar]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef]
- Yadav, A.R.; Mohite, S.K. Applications of nanotechnology in cosmeceuticals. Res. J. Top. Cosmet. Sci. 2020, 11, 83–88. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Mohd-Nasir, H.; Ahmad, A.; Setapar, S.H.M.; Peng, W.L.; Chuo, S.C.; Khatoon, A.; Umar, K.; Yaqoob, A.A.; Ibrahim, M.N.M. Role of Nanotechnology for Design and Development of Cosmeceutical: Application in Makeup and Skin Care. Front. Chem. 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Zhang, L. Applications, challenges and development of nanomaterials and nanotechnology. J. Chem. Soc. Pak. 2020, 42, 658–666. [Google Scholar]
- Yadwade, R.; Gharpure, S.; Ankamwar, B. Nanotechnology in cosmetics pros and cons. Nano Express 2021, 2, 022003. [Google Scholar] [CrossRef]
- Abu Hajleh, M.N.; Abu-Huwaij, R.; Al-Samydai, A.; Al-Halaseh, L.K.; Al-Dujaili, E.A. The revolution of cosmeceuticals delivery by using nanotechnology: A narrative review of advantages and side effects. J. Cosmet. Dermatol. 2021, 20, 3818–3828. [Google Scholar] [CrossRef]
- Ferraris, C.; Rimicci, C.; Garelli, S.; Ugazio, E.; Battaglia, L. Nanosystems in Cosmetic Products: A Brief Overview of Functional, Market, Regulatory and Safety Concerns. Pharmaceutics 2021, 13, 1408. [Google Scholar] [CrossRef]
- Beauty and Personal Care Products Market—Growth, Trends, and Forecasts (2023–2028). Available online: https://www.mordorintelligence.com/industry-reports/global-beauty-and-personal-care-products-market-industry (accessed on 8 March 2023).
- Global Beauty Products Market and Luxury Beauty Market 2022—Product Demand, Worldwide Consumption, Top Brands, Competition, Growth Rates, Investments, Production, Supply Chain and Future Stats Projection 2028. Available online: https://www.globenewswire.com/en/news-release/2022/02/21/2388521/0/en/Global-Beauty-Products-Market-and-Luxury-Beauty-Market-2022-Product-Demand-Worldwide-Consumption-Top-Brands-Competition-Growth-Rates-Investments-Production-Supply-Chain-and-Future-.html (accessed on 11 March 2023).
- Demir, N. Nanotechnology in cosmetics: Opportunities and challenges. NanoEra 2021, 1, 19–23. [Google Scholar]
- Cosmeceuticals Market (By Product: Skin Care, Hair Care, Injectable, Others; By Packaging Material: Glass, Plastic, Metal, Others; By Ingredient: Anti-Oxidants, Sunscreens, Botanicals, Peptides & Proteins, Exfoliants, Moisturizers, Retinoids; By Distribution Channel: Online Platforms, Supermarkets and Specialty Stores)—Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2022–2030. Available online: https://www.precedenceresearch.com/cosmeceuticals-market (accessed on 11 March 2023).
- Cosmetic Pigments Market (By Elemental Composition: Inorganic Pigments, Organic Pigments; By Application: Facial Makeup, Eye Makeup, Lip Products, Nail Products, Hair Color Products, Special effect & Special Purpose Products, Others; By Type: Special Effect Pigments, Surface Treated Pigments, Nano Pigments, Natural Colorants)—Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2022–2030. Available online: https://www.precedenceresearch.com/cosmetic-pigments-market (accessed on 11 March 2023).
- Nanomaterials Market (By Product: Carbon Nanotubes, Titanium Nanoparticles, Silver Nanoparticles, Aluminum Oxide Nanomaterials, Gold (Au), Iron (Fe), Copper (Cu), Platinum (Pt), Nickel (Ni), Antimony Tin Oxide, Bismuth Oxide, Others; By Application: Aeros. Available online: https://www.precedenceresearch.com/nanomaterials-market (accessed on 8 March 2023).
- Global Nano Cosmetic Pigments Market by Types (Titanium Dioxide, Zinc Oxide, Carbon Black, Iron Oxide, and Others), Applications (Facial Make-up, Lip Products, Eye Make-Up, Nail Products, Hair Color Products, Special Effect & Special Purpose Products, and Others), Distribution Channels (Online, Supermarkets/Hypermarkets, Specialty Stores, and Others), Age Groups (Teens, Adults, and Seniors), and Regions (Asia Pacific, Europe, North America, Middle East & Africa, and Latin America)—Global Industry Analysis, Growth, Share, Size, Trends, Opportunities, and Forecast From 2023 To 2031. Available online: https://dataintelo.com/report/nano-cosmetic-pigments-market/ (accessed on 11 March 2023).
- Inorganic Cosmetic Pigments Market: Information by Type, Application and Region-Forcast Till 2030. Available online: https://www.marketresearchfuture.com/reports/inorganic-cosmetics-pigments-market-10531 (accessed on 8 March 2023).
- Global Cosmetic Pigments Market Size By Elemental Composition (Organic [Lakes, True Pigments, Toner], Inorganic [Zinc Oxide, Others], By Product [Surface Treated Pigments, Natural Colorants, Nano Pigments, Special Effect Pigments], By Application Facial Make-Up [Foundation, Blusher], Eye Makeup, Lip care, Hair Care Products, Nail Care, Others [Soaps, Toothpaste] Industry Analysis Report, Country Outlook Application Potential, Price Trends, Competitive Market Share & Forecast, 2020–2026. Available online: https://www.gminsights.com/industry-analysis/cosmetic-pigments-market (accessed on 11 March 2023).
- Cosmetics Market (By Category: Skin & sun care products, Hair care products, Deodorants & fragrances, Makeup & color cosmetics; By Gender: Men, Women, Unisex; By Distribution Channel: Hypermarkets/Supermarkets, Specialty Stores, Pharmacies, Online sales channels, Other)—Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2021–2030. Available online: https://www.precedenceresearch.com/cosmetics-market (accessed on 10 March 2023).
- Vaseghi, Z.; Nematollahzadeh, A.; Tavakoli, O. Green methods for the synthesis of metal nanoparticles using biogenic reducing agents: A review. Rev. Chem. Eng. 2018, 34, 529–559. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of Biogenic Capping Agents in the Synthesis of Metallic Nanoparticles and Evaluation of Their Therapeutic Potential. Front. Nanotechnol. 2022, 3, 801620. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Banerjee, K.; AlAjmi, M.F.; Rahman, S. Mechanistic Aspects of Microbe-Mediated Nanoparticle Synthesis. Front. Microbiol. 2021, 12, 638068. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah; Khare, S.K. Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 2021, 9, 626834. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K. Mycogenic nanoparticles and their bio-prospective applications: Current status and future challenges. J. Nanostruct. Chem. 2018, 8, 369–391. [Google Scholar] [CrossRef]
- Karthik, L.; Kumar, G.; Kirthi, A.V.; Rahuman, A.A.; Rao, K.V.B. Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess Biosyst. Eng. 2014, 37, 261–267. [Google Scholar] [CrossRef]
- Eugenio, M.; Müller, N.; Frases, S.; Almeida-Paes, R.; Lima, L.M.T.; Lemgruber, L.; Farina, M.; de Souza, W.; Sant’Anna, C. Yeast-derived biosynthesis of silver/silver chloride nanoparticles and their antiproliferative activity against bacteria. Univ. Glas. 2016, 6, 13–52. [Google Scholar] [CrossRef]
- Gade, A.; Ingle, A.; Whiteley, C.; Rai, M. Mycogenic metal nanoparticles: Progress and applications. Biotechnol. Lett. 2010, 32, 593–600. [Google Scholar] [CrossRef]
- Owaid, M.N.; Ibraheem, I.J. Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur. J. Nanomed. 2017, 9, 5–23. [Google Scholar] [CrossRef]
- Shu, M.; He, F.; Li, Z.; Zhu, X.; Ma, Y.; Zhou, Z.; Yang, Z.; Gao, F.; Zeng, M. Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Yeast Extract as Reducing and Capping Agents. Nanoscale Res. Lett. 2020, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Zaini, M.A.A. Nanomaterials in detergents and cosmetics products: The mechanisms and implications. In Handbook of Nanomaterials for Manufacturing Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 23–49. [Google Scholar] [CrossRef]
- Naz, M.Y.; Shukrullah, S.; Ghaffar, A.; Ali, K.; Sharma, S.K. Synthesis and Processing of Nanomaterials. In Handbook of Materials Structures, Properties, Processing and Performance; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–17. [Google Scholar]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Singaravelu, G.; Arockiamary, J.; Kumar, V.G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces 2007, 57, 97–101. [Google Scholar] [CrossRef]
- Mulens, V.; del Puerto Morales, M.; Barber, D.F. Development of Magnetic Nanoparticles for Cancer Gene Therapy: A Comprehensive Review. ISRN Nanomater. 2013, 2013, 646284. [Google Scholar] [CrossRef]
- Sriondee, M.; Dungsuwan, W.; Thountom, S. Synthesis and characterization of Bi0.5 (Na1−xKx)0.5 TiO3 powders by sol–gel combustion method with glycine fuel. Ceram. Int. 2018, 44, S168–S171. [Google Scholar] [CrossRef]
- Yu, S.; Jing, W.; Tang, M.; Xu, T.; Yin, W.; Kang, B. Fabrication of Nd:YAG transparent ceramics using powders synthesized by citrate sol-gel method. J. Alloys Compd. 2019, 772, 751–759. [Google Scholar] [CrossRef]
- Hao, S.; Lin, T.; Ning, S.; Qi, Y.; Deng, Z.; Wang, Y. Research on cracking of SiO2 nanofilms prepared by the sol-gel method. Mater. Sci. Semicond. Process. 2018, 91, 181–187. [Google Scholar] [CrossRef]
- Xu, H.; Xin, L.; Liu, L.; Pang, D.; Jiao, Y.; Cong, R.; Yu, W. Large area MoS2/Si heterojunction-based solar cell through sol-gel method. Mater. Lett. 2019, 238, 13–16. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah, S.A.; Al-Ansari, T.; Atieh, M.A. A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Kleckley, S.; Wang, H.; Oladeji, I.; Chow, L.; Daly, T.K.; Buseck, P.R.; Solouki, T.; Marshall, A. Fullerenes and Polymers Produced by the Chemical Vapor Deposition Method; ACS Publications: Washington, DC, USA, 1998. [Google Scholar]
- Zhang, Z.; Wang, L.; Xu, X.; Dong, Y.; Zhang, L. Development of a validated HPLC method for the determination of tenofovir disoproxil fumarate using a green enrichment process. Anal. Methods 2015, 7, 6290–6298. [Google Scholar] [CrossRef]
- Wongpratat, U.; Maensiri, S.; Swatsitang, E. EXAFS study of cations distribution dependence of magnetic properties in Co1−xZnxFe2O4 nanoparticles prepared by hydrothermal method. Microelectron. Eng. 2015, 146, 68–75. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Xu, T.; Nie, Y.; Li, H.; Zhang, C. Synthesis and characterization of nanosized MnZn ferrites via a modified hydrothermal method. J. Magn. Magn. Mater. 2017, 439, 228–235. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Viswanath, I.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Qiu, W.; Feng, X.; Zhang, H.; Huang, H. Synthesis and luminescence properties of CaB4O7:Eu3+ via two-step hydrothermal method. Optik 2019, 182, 1039–1045. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.-M.; Niu, H.-L.; Mao, C.-J.; Zhang, S.-Y.; Shen, Y.-H. ZnFe2O4 nanoparticles: Synthesis, characterization, and enhanced gas sensing property for acetone. Sens. Actuators B Chem. 2015, 221, 55–62. [Google Scholar] [CrossRef]
- Rahimi, R.; Maleki, A.; Maleki, S.; Morsali, A.; Rahimi, M.J. Synthesis and characterization of magnetic dichromate hybrid nanomaterials with triphenylphosphine surface modified iron oxide nanoparticles (Fe3O4@SiO2@PPh3@Cr2O72−). Solid State Sci. 2014, 28, 9–13. [Google Scholar] [CrossRef]
- Amiri, S.; Shokrollahi, H. Magnetic and structural properties of RE doped Co-ferrite (REåNd, Eu, and Gd) nano-particles synthesized by co-precipitation. J. Magn. Magn. Mater. 2013, 345, 18–23. [Google Scholar] [CrossRef]
- Azizi, M.; Maleki, A.; Hakimpoor, F.; Firouzi-Haji, R.; Ghassemi, M.; Rahimi, J. Green Approach for Highly Efficient Synthesis of Polyhydroquinolines Using Fe3O4@PEO-SO3H as a Novel and Recoverable Magnetic Nanocomposite Catalyst. Lett. Org. Chem. 2018, 15, 753–759. [Google Scholar] [CrossRef]
- Xing, Y.; Jin, Y.-Y.; Si, J.-C.; Peng, M.-L.; Wang, X.-F.; Chen, C.; Cui, Y.-L. Controllable synthesis and characterization of Fe3O4/Au composite nanoparticles. J. Magn. Magn. Mater. 2015, 380, 150–156. [Google Scholar] [CrossRef]
- Mittapally, S.; Aziz, A.; Student, A.; Afnan, A.A. A review on nanotechnology in cosmetics. Pharma Innov. Int. J. 2019, 8, 668–671. [Google Scholar]
- Drbohlavova, J.; Hrdy, R.; Adam, V.; Kizek, R.; Schneeweiss, O.; Hubalek, J. Preparation and Properties of Various Magnetic Nanoparticles. Sensors 2009, 9, 2352–2362. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Koop-Santa, C.; López-Mena, E.R.; Orozco-Guareño, E.; García-Guaderrama, M. N-doped TiO2 nanoparticles obtained by a facile coprecipitation method at low temperature. Ceram. Int. 2018, 44, 5273–5283. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, J.; Li, W.; Guo, H.; You, B. Fabrication of cobalt aluminate nanopigments by coprecipitation method in threonine waterborne solution. Dyes Pigments 2018, 151, 130–139. [Google Scholar] [CrossRef]
- El Ghandoor, H.; Zidan, H.M.; Khalil, M.M.H.; Ismail, M.I.M. Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 5734–5745. [Google Scholar] [CrossRef]
- Cao, D.; Wang, X.; Pan, L.; Li, H.; Jing, P.; Wang, J.; Liu, Q. Nonmetal sulfur-doped coral-like cobalt ferrite nanoparticles with enhanced magnetic properties. J. Mater. Chem. C 2016, 4, 951–957. [Google Scholar] [CrossRef]
- Dong, H.; Du, S.-R.; Zheng, X.-Y.; Lyu, G.-M.; Sun, L.-D.; Li, L.-D.; Zhang, P.-Z.; Zhang, C.; Yan, C.-H. Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy. Chem. Rev. 2015, 115, 10725–10815. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Deng, F.; Xu, M.; Wang, J.; Wei, Z.; Wang, Y. GO/Cu2O nanocomposite based QCM gas sensor for trimethylamine detection under low concentrations. Sens. Actuators B Chem. 2018, 273, 498–504. [Google Scholar] [CrossRef]
- Mansoureh, G.; Parisa, V. Synthesis of metal nanoparticles using laser ablation technique. In Emerging Applications of Nanoparticles and Architectural Nanostructures: Current Prospects and Future Trends; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 575–596. [Google Scholar]
- Kurland, H.-D.; Grabow, J.; Staupendahl, G.; Andrä, W.; Dutz, S.; Bellemann, M.E. Magnetic iron oxide nanopowders produced by CO2 laser evaporation. J. Magn. Magn. Mater. 2007, 311, 73–77. [Google Scholar] [CrossRef]
- Kurland, H.-D.; Grabow, J.; Staupendahl, G.; Müller, F.A.; Müller, E.; Dutz, S.; Bellemann, M.E. Magnetic iron oxide nanopowders produced by CO2 laser evaporation—‘In situ’ coating and particle embedding in a ceramic matrix. J. Magn. Magn. Mater. 2009, 321, 1381–1385. [Google Scholar] [CrossRef]
- Stötzel, C.; Kurland, H.-D.; Grabow, J.; Dutz, S.; Müller, E.; Sierka, M.; Müller, F.A. Control of the crystal phase composition of FexOy Nanopowders Prepared by CO2 laser vaporization. Cryst. Growth Des. 2013, 13, 4868–4876. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef] [PubMed]
- Nagaich, U. Nanocosmeceuticals: A boon to personal care products. J. Adv. Pharm. Technol. Res. 2016, 7, 1. [Google Scholar] [CrossRef]
- Tiwari, S.; Talreja, M.S. A concept of nanotechnology in cosmetics: A complete overview. Adalya J. 2020, 9, 14–23. [Google Scholar]
- Abbasi, B.H.; Fazal, H.; Ahmad, N.; Ali, M.; Giglioli-Guivarch, N.; Hano, C. Nanomaterials for cosmeceuticals: Nanomaterials-induced advancement in cosmetics, challenges, and opportunities. In Nanocosmetics; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 79–108. [Google Scholar] [CrossRef]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef]
- Milam, E.C.; A Rieder, E. An Approach to Cosmeceuticals. J. Drugs Dermatol. 2016, 15, 452–456. [Google Scholar]
- Milam, E.C.; Rieder, E.A. An approach to cosmeceuticals. In Essential Psychiatry for the Aesthetic Practitioner; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 42–48. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Kouhbanani, M.A.J.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters With Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef] [PubMed]
- Nikam, N.R.; Patil, P.R.; Vakhariya, R.R.; Magdum, C.S. Liposomes: A Novel Drug Delivery System: An Overview. Asian J. Pharm. Res. 2020, 10, 23–28. [Google Scholar] [CrossRef]
- Faria-Silva, A.C.; Costa, A.M.; Ascenso, A.; Ribeiro, H.M.; Marto, J.; Gonçalves, L.M.; Carvalheiro, M.; Simões, S. Nanoemulsions for cosmetic products. Nanocosmetics 2020, 59–77. [Google Scholar] [CrossRef]
- Sonneville-Aubrun, O.; Yukuyama, M.N.; Pizzino, A. Application of Nanoemulsions in Cosmetics. In Nanoemulsions: Formulation, Applications, and Characterization; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 435–475. [Google Scholar] [CrossRef]
- Pandey, V.; Shukla, R.; Garg, A.; Kori, M.L.; Rai, G. Nanoemulsion in cosmetic: From laboratory to market. In Nanocosmetics; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 201, pp. 327–347. [Google Scholar] [CrossRef]
- Marzuki, N.H.C.; Wahab, R.A.; Hamid, M.A. An overview of nanoemulsion: Concepts of development and cosmeceutical applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Micelles and Nanoemulsions. In Nanocosmetics; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 47–72. [Google Scholar] [CrossRef]
- Khan, R.; Irchhaiya, R. Niosomes: A potential tool for novel drug delivery. J. Pharm. Investig. 2016, 46, 195–204. [Google Scholar] [CrossRef]
- Mehrarya, M.; Gharehchelou, B.; Poodeh, S.H.; Jamshidifar, E.; Karimifard, S.; Far, B.F.; Akbarzadeh, I.; Seifalian, A. Niosomal formulation for antibacterial applications. J. Drug Target. 2022, 30, 476–493. [Google Scholar] [CrossRef]
- Suttee, A.; Mishra, V.; Nayak, P.; Singh, M.; Sriram, P. Niosomes: Potential Nanocarriers for Drug Delivery. Int. J. Pharm. Qual. Assur. 2020, 11, 389–394. [Google Scholar] [CrossRef]
- Kaur, D.; Kumar, S. Niosomes: Present Scenario and Future Aspects. J. Drug Deliv. Ther. 2018, 8, 35–43. [Google Scholar] [CrossRef]
- Aparajay, P.; Dev, A. Functionalized niosomes as a smart delivery device in cancer and fungal infection. Eur. J. Pharm. Sci. 2022, 168, 106052. [Google Scholar] [CrossRef]
- Pandey, P.; Purohit, D.; Jalwal, P.; Manchanda, D.; Saini, S.; Verma, R.; Kaushik, D.; Mittal, V.; Kumar, M.; Bhattacharya, T.; et al. Nanocapsules: An Emerging Drug Delivery System. Recent Patents Nanotechnol. 2022. [Google Scholar] [CrossRef]
- Chawla, S.; Thakkar, D.; Rai, P. Utilization of Consumer Nanoproducts for Cosmetics and Their Impacts. In Handbook of Consumer Nanoproducts; Springer: Singapore, 2021; pp. 1–23. [Google Scholar] [CrossRef]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.-M.; Müller, R.; Bégu, S. Liposomes, lipid nanocapsules and smartCrystals®: A comparative study for an effective quercetin delivery to the skin. Int. J. Pharm. 2018, 542, 176–185. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Ni, X.; Liu, D.; Zhang, Y.; Cao, Y. Preparation and evaluation of polymer-encapsulated UV filter nanocapsules with miniemulsion polymerization. J. Dispers. Sci. Technol. 2021, 42, 1593–1600. [Google Scholar] [CrossRef]
- Mohd-Setapar, S.H.; John, C.P.; Mohd-Nasir, H.; Azim, M.M.; Ahmad, A.; Alshammari, M.B. Application of Nanotechnology Incorporated with Natural Ingredients in Natural Cosmetics. Cosmetics 2022, 9, 110. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Das, K.B.; Sahu, M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Ahmad, J. Lipid nanoparticles based cosmetics with potential application in alleviating skin disorders. Cosmetics 2021, 8, 84. [Google Scholar] [CrossRef]
- Formulation, N.F. Sunscreen Boosting Effect by Solid Lipid Nanoparticles-Loaded Fucoxanthin Formulation. Cosmetics 2020, 7, 14. [Google Scholar]
- Jose, J.; Netto, G. Role of solid lipid nanoparticles as photoprotective agents in cosmetics. J. Cosmet. Dermatol. 2019, 18, 315–321. [Google Scholar] [CrossRef]
- Sadhu, V.R.; Beram, N.S.; Kantamneni, P. A review on cubosome: The novel drug delivery system. GSC Biol. Pharm. Sci. 2018, 5, 076–081. [Google Scholar] [CrossRef]
- Chaudhary, K.; Sharma, D. Cubosomes: A Potential Drug Delivery System. Asian J. Pharm. Res. Dev. 2021, 9, 93–101. [Google Scholar] [CrossRef]
- Kaur, S.D.; Singh, G.; Singh, G.; Singhal, K.; Kant, S.; Bedi, N. Cubosomes as Potential Nanocarrier for Drug Delivery: A Comprehensive Review. J. Pharm. Res. Int. 2021, 33, 118–135. [Google Scholar] [CrossRef]
- Gaballa, S.; El Garhy, O.; Abdelkader, H. Cubosomes: Composition, preparation, and drug delivery applications. J. Adv. Biomed. Pharm. Sci. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Dhadwal, A.; Sharma, D.R.; Pandit, V.; Ashawat, M.S.; Kumar, P. Cubosomes: A Novel Carrier for Transdermal Drug Delivery. J. Drug Deliv. Ther. 2020, 10, 123–130. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Kraeling, M.E.; Topping, V.D.; Belgrave, K.R.; Schlick, K.; Simanek, E.; Man, S.; Dadiboyena, S.; Patri, A.K.; Sprando, R.L.; Yourick, J.J. In Vitro Skin Penetration of Dendrimer Nanoparticles. Appl. Vitr. Toxicol. 2019, 5, 134–149. [Google Scholar] [CrossRef]
- Ambre, P.K.; Gupta, C.R.; Martis, E.A.F.; Coutinho, E.C. Chapter 8—Self-assemblies, dendrimers, and nanoparticles. In Handbook on Nanobiomaterials for Therapeutics and Diagnostic Applications; Anand, K., Saravanan, M., Chandrasekaran, B., Kanchi, S., Jeeva Panchu, S., Chen, Q., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 151–189. [Google Scholar] [CrossRef]
- Simonescu, C.M. Introductory Chapter: Dendrimers as Nanoengineered Materials and Their Applications. Dendrimers-Fundam. Appl. 2018, 10–13. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Ghisleni, D.D.M.; Pinto, T.D.J.A.; Bouchacra, N.A. Nanoemulsion: Process selection and application in cosmetics—A review. Int. J. Cosmet. Sci. 2016, 38, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Rozbu, M.R.; Nuzhat, S.; Selvakumar, P.M. Role of Nanotechnology in Cosmeceuticals. In Handbook of Consumer Nanoproducts; Springer Nature: Singapore, 2022; pp. 985–1003. [Google Scholar]
- Elavia, P.F.; Suvarna, V. A review on applications of nanotechnology in cosmetics. Int. Res. J. Pharm. 2018, 9, 1–4. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Grabowska, A.; Chwastowski, J.; Majka, T.M.; Banach, M. Safety of the application of nanosilver and nanogold in topical cosmetic preparations. Colloids Surf. B Biointerfaces 2019, 183, 110416. [Google Scholar] [CrossRef]
- Rudolf, R.; Jelen, Z.; Zadravec, M.; Majeric, P.; Jovic, Z.; Vuksanovic, M.; Stankovic, I.; Matija, L.; Dragicevic, A.; Thompson, N.M.; et al. A gold nanoparticles and hydroxylated fullerene water complex as a new product for cosmetics. Adv. Prod. Eng. Manag. 2022, 17, 89–107. [Google Scholar] [CrossRef]
- Kantorová, V.; Loula, M.; Kaňa, A.; Mestek, O. Determination of silver nanoparticles in cosmetics using single particle ICP-MS. Chem. Pap. 2021, 75, 5895–5905. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, D.H.; Choi, S.; Yang, J.-Y.; Jung, K.; Jeong, J.; Oh, J.H.; Lee, J.H. Skin Sensitization Potential and Cellular ROS-Induced Cytotoxicity of Silica Nanoparticles. Nanomaterials 2021, 11, 2140. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Deng, J.; Hu, L.; Zhang, Z.; Jiang, H.; Li, Y.; Yi, Z.; Ngai, T. Investigation of the stability in Pickering emulsions preparation with commercial cosmetic ingredients. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125082. [Google Scholar] [CrossRef]
- Åhlén, M.; Cheung, O.; Strømme, M. Amorphous Mesoporous Magnesium Carbonate as a Functional Support for UV-Blocking Semiconductor Nanoparticles for Cosmetic Applications. ACS Omega 2019, 4, 4429–4436. [Google Scholar] [CrossRef]
- Kose, O.; Tomatis, M.; Leclerc, L.; Belblidia, N.-B.; Hochepied, J.-F.; Turci, F.; Pourchez, J.; Forest, V. Impact of the Physicochemical Features of TiO2 Nanoparticles on Their In Vitro Toxicity. Chem. Res. Toxicol. 2020, 33, 2324–2337. [Google Scholar] [CrossRef]
- Lu, P.-J.; Huang, S.-C.; Chen, Y.-P.; Chiueh, L.-C.; Shih, D.Y.-C. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef]
- Vinod, T.P.; Jelinek, R. Inorganic Nanoparticles in Cosmetics. In Nanocosmetics: From Ideas to Products; Cornier, J., Keck, C.M., de Voorde, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 29–46. [Google Scholar]
- Draelos, Z.D. The science behind skin care: Cleansers. J. Cosmet. Dermatol. 2017, 17, 8–14. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Cosma, P. Chapter 18—Nanomaterials in sun-care products. In Nanocosmetics; Nanda, A., Nanda, S., Nguyen, T.A., Rajendran, S., Slimani, Y., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 349–373. [Google Scholar]
- Ngoc, L.T.N.; Van Tran, V.; Moon, J.-Y.; Chae, M.; Park, D.; Lee, Y.-C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics 2019, 6, 64. [Google Scholar] [CrossRef]
- Mallakpour, S.; Hussain, C.M.; Gulati, S.; Kumar, S.; Wadhwa, R.; Lamba, S.; Batra, K. Nanocosmeceuticals: Novel and Advanced Self-Care Materials. In Handbook of Consumer Nanoproducts; Springer Nature: Singapore, 2021; pp. 1–26. [Google Scholar]
- Rosen, J.; Landriscina, A.; Friedman, A.J. Nanotechnology-Based Cosmetics for Hair Care. Cosmetics 2015, 2, 211–224. [Google Scholar] [CrossRef]
- Pereira-Silva, M.; Martins, A.M.; Sousa-Oliveira, I.; Ribeiro, H.M.; Veiga, F.; Marto, J.; Paiva-Santos, A.C. Nanomaterials in hair care and treatment. Acta Biomater. 2022, 142, 14–35. [Google Scholar] [CrossRef]
- Hydra Zen Oil-Free Gel Moisturizer with Salicylic Acid. Available online: https://www.lancome.com.au/skincare/face-moisturisers-creams/hydra-zen-moisturizing-gel-cream-day-cream/00206-LAC.html (accessed on 8 March 2023).
- NEOVA SmartSkincare. Available online: https://www.neova.com/ (accessed on 8 March 2023).
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef]
- Nafisi, S.; Maibach, H.I. Nanotechnology in cosmetics. Cosmet. Sci. Technol. Theor. Princ. Appl. 2017, 337–369. [Google Scholar]
- “Kara Vita” the Project of Emerging Nanotechnologies. 2023. Available online: https://www.nanotechproject.tech/cpi/browse/companies/kara-vita/ (accessed on 10 March 2023).
- Zhou, H.; Luo, D.; Chen, D.; Tan, X.; Bai, X.; Liu, Z.; Yang, X.; Liu, W. Current Advances of Nanocarrier Technology-Based Active Cosmetic Ingredients for Beauty Applications. Clin. Cosmet. Investig. Dermatol. 2021, 14, 867–887. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Carbon nanofiber in cosmetics. In Carbon Nanofibers: Fundamentals and Applications; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 341–363. [Google Scholar] [CrossRef]
- Acnel lotion N. Available online: https://www.nanotechproject.tech/cpi/products/acnel-lotion-n/ (accessed on 11 March 2023).
- Clear Complexion. Available online: https://www.beautyexpert.com/campaign/clear-complexion.list (accessed on 11 March 2023).
- Hydralane Ultra Moisturizing Day Cream with Nanospheres 1.7 Oz.—Beauty—Skin Care—Moisturizers & Creams. Available online: https://www.sears.com/hydralane-ultra-moisturizing-day-cream-with-nanospheres-1.7/p-SPM11839942930 (accessed on 10 March 2023).
- Mester, L.; Govyadinov, A.A.; Chen, S.; Goikoetxea, M.; Hillenbrand, R. Subsurface chemical nanoidentification by nano-FTIR spectroscopy. Nat. Commun. 2020, 11, 3359. [Google Scholar] [CrossRef] [PubMed]
- Chaukura, N.; Madzokere, T.C.; Mugocheki, N.; Masilompane, T.M. The impact of nanomaterials in aquatic systems. In The ELSI Handbook of Nanotechnology: Risk, Safety, Elsi and Commercialization; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 205–222. [Google Scholar]
- Favero, J.D.S.; dos Santos, V.; Weiss-Angeli, V.; Gomes, L.B.; Veras, D.G.; Dani, N.; Mexias, A.S.; Bergmann, C.P. Evaluation and characterization of Melo Bentonite clay for cosmetic applications. Appl. Clay Sci. 2019, 175, 40–46. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jiménez, A.J.; Abad-Jordá, M.; Rubio-Moraga, A.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Biogenic Silver Nanoparticles from Iris tuberosa as Potential Preservative in Cosmetic Products. Molecules 2021, 26, 4696. [Google Scholar] [CrossRef]
- Kim, K.-B.; Kwack, S.J.; Lee, J.Y.; Kacew, S.; Lee, B.-M. Current opinion on risk assessment of cosmetics. J. Toxicol. Environ. Health Part B 2021, 24, 137–161. [Google Scholar] [CrossRef]
- Carrouel, F.; Viennot, S.; Ottolenghi, L.; Gaillard, C.; Bourgeois, D. Nanoparticles as Anti-Microbial, Anti-Inflammatory, and Remineralizing Agents in Oral Care Cosmetics: A Review of the Current Situation. Nanomaterials 2020, 10, 140. [Google Scholar] [CrossRef]
- Rieder, E.A.; Fried, R.G. Front Matter. In Essential Psychiatry for the Aesthetic Practitioner; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. i–xvi. [Google Scholar]
- Lee, C.-C.; Lin, Y.-H.; Hou, W.-C.; Li, M.-H.; Chang, J.-W. Exposure to ZnO/TiO2 Nanoparticles Affects Health Outcomes in Cosmetics Salesclerks. Int. J. Environ. Res. Public Health 2020, 17, 6088. [Google Scholar] [CrossRef]
- Filon, F.L.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef]
- Krewski, D.; Andersen, M.; Tyshenko, M.G.; Krishnan, K.; Hartung, T.; Boekelheide, K.; Wambaugh, J.; Jones, D.; Whelan, M.; Thomas, R.; et al. Toxicity testing in the 21st century: Progress in the past decade and future perspectives. Arch. Toxicol. 2020, 94, 1–58. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When I’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, M.T.; Cable, S.; Carmichael, P.L.; Cubberley, R.; Cull, T.; Delagrange, M.; Dent, M.P.; Hatherell, S.; Houghton, J.; Kukic, P.; et al. A Next-Generation Risk Assessment Case Study for Coumarin in Cosmetic Products. Toxicol. Sci. 2020, 176, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Burden, N.; Clift, M.J.D.; Jenkins, G.J.S.; Labram, B.; Sewell, F. Opportunities and Challenges for Integrating New In Vitro Methodologies in Hazard Testing and Risk Assessment. Small 2021, 17, e2006298. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.; Fauzi, M.B. Current Update of Collagen Nanomaterials—Fabrication, Characterisation and Its Applications: A Review. Pharmaceutics 2021, 13, 316. [Google Scholar] [CrossRef]
- Patil, R.M.; Thorat, N.D.; Townley, H. Nanomaterials exposure to human. In Nano-Pharmacokinetics and Theranostics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 55–70. [Google Scholar]
- Bencsik, A.; Lestaevel, P.; Canu, I.G. Nano- and neurotoxicology: An emerging discipline. Prog. Neurobiol. 2018, 160, 45–63. [Google Scholar] [CrossRef]
- Lee, B.-M.; Choi, M.; Shin, I.; Kim, J.; Choi, Z.; Kim, K.; Choi, K.; Yang, S.; So, D.Y.; Ju, S.T.; et al. Risk communication for labeling all ingredients in consumer products. J. Toxicol. Environ. Health Part A 2020, 83, 509–524. [Google Scholar] [CrossRef]
- Niska, K.; Zielinska, E.; Radomski, M.W.; Inkielewicz-Stepniak, I. Metal nanoparticles in dermatology and cosmetology: Interactions with human skin cells. Chem. Biol. Interact. 2018, 295, 38–51. [Google Scholar] [CrossRef]
- Avila, A.M.; Bebenek, I.; Bonzo, J.A.; Bourcier, T.; Davis Bruno, K.L.; Carlson, D.B.; Dubinion, J.; Elayan, I.; Harrouk, W.; Lee, S.-L.; et al. An FDA/CDER perspective on nonclinical testing strategies: Classical toxicology approaches and new approach methodologies (NAMs). Regul. Toxicol. Pharmacol. 2020, 114, 104662. [Google Scholar] [CrossRef]
- Andersen, M.E.; McMullen, P.; Phillips, M.; Yoon, M.; Pendse, S.N.; Clewell, H.J.; Hartman, J.K.; Moreau, M.; Becker, R.A.; Clewell, R.A. Developing context appropriate toxicity testing approaches using new alternative methods (NAMs). Altex 2019, 36, 532–534. [Google Scholar] [CrossRef]
- Barthe, M.; Bavoux, C.; Finot, F.; Mouche, I.; Cuceu-Petrenci, C.; Forreryd, A.; Hansson, A.C.; Johansson, H.; Lemkine, G.; Thénot, J.-P.; et al. Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (NAMs). Cosmetics 2021, 8, 50. [Google Scholar] [CrossRef]
- Gajbhiye, S.; Sakharwade, S. Silver Nanoparticles in Cosmetics. J. Cosmet. Dermatol. Sci. Appl. 2016, 6, 48–53. [Google Scholar] [CrossRef]
- Fragoso, A.; Wajs, E. Nanosponges in Catalysis and Sensing. Nanosponges 2019, 263–282. [Google Scholar] [CrossRef]
- Subramaniam, V.D.; Prasad, S.V.; Banerjee, A.; Gopinath, M.; Murugesan, R.; Marotta, F.; Sun, X.-F.; Pathak, S. Health hazards of nanoparticles: Understanding the toxicity mechanism of nanosized ZnO in cosmetic products. Drug Chem. Toxicol. 2019, 42, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.; Bania, R.; Devi, J.R.; Deka, S. Therapeutic nanostructures and nanotoxicity. J. Appl. Toxicol. 2021, 41, 1494–1517. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar]
- Cohen, D.; Soroka, Y.; Ma’or, Z.; Oron, M.; Portugal-Cohen, M.; Brégégère, F.M.; Berhanu, D.; Valsami-Jones, E.; Hai, N.; Milner, Y. Evaluation of topically applied copper(II) oxide nanoparticle cytotoxicity in human skin organ culture. Toxicol. Vitr. 2013, 27, 292–298. [Google Scholar] [CrossRef]
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef]
- Mohammed, Y.H.; Holmes, A.; Haridass, I.N.; Sanchez, W.Y.; Studier, H.; Grice, J.E.; Benson, H.A.; Roberts, M.S. Support for the Safe Use of Zinc Oxide Nanoparticle Sunscreens: Lack of Skin Penetration or Cellular Toxicity after Repeated Application in Volunteers. J. Investig. Dermatol. 2019, 139, 308–315. [Google Scholar] [CrossRef]
- Liang, Y.; Simaiti, A.; Xu, M.; Lv, S.; Jiang, H.; He, X.; Fan, Y.; Zhu, S.; Du, B.; Yang, W.; et al. Antagonistic Skin Toxicity of Co-Exposure to Physical Sunscreen Ingredients Zinc Oxide and Titanium Dioxide Nanoparticles. Nanomaterials 2022, 12, 2769. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Arora, S.; Rajwade, J.M.; Omray, P.; Khandelwal, S.; Paknikar, K.M. Silver Nanoparticles in Therapeutics: Development of an Antimicrobial Gel Formulation for Topical Use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.T.J.; Nyam, K.L. Evaluation of silver nanoparticles in cosmeceutical and potential biosafety complications. Saudi J. Biol. Sci. 2022, 29, 2085–2094. [Google Scholar] [CrossRef]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The Potential Risks of Nanomaterials: A Review Carried Out for ECETOC. Part. Fibre Toxicol. 2006, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-H.; Bae, H.C.; Jang, Y.; Jeong, S.H.; Na Lee, H.; Ryu, W.-I.; Yoo, M.G.; Kim, Y.-R.; Kim, M.-K.; Lee, J.K.; et al. Effect of the size and surface charge of silica nanoparticles on cutaneous toxicity. Mol. Cell. Toxicol. 2013, 9, 67–74. [Google Scholar] [CrossRef]
- An, S.S.A.; Ryu, H.J.; Seong, N.-W.; So, B.J.; Seo, H.-S.; Kim, J.-H.; Hong, J.-S.; Park, M.-K.; Kim, M.-S.; Kim, Y.-R.; et al. Evaluation of silica nanoparticle toxicity after topical exposure for 90 days. Int. J. Nanomed. 2014, 9, 127–136. [Google Scholar] [CrossRef]
- Ben Haddada, M.; Gerometta, E.; Chawech, R.; Sorres, J.; Bialecki, A.; Pesnel, S.; Spadavecchia, J.; Morel, A.-L. Assessment of antioxidant and dermoprotective activities of gold nanoparticles as safe cosmetic ingredient. Colloids Surf. B Biointerfaces 2020, 189, 110855. [Google Scholar] [CrossRef]
- Arul Prakash, F.; Dushendra Babu, G.J.; Lavanya, M.; Shenbaga Vidhya, K.; Devasena, T. Toxicity studies of aluminium oxide nanoparticles in cell lines. Int. J. Nanotechnol. Appl. 2011, 5, 99–107. [Google Scholar]
- Wani, T.A.; Masoodi, F.A.; Jafari, S.M.; McClements, D.J. Chapter 19—Safety of Nanoemulsions and Their Regulatory Status. In Nanoemulsions; Jafari, S.M., McClements, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 613–628. [Google Scholar]
- Lémery, E.; Briançon, S.; Chevalier, Y.; Bordes, C.; Oddos, T.; Gohier, A.; Bolzinger, M.-A. Skin toxicity of surfactants: Structure/toxicity relationships. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 166–179. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid nanocarriers for dermal delivery of lutein: Preparation, characterization, stability and performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.C.; Weixelbaum, A.; Pagitsch, E.; Löw, M.; Resch, G.P.; Valenta, C. Nanocarriers for dermal drug delivery: Influence of preparation method, carrier type and rheological properties. Int. J. Pharm. 2012, 437, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M.; Kovačević, A.; Müller, R.H.; Savić, S.; Vuleta, G.; Milić, J. Formulation of solid lipid nanoparticles (SLN): The value of different alkyl polyglucoside surfactants. Int. J. Pharm. 2014, 474, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Bokrova, J.; Marova, I.; Matouskova, P.; Pavelkova, R. Fabrication of novel PHB-liposome nanoparticles and study of their toxicity in vitro. J. Nanopart. Res. 2019, 21, 49. [Google Scholar] [CrossRef]
- Van Gheluwe, L.; Buchy, E.; Chourpa, I.; Munnier, E. Three-Step Synthesis of a Redox-Responsive Blend of PEG–block–PLA and PLA and Application to the Nanoencapsulation of Retinol. Polymers 2020, 12, 2350. [Google Scholar] [CrossRef]
- Kumar, S.; Pooja; Trotta, F.; Rao, R. Encapsulation of Babchi Oil in Cyclodextrin-Based Nanosponges: Physicochemical Characterization, Photodegradation, and In Vitro Cytotoxicity Studies. Pharmaceutics 2018, 10, 169. [Google Scholar] [CrossRef]
- Winnicka, K.; Wroblewska, M.; Sosnowska, K.; Car, H.; Kasacka, I. Evaluation of cationic polyamidoamine dendrimers’ dermal toxicity in the rat skin model. Drug Des. Dev. Ther. 2015, 9, 1367–1377. [Google Scholar] [CrossRef]
- Strachan, J.B.; Dyett, B.P.; Nasa, Z.; Valery, C.; Conn, C.E. Toxicity and cellular uptake of lipid nanoparticles of different structure and composition. J. Colloid Interface Sci. 2020, 576, 241–251. [Google Scholar] [CrossRef]
- Hinton, T.M.; Grusche, F.; Acharya, D.; Shukla, R.; Bansal, V.; Waddington, L.J.; Monaghan, P.; Muir, B.W. Bicontinuous cubic phase nanoparticle lipid chemistry affects toxicity in cultured cells. Toxicol. Res. 2013, 3, 11–22. [Google Scholar] [CrossRef]
- Murgia, S.; Falchi, A.M.; Mano, M.; Lampis, S.; Angius, R.; Carnerup, A.M.; Schmidt, J.; Diaz, G.; Giacca, M.; Talmon, Y.; et al. Nanoparticles from Lipid-Based Liquid Crystals: Emulsifier Influence on Morphology and Cytotoxicity. J. Phys. Chem. B 2010, 114, 3518–3525. [Google Scholar] [CrossRef]
- Ali, I.; Shah, M.R.; Yousuf, S.; Ahmed, S.; Shah, K.; Javed, I. Hemolytic and cellular toxicology of a sulfanilamide-based nonionic surfactant: A niosomal carrier for hydrophobic drugs. Toxicol. Res. 2018, 7, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Baranei, M.; Taheri, R.A.; Tirgar, M.; Saeidi, A.; Oroojalian, F.; Uzun, L.; Asefnejad, A.; Wurm, F.R.; Goodarzi, V. Anticancer effect of green tea extract (GTE)-Loaded pH-responsive niosome Coated with PEG against different cell lines. Mater Today Commun. 2021, 26, 101751. [Google Scholar] [CrossRef]
- Le Gal, K.; Schmidt, E.E.; Sayin, V.I. Cellular redox homeostasis. Antioxidants 2021, 10, 1377. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.-N. Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef]
- Yerra, V.G.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Cheng, Y.-T.; Chau, C.-F.; Yen, G.-C. Effect of Diallyl Sulfide on in Vitro and in Vivo Nrf2-Mediated Pulmonic Antioxidant Enzyme Expression via Activation ERK/p38 Signaling Pathway. J. Agric. Food Chem. 2012, 60, 100–107. [Google Scholar] [CrossRef]
- Sun, T.C.; Liu, X.C.; Yang, S.H.; Song, L.L.; Zhou, S.J.; Deng, S.L.; Tian, L.; Cheng, L.Y. Melatonin Inhibits Oxidative Stress and Apoptosis in Cryopreserved Ovarian Tissues via Nrf2/HO-1 Signaling Pathway. Front. Mol. Biosci. 2020, 7, 163. [Google Scholar] [CrossRef]
- Gabbay, J.S. Cosmetic Skin Care Compositions Comprising Insoluble Copper Oxide. Patent WO2012046229A3, 12 April 2012. [Google Scholar]
- Frula Beauty. Available online: https://www.frulabeauty.com/blogs/ingredients/iron-oxides-for-skin-benefits-and-how-to-use (accessed on 28 March 2023).
- Anderson, R.G. Inside Ingredients: Zinc Oxide. 2022. Available online: https://www.cosmeticsandtoiletries.com/formulas-products/formulating-basics/article/22301527/skin-inc-magazine-inside-ingredients-zinc-oxide (accessed on 28 March 2023).
- Silver Nanoparticle Safety. A Fortis Life Sciences Company. 2023. Available online: https://nanocomposix.com/pages/silver-nanoparticle-safety (accessed on 28 March 2023).
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Element Res. 2016, 172, 1–36. [Google Scholar] [CrossRef]
- Silica Silylate. Available online: https://cosmetics.specialchem.com/inci-ingredients/silica-silylate (accessed on 28 March 2023).
- Anderson, K. Assessing Use of Gold Nanoparticles. 2013. Available online: https://www.cosmeticsandtoiletries.com/research/tech-transfer/blog/21837628/assessing-use-of-gold-nanoparticles (accessed on 28 March 2023).
- Rodriguez, C.; Magniez, H.; Coletta, D.; Quillet, S.; Swoboda, B. Cosmetic Nanoemulsion. Patent WO2016177704A1, 10 November 2016. [Google Scholar]
- Wissing, S.A.; Müller, R.H. Cosmetic applications for solid lipid nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef]
- Biotechnology Innovation Organisation. Liposomes for Cosmetics. Creative Biostructure. 2023. Available online: https://www.creative-biostructure.com/liposomes-for-cosmetics-487.htm (accessed on 28 March 2023).
- Bahary, W.S.; Hogan, M.P. Cleansing Compositions with Dendrimers as Mildness Agents. Patent US5658574A, 19 August 1997. [Google Scholar]
- Pandita, D. Global Regulatory Overview of Nanopharmaceuticals and Nanocosmetics. Appl. Clin. Res. Clin. Trials Regul. Aff. 2018, 5, 73. [Google Scholar] [CrossRef]
- Pastrana, H.; Avila, A.; Tsai, C.S.J. Nanomaterials in Cosmetic Products: The Challenges with regard to Current Legal Frameworks and Consumer Exposure. Nanoethics 2018, 12, 123–137. [Google Scholar] [CrossRef]
- Malik, R.; Patil, S. Nanotechnology: Regulatory outlook on nanomaterials and nanomedicines in United States, Europe and India. Appl. Clin. Res. Clin. Trials Regul. Aff. 2020, 7, 225–236. [Google Scholar] [CrossRef]
- Dave, V.; Sur, S.; Gupta, N. Current Framework, Ethical Consideration and Future Challenges of Regulatory Approach for Nano-Based Products. Nanopharm. Adv. Deliv. Syst. 2021, 447–472. [Google Scholar] [CrossRef]
- Bom, S.; Ribeiro, H.M.; Marto, J. Sustainability Calculator: A Tool to Assess Sustainability in Cosmetic Products. Sustainability 2020, 12, 1437. [Google Scholar] [CrossRef]
- Csóka, I.; Ismail, R.; Jójárt-Laczkovich, O.; Pallagi, E. Regulatory Considerations, Challenges and Risk-based Approach in Nanomedicine Development. Curr. Med. Chem. 2021, 28, 7461–7476. [Google Scholar] [CrossRef]
- Ruhela, M.; Nagar, L.; Gupta, A.; Popli, H. Cosmetics: Regulatory and market scenario for us and India. Pharma Innov. J. 2018, 7, 164–169. [Google Scholar]
- Riccolo, A. The lack of regulation in preventing greenwashing of cosmetics in the US. J. Legis. 2021, 47, 133. [Google Scholar]
- Morganti, P.; Chen, H.-D. Nanocosmetics: Future perspective. Nanocosmetics 2020, 455–481. [Google Scholar] [CrossRef]
- Ahmad, U.; Ahmad, Z.; Khan, A.A.; Akhtar, J.; Singh, S.P.; Ahmad, F.J. Strategies in Development and Delivery of Nanotechnology Based Cosmetic Products. Drug Res. 2018, 68, 545–552. [Google Scholar] [CrossRef]
- Morganti, P.; Paglialunga, S. EU borderline cosmetic products review of current regulatory status. Clin. Dermatol. 2008, 26, 392–397. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).