Mesoporous Silica-Based Catalysts for Biodiesel Production: A Review
Abstract
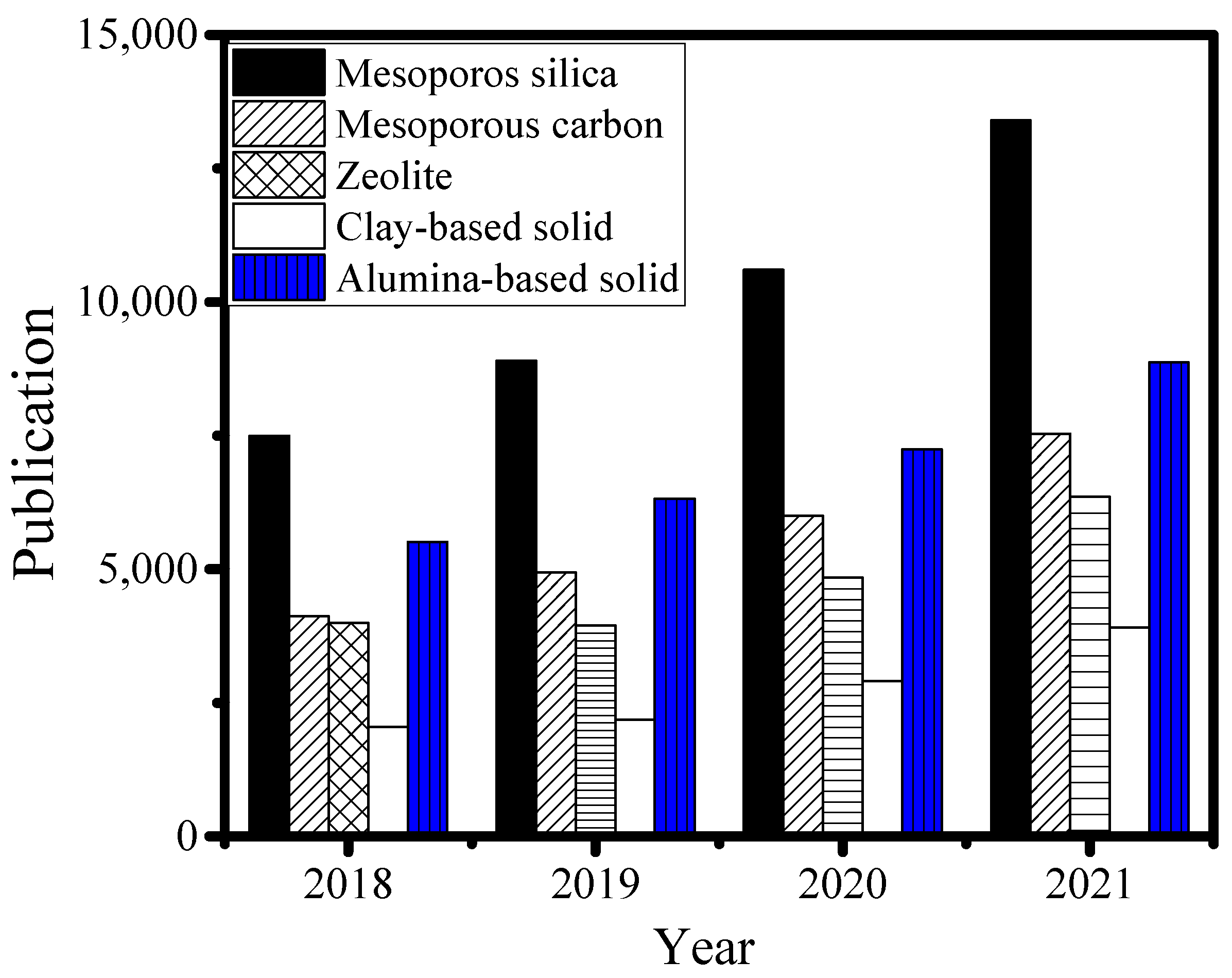
1. Introduction
2. Various Types of Mesoporous Catalysts
3. Mesoporous Silica Materials and Modified Forms
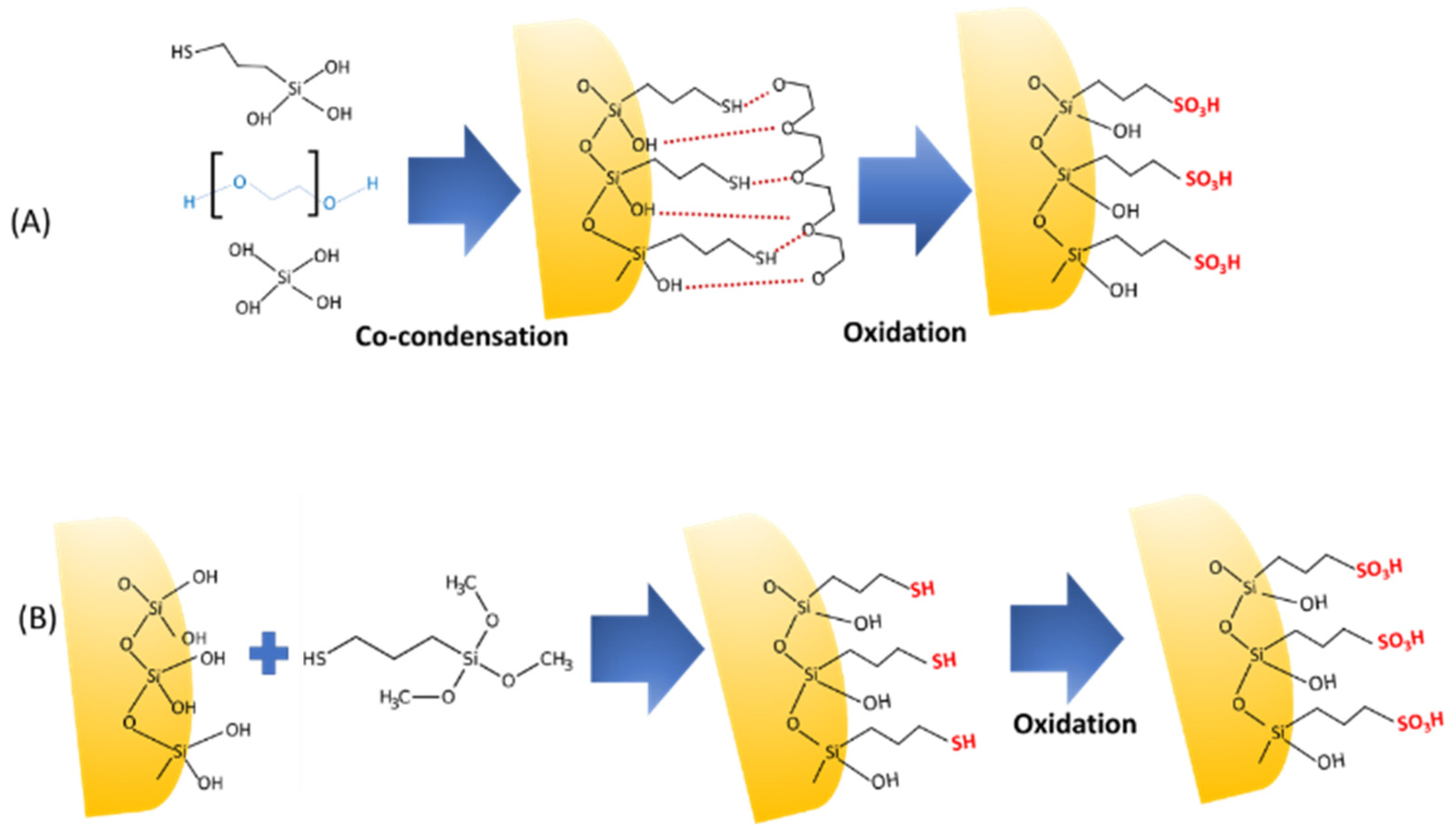
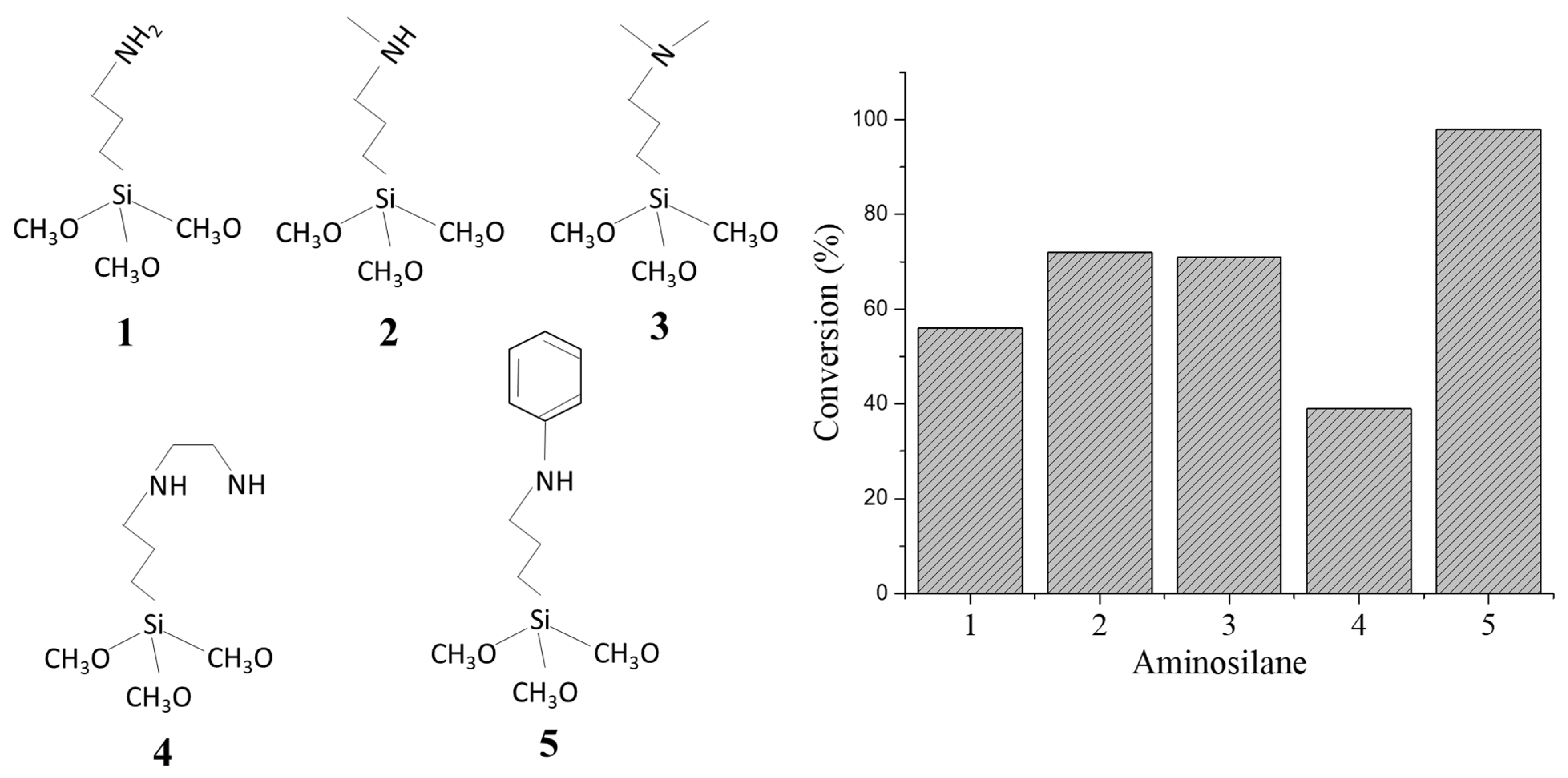
3.1. Acid- or Base-Functionalized Mesoporous Silica
| Catalyst | Preparation Method | Transesterification Reaction | Remark | Reference |
|---|---|---|---|---|
| Ar-SO3H/SBA-15 | Aryl-sulfonic acid-functionalized SBA-15 material was syn-thesized by following the one-step co-condensation procedure. | Crude soybean oil transesterification | Catalyst showed recyclability until third use without any significant yield reduction. Arene structure gave higher surface acidity and tends to give better catalyst stability. | [34] |
| SO3H/SBA-15 | SO3H/SBA-15 was prepared through the co-condensation method. | Olive pomace oil transesterification | Increased conversion of oleic acid was reported. The catalyst has reusability properties. | [36] |
| Propyl sulfonic-KIT-6 | KIT-6 silica functionalized with sulfonic acid through the co-condensation method. | Cashew nut oil transesterification by butanol | The KIT-6 propylsulfonic acid catalyst was able to produce a 70% butyl ester yield. | [39] |
| SO3H/MCM-41 | Material was prepared by using polystyrene as a template and p-toluenesulfonic acid (TsOH) as a carbon precursor and −SO3H source. | Oleic oil transesterification | Catalyst showed recyclability until fifth use without any significant conversion reduction. | [37] |
| Propyl sulfinic-KIT 6 | KIT was prepared by using pluronic acid P123: TEOS: BuOH:HCl:H2O = 0.017:1:0.31:1.83: 195. After calcination, silicas were functionalized with sulfonicacid groups by post-grafting using mercaptopropyl trimethoxysilane (MPTS 95%) and the thiol was converted by oxidation using H2O2. | Propanoic and hexanoic esterification | The enhancements in turnover frequency (TOF) toward propanoic and hexanoic acid esterification were 40 and 70%, respectively. | [43] |
| 12-Tungstophosphoric acid anchored to MCM-41 | MCM-41 was synthesized through the sol–gel method using surfactant cetyl trimethyl ammonium bromide (CTAB), NaOH, and TEOS. 12 Tungstophosphoric acid (12-TPA) was impregnated by stirring at 100 °C for 10 h. | Transesterification of palmitic acid | The catalyst shows high activity in terms of 100% conversion toward palmitic acid and a high turnover number of 1992. | [44] |
| 12-TPA/MCM-48 | MCM-48 was prepared through the sol–gel method with composition of 1 M TEOS: 12.5 M NH4OH:54 M EtOH: 0.4 M CTAB: 174 M H2O. 12-TPA was impregnated by incipient impregnation. | Transesterification of jatropha oil (JO) | The uniform dispersion of HPA inside the 3D channels of MCM-48 influenced the increasing activity for the esterification of oleic acid under mild conditions. The catalyst could be used for biodiesel production from WCO and JO with very high conversion: 95% and 93%, respectively. | [48] |
| HPA/KIT-6 | KIT-6 was synthesized through hydrothermal condensation using precursor at a molar ratio of 1 TEOS: 0.017 P123:1.83 HCl (35%):1.3 n-BuOH: 195 H2O. HPA functionalization to KIT-6 was conducted by impregnation. | Transesterification of neem oil | The conversion of neem oil depends on Brønsted acid sites, large surface area, pore size, and the fine dispersion of HPA in the composite. The optimum HPA content in the composite is 20%. | [50] |
| 1,5,7-triazabicyclo [4.4.0]dec-5-ene (TBD)/SBA-15 | SBA-15 was prepared using a P123 templating agent. 1,5,7-triazabicyclo [4.4.0]dec-5-ene [TBD] was functionalized through the adsorption method in a nitrogen environment. | Transesterification of soybean oil | The higher the grafted base amount, the higher the FAME yield. | [51] |
| TBD/MCM-41 | Material was prepared through the post-synthesis method. TBD was anchored by immersing MCM-41 in TBD using tetrahydrofuran (THF) as a solvent, followed by filtration. | Transesterification of soybean oil | The TON was 57, higher than that of MCM-41 (48). The catalyst is reusable. | [52] |
| Piperazine/MCM-41 | Material was prepared through the post-synthesis method. Piperazine was anchored by using the reflux method in dry toluene and propylamine in a N2 atmosphere. | Transesterification of soybean oil | The TON was 1270, higher than that of MCM-41 (48). The catalyst is reusable without any activity loss in the second cycle. | [52] |
| Amine-functionalized SBA-15 and MCM-41 | Material was prepared through the post-synthesis method. Amine functionalization was conducted by grafting in anhydrous toluene under argon. | Transesterification of glyceryl tributyrate | The aniline-functionalized OMS materials display the highest conversion in transesterification. | [53] |
| Diphenylamine(DPA)/SBA-15 and DPA/MCM-48 | Material was prepared through the co-condensation method. | Transesterification of oleic acid | Diphenylammonium salts were immobilized onto meso-porous silicas using either the co-condensation or grafting technique. The resulting catalysts were highly effective at esterifying the FFA in greases (12–40 wt% FFA) to FAME but displayed only minimal activity in transesterifying glycerides. | [54] |
| Sulfonated phosphotungstic acid-modified ordered mesoporous silica (HPW/OMS-SO3H) | HPW/OMS-SO3H was prepared through the co-condensation method in non-hydrochloric acid solution. | Transesterification of oleic acid | The catalyst showed very high hydrothermal stability and recycling performance. The reaction catalyzed by 0.3HPW/OMS-SO3H-5 followed pseudo-first-order kinetics, and Ea was found to be 22.46 kJ/mol. | [49] |
3.2. Metal- or Metal Oxide-Impregnated Mesoporous Silica
| Catalyst | Transesterification Reaction | Remark | Reference |
|---|---|---|---|
| Zirconium-doped MCM-41-supported WO3. | Transesterification of oleic oil | High conversion was maintained at 97% even at in condition of the presence of 5.5 wt% of water, suggesting that water is not adsorbed on the active centers of the catalyst and oleic acid molecules. | [46] |
| CaO/SBA-15 | Transesterification of sunflower oil and castor oil by using methanol | The conversion was 65.7 and 95% for sunflower oil and castor oil, respectively. | [63] |
| MgO/SBA-15 | Transesterification of lauric acid with butanol | Incorporation of Mg into mesoporous silica does not affect the structure. The catalysts were able to promote the esterification of lauric acid with 1-butanol, giving good yields at ambient pressure. | [80] |
| MgO/ZSM-5 | Transesterification of Spirulina oil | Catalyst is reusable until the fifth cycle. | [67] |
| MgO/KIT 6 | Transesterification of vegetable oil | Reaction conversion of 96%. | [66] |
| Cs/SBA-15 | Transesterification of canola oil | A conversion of 99% was achieved with the pressure of 3 MPa and reaction temperature of 260 °C. | [81] |
| Ce/MCM-41 | Transesterification of sunflower oil | Catalyst shows stability, which is related to the homogeneous distribution of Ce in the nanocomposite. | [76] |
| Zr/MCM-41 | Transesterification of sunflower oil | Catalyst was prepared through ultrasound irradiation. It was found that the frequency of ultrasound influenced the Zr distribution and specific surface area, thus affecting the catalyst stability. | [78] |
| ZnO/MCM-41 | Jatropha oil transesterification | Catalyst was prepared using orange peel extract as a green reductor of ZnO nanoparticles. Catalyst showed high activity (97% conversion). | [79] |
| TiO2/MCM-48 | Palmitic acid photocatalytic transesterification | The prepared material shows photocatalytic activity for the photocatalytic esterification of palmitic acid, and the material is recyclable until the 10th cycle. | [77] |
| Cr/SiO2 | Palmitic acid photocatalytic transesterification | The prepared material shows photocatalytic activity for the photocatalytic esterification of palmitic acid under solar irradiation, and the material is recyclable until the 10th cycle. | [82] |
3.3. Mesoporous Silica-Immobilized Lipase
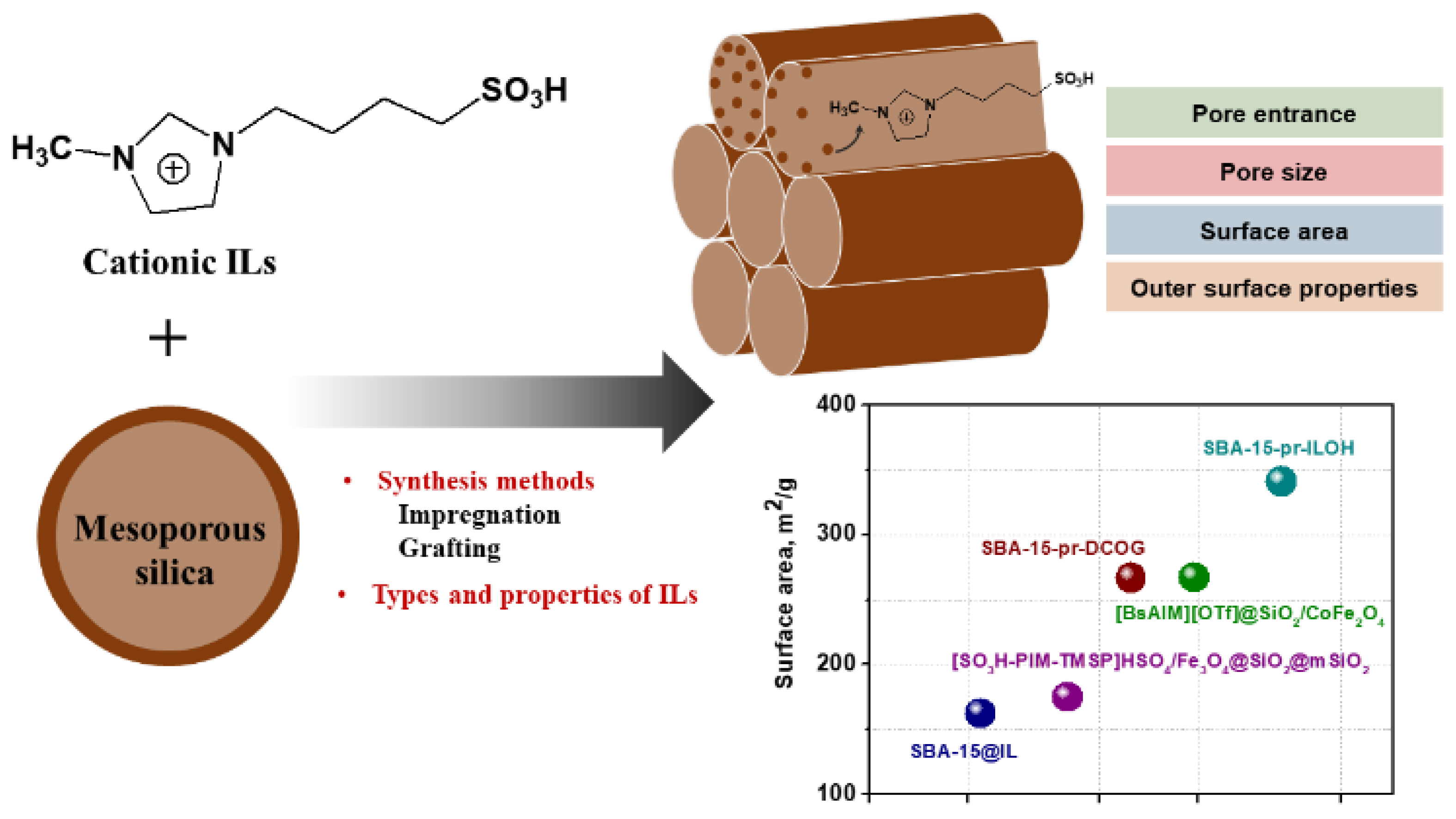
3.4. Mesoporous Silica-Supported Ionic Liquids
4. Environmental Impact and Circular Economy Analysis
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millward-Hopkins, J.; Zwirner, O.; Purnell, P.; Velis, C.A.; Iacovidou, E.; Brown, A. Resource Recovery and Low Carbon Transitions: The Hidden Impacts of Substituting Cement with Imported ‘Waste’ Materials from Coal and Steel Production. Glob. Environ. Chang. 2018, 53, 146–156. [Google Scholar] [CrossRef]
- Xu, H.; Ou, L.; Li, Y.; Hawkins, T.R.; Wang, M. Life Cycle Greenhouse Gas Emissions of Biodiesel and Renewable Diesel Production in the United States. Environ. Sci. Technol 2022, 56, 7512–7521. [Google Scholar] [CrossRef] [PubMed]
- Chanthon, N.; Ngaosuwan, K.; Kiatkittipong, W.; Wongsawaeng, D.; Appamana, W.; Assabumrungrat, S. A Review of Catalyst and Multifunctional Reactor Development for Sustainable Biodiesel Production. ScienceAsia 2021, 47, 531–541. [Google Scholar] [CrossRef]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Biodiesel Feasibility Study: An Evaluation of Material Compatibility; Performance; Emission and Engine Durability. Renew. Sustain. Energy Rev. 2011, 15, 1314–1324. [Google Scholar] [CrossRef]
- Jothiramalingam, R.; Wang, M.K. Review of Recent Developments in Solid Acid, Base, and Enzyme Catalysts (Heterogeneous) for Biodiesel Production via Transesterification. Ind. Eng. Chem. Res. 2009, 48, 6162–6172. [Google Scholar] [CrossRef]
- Parangi, T.; Mishra, M.K. Solid Acid Catalysts for Biodiesel Production. Comments Inorg. Chem. 2020, 40, 176–216. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-Catalyzed Process for Biodiesel Production: Enzyme Immobilization, Process Simulation and Optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Wang, C.; Hong, H.; Lin, Z.; Yuan, Y.; Liu, C.; Ma, X.; Cao, X. Correction: Tethering Silver Ions on Amino-Functionalized Mesoporous Silica for Enhanced and Sustained Antibacterial Properties. RSC Adv. 2016, 6, 8329. [Google Scholar] [CrossRef]
- Essamlali, Y.; Amadine, O.; Larzek, M.; Len, C.; Zahouily, M. Sodium Modified Hydroxyapatite: Highly Efficient and Stable Solid-Base Catalyst for Biodiesel Production. Energy Convers. Manag. 2017, 149, 355–367. [Google Scholar] [CrossRef]
- Zhang, M.; Jun, S.-H.; Wee, Y.; Kim, H.S.; Hwang, E.T.; Shi, J.; Hwang, S.Y.; Lee, J.; Kim, J. Activation of Crosslinked Lipases in Mesoporous Silica via Lid Opening for Recyclable Biodiesel Production. Int. J. Biol. Macromol. 2022, 222, 2368–2374. [Google Scholar] [CrossRef]
- Al-Jammal, N.; Al-Hamamre, Z.; Alnaief, M. Manufacturing of Zeolite Based Catalyst from Zeolite Tuft for Biodiesel Production from Waste Sunflower Oil. Renew. Energy 2016, 93, 449–459. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Haj, L.; Al-Muhtaseb, A.H.; Al-Hinai, M.A.; Baawain, M.; Rashid, U.; Ahmad, M.N.M. Current Scenario of Catalysts for Biodiesel Production: A Critical Review. Rev. Chem. Eng. 2018, 34, 267–297. [Google Scholar] [CrossRef]
- Li, L.; Cani, D.; Pescarmona, P.P. Metal-Containing TUD-1 Mesoporous Silicates as Versatile Solid Acid Catalysts for the Conversion of Bio-Based Compounds into Valuable Chemicals. Inorg. Chim. Acta 2015, 431, 289–296. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, T.; Meena, K.R.; Kanwar, S.S. Physical Adsorption of Lipase onto Mesoporous Silica. Int. J. Curr. Adv. Res. 2017, 6, 3837–3841. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, G.; Tang, Z.; Zhang, H. Preparation and Application of Ordered Mesoporous Metal Oxide Catalytic Materials. Catal. Surv. Asia 2020, 24, 38–58. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Yang, C.; Lu, J.; Zheng, L.; Liang, H.-P. Mesoporous Carbon Promoting the Efficiency and Stability of Single Atomic Electrocatalysts for Oxygen Reduction Reaction. Carbon 2022, 191, 393–402. [Google Scholar] [CrossRef]
- Xu, W.; Thapa, K.B.; Ju, Q.; Fang, Z.; Huang, W. Heterogeneous Catalysts Based on Mesoporous Metal–Organic Frameworks. Coord. Chem. Rev. 2018, 373, 199–232. [Google Scholar] [CrossRef]
- Gabriel, R.; De Carvalho, S.H.; da Silva Duarte, J.L.; Oliveira, L.M.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; Soletti, J.I.; Meili, L. Mixed Metal Oxides Derived from Layered Double Hydroxide as Catalysts for Biodiesel Production. Appl. Catal. A Gen. 2022, 630, 118470. [Google Scholar] [CrossRef]
- Sha, X.; Dai, Y.; Song, X.; Liu, S.; Zhang, S.; Li, J. The Opportunities and Challenges of Silica Nanomaterial for Atherosclerosis. Int. J. Nanomed. 2021, 16, 701–714. [Google Scholar] [CrossRef]
- Cheah, W.K.; Sim, Y.L.; Yeoh, F.Y. Amine-Functionalized Mesoporous Silica for Urea Adsorption. Mater. Chem. Phys. 2016, 175, 151–157. [Google Scholar] [CrossRef]
- Telalović, S.; Ramanathan, A.; Mul, G.; Hanefeld, U. TUD-1: Synthesis and Application of a Versatile Catalyst, Carrier, Material. J. Mater. Chem. 2010, 20, 642–658. [Google Scholar] [CrossRef] [PubMed]
- Van Der Voort, P.; Mathieu, M.; Mees, F.; Vansant, E.F. Synthesis of High-Quality MCM-48 and MCM-41 by Means of the GEMINI Surfactant Method. J. Phys. Chem. B 1998, 102, 8847–8851. [Google Scholar] [CrossRef]
- Wang, S.; Wu, D.; Sun, Y.; Zhong, B. The Synthesis of MCM-48 with High Yields. Mater. Res. Bull. 2001, 36, 1717–1720. [Google Scholar] [CrossRef]
- Galarneau, A.; Mureseanu, M.; Atger, S.; Renard, G.; Fajula, F. Immobilization of Lipase on Silicas. Relevance of Textural and Interfacial Properties on Activity and Selectivity. New J. Chem. 2006, 30, 562–571. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, S.K.; Kim, D.Y. Synthesis and Characterization of Mesoporous SiO2 Nanoparticles Synthesized from Biogenic Rice Husk Ash for Optoelectronic Applications. Int. J. Eng. Sci. 2016, 17, 353–358. [Google Scholar]
- Viscardi, R.; Barbarossa, V.; Maggi, R.; Pancrazzi, F. Effect of Acidic MCM-41 Mesoporous Silica Functionalized with Sulfonic Acid Groups Catalyst in Conversion of Methanol to Dimethyl Ether. Energy Rep. 2020, 6, 49–55. [Google Scholar] [CrossRef]
- Ravi, S.; Roshan, R.; Tharun, J.; Kathalikkattil, A.C.; Park, D.W. Sulfonic Acid Functionalized Mesoporous SBA-15 as Catalyst for Styrene Carbonate Synthesis from CO2 and Styrene Oxide at Moderate Reaction Conditions. J. CO2 Util. 2015, 10, 88–94. [Google Scholar] [CrossRef]
- Kohns, R.; Meyer, R.; Wenzel, M.; Matysik, J.; Enke, D.; Tallarek, U. In Situ Synthesis and Characterization of Sulfonic Acid Functionalized Hierarchical Silica Monoliths. J. Solgel Sci. Technol. 2020, 96, 67–82. [Google Scholar] [CrossRef]
- Luštická, I.; Vrbková, E.; Vyskočilová, E.; Paterová, I.; Červený, L. Acid Functionalized MCM-41 as a Catalyst for the Synthesis of Benzal-1,1-Diacetate. React. Kinet. Mech. Catal. 2013, 108, 205–212. [Google Scholar] [CrossRef]
- Shah, K.A.; Parikh, J.K.; Maheria, K.C. Biodiesel Synthesis from Acid Oil over Large Poresulfonic Acid-Modified Mesostructured SBA-15:Process Optimization and Reaction Kinetics. Catal. Today 2014, 237, 29–37. [Google Scholar] [CrossRef]
- Sherry, L.; Sullivan, J.A. The Reactivity of Mesoporous Silica Modified with Acidic Sites in the Production of Biodiesel. Catal. Today 2011, 175, 471–476. [Google Scholar] [CrossRef]
- Musterman, M.; Placeholder, P. Study About Naphthoquinone Schiff What Is So Different About Was Ist so Anders Am Neuroenhancement ? Open Chem. 2018, 1, 2–7. [Google Scholar]
- Zuo, D.; Lane, J.; Culy, D.; Schultz, M.; Pullar, A.; Waxman, M. Sulfonic Acid Functionalized Mesoporous SBA-15 Catalysts for Biodiesel Production. Appl Catal B 2013, 129, 342–350. [Google Scholar] [CrossRef]
- Melero, J.A.; Bautista, L.F.; Morales, G.; Iglesias, J.; Sánchez-vázquez, R. Biodiesel Production from Crude Palm Oil Using Sulfonic Acid-Modified Mesostructured Catalysts. Chem. Eng. J. 2010, 161, 323–331. [Google Scholar] [CrossRef]
- Mostafa Marzouk, N.; Abo El Naga, A.O.; Younis, S.A.; Shaban, S.A.; El Torgoman, A.M.; El Kady, F.Y. Process Optimization of Biodiesel Production via Esterification of Oleic Acid Using Sulfonated Hierarchical Mesoporous ZSM-5 as an Efficient Heterogeneous Catalyst. J. Env. Chem. Eng. 2021, 9, 105035. [Google Scholar] [CrossRef]
- Alrouh, F.; Karam, A.; Alshaghel, A.; El-Kadri, S. Direct Esterification of Olive-Pomace Oil Using Mesoporous Silica Supported Sulfonic Acids. Arab. J. Chem. 2017, 10, S281–S286. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Tan, M.; Jiang, B.; Zheng, J.; Tsubaki, N.; Wu, M. Monodispersed Hollow SO3H-Functionalized Carbon/Silica as Efficient Solid Acid Catalyst for Esterification of Oleic Acid. ACS Appl. Mater. Interfaces 2015, 7, 26767–26775. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, M.; Tsunoji, N.; Bandyopadhyay, R. Comparison of Sulfonic Acid Loaded Mesoporous Silica in Transesterification of Triacetin. React. Kinet. Mech. Catal. 2019, 126, 167–179. [Google Scholar] [CrossRef]
- Uchoa, A.F.; do Valle, C.P.; Moreira, D.R.; Bañobre-López, M.; Gallo, J.; Dias, F.S.; Anderson, M.W.; Ricardo, N.M.P.S. Synthesis of a KIT-6 Mesoporous Sulfonic Acid Catalyst to Produce Biodiesel from Cashew Nut Oil. Braz. J. Chem. Eng. 2022, 39, 1001–1011. [Google Scholar] [CrossRef]
- Jackson, M.A.; Mbaraka, I.K.; Shanks, B.H. Esterification of Oleic Acid in Supercritical Carbon Dioxide Catalyzed by Functionalized Mesoporous Silica and an Immobilized Lipase. Appl. Catal. A Gen. 2006, 310, 48–53. [Google Scholar] [CrossRef]
- Jaroszewska, K.; Nowicki, J.; Nosal-kovalenko, H.; Grzechowiak, J.; Lewandowski, M.; Kaczmarczyk, J. Selected Acid and Basic Functionalized Ordered Mesoporous Materials as Solid Catalysts for Transesterification of Canola Oil: A Comparative Study. Fuel 2022, 325, 124902. [Google Scholar] [CrossRef]
- Ghoreishi, K.B.; Asim, N.; Yarmo, M.A.; Samsudin, M.W. Mesoporous Phosphated and Sulphated Silica as Solid Acid Catalysts for Glycerol Acetylation. Chem. Pap. 2014, 68, 1194–1204. [Google Scholar] [CrossRef]
- Pirez, C.; Caderon, J.; Dacquin, J.; Lee, A.F.; Wilson, K. Tunable KIT-6 Mesoporous Sulfonic Acid Catalysts for Fatty Acid Esteri Fi Cation. ACS Catal. 2012, 2, 1607–1614. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Patel, A. Biodiesel Production by Esterification of Free Fatty Acids over 12-Tungstophosphoric Acid Anchored to MCM-41. Ind. Eng. Chem. Res. 2011, 50, 6620–6628. [Google Scholar] [CrossRef]
- Sakthivel, A.; Komura, K.; Sugi, Y. MCM-48 Supported Tungstophosphoric Acid: An Efficient Catalyst for the Esterification of Long-Chain Fatty Acids and Alcohols in Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2008, 48, 2538–2544. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Zirconium Doped MCM-41 Supported WO3 Solid Acid Catalysts for the Esterification of Oleic Acid with Methanol. Appl. Catal. A Gen. 2010, 379, 61–68. [Google Scholar] [CrossRef]
- Esmi, F.; Masoumi, S.; Dalai, A.K. Comparative Catalytic Performance Study of 12-Tungstophosphoric Heteropoly Acid Supported on Mesoporous Supports for Biodiesel Production from Unrefined. Catalyst 2022, 12, 658. [Google Scholar] [CrossRef]
- Singh, S.; Patel, A. 12-Tungstophosphoric Acid Supported on Mesoporous Molecular Material: Synthesis, Characterization and Performance in Biodiesel Production. J. Clean Prod. 2014, 72, 46–56. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Y.; Duan, J.; Chen, X.; Piao, M.; Hu, J.; Shi, F. One-Pot Synthesis of Propyl-Sulfonic Phosphotungstic Dual-Acid Functionalized Mesoporous Silica in Non-Hydrochloric Acid Solution: Reusable Catalyst for Efficient Biodiesel Production. ChemistrySelect 2020, 5, 14666–14669. [Google Scholar] [CrossRef]
- Pandurangan, P.S.A. Heteropolyacid (H3PW12O40)—Impregnated Mesoporous KIT-6 Catalyst for Green Synthesis of Bio-Diesel Using Transesterification of Non-Edible Neem Oil. Mater. Renew. Sustain. Energy 2019, 8, 22. [Google Scholar] [CrossRef]
- Meloni, D.; Monaci, R.; Zedde, Z.; Cutrufello, M.G.; Fiorilli, S.; Ferino, I. Transesterification of Soybean Oil on Guanidine Base-Functionalized SBA-15 Catalysts. Appl. Catal. B 2011, 102, 505–514. [Google Scholar] [CrossRef]
- de Lima, A.L.; Mbengue, A.; San, R.A.S.; Ronconi, C.M.; Mota, C.J.A. Synthesis of Amine-Functionalized Mesoporous Silica Basic Catalysts for Biodiesel Production. Catal. Today 2014, 226, 210–216. [Google Scholar] [CrossRef]
- Guerrero, V.V.; Shantz, D.F. Amine-Functionalized Ordered Mesoporous Silica Transesterification Catalysts. Ind. Eng. Chem. 2009, 48, 10375–10380. [Google Scholar] [CrossRef]
- Ngo, H.L.; Zafiropoulos, N.A.; Foglia, T.A.; Samulski, E.T.; Lin, W. Mesoporous Silica-Supported Diarylammonium Catalysts for Esterification of Free Fatty Acids in Greases. J. Am. Oil Chem. Soc. 2010, 87, 445–452. [Google Scholar] [CrossRef]
- Moradi, G.; Mohadesi, M.; Hojabri, Z. Biodiesel Production by CaO/SiO2 Catalyst Synthesized by the Sol–Gel Process. React. Kinet. Mech. Catal. 2014, 113, 169–186. [Google Scholar] [CrossRef]
- Elimbinzi, E.; Nyandoro, S.S.; Mubofu, E.B.; Osatiashtiani, A.; Jinesh, C. Synthesis of Amine Functionalized Mesoporous Silicas Templated by Castor Oil for Transesterification. MRS Adv. 2018, 3, 2261–2269. [Google Scholar] [CrossRef]
- Chermahini, A.N.; Azadi, M.; Tafakori, E.; Teimouri, A.; Sabzalian, M. Amino-functionalized mesoporous silica as solid base catalyst for regioselective aza-Michael reaction of aryl tetrazoles. J.Porous Mater. 2016, 23, 441–451. [Google Scholar] [CrossRef]
- Xie, W.; Hu, L. Mesoporous SBA-15 Silica-Supported Diisopropylguanidine: An Efficient Solid Catalyst for Interesterification of Soybean Oil with Methyl Octanoate or Methyl Decanoate. J. Oleo Sci. 2016, 65, 803–813. [Google Scholar] [CrossRef]
- Xie, W.; Yang, X.; Fan, M. Novel Solid Base Catalyst for Biodiesel Production: Mesoporous SBA-15 Silica Immobilized with 1, 3-Dicyclohexyl-2-Octylguanidine. Renew. Energy 2015, 80, 230–237. [Google Scholar] [CrossRef]
- Mary Anjalin; Kanagathara, N.; Baby Suganthi, A.R. A Brief Review on Aniline and Its Derivatives. Mater. Today Proc. 2020, 33, 4751–4755. [Google Scholar] [CrossRef]
- Santos, E.C.S.; dos Santos, T.C.; Guimarães, R.B.; Ishida, L.; Freitas, R.S.; Ronconi, C.M. Guanidine-Functionalized Fe3O4 Magnetic Nanoparticles as Basic Recyclable Catalysts for Biodiesel Production. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef]
- Abdullahi, M.; Panneerselvam, P.; Imam, S.S.; Ahmad, L.S. Removal of Free Fatty Acids in Neem Oil Using Diphenylamine Functionalized Magnetic Mesoporous Silica SBA-15 for Biodiesel Production. J. Pet. Technol. Altern. Fuels 2016, 7, 31–37. [Google Scholar] [CrossRef]
- Albuquerque, C.G.; Jime, I.; Me, J.M.; Jime, A.; Maireles-torres, P. CaO Supported on Mesoporous Silicas as Basic Catalysts for Transesterification Reactions. Appl. Catal. A Gen. 2008, 334, 35–43. [Google Scholar] [CrossRef]
- Méndez, J.C.; Arellano, U.; Solis, S.; Asomo, M.; Lara, H.; Padilla, A.J.; Wang, J.A. Synthesis of Hybrid Materials, Immobilization of Lipase in SBA-15 Modified with CaO. J. Appl. Res. Technol. 2018, 16, 498–510. [Google Scholar] [CrossRef]
- Kolo, L.; Firdaus; Taba, P.; Zakir, M.; Soekamto, N.H. Selectivity of the New Catalyst ZnO-MCM-48-CaO in Esterification of Calophyllum Inophyllum Oil. Automot. Exp. 2022, 5, 217–229. [Google Scholar] [CrossRef]
- Li, E.; Rudolph, V. Transesterification of Vegetable Oil to Biodiesel over MgO-Functionalized Mesoporous Catalysts. Energy Fuels 2008, 22, 145–149. [Google Scholar] [CrossRef]
- Qu, S.; Chen, C.; Guo, M.; Lu, J.; Yi, W.; Ding, J.; Miao, Z. Synthesis of MgO/ZSM-5 Catalyst and Optimization of Process Parameters for Clean Production of Biodiesel from Spirulina Platensis. J. Clean Prod. 2020, 276, 123382. [Google Scholar] [CrossRef]
- Sani, Y.M.; Alaba, P.A.; Raji-Yahya, A.O.; Abdul Aziz, A.R.; Daud, W.M.A.W. Facile Synthesis of Sulfated Mesoporous Zr/ZSM-5 with Improved Brønsted Acidity and Superior Activity over SZr/Ag, SZr/Ti, and SZr/W in Transforming UFO into Biodiesel. J. Taiwan Inst. Chem. Eng. 2016, 60, 247–257. [Google Scholar] [CrossRef]
- Peruzzolo, T.M.; Stival, J.F.; Baika, L.M.; Ramos, L.P.; Grassi, M.T.; Rocco, M.L.M.; Nakagaki, S. Efficient Esterification Reaction of Palmitic Acid Catalyzed by WO3-x/Mesoporous Silica. Biofuels 2022, 13, 383–393. [Google Scholar] [CrossRef]
- Vardast, N.; Haghighi, M.; Dehghani, S. Sono-Dispersion of Calcium over Al-MCM-41Used as a Nanocatalyst for Biodiesel Production from Sunflower Oil: Influence of Ultrasound. Renew. Energy 2018, 132, 979–988. [Google Scholar] [CrossRef]
- Sahel, F.; Sebih, F.; Bellahouel, S.; Bengueddach, A.; Hamacha, R. Synthesis and Characterization of Highly Ordered from Bentonite as Efficient Catalysts for the Production. Res. Chem. Intermed. 2020, 46, 133–148. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Zhang, L.; Yang, Q.; Wei, Z.; Feng, Z.; Li, C. Direct Synthesis of Al—SBA-15 Mesoporous Materials via Hydrolysis-Controlled Approach. J. Phys. Chem. B 2004, 108, 9739–9744. [Google Scholar] [CrossRef]
- Fedyna, M.; Jaroszewska, K.; Leszek, K.; Trawczy, J. Procedure for the Synthesis of AlSBA-15 with High Aluminium Content: Characterization and Catalytic Activity. Microporous Mesoporous Mater. 2020, 292, 109701. [Google Scholar] [CrossRef]
- Kumar, P.; Mal, N.K.; Oumi, Y.; Sano, T.; Yamana, K. Synthesis and Characterization of Al-MCM-48 Type Materials Using Coal Fly Ash. Stud. Surf. Catal. 2002, 142, 1229–1236. [Google Scholar]
- Juwono, H.; Wahyuni, E.T.; Ulfin, I.; Kurniawan, F. Production of Biodiesel from Seed Oil of Nyamplung (Calophyllum Inophyllum) by Al-MCM-41 and Its Performance in Diesel Engine. Indones. J. Chem. 2017, 17, 316–321. [Google Scholar] [CrossRef]
- Dehghani, S.; Haghighi, M. Structural/Texture Evolution of CaO/MCM—41 Nanocatalyst by Doping Various Amounts of Cerium for Active and Stable Catalyst: Biodiesel Production from Waste Vegetable Cooking Oil. Int. J. Energy Res. 2019, 43, 3779–3793. [Google Scholar] [CrossRef]
- Guliani, D.; Sobti, A.; Toor, A.P. Titania Impregnated Mesoporous MCM-48 as a Solid Photo-Catalyst for the Synthesis of Methyl Palmitate: Reaction Mechanism and Kinetics. Renew. Energy 2022, 191, 405–417. [Google Scholar] [CrossRef]
- Dehghani, S.; Haghighi, M. Sono-Sulfated Zirconia Nanocatalyst Supported on MCM-41 for Biodiesel Production from Sunflower Oil: Influence of Ultrasound Irradiation Power on Catalytic Properties and Performance. Ultrason. Sonochemistry 2017, 35, 142–151. [Google Scholar] [CrossRef]
- Mathimani, T.; Rene, E.R.; Sindhu, R.; Al-Ansari, M.M.; Al-Humaid, L.A.; Jhanani, G.K.; Chi, N.T.L.; Shanmuganathan, R. Biodiesel Production and Engine Performance Study Using One-Pot Synthesised ZnO/MCM-41. Fuel 2023, 336, 126830. [Google Scholar] [CrossRef]
- Barros, S.D.T.; Coelho, A.V.; Lachter, E.R.; San, R.A.S.; Dahmouche, K.; Isabel, M.; Souza, A.L.F. Esteri Fi Cation of Lauric Acid with Butanol over Mesoporous Materials. Renew. Energy 2013, 50, 585–589. [Google Scholar] [CrossRef]
- Kazemian, H.; Turowec, B.; Siddiquee, M.N.; Rohani, S. Biodiesel Production Using Cesium Modified Mesoporous Ordered Silica as Heterogeneous Base Catalyst. Fuel 2013, 103, 719–724. [Google Scholar] [CrossRef]
- Corro, G.; Sánchez, N.; Pal, U.; Cebada, S.; Fierro, J.L.G. Solar-Irradiation Driven Biodiesel Production Using Cr/SiO2 Photocatalyst Exploiting Cooperative Interaction between Cr6+ and Cr3+ Moieties; Elsevier: Amsterdam, The Netherlands, 2017; Volume 203, ISBN 2295500729. [Google Scholar]
- Carlucci, C.; Degennaro, L.; Luisi, R. Titanium Dioxide as a Catalyst in Biodiesel Production. Catalysts 2019, 9, 75. [Google Scholar] [CrossRef]
- Huang, J.; Jian, Y.; Zhu, P.; Abdelaziz, O.; Li, H. Research Progress on the Photo-Driven Catalytic Production of Biodiesel. Front. Chem. 2022, 10, 904251. [Google Scholar] [CrossRef] [PubMed]
- Welter, R.A.; Santana, H.; de la Torre, L.G.; Robertson, M.; Taranto, O.P.; Oelgemöller, M. Methyl Oleate Synthesis by TiO2 Photocatalytic Esterification of Oleic Acid: Optimisation by Response Surface Quadratic Methodology, Reaction Kinetics and Thermodynamics. ChemPhotoChem 2022, 6, e202200007. [Google Scholar] [CrossRef]
- Bhuyan, M.S.U.S.; Alam, A.H.M.A.; Chu, Y.; Seo, Y.C. Biodiesel Production Potential from Littered Edible Oil Fraction Using Directly Synthesized S-TiO2/MCM-41 Catalyst in Esterification Process via Non-Catalytic Subcritical Hydrolysis. Energies 2017, 10, 1290. [Google Scholar] [CrossRef]
- Trisunaryanti, W.; Larasati, S.; Triyono, T.; Santoso, N.R.; Paramesti, C. Selective Production of Green Hydrocarbons from the Hydrotreatment of Waste Coconut Oil over Ni- And NiMo-Supported on Amine-Functionalized Mesoporous Silica. Bull. Chem. React. Eng. Catal. 2020, 15, 415–431. [Google Scholar] [CrossRef]
- Suryajaya, S.K.; Mulyono, Y.R.; Santoso, S.P.; Yuliana, M.; Kurniawan, A.; Ayucitra, A.; Sun, Y.; Hartono, S.B.; Soetaredjo, F.E.; Ismadji, S. Iron (II) Impregnated Double-Shelled Hollow Mesoporous Silica as Acid-Base Bifunctional Catalyst for the Conversion of Low-Quality Oil to Methyl Esters. Renew. Energy 2021, 169, 1166–1174. [Google Scholar] [CrossRef]
- Bhan, C.; Singh, J. Role of Microbial Lipases in Transesterification Process for Biodiesel Production. Environ. Sustain. 2020, 3, 257–266. [Google Scholar] [CrossRef]
- Nikolić, M.P.; Srdić, V.V.; Antov, M.G. Immobilization of Lipase into Mesoporous Silica Particles by Physical Adsorption. Biocatal. Biotransform. 2009, 27, 254–262. [Google Scholar] [CrossRef]
- Katiyar, M.; Ali, A. Immobilization of Candida Rugosa Lipase on MCM-41 for the Transesterification of Cotton Seed Oil. J. Oleo Sci. 2012, 61, 469–475. [Google Scholar] [CrossRef]
- Ali, Z.; Tian, L.; Zhao, P.; Zhang, B.; Ali, N.; Khan, M.; Zhang, Q. Immobilization of Lipase on Mesoporous Silica Nanoparticles with Hierarchical Fibrous Pore. J. Mol. Catal. B Enzym. 2016, 134, 129–135. [Google Scholar] [CrossRef]
- Thangaraj, B.; Jia, Z.; Dai, L.; Liu, D.; Du, W. Effect of Silica Coating on Fe3O4 Magnetic Nanoparticles for Lipase Immobilization and Their Application for Biodiesel Production. Arab. J. Chem. 2019, 12, 4694–4706. [Google Scholar] [CrossRef]
- Tran, D.T.; Chen, C.L.; Chang, J.S. Immobilization of Burkholderia Sp. Lipase on a Ferric Silica Nanocomposite for Biodiesel Production. J. Biotechnol. 2012, 158, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Esmi, F.; Nematian, T.; Salehi, Z.; Khodadadi, A.A.; Dalai, A.K. Amine and Aldehyde Functionalized Mesoporous Silica on Magnetic Nanoparticles for Enhanced Lipase Immobilization, Biodiesel Production, and Facile Separation. Fuel 2021, 291, 120126. [Google Scholar] [CrossRef]
- Shinde, S.S.; Chi, H.M.; Lee, B.S.; Chi, D.Y. Tert-Alcohol-Functionalized Imidazolium Ionic Liquid: Catalyst for Mild Nucleophilic Substitution Reactions at Room Temperature. Tetrahedron Lett. 2009, 50, 6654–6657. [Google Scholar] [CrossRef]
- Bender, J.; Jepkens, D.; Hüsken, H. Ionic Liquids as Phase-Transfer Catalysts: Etherification Reaction of 1-Octanol with 1-Chlorobutane. Org. Process Res. Dev. 2010, 14, 716–721. [Google Scholar] [CrossRef]
- Santiago, C.C.; Lafuente, L.; Bravo, R.; Díaz, G.; Ponzinibbio, A. Ionic Liquids as Phase Transfer Catalysts: Enhancing the Biphasic Extractive Epoxidation Reaction for the Selective Synthesis of β-O-Glycosides. Tetrahedron Lett. 2017, 58, 3739–3742. [Google Scholar] [CrossRef]
- Szepiński, E.; Smolarek, P.; Milewska, M.J.; Łuczak, J. Application of Surface Active Amino Acid Ionic Liquids as Phase-Transfer Catalyst. J. Mol. Liq. 2020, 303, 112607. [Google Scholar] [CrossRef]
- Yuan, C.; Huang, Z.; Chen, J. Basic Ionic Liquid Supported on Mesoporous SBA-15: An Efficient Heterogeneous Catalyst for Epoxidation of Olefins with H 2O 2 as Oxidant. Catal. Commun. 2012, 24, 56–60. [Google Scholar] [CrossRef]
- Karimi, B.; Vafaeezadeh, M. SBA-15-Functionalized Sulfonic Acid Confined Acidic Ionic Liquid: A Powerful and Water-Tolerant Catalyst for Solvent-Free Esterifications. Chem. Commun. 2012, 48, 3327–3329. [Google Scholar] [CrossRef]
- Yao, J.; Sheng, M.; Bai, S.; Su, H.; Shang, H.; Deng, H.; Sun, J. Ionic Liquids Grafted Mesoporous Silica for Chemical Fixation of CO2 to Cyclic Carbonate: Morphology Effect. Catal. Lett. 2022, 152, 781–790. [Google Scholar] [CrossRef]
- Xu, Q.-Q.; Yin, J.-Z.; Zhou, X.-L.; Yin, G.-Z.; Liu, Y.-F.; Cai, P.; Wang, A.-Q. Impregnation of Ionic Liquids in Mesoporous Silica Using Supercritical Carbon Dioxide and Co-Solvent. RSC Adv. 2016, 6, 101079–101086. [Google Scholar] [CrossRef]
- Gholami, A.; Pourfayaz, F.; Maleki, A. Recent Advances of Biodiesel Production Using Ionic Liquids Supported on Nanoporous Materials as Catalysts: A Review. Front. Energy Res. 2020, 8, 144. [Google Scholar] [CrossRef]
- Watanabe, Y.; Amitani, N.; Yokoyama, T.; Ueda, A.; Kusakabe, M.; Unami, S.; Odashima, Y. Synthesis of Mesoporous Silica from Geothermal Water. Sci. Rep. 2021, 11, 23811. [Google Scholar] [CrossRef]
- El-Nahas, S.; Osman, A.I.; Arafat, A.S.; Al-Muhtaseb, A.H.; Salman, H.M. Facile and Affordable Synthetic Route of Nano Powder Zeolite and Its Application in Fast Softening of Water Hardness. J. Water Process Eng. 2020, 33, 101104. [Google Scholar] [CrossRef]
- Aneu, A.; Pratika, R.A.; Hasanudin; Gea, S.; Wijaya, K.; Oh, W.-C. Silica-Based Catalysts for Biodiesel Production: A Brief Review. Silicon 2023. [Google Scholar] [CrossRef]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of Crude Glycerol to Value-Added Products: Perspectives of Process Technology, Economics and Environmental Issues. Biotechnol. Rep. 2020, 27, e00487. [Google Scholar] [CrossRef]
- Osman, A.I.; Qasim, U.; Jamil, F.; Al-Muhtaseb, A.H.; Jrai, A.A.; Al-Riyami, M.; Al-Maawali, S.; Al-Haj, L.; Al-Hinai, A.; Al-Abri, M.; et al. Bioethanol and Biodiesel: Bibliometric Mapping, Policies and Future Needs. Renew. Sustain. Energy Rev. 2021, 152, 111677. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, H.; Shi, Y.; Yuan, S. Low Cost Synthesis of Mesoporous Molecular Sieve MCM-41 from Wheat Straw Ash Using CTAB as Surfactant. Mater. Res. Bull. 2016, 77, 258–264. [Google Scholar] [CrossRef]
- Rangaraj, S.; Venkatachalam, R. A Lucrative Chemical Processing of Bamboo Leaf Biomass to Synthesize Biocompatible Amorphous Silica Nanoparticles of Biomedical Importance. Appl. Nanosci. 2017, 7, 145–153. [Google Scholar] [CrossRef]
- Fatimah, I.; Taushiyah, A.; Najah, F.B.; Azmi, U. ZrO2/Bamboo Leaves Ash (BLA) Catalyst in Biodiesel Conversion of Rice Bran Oil. In Proceedings of the IOP Conference Series: Materials Science and Engineering, the 12th Joint Conference on Chemistry, Semarang, Indonesia, 19–20 September 2017; Volume 349. [Google Scholar]
- Tadjarodi, A.; Haghverdi, M.; Mohammadi, V. Preparation and Characterization of Nano-Porous Silica Aerogel from Rice Husk Ash by Drying at Atmospheric Pressure. Mater. Res. Bull. 2012, 47, 2584–2589. [Google Scholar] [CrossRef]
- Fatimah, I.; Purwiandono, G.; Sahroni, I.; Sagadevan, S.; Chun-Oh, W.; Ghazali, S.A.I.S.M.; Doong, R. Recyclable Catalyst of ZnO/SiO2 Prepared from Salacca Leaves Ash for Sustainable Biodiesel Conversion. S. Afr. J. Chem. Eng. 2022, 40, 134–143. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatimah, I.; Fadillah, G.; Sagadevan, S.; Oh, W.-C.; Ameta, K.L. Mesoporous Silica-Based Catalysts for Biodiesel Production: A Review. ChemEngineering 2023, 7, 56. https://doi.org/10.3390/chemengineering7030056
Fatimah I, Fadillah G, Sagadevan S, Oh W-C, Ameta KL. Mesoporous Silica-Based Catalysts for Biodiesel Production: A Review. ChemEngineering. 2023; 7(3):56. https://doi.org/10.3390/chemengineering7030056
Chicago/Turabian StyleFatimah, Is, Ganjar Fadillah, Suresh Sagadevan, Won-Chun Oh, and Keshav Lalit Ameta. 2023. "Mesoporous Silica-Based Catalysts for Biodiesel Production: A Review" ChemEngineering 7, no. 3: 56. https://doi.org/10.3390/chemengineering7030056
APA StyleFatimah, I., Fadillah, G., Sagadevan, S., Oh, W.-C., & Ameta, K. L. (2023). Mesoporous Silica-Based Catalysts for Biodiesel Production: A Review. ChemEngineering, 7(3), 56. https://doi.org/10.3390/chemengineering7030056










