Abstract
Corn cob is an abundant agricultural residue worldwide, with high potential and interesting composition, and its valorization still needs to be studied. Selectively fractionating its structural components (hemicellulose, cellulose, and lignin), value-added products can be produced, eliminating waste. In this work, integrated fractionation approaches were developed and evaluated. First, an organosolv process was optimized (ethanol:water, 50:50, w/w). Then, as a comparative method, alkaline delignification (using NaOH, 1–2%) was also studied. The organosolv process allowed a significant delignification of the material (79% delignification yield) and, at the same time, a liquid phase containing a relevant concentration (14.6 g/L) of xylooligosaccharides (XOS). The resulting solid fraction, rich in cellulose, showed an enzymatic digestibility of 90%. The alkaline process increased the delignification yield to 94%, producing a solid fraction with a cellulose enzymatic digestibility of 83%. The two later techniques were also used in a combined strategy of hydrothermal processing (autohydrolysis) followed by delignification. The first allowed the selective hydrolysis of hemicellulose to produce XOS-rich hydrolysates (26.8 g/L, 67.3 g/100 g initial xylan). The further delignification processes, alkaline or organosolv, led to global delignification yields of 76% and 93%, respectively. The solid residue, enriched in glucan (above 75% for both combined processes), also presented high enzymatic saccharification yields, 89% and 90%, respectively. The fractionation strategies proposed, and the results obtained are very promising, enabling the integrated upgrading of this material into a biorefinery framework.
1. Introduction
The current environmental and climate crisis is pushing the transition to a more sustainable economy based on low-carbon technologies, renewable energies, energy efficiency, sustainable mobility, and a circular (bio)economy [1]. The sustainability of current economic strategies and utilization patterns is changing towards a bio-circular-green-economy model [2,3]. These strategies are being adopted by both developed and developing countries. In Europe, the European Green Deal sets out how to make Europe the first climate-neutral continent by 2050, boosting the economy, improving people’s health and quality of life, caring for nature, and leaving no one behind [4]. Through the 2030 Climate Target Plan, the European Commission proposes to raise the EU’s ambition to reduce greenhouse gas emissions (GGE) to at least 55% below 1990 levels by 2030. The objective is to set a more ambitious and cost-effective path to achieving climate neutrality by 2050, stimulate the creation of green jobs, continue the EU’s track record of cutting GGE while growing its economy, encourage international partners to increase their ambition to limit the rise in global temperature to 1.5 °C and avoid the most severe consequences of climate change [5,6]. This is also pursued in other world regions, most noteworthy in the US, South America, and the Asia-Pacific Economic region as promoted by the APEC. All these objectives are fully aligned with the UN’s Sustainable Development Goals, especially those related to climate change. The bioeconomy, an economy where the basic building blocks for materials, chemicals, and energy are derived from renewable biological resources, plays a strategic role in reaching these goals.
Among the several possible lignocellulosic feedstocks, non-edible, agricultural-derived byproducts have the advantage of being abundant, renewable, low-cost, and available worldwide [7]. However, although very relevant, upgrading some of these materials is not straightforward, and several significant (technical, environmental, political, and economic) bottlenecks still exist that limit their industrial use. One of these bottlenecks, specifically on the technical side, is still the development of efficient fractionation processes [8,9] that would enable the selective separation of the main biomass components (hemicellulose, cellulose, and lignin) and recover their derivatives with high yield.
Diverse physical, chemical, physicochemical, and/or biological processes have been developed [8,9] targeting either the removal and (partial) depolymerization of hemicellulose, lignin removal, or the reduction of the cellulose crystallinity [9,10,11]. Among these, process options that use water (liquid or steam), acids, and alkaline agents are the most common/susceptible for industrial application. Their discussion can be found in detail in Gírio et al. and Galbe and Wallberg [9,10], but it is important to stress that “neutral” (aqueous) and acidic processes favor hemicellulose removal and that alkaline and organosolv processes increase lignin removal. Organosolv processes are based on utilizing aqueous mixtures of organic solvents, most noteworthy ethanol, a low-boiling point solvent, easy to recover by distillation. These processes are interesting delignification alternatives since they enable high-purity lignins, together with the partial solubilization of hemicelluloses [12,13]. In addition, cellulose-rich solids with enhanced enzymatic hydrolysis rate and yield are also produced [12] due to the increase of porosity, the surface area of the substrate, and the decrease in cellulose crystallinity, and degree of polymerization, as well as a reduction of lignin and hemicellulose contents [13]. Alkaline pretreatments are also typically very effective for lignin solubilization exhibiting only minor cellulose and slightly higher hemicellulose solubilization [8]. These pretreatments are particularly effective for low lignin content biomass such as agricultural residues. Sodium hydroxide pretreatments gained particular attention due to their potential for delignification and reducing the crystallinity of cellulose, increasing enzymatic saccharification of cellulose [13]. In addition, these processes have the advantage that they can be operated at relatively low temperatures and do not require complex reactors.
On the other hand, residence time can be longer than in other processes, and there is a need for neutralization of the pretreated slurry [14,15]. However, regardless of the method, careful optimization must be carried out for any specific raw material. Otherwise, significant economic losses may arise due to the formation of undesirable by-products and/or by provoking extensive chemical degradation of the remaining fractions [16]. Furthermore, the approaches available impose, in general, the use of unattractive and/or high energy and catalyst costs, leading to high wastewater generation and equipment corrosion, implying substantial negative impacts on process economics and environmental issues. However, other pretreatments or combinations may have a different impact on biomass recalcitrance and cellulose saccharification using cellulolytic enzymes. To obtain a selective and sequential fractionation, a combination of processes that are more selective for hemicelluloses, e.g., hydrothermal treatments and dilute-acid hydrolysis, with others that are more efficient for lignin removal, i.e., organosolv and alkali treatments, can be interesting approaches. These have already been tested for some wood and agro-industrial residues [17,18]. However, the advantages of using a single or combined process will depend not only on the type of biomass but also on the products that can be obtained.
Crop residues are adequate raw materials for biorefinery applications because of their immediate availability, low cost, and relatively concentrated location in the major grain-growing regions. The primary residues from corn (Zea mays L.) culture are stalks, leaves, and cobs that contribute to the largest quantities of agricultural residues in the USA and are abundant in China, Brazil, and Europe, presenting a very significant and growing amount in Portugal. Corn residues, i.e., corn stover, are one of the most relevant feedstocks for lignocellulosic ethanol production [7], although in some countries, as is the case of Portugal, despite the use of straw for feed purposes, or partial straw returning to the field to reduce the use of chemical fertilizers, cobs are usually not recovered and can reach as high as 180 kg of cobs per ton of grain [19,20]. This is relevant in Portuguese agriculture, as corn represents 40% of the arable crops and is the most produced crop in agricultural explorations. It is estimated to have more than 75,000 production units in 150,000 Ha [21]. Furthermore, despite a steady increase, it is expected to be a culture whose production continues to grow. However, it is still insufficient to meet increasing demand and worrying world stock reductions.
In this work, corn cobs were used as feedstock to develop a valorization strategy within the biorefinery approach. Furthermore, different pretreatments were used to obtain efficient hemicellulose removal and delignification and to produce upgradable cellulose and added-value marketable products, i.e., xylooligosaccharides from hemicellulose and lignin derivatives.
2. Materials and Methods
2.1. Raw Material
Corn cobs (P1574 variety) were kindly provided by AgroMais, Golegã (Portugal), with a moisture content of around 15%. Before preparation, the material was dried at room temperature and stored in 200 L plastic drums inside a warehouse. When required, the material was shredded (Viking GE 355, Langkampfen, Austria) and then ground using a knife mill (Fritsch GmBH, Idar-Oberstein, Germany) to pass a 6 mm sieve. Milled corn cobs were homogenized and stored at room temperature in plastic containers before use.
2.2. Autohydrolysis
Autohydrolysis treatments were carried out in a stainless-steel reactor (Parr Instruments Company, Moline, IL, USA) with a total volume of 2 L. Temperature was controlled through a Parr PID controller (model 4842). The raw material was mixed with water in the reactor to reach a liquid-to-solid ratio of 7 (g water/g dry biomass). The reactor was heated to the final temperatures of 180 °C to 220 °C. Heating profiles are shown in Figure S3 (Supplementary Material). After reaching the desired temperature, the reactor was rapidly cooled down by water passing the internal cooling coil, and by placing the reactor in an ice bath (cooling time average until 100 °C below 3 min), the liquid and solid phases were separated by pressing, and the solid phase was washed with water and then dried at 50 °C. Finally, the solid phase was milled (<0.5 mm) for further composition analysis and enzymatic digestibility determination. The hydrolyzates were vacuum filtered (Quantitative filter paper, 20–25 µm, Filter-Lab) and stored at 4 °C before analysis. After selecting the optimized conditions (195 °C), several pretreatment batches were performed to produce enough solids for organosolv and alkaline delignification studies. These pre-treated solids were thoroughly mixed into a homogenized lot before use.
2.3. Organosolv
Organosolv treatments were carried out similarly to those described above for autohydrolysis treatment with the following variations. Instead of water, an ethanol solution of 50% (w/w) was added to biomass to reach an LSR of 7 (w/w). The process was studied under isothermal conditions for 2 h in temperatures ranging from 130 °C to 210 °C. At the temperature of 190 °C, treatments were performed under non-isothermal and isothermal conditions (1–4 h). Heating profiles are shown in Figure S4 (Supplementary Material). Upon the completion of the reaction, the reactor was cooled down, and the solid and liquid fractions were separated by pressing as described for autohydrolysis. Upon pressing, each solid phase was washed with 2 L of ethanol solution 50% or 4 L (190–210 °C) assays. The hydrolyzates were vacuum filtered (Quantitative filter paper, 20–25 µm, Filter-Lab), and part of the liquid phase was concentrated twice for ethanol evaporation and stored at 4 °C until further analysis. Vacuum evaporation of ethanol was carried out in an evaporation system (Büchi, Flawil, Switzerland) that includes a vacuum pump VAC® v-500 and a vacuum controller B-721 set at 175 mbar, and a water bath set at 40 °C. The solid phase obtained was washed with ethanol solution of 50% (w/w) followed by water and then dried at 50 °C and milled as referred for autohydrolysis treatments and stored for further composition analysis and enzymatic digestibility determination as described below.
2.4. Alkaline Treatment
Alkaline treatments were performed in 500 mL Schott flasks at 120 °C in an autoclave (Uniclave, Portugal) under isothermal conditions (30, 60, or 120 min). Sodium hydroxide solutions at 1%, 1.5%, or 2% (w/v) were added to 20 g corn cobs to reach an LSR of 7 (w/w). After cooling, as described above, the samples were vacuum filtered (Quantitative filter paper, 20–25 µm, Filter-Lab). The liquid phase was collected, and the pH was adjusted to approximately 4 before analysis. The solids were washed with 1 L of distilled water, dried, and stored as described above.
2.5. Analytical Methods
2.5.1. Chemical Characterization of Solid Samples
For chemical characterization, raw material and processed solids were ground in a knife mill (IKA, Königswinter, Germany) to a particle size smaller than 0.5 mm, and the moisture content was determined by oven-drying at 100 °C to constant weight. Ash content was determined at 550 °C using NREL/TP-510-42622 protocol [22]. Extractives determination was carried out based on the successive Soxhlet extraction with dichloromethane (6 h), ethanol (18 h), and water (18 h). The samples were analyzed for glucan, xylan, arabinan, and acetyl groups after quantitative acid hydrolysis with 72% (w/w) H2SO4 followed by hydrolysis with 4% (w/w) H2SO4. The acid-insoluble residue was considered as Klason lignin after correction for ash. Monosaccharides (glucose, xylose, arabinose) and acetic acid were analyzed by HPLC (Agilent Technologies Liquid Chromatographer 1100 Series System, Santa Clara, CA, USA) equipped with a diode array detector (DAD) and a refractive index detector (RI) using an Aminex HPX-87H column (Bio-Rad, USA) in combination with a cation H+-guard column (Bio-Rad). Elution occurred at 50 °C with 5 mmol/L H2SO4 at a flow rate of 0.4 mL/min. Protein content was determined according to the AOAC protocol [23]. Samples were digested with concentrated H2SO4 and catalyst mixture (potassium sulfate, copper sulfate, titanium oxide, and stearic acid). The tubes were placed in a digestion system (Tecator, Model 1007, Höganäs, Sweden) and kept for 1.5 h at the set temperature. After cooling, water was added, and the tubes were placed in the distillation unit (Tecator, Model 1026, Höganäs, Sweden), which automatically added 50% (w/v) NaOH. The released ammonia was distilled by steam and collected using 4% (w/v) boric acid. Ammonia quantification was determined by acid-base titration using 0.1 N hydrochloric acid as titrant and Tashiro’s indicator.
2.5.2. Chemical Characterization of the Liquors
The liquors were analyzed for monomeric sugars, organic acids, and furan derivatives by HPLC, as described above. Elution occurred at 50 °C with 5 mmol/L H2SO4 at a flow rate of 0.6 mL/min. For oligosaccharides quantification, another sample was hydrolyzed with 4% (w/w) H2SO4. Oligosaccharide concentrations were calculated from the increase in sugar monomers after acid post-hydrolysis and analyzed by HPLC under the conditions described above. Total phenolic compounds were determined by the Folin–Ciocalteu colorimetric method according to [24] as described in Moniz et al. [25]. Total phenolic compounds are expressed as mg GAE mL−1 (gallic acid equivalents).
2.5.3. Enzymatic Hydrolysis
The enzymatic digestibility of the untreated and selected treated samples of corn cobs was evaluated based on the NREL/TP-510-42629 protocol [26]. The reaction mixture contained 0.15 g of dried biomass (dry weight basis), 5 mL of sodium citrate buffer (0.1 M, pH 4.8), 100 μL of sodium azide solution (2% w/v), and Celluclast® 1.5L and Novozyme 188® enzymes in necessary amounts to obtain 60 FPU/g and 64 pNPGU/g of dry biomass, respectively. The total volume was adjusted to 10 mL with water. Samples were tested, at least, in duplicate carrying out proper blank assays (without enzymes and biomass) to correct the results whenever required. Hydrolysis was carried out in an orbital shaker at 50 °C and 250 rpm for 72 h (Comecta 200D, Barcelona, Spain). After this period, the samples were immersed in a water bath at 90 °C for 5 min to inactivate the enzymes. The samples were filtered (0.22 μm syringe filters, Whatman®) and analyzed by HPLC as previously described. All reported results for enzymatic digestibility data refer to the glucan content of the specific sample under test.
2.6. Empirical Modeling
The experimental data were fitted iteratively to empirical polynomial models implemented in MSExcel© 2016. The determination of the best operational conditions maximizing both the cellulose enzymatic hydrolysis and XOS production was obtained using the Solver function, also built-in in MSExcel© 2016 based on 2nd and 3rd-order polynomial models, generically shown in Equation (1):
where y is the studied variable, are coefficients of the polynomial, and is the severity factor (Log Ro) [27] as calculated from the following equation taking into consideration the heating and cooling stages:
where T is the temperature (°C), t is time (min), and 14.75 is an empirical parameter characteristic of the process.
3. Results
3.1. Raw Material Characterization
A granulometric separation was performed after the size reduction of corn cobs to particles inferior to 6 mm into seven fractions. The fraction with particle sizes ranging from 1.6 to 2.36 mm corresponds to 37.2% of the total mass, being the predominant one. Larger fractions (>3.55 mm) represented only 1.30% of the total, while the smallest (<0.25 mm) accounted for 5.5% (Figure S1, Supplementary Material). The latest value is in agreement with corn straw granulometric characterization [28]. The different granulometric fractions were also chemically characterized for glucan, xylan, arabinan, acetyl groups, acid-insoluble lignin, and ash (Figure S2, Supplementary Material). Their chemical composition was similar, with the main difference in xylan ranging from 21.9 to 28.4%. In light of these results, the feedstock used for pretreatment comprised all fractions, and the chemical composition of the lot used in this work is shown in Table 1. Cellulose, estimated from glucan content, and hemicellulose, estimated from xylan, arabinan, and acetyl groups, present similar content. The cellulose content found is higher than reported by Tada et al. [29] but lower than reported by Barl et al. [30] for corn cobs. Lignin content is in the range of the values usually reported for this material [31,32]. The protein content (3.3%) is very similar to Kaliyan and Morey [33], as well as the ash content. The percentage of total extractives content is similar to corn straw [28].

Table 1.
Chemical composition of corn cobs.
3.2. One-Step Process toward the Delignification of Corn Cobs
3.2.1. Organosolv
The behavior of the three main macromolecular compounds, cellulose, hemicelluloses, and acid-insoluble lignin, after the ethanol organosolv treatment are shown in Table 2 (panel I). Lignin, as expected, is the compound mainly affected, with 80% being removed from the solid fraction at 190 °C-2 h assay. This delignification yield is higher than those reported by some authors for other lignocellulosic materials [34,35], even though, in contrast, no catalyst was used in the present work.

Table 2.
Yield of lignin, xylan, glucan, and their derivatives for different assays.
Besides lignin, the hemicellulosic fraction is also significantly affected by organosolv treatment. As a result, a sharp decrease in xylan content, together with the increased production of xylooligosaccharides (XOS), was observed. Xylose and furfural, in particular, as a product of degradation reactions, also increased at higher temperatures and longer reaction times. The highest XOS yield was obtained in the same operational conditions as the maximum delignification, corresponding to a production of 41 g XOS/100 g initial xylan. Glucan essentially remained in the solid phase (maximum solubilization of 10.5%, reaching a content higher than 75%). However, some glucooligosaccharides (GlcOS) production was noted, reaching a maximum of 3.72 g/100 g initial glucan (180 °C-2 h). Glucose yield never surpassed 1 g/100 g initial glucan. In addition, minor formation of HMF, due to degradation reactions, was found. This profile of glucan is typical for organosolv treatments of other lignocellulosic materials [12,36].
The composition of the liquors obtained from organosolv treatments (O) is shown in Table 3 (panel I). These liquors contain a mixture of oligomeric compounds, mainly XOS, monosaccharides (xylose, arabinose, and glucose), acetic acid, and products resulting from degradation reactions of sugars, as well as lignin-derived phenolic compounds. Sugars are mainly present in the oligomeric form, with a maximum XOS concentration of 16.23 g/L. On the contrary, sugar monomers were only present in low concentrations, never surpassing 1.51 g/L. Degradation products were present especially for severest conditions (high temperatures and longer treatments), reaching 1.32 g/L for formic acid, 0.60 g/L for HMF, and 3.19 g/L for furfural. The decrease in pH is coherent with the increasing concentration of acetic acid along the set of treatments. Total phenolic showed an increasing concentration for more severe conditions, although not corresponding to the highest delignification yield. This was found because the methods used to quantify phenolic compounds mainly detected phenolic acids, and lignin itself may be degraded at high temperatures, giving rise to more phenolic acids. In addition, the Folin–Ciocalteu method depends on the reducing power of phenolic hydroxyl groups to estimate the total phenolic compounds (TPC). This is because it accounts for all the phenols and their degraded products. This lack of specificity often results in an overestimation of TPC. As for higher reaction temperatures, hemicellulose hydrolysis also increases the fractionation of lignocellulosic biomass and releases not just phenolic materials but also reducing agents and sugars [37]. These additional compounds could also be detected by the Folin–Ciocalteu assay causing further interferences in the estimation of TPCs.

Table 3.
Composition of hydrolysates obtained from organosolv, alkaline, autohydrolysis, and combined treatments applied to corn cobs.
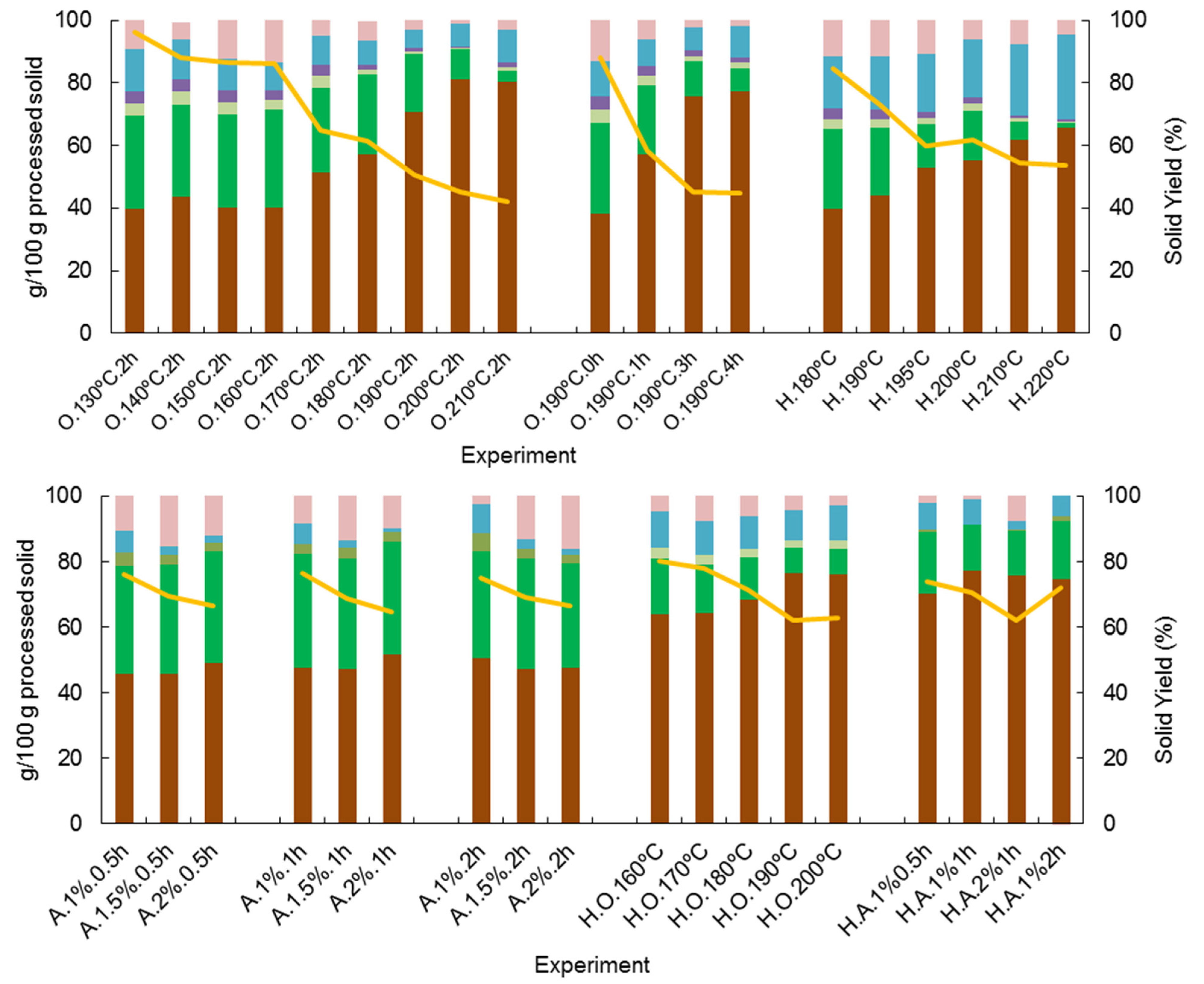
Figure 1 (panel I) shows the composition of the solid phase obtained for the different operational conditions assayed. Solid solubilization was low for milder conditions but increased along with the solubilization of hemicelluloses and lignin for higher temperatures and operation times. Glucan content showed an increasing trend both with time and temperature, which is related to its non-solubilization reaching up to 77% solubilization. In contrast, xylan and acetyl groups are significantly reduced, and arabinan completely disappears from the solid residue for harsh conditions. Lignin, the target component of organosolv treatments, decreased its content to 6.12%. However, for higher temperatures, some lignin condensation together with sugar and/or sugar degradation products may occur, giving insoluble reaction products, and thus its content increased in the solid phase [38].
Figure 1.
Effect of the operational conditions on the solid yield (SY) and polymeric composition of processed solids obtained after pretreatments of corn cob. (■ glucan; ■ xylan; ■ arabinan; ■ acetyl groups; ■ lignin; ■ ash; and ■ others (by difference)). (H.—autohydrolysis, O.—Organosolv, A.—Alkaline treatment, H.O.—autohydrolysis+ organosolv, H.A.—autohydrolysis+alkaline treatment). Deviations < 5%.
In order to try to model the delignification and XOS production during organosolv treatments, a quadratic model as a function of Log Ro (an empiric severity parameter that combines temperature and time) [27] was tested. The results obtained were as follows:
and
where y1 and y2 were the delignification yield and XOS production, respectively, and x corresponds to Log Ro.
A similar fitting was also established for xylan removal (y3) using a cubic model:
The fitting between experimental data and the adjustments obtained by the models (always R2 > 0.90) showed that these models seem to adequately represent the organosolv profile.
Under the operational conditions tested, the optimal temperature for organosolv treatment (during 2 h) can be pointed as 190 °C, resulting in a liquid phase rich in lignin and XOS and cellulose-enriched solid suitable for enzymatic hydrolysis. These results are very interesting and promising since it is rare for the same treatment to achieve such a high delignification yield together with a high production of XOS, with both the highest attained under the same operational conditions [39,40]. Fractionation in a single step has several advantages over two or more step processes, mainly due to the economy of the process (in energy, reagents, residence time, and equipment). However, it might impose higher costs associated with downstream processing for separating XOS and lignin-derived products.
3.2.2. Alkaline Treatment
The delignification yield obtained with sodium hydroxide treatment (Alkali, A) is shown in Table 2 (panel II). NaOH concentration plays a major influence on lignin behavior during the pretreatments. For example, 2% NaOH produces a delignification yield above 90% for all reaction times tested. In contrast, for 1% NaOH, the yield was just above 50%. In general, the data reported in the literature only achieved these delignification yields for higher NaOH concentrations. This is the case with rice husks [41], Cistus ladanifer [42], or even corn cobs [43]. The maximum delignification yield (94.4%) was obtained for treatment with 2% NaOH for 1 h. However, with milder conditions (1.5% NaOH and 0.5 h), a significant delignification yield (88.2%) was also observed. Compared with the previous organosolv assays, alkaline treatment, using a significantly lower temperature (120 °C vs. 190 °C), displayed better delignification results. The differences in the delignification yields achieved may be related to the mild conditions of the NaOH process, which prevents lignin condensation, resulting in high lignin solubility, particularly for low-lignin biomass [15], as is the case with corn cobs.
The liquors obtained from alkaline treatment showed residual monomeric sugar content (Table 3, panel II). In this case, the material balances for xylan also suggest a limited degradation of this polymer into XOS. In addition, lactic acid and glycerol also appear as degradation products, whereas no HMF or furfural was detected.
As for the solid composition, shown in Figure 1 (panel II), glucan was virtually unaffected by alkaline treatment, so its content increased, ranging from 45 to 51%. The hemicellulosic fraction was also slightly affected by alkali compared to organosolv, although acetyl groups were completely removed from the solid. Lignin content decreased with the increase of NaOH concentration, leading to solids with a residual content of lignin (1.25 to 2.16%).
The alkaline treatment proved to be an effective method to remove lignin from corn cobs at mild temperature conditions, while cellulose and hemicellulose were mainly retained in the solid phase. Other authors reported similar properties for the alkaline treatment of corncobs [43] and corn stover [44].
3.3. Autohydrolysis Pretreatment
The yield of xylan, arabinan, glucan, and lignin in the solid fraction, as well as the yield of oligosaccharides (XOS and GlcOS), monosaccharides (xylose, arabinose, and glucose), and furan derivatives (HMF and furfural) obtained in the liquid fraction for hydrothermal treatments (H, autohydrolysis), is shown in Table 2 (panel III).
The profile for lignin recovery in the solid phase showed relatively low solubilization of this fraction (maximum 23%, attained at 195 °C). On the other hand, hemicellulosic components were significantly affected by autohydrolysis. As a result, Xylan is almost completely hydrolyzed, and XOS production achieved a maximum of 67.3 g/100 g initial xylan (obtained at 195 °C) together with some sugar monomers, for higher temperatures, degradation reactions occurred leading to different materials, such as wheat straw, olive tree pruning, and eucalyptus residues [45].
Glucan was mainly retained in the solid phase with minor production of oligosaccharides, glucose, or HMF. The liquid phase resulting from these treatments was analyzed, and its composition is shown in Table 3 (panel III). XOS were the compounds present in higher concentrations (26.8 g/L), together with some sugar monomers. This value is quite remarkable, being higher than the reported for autohydrolysis treatments of corn straw [28], rice straw [46], or wheat straw [47], and points out corn cobs as one of the most interesting substrates for added-value XOS.
The pH of the liquors tended to decrease, mainly related to the hydrolysis of acetyl groups, leading to the increase of acetic acid concentration. The total phenolic profile did not show a defined trend, although an increase was noted.
The chemical characterization of the hydrothermally treated solids is shown in Figure 1 (panel III). The solid resulting from the severest assay mainly consists of glucan (65.7%) and lignin (27.1%), progressively increasing from the mildest to the severest assay. However, solid yield decreased from 84.5 to 53.8 due to associated hemicellulose hydrolysis. Compared to organosolv and alkali fractionation, glucan content of the pretreated solids is lower in the case of autohydrolysis-treated materials, and lignin content is much higher, which is in agreement with the typical effects of these processes, i.e., hydrothermal processes are much more effective on hemicellulose hydrolysis, whereas lignin dissolution is particularly effective with the former pretreatments.
Polynomial models for delignification yield (y1), xylan removal (y3), and XOS production (y2) during autohydrolysis were also performed, as a function of Log Ro, with the following results, respectively:
The models obtained represent the parameters studied and are also useful for determining non-assayed conditions. The results obtained for the autohydrolysis of corn cobs showed that this process mainly affected hemicellulose, producing oligosaccharides as the main products, remaining a solid residue enriched in glucan and lignin.
3.4. Two-Step Process toward the Fractionation of Hemicelluloses and Lignin
Since autohydrolysis proved to be an adequate process for XOS production and organosolv and alkaline treatments are efficient delignification processes, their combination to achieve a two-step selective fractionation of hemicelluloses and lignin was proposed. For this, a hydrothermally treated solid at 195 °C was used for combined processes.
3.4.1. Autohydrolysis Followed by Organosolv
The data for the macromolecular composition of corn cobs obtained after autohydrolysis and organosolv are shown in Table 2. In this two-stage process, the maximum delignification of the hydrothermally pretreated solid reached 65.4% (190 °C). The assays at lower temperatures exhibited lower delignification yields, although it was possible to attain 47.2% delignification at the lowest temperature (160 °C). Accounting for the global delignification yield (autohydrolysis+organosolv), a value between 42.3% (30 °C assay, data not shown) and 75.6% (190 °C) was achieved. At lower operation temperatures (more adequate for industrial applications), the combined process yields exceed those obtained for the direct process, which can be explained by the higher hydrolysis of hemicelluloses during autohydrolysis and so the disruption of the lignin-carbohydrate linkages and the macromolecular cell wall arrangements facilitating access and reactivity to the subsequent process [48]. A lignin removal of 81% was also reported for Eucalyptus globulus after a sequential treatment of autohydrolysis and organosolv [17].
Although a significant part of the hemicellulose fraction was already removed by autohydrolysis, the second step further reduced the xylan content to 24% of the initial compound in the pretreated solid. In addition, it released some monomeric pentoses (maximum 9.9 g/100 g initial xylan). Glucan fraction remains virtually unaffected in all assays performed, favorably compared with Romero et al. [49].
The composition of the liquors obtained is shown in Table 3 (panel IV). The concentration of XOS in the liquors is quite remarkable for a process after autohydrolysis since its value reached 10.24 g/L. Minor monomeric sugar release was observed for glucose, xylose, and arabinose. For the severest assay, degradation products (formic acid, HMF, and furfural) reached a total concentration of 2.78 g/L.
Solid phase composition resulting from the two-step process is shown in Figure 1 (panel IV). It is noticeable that glucan is the major compound ranging from 51.7% (30 °C) to 76.6% (200 °C). Hemicellulosic fraction, together with lignin, show some decrease in their content due to solubilization, also noted by a decrease in the solid yield.
The optimal condition for the second step of the combined treatment is 180–190 °C. This produces around 10 g/L of XOS together with a solid phase containing 77% glucan, 9% hemicellulose, and only 9% lignin, a similar trend to Romani et al. [17] for Eucalyptus globulus fractionation by autohydrolysis and organosolv. However, in this work, the lignin content obtained was lower.
3.4.2. Autohydrolysis Followed by Alkaline Treatment
Mass balances regarding each macromolecular component for alkaline treatment of the hydrothermally treated solids can be seen in Table 2 (panel V). Most of the glucan and a considerable fraction of xylan were recovered in the solid phase. Thus lignin was, as expected, the main component affected. The delignification yield for this process reached 90% (2% NaOH, 1 h). Comparing delignification yields of direct alkaline treatment with the global one (autohydrolysis+alkaline), it was noted that, in general, the yield is higher for the global process except for the maximum attained (94.4% for direct process vs. 92.7% for the global process at the same conditions). However, these yields are higher than the ones reported for the two-step treatment of Miscanthus [50] or rape straw [49].
The composition of the liquors for this treatment is shown in Table 3 (panel V). As observed for the direct process, no major sugar monomers were detected in the liquid phase. However, a significant amount of formic acid was produced (maximum 4.92 g/L) together with some glycerol and lactic acid, which are degradation products that can occur due to the degradation reaction of polysaccharides in an alkaline medium [51].
Solids resulting from the second step treatment were chemically characterized (Figure 1, panel V). Their glucan content is high, ranging from 70 to 77%, with a moderate xylan content, around 15%, and minor lignin content (minimum 2.8%).
The combined autohydrolysis+alkaline treatment is adequate for a complete fractionation of corn cobs, removing part of hemicellulose in the first step and leading to a high XOS production together with major lignin removal in the second step. The remaining solid, largely enriched in glucan, seems suitable for enzymatic hydrolysis and subsequent fermentation.
3.5. Enzymatic Hydrolysis
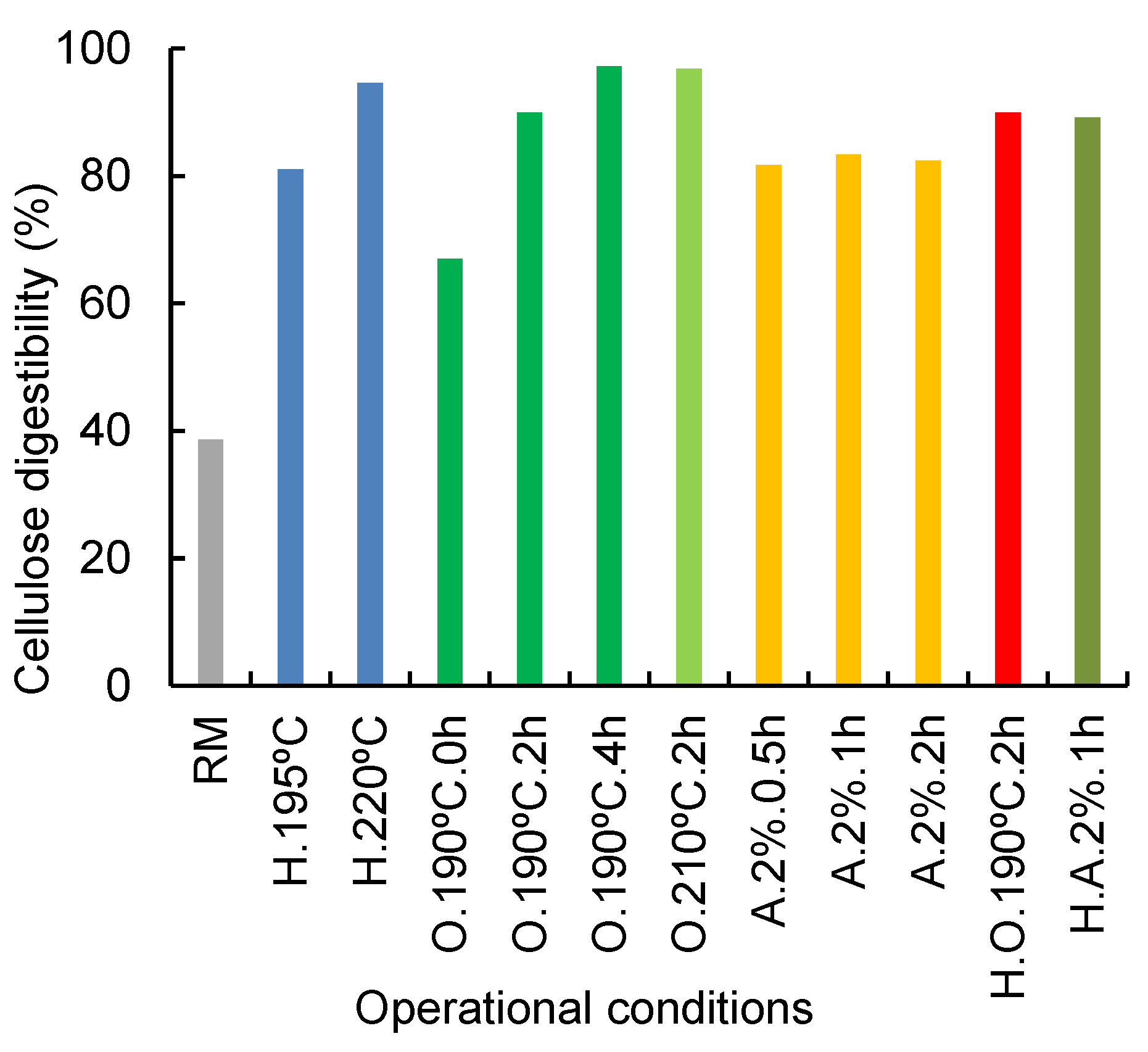
The effects of the different pretreatments (direct organosolv, direct alkaline treatment, autohydrolysis, combined autohydrolysis+ organosolv, and combined autohydrolysis+alkaline treatment) on the cellulose digestibility were evaluated by enzymatic saccharification of the remaining solid fractions (Figure 2). The most interesting samples were selected based on the optimum values reported for each treatment. A comparative solid for the same pretreatment at lower and higher severity conditions was also analyzed whenever possible. Raw corn cobs showed an enzymatic digestibility of 38.6% which is in agreement with previous studies for corn stover [52] and corn straw [28]. For organosolv-pretreated solid residue, enzymatic digestibility varied from 67.1% (190 °C, 0 h) to 97.3 (190 °C, 4 h), so it is clear that the longest organosolv pretreatments contributed to an increased enzymatic digestibility, although no increase in delignification was observed. An increase in cellulose enzymatic hydrolysis was also noted when the temperature was increased for organosolv treatments. Compared to cellulose, the enzymatic digestibility of the remaining xylan was typically slightly lower, except for the most severe conditions, where the low xylan content was completely hydrolyzed. For hydrothermally treated solids, the same dependence was obtained, analyzing H.195 °C, 0 h and H.220 °C, 0 h assays; an increase from 81.1% to 94.6% was verified. These results are in agreement with those found for pretreated corn cobs with 1% H2SO4 at 108 °C, which reached 83.9% hydrolysis [53].
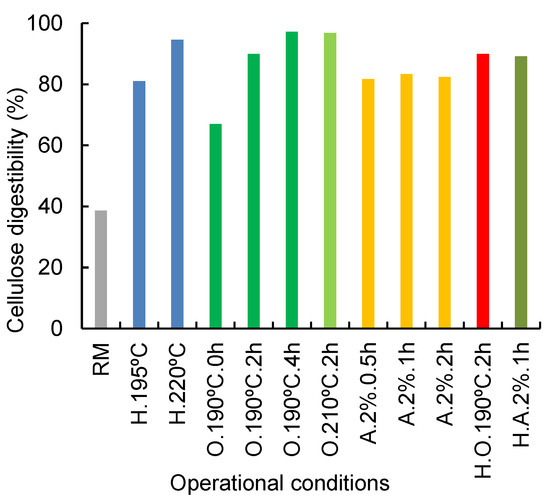
Figure 2.
Enzymatic digestibility (g/100 g glucan) of raw corn cobs and selected solids resulting from several assays. (RM—Raw material; H.—autohydrolysis, O.—Organosolv, A.—Alkaline treatment, H.O.—autohydrolysis+organosolv, H.A.—autohydrolysis+alkaline treatment). Deviations < 5%.
For alkaline treatment, enzymatic hydrolysis yields of around 82% were obtained for all three assays, with no significant differences. The same behavior was found to happen for delignification yield. The results for enzymatic digestibility of alkaline treated solids are lower than those obtained for organosolv, which can be related to the amount of xylan still present in the samples since organosolv, besides delignification also had a notorious effect on hemicellulose removal, contrary to alkaline treatment. The same behavior was observed for solid residues from combined alkaline treatments: enzymatic yield increased from 83.4% for direct pretreatment to 89.2% for combined. No differences were found regarding organosolv treatment, direct and combined pretreatments, with both values around 90% for cellulose enzymatic hydrolysis.
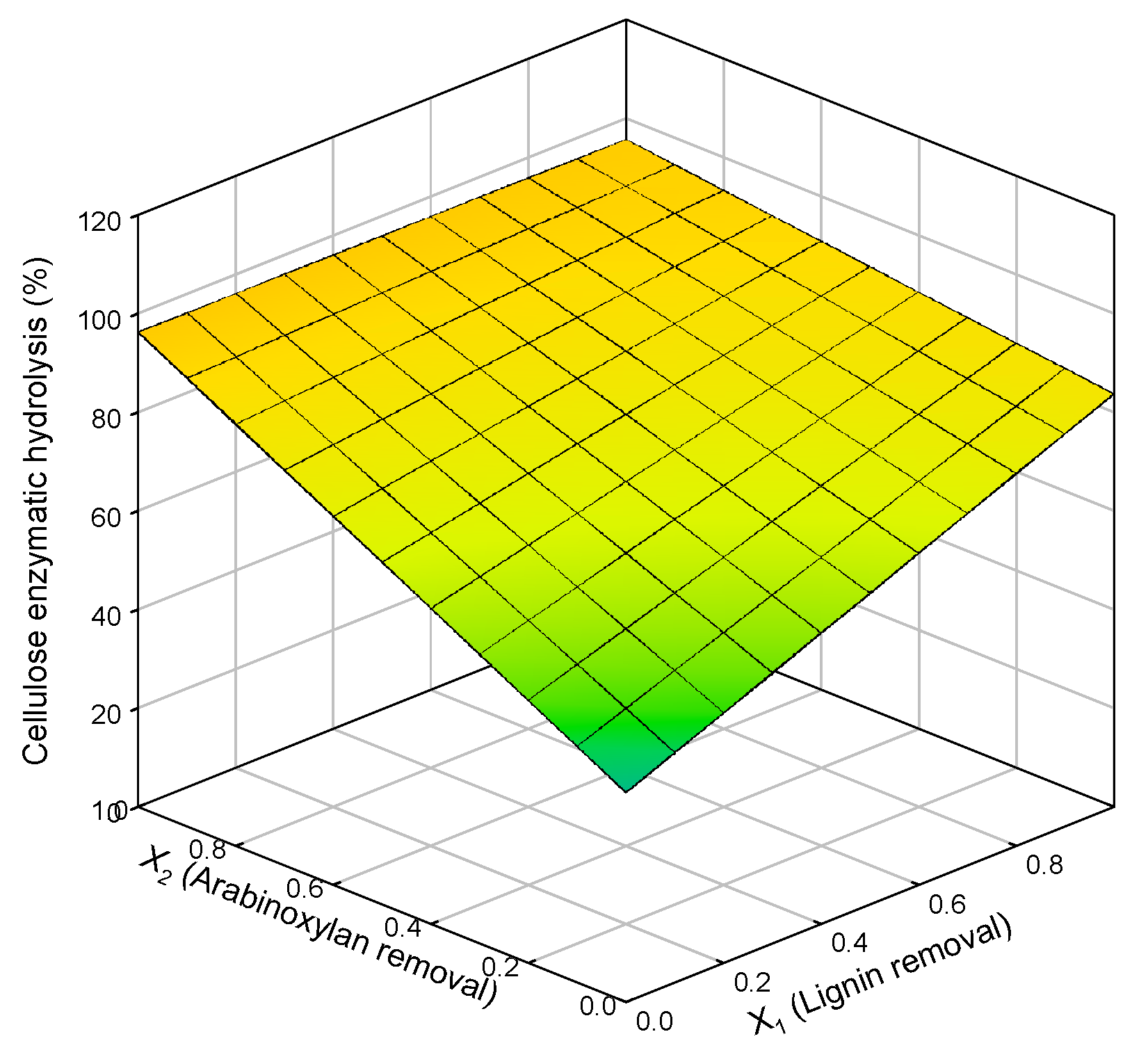
A model correlating the extent of biomass pretreatment (measured by lignin and xylan removal) and cellulose enzymatic digestibility was tested. The results of the statistical multi-linear regression plot are presented in Figure 3, and the statistically relevant parameters (90% significance level) are presented below (Equation (9)).
Cellulose enzymatic hydrolysis (%) = 42.53 + 41.23 × lignin removal (%) + 53.76 ×
xylan removal (%) − 41.76 × lignin removal (%) × xylan removal (%) (R2 = 0.9585)
xylan removal (%) − 41.76 × lignin removal (%) × xylan removal (%) (R2 = 0.9585)
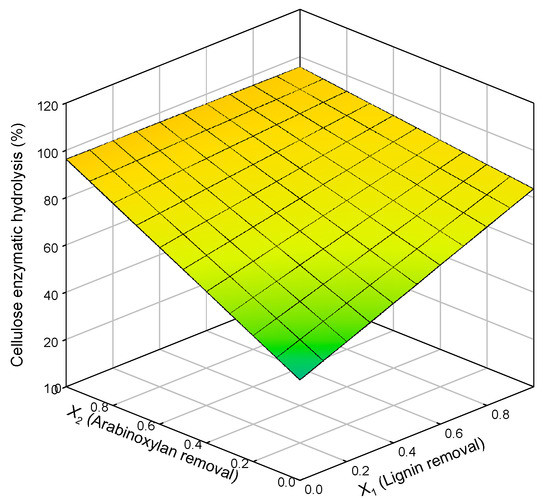
Figure 3.
Response surface for cellulose enzymatic hydrolysis in relation to lignin removal (X1) and arabinoxylan removal (X2).
The fitting of this equation to data derived from the organosolv or autohydrolysis experiments is represented in Figure 3.
A significant fit and correlation were found (R2 = 0.9790) between cellulose enzymatic hydrolysis and both xylan and lignin. For lower percentages of removal, residual enzymatic hydrolysis was verified as expected. On the other hand, when xylan removal is maximum, there is no need for lignin removal because enzymatic hydrolysis is already at its maximum. For lignin removal, the same cannot be said since for complete lignin removal, the enzymatic hydrolysis is not maximal; just when xylan removal increases, a maximum of hydrolysis can be achieved. This data proposes that hemicellulose removal seems to have a stronger impact than delignification on cellulose enzymatic hydrolysis. In contrast, other studies referred that delignification has a stronger effect than hemicellulose removal on cellulose saccharification [54]. Still, others show that hemicellulose and lignin removal significantly impact enzymatic hydrolysis, and their effects cannot be separated [55,56].
The model above can be coupled to the models described for the autohydrolysis and organosolv treatments and used to predict the best trade-off between increased cellulose enzymatic hydrolysis and oligosaccharides production for both processes. The optimized values are shown in Table 4.

Table 4.
Values obtained for Log Ro, delignification, xylan removal, and XOS production for autohydrolysis and organosolv treatment after optimization using the Solver function.
Autohydrolysis was identified as the more efficient process as it presents higher oligosaccharides and glucose yields at lower operational severities. However, it is important to note that the organosolv treatment enables a faster and potentially higher value for lignin recovery.
4. Conclusions
The proposed set of strategies for the valorization of corn cobs showed very interesting results for both liquid and solid phases. Direct organosolv treatments enabled a significant delignification, yielding a liquor rich in lignin-derived products and containing a considerable amount of XOS that might require further purification. In addition, the resulting solid phase was enriched in glucan. Alkaline treatments were also very efficient and almost completely removed lignin, producing a solid where hemicellulose, especially cellulose, is mainly retained.
In the first step (autohydrolysis treatment), the two-step processes enabled obtaining a hydrolysate with a high concentration of XOS and a solid fraction enriched in lignin and glucan, with a high potential for further fractionation. This later fraction was efficiently delignified by organosolv or alkaline treatments to produce liquors from which lignin-derived products can be easily obtained and solids further enriched in glucan. Promising saccharification yields of the solids were also obtained, aiming at a complete valorization of the fractions and integration in a biorefinery framework.
Based on a strategy for hemicellulose removal, the two-step processes were first demonstrated to be an efficient strategy for corn cobs valorization. They combine the advantages of both processes, increasing the range of the products obtained and their easier subsequent processing.
Supplementary Materials
Extra supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemengineering7020035/s1. Figure S1: Particle size distribution for corn cobs. Figure S2: Chemical composition of the different corn cobs fractions. Figure S3: Temperature and pressure profiles for the autohydrolysis of corn cobs. Figure S4: Temperature and pressure profiles organosolv profiles for the organosolv fractionation of corn cobs.
Author Contributions
Conceptualization, F.C., and L.C.D.; methodology, J.F., and P.M. validation: F.C. and L.C.D.; formal analysis, F.C., P.M., and L.C.D.; investigation: J.F. and P.M.; resources, L.C.D.; data curation, F.C., L.C.D., and P.M.; writing—original draft preparation, J.F.; writing—review and editing, F.C., P.M. and L.C.D.; visualization, F.C., and L.C.D.; Supervision, F.C.; project administration, L.C.D., and F.C.; funding acquisition: L.C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was developed under the framework of the project “SecMilho—Use of corn cobs and straw as an energy source, Operation PRODER 43316, Cooperation for Innovation” and it was carried out in the Biomass and Bioenergy Research Infrastructure (BBRI-LISBOA-01-0145-FEDER-022059) that is supported by the Operational Programme for Competitiveness and Internationalization (PORTUGAL 2020), by Lisbon Portugal Regional Operational Programme (Lisboa 2020) and by North Portugal Regional Operational Programme (Norte 2020) under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Céu Penedo and Belina Ribeiro for laboratory help. The authors also gratefully acknowledge AgroMais, Golegã (Portugal), for providing corn cobs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moreira da Silva, J.; Byrne, C.; Rocha, I.; Rodrigues, J.P.; Lemos, P.; Malheiro, S. Combater as alterações climáticas liderar a revolução energética e a economia circular. Policy Paper, PCS. 2023. Available online: https://www.crescimentosustentavel.org/media/PolicyPaperClimaEnergiaCircularidade1_PDFfinal.pdf (accessed on 16 March 2023).
- Cheng, Y.-S.; Mutrakulcharoen, P.; Chuetor, S.; Cheenkachorn, K.; Tantayotai, P.; Panakkal, E.J.; Sriariyanun, M. Recent situation and progress in biorefining process of lignocellulosic biomass: Toward Green Economy. Appl. Sci. Eng. Prog. 2020, 13, 299–311. [Google Scholar] [CrossRef]
- Asia-Pacific Economic Cooperation (APEC). Understanding the Bio-Circular-Green (BCG) Economy Model. 2022. Available online: https://www.apec.org/publications/2022/08/understanding-the-bio-circular-green-(bcg)-economy-model (accessed on 17 March 2023).
- The European Green Deal. Communication from the Commission. 2019. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_19_6691 (accessed on 17 March 2023).
- A European Green Deal. Striving to Be the First Climate-Neutral Continent. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 17 March 2023).
- The Paris Agreement. United Nations Climate Change. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement (accessed on 17 March 2023).
- Zhao, Y.; Damgaard, A.; Christensen, T.H. Bioethanol from corn stover—A review and technical assessment of alternative biotechnologies. Prog. Energy Combust. Sci 2018, 67, 275–291. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Girio, F.M. Hemicellulose biorefineries: A review on biomass pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864. [Google Scholar]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Pires, F.; Van-Dúnem, V.; Sanfins, L.; Roseiro, L.B.; Gírio, F. Effective mild ethanol-based organosolv pre-treatment for the selective valorization of polysaccharides and lignin from agricultural and forestry residues. Energies 2022, 15, 5654. [Google Scholar] [CrossRef]
- Jose, D.; Kitiborwornkul, N.; Sriariyanun, M.; Keerthi, K. A Review on chemical pretreatment methods of lignocellulosic biomass: Recent advances and progress. Appl. Sci. Eng. Prog. 2022, 15, 6210. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Norrahim, M.N.F.; Ilyas, R.A.; Nurazzi, M.N.; Rani, M.S.A.; Atikah, M.S.N.; Shazleen, S.S. Chemical pretreatment of lignocellulosic biomass for the production of bioproducts: An overview. Appl. Sci. Eng. Prog. 2021, 14, 588–605. [Google Scholar]
- Jönsson, L.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Romani, A.; Garrote, G.; Lopez, F.; Parajó, J.C. Eucalyptus globulus wood fractionation by autohydrolysis and organosolv delignification. Bioresour. Technol. 2011, 102, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Rascón, C.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Ruiz, E.; Castro, E. An integrated olive stone biorefinery based on a two-step fractionation strategy. Ind. Crops Prod. 2022, 187, 115157. [Google Scholar] [CrossRef]
- Pordesimo, L.O.; Edens, W.C.; Sokhansanj, S. Distribution of aboveground biomass in corn stover. Biomass Bioenerg 2004, 26, 337–343. [Google Scholar] [CrossRef]
- Moniz, P. Processos de fracionamento de resíduos agroindustriais para obtenção de hemiceluloses e lenhina de elevada qualidade para aproveitamento integrado no âmbito de uma biorrefinaria. Ph.D. Thesis, University of Lisboa, Lisboa, Portugal, 2014; p. 197. [Google Scholar]
- Anpromis. Available online: www.anpromis.pt (accessed on 22 February 2023).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.J.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; NREL/TP-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- AOAC. AOAC Official Methods of Analysis; AOAC: Washington, DC, USA, 1975. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Moniz, P.; Lino, J.; Duarte, L.C.; Roseiro, L.B.; Boeriu, C.G.; Pereira, H.; Carvalheiro, F. Fractionation of hemicelluloses and lignin from rice straw by combining autohydrolysis and optimised mild organosolv delignification. BioResources 2015, 10, 2626–2641. [Google Scholar] [CrossRef]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass; NREL/TP-510-42629; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of Lignocellulosics by steam-aqueous pretreatments. Phil. Trans. R. Soc. Lond. A 1987, 321, 523–536. [Google Scholar]
- Moniz, P.; Pereira, H.; Quilhó, T.; Carvalheiro, F. Characterisation and hydrothermal processing of corn straw towards the selective fractionation of hemicelluloses. Ind. Crops Prod. 2013, 50, 145–153. [Google Scholar] [CrossRef]
- Tada, K.; Horiuchi, J.-I.; Kanno, T.; Kobayashi, M. Microbial xylitol production from corn cobs using Candida magnoliae. J. Biosci. Bioeng. 2004, 98, 228–230. [Google Scholar] [CrossRef]
- Barl, B.; Biliaderis, C.G.; Murray, E.D.; Macgregor, A.W. Combined chemical and enzymic treatments of corn husk lignocellulosics. J. Sci. Food Agric. 1991, 56, 195–214. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Kinetic modelling of corncob autohydrolysis. Process Biochem. 2001, 36, 571–578. [Google Scholar] [CrossRef]
- Moura, P.; Barata, R.; Carvalheiro, F.; Gírio, F.; Loureiro-Dias, M.C.; Esteves, M.P. In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains. Food Sci. Technol. 2007, 40, 963–972. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V. Densification characteristics of corn cobs. Fuel Process. Technol. 2010, 91, 559–565. [Google Scholar] [CrossRef]
- Brosse, N.; Hage, R.; Sannigrahi, P.; Ragauskas, A. Dilute sulphuric acid and ethanol organosolv pretreatment of Miscanthus x Giganteus. Cellul. Chem. Technol. 2010, 44, 71–78. [Google Scholar]
- Hallac, B.B.; Sannigrahi, P.; Pu, Y.; Ray, M.; Murphy, R.J.; Ragauskas, A.J. Effect of ethanol organosolv pretreatment on enzymatic hydrolysis of Buddleja davidii stem biomass. Ind. Eng. Chem. Res. 2010, 49, 1467–1472. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Smit, A.T.; Reith, J.H.; den Uil, H. Catalytic organosolv fractionation of willow wood and wheat straw as pretreatment for enzymatic cellulose hydrolysis. J. Chem. Technol. Biotechnol. 2011, 86, 1428–1438. [Google Scholar] [CrossRef]
- Maillard, M.-N.; Berset, C. Evolution of antioxidant activity during kilning: Role of insoluble bound phenolic acids of barley and malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- Ramos, L.P. The chemistry involved in the steam treatment of lignocellulosic materials. Quim. Nova 2003, 26, 863–871. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Reith, J.H.; den Uil, H. Pretreatment and fractionation of wheat straw by an acetone-based organosolv process. Ind. Eng. Chem. Res. 2010, 49, 10132–10140. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, S. Efficient sugar release by acetic acid ethanol-based organosolv pretreatment and enzymatic saccharification. J. Agric. Food Chem. 2014, 62, 11681–11687. [Google Scholar] [CrossRef]
- Ekwe, N.B. The effect of delignification on the saccharification on of abakaliki rice husk. Adv. Appl. Sci. Res. 2012, 3, 3902–3908. [Google Scholar]
- Alves-Ferreira, J.; Lourenço, A.; Morgado, F.; Duarte, L.C.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Delignification of Cistus ladanifer biomass by organosolv and alkali processes. Energies 2021, 14, 1127. [Google Scholar] [CrossRef]
- Mafa, M.S.; Malgas, S.; Bhattacharya, A.; Rashamuse, K.; Pletschke, B.I. The effects of alkaline pretreatment on agricultural biomasses (corn cob and sweet sorghum bagasse) and their hydrolysis by a termite-derived enzyme cocktail. Agronomy 2020, 10, 1211. [Google Scholar] [CrossRef]
- He, X.; Miao, Y.; Jiang, X. Enhancing the enzymatic hydrolysis of corn stover by an integrated wet-milling and alkali pretreatment. Appl. Biochem. Biotechnol. 2010, 160, 2449–2457. [Google Scholar] [CrossRef]
- Silva-Fernandes, T.; Duarte, L.C.; Carvalheiro, F.; Marques, S.; Loureiro-Dias, M.C.; Fonseca, C.; Gírio, F. Biorefining strategy for maximal monosaccharide recovery from three different feedstocks: Eucalyptus residues, wheat straw and olive tree pruning. Bioresour. Technol. 2015, 183, 203–212. [Google Scholar] [CrossRef]
- Moniz, P.; Pereira, H.; Duarte, L.C.; Carvalheiro, F. Hydrothermal production and gel filtration purification of xylo-oligosaccharides from rice straw. Ind. Crops Prod. 2014, 62, 460–465. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Silva-Fernandes, T.; Duarte, L.C.; Girio, F.M. Wheat straw autohydrolysis: Process optimization and products characterization. Appl. Biochem. Biotechnol. 2009, 153, 84–93. [Google Scholar] [CrossRef]
- Obama, P.; Ricochon, G.; Muniglia, L.; Brosse, N. Combination of enzymatic hydrolysis and ethanol organosolv pretreatments: Effect on lignin structures, delignification yields and cellulose-to-glucose conversion. Bioresour. Technol. 2012, 112, 156–163. [Google Scholar] [CrossRef]
- Romero, I.; López-Linares, J.C.; Delgado, Y.; Cara, C.; Castro, E. Ethanol production from rape straw by a two-stage pretreatment under mild conditions. Bioprocess Biosyst. Eng. 2015, 38, 1469–1478. [Google Scholar] [CrossRef]
- Liu, Z.; Padmanabhan, S.; Cheng, K.; Xie, H.; Gokhale, A.; Afzal, W.; Na, H.; Pauly, M.; Bell, A.T.; Prausnitz, J.M. Two-step delignification of Miscanthus to enhance enzymatic hydrolysis: Aqueous ammonia followed by sodium hydroxide and oxidants. Energ Fuels 2014, 28, 542–548. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood Chemistry, Ultraestructure, Reactions; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1984. [Google Scholar]
- Kim, S.; Holtzapple, M.T. Effect of structural features on enzyme digestibility of corn stover. Bioresour. Technol. 2006, 97, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xia, L.; Xue, P. Enzymatic hydrolysis of corncob and ethanol production from cellulosic hydrolysate. Int. Biodeterior. Biodegrad. 2007, 59, 85–89. [Google Scholar] [CrossRef]
- Pires, F.; Van-Dunem, V.; Sanfins, L.; Duarte, L.C.; Gírio, F.; Carvalheiro, F. Optimization of a mild organosolv ethanol-based process for the selective fraction of Eucalyptus globulus residues. In Proceedings of the 28th European Biomass Conference & Exhibition, (EUBCE Proceedings, 2020), Marseille, France, 6–9 July 2020. [Google Scholar]
- Mussatto, S.I.; Fernandes, M.; Milagres, A.M.F.; Roberto, I.C. Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain. Enzyme Microb. Technol. 2008, 43, 124–129. [Google Scholar] [CrossRef]
- Yoshida, M.; Liu, Y.; Uchida, S.; Kawarada, K.; Ukagami, Y.; Ichinose, H.; Kaneko, S.; Fukuda, K. Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Biosci. Biotechnol. Biochem. 2008, 72, 805–810. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).