Mathematical Modeling and Experiments on Pyrolysis of Walnut Shells Using a Fixed-Bed Reactor
Abstract
1. Introduction
2. Materials and Methods
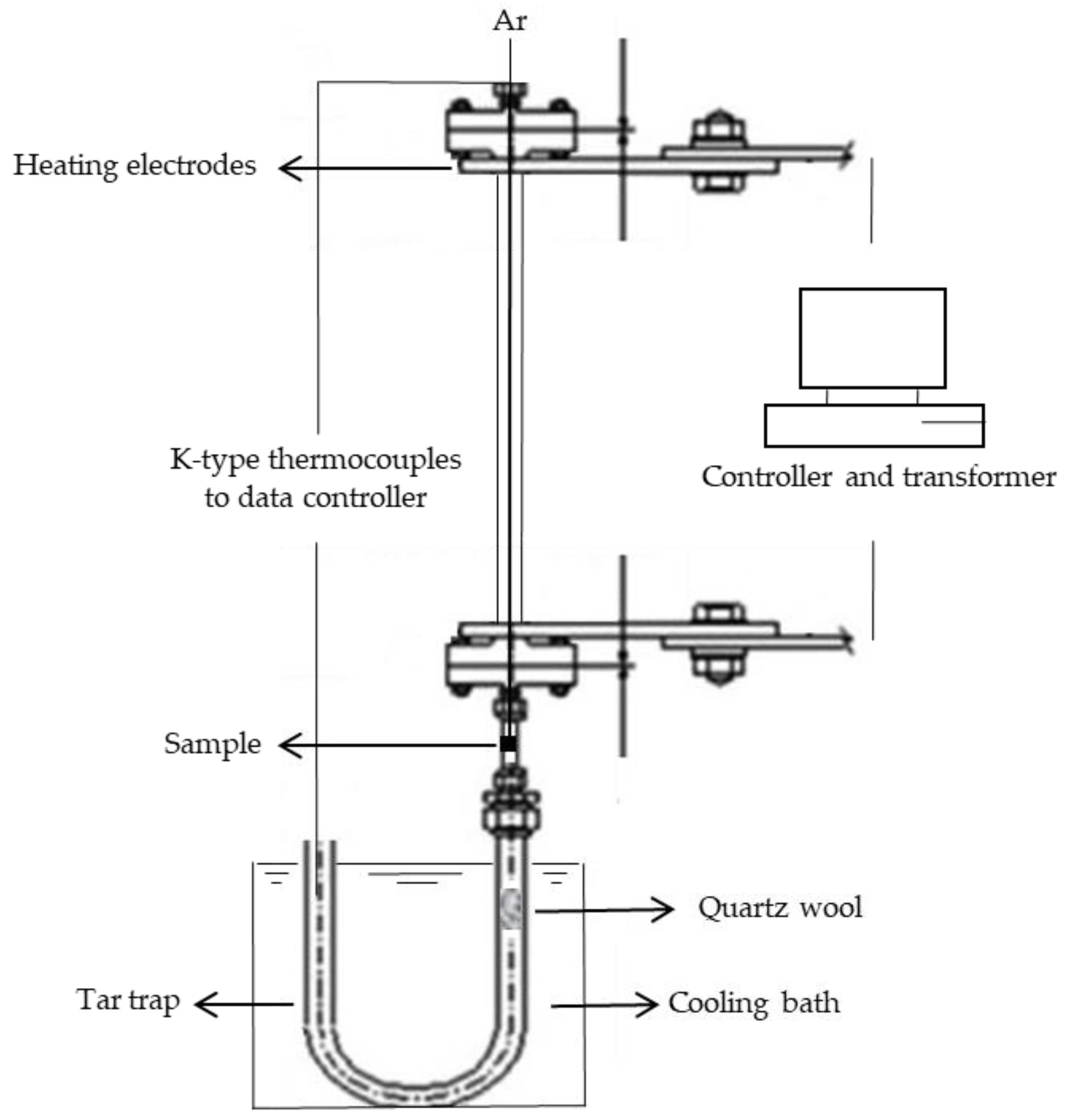
2.1. Pyrolysis Experiment
Product Yield Calculations
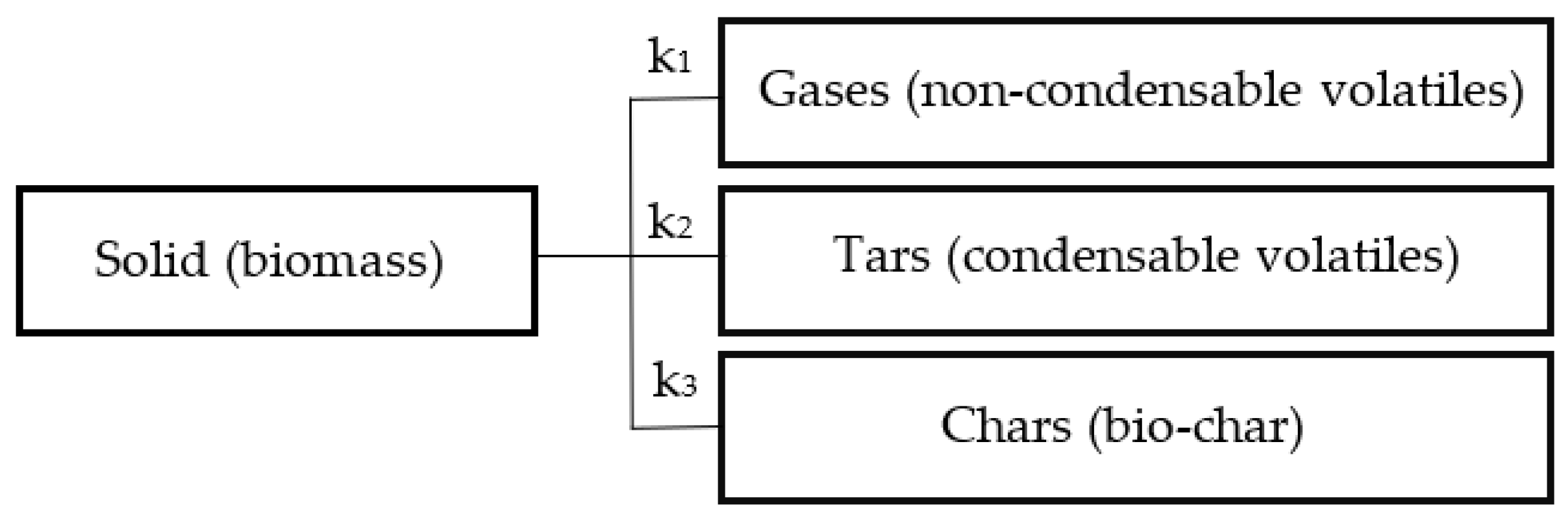
2.2. Mathematical Modeling
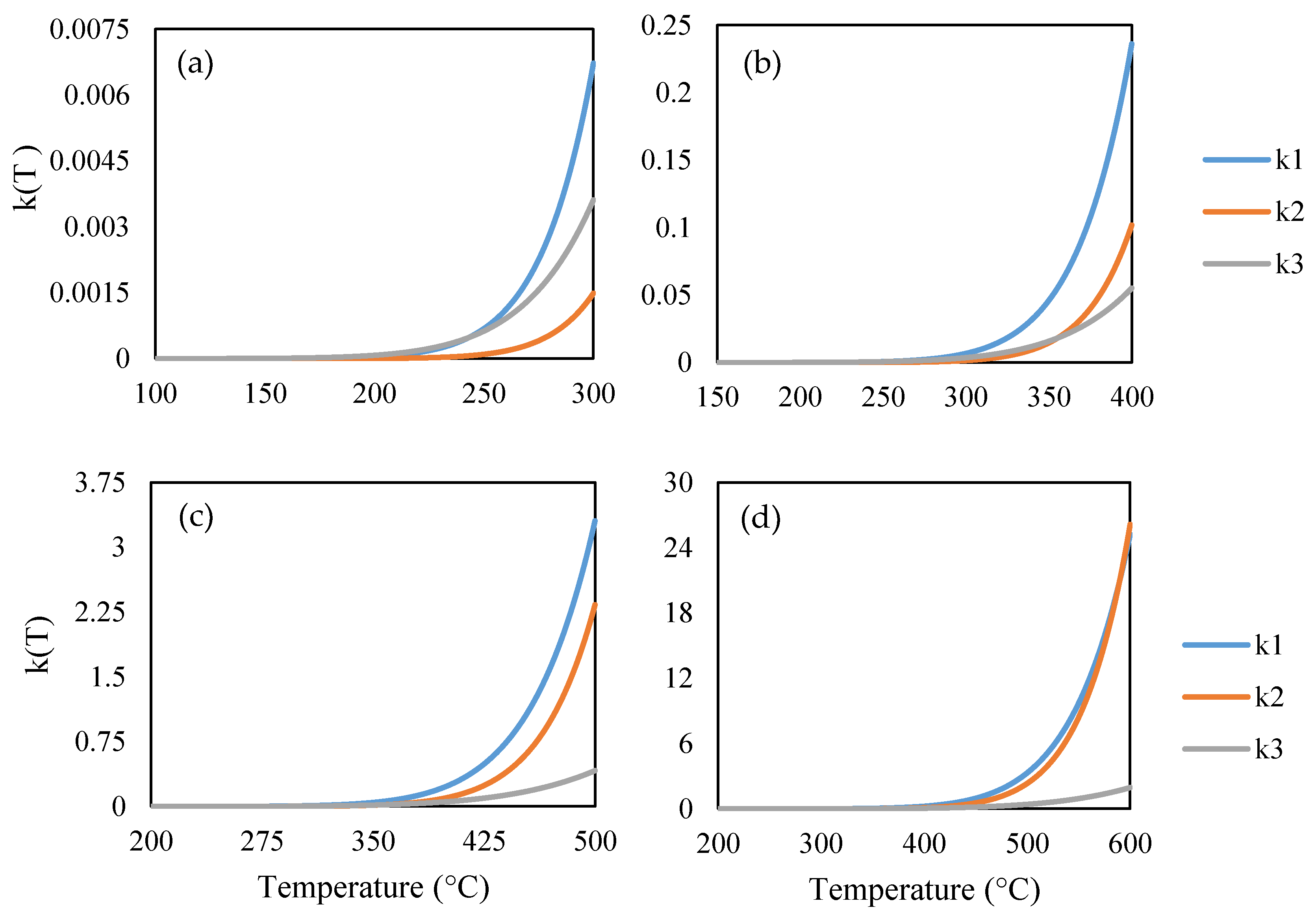
3. Results and Discussion
3.1. Pyrolysis Experiments
3.1.1. Vapor-Condensing Temperature and Condensing Efficiency
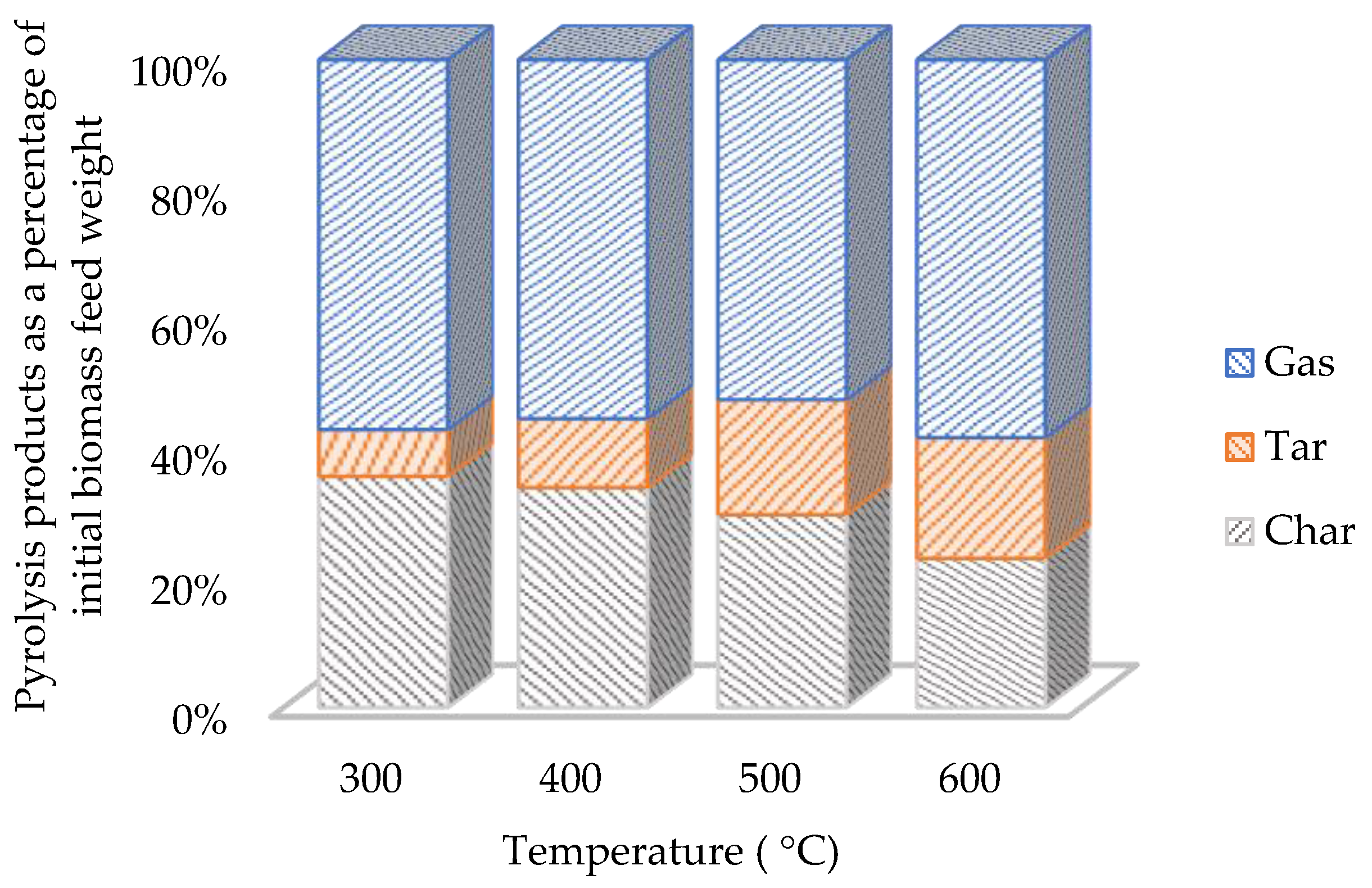
3.1.2. Pyrolysis Products and the Effect of Temperature
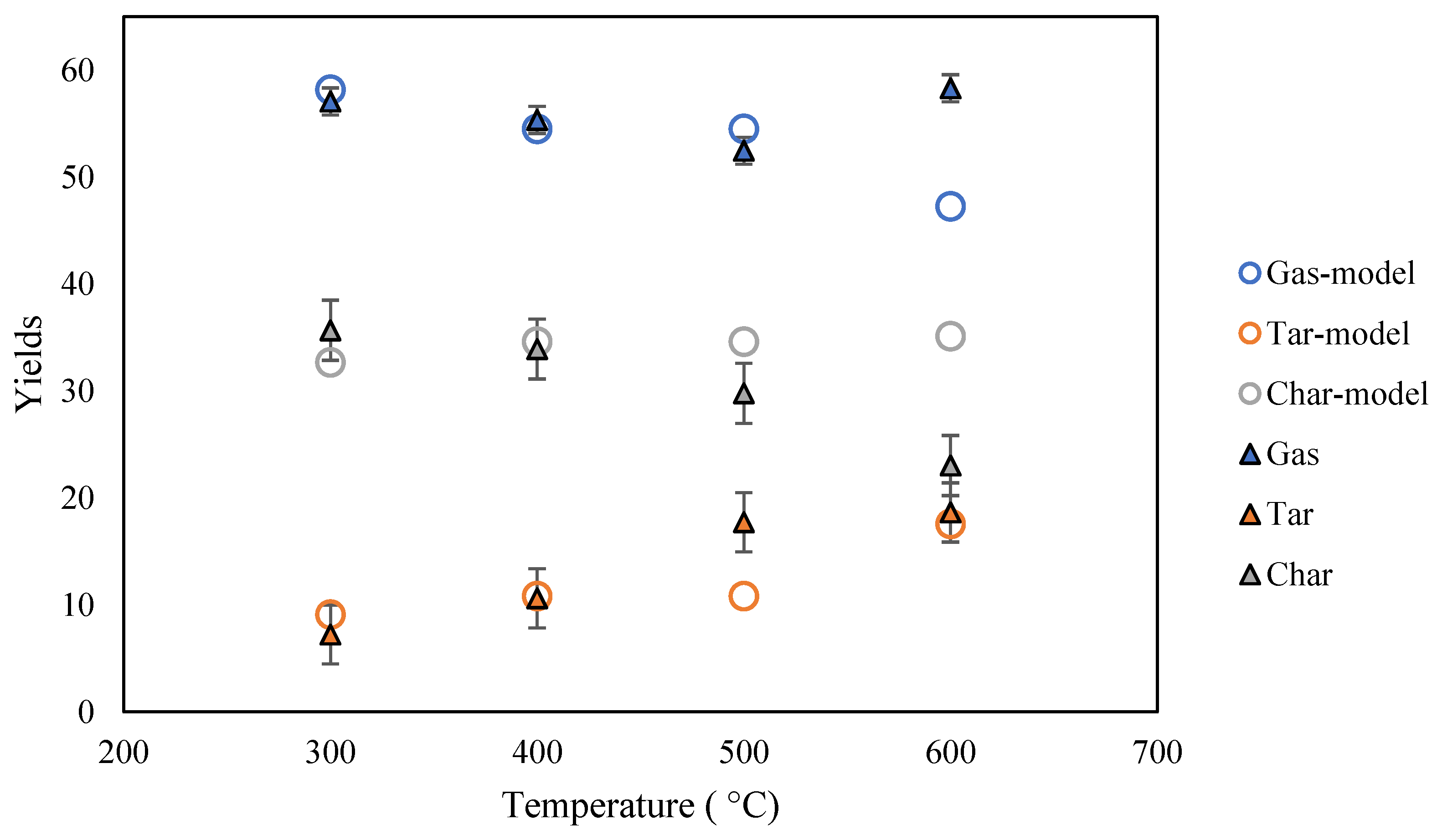
3.2. Pyrolysis Modeling
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Safavi, S.M.; Unnthorsson, R. Methane yield enhancement via electroporation of organic waste. Waste Manag. 2017, 66, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Safavi, S.M.; Unnthorsson, R. Enhanced methane production from pig slurry with pulsed electric field pre-treatment. Environ. Technol. 2017, 39, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Birol, G.; Önsan, Z.İ.; Kırdar, B.; Oliver, S.G. Ethanol production and fermentation characteristics of recombinant saccharomyces cerevisiae strains grown on starch. Enzyme Microb. Technol. 1998, 22, 672–677. [Google Scholar] [CrossRef]
- Moliner, C.; Bove, D.; Arato, E. Co-Incineration of Rice Straw-Wood Pellets: A Sustainable Strategy for the Valorisation of Rice Waste. Energies 2020, 13, 5750. [Google Scholar] [CrossRef]
- Monteiro Nunes, S.; Paterson, N.; Dugwell, D.R.; Kandiyoti, R. Tar formation and destruction in a simulated downdraft, fixed-bed gasifier: Reactor design and initial results. Energy Fuels 2007, 21, 3028–3035. [Google Scholar] [CrossRef]
- Ouadi, M.; Brammer, J.G.; Kay, M.; Hornung, A. Fixed bed downdraft gasification of paper industry wastes. Appl. Energy 2013, 103, 692–699. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Williams, O. Experiments on torrefied wood pellet: Study by gasification and characterization for waste biomass to energy applications. R. Soc. Open Sci. 2016, 3, 150578. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Yang, G.-X.; Jiang, H.; Liu, W.-J.; Ding, H.-S. Mass production of chemicals from biomass-derived oil by directly atmospheric distillation coupled with co-pyrolysis. Sci. Rep. 2013, 3, 1120. [Google Scholar] [CrossRef]
- Safavi, S.M.; Richter, C.; Unnthorsson, R. Dioxin and Furan Emissions from Gasification. In Gasification; IntechOpen: London, UK, 2021. [Google Scholar]
- Safavi, A.; Richter, C.; Unnthorsson, R. Dioxin Formation in Biomass Gasification: A Review. Energies 2022, 15, 700. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction Practical Design and Theory, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Çepelioğullar, Ö.; Haykiri-Açma, H.; Yaman, S. Kinetic modelling of RDF pyrolysis: Model-fitting and model-free approaches. Waste Manag. 2016, 48, 275–284. [Google Scholar] [CrossRef]
- Gouws, S.M.; Carrier, M.; Bunt, J.R.; Neomagus, H.W.J.P. Lumped chemical kinetic modelling of raw and torrefied biomass under pressurized pyrolysis. Energy Convers. Manag. 2022, 253, 115199. [Google Scholar] [CrossRef]
- Koufopanos, C.A.; Lucchesi, A.; Maschio, G. Kinetic modelling of the pyrolysis of biomass and biomass components. Can. J. Chem. Eng. 1989, 67, 75–84. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C. Kinetics of Primary Product Formation from Wood Pyrolysis. Ind. Eng. Chem. Res. 2001, 40, 5547–5556. [Google Scholar] [CrossRef]
- Wagenaar, B.M.; Prins, W.; van Swaaij, W.P.M. Flash pyrolysis kinetics of pine wood. Fuel Process. Technol. 1993, 36, 291–298. [Google Scholar] [CrossRef]
- Ranzi, E.; Dente, M.; Goldaniga, A.; Bozzano, G.; Faravelli, T. Lumping procedures in detailed kinetic modeling of gasification, pyrolysis, partial oxidation and combustion of hydrocarbon mixtures. Prog. Energy Combust. Sci. 2001, 27, 99–139. [Google Scholar] [CrossRef]
- Vikram, S.; Rosha, P.; Kumar, S. Recent modeling approaches to biomass pyrolysis: A review. Energy Fuels 2021, 35, 7406–7433. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 2007, 6, 183–195. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Al-Fatesh, A.S.; Fakeeha, A.H.; Rooney, D.W. Kinetic modelling for pyrolytic conversion of dedicated short rotation woody crop with predictions for isothermal, non-isothermal and stepwise heating regimes. Appl. Energy Combust. Sci. 2022, 9, 100048. [Google Scholar] [CrossRef]
- Vo, T.A.; Ly, H.V.; Tran, Q.K.; Kwon, B.; Kim, S.S.; Kim, J. Lumped-kinetic modeling and experiments on co-pyrolysis of palm kernel cake with polystyrene using a closed-tubing reactor to upgrade pyrolysis products. Energy Convers. Manag. 2021, 249, 114879. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Zubkova, V.; Strojwas, A.; Bielecki, M.; Kieush, L.; Koverya, A. Comparative study of pyrolytic behavior of the biomass wastes originating in the Ukraine and potential application of such biomass. Part 1. Analysis of the course of pyrolysis process and the composition of formed products. Fuel 2019, 254, 115688. [Google Scholar] [CrossRef]
- Yi, L.; Liu, H.; Li, S.; Li, M.; Wang, G.; Man, G.; Yao, H. Catalytic pyrolysis of biomass wastes over Org-CaO/Nano-ZSM-5 to produce aromatics: Influence of catalyst properties. Bioresour. Technol. 2019, 294, 122186. [Google Scholar] [CrossRef] [PubMed]
- Noszczyk, T.; Dyjakon, A.; Koziel, J.A. Kinetic parameters of nut shells pyrolysis. Energies 2021, 14, 682. [Google Scholar] [CrossRef]
- Sheth, P.; Babu, B. Pyrolysis of Hazelnut Shell: Kinetic Modeling And Simulation; Chemical Engineering Group, Birla Institute of Technology and Science: Rajasthan, India, 2007. [Google Scholar]
- Koufopanos, C.A.; Papayannakos, N.; Maschio, G.; Lucchesi, A. Modelling of the pyrolysis of biomass particles. Studies on kinetics, thermal and heat transfer effects. Can. J. Chem. Eng. 1991, 69, 907–915. [Google Scholar] [CrossRef]
- Demirbaş, A. Kinetics for non-isothermal flash pyrolysis of hazelnut shell. Bioresour. Technol. 1998, 66, 247–252. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Thurner, F.; Mann, U. Kinetic investigation of wood pyrolysis. Ind. Eng. Chem. Process Des. Dev. 1981, 20, 482–488. [Google Scholar] [CrossRef]
- Demirbaş, A. Fuel Characteristics of Olive Husk and Walnut, Hazelnut, Sunflower, and Almond Shells. Energy Sources 2002, 24, 215–221. [Google Scholar] [CrossRef]
- Harris, H.H.; Lee, D.W.; Jensen, C.M. Cost-Effective Teacher Dry-Ice Bath Based on Ethylene Glycol Mixtures. J. Chem. Educ. 2000, 77, 2000. [Google Scholar]
- Nunes, S.M.; Paterson, N.; Herod, A.A.; Dugwell, D.R.; Kandiyoti, R. Tar Formation and Destruction in a Fixed Bed Reactor Simulating Downdraft Gasification: Optimization of Conditions. Energy Fuels 2008, 22, 1955–1964. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A review on the pyrolysis of woody biomass to bio-oil: Focus on kinetic models. Renew. Sustain. Energy Rev. 2015, 52, 1580–1595. [Google Scholar] [CrossRef]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 3rd ed.; Pearson Educacion: Singapore, 2004; ISBN 81-203-2234-7. [Google Scholar]
- Varhegyi, G.; Antal, M.J.; Szekely, T.; Szabo, P. Kinetics of the thermal decomposition of cellulose, hemicellulose, and sugarcane bagasse. Energy Fuels 1989, 3, 329–335. [Google Scholar] [CrossRef]
- Várhegyi, G.; Antal, M.J.; Jakab, E.; Szabó, P. Kinetic modeling of biomass pyrolysis. J. Anal. Appl. Pyrolysis 1997, 42, 73–87. [Google Scholar] [CrossRef]
- Chan, W.-C.R.; Kelbon, M.; Krieger, B.B. Modelling and experimental verification of physical and chemical processes during pyrolysis of a large biomass particle. Fuel 1985, 64, 1505–1513. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling and simulation of combustion processes of charring and non-charring solid fuels. Prog. Energy Combust. Sci. 1993, 19, 71–104. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Z.; Diao, R.; Zhu, X. Study on the effect of condensing temperature of walnut shells pyrolysis vapors on the composition and properties of bio-oil. Bioresour. Technol. 2019, 285, 121370. [Google Scholar] [CrossRef]
- Bhoi, P.R.; Ouedraogo, A.S.; Soloiu, V.; Quirino, R. Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew. Sustain. Energy Rev. 2020, 121, 109676. [Google Scholar] [CrossRef]
- Efeovbokhan, V.E.; Akinneye, D.; Ayeni, A.O.; Omoleye, J.A.; Bolade, O.; Oni, B.A. Experimental dataset investigating the effect of temperature in the presence or absence of catalysts on the pyrolysis of plantain and yam peels for bio-oil production. Data Br. 2020, 31, 105804. [Google Scholar] [CrossRef]
- Dhar, S.A.; Sakib, T.U.; Hilary, L.N. Effects of pyrolysis temperature on production and physicochemical characterization of biochar derived from coconut fiber biomass through slow pyrolysis process. Biomass Convers. Biorefinery 2022, 12, 2631–2647. [Google Scholar] [CrossRef]
- Sarkar, J.K.; Wang, Q. Different Pyrolysis Process Conditions of South Asian Waste Coconut Shell and Characterization of Gas, Bio-Char, and Bio-Oil. Energies 2020, 13, 1970. [Google Scholar] [CrossRef]
- Sheth, P.; Babu, B. Kinetic Modeling, Simulation and Optimization of Pyrolysis. In Proceedings of the International Symposium & 61st Annual Session of IIChE in Association with International Partners (CHEMCON-2008), Panjab University, Chandigarh, India, 27 December 2008; pp. 27–30. [Google Scholar]
- Park, W.C.; Atreya, A.; Baum, H.R. Experimental and theoretical investigation of heat and mass transfer processes during wood pyrolysis. Combust. Flame 2010, 157, 481–494. [Google Scholar] [CrossRef]
- Xia, C.; Cai, L.; Zhang, H.; Zuo, L.; Shi, S.Q.; Lam, S.S. A review on the modeling and validation of biomass pyrolysis with a focus on product yield and composition. Biofuel Res. J. 2021, 8, 1296–1315. [Google Scholar] [CrossRef]
- Mate, M. Numerical Modelling of Wood Pyrolysis; Royal Institute of Technology: Stockholm, Sweden, 2016. [Google Scholar]
- Babu, B.V.; Chaurasia, A.S. Modeling for pyrolysis of solid particle: Kinetics and heat transfer effects. Energy Convers. Manag. 2003, 44, 2251–2275. [Google Scholar] [CrossRef]

| Parameters | Values |
|---|---|
| A1 (s−1) | 1.30 × 108 |
| A2 (s−1) | 2.00 × 108 |
| A3 (s−1) | 1.08 × 107 |
| E1 (kJ·mol−1) | 140 |
| E2 (kJ·mol−1) | 133 |
| E3 (kJ·mol−1) | 121 |
| R (kJ·mol−1) | 8.314 × 10−3 |
| Heating rate (°C·s−1) | 0.25 |
| Parameters | Values |
|---|---|
| A1 (s−1) | 1.70 × 108 |
| A2 (s−1) | 3.35 × 109 |
| A3 (s−1) | 3.29 × 105 |
| E1 (kJ·mol−1) | 114.14 |
| E2 (kJ·mol−1) | 135.54 |
| E3 (kJ·mol−1) | 87.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safavi, A.; Richter, C.; Unnthorsson, R. Mathematical Modeling and Experiments on Pyrolysis of Walnut Shells Using a Fixed-Bed Reactor. ChemEngineering 2022, 6, 93. https://doi.org/10.3390/chemengineering6060093
Safavi A, Richter C, Unnthorsson R. Mathematical Modeling and Experiments on Pyrolysis of Walnut Shells Using a Fixed-Bed Reactor. ChemEngineering. 2022; 6(6):93. https://doi.org/10.3390/chemengineering6060093
Chicago/Turabian StyleSafavi, Aysan, Christiaan Richter, and Runar Unnthorsson. 2022. "Mathematical Modeling and Experiments on Pyrolysis of Walnut Shells Using a Fixed-Bed Reactor" ChemEngineering 6, no. 6: 93. https://doi.org/10.3390/chemengineering6060093
APA StyleSafavi, A., Richter, C., & Unnthorsson, R. (2022). Mathematical Modeling and Experiments on Pyrolysis of Walnut Shells Using a Fixed-Bed Reactor. ChemEngineering, 6(6), 93. https://doi.org/10.3390/chemengineering6060093







