Solubility of Rosmarinic Acid in Supercritical Carbon Dioxide Extraction from Orthosiphon stamineus Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Supercritical Carbon Dioxide (SCCO2) Extraction
2.3. Quantification of RA
2.4. Solubility Measurement and Correlation
2.5. Extraction Rate
2.6. Validation
3. Results and Discussion
3.1. Solubility of RA in the SCCO2 System
3.2. Solubility Correlation of RA in SCCO2 System
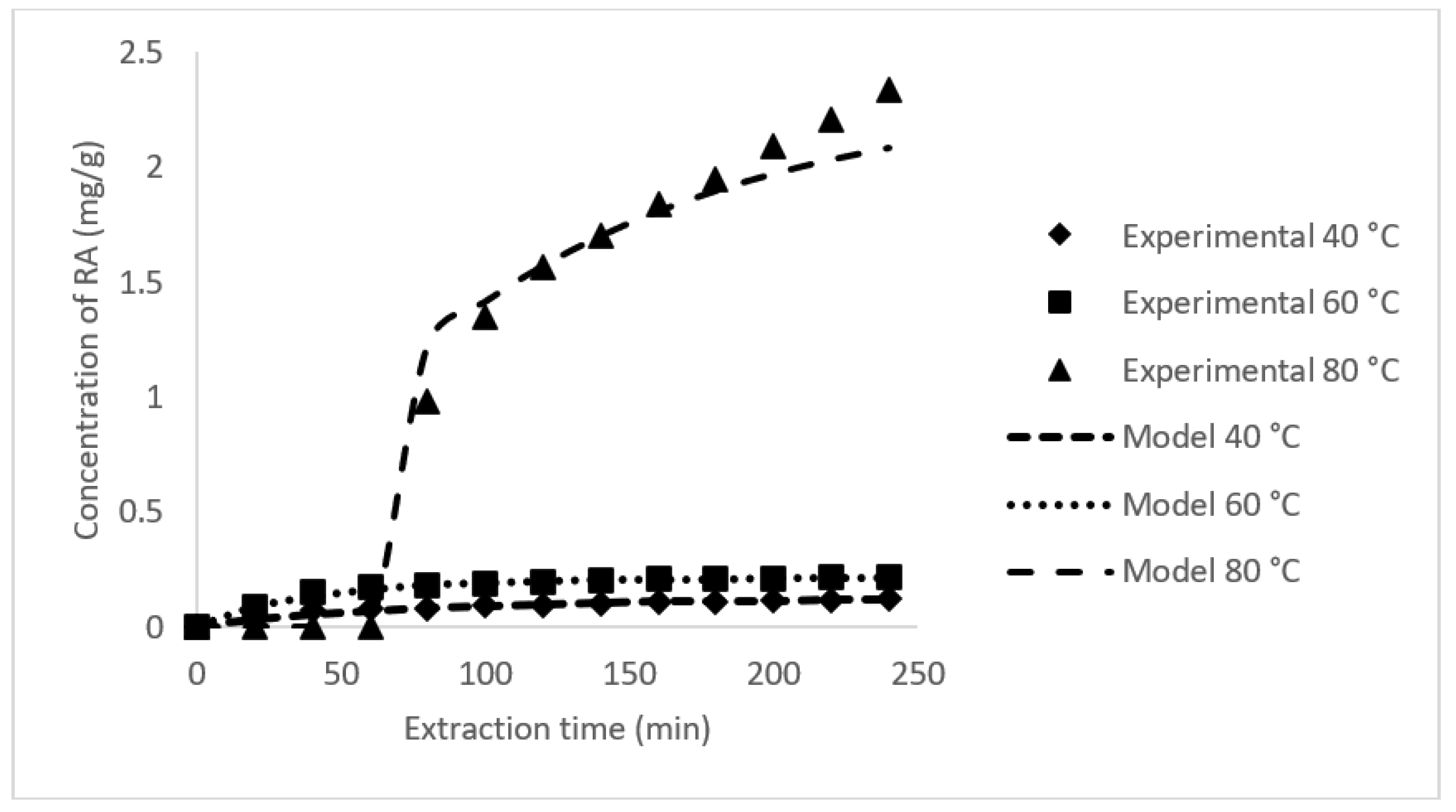
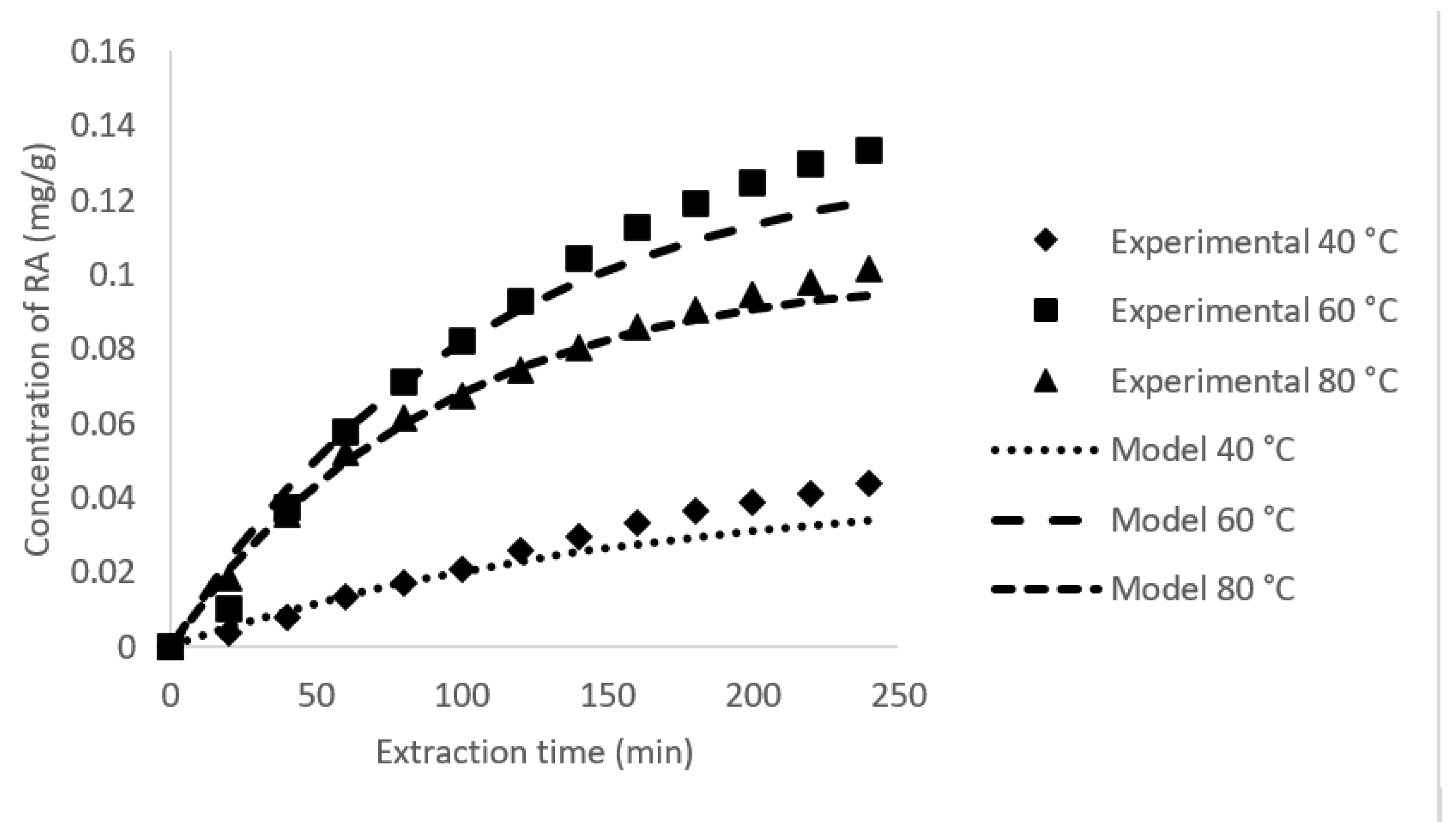
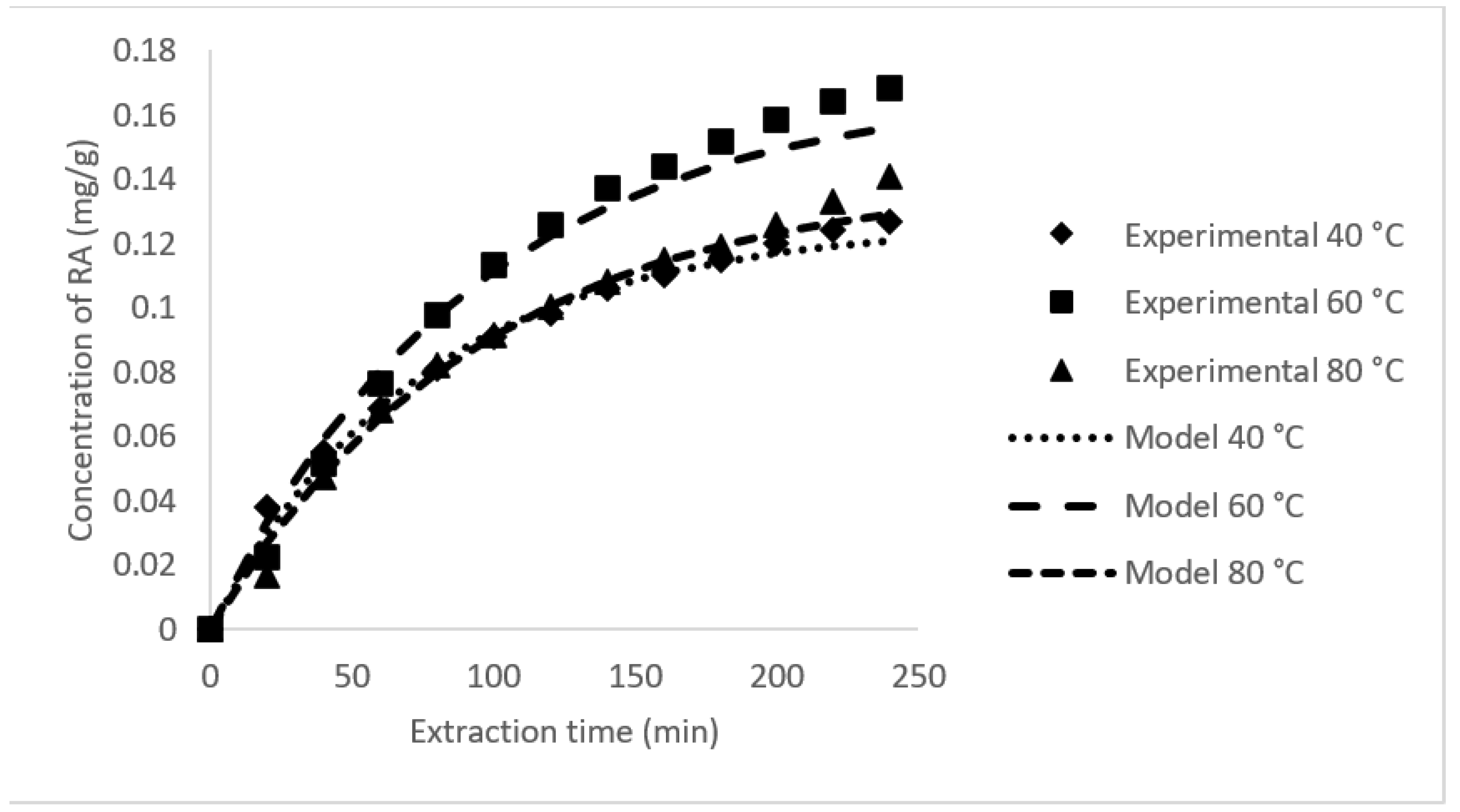
3.3. Rate of RA Extraction and Its Mass Transfer Coefficient
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamaruddin, M.J.; Hamid, S.R.A.; Othman, S.I.A.; Alam, M.N.H.Z.; Zaini, M.A.A.; Zakaria, Z.Y. The effects of conventional and microwave heating techniques on extraction yield of Orthosiphon stamineus leaves. Chem. Eng. Trans. 2018, 63, 601–606. [Google Scholar]
- Himani, B.; Seema, B.; Bhole, N.; Mayank, Y.; Vinod, S.; Mamta, S. Misai kuching: A glimpse of maestro. Int. J. Pharmaceut. Sci. Rev. Res. 2013, 22, 55–59. [Google Scholar]
- Aziz, A.H.A.; Yunus, M.A.C.; Yian, L.N.; Idham, Z.; Rithwan, F.; Hadzri, H.M.; Mustapha, A.N. Enhancement and optimization of sinensetin extract from Orthosiphon stamineus using supercrtitical carbon dioxide extraction. Malays. J. Anal. Sci. 2018, 22, 867–876. [Google Scholar]
- Al-Suede, F.S.R.; Khadeer Ahamed, M.B.; Abdul Majid, A.S.; Baharetha, H.M.; Hassan, L.E.A.; Kadir, M.O.A.; Nassar, Z.D.; Abdul Majid, A.M.S. Optimization of Cat’s Whiskers Tea (Orthosiphon stamineus) Using Supercritical Carbon Dioxide and Selective Chemotherapeutic Potential against Prostate Cancer Cells. Evid.-Based Complem. Altern. Med. 2014, 2014, 396016. [Google Scholar] [CrossRef] [Green Version]
- Ghedira, K.; Goetz, P. Orthosiphon stamineus Benth.: Orthosiphon (Lamiaceae). Phytothérapie 2015, 13, 39–44. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Hong, B.-H.; Yu, Y.-S.; Yen, G.-C. Antioxidant and anti-inflammatory effects of Orthosiphon aristatus and its bioactive compounds. J. Agric. Food Chem. 2010, 58, 2150–2156. [Google Scholar] [CrossRef]
- Adnyana, I.K.; Setiawan, F.; Insanu, M. From Ethnopharmacology to Clinical Study of Orthosiphon Stamineus Benth. Int. J. Pharm. Pharm. Sci. 2013, 5, 66–73. [Google Scholar]
- Cher, H.L.; Lee, S.C.; Chew, T.L.; Ramlan, A. Optimization and Kinetic Modeling of Rosmarinic Acid Extraction from Orthosiphon stamineus. Curr. Bioact. Comp. 2014, 10, 271–285. [Google Scholar]
- Abd Razak, N.; Yeap, S.K.; Alitheen, N.B.; Ho, W.Y.; Yong, C.Y.; Tan, S.W.; Tan, W.S.; Long, K. Eupatorin Suppressed Tumor Progression and Enhanced Immunity in a 4T1 Murine Breast Cancer Model. Integr. Cancer Ther. 2020, 19, 153473542093562. [Google Scholar] [CrossRef]
- Al-Dulaimi, D.W.; Shah Abdul Majid, A.; Baharetha, M.H.; Ahamed, M.B.K.; Faisal, S.F.; Al Zarzour, R.H.; Ein Oon, C.; Abdul Majid, A.M.S.; Ahmed Hassan, L.E. Anticlastogenic, antimutagenic, and cytoprotective properties of Orthosiphon stamineus ethanolic leaves extract. Drug Chem. Toxicol. 2022, 45, 641–650. [Google Scholar] [CrossRef]
- Pauzi, N.; Mohd, K.S.; Abdul Halim, N.H.; Ismail, Z. Orthosiphon stamineus Extracts Inhibits Proliferation and Induces Apoptosis in Uterine Fibroid Cells. Asian Pac. J. Cancer Prev. 2018, 19, 2737–2744. [Google Scholar] [PubMed]
- Basheer, M.K.A.; Majid, A.M.S.A. Medicinal potentials of Orthosiphon stamineus Benth. Webmedcentr. Cancer 2010, 1, WMC001361. [Google Scholar]
- Othman, N.F.; Ya’acob, M.E.; Abdul-Rahim, A.S.; Hizam, H.; Farid, M.M.; Abd Aziz, S. Inculcating herbal plots as effective cooling mechanism in urban planning. Acta Horticult. 2017, 1152, 235–242. [Google Scholar] [CrossRef]
- Sik, B.; Hanczné, E.L.; Kapcsándi, V.; Ajtony, Z. Conventional and nonconventional extraction techniques for optimal extraction processes of rosmarinic acid from six Lamiaceae plants as determined by HPLC-DAD measurement. J. Pharmaceut. Biomed. Anal. 2020, 184, 113173. [Google Scholar] [CrossRef]
- Soraki, R.K.; Gerami, M.; Ramezani, M. Effect of graphene / metal nanocomposites on the key genes involved in rosmarinic acid biosynthesis pathway and its accumulation in Melissa officinalis. BMC Plant Biol. 2021, 21, 260. [Google Scholar] [CrossRef]
- Jurić, T.; Mićić, N.; Potkonjak, A.; Milanov, D.; Dodić, J.; Trivunović, Z.; Popović, B.M. The evaluation of phenolic content, in vitro antioxidant and antibacterial activity of Mentha piperita extracts obtained by natural deep eutectic solvents. Food Chem. 2021, 362, 130226. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.V.; Savova, M.S.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Rosmarinic acid attenuates obesity and obesity-related inflammation in human adipocytes. Food Chem. Toxicol. 2021, 149, 112002. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Zhari, I. Effect of Extraction Temperature on Stability of Major Polyphenols and Antioxidant Activity of Orthosiphon stamineus Leaf. J. Herbs Spices Med. Plants 2010, 16, 160–166. [Google Scholar] [CrossRef]
- Abdul Aziz, A.H.; Putra, N.R.; Kong, H.; Che Yunus, M.A. Supercritical Carbon Dioxide Extraction of Sinensetin, Isosinensetin, and Rosmarinic Acid from Orthosiphon stamineus Leaves: Optimization and Modeling. Arab. J. Sci. Eng. 2020, 45, 7467–7476. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Cizauskaite, U.; Ivanauskas, L.; Jakstas, V.; Kalveniene, Z.; Kopustinskiene, D.M. Novel approaches to optimize extraction processes of ursolic, oleanolic and rosmarinic acids from Rosmarinus officinalis leaves. Ind. Crops Prod. 2016, 84, 72–79. [Google Scholar] [CrossRef]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci. Rep. 2020, 10, 10300. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Nguyen, H.N.T.; Huang, M.-Y.; Lin, K.-H.; Pham, D.-C.; Tran, Y.B.; Su, C.-H. Optimization of aqueous enzyme-assisted extraction of rosmarinic acid from rosemary (Rosmarinus officinalis L.) leaves and the antioxidant activity of the extract. J. Food Process. Preserv. 2021, 45, e15221. [Google Scholar] [CrossRef]
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.; Hamedani, M. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharm. Mag. 2012, 8, 37–41. [Google Scholar]
- Razboršek, M.I. Stability studies on trans-rosmarinic acid and GC–MS analysis of its degradation product. J. Pharmaceut. Biomed. Anal. 2011, 55, 1010–1016. [Google Scholar] [CrossRef]
- Ramli, W.N.D.; Yunus, M.A.C.; Yian, L.N.; Idham, Z.; Aziz, A.H.A.; Aris, N.A.; Putra, N.R.; Sham, S.K. Extraction of squalene from Aquilaria malaccensis leaves using different extraction methods. Mal. J. Anal. Sci. 2018, 22, 973–983. [Google Scholar]
- Priyadarsani, S.; Patel, A.S.; Kar, A.; Dash, S. Process optimization for the supercritical carbon dioxide extraction of lycopene from ripe grapefruit (Citrus paradisi) endocarp. Sci. Rep. 2021, 11, 10273. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Machmudah, S.; Yunus, M.A.C. Solubility of catechin and epicatechin from Arachis Hypogea skins wastes by using supercritical carbon dioxide-ethanol and its optimization. J. Food Measur. Charact. 2021, 15, 2031–2038. [Google Scholar] [CrossRef]
- Abdul Aziz, A.H.; Putra, N.R.; Nian Yian, L.; Mohd Rasidek, N.A.; Che Yunus, M.A. Parametric and kinetic study of supercritical carbon dioxide extraction on sinensetin from Orthosiphon stamineus Benth. leaves. Separ. Sci. Technol. 2021, 57, 444–453. [Google Scholar] [CrossRef]
- Idham, Z.; Putra, N.R.; Aziz, A.H.A.; Zaini, A.S.; Rasidek, N.A.M.; Mili, N.; Yunus, M.A.C. Improvement of extraction and stability of anthocyanins, the natural red pigment from roselle calyces using supercritical carbon dioxide extraction. J. CO2 Utiliz. 2022, 56, 101839. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.; Gwon, H.; Lee, K.-Y.; Choi, S.-G.; Atiqual Islam, M.; Chun, J.; Hwang, J. Phytochemical profile and antioxidant activity of Dracocephalum moldavica L. seed extracts using different extraction methods. Food Chem. 2021, 350, 128531. [Google Scholar] [CrossRef]
- Bakota, E.L.; Winkler-Moser, J.K.; Berhow, M.A.; Eller, F.J.; Vaughn, S.F. Antioxidant Activity and Sensory Evaluation of a Rosmarinic Acid-Enriched Extract of Salvia officinalis. J. Food Sci. 2015, 80, C711–C717. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Sequential extraction of carnosic acid, rosmarinic acid and pigments (carotenoids and chlorophylls) from Rosemary by online supercritical fluid extraction-supercritical fluid chromatography. J. Chromatogr. A 2021, 1639, 461709. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Machmudah, S.; Jumakir, J.; Waluyo, W.; Che Yunus, M.A. Procyanidin and proanthocyanidin extraction from Arachis hypogaea skins by using supercritical carbon dioxide: Optimization and modeling. J. Food Process. Preserv. 2021, 18, e15689. [Google Scholar] [CrossRef]
- Sato, T.; Ikeya, Y.; Adachi, S.-I.; Yagasaki, K.; Nihei, K.-I.; Itoh, N. Extraction of strawberry leaves with supercritical carbon dioxide and entrainers: Antioxidant capacity, total phenolic content, and inhibitory effect on uric acid production of the extract. Food Bioprod. Process. 2019, 117, 160–169. [Google Scholar] [CrossRef]
- Reddy, V.; Saharay, M. Solubility of Caffeine in Supercritical CO2: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2019, 123, 9685–9691. [Google Scholar] [CrossRef] [PubMed]
- Lemert, R.M.; Johnston, K.P. Solubilities and selectivities in supercritical fluid mixtures near critical end points. Fluid Phase Equilibr. 1990, 59, 31–55. [Google Scholar] [CrossRef]
- Huang, Z.; Chiew, Y.C.; Lu, W.-D.; Kawi, S. Solubility of aspirin in supercritical carbon dioxide/alcohol mixtures. Fluid Phase Equilibr. 2005, 237, 9–15. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Filho, W.A.R.; Paula, J.T.; Garmus, T.T.; Cabral, F.A. Solubility of γ-oryzanol in supercritical carbon dioxide and extraction from rice bran. J. Supercrit. Fluids 2016, 107, 196–200. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Idham, Z.; Rizkiyah, D.N.; Jumakir, J.; Mastaza, M.H.A.; Yunus, M.A.C. Kinetic modelling and extraction of Orthosiphon stamineus stems and leaves by SC-CO2. Malays. J. Fund. Appl. Sci. 2020, 16, 602–608. [Google Scholar]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Idham, Z.; Qomariyah, L.; Che Yunus, M.A. Extraction rate of Valuable Compounds from Peanut Skin Waste by Ethanol-Assisted Supercritical Carbon Dioxide: Modelling and Optimization. Malays. J. Fund. Appl. Sci. 2022, 18, 157–170. [Google Scholar] [CrossRef]
- Sodeifian, G.; Alwi, R.S.; Razmimanesh, F.; Tamura, K. Solubility of Quetiapine hemifumarate (antipsychotic drug) in supercritical carbon dioxide: Experimental, modeling and Hansen solubility parameter application. Fluid Phase Equilibr. 2021, 537, 113003. [Google Scholar] [CrossRef]
- Antonie, P.; Pereira, C.G. Solubility of functional compounds in supercritical CO2: Data evaluation and modelling. J. Food Eng. 2019, 245, 131–138. [Google Scholar] [CrossRef]
- Putra, N.; Aziz, A.A.; Zaini, A.; Idham, Z.; Idrus, F.; Zullyadini, M.B.; Yunus, M.C. Optimization of Soybean Oil by Modified Supercritical Carbon Dioxide. Int. J. Chem. Mol. Eng. 2018, 12, 495–500. [Google Scholar]
- Cheng, J.; Han, S.; Song, J.; Wang, W.; Jiao, Z. Solubility of Vitamin E Acetate in Supercritical Carbon Dioxide with Ethanol as Cosolvent. J. Chem. Eng. Data 2018, 63, 4248–4255. [Google Scholar] [CrossRef]
- Mouahid, A.; Bombarda, I.; Claeys-Bruno, M.; Amat, S.; Myotte, E.; Nisteron, J.-P.; Crampon, C.; Badens, E. Supercritical CO2 extraction of Moroccan argan (Argania spinosa L.) oil: Extraction kinetics and solubility determination. J. CO2 Utiliz. 2021, 46, 101458. [Google Scholar] [CrossRef]
- Hazaveie, S.M.; Sodeifian, G.; Sajadian, S.A. Measurement and thermodynamic modeling of solubility of Tamsulosin drug (anti cancer and anti-prostatic tumor activity) in supercritical carbon dioxide. J. Supercrit. Fluids 2020, 163, 104875. [Google Scholar] [CrossRef]
- Rosales-García, T.; Rosete-Barreto, J.M.; Pimentel-Rodas, A.; Davila-Ortiz, G.; Galicia-Luna, L.A. Solubility of Squalene and Fatty Acids in Carbon Dioxide at Supercritical Conditions: Binary and Ternary Systems. J. Chem. Eng. Data 2017, 63, 69–76. [Google Scholar] [CrossRef]
- Guo, L.; Qu, M.; Jin, J.; Meng, H. Solubility of cinnamic acid in supercritical carbon dioxide and subcritical 1,1,1,2-tetrafluoroethane: Experimental data and modelling. Fluid Phase Equilibr. 2019, 480, 66–80. [Google Scholar] [CrossRef]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Adachi, Y.; Lu, B.C.Y. Supercritical fluid extraction with carbon dioxide and ethylene. Fluid Phase Equilibr. 1983, 14, 147–156. [Google Scholar] [CrossRef]
- Kumar, S.K.; Johnston, K.P. Modelling the solubility of solids in supercritical fluids with density as the independent variable. J. Supercrit. Fluids 1988, 1, 15–22. [Google Scholar] [CrossRef]
- Sung, H.-D.; Shim, J.-J. Solubility of C. I. Disperse Red 60 and C. I. Disperse Blue 60 in Supercritical Carbon Dioxide. J. Chem. Eng. Data 1999, 44, 985–989. [Google Scholar] [CrossRef]
- Méndez-Santiago, J.; Teja, A.S. The solubility of solids in supercritical fluids. Fluid Phase Equilibr. 1999, 158–160, 501–510. [Google Scholar] [CrossRef]
- Bartle, K.D.; Clifford, A.A.; Jafar, S.A.; Shilstone, G.F. Solubilities of Solids and Liquids of Low Volatility in Supercritical Carbon Dioxide. J. Phys. Chem. Ref. Data 1991, 20, 713–756. [Google Scholar] [CrossRef]
- González, J.C.; Vieytes, M.R.; Botana, A.M.; Vieites, J.M.; Botana, L.M. Modified mass action law-based model to correlate the solubility of solids and liquids in entrained supercritical carbon dioxide. J. Chromatogr. A 2001, 910, 119–125. [Google Scholar] [CrossRef]
- Sovová, H. Solubility of ferulic acid in supercritical carbon dioxide with ethanol as cosolvent. J. Chem. Eng. Data 2001, 46, 1255–1257. [Google Scholar] [CrossRef]
- Tang, Z.; Jin, J.-s.; Zhang, Z.-t.; Yu, X.-y.; Xu, J.-N. Solubility of 3,5-Dinitrobenzoic Acid in Supercritical Carbon Dioxide with Cosolvent at Temperatures from (308 to 328) K and Pressures from (10.0 to 21.0) MPa. J. Chem. Eng. Data 2010, 55, 3834–3841. [Google Scholar] [CrossRef]
- Sauceau, M.; Letourneau, J.-J.; Richon, D.; Fages, J. Enhanced density-based models for solid compound solubilities in supercritical carbon dioxide with cosolvents. Fluid Phase Equilibr. 2003, 208, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Zhan, S.; Li, S.; Zhao, Q.; Wang, W.; Wang, J. Measurement and Correlation of Curcumin Solubility in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2017, 62, 1257–1263. [Google Scholar] [CrossRef]
- Jin, J.-S.; Wang, Y.-W.; Zhang, H.-F.; Fan, X.; Wu, H. Solubility of 4-Hydroxybenzaldehyde in Supercritical Carbon Dioxide with and without Cosolvents. J. Chem. Eng. Data 2014, 59, 1521–1527. [Google Scholar] [CrossRef]
- Wei, M.-C.; Xiao, J.; Yang, Y.-C. Extraction of α-humulene-enriched oil from clove using ultrasound-assisted supercritical carbon dioxide extraction and studies of its fictitious solubility. Food Chem. 2016, 210, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.-F.; Zabihi, F.; Gao, Y.-H.; Zhao, Y.-P. Solubility of Glycyrrhizin in Supercritical Carbon Dioxide with and without Cosolvent. J. Chem. Eng. Data 2015, 60, 1744–1749. [Google Scholar] [CrossRef]
- Soetaredjo, F.E.; Ismadji, S.; Yuliana Liauw, M.; Angkawijaya, A.E.; Ju, Y.-H. Catechin sublimation pressure and solubility in supercritical carbon dioxide. Fluid Phase Equilibr. 2013, 358, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Abdul Aziz, A.H.; Putra, N.R.; Zaini, A.S.; Idham, Z.; Ahmad, M.Z.; Che Yunus, M.A. Solubility of sinensetin and isosinensetin from Cat’s Whiskers (Orthosiphon stamineus) leaves in ethanol-assisted supercritical carbon dioxide extraction: Experimental and modeling. Chem. Papers 2021, 75, 6557–6563. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Idham, Z.; Jumakir, J.; Waluyo, W.; Che Yunus, M.A. Application of Drying Model to Determine Extraction Behaviours on Peanut Skin Oil Recovery by Supercritical Carbon Dioxide-Ethanol. Malays. J. Fund. Appl. Sci. 2021, 17, 114–127. [Google Scholar] [CrossRef]
- Ruslan, M.S.H.; Idham, Z.; Nian Yian, L.; Ahmad Zaini, M.A.; Che Yunus, M.A. Effect of operating conditions on catechin extraction from betel nuts using supercritical CO2-methanol extraction. Separ. Sci. Technol. 2018, 53, 662–670. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Aguilera, J.M. An improved equation for predicting the solubility of vegetable oils in supercritical carbon dioxide. Ind. Eng. Chem. Res. 1988, 27, 1551–1553. [Google Scholar] [CrossRef]
- Patricelli, A.; Assogna, A.; Casalaina, A.; Emmi, E.; Sodini, G. Fattoriche influenzano l’estrazione dei lipidi da semi decorticati di girasole. La Revista Italiana Delle Sostanze Grasse 1979, 56, 151–154. [Google Scholar]
- Paviani, L.C.; Dariva, C.; Marcucci, M.C.; Cabral, F.A. Supercritical carbon dioxide selectivity to fractionate phenolic compounds from the dry ethanolic extract of propolis. J. Food Process Eng. 2010, 33, 15–27. [Google Scholar] [CrossRef]

| Properties | Rosmarinic Acid |
|---|---|
| Chemical Structure | 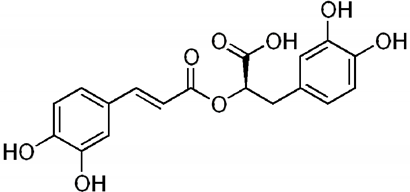 |
| Formula | C18H16O8 |
| Molecular weight | 360.31 g/mol |
| Melting point | 231.38 °C |
| Boiling point | 495.29 °C |
| Critical temperature | 678.26 °C |
| Critical pressure | 4.427 MPa |
| Molar Volume | 250.79 cm3/mol |
| Solubility parameter | 35.02 MPa1/2 |
| Dipole moment | 5.3023 Debye |
| Temperature (°C) | Pressure (MPa) | Density (g/L) | Experimental RA Solubility (mg/L solvent) | Predicted RA Solubility (mg/L) |
|---|---|---|---|---|
| 40 | 10 | 634.603 | 0.385 | 0.282 |
| 40 | 20 | 836.246 | 0.056 | 0.212 |
| 40 | 30 | 901.966 | 0.144 | 0.196 |
| 60 | 10 | 299.201 | 0.677 | 0.677 |
| 60 | 20 | 725.224 | 0.192 | 0.271 |
| 60 | 30 | 825.800 | 0.251 | 0.237 |
| 80 | 10 | 229.611 | 2.004 | 0.970 |
| 80 | 20 | 599.789 | 0.174 | 0.360 |
| 80 | 30 | 745.349 | 0.306 | 0.288 |
| Models | Model Parameters | Model Coefficient |
|---|---|---|
| Chrastil | k | −1.012 |
| −446.398 | ||
| b | −0.187 | |
| AARD (%) | 5.245 | |
| dVA | k | −1.033 |
| −391.280 | ||
| b | −19,889.733 | |
| c | −0.057 | |
| AARD (%) | 5.184 | |
| Gonzalez | k | −0.100 |
| γ | −0.888 | |
| −576.936 | ||
| b | 7.193 × 10−4 | |
| AARD (%) | 8.566 |
| T (°C) | P (MPa) | Mass Transfer Coefficient (min−1) | RA (mg/g) | Extraction Rate(mg/g min−1) | Equilibrium RA(mg/g) | AARD (%) | R2 | ||
| 40 | 10 | 0.0147 | 0.0135 | 0.0683 | 0.0522 | 0.0017 | 0.1205 | 3.6219 | 0.9907 |
| 60 | 10 | 0.0321 | 0.0127 | 0.1545 | 0.0606 | 0.0057 | 0.2151 | 1.7715 | 0.9960 |
| 80 | 10 | 0.0094 | 0.0093 | 1.3453 | 0.9914 | 0.0218 | 2.3367 | 4.5450 | 0.9652 |
| 40 | 20 | 0.0062 | 0.0063 | 0.0257 | 0.0180 | 0.0003 | 0.0437 | 14.7454 | 0.9930 |
| 60 | 20 | 0.0095 | 0.0095 | 0.0709 | 0.0625 | 0.0013 | 0.1334 | 14.9458 | 0.9957 |
| 80 | 20 | 0.0112 | 0.0111 | 0.0523 | 0.0493 | 0.0011 | 0.1016 | 3.3129 | 0.9979 |
| 40 | 30 | 0.0151 | 0.0102 | 0.0808 | 0.0460 | 0.0017 | 0.1268 | 3.5445 | 0.9963 |
| 60 | 30 | 0.0108 | 0.0108 | 0.1132 | 0.0552 | 0.0018 | 0.1684 | 8.1098 | 0.9974 |
| 80 | 30 | 0.0104 | 0.0104 | 0.0915 | 0.0491 | 0.0015 | 0.1406 | 6.2773 | 0.9963 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Aziz, A.H.; Mohd Idrus, N.F.; Putra, N.R.; Awang, M.A.; Idham, Z.; Mamat, H.; Che Yunus, M.A. Solubility of Rosmarinic Acid in Supercritical Carbon Dioxide Extraction from Orthosiphon stamineus Leaves. ChemEngineering 2022, 6, 59. https://doi.org/10.3390/chemengineering6040059
Abdul Aziz AH, Mohd Idrus NF, Putra NR, Awang MA, Idham Z, Mamat H, Che Yunus MA. Solubility of Rosmarinic Acid in Supercritical Carbon Dioxide Extraction from Orthosiphon stamineus Leaves. ChemEngineering. 2022; 6(4):59. https://doi.org/10.3390/chemengineering6040059
Chicago/Turabian StyleAbdul Aziz, Ahmad Hazim, Nor Faadila Mohd Idrus, Nicky Rahmana Putra, Mohd Azrie Awang, Zuhaili Idham, Hasmadi Mamat, and Mohd Azizi Che Yunus. 2022. "Solubility of Rosmarinic Acid in Supercritical Carbon Dioxide Extraction from Orthosiphon stamineus Leaves" ChemEngineering 6, no. 4: 59. https://doi.org/10.3390/chemengineering6040059
APA StyleAbdul Aziz, A. H., Mohd Idrus, N. F., Putra, N. R., Awang, M. A., Idham, Z., Mamat, H., & Che Yunus, M. A. (2022). Solubility of Rosmarinic Acid in Supercritical Carbon Dioxide Extraction from Orthosiphon stamineus Leaves. ChemEngineering, 6(4), 59. https://doi.org/10.3390/chemengineering6040059







