An Overview on the Recent Advances in Alternative Solvents as Stabilizers of Proteins and Enzymes
Abstract
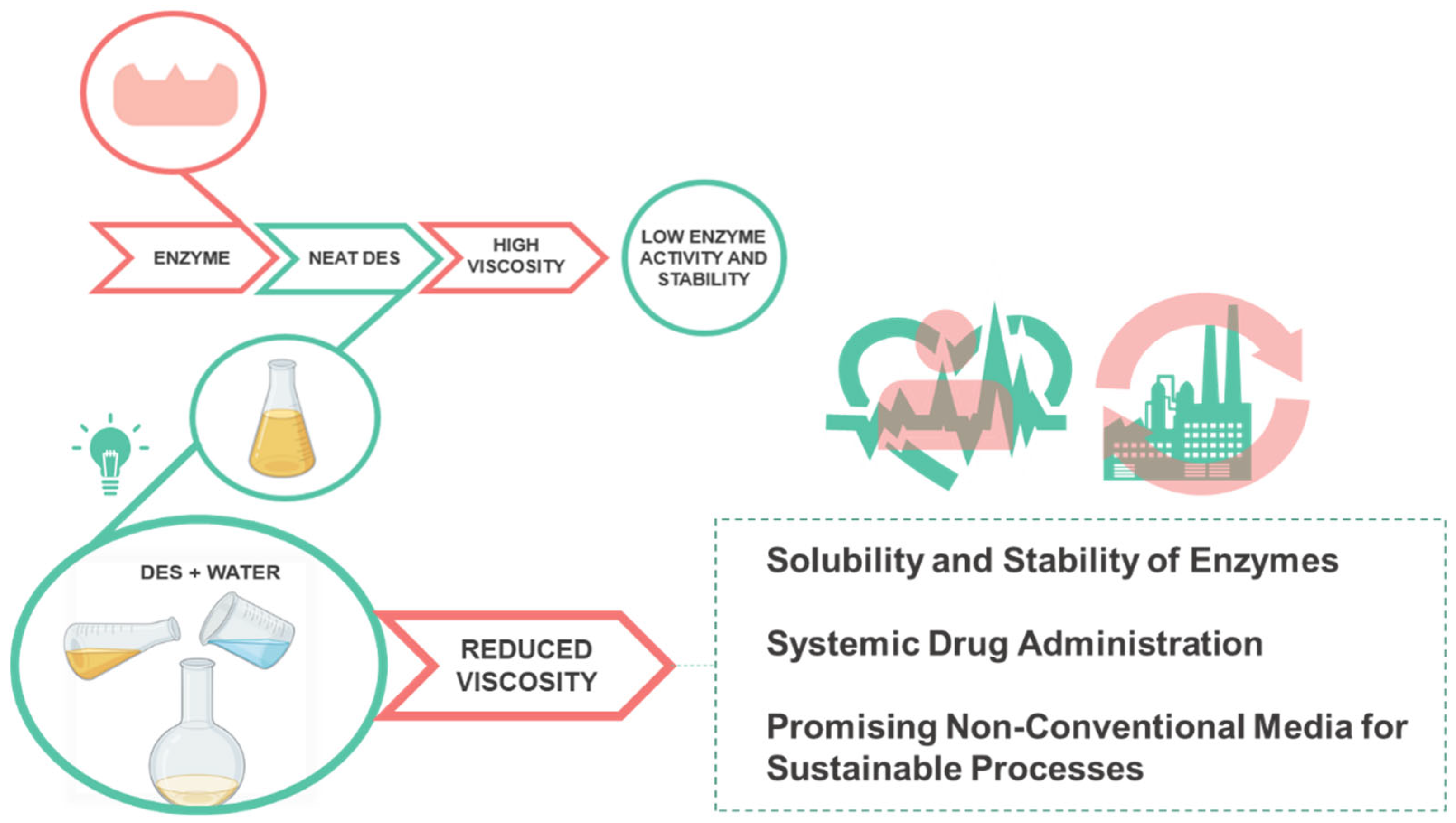
:1. Introduction
2. Stability of Proteins in Alternative Solvents
2.1. Stability of Proteins in Ionic Liquids
2.2. Stability of Proteins in Deep Eutectic Solvents
3. Stability of Enzymes in Alternative Solvents
3.1. Stability of Enzymes in Ionic Liquids
3.2. Stability of Enzymes in Deep Eutectic Solvents
4. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Bisht, M.; Venkatesu, P. Biocompatibility of ionic liquids towards protein stability: A comprehensive overview on the current understanding and their implications. Int. J. Biol. Macromol. 2017, 96, 611–651. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kazlauskas, R. Biocatalysis in ionic liquids—Advantages beyond green technology. Curr. Opin. Biotechnol. 2003, 14, 432–437. [Google Scholar] [CrossRef]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H. DNA stability in ionic liquids and deep eutectic solvents. J. Chem. Technol. Biotechnol. 2015, 90, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Capela, E.V.; Valente, A.I.; Nunes, J.C.; Magalhães, F.; Rodríguez, O.; Soto, A.; Freire, M.G.; Tavares, A.P. Insights on the laccase extraction and activity in ionic-liquid-based aqueous biphasic systems. Sep. Purif. Technol. 2020, 248, 117052. [Google Scholar] [CrossRef]
- Arumugam, V.; Rajamanikandan, R.; Ilanchelian, M.; Moodley, K.G.; Redhi, G.G. Investigation of binding interactions between BSA and [EPMpyr][Sal] through spectroscopy studies, thermophysical and thermodynamic properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 299–307. [Google Scholar] [CrossRef]
- Sindhu, A.; Mogha, N.K.; Venkatesu, P. Insight into impact of choline-based ionic liquids on bovine β-lactoglobulin structural analysis: Unexpected high thermal stability of protein. Int. J. Biol. Macromol. 2019, 126, 1–10. [Google Scholar] [CrossRef]
- Shire, S. Monoclonal Antibodies: Meeting the Challenges in Manufacturing, Formulation, Delivery and Stability of Final Drug Product; Woodhead Publishing: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Wang, Y.; Hanson, M.A. Parenteral formulations of proteins and peptides: Stability and stabilizers. J. Parenter. Sci. Technol. 1988, 42, 4–26. [Google Scholar]
- Weinbuch, D.; Cheung, J.K.; Ketelaars, J.; Filipe, V.; Hawe, A.; Den Engelsman, J.; Jiskoot, W. Nanoparticulate Impurities in Pharmaceutical-Grade Sugars and their Interference with Light Scattering-Based Analysis of Protein Formulations. Pharm. Res. 2015, 32, 2419–2427. [Google Scholar] [CrossRef] [Green Version]
- Roberts, C.J. Therapeutic protein aggregation: Mechanisms, design, and control. Trends Biotechnol. 2014, 32, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Gad, S.C. (Ed.) Handbook of Pharmaceutical Biotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Lin, S. Solubility and dissolution enhancement strategies: Current understanding and recent trends. Drug Dev. Ind. Pharm. 2015, 41, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Stefanuto, V.; Tojo, E. New Active Pharmaceutical Ingredient-Ionic Liquids (API-ILs) Derived from Indomethacin and Mebendazole. Proceedings 2019, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Shire, S.J.; Shahrokh, Z.; Liu, J. Challenges in the development of high protein concentration formulations. J. Pharm. Sci. 2004, 93, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Jezek, J.; Rides, M.; Derham, B.; Moore, J.; Cerasoli, E.; Simler, R.; Perez-Ramirez, B. Viscosity of concentrated therapeutic protein compositions. Adv. Drug Deliv. Rev. 2011, 63, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Wang, W.; Arakawa, T.; Ohtake, S. Developments and Challenges for mAb-Based Therapeutics. Antibodies 2013, 2, 452–500. [Google Scholar] [CrossRef] [Green Version]
- Magina, S.; Barros-Timmons, A.; Ventura, S.P.M.; Evtuguin, D.V. Evaluating the hazardous impact of ionic liquids—Challenges and opportunities. J. Hazard. Mater. 2021, 412, 125215. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Liu, P.; Hao, J.-W.; Mo, L.-P.; Zhang, Z.-H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Teles, A.R.R.; Capela, E.V.; Carmo, R.S.; Coutinho, J.A.; Silvestre, A.J.; Freire, M.G. Solvatochromic parameters of deep eutectic solvents formed by ammonium-based salts and carboxylic acids. Fluid Phase Equilibria 2017, 448, 15–21. [Google Scholar] [CrossRef]
- Macário, I.P.; Jesus, F.; Pereira, J.L.; Ventura, S.P.; Gonçalves, A.M.; Coutinho, J.A.; Gonçalves, F.J. Unraveling the ecotoxicity of deep eutectic solvents using the mixture toxicity theory. Chemosphere 2018, 212, 890–897. [Google Scholar] [CrossRef]
- Macário, I.; Oliveira, H.; Menezes, A.C.; Ventura, S.; Pereira, J.L.; Gonçalves, A.M.M.; Coutinho, J.; Gonçalves, F.J.M. Cytotoxicity profiling of deep eutectic solvents to human skin cells. Sci. Rep. 2019, 9, 3932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedro, S.N.; Freire, M.G.; Freire, C.; Silvestre, A.J.D. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems. Expert Opin. Drug Deliv. 2019, 16, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep Eutectic Solvents as Efficient Solvents in Biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.B.; Kumar, V.S.; Chaudhary, M.; Singh, P. A mini review on synthesis, properties and applications of deep eutectic solvents. J. Indian Chem. Soc. 2021, 98, 100210. [Google Scholar] [CrossRef]
- Colmenarejo, G.; Alvarez-Pedraglio, A.; Lavandera, J.-L. Cheminformatic Models to Predict Binding Affinities to Human Serum Albumin. J. Med. Chem. 2001, 44, 4370–4378. [Google Scholar] [CrossRef] [Green Version]
- Ossowicz, P.; Kardaleva, P.; Guncheva, M.; Klebeko, J.; Świątek, E.; Janus, E.; Yancheva, D.; Angelov, I. Ketoprofen-Based Ionic Liquids: Synthesis and Interactions with Bovine Serum Albumin. Molecules 2020, 25, 90. [Google Scholar] [CrossRef] [Green Version]
- Arques, S. Human serum albumin in cardiovascular diseases. Eur. J. Intern. Med. 2018, 52, 8–12. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Khan, J.M.; Alsenaidy, M.A.; Alsenaidy, A.M.; Khan, M.S.; Husain, F.M.; Khan, M.R.; Naseem, M.; Sen, P.; Alam, P.; et al. Unveiling the stimulatory effects of tartrazine on human and bovine serum albumin fibrillogenesis: Spectroscopic and microscopic study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 116–124. [Google Scholar] [CrossRef]
- Han, X.-L.; Tian, F.-F.; Ge, Y.-S.; Jiang, F.-L.; Lai, L.; Li, D.-W.; Yu, Q.-L.; Wang, J.; Lin, C.; Liu, Y. Spectroscopic, structural and thermodynamic properties of chlorpyrifos bound to serum albumin: A comparative study between BSA and HSA. J. Photochem. Photobiol. B Biol. 2012, 109, 1–11. [Google Scholar] [CrossRef]
- Ni, R.; Wang, Y.; Wei, X.; Chen, J.; Meng, J.; Xu, F.; Liu, Z.; Zhou, Y. Magnetic carbon nanotube modified with polymeric deep eutectic solvent for the solid phase extraction of bovine serum albumin. Talanta 2020, 206, 120215. [Google Scholar] [CrossRef]
- Geng, F.; Zheng, L.; Liu, J.; Yu, L.; Tung, C. Interactions between a surface active imidazolium ionic liquid and BSA. Colloid Polym. Sci. 2009, 287, 1253–1259. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B. Bovine Serum Albumin as a Versatile Platform for Cancer Imaging and Therapy. Curr. Med. Chem. 2018, 25, 2938–2953. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K. The history of monoclonal antibody development–Progress, remaining challenges and future innovations. Ann. Med. Surg. 2014, 3, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef]
- Satish, L.; Millan, S.; Sahoo, H. Spectroscopic insight into the interaction of bovine serum albumin with imidazolium-based ionic liquids in aqueous solution. Luminescence 2017, 32, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Lipman, N.S.; Jackson, L.R.; Trudel, L.J.; Weis-Garcia, F. Monoclonal Versus Polyclonal Antibodies: Distinguishing Characteristics, Applications, and Information Resources. ILAR J. 2005, 46, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. mAbs 2010, 2, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Laptoš, T.; Omersel, J. The importance of handling high-value biologicals: Physico-chemical instability and immunogenicity of monoclonal antibodies (Review). Exp. Ther. Med. 2018, 15, 3161–3168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, Y.; Zhang, H.; Cao, J.; Fei, Z.; Wang, Y. Impact of the alkyl chain length on binding of imidazolium-based ionic liquids to bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 323–333. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Coutinho, J.A.P.; Fernandes, A.M. Hydrolysis of Tetrafluoroborate and Hexafluorophosphate Counter Ions in Imidazolium-Based Ionic Liquids. J. Phys. Chem. A 2009, 114, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Barik, S.; Sarkar, M. Probing the Interactions of 1-Alkyl-3-methylimidazolium Tetrafluoroborate (Alkyl = Octyl, Hexyl, Butyl, and Ethyl) Ionic Liquids with Bovine Serum Albumin: An Alkyl Chain Length-Dependent Study. J. Phys. Chem. B 2019, 123, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.R.; Shanmugam, G.; Madhan, B.; Kumar, B.V.N.P. Selective binding and dynamics of imidazole alkyl sulfate ionic liquids with human serum albumin and collagen–A detailed NMR investigation. Phys. Chem. Chem. Phys. 2018, 20, 9256–9268. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Figueiredo, A.M.; Cabrita, E.J. Epitope mapping of imidazolium cations in ionic liquid–protein interactions unveils the balance between hydrophobicity and electrostatics towards protein destabilisation. Phys. Chem. Chem. Phys. 2014, 16, 23394–23403. [Google Scholar] [CrossRef]
- Shu, Y.; Liu, M.; Chen, S.; Chen, X.; Wang, J. New Insight into Molecular Interactions of Imidazolium Ionic Liquids with Bovine Serum Albumin. J. Phys. Chem. B 2011, 115, 12306–12314. [Google Scholar] [CrossRef]
- Satish, L.; Millan, S.; Sasidharan, V.V.; Sahoo, H. Molecular level insight into the effect of triethyloctylammonium bromide on the structure, thermal stability, and activity of Bovine serum albumin. Int. J. Biol. Macromol. 2018, 107, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Satish, L.; Millan, S.; Bera, K.; Mohapatra, S.; Sahoo, H. A spectroscopic and molecular dynamics simulation approach towards the stabilizing effect of ammonium-based ionic liquids on bovine serum albumin. New J. Chem. 2017, 41, 10712–10722. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef]
- Frizzo, C.P.; Wust, K.; Tier, A.Z.; Beck, T.S.; Rodrigues, L.V.; Vaucher, R.A.; Bolzan, L.P.; Terra, S.; Soares, F.; Martins, M.A.P. Novel ibuprofenate- and docusate-based ionic liquids: Emergence of antimicrobial activity. RSC Adv. 2016, 6, 100476–100486. [Google Scholar] [CrossRef]
- Kumar, S.; Kukutla, P.; Devunuri, N.; Venkatesu, P. How does cholinium cation surpass tetraethylammonium cation in amino acid-based ionic liquids for thermal and structural stability of serum albumins? Int. J. Biol. Macromol. 2020, 148, 615–626. [Google Scholar] [CrossRef]
- Dhiman, D.; Bisht, M.; Tavares, A.P.M.; Freire, M.G.; Venkatesu, P. Cholinium-Based Ionic Liquids as Efficient Media for Improving the Structural and Thermal Stability of Immunoglobulin G Antibodies. ACS Sustain. Chem. Eng. 2022, 10, 5404–5420. [Google Scholar] [CrossRef]
- Reslan, M.; Ranganathan, V.; Macfarlane, D.R.; Kayser, V. Choline ionic liquid enhances the stability of Herceptin® (trastuzumab). Chem. Commun. 2018, 54, 10622–10625. [Google Scholar] [CrossRef] [PubMed]
- Mazid, R.R.; Vijayaraghavan, R.; MacFarlane, D.R.; Cortez-Jugo, C.; Cheng, W. Inhibited fragmentation of mAbs in buffered ionic liquids. Chem. Commun. 2015, 51, 8089–8092. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fernandez, A.; Edler, K.J.; Arnold, T.; Venero, D.A.; Jackson, A.J. Protein conformation in pure and hydrated deep eutectic solvents. Phys. Chem. Chem. Phys. 2017, 19, 8667–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.-J.; Sun, C.; Xu, X.-B.; Zhou, D.-Y.; Song, L.; Zhu, B.-W. Improving the functional properties of bovine serum albumin-glucose conjugates in natural deep eutectic solvents. Food Chem. 2020, 328, 127122. [Google Scholar] [CrossRef]
- Bhakuni, K.; Yadav, N.; Venkatesu, P. A novel amalgamation of deep eutectic solvents and crowders as biocompatible solvent media for enhanced structural and thermal stability of bovine serum albumin. Phys. Chem. Chem. Phys. 2020, 22, 24410–24422. [Google Scholar] [CrossRef]
- Kumari, M.; Kumari, P.; Kashyap, H.K. Structural adaptations in the bovine serum albumin protein in archetypal deep eutectic solvent reline and its aqueous mixtures. Phys. Chem. Chem. Phys. 2022, 24, 5627–5637. [Google Scholar] [CrossRef]
- Gorke, J.; Srienc, F.; Kazlauskas, R. Toward advanced ionic liquids. Polar, enzyme-friendly solvents for biocatalysis. Biotechnol. Bioprocess Eng. 2010, 15, 40–53. [Google Scholar] [CrossRef]
- Patel, N.; Rai, D.; Shivam, S.; Shahane, S.; Mishra, U. Lipases: Sources, Production, Purification, and Applications. Recent Pat. Biotechnol. 2019, 13, 45–56. [Google Scholar] [CrossRef]
- Bancerz, R. Industrial application of lipases. Postepy Biochem. 2017, 63, 335–341. [Google Scholar]
- Sharma, R.; Sharma, N. Microbial Lipase Mediated by Health Beneficial Modification of Cholesterol and Flavors in Food Products: A Review. Recent Pat. Biotechnol. 2018, 12, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ercan, D.; Demirci, A. Recent advances for the production and recovery methods of lysozyme. Crit. Rev. Biotechnol. 2015, 36, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Manickam, P.; Kaushik, A.; Karunakaran, C.; Bhansali, S. Recent advances in cytochrome c biosensing technologies. Biosens. Bioelectron. 2017, 87, 654–668. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.; Shahane, S.; Shivam; Majumdar, R.; Mishra, U. Mode of Action, Properties, Production, and Application of Laccase: A Review. Recent Pat. Biotechnol. 2019, 13, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Shrivastava, R.; Agrawal, P.K. Bioprospecting and biotechnological applications of fungal laccase. 3 Biotech. 2016, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Pezzella, C.; Guarino, L.; Piscitelli, A. How to enjoy laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef]
- Reslan, M.; Kayser, V. Ionic liquids as biocompatible stabilizers of proteins. Biophys. Rev. 2018, 10, 781–793. [Google Scholar] [CrossRef]
- Schindl, A.; Hagen, M.L.; Muzammal, S.; Gunasekera, H.A.D.; Croft, A.K. Proteins in Ionic Liquids: Reactions, Applications, and Futures. Front. Chem. 2019, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Bhakuni, K.; Venkatesu, P. Strategic planning of proteins in ionic liquids: Future solvents for the enhanced stability of proteins against multiple stresses. Phys. Chem. Chem. Phys. 2019, 21, 23269–23282. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, T.A.; Singh, L.R.; Rather, G.M.; Bhat, M.A. Structural-functional integrity of lysozyme in imidazolium based surface active ionic liquids. Int. J. Biol. Macromol. 2020, 156, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.S.; Freire, C.; de Souza, R.L.; Cabrera-Padilla, R.Y.; Pereira, M.M.; Freire, M.G.; Lima, Á.S.; Soares, C.M.F. Effects of phosphonium-based ionic liquids on the lipase activity evaluated by experimental results and molecular docking. Biotechnol. Prog. 2019, 35, e2816. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, P.A.M.; Pereira, J.F.B.; Santos-Ebinuma, V.D.C. Insights into the effect of imidazolium-based ionic liquids on chemical structure and hydrolytic activity of microbial lipase. Bioprocess Biosyst. Eng. 2019, 42, 1235–1246. [Google Scholar] [CrossRef]
- Shahriari, S.; Tomé, L.C.; Araújo, J.M.M.; Rebelo, L.P.N.; Coutinho, J.A.P.; Marrucho, I.M.; Freire, M.G. Aqueous biphasic systems: A benign route using cholinium-based ionic liquids. RSC Adv. 2012, 3, 1835–1843. [Google Scholar] [CrossRef]
- Nascimento, P.A.M.; Picheli, F.P.; Lopes, A.M.; Pereira, J.F.B.; Santos-Ebinuma, V.D.C. Effects of cholinium-based ionic liquids on Aspergillus niger lipase: Stabilizers or inhibitors. Biotechnol. Prog. 2019, 35, e2838. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Harter, G.A.; Martin, C.J. “Water-like” Dual-Functionalized Ionic Liquids for Enzyme Activation. ACS Omega 2019, 4, 15234–15239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, S.P.; Marques, C.S.; Rosatella, A.A.; Afonso, C.A.; Gonçalves, F.; Coutinho, J.A. Toxicity assessment of various ionic liquid families towards Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2012, 76, 162–168. [Google Scholar] [CrossRef]
- Arunkumar, R.; Drummond, C.; Greaves, T.L. FTIR Spectroscopic Study of the Secondary Structure of Globular Proteins in Aqueous Protic Ionic Liquids. Front. Chem. 2019, 7, 74. [Google Scholar] [CrossRef]
- Attri, P.; Choi, S.; Kim, M.; Shiratani, M.; Cho, A.E.; Lee, W. Influence of alkyl chain substitution of ammonium ionic liquids on the activity and stability of tobacco etch virus protease. Int. J. Biol. Macromol. 2020, 155, 439–446. [Google Scholar] [CrossRef]
- Attri, P.; Venkatesu, P. Exploring the thermal stability of α-chymotrypsin in protic ionic liquids. Process Biochem. 2013, 48, 462–470. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Jena, S.; Tulsiyan, K.D.; Dutta, J.; Chakrabarty, S.; Biswal, H.S. Amino-Acid-Based Ionic Liquids for the Improvement in Stability and Activity of Cytochrome c: A Combined Experimental and Molecular Dynamics Study. J. Phys. Chem. B 2019, 123, 10100–10109. [Google Scholar] [CrossRef] [PubMed]
- Nian, B.; Cao, C.; Liu, Y. Activation and stabilization of Candida antarctica lipase B in choline chloride-glycerol-water binary system via tailoring the hydrogen-bonding interaction. Int. J. Biol. Macromol. 2019, 136, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, N.; Schrebler, R.A.; de María, P.D. From batch to fed-batch and to continuous packed-bed reactors: Lipase-catalyzed esterifications in low viscous deep-eutectic-solvents with buffer as cosolvent. Bioresour. Technol. 2019, 273, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Gojun, M.; Bačić, M.; Ljubić, A.; Šalić, A.; Zelić, B. Transesterification in Microreactors—Overstepping Obstacles and Shifting Towards Biodiesel Production on a Microscale. Micromachines 2020, 11, 457. [Google Scholar] [CrossRef]
- Huang, L.; Bittner, J.P.; de María, P.D.; Jakobtorweihen, S.; Kara, S. Modeling Alcohol Dehydrogenase Catalysis in Deep Eutectic Solvent/Water Mixtures. ChemBioChem 2019, 21, 811–817. [Google Scholar] [CrossRef]
- Khodaverdian, S.; Dabirmanesh, B.; Heydari, A.; Dashtban-Moghadam, E.; Khajeh, K.; Ghazi, F. Activity, stability and structure of laccase in betaine based natural deep eutectic solvents. Int. J. Biol. Macromol. 2018, 107, 2574–2579. [Google Scholar] [CrossRef]
- Toledo, M.L.; Pereira, M.M.; Freire, M.G.; Silva, J.P.A.; Coutinho, J.A.P.; Tavares, A.P.M. Laccase Activation in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 11806–11814. [Google Scholar] [CrossRef]
- Ma, Z.; Mi, Y.; Han, X.; Li, H.; Tian, M.; Duan, Z.; Fan, D.; Ma, P. Transformation of ginsenoside via deep eutectic solvents based on choline chloride as an enzymatic reaction medium. Bioprocess Biosyst. Eng. 2020, 43, 1195–1208. [Google Scholar] [CrossRef]
- Miranda-Molina, A.; Xolalpa, W.; Strompen, S.; Arreola-Barroso, R.; Olvera, L.; López-Munguía, A.; Castillo, E.; Saab-Rincon, G. Deep Eutectic Solvents as New Reaction Media to Produce Alkyl-Glycosides Using Alpha-Amylase from Thermotoga maritima. Int. J. Mol. Sci. 2019, 20, 5439. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.-N.; Dou, Y. Deep eutectic solvents for biocatalytic transformations: Focused lipase-catalyzed organic reactions. Appl. Microbiol. Biotechnol. 2020, 104, 1481–1496. [Google Scholar] [CrossRef]
| ILs | Proteins | Remarks | Ref. |
|---|---|---|---|
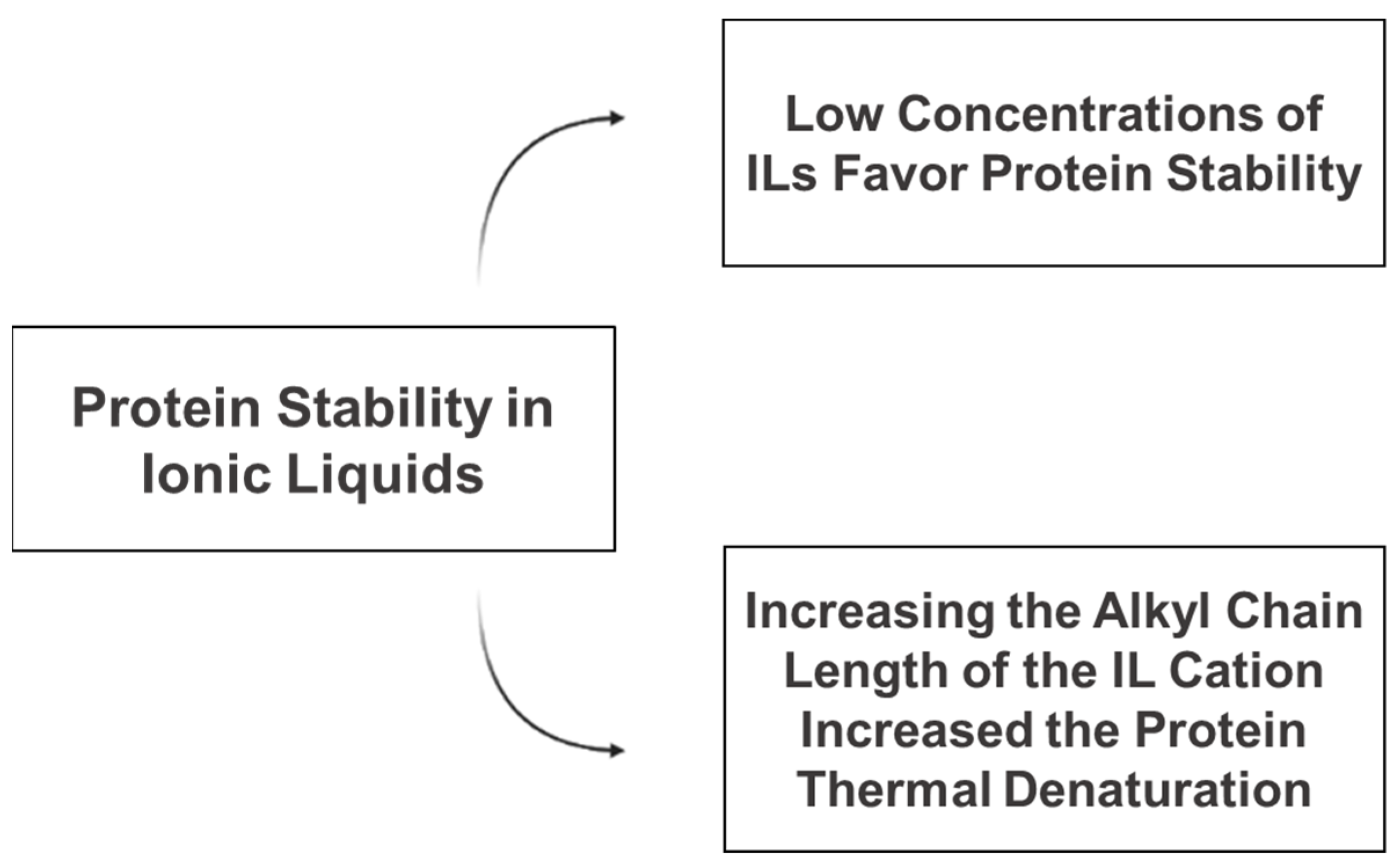
| [C14mim]Br | BSA | BSA secondary structure was stabilized at a low concentration of IL but destroyed at a high concentration. | [33] |
| [C2mim][EtSO4], [C2mim]Cl, [C4mim]Cl | BSA | Destabilization was due to hydrophobicity. In the presence of more hydrophobic IL, destabilization increased fast as a function of IL concentration. | [38] |
| [C2mim]Cl, [C4mim]Cl, [C6mim]Cl, [C8mim]Cl, [C10mim]Cl, [C12mim]Cl | BSA | Longer alkyl chains have a stronger binding interaction with BSA and larger conformational damage to the protein. | [42] |
| [C8mim][BF4], [C2mim][BF4], [C4mim][BF4], [C6mim][BF4] | BSA | Shorter alkyl chain-containing ILs did not bind at all with the proteins, and the binding interactions are initiated in the presence of [C6mim][BF4] and reach the maximum in the case of [C8mim][BF4]. | [44] |
| [C2mim]Cl, [C2mim][dca], [C4mim]Cl, [C4mim][dca], [C2OHmim]Cl, [C2OHmim][dca], [C4dmim]Cl, [C3Omim]Cl | HSA | Increasing the alkyl chain length increased the denaturation. | [46] |
| [C4mim][HSO4], [C4mim][C1SO4], [C4mim][C8SO4], [C4mim][C12SO4] | HSA | The binding affinity between IL and HSA was enhanced with an increase in the alkyl chain length of the anionic moiety of the IL. | [45] |
| [EPMpyr][Sal] | BSA | Increasing the alkyl chain length of the IL enhanced the thermal denaturation of BSA. | [6] |
| [N2888]Br | BSA | BSA was stable in the IL concentration up to <0.02 M. At a higher concentration of IL, there was a destabilizing effect. | [48] |
| [L−LeuOEt][KETO], [L−ValOEt][KETO], [L−ValOiPr][KETO], [L−ValOPr][KETO], [L−ValOBu][KETO] | BSA | The affinity of the ILs to BSA is within the range of the estimated KA (105 L mol−1), which indicates strong IL-BSA interactions. | [28] |
| [Ch][Trp], [TEA][Trp] | BSA, HSA | Both AAILs increased thermal stability in BSA and HSA. | [52] |
| [Ch][Ac], [Ch]Cl, [Ch][Dhc], [Ch][Dhp] | IgG | Thermal and structural stability of IgG enhanced | [53] |
| [Ch][Dhp] | Trastuzumab | [Ch][Dhp] stabilizes trastuzumab at high concentrations and in combination with other excipients, against unfolding and irreversible aggregation. | [54] |
| b[Ch][Dhp] | EGFR mAb | mAbs display extended continual stability in ILs under non-ideal storage conditions at high temperatures and in the presence of contaminants. | [55] |
| ILs | Proteins | Remarks | Ref. |
|---|---|---|---|
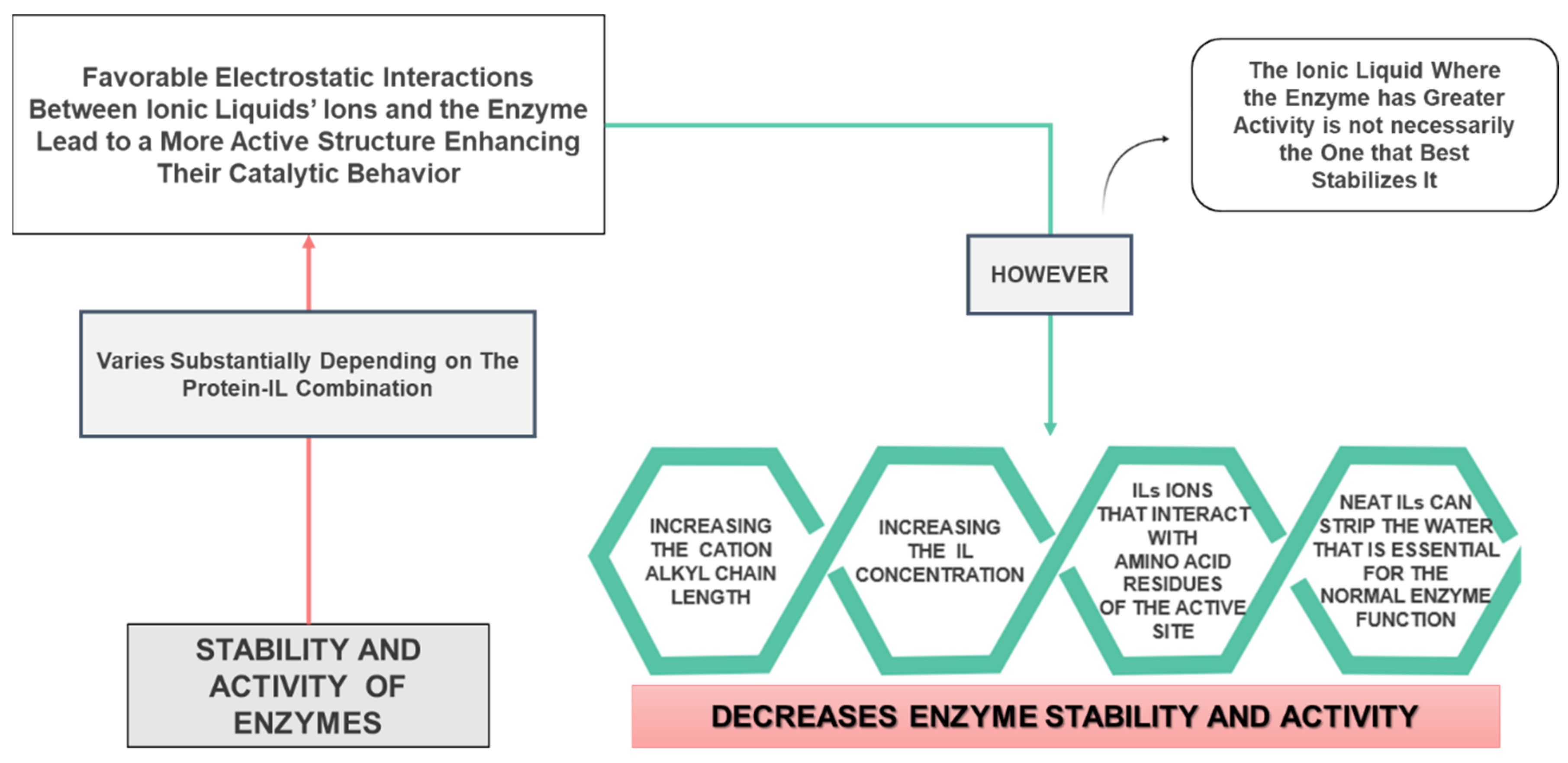
| [P4444]+, [P444(14)]+, [P666(14)]+ cations combined with Cl−, Br−, [Deca]−, [Phosp]−, [NTf2]− anions | BCL | Maximum activity of BCL in the IL [P666(14)][NTf2]. ILs with [Phosp]− and [NTf2]− anions increase BCL activity, while the remaining display a negative effect. Enzyme activity decreases with the increase in the cation alkyl chain length. | [74] |
| [Cnmim]Cl (n = 4, 6, 8, 10 and 12) | Aspergillus niger lipase | Enzyme activity decreases with the increase in the cation alkyl chain length. For n = 4 and 6, the ILs stabilized and promoted lipase activity. For n ≥ 8, ILs could maintain, decrease or suppress the enzyme activity, depending on the IL concentration. | [75] |
| [Ch]Cl, [Ch][Ac] [Ch][Prop], [Ch][But], [Ch][Pent] and [Ch][Hex] | Aspergillus niger lipase | Enzymatic activity maintained or enhanced below 0.1 M of all ILs. Lipase conformational integrity decreased with the increase in the anion alkyl chain length. The enzyme biocatalytic behavior was maintained by [Ch][Ac] and unaffected by the IL concentration and incubation time. | [77] |
| [Et-Im-t-BuOH][Tf2N], [Bu-Im-t-BuOH][Tf2N], [CH3OCH2CH2-Im-Et][Tf2N] and [CH3OCH2CH2-Im-t-BuOH][Tf2N] | CALB | Higher CALB activities than the nonfunctionalized imidazolium-based ILs. Enzymatic activity is significantly affected by small changes in water content. Enhanced thermal stability of CALB in [CH3OCH2CH2-Im-Et][Tf2N] and [CH3OCH2CH2-Im-t-BuOH][Tf2N]. | [78] |
| ([C8mim][DBS] and [C12mim][DBS] | Lysozyme | [C8mim][DBS] promoted the enzyme stability at the concentration range of 0.5 mM–1.35 Mm. [C12mim][DBS] destabilizes the enzyme. | [73] |
| EAA, EtAA, DetAA, TetAA, EAN and EAF. | Lysozyme, trypsin, β-lactoglobulin and α-amylase | Lysozyme and trypsin are more stable in IL with formate/nitrate anions (EAN and EAF) at the most diluted concentrations. Both α-amylase and β-lactoglobulin present reduced stability and solubility in the IL solutions. | [80] |
| TEAP and DEAP | TEV protease | Increasing the IL concentration led to decreased stability of TEVp. Enhanced thermal stability in both ILs at 10% (v/v). TEAP stabilized TEVp better than DEAP but TEVp was only active in DEAP. | [81] |
| [Pro][NO3] and [Ch][Pro] | Cyt-C | Both ILs at low concentration (1 mM) stabilized cyt-c and allowed long-term storage. Cyt-C was not denatured in neat [Ch][Pro]. | [83] |
| ILs | Proteins | Remarks | Ref. |
|---|---|---|---|
| ChCl:Gly (1:2) | CALB | Increasing the molar fraction of water increased the polarity and decreased the viscosity of the DES. A molar ration of water of 0.3 added to the DES led to an increase of 57% in CALB activity. Higher amounts of water (<0.7) denature the DES and reduce the enzyme stability and activity. | [84] |
| ChCl:Gly (1:2) | CALB | The DES was used as a reaction media and substrate. The conversion rate increased by 54% in seven cycles of operation. | [85] |
| ChCl:Gly (1:3) | TIL | Adding water up to 4 wt% to the DES increased the biodiesel yield. Above 8 wt% of water added, the yield and productivity were lower than in neat DES. | [86] |
| ChCl:Gly (1:2) | ADH | The enzyme activity was reduced in the DES even with high water content (20% (v/v)) added. | [87] |
| HBA: ChCl and Bet HBD: sorbitol, Gly, urea, citric, malic and oxalic acid | Laccase | ChCl-based DESs deactivated the enzyme. The highest enzymatic activity and stability were observed in Bet:Gly (1:2). | [88] |
| HBA: ChCl, ChDHP, ChDHC and Bet HBD: EtG, Gly, Ery and Xyl | Laccase | ChCl-based DESs deactivated the enzyme. Enzyme activity was enhanced by HBDs with an increasing number of hydroxyl groups. ChDHC:Ery and ChDH:Xyl increased the laccase activity up to 200%. A maximum activity enhancement of 20% was observed in all Bet-based DESs. ChDHP:Xyl provided remarkable long-term storage at −80 °C. | [89] |
| ChCl:EtG (2:1) | β-glucosidase | The half-life of β-glucosidase was increased by 96%. At 60 °C, the conversion yield was increased by 80.6% compared with the yield in the buffer. | [90] |
| ChCl-based DES | Amy A | Neat DESs completely inhibited enzyme activity. Aqueous solutions ChCl-based DESs composed of HBDs consisting of alcohols, sugars, and amides showed promising results. | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, J.S.; Capela, E.V.; Loureiro, A.M.; Tavares, A.P.M.; Freire, M.G. An Overview on the Recent Advances in Alternative Solvents as Stabilizers of Proteins and Enzymes. ChemEngineering 2022, 6, 51. https://doi.org/10.3390/chemengineering6040051
Almeida JS, Capela EV, Loureiro AM, Tavares APM, Freire MG. An Overview on the Recent Advances in Alternative Solvents as Stabilizers of Proteins and Enzymes. ChemEngineering. 2022; 6(4):51. https://doi.org/10.3390/chemengineering6040051
Chicago/Turabian StyleAlmeida, Jéssica S., Emanuel V. Capela, Ana M. Loureiro, Ana P. M. Tavares, and Mara G. Freire. 2022. "An Overview on the Recent Advances in Alternative Solvents as Stabilizers of Proteins and Enzymes" ChemEngineering 6, no. 4: 51. https://doi.org/10.3390/chemengineering6040051
APA StyleAlmeida, J. S., Capela, E. V., Loureiro, A. M., Tavares, A. P. M., & Freire, M. G. (2022). An Overview on the Recent Advances in Alternative Solvents as Stabilizers of Proteins and Enzymes. ChemEngineering, 6(4), 51. https://doi.org/10.3390/chemengineering6040051










