Abstract
In recent decades, the environmental impact caused by greenhouse gases, especially CO2, has driven many countries to reduce the concentration of these gases. The study and development of new designs that maximise the efficiency of CO2 capture continue to be topical. This paper presents a review of the application of hydrotalcites as CO2 sinks. There are several parameters that can make hydrotalcites suitable for use as CO2 sinks. The first question is the use of calcined or uncalcined hydrotalcite as well as the temperature at which it is calcined, since the calcination conditions (temperature, rate and duration) are important parameters determining structure recovery. Other aspects were also analysed: (i) the influence of the pH of the synthesis; (ii) the molar ratio of its main elements; (iii) ways to increase the specific area of hydrotalcites; (iv) pressure, temperature, humidity and time in CO2 absorption; and (v) combined use of hydrotalcites and cement-based materials. A summary of the results obtained so far in terms of CO2 capture with the parameters described above is presented. This work can be used as a guide to address CO2 capture with hydrotalcites by showing where the information gaps are and where researchers should apply their efforts.
1. Introduction
The incessant consumption of energy, which goes hand in hand with modern life in developed countries, has negative effects on the quality of the environment and ecosystems. These impacts, caused by greenhouse gases (GHGs), are leading many countries to adopt responsible environmental policies. Carbon dioxide (CO2) is the dominant anthropogenic greenhouse gas (76%), responsible for global warming [1]. Before the industrial revolution (1760s), the CO2 level was about 280 ppm [2], while today it could be considered at an average level of 400 ppm [3,4]. According to the International Energy Agency (IEA, 2017), the global mean temperature has increased by 1 °C above the preindustrial level due to anthropogenic greenhouse gas emissions [5]. The increase in global average temperature is expected to reach 1.5 °C by the end of 2040 [6,7], and it is therefore necessary to take measures to reduce these CO2 levels.
To reduce these levels, two main carbon capture (CC) technologies are being presented [6,8]: (i) carbon capture, transport and storage technologies (CCS); and (ii) carbon capture and utilisation technologies (CCU). CCSs are primarily aimed at mitigating GHGs when fossil fuels are used for energy generation. CCS technologies are classified into three types: pre-combustion, post-combustion, and oxy-fuel combustion capture [6]. CCS technologies would remove around 20% of GHG emission by 2050 [9]. Captured CO2 can be a source of recycled carbon, and CCU can provide more services and greater climate change savings than capturing and storing CO2 underground [10]. The use of CO2 gives an added value to these GHGs, which, together with the circular economy concept, can mitigate climate change [11,12]
Therefore, CO2 capture is expected to play an important role in the commercialisation of future CCS technologies [13]. Many countries and research teams are considering various candidate processes and materials [14], such as absorption [15], adsorption [16,17]; membranes [18] and cryogenic distillation systems [19]. Solid sorbents (carbon, silica, calcium oxide, among others) are expanding as an emerging alternative for CO2 capture, due to their great characteristics for such a task [20,21,22]. According to their temperature of use, solid sorbents can be classified as follows [23]: (a) low temperature (<200 °C) [24,25,26,27]; (b) intermediate temperature (between 200–400 °C) [28,29]; and (c) high-temperature (> 400 °C).
Hydrotalcites are brucite-like layered materials, that have been known for over 150 years with a general formula of , where M2+ and M3+ represent divalent and trivalent cations, respectively, and A represents the interlayered anion [30,31,32,33,34,35]. Hydrotalcites are known in the bibliography as LDHs (Layered double hydroxides). The layer charge is determined by the molar ratio, that is x = M3+/(M3+ + M2+), and it varies between 0.2 and 0.4 [36,37,38]. The Mg3AlCO3 hydrotalcite is a type of LDH commonly found in nature. Reviewing the literature, a wide range of applications of these materials can be found [3]: catalytic applications [36,39,40,41,42], medical applications [43,44,45,46] as additives for polymers [47,48], for adsorption of pollutants [49,50,51], water decontamination [52,53], waste barriers [54,55,56,57,58], among various other applications. Several chemical companies (e.g., BASF, SASOL, Clariant, Kisuma Chemicals, Sakai Chemicals, etc.) produce several thousands of tonnes yearly, so it is an easily-available product [19]. Hydrotalcites, as such, are not good CO2 absorbents due to poor basic properties and presence of entities that hinder CO2 adsorption and are therefore subjected to thermal treatment (around 500 °C) to obtain nearly amorphous metastable mixed solid solutions known as calcined layered double hydroxides (CLDHs) [19]. In CLDHs, there is a loss of mass and a breakdown of its structure, forming an oxide, according to Equation (1) [59]:
The CO2 emitted during calcination (Equation (1)) is identical to the CO2 captured during the synthesis of hydrotalcite (Equation(2)):
Consequently, the CO2 balance of the calcined hydrotalcite is 0 (Equations (1) and (2)). In this sense, all the CO2 captured by the calcined hydrotalcite represents a negative CO2 balance. This will reduce the carbon footprint of those materials to which calcined hydrotalcite is added.
Another characteristic of hydrotalcite is the memory effect, which allows the reconstruction of the original shape of hydrotalcite when it is in a humid environment and in the presence of CO2. CLDHs in a CO2 environment return to its initial state of LDHs [60,61,62,63]. The CO2 capture balance by the hydrotalcite in its reconstruction is positive and hence the interest of using this material as a CO2 capture material is very great (in the last decade) [64]. Nowadays, the challenge is to develop new types of hydrotalcites with higher CO2 sorption capacities, higher sorption/desorption kinetics, and good stability throughout consecutive reutilisation cycles in similar operation conditions as those applied in a sorption-enhanced steam reforming process [65].
These hydrotalcites (in their LDH or CLDHs form) can also be found as additives to cement-based materials to improve resistance to chloride attack [66], durability [67,68,69,70] and even the use of LDH as additives to improve the thermal insulation of the intumescent fire retardant (IFR) coating [71]. Wu et al. [72] indicated that the structure regeneration of CLDHs in a cement paste environment had also been revealed [69]. After calcination, a large number of active sites produced what in favour of the improvement effect of CLDH on cement [72]. Since hydrotalcites may be incorporated into various building material mixtures, mortars, concretes and backfills, their application as accessible and affordable materials is prospective [73,74,75]. However, research in the field of hydrotalcites and cement-based materials is still insufficient [72,76]. It is also difficult to find studies that add LDH or CLDH to alkaline-activated materials [77,78].
It is even more difficult to find studies in which CLDHs are used as additives in order to increase the CO2 capture capacity of cement-based materials. Ma et al. [79] reported the adsorption of CO32− by CLDH seven times faster than LDH due to the release of anions after calcination, which can be very beneficial for CO2 capture. Suescum-Morales et al. [3,59] added different percentages of CLDH (calcined Mg3AlCO3) in a one-coat mortar in order to increase the CO2 capture capacity. Adding 5% of calcined hydrotalcite increased the CO2 capture capacity by 8.52% with respect to the reference mortar.
This paper presents a review of the application of hydrotalcites as CO2 sinks. Different aspects were analysed: pH of the synthesis, the molar ratio (Mg/Al), the specific area, pressure, temperature and time in CO2 absorption. A summary of the results obtained so far in terms of CO2 capture with the parameters described above is presented. This work can be used as a guide to address CO2 capture with hydrotalcites by showing where the information gaps are and where researchers should apply their efforts.
2. Calcined or Uncalcined Hydrotalcite to Capture CO2?
The answer is immediate: calcined hydrotalcite or its use under high temperatures (around 400 °C so that the hydrotalcite becomes oxide and can be rebuilt in contact with CO2). LDH are poor CO2 adsorbents in their natural or unburned form, which is due to a poor basic property and the presence of entities that hinder CO2 adsorption. Hence, they are subjected to thermal treatment to obtain nearly amorphous metastable mixed solid solutions (CLDHs) [19]. Figure 1 shows two diagrams representing what happens during the calcination of hydrotalcite of Mg3AlCO3 as shown (A) by W.J. Long et al. [77] and (B) by Lauermannová et al. [73]. Both schemes attempt to represent the collapse of the structure due to the loss of interlayer anions and moisture.
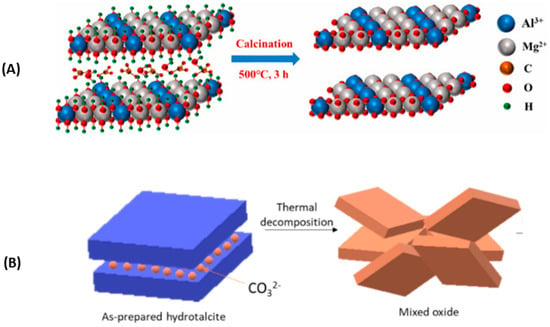
Figure 1.
Structural changes of LDH after calcination according to adapted from W.J. Long et al. [77] (A) and Lauermannová et al. [73] (B) (open Access).
2.1. Thermal Behaviour of Hydrotalcite by TGA/DTA
LDH undergoes several stages until it reaches CLDHs. There is even research that attempts to explain this process in great detail and focuses on it alone [80,81]. It is very important to choose a suitable calcination temperature, avoiding it being too high (to avoid higher energy consumption), or being too low (not producing the collapse of the structure and hindering a higher CO2 capture). To distinguish the different stages, it is useful to rely on thermogravimetric analysis (TGA) and differential thermal analysis (DTA) carried out by Suescum-Morales et al. [33], shown in Figure 2. It should be noted that different variations in temperature ranges may be encountered, approximately as shown below.
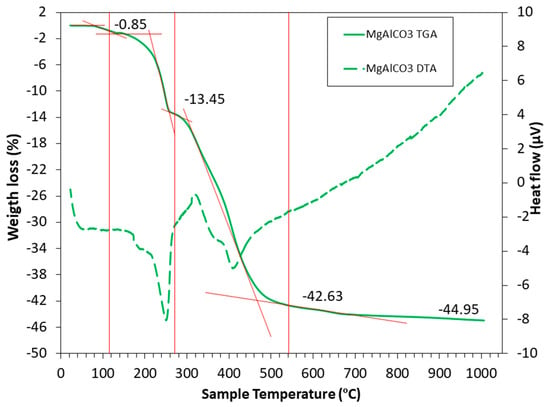
Figure 2.
TGA (solid lines) and DTA (dotted lines) for commercial hydrotalcite of MgAlCO3 [33].
First of all, a loss of humidity is observed, up to a temperature of about 105 °C. The second stage occurs from 105 to 270 °C, where the water hydration of the hydrotalcite structure is lost (leading to a decrease in basal spacing) [82,83,84]. There are authors who indicate that between 200 and 300 °C, the OH− groups attached to Al3+ are lost [19]. The third stage, from 270 to 540 °C, is where the dehydroxilation of the hydrotalcite takes place and the loss of the carbonate anion of the interlayer in the form of CO2 occurs. The layer structure collapses (Figure 1), and the LDH converts to a mixed-oxide MgO-like phase [85]. In the last stage, from 540 to 1000 °C, very small weight losses are observed, attributed to the loss of residual OH− groups.
From the above, it can be seen that the calcination temperature affects the capacity to capture CO2 in CLDHs. Different structural characteristics are presented in the different stages of thermal decomposition. From this analysis, the ideal calcination temperature of the hydrotalcite under study can be determined, which is characteristic and unique depending on the type of hydrotalcite. Most research indicates that the calcination temperature of a Mg-Al LDH is around 400 °C [86,87,88]. However, the performance of a TGA/DTA for each specific case would allow observing the exact calcination temperature. Therefore, specific experimental tests are necessary to further verify the influence of the calcination temperature on adsorption. This is discussed in the following sections of this review, using XRD, SEM/TEM, pH measurements in the synthesis, influence of molar ratio, and specific surface area measurements.
2.2. Thermal Behaviour of Hydrotalcite by XRD
The appropriate calcination temperature can also be determined by XRD temperature variation analysis. The thermal decomposition sequence of Mg-Al-CO3 hydrotalcite is well documented. Miyata, 1980 and Hibino et al., 1995 [82,89] studied the XRD variation at different temperatures, shown in Figure 3 (non-calcined, 150, 250, 350, 500, 850 and 1000 °C). The diffraction patterns of MgO can be identified between 400 °C and 850 °C, given the amorphous nature of Al2O3 at this temperature. For 900 °C the spinal phase (MgAl2O3) was formed. Given these experiences, the following chemical equations can be posed, with the different temperature ranges, to help us understand the process (Equations (3)–(5)):
Figure 4 shows the similar XRD obtained by several authors by calcination at 500 °C for different periods: firstly, the XRD obtained by W.J. Long et al. [77] (Figure 4a) shows how at a temperature of 500 °C for 3 h the layered structure collapses and also shows the production of mixed oxides. Figure 4b shows a similar result, but in this case using a calcination time of 2 h, and the same temperature (500 °C) [33]. This leads to a large saving of energy in the calcination of LDH, with a consequent lowering of the carbon footprint. Already, Z. Yang et al. [67] calcined at 500 °C for 3 h, obtaining a similar result. Even Q.Tao et al. [90] heated at 500 °C for 4 h, obtaining similar results (Figure 4c). Similar results in XRD were obtained by S.I. Garcés Polo et al. [91] for CLDH. None of the previous authors [33,67,77,91] indicated the amount of sample used in the calcination, which may have led to these observed differences. Although different calcination temperatures have been used with similar results, there are no economic studies of the cost of calcination; studies of this type, with times, temperatures and quantities of LDH to be calcined, would be very useful for these materials in industrial applications. It would also be very important to carry out a real carbon footprint calculation (UNE EN 15804:2012), which determines the real CO2 sink capacity in each specific case (amount of hydrotalcite, type of furnace, etc.). Only two studies have been found that indicate the amount of hydrotalcite calcined (100 and 1 g respectively) [92,93]. Annotations of this type, i.e., what quantity is fed into the LDH kiln, are very important in order to maximise the efficiency of the calcination process.
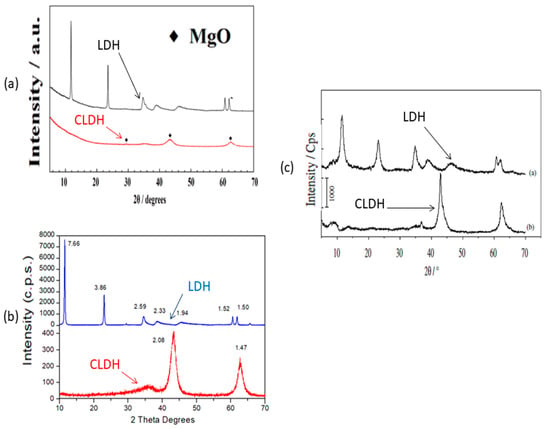
Figure 4.
XRD patterns of (a) LDH and CLDH adapted from W.J. Long et al. [77]; (b) LDH and CLDH of Suescum-Morales et al. [33]; (c) LDH and CLDH adapted from Q.Tao et al. [90].
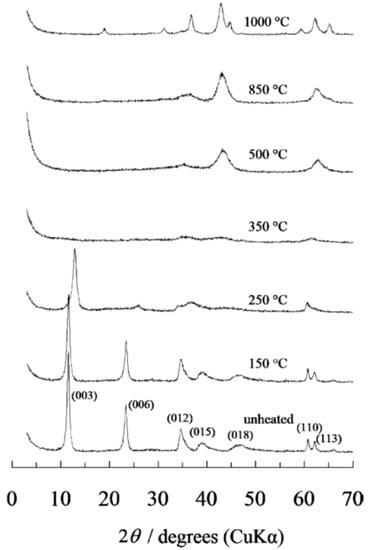
Figure 3.
XRD of MgAlCO3 formed between room temperature and 1000 °C [94].
2.3. Thermal Behaviour of Hydrotalcite by SEM/TEM
Figure 5a shows SEM images of commercial LDH and CLDH Mg-Al from W.J. Long et al. [77]. After 3 h at 500 °C, the structure collapses. A decrease in size was also observed. P. Cai et al. [95] obtained similar results (Figure 5b) with the same temperature and time of calcination. After 4 h at 450 °C on Mg-Al LDH, C. Geng et al. [96] observed a decrease in particle size, and the hexagonal shape was hardly noticeable (Figure 5c). No other studies using different temperatures (different at 500 °C) and calcination times have been found that show SEM images of LDH and CLDH. Studies along these lines could fill this information gap.
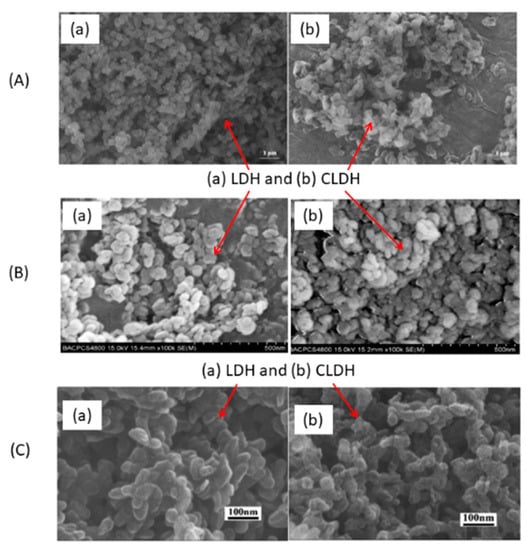
Figure 5.
SEM images of LDH and CLDH Mg-Al of (A) adapted from W.J. Long et al. [77] and (B) adapted from P. Cai et al. [95] and (C) adapted from C. Geng et al. [96].
Figure 6A shows the TEM images of LDH and CLDH, after 2 h at 500 °C, obtained by Suescum-Morales et al. [33]. In CLDH, small pores were formed, attributable to the dehydration process, dehydroxilation of the OH− groups, and to the decomposition of the interlayer carbonate. C Hobbs et al. [97] studied the evolution of a Mg-Al LDH under different temperatures using a rate of 10 °C/min (Figure 6B). At a temperature of 20 °C (LDH), they have a well-defined platelet shape; the porous structure is clearly visible at 850 °C. S Luo et al. [98] also obtained the same porous structure in CLDH, as shown in Figure 6C.
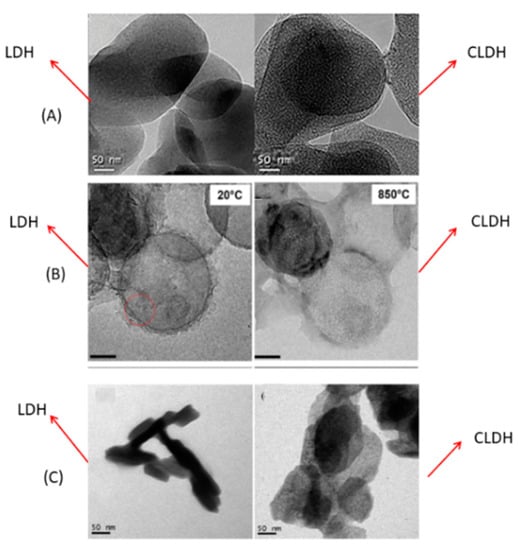
Figure 6.
TEM images of LDH and CLDH Mg-Al of (A) Suescum-Morales et al. [33]; (B) adapted from C Hobbs et al. [97] (open access) and (C) adapted from S Luo et al. [98].
Different heating rates have been used in LDH calcination; a heating rate of 10 °C/min was used in Mg/Al LDH by several authors [99,100,101,102] and a heating rate of 5 °C /min was used by [103]. It is known that if the heating rate is slower, the thermal effects are better observed (better porous structure). However, if the ramp is slower, it takes more time and wastes more energy; if the heating is too fast, the porous structure will be worse. These differences should be extensively studied, both from an energy point of view (higher consumption and, therefore, higher carbon footprint produced by the kiln when the larger ramp is used), and from the point of view of the efficiency of the CLDH itself. Another very important aspect is the kinetics of LDH, which has been extensively discussed in other research [104,105].
3. Influence of the pH Used in the Synthesis in CO2 Capture
Generally, LDH used for CO2 capture has been synthesised by the coprecipitation method [33,34]. In this respect, Wang et al. [106] studied the effect of pH variation on the synthesis (coprecipitation method) in the range of 6.5–14. Subsequently, they studied the effect of the variation of this parameter on CO2 capture. The pH that produced the best adsorption was 12 (23.76 mg/g). In other previous research, Wang et al. [88] indicated that the best pH value used for the synthesis of hydrotalcite oriented for CO2 capture was between 10 and 12.
The crystallinity of the HT samples increases with the pH value of the synthesis, while the BET surface area decreases with increasing synthesis pH (in the range of 6.5–9 pH values). However, from a pH value of 10, the BET surface area increases suddenly, which can be seen in Figure 7, taken from Wang et al. [88].
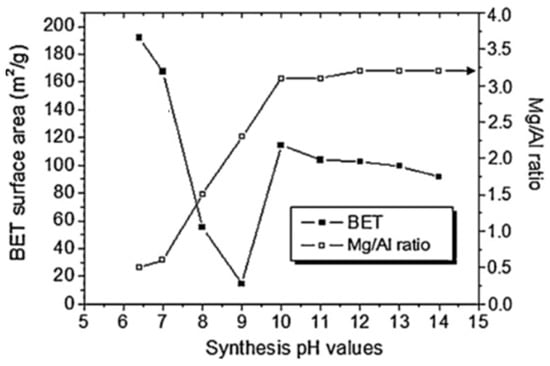
Figure 7.
BET surface area and Mg/Al ratio as function of synthesis pH values adapted from Wang et al [88].
In other research studies, a pH of 7 was used by León et al. [25] and Rossi et al. [27], a pH of 9 was used by Torres-Rodriguez et al. [26], and a pH of 8 by Suescum-Morales et al. [33]. In summary, we must take into account that the pH value in the synthesis is fundamental in the development of the morphology, porous structure, as well as in the chemical composition (Mg/Al ratio).
4. Influence of the Molar Ratio (Mg/Al) of Its Main Elements in CO2 Capture
The properties of LDH are also strongly influenced by the M2+/M3+ ratio, cation type and anion type. For a higher capture capacity of CO2, large interlayer spacing, high layer charge density and a greater number of basic sites are desirable. A high Al content increases the density, but decreases the layer spacing. A high Mg content increases the number of basic sites. In the consulted bibliography, optimal Mg/Al ratios are considered to vary from 1:1 to 3:1 [19,106,107,108,109].
Kim et al. [110] studied the effect of high Mg/Al ratios on the CO2 sorption with hydrotalcites prepared with the coprecipitation method using ratios between 3 and 30. The highest CO2 capture capacity was obtained (407.9 mg/g) for an Mg/Al ratio of 20, using temperatures of 240 °C.
M. Salomé Macedo et al. [111] studied the influence of different Mg/Al ratios (from 2 to 20) to be used as CO2 sorbents at high temperature. The best results were obtained for an Mg/Al ratio of 7 at 1 bar of pressure and 300 °C (71.3 mg/g). These authors indicated that, in general, one observes a gradual increase in the sorption capacity for the same synthesis pH with the increase in the Mg/Al ratio.
The relationship between the pH of the synthesis, the molar ratio and the specific surface area obtained is very important. For example, Kim et al. [110] obtained the highest surface area with a molar ratio of 3 (256 m2/g−). M. Salomé Macedo et al. [111] showed that the BET specific surface area and total pore volume of samples clearly depend on the Mg/Al molar ratio. Suescum-Morales et al. [33] showed that the higher the specific surface area, the higher the CO2 capture capacity. Therefore, the specific surface area seems to be a very important factor in determining the CO2 capture.
As a summary, it can be said that although the molar ratio is very important in terms of CO2 capture, there are also many other factors that affect this parameter: the pH of the synthesis, the nature of the anion, pressure used, and absorption temperature, among others, are the most important.
5. Ways to Increase the Specific Area of Hydrotalcites
The extensive literature presented in this paper highlights the significant interest of researchers in using LDH and CLDH as CO2 adsorbents. Several strategies have been implemented in order to increase the CO2 capture capacities: replacing cations or anions [88], different preparation methods [112], calcination, working temperatures and pressures [113] and alkali doping [29,114], among others.
A good strategy would be to increase the specific surface area, as a larger specific surface area would lead to a higher CO2 capture capacity. Wang et al. [115] intercalated long carbon-chain organic anions, and it increased the CO2 capture capacity. This was due to the increase in the interlayer distance from 0.78 to 3.54 nm (Figure 8). A similar strategy was followed by Li et al. [116], except that in this case they used K2CO3 in the LDH precursor of Mg3Al-stearate, again increasing the interlayer distance, and achieving a higher CO2 capture capacity. A similar strategy was also followed by A. Hanif et al. [117].
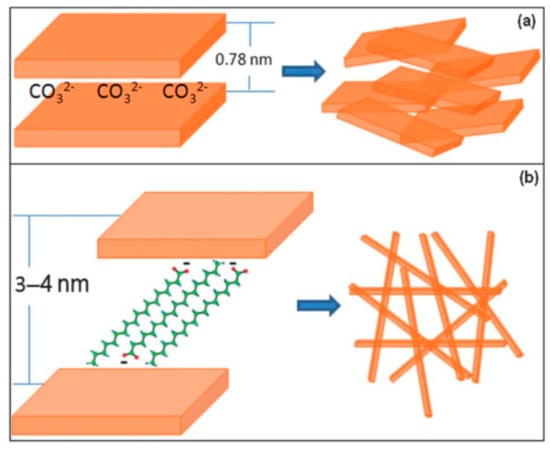
Figure 8.
General schemes of the structural changes of (a) Mg3Al-CO3 and (b) with organic anions adopted from abstract of Wang et al [115].
Another way to increase the specific surface area would be to directly exfoliate the LDH in layers using bottom-up or top-down methods [118], although some authors indicate that the layers could be restacked after drying [119]. To avoid this, García-Gallastegui et al. [86] used graphene oxide as a support, where the negatively charged graphene oxide flakes were well dispersed in the positively charged LDH layers. Another strategy was followed by Othman et al. [120], who coated a MgAlCO3 hydrotalcite with zeolites in order to increase the CO2 capture capacity. K. Wu et al. [121] were the first to use mesoporous alumina to load LDH using the coprecipitation method, obtaining a large BET surface area (278–378 m2/g).
A new method, called aqueous miscible organic solvent treatment (AMOST), uses solvents such as acetone and methanol to wash the LDH wet slurry and remove the intercalated water [119,122]. This method achieves larger surface areas and larger pore volumes [119]. Figure 9 shows the diagram of the synthetic process used for AMOST using acetone and K2CO3.
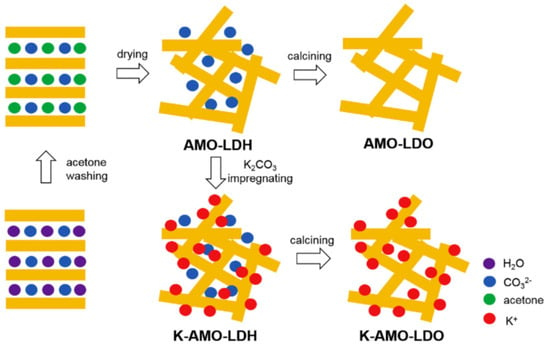
Figure 9.
General scheme of synthetic process of AMOST method using by adopted from Zhu et al [122].
6. Pressure, Temperature, and Capacity in CO2 Absorption and Use of CO2 Captured
There are several parameters that can vary the CO2 capture capacity of a hydrotalcite; among the most important are temperature and pressure [33]. Table 1 shows a comparison of the capture capacity of different types of hydrotalcite under different conditions.

Table 1.
Maximum adsorption capacities of CO2 for hydrotalcites reported in the literature.
As can be seen in Table 1, it is uncommon to use pressures higher than atmospheric pressure to measure CO2 capture capacity, except in some research [2,22,29,38,64,91,92,114,117]. It is much more unusual to find research using high pressures and low temperatures (between 0 and 40 °C) [33,34]. At a pressure of 35 atm, an amount of 1.34 g of CLDH reduce CO2 in 1 m3 of air to preindustrial level [34]. Therefore, researchers have to pool their efforts to study CO2 capture at high pressures and low application temperatures with calcined hydrotalcites.
Another very important factor to take into account will be the analysis of the reversibility conditions of CO2 adsorption–desorption. For those materials in which it is reversible, these could be used to purify different gas streams in cyclic adsorption–desorption processes, as in the case of the use of CaO [21]. CaO could be used to capture CO2 from thermal power plants or cement plants, to be stored and subsequently sold for use in different industrial processes. There is research in which CO2 is used as a curing gas [11], capturing CO2 (5.55 kg CO2/t mix), improving mechanical properties and decreasing curing times (1 day in a CO2 chamber is similar to 7 days in a conventional chamber), which can lead to an improvement in productivity. In addition, in order to avoid the inconvenience of using an accelerated carbonation chamber, an unprecedented strategy has been introduced, where an aqueous solution with injected CO2 is used as kneading water, with very promising results [12]. In the case of irreversible processes, they could be used as CO2 sinks, either alone or incorporated into other materials.
7. Combined Use of Hydrotalcites and Cement-Based Materials
The good and promising results obtained by the scientific community as adsorbents of hydrotalcites suggest the idea of incorporating them into construction materials, such as cement-based materials [66,67,68,69,70]. The ion exchange is the key feature that makes the use of LDH/CLDH in building materials attractive. These, together with the memory effect of CLDH, are the two suitable factors for the ion exchange and capture mechanism shown in Figure 10 [75].
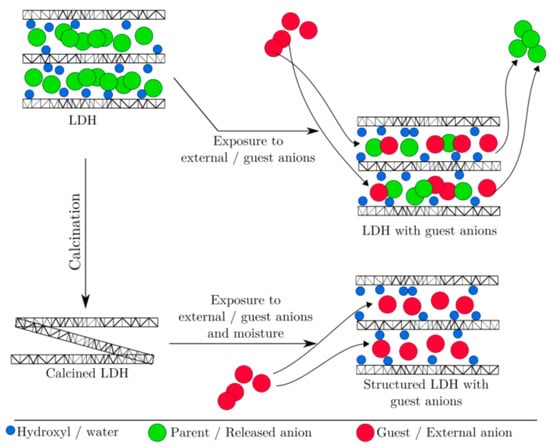
Figure 10.
Schematic representation of the LDH/CLDH structure and ion exchange/captured mechanism represented by Zahid M. Mir [75] (open access).
In recent years, LDH/CLDH have emerged as a new class of engineering materials [75], which can aid in the corrosion control of concrete structures and potentially prolong their service life. Calcined hydrotalcite improves the durability of cement-base materials. One of the first findings mixing hydrotalcites with concrete dates back to 2004 [126]. In 2013, there were two very important contributions in this field [69,126].
This includes the use of LDH as an additive to improve the thermal insulation of the intumescent fire retardant (IFR) coating [71]. Wu et al. [72] indicated that the structure regeneration of CLDHs in a cement paste environment was also revealed [69]. After calcination, a large number of active sites produced were improving the effect of CLDH on cement [72]. Since hydrotalcites may be incorporated into various building materials mixtures, mortars, concretes and backfills, their applications as accessible and affordable materials is prospective [73,74,75]. However, research in the field of hydrotalcites and cement-based materials is still insufficient [72,76].
It is also difficult to find studies that add LDH or CLDH to alkaline activated materials [77,78]. Mixing LDH/CLDH in alkali-activated materials to improve the durability of these materials may be a very interesting field for the scientific community.
It is even more difficult to find studies in which CLDHs are used as additives in order to increase the CO2 capture capacity of cement-based materials. Ma et al. [79] reported the adsorption of CO32− by CLDH seven times faster than LDH due to the release of anions after calcination, which can be very beneficial for CO2 capture. Suescum-Morales et al. [3,59] added different percentages of CLDH (Mg3AlCO3) in a one-coat mortar in order to increase the CO2 capture capacity. Adding 5% of calcined hydrotalcite increased the CO2 capture capacity by 8.52% with respect to the reference mortar. One m2 of one-coat mortar with 5% of calcined hydrotalcites cleans 5540 m3 of air [3]. The use of these one-coat mortars in building facades is a very promising strategy due to the large surface area exposed to the atmosphere. Studies along these lines should be reinforced to improve this information gap.
8. Conclusions
Application of hydrotalcites as CO2 sinks and climate change mitigation is an emerging line of research. In this review, a guide on CO2 capture by hydrotalcites is presented.
Firstly, the behaviour of hydrotalcites and the structural change from LDH to CLDH and their properties with the calcination of LDH are presented. After a review of the literature, although the calcination temperatures are roughly similar, the times required to produce these changes, as well as the amount of hydrotalcite to be calcined, are somewhat diverse. Researchers must work together to clarify these factors so that they are not ambiguous data. These data would be very important since, for industrial use and a real application of these materials as CO2 sinks, technical and economic calculations are necessary, which indicate the feasibility of using these products by companies. On the other hand, hydrotalcites can also be excessively calcined, with the consequent waste of energy (in time or temperature).
Secondly, the pH value in the synthesis is very important in the development of the morphology, porous structure, as well as in the chemical composition. The molar ratio (Mg/Al) is very important in terms of CO2 capture, although there are also many other factors that affect this parameter: pH of the synthesis, nature of the anion, pressure used and absorption temperature.
It is also important to develop strategies that increase the specific surface area of hydrotalcites, as this is one of the most important factors in CO2 absorption capacity. After a comparison of different studies (32 papers) on the capture capacity of LDH and CLDH, a gap in information on CO2 capture capacities at high pressure and low temperature has been observed. Therefore, researchers have to pool their efforts to study CO2 capture at high pressures and low application temperatures using calcined hydrotalcites.
It is very difficult to find studies in which CLDHs are used as additives in order to increase the CO2 capture capacity of cement-based materials. The ion exchange and the memory effect of CLDH are key to the use of hydrotalcites as CO2 capture additives in cement-based materials. Studies along these lines should be reinforced to improve this information gap and mitigate climate change.
Author Contributions
Conceptualization, D.S.-M. and J.M.F.-R.; Methodology, J.R.J.; writing—original draft preparation, D.S.-M.; writing—review and editing, J.M.F.-R. and J.R.J.; supervision, J.M.F.-R.; project administration, J.M.F.-R. and J.R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been supported from the Andalusian Regional Government, Spain (UCO-FEDER 20 REF. 1381172-R).
Data Availability Statement
Not applicable.
Acknowledgments
D. Suescum-Morales also acknowledges funding from MECD-Spain (http://www.mecd.gob.es/educacion-mecd/) FPU 17/04329 (accessed on 1 April 2022). The authors wish to thank the IE 57164 project for the implementation and improvement of scientific and technological infrastructures and equipment supported by the Andalusian Regional Government (FEDER 2011).
Conflicts of Interest
The authors declare no conflict of interest.
References
- USEP Agency. Global Greenhouse Gas Emissions Data. 2022. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 24 March 2022).
- Van Selow, E.R.; Cobden, P.D.; Verbraeken, P.A.; Hufton, J.R.; Brink, R.W.V.D. Carbon Capture by Sorption-Enhanced Water−Gas Shift Reaction Process using Hydrotalcite-Based Material. Ind. Eng. Chem. Res. 2009, 48, 4184–4193. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.R.; Fernández, J.M. Potential CO2 capture in one-coat limestone mortar modified with Mg3Al–CO3 calcined hydrotalcites using ultrafast testing technique. Chem. Eng. J. 2021, 415, 129077. [Google Scholar] [CrossRef]
- Martins, V.F.D.; Miguel, C.V.; Gonçalves, J.C.; Rodrigues, A.E.; Madeira, L.M. Modeling of a cyclic sorption–desorption unit for continuous high temperature CO2 capture from flue gas. Chem. Eng. J. 2022, 434, 134704. [Google Scholar] [CrossRef]
- IEA. Global Energy and CO2 Status Report 2017. Glob. Energy CO2 Status Rep. 2017. Available online: https://www.iea.org/publications/freepublications/publication/GECO2017.pdf (accessed on 1 April 2022).
- Yadav, S.; Mondal, S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2021, 308, 122057. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.; Madeira, L.M. Effect of interlayer anion on the CO2 capture capacity of hydrotalcite-based sorbents. Sep. Purif. Technol. 2019, 219, 290–302. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Mondal, S.S. A complete review based on various aspects of pulverized coal combustion. Int. J. Energy Res. 2019, 43, 3134–3165. [Google Scholar] [CrossRef]
- Christensen, T.H.; Bisinella, V. Climate change impacts of introducing carbon capture and utilisation (CCU) in waste incineration. Waste Manag. 2021, 126, 754–770. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Kalinowska-Wichrowska, K.; Fernández, J.M.; Jiménez, J.R. Accelerated carbonation of fresh cement-based products containing recycled masonry aggregates for CO2 sequestration. J. CO2 Util. 2021, 46, 101461. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Fernández-Rodríguez, J.M.; Jiménez, J.R. Use of carbonated water to improve the mechanical properties and reduce the carbon footprint of cement-based materials with recycled aggregates. J. CO2 Util. 2022, 57, 101886. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2013, 7, 130–189. [Google Scholar] [CrossRef]
- Jung, W.; Lee, J. Economic evaluation for four different solid sorbent processes with heat integration for energy-efficient CO2 capture based on PEI-silica sorbent. Energy 2021, 238, 121864. [Google Scholar] [CrossRef]
- Jung, W.; Lee, M.; Hwang, G.S.; Kim, E.; Lee, K.S. Thermodynamic modeling and energy analysis of a polyamine-based water-lean solvent for CO2 capture. Chem. Eng. J. 2020, 399, 125714. [Google Scholar] [CrossRef]
- Jung, W.; Park, J.; Won, W.; Lee, K.S. Simulated moving bed adsorption process based on a polyethylenimine-silica sorbent for CO2 capture with sensible heat recovery. Energy 2018, 150, 950–964. [Google Scholar] [CrossRef]
- Jung, W.; Park, S.; Lee, K.S.; Jeon, J.-D.; Lee, H.K.; Kim, J.-H.; Lee, J.S. Rapid thermal swing adsorption process in multi-beds scale with sensible heat recovery for continuous energy-efficient CO2 capture. Chem. Eng. J. 2019, 392, 123656. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.-K. Process-integrated design of a sub-ambient membrane process for CO2 removal from natural gas power plants. Appl. Energy 2019, 260, 114255. [Google Scholar] [CrossRef]
- Bhatta, L.K.G.; Subramanyam, S.; Chengala, M.D.; Olivera, S.; Venkatesh, K. Progress in hydrotalcite like compounds and metal-based oxides for CO2 capture: A review. J. Clean. Prod. 2015, 103, 171–196. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Quesada Carballo, L.; Perez Perez, M.; Cantador Fernández, D.; Caballero Amores, A.; Fernández Rodríguez, J.M. Optimum Particle Size of Treated Calcites for CO2 Capture in a Power Plant. Materials 2019, 12, 1284. [Google Scholar] [CrossRef] [Green Version]
- Halabi, M.; de Croon, M.; van der Schaaf, J.; Cobden, P.; Schouten, J. High capacity potassium-promoted hydrotalcite for CO2 capture in H2 production. Int. J. Hydrog. Energy 2012, 37, 4516–4525. [Google Scholar] [CrossRef]
- Kou, X.; Guo, H.; Ayele, E.G.; Li, S.; Zhao, Y.; Wang, S.; Ma, X. Adsorption of CO2 on MgAl-CO3 LDHs-Derived Sorbents with 3D Nanoflower-like Structure. Energy Fuels 2018, 32, 5313–5320. [Google Scholar] [CrossRef]
- Williams, G.R.; O’Hare, D. Towards understanding, control and application of layered double hydroxide chemistry. J. Mater. Chem. 2006, 16, 3065–3074. [Google Scholar] [CrossRef]
- León, M.; Díaz, E.; Bennici, S.; Vega, A.; Ordóñez, S.; Auroux, A. Adsorption of CO2 on Hydrotalcite-Derived Mixed Oxides: Sorption Mechanisms and Consequences for Adsorption Irreversibility. Ind. Eng. Chem. Res. 2010, 49, 3663–3671. [Google Scholar] [CrossRef]
- Torres-Rodríguez, D.A.; Lima, E.; Valente, J.S.; Pfeiffer, H. CO2 Capture at Low Temperatures (30–80 °C) and in the Presence of Water Vapor over a Thermally Activated Mg–Al Layered Double Hydroxide. J. Phys. Chem. A 2011, 115, 12243–12250. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.M.; Campos, J.; Souza, M.M.V.M. CO2 capture by Mg–Al and Zn–Al hydrotalcite-like compounds. Adsorption 2015, 22, 151–158. [Google Scholar] [CrossRef]
- Hutson, N.D.; Attwood, B.C. High temperature adsorption of CO2 on various hydrotalcite-like compounds. Adsorption 2008, 14, 781–789. [Google Scholar] [CrossRef]
- Oliveira, E.L.; Grande, C.A.; Rodrigues, A.E. CO2 sorption on hydrotalcite and alkali-modified (K and Cs) hydrotalcites at high temperatures. Sep. Purif. Technol. 2008, 62, 137–147. [Google Scholar] [CrossRef]
- Allmann, R. The crystal structure of pyroaurite. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1968, 24, 972–977. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Crystal structures of some double hydroxide minerals. Miner. Mag. 1973, 39, 377–389. [Google Scholar] [CrossRef] [Green Version]
- Miyata, S. The syntheses of hydrotalcite-like compounds and their structure and physico-chemical properties—I: The systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Mg2+-Al3+-ClO4−, Ni2+-Al3+-Cl− and Zn2+-Al3+-Cl−. Clays Clay Miner. 1975, 23, 369–375. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.; Fernández, J. Mitigation of CO2 emissions by hydrotalcites of Mg3Al-CO3 at 0 °C and high pressure. Appl. Clay Sci. 2020, 202, 105950. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Morales, D.S.; Jiménez, J.R.; Fernández-Rodriguez, J.M. CO2 adsorption by organohydrotalcites at low temperatures and high pressure. Chem. Eng. J. 2021, 431, 134324. [Google Scholar] [CrossRef]
- Faria, A.C.; Trujillano, R.; Rives, V.; Miguel, C.; Rodrigues, A.; Madeira, L.M. Alkali metal (Na, Cs and K) promoted hydrotalcites for high temperature CO2 capture from flue gas in cyclic adsorption processes. Chem. Eng. J. 2021, 427, 131502. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Costantino, U.; Nocchetti, M.; Sisani, M.; Vivani, R. Recent progress in the synthesis and application of organically modified hydrotalcites. Zeitschrift für Kristallographie 2009, 224, 273–281. [Google Scholar] [CrossRef]
- Ding, Y.; Alpay, E. Equilibria and kinetics of CO2 adsorption on hydrotalcite adsorbent. Chem. Eng. Sci. 2000, 55, 3461–3474. [Google Scholar] [CrossRef]
- Schulze, K.; Makowski, W.; Chyzy, R. Nickel doped hydrotalcites as catalyst precursors for the partial oxidation of light paraffins. Appl. Clay Sci. 2001, 18, 59–69. [Google Scholar] [CrossRef]
- Reichle, W. Catalytic reactions by thermally activated, synthetic, anionic clay minerals. J. Catal. 1985, 94, 547–557. [Google Scholar] [CrossRef]
- Vaccari, A. Preparation and catalytic properties of cationic and anionic clays. Catal. Today 1998, 41, 53–71. [Google Scholar] [CrossRef]
- Rives, V.; Ulibarri, M.A. Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates. Coord. Chem. Rev. 1999, 181, 61–120. [Google Scholar] [CrossRef]
- Ishihara, Y.; Okabe, S. Effects of cholestyramine and synthetic hydrotalcite on acute gastric or intestinal lesion formation in rats and dogs. Am. J. Dig. Dis. 1981, 26, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Barlattani, M.; Mantera, G.; Fasani, R.; Carosi, M. Efficacy of antacid treatment in peptic ulcerative patients: Therapeutic value of synthetic hydrotalcite (Talcid). Clin. Trials J. 1982, 19, 359–367. [Google Scholar]
- Ookubo, A.; Ooi, K.; Hayashi, H. Hydrotalcites as Potential Adsorbents of Intestinal Phosphate. J. Pharm. Sci. 1992, 81, 1139–1140. [Google Scholar] [CrossRef] [PubMed]
- Rives, V.; Carriazo, D.; Martín, C. Heterogeneous Catalysis by Polyoxometalate-Intercalated Layered Double Hydroxides. In Pillared Clays and Related Catalysts; Springer: New York, NY, USA, 2010. [Google Scholar]
- Mori, K.; Nakamura, Y.; Kikuchi, I. Modification of poly(vinyl chloride). XLI. Effect of hydrotalcite on the stabilization of poly(vinyl chloride) by 6-anilino-1,3,5-triazine-2,4-dithiol and zinc stearate. J. Polym. Sci. Part C Polym. Lett. 1981, 19, 623–628. [Google Scholar] [CrossRef]
- Van der Ven, L.; van Gemert, M.; Batenburg, L.; Keern, J.; Gielgens, L.; Koster, T.; Fischer, H. On the action of hydrotalcite-like clay materials as stabilizers in polyvinylchloride. Appl. Clay Sci. 2000, 17, 25–34. [Google Scholar] [CrossRef]
- Hermosín, M.; Pavlovic, I.; Ulibarri, M.; Cornejo, J. Hydrotalcite as sorbent for trinitrophenol: Sorption capacity and mechanism. Water Res. 1996, 30, 171–177. [Google Scholar] [CrossRef]
- Cheng, W.; Wan, T.; Wang, X.; Wu, W.; Hu, B. Plasma-grafted polyamine/hydrotalcite as high efficient adsorbents for retention of uranium (VI) from aqueous solutions. Chem. Eng. J. 2018, 342, 103–111. [Google Scholar] [CrossRef]
- Ogata, F.; Ueta, E.; Kawasaki, N. Characteristics of a novel adsorbent Fe–Mg-type hydrotalcite and its adsorption capability of As(III) and Cr(VI) from aqueous solution. J. Ind. Eng. Chem. 2018, 59, 56–63. [Google Scholar] [CrossRef]
- Miyata, S. Anion-Exchange Properties of Hydrotalcite-Like Compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Abdelouas, A. Formation of Hydrotalcite-like Compounds During R7T7 Nuclear Waste Glass and Basaltic Glass Alteration. Clays Clay Miner. 1994, 42, 526–533. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Faucon, P.; Le Bescop, P.; Adenot, F.; Bonville, P.; Jacquinot, J.; Pineau, F.; Felix, B. Leaching of cement: Study of the surface layer. Cem. Concr. Res. 1996, 26, 1707–1715. [Google Scholar] [CrossRef]
- Paul, M.; Glasser, F. Impact of prolonged warm (85 °C) moist cure on Portland cement paste. Cem. Concr. Res. 2000, 30, 1869–1877. [Google Scholar] [CrossRef]
- Scheidegger, A.M.; Wieland, E.; Scheinost, A.; Dähn, R.; Tits, J.; Spieler, P. Ni phases formed in cement and cement systems under highly alkaline conditions: An XAFS study. J. Synchrotron Radiat. 2001, 8, 916–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suescum-Morales, D.; Fernández, D.C.; Fernández, J.M.; Jiménez, J.R. The combined effect of CO2 and calcined hydrotalcite on one-coat limestone mortar properties. Constr. Build. Mater. 2021, 280, 122532. [Google Scholar] [CrossRef]
- He, P.; Shi, C.; Tu, Z.; Poon, C.S.; Zhang, J. Effect of further water curing on compressive strength and microstructure of CO2-cured concrete. Cem. Concr. Compos. 2016, 72, 80–88. [Google Scholar] [CrossRef]
- Erans, M.; Jeremias, M.; Zheng, L.; Yao, J.G.; Blamey, J.; Manovic, V.; Fennell, P.S.; Anthony, E.J. Pilot testing of enhanced sorbents for calcium looping with cement production. Appl. Energy 2018, 225, 392–401. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Shoro, D.; Murakami, K.; Honda, M.; Kawabata, T.; Takaki, K. Preparation of egg-shell type Ni-loaded catalyst by adopting “Memory Effect” of Mg–Al hydrotalcite and its application for CH4 reforming. Catal. Commun. 2004, 5, 209–213. [Google Scholar] [CrossRef]
- Gao, Z.; Sasaki, K.; Qiu, X. Structural Memory Effect of Mg–Al and Zn–Al layered Double Hydroxides in the Presence of Different Natural Humic Acids: Process and Mechanism. Langmuir 2018, 34, 5386–5395. [Google Scholar] [CrossRef]
- Yong, Z.; Rodrigues, E. Hydrotalcite like compounds as adsorbents for carbon dioxide. Energy Convers. Manag. 2002, 43, 1865–1876. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.; Madeira, L.M. Doping of hydrotalcite-based sorbents with different interlayer anions for CO2 capture. Sep. Purif. Technol. 2019, 235, 116140. [Google Scholar] [CrossRef]
- Qu, Z.; Yu, Q.; Brouwers, H. Relationship between the particle size and dosage of LDHs and concrete resistance against chloride ingress. Cem. Concr. Res. 2018, 105, 81–90. [Google Scholar] [CrossRef]
- Yang, Z.; Fischer, H.; Cerezo, J.; Mol, J.M.C.; Polder, R. Aminobenzoate modified MgAAl hydrotalcites as a novel smart additive of reinforced concrete for anticorrosion applications. Constr. Build. Mater. 2013, 47, 1436–1443. [Google Scholar] [CrossRef]
- Yang, Z.; Fischer, H.; Polder, R. Synthesis and characterization of modified hydrotalcites and their ion exchange characteristics in chloride-rich simulated concrete pore solution. Cem. Concr. Compos. 2014, 47, 87–93. [Google Scholar] [CrossRef]
- Yang, Z.; Fischer, H.; Polder, R. Modified hydrotalcites as a new emerging class of smart additive of reinforced concrete for anticorrosion applications: A literature review. Mater. Corros. 2013, 64, 1066–1074. [Google Scholar] [CrossRef]
- Lozano-Lunar, A.; Álvarez, J.I.; Navarro-Blasco, Í.; Jiménez, J.R.; Fernández-Rodriguez, J.M. Optimisation of mortar with Mg-Al-Hydrotalcite as sustainable management strategy lead waste. Appl. Clay Sci. 2021, 212, 106218. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Fireproof performance of the intumescent fire retardant coatings with layered double hydroxides additives. Constr. Build. Mater. 2020, 256, 119445. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, P.; Yan, C. Role of layered double hydroxides in setting, hydration degree, microstructure and compressive strength of cement paste. Appl. Clay Sci. 2018, 158, 123–131. [Google Scholar] [CrossRef]
- Lauermannová, A.-M.; Paterová, I.; Patera, J.; Skrbek, K.; Jankovský, O.; Bartůněk, V. Hydrotalcites in Construction Materials. Appl. Sci. 2020, 10, 7989. [Google Scholar] [CrossRef]
- Gomes, C.; Mir, Z.; Sampaio, R.; Bastos, A.; Tedim, J.; Maia, F.; Rocha, C.; Ferreira, M. Use of ZnAl-Layered Double Hydroxide (LDH) to Extend the Service Life of Reinforced Concrete. Materials 2020, 13, 1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, Z.M.; Bastos, A.; Höche, D.; Zheludkevich, M.L. Recent Advances on the Application of Layered Double Hydroxides in Concrete—A Review. Materials 2020, 13, 1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Guo, J.; Tian, J.; Xu, Y.; Hu, M.; Wang, M.; Fan, J. Preparation of Ca/Al-Layered Double Hydroxide and the influence of their structure on early strength of cement. Constr. Build. Mater. 2018, 184, 203–214. [Google Scholar] [CrossRef]
- Long, W.-J.; Xie, J.; Zhang, X.; Fang, Y.; Khayat, K.H. Hydration and microstructure of calcined hydrotalcite activated high-volume fly ash cementitious composite. Cem. Concr. Compos. 2021, 123, 104213. [Google Scholar] [CrossRef]
- Long, W.; Xie, J.; Zhang, X.; Kou, S.; Xing, F.; He, C. Accelerating effect of calcined hydrotalcite-Na2SO4 binary system on hydration of high volume fly ash cement. Constr. Build. Mater. 2022, 328, 127068. [Google Scholar] [CrossRef]
- Ma, J.; Duan, P.; Ren, D.; Zhou, W. Effects of layered double hydroxides incorporation on carbonation resistance of cementitious materials. J. Mater. Res. Technol. 2019, 8, 292–298. [Google Scholar] [CrossRef]
- Vágvölgyi, V.; Palmer, S.J.; Kristóf, J.; Frost, R.L.; Horváth, E. Mechanism for hydrotalcite decomposition: A controlled rate thermal analysis study. J. Colloid Interface Sci. 2007, 318, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Yahyaoui, R.; Jimenez, P.E.S.; Maqueda, L.A.P.; Nahdi, K.; Luque, J.M.C. Synthesis, characterization and combined kinetic analysis of thermal decomposition of hydrotalcite (Mg6Al2(OH)16CO3·4H2O). Thermochim. Acta 2018, 667, 177–184. [Google Scholar] [CrossRef]
- Miyata, S. Physico-Chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Kannan, V.R.S.; Velu, S. Synthesis and physicochemical properties of cobalt aluminium hydrotalcites. J. Mater. Sci. 1995, 30, 1462–1468. [Google Scholar] [CrossRef]
- Cocheci, L.; Barvinschi, P.; Pode, R.; Popovici, E.; Seftel, E.M. Structural Characterization of Some Mg/Zn-Al Type Hy-drotalcites Prepared for Chromate Sorption from Wastewater. Chem. Bull. 2010, 55, 40–45. [Google Scholar]
- Palmer, S.J.; Spratt, H.J.; Frost, R.L. Thermal decompostition of hydrotalcites with variable cationic ratios. J. Therm. Anal. Calorim. 2009, 95, 123–129. [Google Scholar] [CrossRef]
- Garcia-Gallastegui, A.; Iruretagoyena, D.; Gouvea, V.; Mokhtar, M.; Asiri, A.M.; Basahel, S.N.; Al-Thabaiti, S.A.; Alyoubi, A.O.; Chadwick, D.; Shaffer, M.S.P. Graphene Oxide as Support for Layered Double Hydroxides: Enhancing the CO2 Adsorption Capacity. Chem. Mater. 2012, 24, 4531–4539. [Google Scholar] [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; Lu, G.Q.; Diniz da Costa, J.C. Layered Double Hydroxides for CO2 Capture: Structure Evolution and Regeneration. Ind. Eng. Chem. Res. 2006, 45, 7504–7509. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Z.; Tay, H.H.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. High temperature adsorption of CO2 on Mg–Al hydrotalcite: Effect of the charge compensating anions and the synthesis pH. Catal. Today 2011, 164, 198–203. [Google Scholar] [CrossRef]
- Hibino, T. Decarbonation Behavior of Mg-Al-CO3 Hydrotalcite-like Compounds during Heat Treatment. Clays Clay Miner. 1995, 43, 427–432. [Google Scholar] [CrossRef]
- Tao, Q.; Zhang, Y.; Zhang, X.; Yuan, P.; He, H. Synthesis and characterization of layered double hydroxides with a high aspect ratio. J. Solid State Chem. 2006, 179, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Garcés-Polo, S.; Villarroel-Rocha, J.; Sapag, K.; Korili, S.; Gil, A. Adsorption of CO2 on mixed oxides derived from hydrotalcites at several temperatures and high pressures. Chem. Eng. J. 2018, 332, 24–32. [Google Scholar] [CrossRef]
- Martunus; Othman, M.R.; Fernando, W.J.N. Elevated temperature carbon dioxide capture via reinforced metal hydrotalcite. Microporous Mesoporous Mater. 2011, 138, 110–117. [Google Scholar] [CrossRef]
- Aschenbrenner, O.; McGuire, P.; Alsamaq, S.; Wang, J.; Supasitmongkol, S.; Al-Duri, B.; Styring, P.; Wood, J. Adsorption of carbon dioxide on hydrotalcite-like compounds of different compositions. Chem. Eng. Res. Des. 2011, 89, 1711–1721. [Google Scholar] [CrossRef] [Green Version]
- Forano, C.T.-G.C.; Hibino, T.; Leroux, F. Layered double hydroxides. In Hand-Book of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Newnes, Australia, 2006; ISBN 978-0-08-044183-2. [Google Scholar]
- Cai, P.; Zheng, H.; Wang, C.; Ma, H.; Hu, J.; Pu, Y.; Liang, P. Competitive adsorption characteristics of fluoride and phosphate on calcined Mg–Al–CO3 layered double hydroxides. J. Hazard. Mater. 2012, 213–214, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Xu, T.; Li, Y.; Chang, Z.; Sun, X.; Lei, X. Effect of synthesis method on selective adsorption of thiosulfate by calcined MgAl-layered double hydroxides. Chem. Eng. J. 2013, 232, 510–518. [Google Scholar] [CrossRef]
- Hobbs, C.; Jaskaniec, S.; McCarthy, E.K.; Downing, C.; Opelt, K.; Güth, K.; Shmeliov, A.; Mourad, M.C.D.; Mandel, K.; Nicolosi, V. Structural transformation of layered double hydroxides: An in situ TEM analysis. npj 2D Mater. Appl. 2018, 2, 4. [Google Scholar] [CrossRef]
- Luo, S.; Guo, Y.; Yang, Y.; Zhou, X.; Peng, L.; Wu, X.; Zeng, Q. Synthesis of calcined La-doped layered double hydroxides and application on simultaneously removal of arsenate and fluoride. J. Solid State Chem. 2019, 275, 197–205. [Google Scholar] [CrossRef]
- Elhalil, A.; Qourzal, S.; Mahjoubi, F.; Elmoubarki, R.; Farnane, M.; Tounsadi, H.; Sadiq, M.; Abdennouri, M.; Barka, N. Defluoridation of groundwater by calcined Mg/Al layered double hydroxide. Emerg. Contam. 2016, 2, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Harizi, I.; Chebli, D.; Bouguettoucha, A.; Rohani, S.; Amrane, A. A New Mg–Al–Cu–Fe-LDH Composite to Enhance the Adsorption of Acid Red 66 Dye: Characterization, Kinetics and Isotherm Analysis. Arab. J. Sci. Eng. 2018, 44, 5245–5261. [Google Scholar] [CrossRef]
- Chebli, D.; Bouguettoucha, A.; Reffas, A.; Tiar, C.; Boutahala, M.; Gulyas, H.; Amrane, A. Removal of the anionic dye Biebrich scarlet from water by adsorption to calcined and non-calcined Mg–Al layered double hydroxides. Desalin. Water Treat. 2016, 57, 22061–22073. [Google Scholar] [CrossRef]
- Li, R.; Zhan, W.; Song, Y.; Lan, J.; Guo, L.; Zhang, T.C.; Du, D. Template-free synthesis of an eco-friendly flower-like Mg/Al/Fe-CLDH for efficient arsenate removal from aqueous solutions. Sep. Purif. Technol. 2021, 282, 120011. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Yu, Q.; Fan, J.; Brouwers, H. Effect of MgO, Mg-Al-NO3 LDH and calcined LDH-CO3 on chloride resistance of alkali activated fly ash and slag blends. Constr. Build. Mater. 2020, 250, 118865. [Google Scholar] [CrossRef]
- Singh, R.; Reddy, M.R.; Wilson, S.; Joshi, K.; da Costa, J.C.D.; Webley, P. High temperature materials for CO2 capture. Energy Procedia 2009, 1, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Ebner, A.D.; Reynolds, S.P.; Ritter, J.A. Nonequilibrium Kinetic Model That Describes the Reversible Adsorption and Desorption Behavior of CO2 in a K-Promoted Hydrotalcite-like Compound. Ind. Eng. Chem. Res. 2007, 46, 1737–1744. [Google Scholar] [CrossRef]
- Wang, Q.; Tay, H.H.; Guo, Z.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. Morphology and composition controllable synthesis of Mg–Al–CO3 hydrotalcites by tuning the synthesis pH and the CO2 capture capacity. Appl. Clay Sci. 2012, 55, 18–26. [Google Scholar] [CrossRef]
- Yong, Z.; Mata, V.; Rodrigues, E. Adsorption of Carbon Dioxide onto Hydrotalcite-like Compounds (HTlcs) at High Temperatures. Ind. Eng. Chem. Res. 2001, 40, 204–209. [Google Scholar] [CrossRef]
- Yang, W.; Kim, Y.; Liu, P.K.T.; Sahimi, M.; Tsotsis, T.T. A study by in situ techniques of the thermal evolution of the structure of a Mg–Al–CO3 layered double hydroxide. Chem. Eng. Sci. 2002, 57, 2945–2953. [Google Scholar] [CrossRef]
- Peng, J.; Iruretagoyena, D.; Chadwick, D. Hydrotalcite/SBA15 composites for pre-combustion CO2 capture: CO2 adsorption characteristics. J. CO2 Util. 2018, 24, 73–80. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, S.G.; Lee, K.B. High-Temperature CO2 Sorption on Hydrotalcite Having a High Mg/Al Molar Ratio. ACS Appl. Mater. Interfaces 2016, 8, 5763–5767. [Google Scholar] [CrossRef]
- Macedo, M.S.; Soria, M.; Madeira, L.M. High temperature CO2 sorption using mixed oxides with different Mg/Al molar ratios and synthesis pH. Chem. Eng. J. 2021, 420, 129731. [Google Scholar] [CrossRef]
- Silva, J.; Trujillano, R.; Rives, V.; Soria, M.; Madeira, L.M. High temperature CO2 sorption over modified hydrotalcites. Chem. Eng. J. 2017, 325, 25–34. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Wu, J.; Yi, X.; Zheng, A.; Umar, A.; O’Hare, D.; Wang, Q. Comprehensive investigation of CO2 adsorption on Mg–Al–CO3 LDH-derived mixed metal oxides. J. Mater. Chem. A 2013, 1, 12782–12790. [Google Scholar] [CrossRef]
- Walspurger, S.; de Munck, S.; Cobden, P.; Haije, W.; Brink, R.V.D.; Safonova, O. Correlation between structural rearrangement of hydrotalcite-type materials and CO2 sorption processes under pre-combustion decarbonisation conditions. Energy Procedia 2011, 4, 1162–1167. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Tay, H.H.; Zhong, Z.; Luo, J.; Borgna, A. Synthesis of high-temperature CO2 adsorbents from organo-layered double hydroxides with markedly improved CO2 capture capacity. Energy Environ. Sci. 2012, 5, 7526–7530. [Google Scholar] [CrossRef]
- Li, S.; Shi, Y.; Yang, Y.; Zheng, Y.; Cai, N. High-Performance CO2 Adsorbent from Interlayer Potassium-Promoted Stearate-Pillared Hydrotalcite Precursors. Energy Fuels 2013, 27, 5352–5358. [Google Scholar] [CrossRef]
- Hanif, A.; Dasgupta, S.; Divekar, S.; Arya, A.; Garg, M.O.; Nanoti, A. A study on high temperature CO2 capture by improved hydrotalcite sorbents. Chem. Eng. J. 2014, 236, 91–99. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Large-scale synthesis of highly dispersed layered double hydroxide powders containing delaminated single layer nanosheets. Chem. Commun. 2013, 49, 6301–6303. [Google Scholar] [CrossRef]
- Othman, M.; Rasid, N.; Fernando, W. Mg–Al hydrotalcite coating on zeolites for improved carbon dioxide adsorption. Chem. Eng. Sci. 2006, 61, 1555–1560. [Google Scholar] [CrossRef]
- Wu, K.; Ye, Q.; Wang, L.; Meng, F.; Dai, H. Mesoporous alumina-supported layered double hydroxides for efficient CO2 capture. J. CO2 Util. 2022, 60, 101982. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, C.; Suo, H.; Wang, Q.; Shi, Y.; O’Hare, D.; Cai, N. Synthesis of elevated temperature CO2 adsorbents from aqueous miscible organic-layered double hydroxides. Energy 2018, 167, 960–969. [Google Scholar] [CrossRef]
- Meis, N.N.A.H.; Bitter, J.H.; de Jong, K.P. On the Influence and Role of Alkali Metals on Supported and Unsupported Activated Hydrotalcites for CO2 Sorption. Ind. Eng. Chem. Res. 2010, 49, 8086–8093. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Shinall, B.D.; Iretskii, A.V.; White, M.G.; Golden, T.; Atilhan, M.; Ford, P.C.; Stucky, G.D. Markedly Improved CO2 Capture Efficiency and Stability of Gallium Substituted Hydrotalcites at Elevated Temperatures. Chem. Mater. 2009, 21, 3473–3475. [Google Scholar] [CrossRef]
- Lwin, Y.; Abdullah, F. High temperature adsorption of carbon dioxide on Cu–Al hydrotalcite-derived mixed oxides: Kinetics and equilibria by thermogravimetry. J. Therm. Anal. 2009, 97, 885–889. [Google Scholar] [CrossRef]
- Raki, L.; Beaudoin, J.; Mitchell, L. Layered double hydroxide-like materials: Nanocomposites for use in concrete. Cem. Concr. Res. 2004, 34, 1717–1724. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).