Abstract
Currently, the use of alternative solvents is increasing, namely ionic liquids (ILs) and deep eutectic solvents (DESs) in diverse fields of knowledge, such as biochemistry, chemistry, chemical engineering, biotechnology and biomedicine. Particularly, when compared to traditional solvents, these alternative solvents have great importance for biomolecules due to the enhanced solubility, structure stability and the biological activity of biomolecules, such as protein and enzymes. Thus, in this review article, the recent developments and efforts on the technological developments carried out with ILs and DESs for the stabilization and activation of proteins and enzymes are provided. The most studied IL- and DES-based formulations for proteins and enzymes are discussed and the molecular mechanisms and interactions related to the increased stability promoted by these alternative solvents are disclosed, while emphasizing their main advantages.
1. Introduction
When developing a formulation for proteins, including their use as biopharmaceuticals, there are some variables that should be accounted for, such as stability and biological activity, clinical needs, patient compliance, delivery method, storage and distribution, and market competitiveness [1,2]. Knowing the end goal of the protein-based formulation will help design the formulation that better suits the expected needs [1,2]. The main drawbacks of protein formulation are represented in Figure 1. Preserving the structural integrity and biological activities of these macromolecules, as well as improving their bioavailability or targeting them to specific sites in the body are key ideas to consider when developing a formulation [3]. Due to their various levels of structural complexity (primary, secondary, tertiary, quaternary), protein-based biopharmaceuticals are vulnerable to degradation or denaturation [3]. The primary structure (amino acid sequence) and secondary structure (α-helix or β-sheet) contribute to the tertiary structure of a protein. The tertiary structure of biomolecules, such as proteins is the one that needs to be stabilized against the disrupting forces [4]. During protein storage, thermal denaturation must also be accounted for, as it is the main source of denaturation [5]. As proteins are only marginally stable at room temperature, all types of molecular interactions are to be accounted for, even small interactions can contribute significantly to the stability of proteins [5]. The main type of interactions that contribute to maintaining the three-dimensional structure of proteins is hydrogen bonding, hydrophobic, and ionic interactions [6]. The native conformation of a protein depends on the intramolecular interactions between the functional groups and the intermolecular interactions between solvents and protein functional groups. Besides the temperature, there are also other factors that contribute to changes in protein conformation that may lead to denaturation, namely the addition of polymers, organic solvents, changes in pH or pressure, or the addition of other co-solvents or molecules [6,7].
Figure 1.
Main drawbacks of current protein formulations.
In terms of dosage form assessment, liquid formulations are more convenient for the end user, since there is no need for product reconstitution and errors of volume added for its reconstitution [8]. Unfortunately, liquid forms are less stable than solid ones due to hydrolytically driven chemical degradation routes; this may result in reduced shelf life and the need for a more careful approach during manufacture, shipping, storage, and use [8]. It is possible to minimize the factors that cause degradation, but the stability of protein liquid formulations is more difficult to manage since proteins are sensitive and might denature or aggregate if they are not well protected [8]. Usually, aggregation is caused by shaking or heat denaturation [9].
The chemical and physical stability of a therapeutic protein will condition its safety and efficacy. In fact, degradation and aggregation can decrease the availability of the biological activity of proteins, influencing their pharmacokinetic characteristics [10], which can ultimately lead to adverse effects, such as unwanted immunogenicity [10]. When a patient develops an immune response to the active protein monomer, the drug might no longer be effective for the patient or it might even lead to serious safety issues [11]. Drug tolerance is especially damaging to patients with chronic, life-altering, or life-threatening diseases, and as there are not many drug competitors to treat the diseases, it becomes urgent to avoid aggregation [11]. Aggregation can, therefore, result in deep problems, from the reduction of biological potency to the blocking of tubing, membranes, or pumps in an infusion set [9]. However, in some cases, aggregation is not a problem because the effectiveness of the product is not affected [9].
The aqueous solubility of a protein comes from the interaction of its polar residues with water, whereas the non-polar side chains and peptide groups of the folded protein are normally facing inwards, maintaining the native state [12]. The isoelectric point (pI), where the protein has no electrical charge, is when the minimum protein solubility occurs [12]. Proteins should not be buffered at or near their pI to ensure adequate solubility in water. However, when selecting an appropriate pH, considerations should also be given to the chemical integrity and activity of the proteins, as these are also pH-dependent [12]. One of the problems when developing a biopharmaceutical drug is the poor aqueous solubility and dissolution rate of compounds [13]. For a biopharmaceutical to access its pharmacological target in the body, it will have to solubilize in body fluids and go through membranes, and when the solubility is low, so will the dissolution rate and absorption [14]. This leads to a need for a higher dose of the pharmaceutical to achieve the therapeutic effect [14]. For some proteins, that have limited solubility, achieving high concentration formulations may require the use of solubility enhancers [15].
Therefore, the need for high soluble protein concentrations in liquid form is important in the pharmaceutical industry [16]. For example, this is especially important regarding the development of monoclonal antibodies (mAbs) therapeutics [16]. To achieve efficacy, a relatively high dose of mAbs is required and mAbs are usually prepared at very high concentrations [17]. Unfortunately, higher concentrations further promote aggregation, and consequently, this could favor high viscosity and turn the handling/processing complex [17]. Increased viscosity is a key factor to take into account when developing high-concentration protein formulations since it will affect the production process and drug administration [16].
Enzymes are a special type of proteins that have the ability to catalyze or speed up chemical reactions in biological organisms [1]. During the past decades, successful discoveries of novel and effective biocatalysts for a diverse range of applications have attracted huge interest from academic and industrial sectors [1]. However, a bottleneck common to enzymes is how to maintain or increase their stability and activity for application in the industrial sector. At present, enzyme catalysis is, in some cases, used at high temperatures, in order to obtain a high product yield [1]. However, the enzyme must be stabilized and using an appropriated non-aqueous mixture, the stability of enzymes can be improved at higher temperatures, where the biomolecule would exist in a denatured state [1]. Aiming at increasing the diversity of the use of enzymes, the reaction in an aqueous medium became limiting [1]. Therefore, the use of the non-aqueous medium for enzyme reaction has attracted unique opportunities in enzymology [1]. However, organic solvents are usually not suitable for enzymes due to their volatility, toxicity and low solubilities which restrict the use of enzymes in non-aqueous media [1].
Recent literature shows that traditional organic solvents can be replaced by properly designed Ionic Liquids (ILs) and Deep Eutectic Solvents (DESs) with remarkable advantages and improvements in protein and enzyme stability and activity [1]. When properly designed, ILs appear as a new class of alternative solvents that can potentially decrease the process costs while avoiding environmental pollution when compared to volatile organic solvents [1,2,3,18]. ILs are constituted by an organic cation combined with an organic or inorganic anion that remains liquid at temperatures below 100 °C [3]. These non-aqueous solvents present a wide range of desired properties, such as no vapor pressure, low flammability, improved thermal and ionic conductivity, high capacity for dissolution of many compounds, and high thermal and chemical stability [3,4,5]. ILs are known as designer solvents due to the different combinations of cations and anions; thus, ILs can be specifically designed for different purposes and applications [3,4,5]. Various applications of ILs have been reported in the literature [19]. The significant usage of ILs has been observed in the fields of electrochemistry, extraction and separation processes, biodiesel production, spectrometry, carbon capture processes, treatment of nuclear wastes as well as fuel purification [19]. Despite all this, their use as solvents as well as catalysts have been described in various types of organic reactions, nanoparticle synthesis, and enzymatic reactions [19].
DESs are also part of the novel class of alternative solvents. They are eutectic, homogeneous mixtures of at least two compounds with a lower melting point than the respective individual constituents. DESs are prepared by mixing a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD) under heating and agitation until a liquid is formed [20,21]. In addition to sharing many of the desirable properties of ILs mentioned above, if properly designed, DESs may also present lower costs and enhanced biodegradability, biocompatibility and sustainability [22,23,24,25]. Moreover, DESs are easily prepared and have a huge number of diverse starting constituents that can be used to prepare an adequate DES for a target application [25]. The elevated polarity makes DESs a proper alternative solvent to be used in the purification of bio-diesel [26]. DESs have high solute distribution coefficients and are selectively able to dissolve gases and metal oxides [26]. Moreover, DESs also dissolve organic macromolecules with important properties in pharmacological applications [26]. Thus, a diversity of organic synthesis has been conducted using DESs as an alternative solvent with the advantage of being recovered at the end of the process [26].
ILs and DESs are promising alternatives and replacers for the conventional organic solvents used as media for proteins and enzymes. In this context, studies addressing how ILs and DESs can better stabilize and activate proteins and enzymes with biotechnological relevance are discussed in this review.
2. Stability of Proteins in Alternative Solvents
Serum albumins (bovine—BSA and human—HAS, Figure 2) and antibodies are the most studied proteins regarding their interaction with alternative solvents namely ILs and DESs.
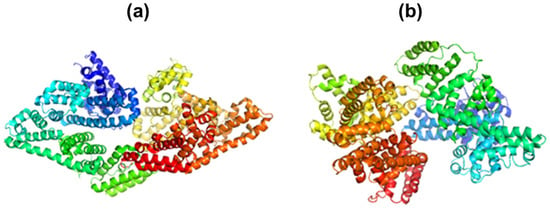
Figure 2.
Crystallographic structures from protein data bank (PDB) of: (a) BSA molecule obtained through X-ray diffraction (PDB ID: 3V03); (b) HSA molecule obtained through X-ray diffraction (PDB ID: 1AO6)). Each albumin domain is marked with a different color. The albumin structures have three domains (red, green, and blue) which are divided into two subdomains (light red, light green, and light blue, respectively).
Serum albumin is the main protein present in the plasma [27] and the most well-studied protein due to its large quantity found in plasma, elevated structural stability, reduced cost and simple purification [27]. Serum albumins control the transport of exogenous and endogenous molecules, such as hormones, fatty acids, steroids, inorganic ions, bilirubin, vitamins, and drugs. Albumin also has an essential function in the blood osmotic pressure and pH maintenance in mammalians [28,29]. Although the anti-inflammatory activity of serum albumin is still not well understood, this protein is considered the most important antioxidant in the whole blood [29].
BSA and HSA share about 76% sequence homology, and they are both multifunctional proteins in plasma [30]. Even though they are homologous, BSA and HSA present some differences, such as HSA has one tryptophan (Trp-214) residue and BSA has two, Trp-212, which is placed within a hydrophobic binding pocket of the protein and Trp-134, which is placed on the surface of the protein. In BSA, the two tryptophan residues have intrinsic fluorescence [31]. BSA is a water-soluble globulin, with a heart-shaped helical structure, composed of one polypeptide chain containing 583 amino acid residues (MW of 66 kDa). The BSA structure has three homologous domains (red, green, and blue) which are divided into two subdomains (light red, light green, and light blue) [32]. As BSA structure and physicochemical characteristics are well defined and established, it is considered a model globular protein. This molecule can attach to diverse types of amphiphilic biological compounds, that have a special role in some physiological functions [33]. From the wide variety of BSA applications, being used as drug vehicles to deliver anticancer drugs and therapeutic agents can be highlighted [34]. HSA also presents a wide variety of applications, namely as a biomarker in several diseases, for instance, cancer, ischemia, rheumatoid arthritis, and severe acute graft-versus-host disease, among others [35]. In addition, HSA has been used as a biopharmaceutical in the clinical treatment of diverse diseases, such as hypovolemia, hemorrhage, shock, trauma, burns, surgical blood loss, cardiopulmonary bypass, etc. [35]. In biotechnology, HSA has been used in biomaterials, adhesives for surgery, chromatography, ligand trapping, and protein of fusion [35].
Monoclonal antibodies (mAbs) are presently applied in clinical practice for treating diseases and conditions, such as cancer, inflammatory, transplantation, infectious and cardiovascular diseases [36]. The production of antibodies, which are glycoproteins, is a response mechanism triggered by the presence of foreign molecules or other agents, known as antigens, in a host organism. This response plays a fundamental role in organism protection [37,38]. In particular, mAbs play a major part in medicine and are promising therapeutic tools [39,40]. The demonstration of immunogenic responses to mAbs is a setback in both their safety and efficiency [40]. There are many reasons which can affect the medicinal products for immunogenicity or preparations containing mAbs biopharmaceuticals, i.e., from product to patient related factors. In particular, the aggregation of mAbs can trigger mechanisms of immunogenicity [41]. Aggregation, or potential for self-association, is fairly common in antibodies since they are not very stable relative to small molecule drugs [17]. Another challenge in their development is due to the large size of mAbs and their surface-exposed functional groups, which are susceptible to chemical degradation [17]. Both chemical and physical degradation routes have an impact on the stability of mAbs [8]. To achieve efficacy, a relatively high dose of mAbs is required and they are usually produced at an elevated concentration. Higher concentrations further promote aggregation, and consequently, this may lead to high viscosity and difficulty in handling/processing [17]. Therefore, there has been an effort to develop more stable and promising formulations of mAbs-based biopharmaceuticals in order to overcome such drawbacks.
In summary, BSA, HSA and antibodies have a wide range of applications that make them extremely relevant. For this reason, coupled with the fact of being widely studied in the literature, this review will be focusing on their stability in formulations composed of alternative solvents as detailed in the next sections.
2.1. Stability of Proteins in Ionic Liquids
Proteins are complex molecules and extremely sensitive to their environment. The properties and applications of BSA, HSA and antibodies are highly valuable; they are among the most studied proteins in the scientific community. Therefore, the most recent advances made in the development of stabilizing IL-based formulations for BSA, HSA and antibodies, and the main molecular mechanisms behind that increased stability are reviewed in this section.
Geng et al. [33] studied the interactions that occurred between BSA and IL 1-tetradecyl-3-methylimidazolium bromide ([C14mim]Br). The effect of [C14mim]Br on the BSA structure was evaluated [33]. It was found that, when using low concentrations of [C14mim]Br, they can bind with BSA by electrostatic interactions, and at higher IL concentrations they can bind by hydrophobic interactions [33]. The secondary structure of BSA was stabilized at the IL concentration below the CMC, however, at a concentration above the CMC, an unfolding of the BSA was observed leading to BSA denaturation [33].
Satish et al. [38] studied the interaction of BSA with different imidazolium-based ILs namely 1-ethyl-3-methyl-imidazolium ethyl sulfate ([C2mim][EtSO4]), 1-ethyl-3-methyl-imidazolium chloride ([C2mim]Cl) and 1-butyl-3-methyl-imidazolium chloride ([C4mim]Cl), applying different spectroscopic techniques. The thermal stability of BSA in the ILs followed the trend [C2mim][EtSO4] > [C2mim]Cl > [C4mim]Cl. It was concluded that the main interactions were the hydrophobic interactions between BSA and IL, which had a negative effect on the stability of the BSA [38]. The more hydrophobic IL destabilized BSA with the increase in the IL concentration. As previously described, the same results were observed by Geng et al. [33], as BSA was found to be stabilized at low concentrations of IL, but destroyed at elevated IL concentrations [33]. Moreover, the anion had a mandatory positive thermal effect in stabilizing BSA. Regarding hydrophobicity of the cation in the ILs, it affected the destabilization of BSA [38]. The interaction of BSA with the imidazolium cation increased with the increase in alkyl chain length in the order as [C4mim]+ > [C2mim]+. The stability of BSA was affected by increasing the hydrophobic moiety of the IL [38]. The lower stability of the protein was due to the interactions between the hydrophobic part of BSA with the IL hydrophobic moieties. On the other hand, the stabilization of BSA in the presence of [C2mim]+ was probably due to the interaction between the charged amino acids of the protein with the IL cation via electrostatic and hydrophobic interactions. For the IL [C2mim][EtSO4], the interaction occurred between the lysine residues of the protein and IL anion leading a protein stabilization [38].
Zhang et al. [42] also evaluated the structure and functions of BSA using different alkyl chain length imidazolium ILs, namely: [C2mim]Cl, [C4mim]Cl, 1-hexyl-3- methylimidazolium chloride ([C6mim]Cl), 1-octyl-3-methylimidazolium chloride ([C8mim]Cl), 1-decyl-3-methylimidazolium chloride ([C10mim]Cl), and 1-dodecyl-3-methylimidazolium chloride ([C12mim]Cl). A strong interaction was observed between BSA and the longer alkyl chain length, leading to conformational destruction of the BSA structure, which is in agreement with what was observed by Geng et al. [33] and Satish et al. [38]. The binding interaction of ILs was related to the different lengths of the alkyl chain which promoted different impacts on the BSA tertiary structure. The unfolding of the protein followed the order [C12mim]Cl > [C10mim]Cl > [C8mim]Cl > [C6mim]Cl > [C4mim]Cl > [C2mim]Cl. Among them, [C12mim]Cl presented the highest destruction of the protein secondary structure. A decrease was observed in the transition temperature (Tm), meaning that in the ILs at high concentration, the thermal stability of the protein was decreased by the acceleration of the thermal process. Molecular modeling studies showed that ILs with longer hydrophobic chains are susceptible to binding with BSA by the polar interaction and hydrophobic forces [42].
Besides, ILs based on tetrafluoroborate are not water-stable compounds since they hydrolyze [43], some works have reported their use as a medium for proteins. For instance, Islam et al. [44] studied the interaction of BSA with ILs (3-methyl-1-octylimidazolium tetrafluoroborate ([C8mim][BF4]), 1-ethyl-3-methylimidazolium tetrafluoroborate ([C2mim][BF4]), 1-butyl-3-methylimidazolium tetrafluoroborate ([C4mim][BF4]), 1-hexyl-3-methylimidazolium tetrafluoroborate ([C6mim][BF4])) [44]. It was observed that the protein quenching of fluorescence was induced by [C8mim][BF4] and [C6mim][BF4], while ILs with shorter alkyl chains did not quench the fluorescence [44]. The authors conclude that the interaction between BSA and IL is enthalpy-driven [44]. Moreover, ILs with shorter alkyl chains do not bind with the BSA. The binding interactions with BSA occurred in the presence of [C6mim][BF4] and [C8mim][BF4] [44] and it was due to hydrogen bonding and van der Waals interactions [44]. The work proved the importance of hydrophobic interactions on BSA−IL binding and of the alkyl chain length of ILs [44].
Reddy et al. [45] investigated in aqueous media the interaction HSA with the ILs, namely 1-butyl-3-methylimidazolium hydrogen sulfate ([C4mim][HSO4]), 1-butyl-3-methylimidazolium methyl sulfate ([C4mim][C1SO4]), 1-butyl-3-methylimidazolium octyl sulfate ([C4mim][C8SO4]), and 1-butyl-3-methylimidazolium dodecyl sulfate ([C4mim][C12SO4]). It was determined that the interaction between IL and HSA increases in the following order: [C4mim][HSO4] < [C4mim][C1SO4] < [C4mim][C8SO4] < [C4mim][C12SO4]. These results show that the interactions increase with an increase in the alkyl chain length of the IL, proving that the hydrophobic interactions are the mandatory [45].
Silva et al. [46] investigated the level of cation interactions between HSA and imidazolium-based ILs: ([C2mim]Cl, 1-ethyl-3-methylimidazolium dicyanamide ([C2mim][dca]), [C4mim]Cl, 1-butyl-3-methylimidazolium dicyanamide ([C4mim][dca]), 1-(2-hydroxyethyl)-3-methylimidazolium chloride ([C2OHmim]Cl), 1-(2-hydroxyethyl)-3-methylimidazolium dicyanamide ([C2OHmim][DCA]), 1-butyl-2,3-dimethylimidazolium chloride ([C4dmim]Cl) and 1-(2-methoxyethyl)-3-methylimidazolium chloride ([C3Omim]Cl)). It was found that the most hydrophobic ILs (longer alkyl side chains of imidazolium-based ILs) destabilized HSA, while less hydrophobic ILs (short-alkyl groups) stabilized HSA [46].
Shu et al. [47] evaluated the interactions between HSA and imidazolium ILs, namely 1, 3-bibutylimidazolium chloride ([Bbim]Cl), 1-butyl-3-methylimidazolium chloride ([Bmim]Cl), and 1-butyl-3-methylimidazolium nitrate ([Bmim][NO3]). [Bbim]Cl has a higher hydrophobicity than [Bmim]Cl due to the longer alkyl side chains. However, the UV-vis spectra were very similar, meaning that the imidazolium ring side chain did not affect the interactions between ILs and protein [47]. On the other hand, even though [Bmim]Cl and [Bmim][NO3] have the same cationic imidazolium moieties, the interactions of these ILs with BSA led to different UV-vis spectra, due to the difference of IL anionic moieties [47]. Moreover, among all the ILs evaluated, it was observed that [Bmim][NO3] and BSA have the strongest interaction [47]. In addition, a high decrease of the HAS helical contents was observed with the increase in the ILs concentration [47].
Spectroscopy studies, thermophysical and thermodynamic properties were the techniques used to evaluate the interactions between 2′,3′-epoxy propyl-N-methyl-2-oxopyrrolidinium salicylate (II) ([EPMpyr][Sal]) IL and BSA [6]. It was observed that BSA presented conformational changes in the presence of the IL and that the IL concentration was crucial for the BSA secondary structure. The optimum IL concentration and the IL anion were important to protect BSA at high temperatures and avoid thermal denaturation [6]. The increase in the IL alkyl chain length enhanced the thermal denaturation of BSA which was confirmed by CD analysis. A decrease in the helical content of BSA in the presence of the IL was from 39% to 36%, due to the exposure of hydrophobic cavities and changes in the microstructures in the vicinity of amino acid residues. Moreover, the damage to the BSA tertiary structure was due to the interaction between the hydrophobic moiety of IL with similar moieties in BSA [6].
Satish et al. [48] studied the impact of the IL triethyloctylammonium bromide ([N2888]Br), regarding their concentration on the structure, stability, and activity of BSA using fluorescence spectroscopy, CD spectroscopy, dynamic light scattering measurements and the esterase-like activity assay. In their study, the hydrophobicity of the cationic part of the IL was increased by taking one of the alkyl groups as octyl. In previous work of the same research group [49], it was found that IL hydrophobicity was essential for determining the stabilizing effect on the protein. High concentrations of [N2888]Br affected the BSA stability and activity [48]. BSA was stable up to an IL concentration of 0.02 M. The results proved some hydrophobicity in the cationic moiety of the IL is crucial in stabilizing BSA, especially against thermal unfolding and aggregation. However, beyond that limit, the same family of IL with higher hydrophobicity destabilizes BSA [48].
As mentioned before, the limited solubility of the protein in an aqueous solution is a problem when developing pharmaceutical drugs based on proteins. The introduction of ILs based on active pharmaceutical ingredients (API-ILs) is a promising alternative for problems, such as this one [28]. In addition, API-ILs can also be an alternative to solid and/or hydrophobic drugs with low bioavailability, and polymorphism and as an alternative way of administration [28]. Ossowicz et al. [28] studied novel ILs containing amino acid esters as the cation and ketoprofen (KETO-ILs) as the anion and their interactions with BSA using fluorescence spectroscopy and FTIR spectroscopy methods. KETO is a non-steroidal anti-inflammatory drug. ILs constituted by biologically active ions have been evaluated and they were reported to be able to maintain the biological activities of both the API [50,51]. The ILs tested were L-Leucine Ethyl Ester Ketoprofenate ([L-LeuOEt][KETO]), L-Valine Ethyl Ester Ketoprofenate ([L-ValOEt][KETO]), L-Valine Isopropyl Ester Ketoprofenate ([L-ValOiPr][KETO]), L-Valine Propyl Ester Ketoprofenate ([L-ValOPr][KETO]), L-Valine Butyl Ester Ketoprofenate ([L-ValOBu][KETO]). It was observed that all evaluated constituents caused a rearrangement in the BSA structure. KETO-ILs favored the partial unfolding of the protein and a decrease in the α-helical content and an increase in antiparallel β-sheets and aggregates in this BSA-IL complex was observed [28]. Among them, [L-ValOiPr][KETO] led to the stronger modifications in the BSA secondary structure, a significant loss of helical content and aggregation was observed. The determined binding constant (KA) was 105 L mol−1, which means a strong interaction between the IL and BSA. Regarding the ketoprofen-BSA system, a high affinity between the ILs containing the cations [L-LeuOEt], [L-ValOEt] and [L-ValOBu] and BSA was observed [28]. The cytotoxicity effect of the compounds was evaluated, and it was shown that the constituents were not dangerous for the immune murine macrophages RAW 264.7 cells at the evaluated concentrations [28].
Kumar et al. [52] studied the effect of various concentrations of aromatic amino acid based ILs (AAILs) on BSA and HSA stability. The AAILs were cholinium tryptophan ([Ch][Trp]) and tetraethylammonium tryptophan ([TEA][Trp]). It was stated that AAILs improved the thermal stability of BSA and HSA, i.e., for [Ch][Trp]—the Tm of BSA increased from 65.51 to 72.46 °C and for HSA, the Tm increased from 65.46 to 75.97 °C. For [TEA][Trp]—BSA Tm increased from 65.51 to 69.75 °C and for HAS, the Tm increased from 65.46 to 72.08 °C [52].
As mentioned before, to achieve efficacy in mAbs therapeutics, a relatively high dose of mAbs is required, and they are frequently produced at high concentrations [17]. Therefore, the recent advances in the few studies available on the development of stabilizing IL-based formulations for mAbs are reviewed.
Reslan et al. [43] investigated the impact of high concentrations of cholinium dihydrogen phosphate ([Ch][Dhp]) IL on the stability of the mAb Herceptin® (trastuzumab). It was shown that [Ch][Dhp] repressed the unfolding and aggregation of mAb Herceptin®. At low concentrations of [Ch][Dhp], the unfolding of trastuzumab was not as strongly repressed, and an irreversible aggregation was observed [43]. On the other hand, at the high concentration of [Ch][Dhp] (53%), unfolding and irreversible aggregation was avoided, resulting in decreased monomer loss [43]. It was also concluded that the [Ch][Dhp] has a stabilizing effect when combined with other stabilizing excipients [43].
Mazid et al. [44] explored the use of cholinium-based buffered ILs (BILs) in the biological activity and structural stability of the epidermal growth factor receptor mAb (EGFR mAb) under proteinase-contaminated conditions. The results demonstrated that b[Ch][Dhp] maintained the EGFR mAb α-helix conformation and its stability and activity in the presence of proteinases [44]. In addition, the biologically activity of EGFR mAb stored in b[Ch][Dhp] was maintained for a longer period. Moreover, the binding efficacy was improved in the presence of b[Ch][Dhp] in 20%. After storage at 37 °C for 7 days, the retained binding was improved in b[Ch][Dhp] (50% (w/w)) even in the presence of proteinase K [44].
The recent work of Dhiman et al., [53] evaluated the presence of cholinium-based ILs as a promisor medium to enhance the thermal and structural stability of IgG. The evaluated compounds included cholinium acetate ([Ch][Ac]), cholinium chloride ([Ch]Cl), cholinium dihydrogen citrate ([Ch][Dhc]) and [Ch][Dhp] aqueous solution. Among them, the results show an increase in Tm when [Ch][Ac] and [Ch]Cl were added. The fluorescence thermodynamic parameters obtained were compared with UV, fluorescence, CD and FT-IR spectroscopies, and SE-HPLC and SDS-PAGE structural stability of IgG. The obtained data showed that all results were corroborated. Molecular docking (Molegro Virtual Docker (MVD)) reinforced the main interactions, ruling the IgG stability in these ILs, validating their potential for IgG formulations since their thermal and structural stability was improved and preserved [53].
The studies reporting the stabilization of proteins in hydrated ILs are summarized in Table 1 and the main conclusions are presented in Figure 3.

Table 1.
Summary of the works reporting the stabilization of proteins in ILs.
Figure 3.
Main factors affecting the stability of proteins in the presence of ionic liquids.
2.2. Stability of Proteins in Deep Eutectic Solvents
DESs share many of the particular properties of ILs, allied with several other interesting properties. Thus, DESs are promising alternative solvents to the traditional ones for protein stability. However, since they started to be recently featured, the literature available on the use of DESs for protein stabilization, such as BSA and HSA, is still limited when compared to ILs.
Sanchez-Fernandez et al. [56] investigated BSA structure in choline chloride: glycerol, and choline chloride: glycerol/water mixtures (two solvents: 75 wt% and 50 wt% of DES) and buffer (phosphate buffered saline, 0.01 M, pH = 7.4). It was shown that the BSA secondary structure was not changed. The BSA CD spectra showed that the protein folding was similar in buffer and DES/water mixtures. A slight difference between the tertiary structure of the protein in these solvents was observed. On the other hand, the CD spectrum of BSA in pure DESs was significantly different from the solvents containing water. A change in the tertiary structure of BSA was found, and regarding its denaturation above 80 °C, an irreversible change to its conformation was detected, showing that the DESs evaluated did not provide any improvement in BSA thermostability [56].
The work of Fu et al. [57] evaluated the glycation of BSA with glucose using a natural deep eutectic solvent (NADES), namely choline chloride/glucose. Compared to the aqueous medium, glycated BSA in NADES presented more—OH groups, lower intrinsic fluorescence intensity, more disordered secondary structures, and higher ultraviolet–visible absorption [57]. This study promoted glycated BSA functional activities, making it appropriate for the food sector.
The thermal stability of BSA was improved in the presence of choline chloride-urea and choline chloride-glycerol [58]. Comparing both DES, and choline chloride-urea lead to the superior thermal stability of the BSA structure. Moreover, the combination of chloride-urea with PEG led to better results, as the thermal stability of BSA was increased at 16 °C [58].
More recently, the effect of pure and hydrated reline choline chloride/urea DES in the structural stability of BSA using all-atom molecular dynamics simulations was investigated by Kumari et al. [59]. A considerable change in the BSA structure and an increment in the accessible surface area of the solvent were found. These induced structural changes were evident in the reline–water mixtures. Rigidity in the protein structure is also noted in the presence of pure reline. The authors concluded that, despite the expansion, the tertiary structure of BSA in pure reline was similar to the native protein structure [59].
3. Stability of Enzymes in Alternative Solvents
Most enzymatic reactions occur in an aqueous medium. Organic substrates can be used to boost the solubility of hydrophobic substrates; however, these solvents are usually toxic and flammable. Due to the outstanding properties of alternative solvents, the traditional organic solvents used in biocatalysis are gradually being replaced by DESs or ILs aiming to promote the activity and stability of enzymes, increase substrate solubility, conversion rate and ultimately decrease environmental pollution [60].
Hydrolases, such as lipases, are the most used and explored enzymes in industrial biocatalysis. Lipases are naturally found in the stomach and pancreatic juice and are usually stable in organic solvents and active under mild conditions [61]. Their main biological functions consist of fat and lipid digestion and contributing to the correct operation of the gallbladder [61]. Lipases are widely used in several industries ranging from the oleochemical and food industries to environment management and biosensors. [61]. In addition, the employment of lipases in the synthesis of enantiomeric pharmaceuticals, agrochemicals and flavoring compounds was recently described [62]. Moreover, the therapeutic activity of lipase was also enhanced through the modification of its physical, chemical and biochemical properties when added as a supplement to food products [63]. This innovative strategy benefits human health due to its ability to lower serum cholesterol whilst enhancing flavor in diverse food products [63].
Another enzyme with exceptional value is lysozyme, an antimicrobial enzyme with various applications in the fields of cosmetics, food and medicine [64]. The enzyme’s applications range from its use in hurdle food preservation techniques to roles of great importance in the treatment of allergies, colitis, various pains, inflammations and assorted bacterial or viral (zona, herpes zoster) infections [64]. Lysozyme exerts an anti-inflammatory activity making it efficient against inflammatory processes related to cancer, healing and recovery of ulcers in arteriopathy and post-radiation therapy [64]. Furthermore, the literature reports that lysozyme was able to suppress the development of HIV-1 in vitro and help in several other medical conditions [64].
Moreover, a protein that belongs to class 1 of the c-type cytochrome family, cytochrome c (Cyt-c), can be defined as a highly multifunctional protein, due to its diverse roles according to its localization in the cell and the circumstances in which it operates. [65]. Cyt-c intervenes in the electron-transfer in the respiratory chain and exerts antioxidant activity by disposing of reactive oxygen species (ROS). This enzyme also takes part in cell apoptosis and can be used as a biomarker in clinal diagnostics applications through its detection in the cytoplasm [65]. Recently, Cyt-c biosensors were developed and allow for the real-time informatics needed to evaluate the death process, disease progression, therapeutics and processes related to mitochondrial injury [66].
A further example is laccases, enzymes extensively distributed in fungi and higher plants belonging to the blue multi-copper oxidases with a wide range of applications in the food, textile and pulp and paper industries [67]. Recently, laccases are being employed in the development of biosensors, the design of biofuel cells and medical diagnostics tools [67]. In the last few decades, laccases have peaked the attention of researchers due to their potent ability in cleaning up herbicides, pesticides and certain explosives in the soil, thus, becoming quite relevant in bioremediation strategies [67]. These enzymes are also being used in the development of drugs against cancer, Hepatitis C and HIV-1, and in the synthesis of complex medical compounds, such as anesthetics, anti-inflammatories, antibiotics and sedatives, thus, becoming increasingly relevant in the imminent field of medicinal biotechnology [68,69].
3.1. Stability of Enzymes in Ionic Liquids
As previously discussed, ILs are capable of rising or reducing the stability of proteins, according to the type of IL, its concentration and the type of protein [1,70,71,72,73]. The capacity of ILs to stabilize enzymes diverge and is usually influenced by the IL cation and anion combination [1,70,71,72]. The activation and stabilization of enzymes in pure ILs are influenced not only by the type of ions that constitute the IL but also by the enzyme arrangement of functional groups [1,70,71]. On the other hand, the fact that there are more studies addressing the stability of enzymes in hydrated ILs, is also advantageous from a monetary and ecological point of view, since a small concentration of IL is needed and the greenest and cheapest solvent of all is water. Moreover, it is essential to point out that aqueous solutions of ILs are also remarkably advantageous concerning systemic drug administration because they implicate less viscosity when compared with neat ILs. Despite the advantages associated with aqueous solutions of ILs, enzyme catalysis can be performed at high temperatures in order to optimize the product yield. [1]. This can only be achieved by accomplishing enzyme stability in proper non-aqueous mixtures at elevated temperatures [1]. In order to achieve this goal, it is also crucial to identify neat ILs that have the ability to dissolve, stabilize and activate enzymes. A scheme showing the advantages and disadvantages of using ILs as reaction media for enzymes is presented in Figure 4.
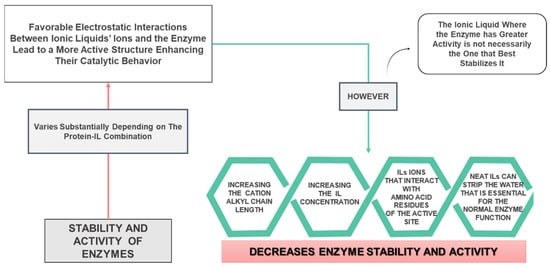
Figure 4.
Scheme summarizing advantages and disadvantages of ionic liquids as reaction media for enzymes.
The activity of lipase from Burkholderia cepacia (BCL) in diverse phosphonium-based ILs was evaluated by Barbosa et al. [74] through experimental and molecular docking studies. ILs containing a variety of different cations (trihexyltetradecylphosphonium ([P666(14)]+), tetrabutylphosphonium ([P4444]+), tributyltetradecylphosphonium ([P444(14)]+)), combined with several anions (bis(trifluoromethylsulfonyl)imide ([NTf2]−), bis(2,4,4-trimethylpentyl)phosphinate ([Phosp]−)chloride (Cl−), decanoate ([Deca]−) and bromide (Br−) ) were selected to inspect the separate effect of IL ions on the behavior of BCL [74]. Results from this study demonstrated that BCL activity was found to decrease as the behavior cation alkyl chain length of the IL increased [74]. The molecular docking studies corroborated these findings by demonstrating that the IL cations preferred interactions with the amino acids present in the oxyanion hole of BLC [74]. Regarding the role of the IL anions, [Phosp]− and [NTf2]− favored the activity of BCL, whereas the remaining anions presented adverse effects [74]. the anions [Deca]−,Cl− and Br−, essentially interacted by hydrogen bonding with amino acid residues that were part of the catalytic triad of the enzyme [74]. In contrast, the anions [Phosp]− and [NTf2]−, in addition to having higher binding energies with BCL, interacted mainly with the side chain amino acids of the enzyme and not with residues of the active site [74]. Moreover, the protein secondary structure was analyzed by FTIR spectroscopy which exhibited that a reduction in the α-helix protein content took place in the presence of the ILs resulting in increased enzyme activity [74]. The authors suggested that these modifications in the enzyme structure allowed a wider explosion of the enzyme active site and thus, facilitated access to the substrate and subsequent increased BLC activity [74]. The IL [P666(14)][NTf2] at 0.055 mol L−1 showed the highest potential since it was able to increase BCL activity by 61% [74].
The behavior of Aspergillus niger lipase was also studied in the well-known imidazolium-based ILs by Nascimento et al. [75]. The goal of this study was to investigate the effect of the cation alkyl chain length and different concentrations of the 1-alkyl-n-methylimidazolium chloride ([Cnmim]Cl (n = 4, 6, 8, 10, 12))-based ILs on the enzyme activity and stability [75]. In this study, the samples were incubated at 35 °C for 24 h, and lipase activity and stability were evaluated in aqueous solutions of the ILs prepared with 0.025, 0.05 and 0.15 wt% IL to study the lipase activity; 0.1, 0.3 and 0.5 wt% IL to study if the enzyme was stable in the presence of these ILs [75]. Results show that lipase activity and stability were promoted by the ILs with reduced cation alkyl side chain length, namely [C4mim]Cl and [C6mim]Cl; while [C8mim]Cl, depending on its concentration, maintained or decreased the enzyme activity [75]. In the presence of ILs with longer cation alkyl chain length, such as [C8mim]Cl, the lipase activity was maintained or decreased, depending on its concentration; while [C10mim]Cl and [C12mim]Cl reduced the enzyme activity at 0.1 wt% and could even suppress it at 0.3 wt% [75]. Spectroscopy analysis (fluorescence and circular dichroism (CD)) corroborated the previous results and demonstrated that the activity of lipase was increased by the ILs with shorter alkyl chain lengths due to beneficial electrostatic interactions between IL’s ions and the enzyme, leading to adjustments in lipase structure, benefiting the arrangement of the enzyme–substrate complex and subsequently promoting the catalytic behavior of lipase [75]. Contrary, if the IL presents longer alkyl chain lengths, hydrophobic interactions between the chain and the enzyme active site take place, making the substrate consumption difficult and inhibiting the catalytic performance [75].
The findings of the studies carried out by Barbosa et al. [74] and Nascimento et al. [75] both show that phosphonium and imidazolium-based ILs can promote contrasting behavior on the activity of lipases (enhance, maintain or even inhibit) and structural conformation, depending on the cation alkyl chain length, the IL anion used and the IL relative concentration [74,75]. The key mechanism that usually governs the lipase catalytic behavior is a result of highly balanced competing interactions between the solvent (water), the enzyme surface and IL cation, anion or the ion pair itself [75]. IL ions and water can reach out with non-polar, polar, and charged surface areas of enzymes, according to their various hydrophobic/hydrophilic/amphiphilic characters, subsequently causing opposite beneficial and unfavorable effects depending on how specific and predominant each of these interactions is [75].
Although satisfactory results were obtained from the previous studies, there is an emerging need to find more biocompatible ILs, since phosphonium and imidazolium-based ILs have been reported to raise toxicity and biocompatibility concerns [1]. Since ILs are significantly soluble in water, they can become dangerous pollutants in wastewater and endangered environmental systems [1]. Among the great variety of ILs, choline-based ILs have become remarkably relevant. Their eco-friendly character has entrenched them as a first-choice to study enzyme stability and activity [72,76].
In this context, Nascimento et al. [77] further investigated the stability and activity of A. niger lipase in aqueous solutions of six cholinium-based ILs ([Ch][Ac]; cholinium propanoate ([Ch][Prop]); ([Ch]Cl); cholinium hexanoate ([Ch][Hex])), cholinium butanoate ([Ch][But]) and cholinium pentanoate ([Ch][Pent])) during 24 h [77]. The catalytic activity of lipase was sustained or increased in the lower concentrations (below 0.1 M) of all ILs [77]. The enzymatic activity and biocatalytic behavior of lipase were maintained in all the aqueous solutions of the IL [Ch][Ac], regardless of the time of incubation [77]. However, aqueous solutions with concentrations above 0.1 M of the ILs [Ch][Pent] and [Ch][Hex] led to full catalytic activity suppression of lipase, showing how the conformation of the enzyme is heavily affected by increasing the anionic alkyl chain length [77]. These results allowed us to conclude that, as previously reported [74,75], the hydrophobicity of long alkyl chain lengths (anionic or cationic) and the ILs concentration have a crucial role in the biocatalytic behavior of the enzyme, and these parameters can be regulated by adapting the alkyl chain lengths and adjusting the ILs concentration [74,75,77]. Lipase can be stabilized by ILs with short alkyl chain lengths and these neat ILs can be used as non-aqueous media for reactions that are not possible in typical organic solvents, while ILs with longer alkyl chains can also be used with the pharmaceutical target of inhibiting lipase activity, for instance, to promote the pharmacological suppression of gastric and pancreatic lipases [74,75,77].
Relatively high enzyme activities are achieved in tert-butanol, an organic solvent commonly used for transesterification and ammonolysis reactions catalyzed by lipase [78]. Lipases are highly flexible in tert-butanol facilitating the access of the substrate to the active site while maintaining hydrogen bonds with the solvent [78]. Moreover, non-aqueous biocatalysis is also promoted in some ethers [78]. Despite this, the enzyme activities are still typically lower by several magnitudes in non-aqueous organic solvents when compared to aqueous solutions [78]. Given this information, Zhao et al. [78] designed ILs that combined both features of tert-alcohols and ethers, with the target of creating IL structures that could create water-like environments by having both hydrogen-bond-donating (−OH) and-accepting (R−O−R) properties. A variety of these ILs were designed through the affixation of ether and tert-alcohol functional groups to an imidazolium [78]. In order to evaluate if these ILs were appropriate and could promote enzymatic activity, the activity of lipase B from Candida antarctica (CALB) was investigated, and results revealed that the IL 1-Ethyl-3-(2-hydroxy-2-methylpropyl)imidazolium bis(trifluoromethylsulfonyl)imide ([Et-Im-t-BuOH][Tf2N]) afforded a high lipase activity which could be compared with diisopropyl ether [78]. However, the IL with the longer alkyl chain, 1-butyl-3-(2-hydroxy-2-methylpropyl)imidazolium bis(trifluoromethylsulfonyl)imide ([Bu-Im-t-BuOH][Tf2N]), could not be employed as a solvent for CALB catalytic activity since it remained solid at the temperature needed for the reaction to happen (50 °C) [78]. In contrast, the enzyme activity in the other several dual-functionalized ILs was doubled and even quadruplicated in comparison to the traditional imidazolium based ILs, almost two-fold higher than the activity present in tert-butanol, and enhanced over 40% than that in diisopropyl ether [78]. The authors further explored if the water content had a drastic effect on lipase activity [78]. When comparing trials where the water content was slightly increased a harsh decline in CALB catalytic behavior was observed [78]. Therefore, in order to reach elevated transesterification activities, it is necessary to finely regulate the content of water in ILs solutions [78]. Furthermore, this study investigated if these ILs were able to provide exceptional thermal stability to the enzyme [78]. Fluorescence emission spectra results demonstrated that CALB maintained its conformation after its incubation at 50 °C for 24−48 h in dual-functionalized ILs [78]. In addition, the enzyme thermal stability was much lower in tert-butanol than in the ILs, suggesting that these short-chain-glycol-grafted ILs do not denature or disrupt lipase conformation, and are unique alternative solvents for the stabilization of this enzyme [78].
Although imidazolium-based ILs have been reported to raise toxicity and biocompatibility concerns, a study conducted by Ventura et al. [79] reported that the toxicity of these imidazolium-based ILs can be reduced by grafting oxygenated groups, such as ether and ester, on the IL alkyl chains. Therefore, Zhao et al. [78] not only attained positive results but also developed a truly innovative class of ILs. Nevertheless, experimental ecotoxicity and cytotoxicity assays should be made in order to evaluate the toxic effects that these compounds may represent in nature and ecosystems. This is a valid point for all studies that investigate ILs whose toxicity was not yet accessed since in the current situation it is urgent to design active ILs with little toxicity and great biocompatibility.
Despite all these toxicity and biocompatibility concerns, imidazolium-based ILs continue to be relevant and are still explored as alternative media for enzymes. Since ILs are designer solvents, there are still a high number of different anions combined with imidazolium-based cations that can still be explored. For instance, Rather et al. [73] investigated if the surface active ionic liquids (SAILs), 1-octyl-3-methylimidazolium dodecylbenzenesulfonate ([C8mim][DBS]) and 1-dodecyl-3-methylimidazolium dodecylbenzenesulfonate ([C12mim][DBS]), were able to promote the activation, stabilization and structure of lysozyme. This study demonstrated that the interactions between the enzyme and the SAILs were influenced by the ILs concentration and the cations and anions used to synthesize SAILs [73]. The SAIL with the longer cation alkyl chain length, [C12mim][DBS], exerted a destabilizing effect on the enzyme at all concentrations [73]. In contrast, lysozyme activity, thermal stability and general conformational stability were promoted by [C8mim][DBS] when this IL was in the concentration range of 0.5 mM to 1.35 mM [73]. Thus, [C8mim][DBS] with adjusted concentration could be used as an adjuvant for biotechnologically relevant enzymes, namely lysozyme, promoting their shelf life and stability [73].
The misfolding of proteins is a prejudicial phenomenon that can cause enzymatic inactivation, aggregation, and the production of insoluble protein fibrils called amyloids [80]. In this context, it is important to investigate how protein folding takes place and under which circumstances it can be diminished. As previously addressed, ILs have the ability to enhance or reduce protein and enzyme stability, according to their constitution, concentration and which protein or enzyme is under study [1,70,71]. There is not a specific tendency observed regarding the behavior of enzymes in ILs, since diverse complexities exist in the way they interact [1,70,71,80]. Therefore, there is a need for more and larger studies to enable the selection and optimization of ILs used as solvents for enzymes.
Another class of ILs with remarkable potential as candidates to stabilize and separate enzymes are ammonium-based ILs, consisting of singular molecular structures with hulking ammonium cations and relatively minor anions [72]. In this context, Arunkumar et al. [80] developed a systematic study in which, the stability and secondary structure of different enzymes (lysozyme, trypsin, β-lactoglobulin and α-amylase) were analyzed in hydrated solutions of 10 ILs at variable concentrations of IL. The ILs were made of ethyl- (EE), ethanol- (EtA), diethanol- (DetA) and triethanolammonium (TeTA) cations combined with nitrate, formate, acetate or glycolate anions [80]. Attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy analysis showed that both lysozyme and trypsin maintained their native folded state compatible with their secondary structure in the solutions of ILs composed of formate or nitrate anions at the lower IL concentrations [80]. However, the isoelectric point of α-amylase and β-lactoglobulin was very close to the pH of the solvent causing reduced stability and solubility of these enzymes [80]. Moreover, the IL cations EA, EtA and DetA combined with the anion acetate were not able to solubilize any of the enzymes, only TetA paired with acetate was capable to stabilize α-amylase and trypsin in up to 20 and 30 mol% of IL, respectively [80]. In summary, the authors’ findings demonstrate that these enzymes are not stable in ILs with the acetate anion but ILs containing nitrate and formate anions, such as ethylammonium nitrate (EAN) and ethylammonium formate (EAF), were able to promote enzyme solubility and stability [80]. Once again, this study also evidences that enzyme stability in ILs is highly complex and is influenced by several aspects.
In the scope of ammonium-based ILs, Attri et al. [81] investigated for the first time, the tobacco etch virus protease (TEVp) stability and activity in triethylammoniumhydrogen phosphate (TEAP) and diethylammonium dihydrogen phosphate (DEAP). The enzyme stability was measured for both ILs, the protease was better stabilized in both ILs at 10% (v/v). However, TEVp stability was inversely correlated with the IL concentration, it decreased as the concentration of IL increased [81]. CD studies were performed for both ILs at 10% (v/v) for various temperatures. Results demonstrated that the enzyme was totally denatured at 50 °C in the absence of ILs [81]. Moreover, the structural properties of the enzyme were completely conserved in 10% (v/v) TEAP up to 70 °C. However, TEVp was stabilized in the presence of DEAP only up to 50 °C [81]. MD simulations validated the results obtained from the CD spectrums, clearly showing that the highest stability of TEVp was observed in TEAP [81]. Thus, ILs containing triethylammonium cations are more capable to stabilize enzymes in comparison to ILs with dimethylammonium cations. Despite this, SDS-PAGE analyses demonstrated that TEVp activity was maintained in the presence of DEAP but not in TEAP [81]. Interestingly, the IL that best stabilized TEVp did not allow the enzyme to remain active [81]. The most probable mechanism that justifies the loss of activity, is that the enzyme is stabilized by powerful bonds with TEAP IL; however, the active site of the enzyme is covered, inhibiting the enzyme catalytic behavior [81]. This phenomenon was further backed by a different investigation from the same research group [82], which reported how the TEAP IL was able to stabilize the α-chymotrypsin structure and at the same time completely inhabited its activity [82]. The similar behavior of α-chymotrypsin and TEVp in these ILs could be due to the fact that they are both members of the same family of serine proteases, resulting in analogous IL-enzyme mechanisms. Although it cannot be defined as a general trend, it is necessary to keep in mind that the ILs that best stabilize enzymes are not always the ones that better promote their activity [81,82].
Amino acid ILs (AA-ILs) have emerged as a recent category of ILs with promising relevance as biocompatible alternative solvents for biomolecules since they have low-cost eco-friendly starting materials. In addition to their environmentally compatible nature, AA-ILs have a unique set of properties, such as reduced melting points, and relevance in the biopharmaceutical industry making them exceptional as biocompatible non-traditional solvents for enzyme stabilization and storage [1,83]. For instance, Sahoo et al. [83] investigated the stability of Cyt-c in two cation- and anion-based amino acid ILs (CAAAILs), choline proline ([Ch][Pro]) and proline nitrate ([Pro][NO3]), known for their biocompatible and nontoxic nature. Detailed analysis of CD and ultraviolet-visible (UV-Vis) spectra revealed that low concentrations of IL preserved the stability of secondary and tertiary structures of Cyt-c [83]. On the other hand, this enzyme lost its integrity at elevated concentrations of [Pro][NO3] [83]. In addition to maintaining its thermal stability in CAAAILs, the long-term storage of Cyt-c dissolved in 1 mM CAAAILs was also evidenced after observing that its conformation was unaffected after 2 months of incubation [83]. MD simulations revealed that the ILs stabilized the enzyme through electrostatic and hydrophobic interactions on terminal helices and between the loop of protein surfaces [83]. The IL containing the nitrate anion presented a higher ability to stabilize Cyt-c due to its small size and lack of an alkyl chain on the nitrate anion, which interacted with positively charged amino acid residues [83]. In contrast, the IL [Ch][Pro] showed less potential in this study because the [Pro] anion bound too strongly at the hydrophobic ends of the enzyme causing the loss of its conformational integrity [83]. According to the results observed in this study, the authors suggested that the structural integrity of Cyt-c is promoted by CAAAILs where the anions are amino acids but not where cations are amino acids [83]. In addition to being economic and green solvents, CAAAILs present a high potential for protein dissolution, stability, and long-term [83]. Hence, these ILs could revolutionize protein chemistry being necessary to continuously explore the role of AA-ILs in enzyme stability and activity in order to contribute to their application in the biotechnological and biomedical fields. [83].
The studies reviewed in this section reporting how ILs stabilize and activate enzymes are summarized in Table 2.

Table 2.
Summary of the studies reviewed in this work reporting the stabilization of enzymes in ILs.
3.2. Stability of Enzymes in Deep Eutectic Solvents
Similar to proteins, recent studies addressing the enzymatic stabilization and activation in DESs will also be discussed in this review. Although DESs were contemplated as a promising alternative medium of enzymatic biocatalysis, these solvents are highly viscous and present comparably reduced enzyme activity. Thus, they are scarcely used at industrial scales up to date [84]. However, as summarized in Figure 5, it has been reported that using DESs as cosolvents, by adding small amounts of water, can increase the solubility and stability of enzymes allowing their use in various applications [84].
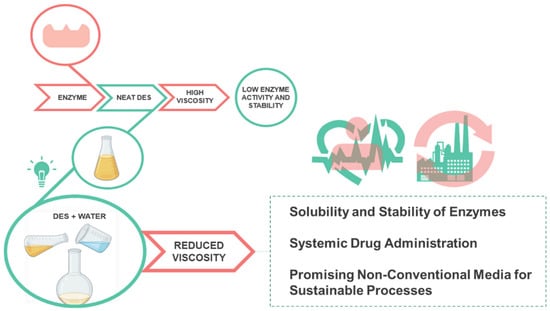
Figure 5.
Scheme representing the main properties and advantages of hydrated deep eutectic solvents for the stability of enzymes.
Inspired by this, B. Nian et al. [84] developed several experiments in which several aqueous solutions of choline chloride-glycerol (ChCl:Gly 1:2) were studied as alternative media to improve CALB biocatalyst behavior. The goal of this study was to drastically reduce the DES viscosity through the addition of water while investigating the behavior of DES in cosolvents [84]. Quantum chemical calculations and MD simulations revealed that the addition of increasing amounts of water was effective for enhancing polarity and reducing the viscosity of ChCl:Gly [84]. The most satisfactory molar ration of water was 0.3, which increased enzymatic activity by 57%. The authors suggested that the increase in CALB activity was due to the formation of favorable hydrogen bonds among CALB active and the glycine present in the DES, which contribute to the equilibrium between the structural elasticity and rigidity of CALB [84]. Although adding water to the DES promoted CALB stability and activity, when higher amounts of water were added, the integrity of the DES was compromised which subsequently led to excessive hydrogen bonding interactions and the disruption of the balanced properties of the enzyme structure, resulting in less activity and stability of CALB [84].
Guajardo et al. [85] also explored the biocatalyst behavior of CALB in ChCl:Gly (1:2), but instead of adding water, the authors used phosphate buffer as cosolvent (10% v/v) in bioreactors [85]. The activity of CALB increased by 54% in seven cycles of operation, demonstrating that lowering the viscosity of DESs by blending them with aqueous cosolvents potentiates them as non-conventional media for sustainable and scale-up processes [85].
Another recent study addressing the behavior of a lipase in DESs is reported by Gojun et al. [86]. An innovative process in which a lipase from Thermomyces lanuginosus (TIL) was applied in the production of biodiesel [86]. The reaction was performed in the neat DES ChCl:Gly in a molar ratio of 1:3; however, the results obtained in the neat DES were not satisfactory [32]. Therefore, Gojun et al. [86] performed experiments in which different amounts of water (1–8 wt%) were added to the DES [86]. The biodiesel reaction was significantly enhanced up to 4 wt% of water added [86]. However, above that, the value of the production experienced a dramatic decline [86]. The authors proposed this yield decrease was caused by more pronounced hydrolysis in the reaction system, a consequence of a higher water content [86].
Although the studies discussed so far present ChCl:Gly as a suitable media for lipases [84,85,86], Huang et al. [87] did not report positive results with ADH in this DES. In this study, the activation and stabilization of this oxidoreductase in ChCl:Gly (1:2)/water solutions with variable quantities of water (0 to 20% (v/v)) were measured [87]. Results indicated that at low water contents (<10% (v/v)) the enzyme did not show any activity on account of being dehydrated and because the attractive interactions between water and the DESs were too powerful [87]. Increasing the water content was not enough to improve ADH since the DES was still very viscous [87]. Overall, despite the negative results, this study represents a comprehensive analysis regarding the biocatalyst behavior of an oxidoreductase in a DES [87]. However, other DESs should be explored as a catalytic medium for ADH.
Natural deep eutectic solvents (NADES) can be produced by cells as a defense mechanism in demanding conditions at extreme temperatures, providing stability to the cell membranes, enzymes and metabolites [88]. Based on this fact, Khodaverdian et al. [88] conducted a study in order to evaluate if Bacillus HR03 laccase could be stabilized and activated in several ammonium-based (choline and betaine) NADES with various combinations of HBDs (sorbitol, glycerol, urea, citric, malic and oxalic acid) with a substrate that has reduced solubility in water [88]. Results showed that in DESs with ChCl as the HBA, an abrupt decline in the activity and stability of laccase was noticed because the choline ion has a detrimental effect on the laccase biocatalyst behavior [88]. On the other hand, laccase exhibited great catalytic behavior in the betaine-based DESs, Bet:sorbitol:water (1:1:1) and Bet:Gly (1:2), relative to the Begley aqueous buffer control at 80 and 90 °C and the ChCl-based DESs [88]. Although Khodaverdian et al. [88] reported interesting findings regarding the behavior of laccase in DES, the molecular mechanisms underlying the enhanced enzymatic stability and activity should be further investigated in order to achieve a deeper knowledge regarding the role played by DESs, their components, ratios and amounts of water added as alternative media for laccase catalytic reactions.
In the same context, Toledo et al. [89] also evaluated laccase performance in a series of NADES composed of ChCl, ChDH, ChDHC and Bet as HBAs combined with EtG, Gly, erytritol (Ery), and xylitol (Xyl) as HBDs. In accordance with Khodaverdian et al. [88], laccase relative activity was reduced by ChCl-based DES. The enzyme activity was even below 40% at the highest concentration tested (50 wt%) of ChCl-based DESs relative to the phosphate buffer control [89]. In addition to the denaturing role of chloride, the authors further suggested that the inhibitory effect in laccase can be attributed to the disruption of intramolecular hydrogen bonds caused by ChCl itself [89]. The replacement of ChCl by ChDHP and ChDHC in the DESs formulations, as expected, enhanced the enzymatic activity relative to the control, with the exceptions of ChDHP:EtG and DESs at 50 wt% [89]. Moreover, a trend was observed where the enzyme activity increased as the number of hydroxyl groups increased in the HBDs of ChDHC and ChDHP-based DES [89]. ChDHC is also composed of three hydroxyl groups which also benefits the activity of laccase [89]. Thus, the authors concluded that the biocatalytic behavior of laccase is favored by DESs with HBDs and HBAs presenting several hydroxyl groups in their chemical formula [89]. Regarding the behavior of laccase in the Bet-based DESs, the highest activity observed was a 20% enhancement relative to the buffer control. Regarding the behavior of laccase in the Bet-based DESs, the highest activity observed was a 20% enhancement relative to the buffer control and this value was very much alike for all the betaine aqueous solutions [89]. Thus, different from the cholinium-based DES analyzed by Toledo et al. [89], betaine did not present polyols synergetic effects for DESs production. Molecular docking calculations showed that the favorable influence of DESs in laccase activity was directly related to interactions between DES components and histidine residues at the enzyme catalytic cluster [89]. In fact, choline and chloride did not present affinity for these HIS residues, presenting one more reason why ChCl-based DES is not beneficial to laccase [89]. Overall, the DESs that contributed the most to laccase activity were ChDHC-based DES combined with Ery or Xyl at concentrations of 25 and 50 wt%, leading to a 200% enhancement in laccase activity [89].
Moreover, Toledo et al. [89] evaluated if ChDHP:Xyl (1:2) at 10 wt%, ChDHP:Xyl (1:2) at 25 wt%, and ChDHC:Xyl (2:1) at 25 wt% could provide long-term stabilization (20 days) to laccase at the extreme temperatures of −80 °C and 60 °C [89]. Although these DES were not able to maintain the stability and activity of laccase incubated at 60 °C, an exceptional effect was observed at −80 °C. These DESs were able to provide remarkable storage thermal stability while also enhancing laccase activity between 130 and 200% for up to 20 days of incubation [89]. After the 20-day period, laccase activity decreased but was still superior to that in the control [89]. The authors concluded that hydrated mixtures of NADES have a powerful effect by avoiding water crystallization/freezing and melting process, decreasing the amount of ice crystals and subsequent cell damage [89]. Therefore, the application of DESs as alternative cryoprotective is extremely relevant for the stabilization of biomolecules at negative temperatures, which is likely an important role played by NADESs at the cellular level [88,89]. Additionally, laccase is widely used in industrial biotechnology, thus; these findings regarding the increased activity and stability of laccase in NADES step up the potential of this enzyme in several industries and put in perspective the possibility to apply these eco-friendly and sustainable solvents to a wide range of oxidative biotransformations with great relevance and importance [88,89].
Ginsenoside compound K (CK) is a compound with great relevance in the biopharmaceutical industry whose production comes from the conversion of ginsenoside Rb1 into ginsenoside CK, a reaction catalyzed by β-glucosidase [90]. However, this reaction has a low yield percentage which limits the industrial application of CK [90]. In this context, the use of ChCl:EtG (2:1) as a reaction medium for the enhanced production of β-glucosidase activity and purity in order to increase the yield of CK was studied by Ma et al. [90]. Results showed a 96% increase in β-glucosidase half-life and a subsequent 54% increase in CK yield in an aqueous solution of ChCl:EtG (2:1) at 30% (v/v), compared with the buffer control [90]. Moreover, raising the temperature to 60 °C led to an enhancement of 80.6% in the conversion of CK [35,90]. In order to verify if the enzyme was still stable in these conditions, fluorescence spectroscopy FTIR, and CD analysis were carried and the results demonstrated that the structure of β-glucosidase was neither altered nor denatured ChCl:EtG (2:1) [90]. Therefore, using this DES could provide an efficient way for CK production [90]. Furthermore, in a study developed by Miranda-Molina et al. [91], a series of 12 DESs were also evaluated as an alternative reaction media for the thermostable Thermotoga maritima amylase, an enzyme that catalyzes the production of alkyl glycosides. The neat DESs completely inhibited the enzyme activity. However, when the amylase activity and stability were tested in aqueous solutions of DESs composed of ChCl as HBA and alcohols, sugars, and amides as HBDs, positive results were achieved [91].
The studies reviewed point out that a suitable choice of DESs as solvents or cosolvents can result in a remarkable biocatalytic behavior. Furthermore, previous reports addressed in this review, allow us to conclude that ChCl was the most common HBA selected and the addition of water (or aqueous buffers) to the DES significantly improves reaction yields [84,85,86,88,89,90,91]. As previously discussed, the major problem of DESs is their viscosity [84]. Notably, neat DESs or ChCl-based DESs can be extremely viscous, and therefore, do not exert a positive effect on enzyme stability and activity [91]. Adding water to DESs allows the water molecules to alter the hydrogen bond network and subsequently create a more favorable environment that promotes the enzyme’s biocatalysis performance [25]. However, adding higher amounts of water could affect the unique properties of the DES and lead to product hydrolysis which drastically decreases the reaction yield [25]. Thus, the role of water in enzymatic reactions performed in DESs is still far from being completely understood and merits further investigation [92]. Moreover, future studies addressing the biocatalytic performance of enzymes in DESs should investigate the molecular mechanisms underlying the influence exerted by DESs of different natures on enzyme stability and activity [92].
An innovative and interesting property of DESs was reviewed by Guajardo et al. [31], which consisted of the possibility of exploiting DESs not only as an alternative solvent but also as the substrate for the enzymatic reaction [25]. Although the potential of DESs for this purpose is not yet fully explored, finding approaches that allowed it is highly relevant since it would grant the improvement of bioprocesses where a great amount of substrate would be available, resulting in elevated product yield. Moreover, more competent, environmentally friendly and less dangerous processes would be available [25].
The studies reviewed in this section, reporting the stabilization of enzymes in DES, are summarized in Table 3.

Table 3.
Summary of the studies reporting the stabilization of enzymes in DESs.
4. Conclusions and Future Prospects
This article reviewed recent works showing that the type of proteins and enzymes, and the association of alternative solvents (ILs and DESs) deal with their stability. Protein properties, such as self-association/aggregation, solubility, and viscosity of the final formulation with alternative solvents pose challenges to developing novel, and economically acceptable formulations and biocatalytic reactions. Overall, when properly designed, ILs have great potential to enhance protein and enzyme stabilization and biological activity. Nevertheless, researchers should continue the study of the unique properties and mechanisms underlying the stabilization proteins and enzymes in ILs as it could allow diverse applications and opportunities in various scientific fields. Moreover, future studies involving ILs and biomolecules should include ecotoxicity and cytotoxicity assays in order to evaluate if the compounds are truly safe and biocompatible. Compared to ILs, few studies were found addressing the stability of proteins and enzymes in DESs probably due to their novelty when compared with ILs that have been deeply studied during the last decades. In addition, having in mind the ecological and health concerns in the employment of new alternative media, some works were found to have already integrated ecotoxicity and cytotoxicity studies of the developed alternative formulations/reaction media, showing for the majority of the cases, that low toxicities are associated with ILs/DES. There is an increasing tendency observed in the last few years, for the replacement of the typical and well-studied imidazolium-based ILs with new more sustainable, biodegradable and biocompatible ILs, obtained from natural and renewable sources, namely cholinium-based alternative solvents. Thus, efforts should continue to be made to develop novel formulations of ILs and DESs based on natural and renewable sources in order to enhance the stability of biomolecules through strategies that can be faithfully considered fully sustainable and biocompatible, allowing further improvements in several applications fields. Therefore, it is expected that, in the future, more studies using this class of solvents could be reported for the processing/stability of a wider spectrum of (bio)molecules. Finally, the possibility of reusing and recycling the ILs/DES after the biomolecule processing should also be taken into consideration in future studies in this field, to fully accomplish the green credentials of the reported strategies.
Author Contributions
Conceptualization, M.G.F. and A.P.M.T.; formal analysis, J.S.A., A.M.L. and E.V.C.; writing—original draft preparation, J.S.A., A.M.L. and E.V.C.; writing—review and editing, E.V.C., M.G.F. and A.P.M.T.; supervision, M.G.F. and A.P.M.T.; funding acquisition, M.G.F. and A.P.M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 and LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC). This work was also financially supported by the project “IL2BioPro” (PTDC/BII-BBF/030840/2017), funded by FEDER, through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI), and by national funds (OE), through FCT/MCTES. E.V. Capela acknowledges FCT for the PhD grant SFRH/BD/126202/2016. A.P.M. Tavares acknowledges FCT for the research contract CEECIND/2020/01867.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, A.; Bisht, M.; Venkatesu, P. Biocompatibility of ionic liquids towards protein stability: A comprehensive overview on the current understanding and their implications. Int. J. Biol. Macromol. 2017, 96, 611–651. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kazlauskas, R. Biocatalysis in ionic liquids—Advantages beyond green technology. Curr. Opin. Biotechnol. 2003, 14, 432–437. [Google Scholar] [CrossRef]
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H. DNA stability in ionic liquids and deep eutectic solvents. J. Chem. Technol. Biotechnol. 2015, 90, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Capela, E.V.; Valente, A.I.; Nunes, J.C.; Magalhães, F.; Rodríguez, O.; Soto, A.; Freire, M.G.; Tavares, A.P. Insights on the laccase extraction and activity in ionic-liquid-based aqueous biphasic systems. Sep. Purif. Technol. 2020, 248, 117052. [Google Scholar] [CrossRef]
- Arumugam, V.; Rajamanikandan, R.; Ilanchelian, M.; Moodley, K.G.; Redhi, G.G. Investigation of binding interactions between BSA and [EPMpyr][Sal] through spectroscopy studies, thermophysical and thermodynamic properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 299–307. [Google Scholar] [CrossRef]
- Sindhu, A.; Mogha, N.K.; Venkatesu, P. Insight into impact of choline-based ionic liquids on bovine β-lactoglobulin structural analysis: Unexpected high thermal stability of protein. Int. J. Biol. Macromol. 2019, 126, 1–10. [Google Scholar] [CrossRef]
- Shire, S. Monoclonal Antibodies: Meeting the Challenges in Manufacturing, Formulation, Delivery and Stability of Final Drug Product; Woodhead Publishing: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Wang, Y.; Hanson, M.A. Parenteral formulations of proteins and peptides: Stability and stabilizers. J. Parenter. Sci. Technol. 1988, 42, 4–26. [Google Scholar]
- Weinbuch, D.; Cheung, J.K.; Ketelaars, J.; Filipe, V.; Hawe, A.; Den Engelsman, J.; Jiskoot, W. Nanoparticulate Impurities in Pharmaceutical-Grade Sugars and their Interference with Light Scattering-Based Analysis of Protein Formulations. Pharm. Res. 2015, 32, 2419–2427. [Google Scholar] [CrossRef] [Green Version]
- Roberts, C.J. Therapeutic protein aggregation: Mechanisms, design, and control. Trends Biotechnol. 2014, 32, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Gad, S.C. (Ed.) Handbook of Pharmaceutical Biotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Lin, S. Solubility and dissolution enhancement strategies: Current understanding and recent trends. Drug Dev. Ind. Pharm. 2015, 41, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Stefanuto, V.; Tojo, E. New Active Pharmaceutical Ingredient-Ionic Liquids (API-ILs) Derived from Indomethacin and Mebendazole. Proceedings 2019, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Shire, S.J.; Shahrokh, Z.; Liu, J. Challenges in the development of high protein concentration formulations. J. Pharm. Sci. 2004, 93, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Jezek, J.; Rides, M.; Derham, B.; Moore, J.; Cerasoli, E.; Simler, R.; Perez-Ramirez, B. Viscosity of concentrated therapeutic protein compositions. Adv. Drug Deliv. Rev. 2011, 63, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Wang, W.; Arakawa, T.; Ohtake, S. Developments and Challenges for mAb-Based Therapeutics. Antibodies 2013, 2, 452–500. [Google Scholar] [CrossRef] [Green Version]
- Magina, S.; Barros-Timmons, A.; Ventura, S.P.M.; Evtuguin, D.V. Evaluating the hazardous impact of ionic liquids—Challenges and opportunities. J. Hazard. Mater. 2021, 412, 125215. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Liu, P.; Hao, J.-W.; Mo, L.-P.; Zhang, Z.-H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Teles, A.R.R.; Capela, E.V.; Carmo, R.S.; Coutinho, J.A.; Silvestre, A.J.; Freire, M.G. Solvatochromic parameters of deep eutectic solvents formed by ammonium-based salts and carboxylic acids. Fluid Phase Equilibria 2017, 448, 15–21. [Google Scholar] [CrossRef]
- Macário, I.P.; Jesus, F.; Pereira, J.L.; Ventura, S.P.; Gonçalves, A.M.; Coutinho, J.A.; Gonçalves, F.J. Unraveling the ecotoxicity of deep eutectic solvents using the mixture toxicity theory. Chemosphere 2018, 212, 890–897. [Google Scholar] [CrossRef]
- Macário, I.; Oliveira, H.; Menezes, A.C.; Ventura, S.; Pereira, J.L.; Gonçalves, A.M.M.; Coutinho, J.; Gonçalves, F.J.M. Cytotoxicity profiling of deep eutectic solvents to human skin cells. Sci. Rep. 2019, 9, 3932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedro, S.N.; Freire, M.G.; Freire, C.; Silvestre, A.J.D. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems. Expert Opin. Drug Deliv. 2019, 16, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep Eutectic Solvents as Efficient Solvents in Biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.B.; Kumar, V.S.; Chaudhary, M.; Singh, P. A mini review on synthesis, properties and applications of deep eutectic solvents. J. Indian Chem. Soc. 2021, 98, 100210. [Google Scholar] [CrossRef]
- Colmenarejo, G.; Alvarez-Pedraglio, A.; Lavandera, J.-L. Cheminformatic Models to Predict Binding Affinities to Human Serum Albumin. J. Med. Chem. 2001, 44, 4370–4378. [Google Scholar] [CrossRef] [Green Version]
- Ossowicz, P.; Kardaleva, P.; Guncheva, M.; Klebeko, J.; Świątek, E.; Janus, E.; Yancheva, D.; Angelov, I. Ketoprofen-Based Ionic Liquids: Synthesis and Interactions with Bovine Serum Albumin. Molecules 2020, 25, 90. [Google Scholar] [CrossRef] [Green Version]
- Arques, S. Human serum albumin in cardiovascular diseases. Eur. J. Intern. Med. 2018, 52, 8–12. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Khan, J.M.; Alsenaidy, M.A.; Alsenaidy, A.M.; Khan, M.S.; Husain, F.M.; Khan, M.R.; Naseem, M.; Sen, P.; Alam, P.; et al. Unveiling the stimulatory effects of tartrazine on human and bovine serum albumin fibrillogenesis: Spectroscopic and microscopic study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 116–124. [Google Scholar] [CrossRef]
- Han, X.-L.; Tian, F.-F.; Ge, Y.-S.; Jiang, F.-L.; Lai, L.; Li, D.-W.; Yu, Q.-L.; Wang, J.; Lin, C.; Liu, Y. Spectroscopic, structural and thermodynamic properties of chlorpyrifos bound to serum albumin: A comparative study between BSA and HSA. J. Photochem. Photobiol. B Biol. 2012, 109, 1–11. [Google Scholar] [CrossRef]
- Ni, R.; Wang, Y.; Wei, X.; Chen, J.; Meng, J.; Xu, F.; Liu, Z.; Zhou, Y. Magnetic carbon nanotube modified with polymeric deep eutectic solvent for the solid phase extraction of bovine serum albumin. Talanta 2020, 206, 120215. [Google Scholar] [CrossRef]
- Geng, F.; Zheng, L.; Liu, J.; Yu, L.; Tung, C. Interactions between a surface active imidazolium ionic liquid and BSA. Colloid Polym. Sci. 2009, 287, 1253–1259. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B. Bovine Serum Albumin as a Versatile Platform for Cancer Imaging and Therapy. Curr. Med. Chem. 2018, 25, 2938–2953. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K. The history of monoclonal antibody development–Progress, remaining challenges and future innovations. Ann. Med. Surg. 2014, 3, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef]
- Satish, L.; Millan, S.; Sahoo, H. Spectroscopic insight into the interaction of bovine serum albumin with imidazolium-based ionic liquids in aqueous solution. Luminescence 2017, 32, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Lipman, N.S.; Jackson, L.R.; Trudel, L.J.; Weis-Garcia, F. Monoclonal Versus Polyclonal Antibodies: Distinguishing Characteristics, Applications, and Information Resources. ILAR J. 2005, 46, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. mAbs 2010, 2, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Laptoš, T.; Omersel, J. The importance of handling high-value biologicals: Physico-chemical instability and immunogenicity of monoclonal antibodies (Review). Exp. Ther. Med. 2018, 15, 3161–3168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, Y.; Zhang, H.; Cao, J.; Fei, Z.; Wang, Y. Impact of the alkyl chain length on binding of imidazolium-based ionic liquids to bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 323–333. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Coutinho, J.A.P.; Fernandes, A.M. Hydrolysis of Tetrafluoroborate and Hexafluorophosphate Counter Ions in Imidazolium-Based Ionic Liquids. J. Phys. Chem. A 2009, 114, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Barik, S.; Sarkar, M. Probing the Interactions of 1-Alkyl-3-methylimidazolium Tetrafluoroborate (Alkyl = Octyl, Hexyl, Butyl, and Ethyl) Ionic Liquids with Bovine Serum Albumin: An Alkyl Chain Length-Dependent Study. J. Phys. Chem. B 2019, 123, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.R.; Shanmugam, G.; Madhan, B.; Kumar, B.V.N.P. Selective binding and dynamics of imidazole alkyl sulfate ionic liquids with human serum albumin and collagen–A detailed NMR investigation. Phys. Chem. Chem. Phys. 2018, 20, 9256–9268. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Figueiredo, A.M.; Cabrita, E.J. Epitope mapping of imidazolium cations in ionic liquid–protein interactions unveils the balance between hydrophobicity and electrostatics towards protein destabilisation. Phys. Chem. Chem. Phys. 2014, 16, 23394–23403. [Google Scholar] [CrossRef]
- Shu, Y.; Liu, M.; Chen, S.; Chen, X.; Wang, J. New Insight into Molecular Interactions of Imidazolium Ionic Liquids with Bovine Serum Albumin. J. Phys. Chem. B 2011, 115, 12306–12314. [Google Scholar] [CrossRef]
- Satish, L.; Millan, S.; Sasidharan, V.V.; Sahoo, H. Molecular level insight into the effect of triethyloctylammonium bromide on the structure, thermal stability, and activity of Bovine serum albumin. Int. J. Biol. Macromol. 2018, 107, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Satish, L.; Millan, S.; Bera, K.; Mohapatra, S.; Sahoo, H. A spectroscopic and molecular dynamics simulation approach towards the stabilizing effect of ammonium-based ionic liquids on bovine serum albumin. New J. Chem. 2017, 41, 10712–10722. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef]
- Frizzo, C.P.; Wust, K.; Tier, A.Z.; Beck, T.S.; Rodrigues, L.V.; Vaucher, R.A.; Bolzan, L.P.; Terra, S.; Soares, F.; Martins, M.A.P. Novel ibuprofenate- and docusate-based ionic liquids: Emergence of antimicrobial activity. RSC Adv. 2016, 6, 100476–100486. [Google Scholar] [CrossRef]
- Kumar, S.; Kukutla, P.; Devunuri, N.; Venkatesu, P. How does cholinium cation surpass tetraethylammonium cation in amino acid-based ionic liquids for thermal and structural stability of serum albumins? Int. J. Biol. Macromol. 2020, 148, 615–626. [Google Scholar] [CrossRef]
- Dhiman, D.; Bisht, M.; Tavares, A.P.M.; Freire, M.G.; Venkatesu, P. Cholinium-Based Ionic Liquids as Efficient Media for Improving the Structural and Thermal Stability of Immunoglobulin G Antibodies. ACS Sustain. Chem. Eng. 2022, 10, 5404–5420. [Google Scholar] [CrossRef]
- Reslan, M.; Ranganathan, V.; Macfarlane, D.R.; Kayser, V. Choline ionic liquid enhances the stability of Herceptin® (trastuzumab). Chem. Commun. 2018, 54, 10622–10625. [Google Scholar] [CrossRef] [PubMed]
- Mazid, R.R.; Vijayaraghavan, R.; MacFarlane, D.R.; Cortez-Jugo, C.; Cheng, W. Inhibited fragmentation of mAbs in buffered ionic liquids. Chem. Commun. 2015, 51, 8089–8092. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fernandez, A.; Edler, K.J.; Arnold, T.; Venero, D.A.; Jackson, A.J. Protein conformation in pure and hydrated deep eutectic solvents. Phys. Chem. Chem. Phys. 2017, 19, 8667–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.-J.; Sun, C.; Xu, X.-B.; Zhou, D.-Y.; Song, L.; Zhu, B.-W. Improving the functional properties of bovine serum albumin-glucose conjugates in natural deep eutectic solvents. Food Chem. 2020, 328, 127122. [Google Scholar] [CrossRef]
- Bhakuni, K.; Yadav, N.; Venkatesu, P. A novel amalgamation of deep eutectic solvents and crowders as biocompatible solvent media for enhanced structural and thermal stability of bovine serum albumin. Phys. Chem. Chem. Phys. 2020, 22, 24410–24422. [Google Scholar] [CrossRef]
- Kumari, M.; Kumari, P.; Kashyap, H.K. Structural adaptations in the bovine serum albumin protein in archetypal deep eutectic solvent reline and its aqueous mixtures. Phys. Chem. Chem. Phys. 2022, 24, 5627–5637. [Google Scholar] [CrossRef]
- Gorke, J.; Srienc, F.; Kazlauskas, R. Toward advanced ionic liquids. Polar, enzyme-friendly solvents for biocatalysis. Biotechnol. Bioprocess Eng. 2010, 15, 40–53. [Google Scholar] [CrossRef]
- Patel, N.; Rai, D.; Shivam, S.; Shahane, S.; Mishra, U. Lipases: Sources, Production, Purification, and Applications. Recent Pat. Biotechnol. 2019, 13, 45–56. [Google Scholar] [CrossRef]
- Bancerz, R. Industrial application of lipases. Postepy Biochem. 2017, 63, 335–341. [Google Scholar]
- Sharma, R.; Sharma, N. Microbial Lipase Mediated by Health Beneficial Modification of Cholesterol and Flavors in Food Products: A Review. Recent Pat. Biotechnol. 2018, 12, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ercan, D.; Demirci, A. Recent advances for the production and recovery methods of lysozyme. Crit. Rev. Biotechnol. 2015, 36, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Manickam, P.; Kaushik, A.; Karunakaran, C.; Bhansali, S. Recent advances in cytochrome c biosensing technologies. Biosens. Bioelectron. 2017, 87, 654–668. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.; Shahane, S.; Shivam; Majumdar, R.; Mishra, U. Mode of Action, Properties, Production, and Application of Laccase: A Review. Recent Pat. Biotechnol. 2019, 13, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Shrivastava, R.; Agrawal, P.K. Bioprospecting and biotechnological applications of fungal laccase. 3 Biotech. 2016, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Pezzella, C.; Guarino, L.; Piscitelli, A. How to enjoy laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef]
- Reslan, M.; Kayser, V. Ionic liquids as biocompatible stabilizers of proteins. Biophys. Rev. 2018, 10, 781–793. [Google Scholar] [CrossRef]
- Schindl, A.; Hagen, M.L.; Muzammal, S.; Gunasekera, H.A.D.; Croft, A.K. Proteins in Ionic Liquids: Reactions, Applications, and Futures. Front. Chem. 2019, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Bhakuni, K.; Venkatesu, P. Strategic planning of proteins in ionic liquids: Future solvents for the enhanced stability of proteins against multiple stresses. Phys. Chem. Chem. Phys. 2019, 21, 23269–23282. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, T.A.; Singh, L.R.; Rather, G.M.; Bhat, M.A. Structural-functional integrity of lysozyme in imidazolium based surface active ionic liquids. Int. J. Biol. Macromol. 2020, 156, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.S.; Freire, C.; de Souza, R.L.; Cabrera-Padilla, R.Y.; Pereira, M.M.; Freire, M.G.; Lima, Á.S.; Soares, C.M.F. Effects of phosphonium-based ionic liquids on the lipase activity evaluated by experimental results and molecular docking. Biotechnol. Prog. 2019, 35, e2816. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, P.A.M.; Pereira, J.F.B.; Santos-Ebinuma, V.D.C. Insights into the effect of imidazolium-based ionic liquids on chemical structure and hydrolytic activity of microbial lipase. Bioprocess Biosyst. Eng. 2019, 42, 1235–1246. [Google Scholar] [CrossRef]
- Shahriari, S.; Tomé, L.C.; Araújo, J.M.M.; Rebelo, L.P.N.; Coutinho, J.A.P.; Marrucho, I.M.; Freire, M.G. Aqueous biphasic systems: A benign route using cholinium-based ionic liquids. RSC Adv. 2012, 3, 1835–1843. [Google Scholar] [CrossRef]
- Nascimento, P.A.M.; Picheli, F.P.; Lopes, A.M.; Pereira, J.F.B.; Santos-Ebinuma, V.D.C. Effects of cholinium-based ionic liquids on Aspergillus niger lipase: Stabilizers or inhibitors. Biotechnol. Prog. 2019, 35, e2838. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Harter, G.A.; Martin, C.J. “Water-like” Dual-Functionalized Ionic Liquids for Enzyme Activation. ACS Omega 2019, 4, 15234–15239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, S.P.; Marques, C.S.; Rosatella, A.A.; Afonso, C.A.; Gonçalves, F.; Coutinho, J.A. Toxicity assessment of various ionic liquid families towards Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2012, 76, 162–168. [Google Scholar] [CrossRef]
- Arunkumar, R.; Drummond, C.; Greaves, T.L. FTIR Spectroscopic Study of the Secondary Structure of Globular Proteins in Aqueous Protic Ionic Liquids. Front. Chem. 2019, 7, 74. [Google Scholar] [CrossRef]
- Attri, P.; Choi, S.; Kim, M.; Shiratani, M.; Cho, A.E.; Lee, W. Influence of alkyl chain substitution of ammonium ionic liquids on the activity and stability of tobacco etch virus protease. Int. J. Biol. Macromol. 2020, 155, 439–446. [Google Scholar] [CrossRef]
- Attri, P.; Venkatesu, P. Exploring the thermal stability of α-chymotrypsin in protic ionic liquids. Process Biochem. 2013, 48, 462–470. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Jena, S.; Tulsiyan, K.D.; Dutta, J.; Chakrabarty, S.; Biswal, H.S. Amino-Acid-Based Ionic Liquids for the Improvement in Stability and Activity of Cytochrome c: A Combined Experimental and Molecular Dynamics Study. J. Phys. Chem. B 2019, 123, 10100–10109. [Google Scholar] [CrossRef] [PubMed]
- Nian, B.; Cao, C.; Liu, Y. Activation and stabilization of Candida antarctica lipase B in choline chloride-glycerol-water binary system via tailoring the hydrogen-bonding interaction. Int. J. Biol. Macromol. 2019, 136, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, N.; Schrebler, R.A.; de María, P.D. From batch to fed-batch and to continuous packed-bed reactors: Lipase-catalyzed esterifications in low viscous deep-eutectic-solvents with buffer as cosolvent. Bioresour. Technol. 2019, 273, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Gojun, M.; Bačić, M.; Ljubić, A.; Šalić, A.; Zelić, B. Transesterification in Microreactors—Overstepping Obstacles and Shifting Towards Biodiesel Production on a Microscale. Micromachines 2020, 11, 457. [Google Scholar] [CrossRef]
- Huang, L.; Bittner, J.P.; de María, P.D.; Jakobtorweihen, S.; Kara, S. Modeling Alcohol Dehydrogenase Catalysis in Deep Eutectic Solvent/Water Mixtures. ChemBioChem 2019, 21, 811–817. [Google Scholar] [CrossRef]
- Khodaverdian, S.; Dabirmanesh, B.; Heydari, A.; Dashtban-Moghadam, E.; Khajeh, K.; Ghazi, F. Activity, stability and structure of laccase in betaine based natural deep eutectic solvents. Int. J. Biol. Macromol. 2018, 107, 2574–2579. [Google Scholar] [CrossRef]
- Toledo, M.L.; Pereira, M.M.; Freire, M.G.; Silva, J.P.A.; Coutinho, J.A.P.; Tavares, A.P.M. Laccase Activation in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 11806–11814. [Google Scholar] [CrossRef]
- Ma, Z.; Mi, Y.; Han, X.; Li, H.; Tian, M.; Duan, Z.; Fan, D.; Ma, P. Transformation of ginsenoside via deep eutectic solvents based on choline chloride as an enzymatic reaction medium. Bioprocess Biosyst. Eng. 2020, 43, 1195–1208. [Google Scholar] [CrossRef]
- Miranda-Molina, A.; Xolalpa, W.; Strompen, S.; Arreola-Barroso, R.; Olvera, L.; López-Munguía, A.; Castillo, E.; Saab-Rincon, G. Deep Eutectic Solvents as New Reaction Media to Produce Alkyl-Glycosides Using Alpha-Amylase from Thermotoga maritima. Int. J. Mol. Sci. 2019, 20, 5439. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.-N.; Dou, Y. Deep eutectic solvents for biocatalytic transformations: Focused lipase-catalyzed organic reactions. Appl. Microbiol. Biotechnol. 2020, 104, 1481–1496. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).