Graphene-Wine Waste Derived Carbon Composites for Advanced Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Physicochemical Characterization
2.3. Electrochemical Characterization
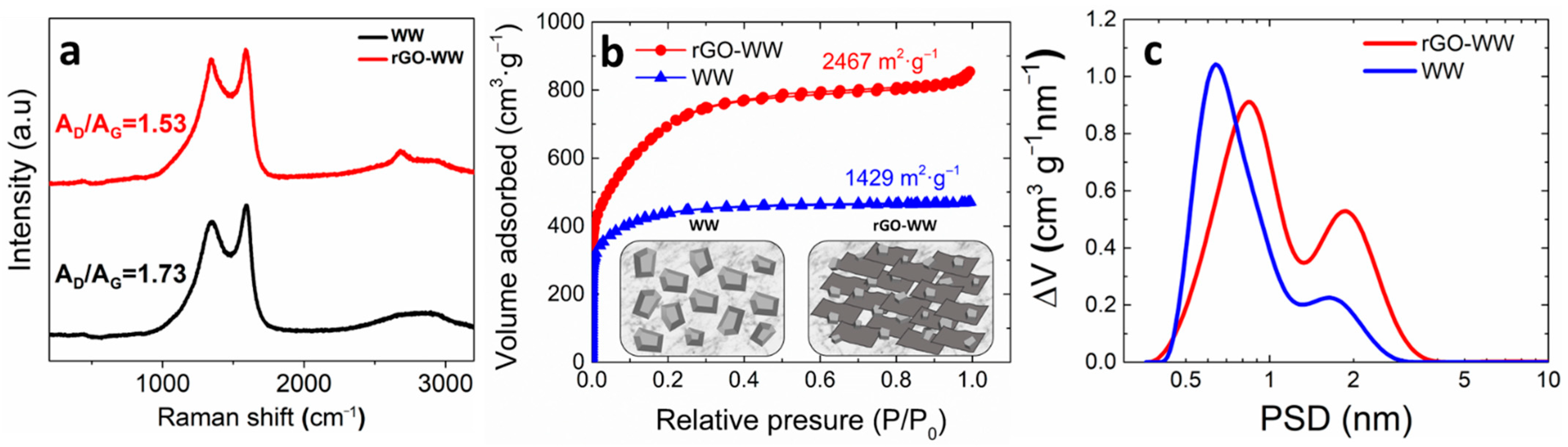
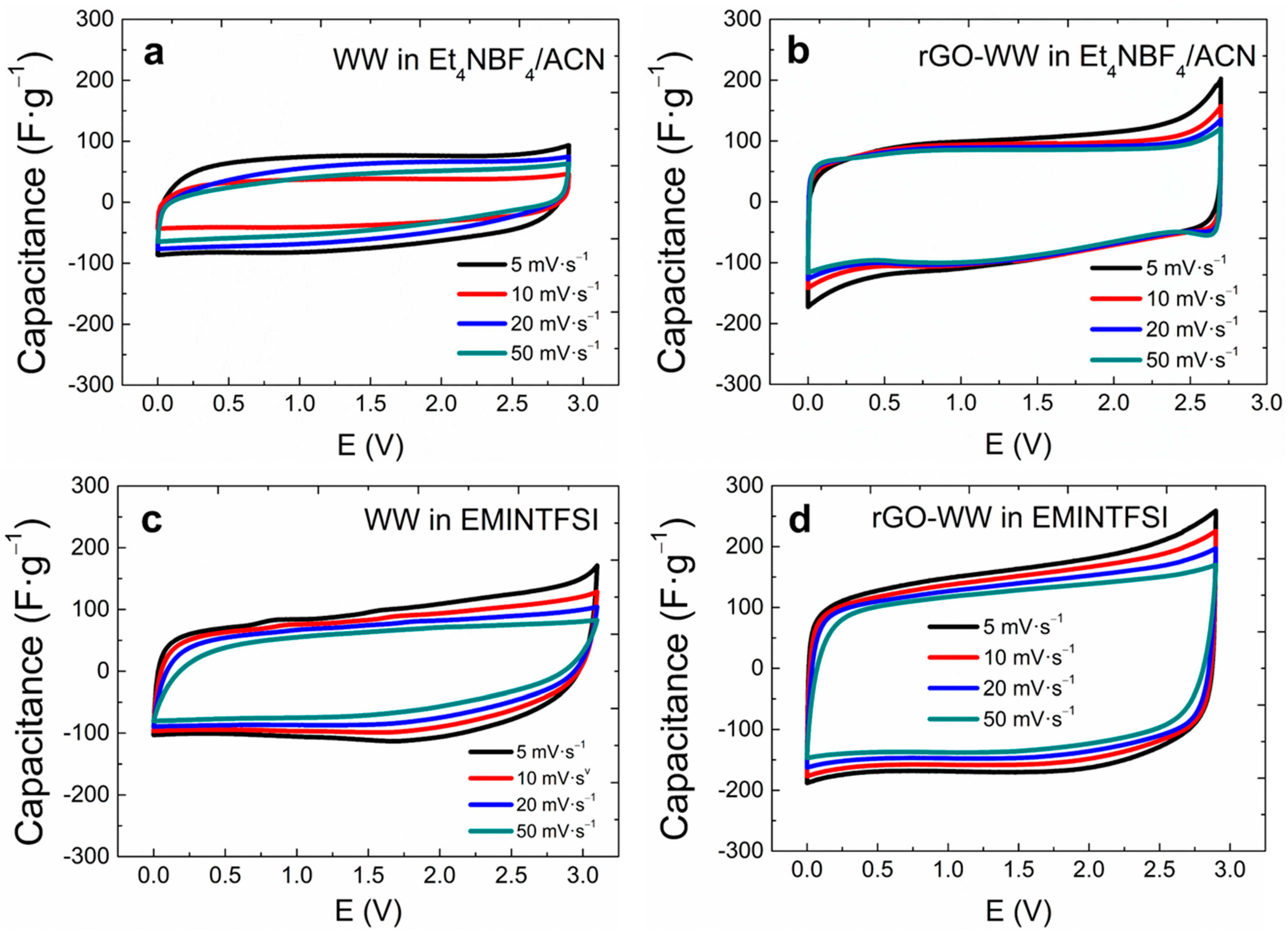
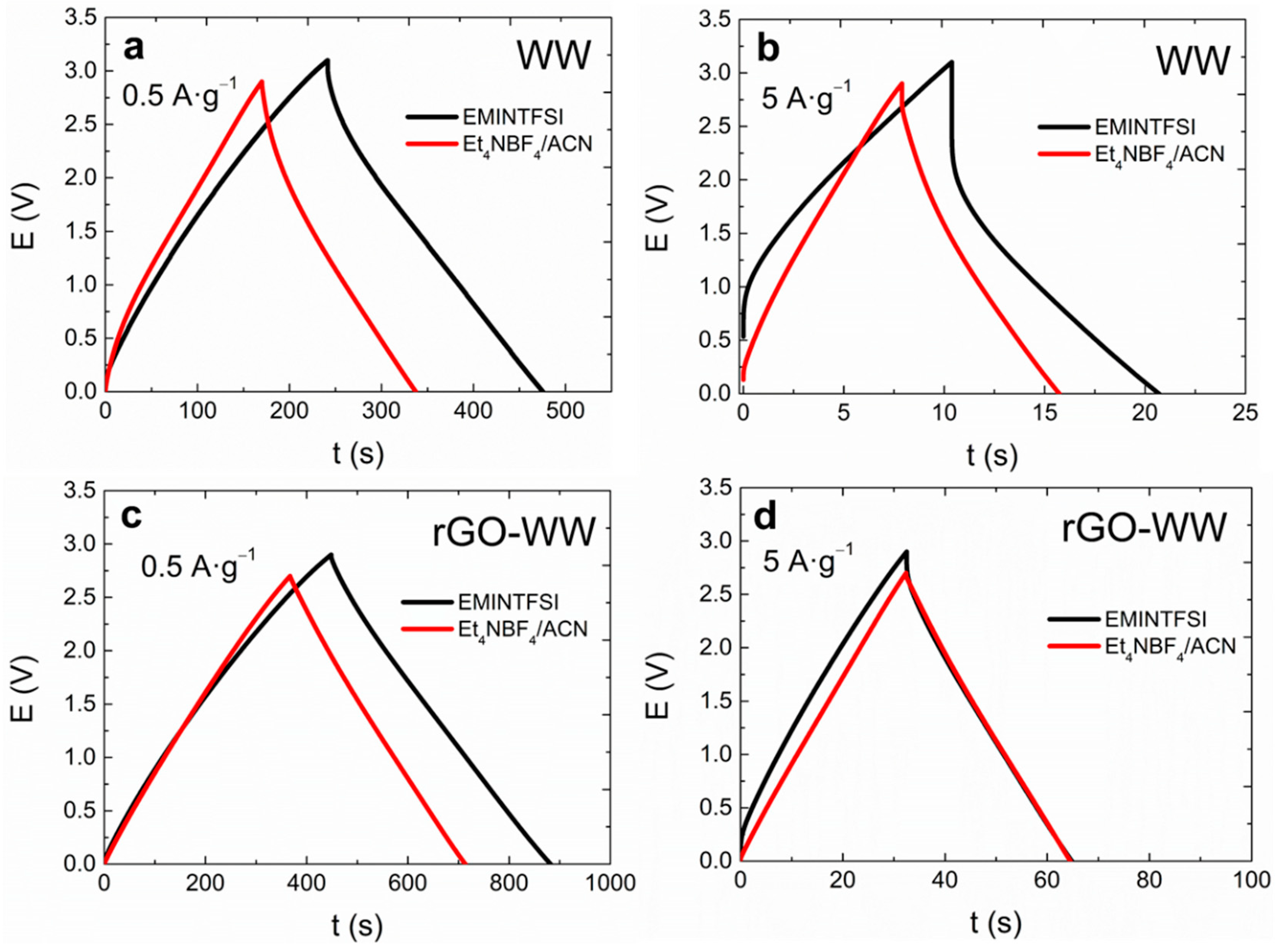
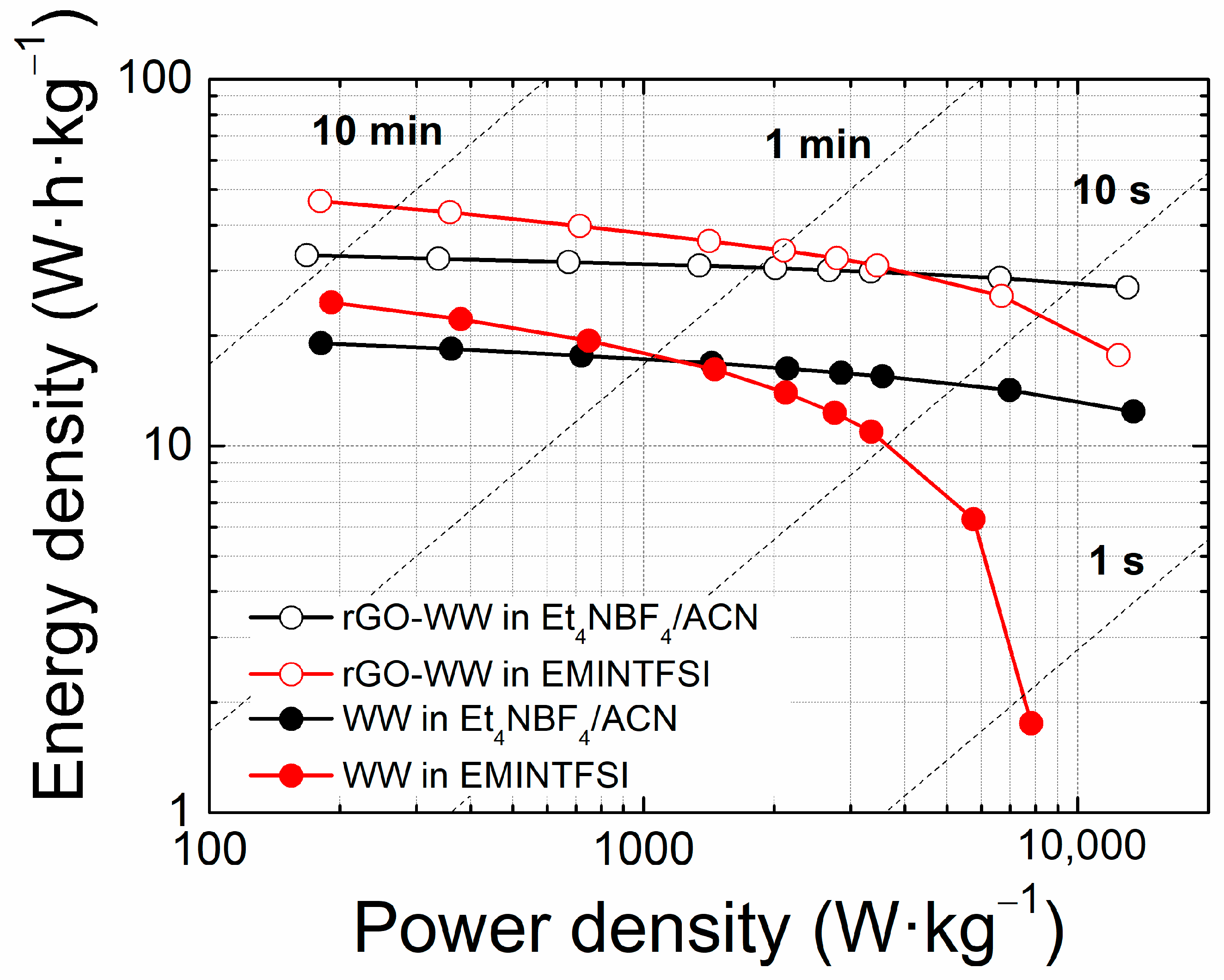
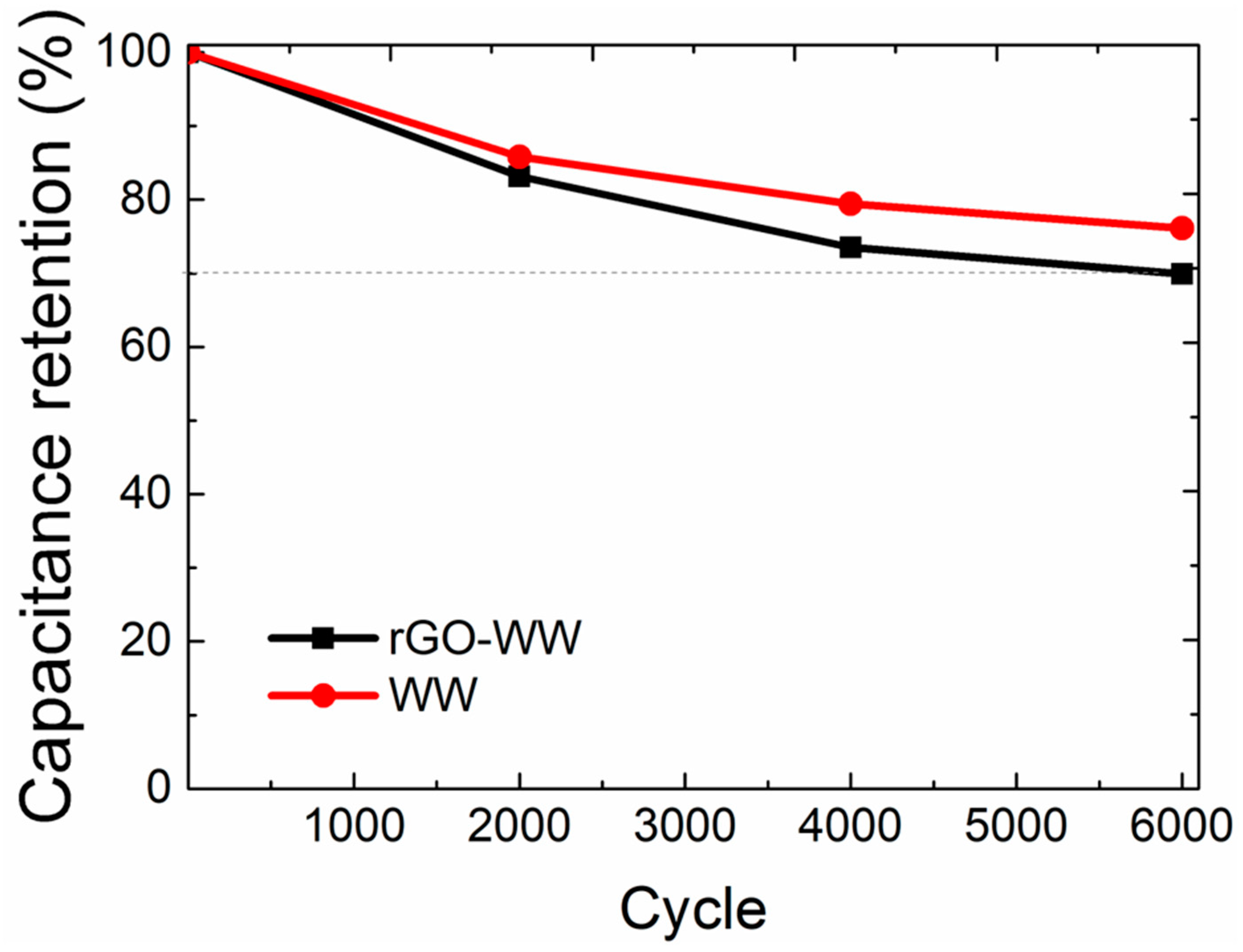
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koohi-Fayegh, S.; Rosen, M.A. A Review of Energy Storage Types, Applications and Recent Developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of Energy Storage Systems for Electric Vehicle Applications: Issues and Challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Burke, A.F. Review on Supercapacitors: Technologies and Performance Evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on Supercapacitors: Technologies and Materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Díez, N.; Botas, C.; Mysyk, R.; Goikolea, E.; Rojo, T.; Carriazo, D. Highly Packed Graphene–CNT Films as Electrodes for Aqueous Supercapacitors with High Volumetric Performance. J. Mater. Chem. A 2018, 6, 3667–3673. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Fernández, G.; Gómez-Urbano, J.L.; Enterría, M.; Rojo, T.; Carriazo, D. Flat-Shaped Carbon–Graphene Microcomposites as Electrodes for High Energy Supercapacitors. J. Mater. Chem. A 2019, 7, 14646–14655. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Urbano, J.L.; Moreno-Fernández, G.; Granados-Moreno, M.; Rojo, T.; Carriazo, D. Nanostructured Carbon Composites from Cigarette Filter Wastes and Graphene Oxide Suitable as Electrodes for 3.4 V Supercapacitors. Batter. Supercaps 2021, 4, 1749–1756. [Google Scholar] [CrossRef]
- Gómez-Urbano, J.L.; Moreno-Fernández, G.; Arnaiz, M.; Ajuria, J.; Rojo, T.; Carriazo, D. Graphene-Coffee Waste Derived Carbon Composites as Electrodes for Optimized Lithium Ion Capacitors. Carbon 2020, 162, 273–282. [Google Scholar] [CrossRef]
- Cheng, F.; Yang, X.; Zhang, S.; Lu, W. Boosting the Supercapacitor Performances of Activated Carbon with Carbon Nanomaterials. J. Power Sources 2020, 450, 227678. [Google Scholar] [CrossRef]
- Guardia, L.; Suárez, L.; Querejeta, N.; Pevida, C.; Centeno, T.A. Winery Wastes as Precursors of Sustainable Porous Carbons for Environmental Applications. J. Clean. Prod. 2018, 193, 614–624. [Google Scholar] [CrossRef]
- Suárez, L.; Centeno, T.A. Unravelling the Volumetric Performance of Activated Carbons from Biomass Wastes in Supercapacitors. J. Power Sources 2020, 448, 227413. [Google Scholar] [CrossRef]
- Jiménez-Cordero, D.; Heras, F.; Gilarranz, M.A.; Raymundo-Piñero, E. Grape Seed Carbons for Studying the Influence of Texture on Supercapacitor Behaviour in Aqueous Electrolytes. Carbon 2014, 71, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Díez, N.; Mysyk, R.; Zhang, W.; Goikolea, E.; Carriazo, D. One-Pot Synthesis of Highly Activated Carbons from Melamine and Terephthalaldehyde as Electrodes for High Energy Aqueous Supercapacitors. J. Mater. Chem. A 2017, 5, 14619–14629. [Google Scholar] [CrossRef]
- Moreno-Fernández, G.; Boulanger, N.; Nordenström, A.; Iakunkov, A.; Talyzin, A.; Carriazo, D.; Mysyk, R. Ball-Milling-Enhanced Capacitive Charge Storage of Activated Graphene in Aqueous, Organic and Ionic Liquid Electrolytes. Electrochim. Acta 2021, 370, 137738. [Google Scholar] [CrossRef]
- Brandt, A.; Pohlmann, S.; Varzi, A.; Balducci, A.; Passerini, S. Ionic Liquids in Supercapacitors. MRS Bull. 2013, 38, 554–559. [Google Scholar] [CrossRef]
- Bahdanchyk, M.; Hashempour, M.; Vicenzo, A. Evaluation of the Operating Potential Window of Electrochemical Capacitors. Electrochim. Acta 2020, 332, 135503. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Guardia, L.; Suárez, L.; Querejeta, N.; Vretenár, V.; Kotrusz, P.; Skákalová, V.; Centeno, T.A. Biomass Waste-Carbon/Reduced Graphene Oxide Composite Electrodes for Enhanced Supercapacitors. Electrochim. Acta 2019, 298, 910–917. [Google Scholar] [CrossRef]
- Zuo, X.; Chang, K.; Zhao, J.; Xie, Z.; Tang, H.; Li, B.; Chang, Z. Bubble-Template-Assisted Synthesis of Hollow Fullerene-like MoS 2 Nanocages as a Lithium Ion Battery Anode Material. J. Mater. Chem. A 2016, 4, 51–58. [Google Scholar] [CrossRef]
- Hoffmann, V.; Jung, D.; Zimmermann, J.; Rodriguez Correa, C.; Elleuch, A.; Halouani, K.; Kruse, A. Conductive Carbon Materials from the Hydrothermal Carbonization of Vineyard Residues for the Application in Electrochemical Double-Layer Capacitors (EDLCs) and Direct Carbon Fuel Cells (DCFCs). Materials 2019, 12, 1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanathan, S.; Moorthy, S.; Ramasundaram, S.; Rajan, H.K.; Vishwanath, S.; Selvinsimpson, S.; Durairaj, A.; Kim, B.; Vasanthkumar, S. Grape Seed Extract Assisted Synthesis of Dual-Functional Anatase TiO 2 Decorated Reduced Graphene Oxide Composite for Supercapacitor Electrode Material and Visible Light Photocatalytic Degradation of Bromophenol Blue Dye. ACS Omega 2021, 6, 14734–14747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; Bai, J.; Xu, M.; Luo, C.; Yang, L.; Bai, L.; Wei, D.; Wang, W.; Yang, H. N-Doped Hierarchically Porous Carbon Derived from Grape Marcs for High-Performance Supercapacitors. J. Alloys Compd. 2021, 854, 157207. [Google Scholar] [CrossRef]
- Babu, B.; Simon, P.; Balducci, A. Fast Charging Materials for High Power Applications. Adv. Energy Mater. 2020, 10, 2001128. [Google Scholar] [CrossRef]
- Pohlmann, S.; Kühnel, R.-S.; Centeno, T.A.; Balducci, A. The Influence of Anion-Cation Combinations on the Physicochemical Properties of Advanced Electrolytes for Supercapacitors and the Capacitance of Activated Carbons. ChemElectroChem 2014, 1, 1301–1311. [Google Scholar] [CrossRef]
- Singh, R.; Rajput, N.N.; He, X.; Monk, J.; Hung, F.R. Molecular Dynamics Simulations of the Ionic Liquid [EMIM+][TFMSI−] Confined inside Rutile (110) Slit Nanopores. Phys. Chem. Chem. Phys. 2013, 15, 16090. [Google Scholar] [CrossRef] [Green Version]
- Merlet, C.; Péan, C.; Rotenberg, B.; Madden, P.A.; Daffos, B.; Taberna, P.-L.; Simon, P.; Salanne, M. Highly Confined Ions Store Charge More Efficiently in Supercapacitors. Nat. Commun. 2013, 4, 2701. [Google Scholar] [CrossRef] [Green Version]
- Momodu, D.; Sylla, N.F.; Mutuma, B.; Bello, A.; Masikhwa, T.; Lindberg, S.; Matic, A.; Manyala, N. Stable Ionic-Liquid-Based Symmetric Supercapacitors from Capsicum Seed-Porous Carbons. J. Electroanal. Chem. 2019, 838, 119–128. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, H.; Mao, N.; Yu, W.; Shi, J.; Huang, M.; Liu, W.; Chen, S.; Wang, X. Tuning the Morphology and Structure of Nanocarbons with Activating Agents for Ultrafast Ionic Liquid-Based Supercapacitors. J. Power Sources 2017, 361, 182–194. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Yang, K.; Zhu, L.; Yang, C.; Zhang, H. Bio-Inspired Beehive-like Hierarchical Nanoporous Carbon Derived from Bamboo-Based Industrial by-Product as a High Performance Supercapacitor Electrode Material. J. Mater. Chem. A 2015, 3, 5656–5664. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Tak, J.K.; Holt, C.M.B.; Tan, X.; Xu, Z.; Amirkhiz, B.S.; Harfield, D.; Anyia, A.; Stephenson, T.; et al. Supercapacitors Based on Carbons with Tuned Porosity Derived from Paper Pulp Mill Sludge Biowaste. Carbon 2013, 57, 317–328. [Google Scholar] [CrossRef]


| Material | % C | % O | % N | % H |
|---|---|---|---|---|
| WW | 87.5 | 11.2 | 0.6 | 0.7 |
| rGO-WW | 91.2 | 8.3 | 0.2 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ureña-Torres, V.; Moreno-Fernández, G.; Gómez-Urbano, J.L.; Granados-Moreno, M.; Carriazo, D. Graphene-Wine Waste Derived Carbon Composites for Advanced Supercapacitors. ChemEngineering 2022, 6, 49. https://doi.org/10.3390/chemengineering6040049
Ureña-Torres V, Moreno-Fernández G, Gómez-Urbano JL, Granados-Moreno M, Carriazo D. Graphene-Wine Waste Derived Carbon Composites for Advanced Supercapacitors. ChemEngineering. 2022; 6(4):49. https://doi.org/10.3390/chemengineering6040049
Chicago/Turabian StyleUreña-Torres, Violeta, Gelines Moreno-Fernández, Juan Luis Gómez-Urbano, Miguel Granados-Moreno, and Daniel Carriazo. 2022. "Graphene-Wine Waste Derived Carbon Composites for Advanced Supercapacitors" ChemEngineering 6, no. 4: 49. https://doi.org/10.3390/chemengineering6040049
APA StyleUreña-Torres, V., Moreno-Fernández, G., Gómez-Urbano, J. L., Granados-Moreno, M., & Carriazo, D. (2022). Graphene-Wine Waste Derived Carbon Composites for Advanced Supercapacitors. ChemEngineering, 6(4), 49. https://doi.org/10.3390/chemengineering6040049







