Hydrogen and CNT Production by Methane Cracking Using Ni–Cu and Co–Cu Catalysts Supported on Argan-Derived Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Catalytic Decomposition of Methane
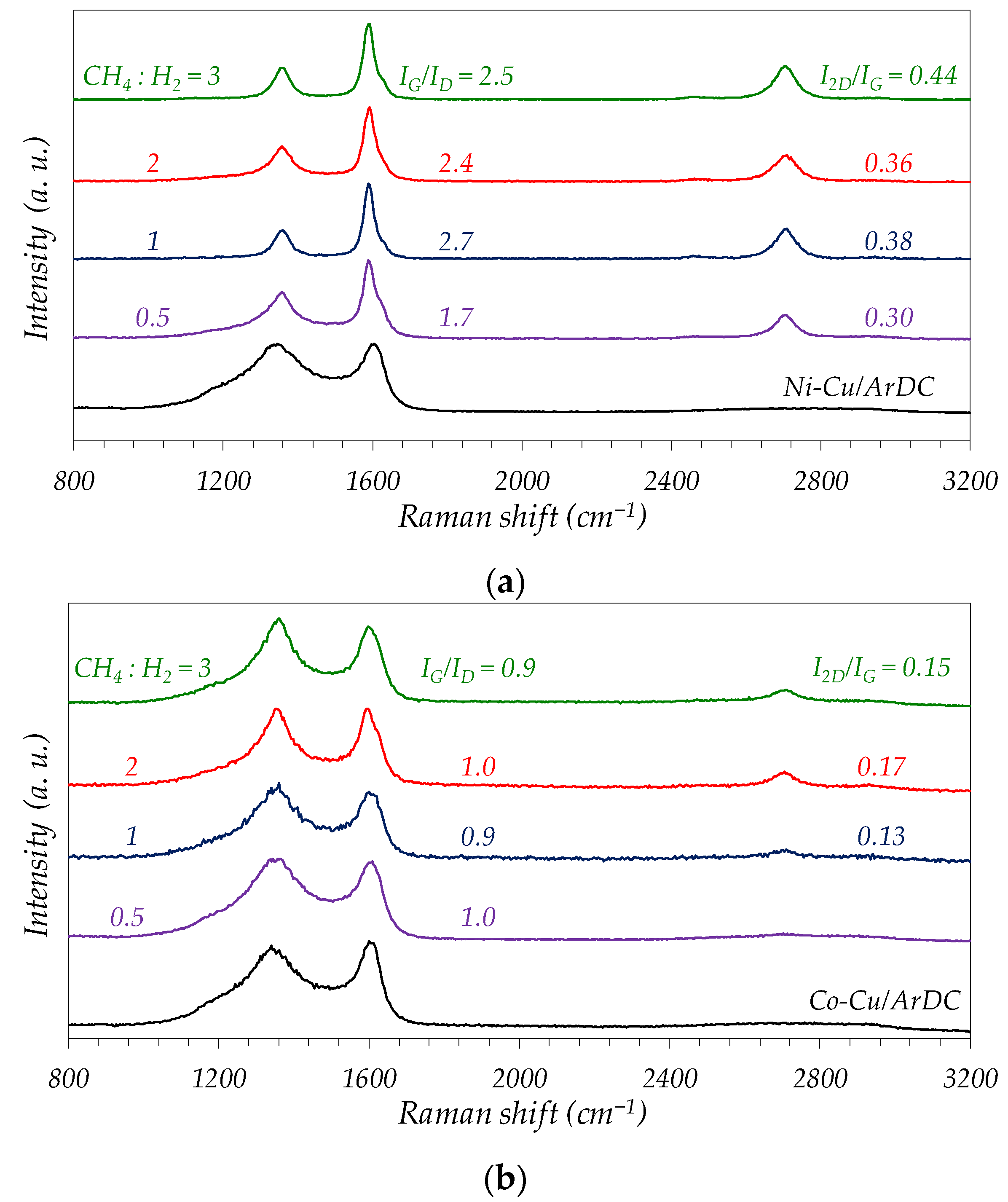
2.3. Catalysts and Carbonaceous Nanomaterials Characterization
3. Results
3.1. Fresh Catalyst Characterization
3.2. Catalytic Decomposition of Methane
3.2.1. Influence of Reaction Temperature


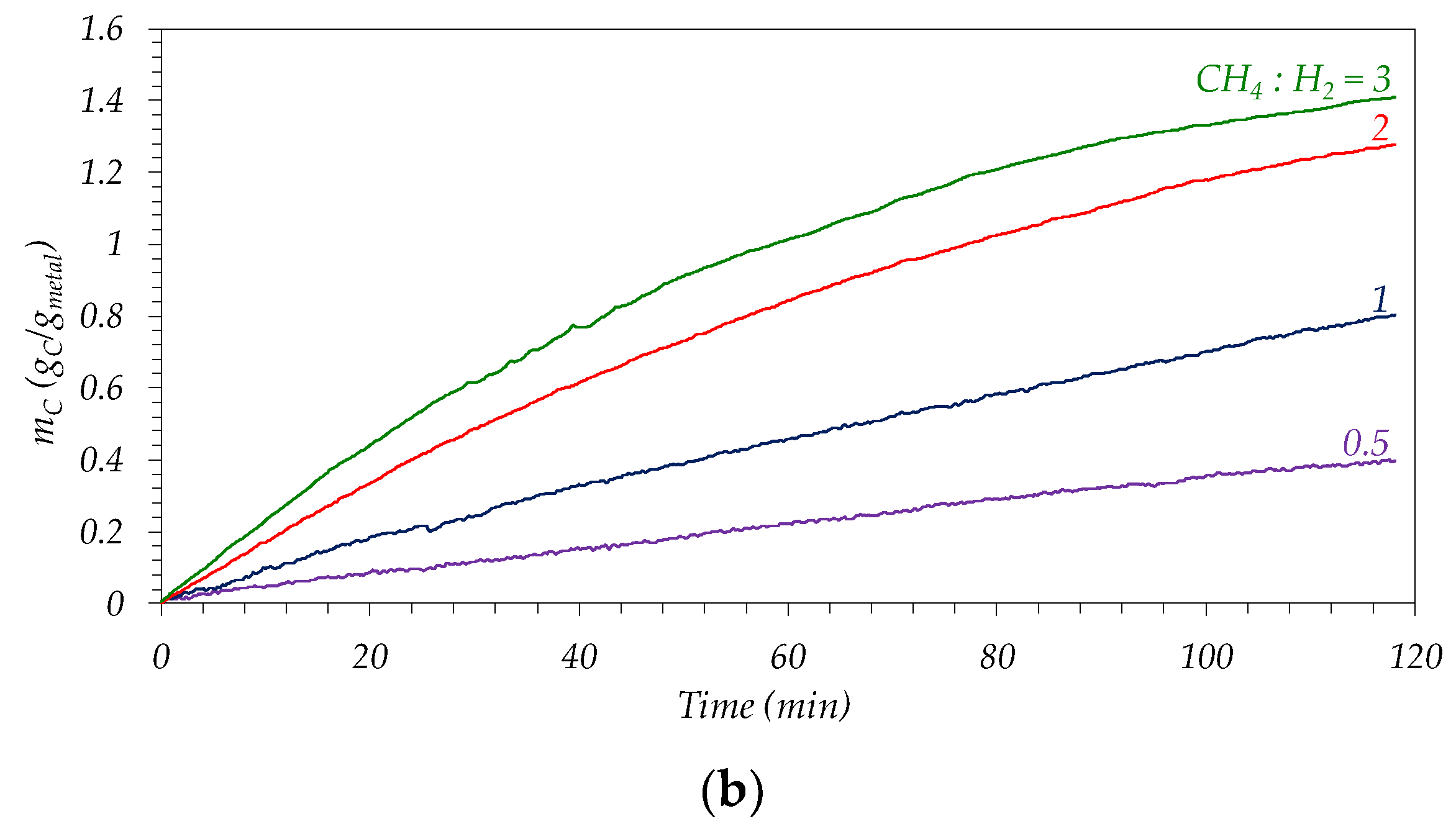
3.2.2. Influence of Feed Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Dresselhaus, G.; Hone, J. (Eds.) Carbon Nanotubes: Synthesis, Structure, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Serp, P.; Corrias, M.; Kalk, P. Carbon nanotubes and nanofibers in catalysis. Appl. Catal. A 2003, 253, 337–358. [Google Scholar] [CrossRef]
- Terrones, M. Science and Technology of the Twenty-First Century: Synthesis, Properties, and Applications of Carbon Nanotubes. Annu. Rev. Mater. Res. 2003, 33, 419–501. [Google Scholar] [CrossRef]
- Ding, S.; Xiang, Y.; Ni, Y.Q.; Kumar Thakur, V.; Wang, X.; Han, B.; Ou, J. In-situ synthesizing carbon nanotubes on cement to develop self-sensing cementitious composites for smart high-speed rail infrastructures. Nano Today 2022, 43, 101438. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Gong, C.; Liu, B.; Wei, G. Production, structural design, functional control, and broad applications of carbon nanofiber-based nanomaterials: A comprehensive review. Chem. Eng. J. 2020, 402, 126189. [Google Scholar] [CrossRef]
- Bai, Y.; Yue, H.; Wang, J.; Shen, B.; Sun, S.; Wang, S.; Wang, H.; Li, X.; Xu, Z.; Zhang, R.; et al. Super-durable ultralong carbon nanotubes. Science 2020, 369, 1104–1106. [Google Scholar] [CrossRef]
- Hills, G.; Lau, C.; Wright, A.; Fuller, S.; Bishop, M.D.; Srimani, T.; Kanhaiya, P.; Ho, R.; Amer, A.; Stein, Y.; et al. Modern microprocessor built from complementary carbon nanotube transistors. Nature 2019, 572, 595–602. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Nam Hui, K.; Hoon Kim, N.; Hee Lee, J. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Hart, A.J. Carbon Nano-tubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [Green Version]
- Pinilla, J.L.; de Llobet, S.; Moliner, R.; Suelves, I. Ni-Co bimetallic catalysts for the simultaneous production of carbon nanofibres and syngas through biogas decomposition. Appl. Catal. B Environ. 2017, 200, 255–264. [Google Scholar] [CrossRef]
- Gómez-Pozuelo, G.; Pizarro, P.; Botas, J.A.; Serrano, D.P. Hydrogen production by catalytic methane decomposition over rice husk derived silica. Fuel 2021, 306, 121697. [Google Scholar] [CrossRef]
- Fan, Z.; Weng, W.; Zhou, J.; Gu, D.; Xiao, W. Catalytic decomposition of methane to produce hydrogen: A review. J. Energy Chem. 2021, 58, 415–430. [Google Scholar] [CrossRef]
- Dipu, A.L. Methane decomposition into COx-free hydrogen over a Ni-based catalyst: An overview. Int. J. Energy Res. 2021, 45, 9858–9877. [Google Scholar] [CrossRef]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Basset, J.M.; Zhou, L. Methane decomposition to pure hydrogen and carbon nano materials: State-of-the-art and future perspectives. Int. J. Hydrogen Energy 2020, 45, 15721–15743. [Google Scholar] [CrossRef]
- Sanchez-Bastardo, N.; Schlogl, R.; Ruland, H. Methane Pyrolysis for CO2-Free H2 Production: A Green Process to Overcome Renewable Energies Unsteadiness. Chem. Ing. Technik. 2020, 92, 1596–1609. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Y.; Tanabe, E.; Shishido, T.; Takehira, K. Carbon fibers prepared by pyrolysis of methane over Ni/MCM-41 catalyst. Micropor. Mesopor. Mat. 2003, 57, 283–289. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef] [Green Version]
- Helveg, S.; López-Cartes, C.; Sehested, J.; Hansen, P.L.; Clausen, B.S.; Rostrup-Nielsen, J.R.; Abild-Pedersen, F.; Nørskov, J.K. Atomic-scale imaging of carbon nanofibre growth. Nature 2004, 427, 426–429. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Methane decomposition over Ni, Co and Fe based monometallic catalysts supported on sol gel derived SiO2 microflakes. Chem. Eng. J. 2015, 262, 1009–1021. [Google Scholar] [CrossRef]
- Alves Silva, J.; Oliveira Santos, J.B.; Torres, D.; Pinilla, J.L.; Suelves, I. Natural Fe-based catalysts for the production of hydrogen and carbon nanomaterials via methane decomposition. Int. J. Hydrogen Energy 2021, 46, 35137–35148. [Google Scholar] [CrossRef]
- Zhou, L.; Reddy Enakonda, L.; Li, S.; Gary, D.; Del-Gallo, P.; Mennemann, C.; Basset, J.M. Iron ore catalysts for methane decomposition to make COx free hydrogen and carbon nano material. J. Taiwan Inst. Chem. Eng. 2018, 87, 54–63. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Aboul-Enein, A.A.; Kandil, U.F.; Reda Taha, M. Facile and large-scale synthesis of high quality few-layered graphene nano-platelets via methane decomposition over unsupported iron family catalysts. Mater. Chem. Phys. 2017, 191, 75–85. [Google Scholar] [CrossRef]
- Latorre, N.; Cazaña, F.; Martínez-Hansen, V.; Royo, C.; Romeo, E.; Monzón, A. Ni-Co-Mg-Al catalysts for hydrogen and carbonaceous nanomaterials production by CCVD of methane. Catal. Today 2011, 172, 143–151. [Google Scholar] [CrossRef]
- Cazaña, F.; Latorre, N.; Tarifa, P.; Labarta, J.; Romeo, E.; Monzón, A. Synthesis of graphenic nanomaterials by decomposition of methane on a Ni-Cu/biomorphic carbon catalyst. Kinetic and characterization results. Catal. Today 2018, 299, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Zhu, H.; Huang, M.; Wan, C.; Li, D.; Jiang, L. Controlled preparation of Ni–Cu alloy catalyst via hydrotalcite-like precursor and its enhanced catalytic performance for methane decomposition. Fuel Process. Technol. 2022, 233, 107271. [Google Scholar] [CrossRef]
- Tezel, E.; Eren Figen, H.; Baykara, S.Z. Hydrogen production by methane decomposition using bimetallic Ni–Fe catalysts. Int. J. Hydrogen Energy 2019, 44, 9930–9940. [Google Scholar] [CrossRef]
- Henao, W.; Cazaña, F.; Tarifa, P.; Romeo, E.; Latorre, N.; Sebastian, V.; Delgado, J.J.; Monzón, A. Selective synthesis of carbon nanotubes by catalytic decomposition of methane using Co-Cu/cellulose derived carbon catalysts: A comprehensive kinetic study. Chem. Eng. J. 2021, 404, 126103. [Google Scholar] [CrossRef]
- Cazaña, F.; Latorre, N.; Tarifa, P.; Royo, C.J.; Sebastián, V.; Romeo, E.; Centeno, M.A.; Monzón, A. Performance of AISI 316L-stainless steel foams towards the formation of graphene related nanomaterials by catalytic decomposition of methane at high temperature. Catal. Today 2022, 383, 236–246. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Zapata-Benabithe, Z.; Carrasco-Marín, F.; Moreno-Castilla, C. Activated carbons from KOH-activation of argan (Argania spinosa) seed shells as supercapacitor electrodes. Bioresour. Technol. 2012, 111, 185–190. [Google Scholar] [CrossRef]
- Azuara, M.; Latorre, N.; Villacampa, J.I.; Sebastián, V.; Cazaña, F.; Romeo, E.; Monzón, A. Use of Ni Catalysts Supported on Biomorphic Carbon Derived From Lignocellulosic Biomass Residues in the Decomposition of Methane. Front. Energy Res. 2019, 7, 34. [Google Scholar] [CrossRef]
- Dahbi, M.; Kiso, M.; Kubota, K.; Horiba, T.; Chafik, T.; Hida, K.; Matsuyama, T.; Komaba, S. Synthesis of hard carbon from argan shells for Na-ion batteries. J. Mater. Chem. A 2017, 5, 9917. [Google Scholar] [CrossRef]
- Harhar, H.; Gharby, S.; Ghanmi, M.; El Monfalouti, H.; Guillaume, D.; Charrouf, Z. Composition of the Essential Oil of Argania spinosa (Sapotaceae) Fruit Pulp. Nat. Prod. Commun. 2010, 5, 1934578X1000500626. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.L.; Mäki-Arvela, P.; Monzón, A.; Murzin, D.Y.; Centeno, M.A. Metal catalysts supported on biochars: Part I synthesis and characterization. Appl. Catal B Environ. 2020, 268, 118423. [Google Scholar] [CrossRef]
- Santos, J.L.; Mäki-Arvela, P.; Wärnå, J.; Monzón, A.; Centeno, M.A.; Murzin, D.Y. Hydrodeoxygenation of vanillin over noble metal catalyst supported on biochars: Part II: Catalytic behaviour. Appl. Catal B Environ. 2020, 268, 118425. [Google Scholar] [CrossRef]
- Cazaña, F.; Jimaré, M.T.; Romeo, E.; Sebastián, V.; Irusta, S.; Latorre, N.; Royo, C.; Monzón, A. Kinetics of liquid phase cyclohexene hydrogenation on Pd–Al/biomorphic carbon catalysts. Catal. Today 2015, 249, 127–136. [Google Scholar] [CrossRef]
- Cazaña, F.; Galetti, A.; Meyer, C.; Sebastián, V.; Centeno, M.A.; Romeo, E.; Monzón, A. Synthesis of Pd-Al/biomorphic carbon catalysts using cellulose as carbon precursor. Catal. Today 2018, 301, 226–238. [Google Scholar] [CrossRef]
- Tarifa, P.; Megías-Sayago, C.; Cazaña, F.; González-Martín, M.; Latorre, N.; Romeo, E.; Delgado, J.J.; Monzón, A. Highly Active Ce- and Mg-Promoted Ni Catalysts Supported on Cellulose-Derived Carbon for Low-Temperature CO2 Methanation. Energy Fuels 2021, 35, 17212–17224. [Google Scholar] [CrossRef]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Will, J.; Zollfrank, C.; Kaindl, A.; Sieber, H.; Grei, P. Biomorphic ceramics: Technologies based on nature. Keram. Z 2010, 62, 114. [Google Scholar]
- Zuo, C.-Y.; Li, Q.S.; Peng, G.R.; Xing, G.Z. Manufacture of biomorphic Al2O3 ceramics using filter paper as template. Prog. Nat. Sci. Mater. Int. 2011, 21, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Xu, H.; Liu, Y.; Chu, W.; Jiang, C.; Zhao, X. A facile approach for the preparation of biomorphic CuO–ZrO2 catalyst for catalytic combustion of methane. Appl. Catal. A Gen. 2012, 423, 121–129. [Google Scholar] [CrossRef]
- Chakraborty, R.; RoyChowdhury, D. Fish bone derived natural hydroxyapatite-supported copper acid catalyst: Taguchi optimization of semibatch oleic acid esterification. Chem. Eng. J. 2013, 215, 491–499. [Google Scholar] [CrossRef]
- Wu, Q.; Duchstein, L.D.L.; Chiarello, G.L.; Christensen, J.M.; Damsgaard, C.D.; Elkjær, C.F.; Wagner, J.B.; Temel, B.; Grunwaldt, J.D.; Jensen, A.D. In Situ Observation of Cu–Ni Alloy Nanoparticle Formationby X-Ray Diffraction, X-Ray Absorption Spectroscopy, and Transmission Electron Microscopy: Influence of Cu/Ni Ratio. Chem. Cat. Chem. 2014, 6, 301–310. [Google Scholar]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Rhim, Y.R.; Zhang, D.; Fairbrother, D.H.; Wepasnick, K.A.; Livi, K.J.; Bodnar, R.J.; Nagle, D.C. Changes in electrical and microstructural properties of microcrystalline cellulose as function of carbonization temperature. Carbon 2010, 48, 1012–1024. [Google Scholar] [CrossRef]
- Latorre, N.; Cazaña, F.; Sebastián, V.; Royo, C.; Romeo, E.; Monzón, A. Effect of the Operating Conditions on the Growth of Carbonaceous Nanomaterials over Stainless Steel Foams. Kinetic and Characterization Studies. Int. J. Chem. React. Eng. 2017, 15, 20170121. [Google Scholar] [CrossRef]
- Ahmad, M.; Silva, S.R.P. Low temperature growth of carbon nanotubes—A review. Carbon 2020, 158, 24–44. [Google Scholar] [CrossRef]
- Galetti, A.; Barroso, M.N.; Monzón, A.; Abello, M.C. Synthesis of Nickel Nanoparticles Supported on Carbon Using a Filter Paper as Biomorphic Pattern for Application in Catalysis. Mater. Res. 2015, 18, 1278–1283. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Christensen, K.O.; Ochoa-Fernández, E.; Yu, Z.; Tøtdal, B.; Latorre, N.; Monzón, A.; Holmen, A. Synthesis of carbon nanofibers: Effects of Ni crystal size during methane decomposition. J. Catal. 2005, 229, 82–96. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, J.; Kwong, F.L.; Ng, D.H.L. Synthesis of bamboo-like carbon nanotubes on a cop-per foil by catalytic chemical vapor deposition from ethanol. Carbon 2012, 50, 2504–2512. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, P.Y.; Fang, F.; Zhou, S.S.; Peng, D.Y. Synthesis and characterization of un-branched and branched multi-walled carbon nanotubes using Cu as catalyst. Solid State Sci. 2013, 18, 71–77. [Google Scholar] [CrossRef]
- Mohana Krishna, V.; Somanathan, T. Efficient strategy to Cu/Si catalyst into vertically aligned carbon nanotubes with Bamboo shape by CVD technique. Bull. Mater. Sci. 2016, 39, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Calizo, I.; Bejenari, I.; Rahman, M.; Liu, G.; Balandin, A.A. Ultraviolet Raman microscopy of single and multilayer graphene. J. Appl. Phys. 2009, 106, 043509. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.L.; Centeno, M.A.; Odriozola, J.A. Reductant atmospheres during slow pyrolysis of cellulose: First approach to obtaining efficient char-based catalysts in one pot. J. Anal. Appl. Pyrolysis 2020, 148, 104821. [Google Scholar] [CrossRef]
- Ramirez, A.; Gevers, L.; Bavykina, A.; Ould-Chikh, S.; Gascon, J. Metal Organic Framework-Derived Iron Catalysts for the Direct Hydrogenation of CO2 to Short Chain Olefins. ACS Catal. 2018, 8, 9174–9182. [Google Scholar] [CrossRef]
- Monzón, A.; Lolli, G.; Cosma, S.; Mohamed, S.B.; Resasco, D.E. Kinetic Modeling of the SWNT Growth by CO Disproportionation on CoMo Catalysts. J. Nanosci. Nanotechnol. 2008, 8, 6141–6152. [Google Scholar] [CrossRef]
- Latorre, N.; Romeo, E.; Cazaña, F.; Ubieto, T.; Royo, C.; Villacampa, J.I.; Monzón, A. Carbon Nanotube Growth by Catalytic Chemical Vapor Deposition: A Phenomenological Kinetic Model. J. Phys. Chem. C 2010, 114, 4773–4782. [Google Scholar] [CrossRef]
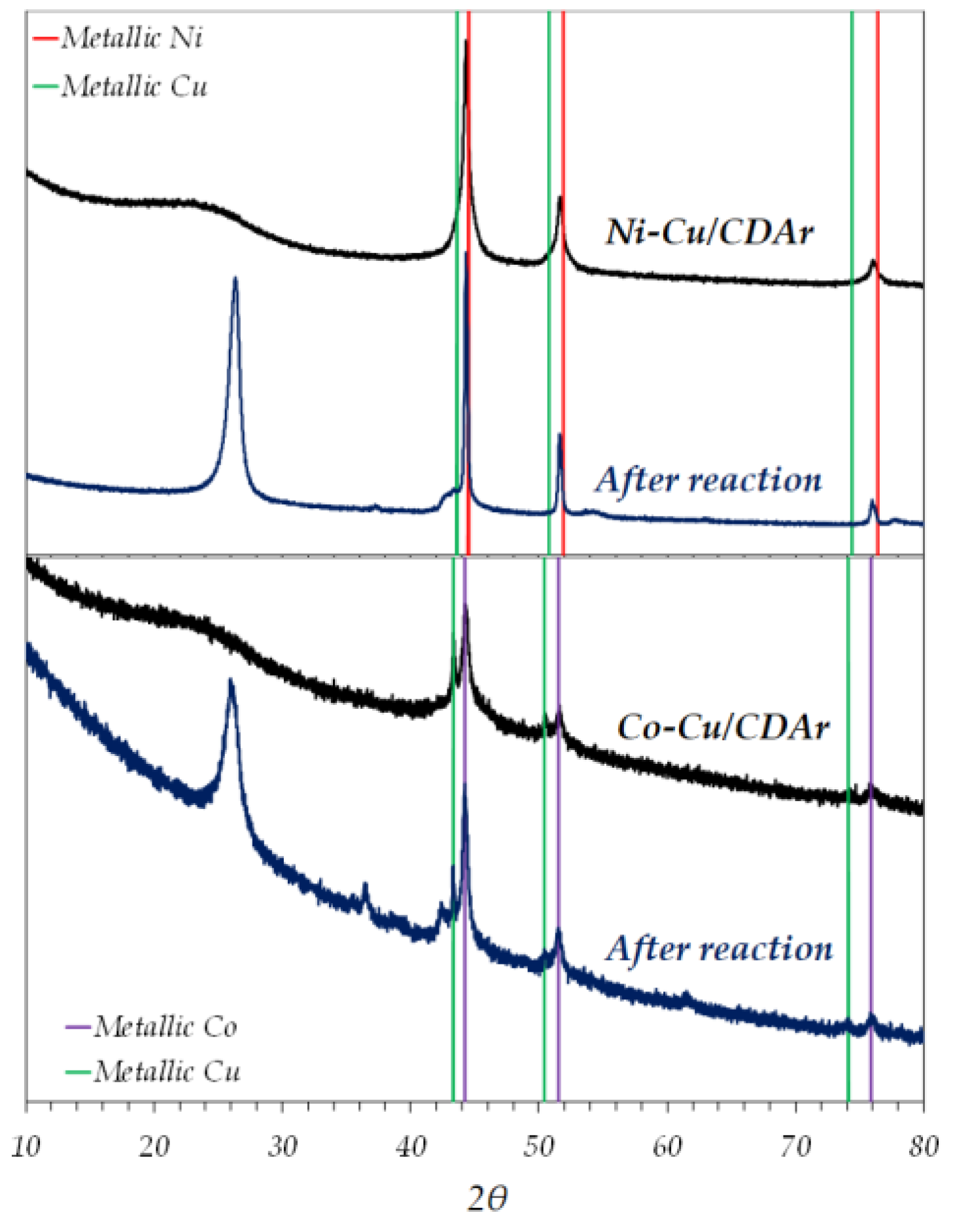
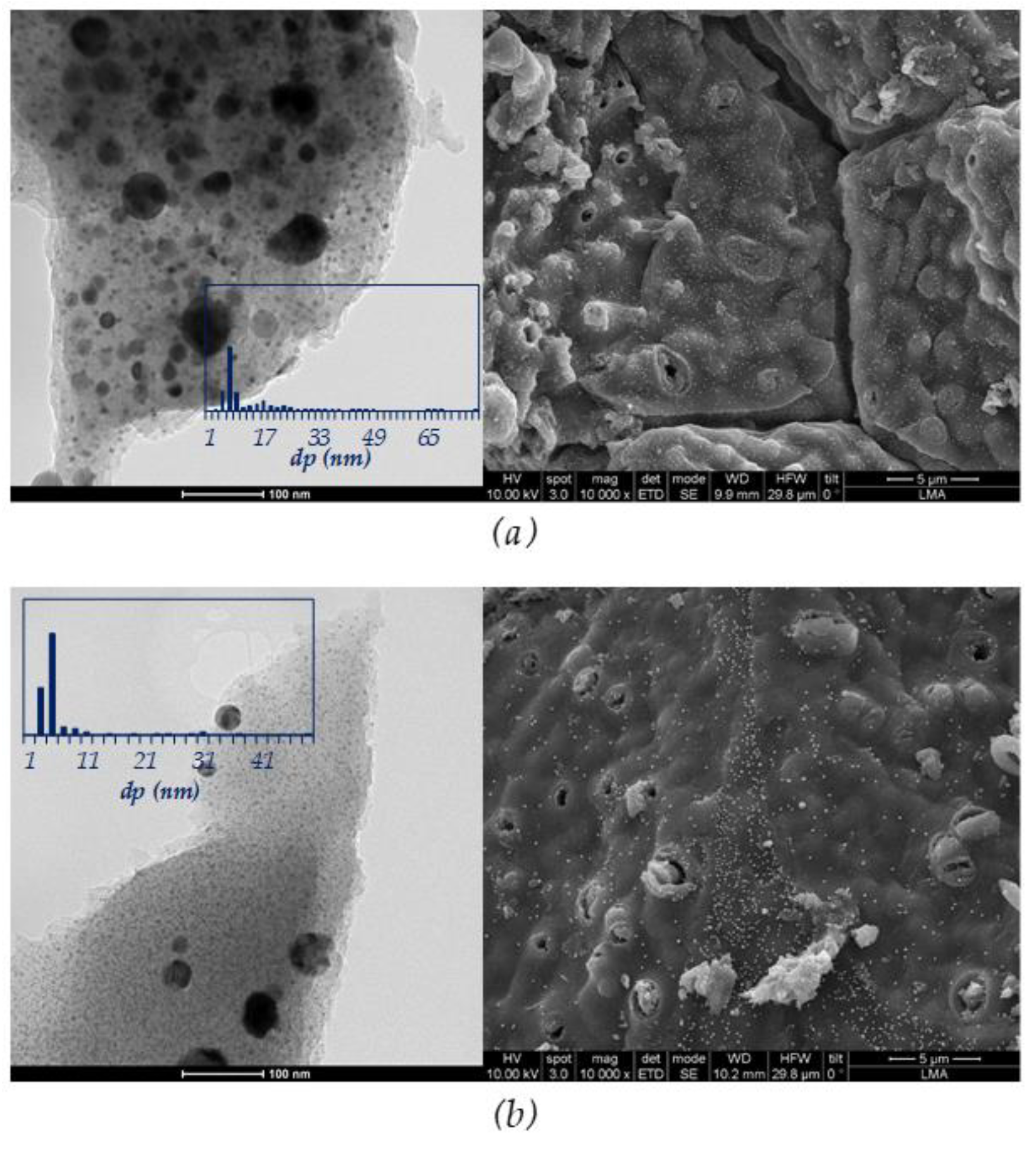
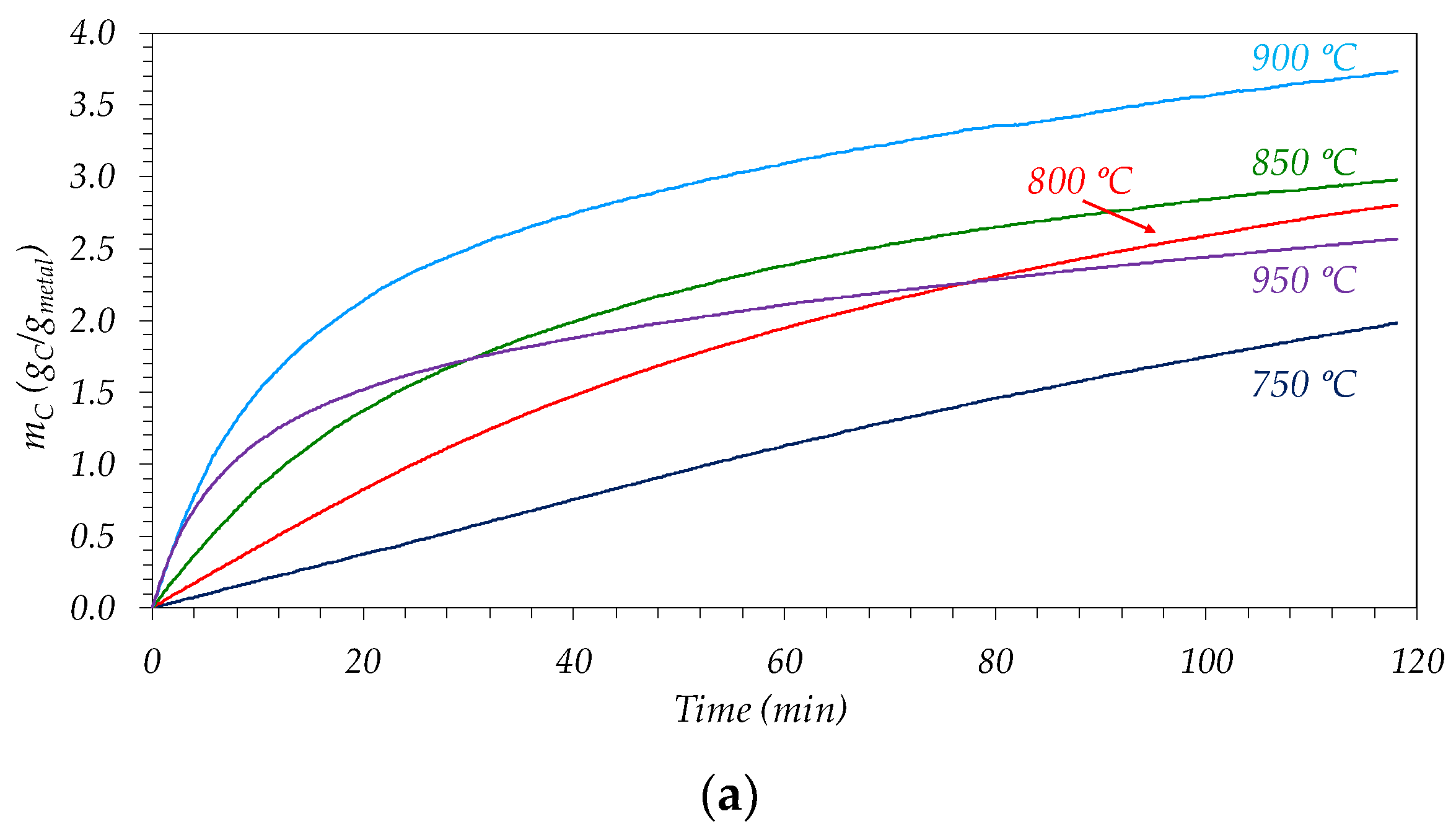


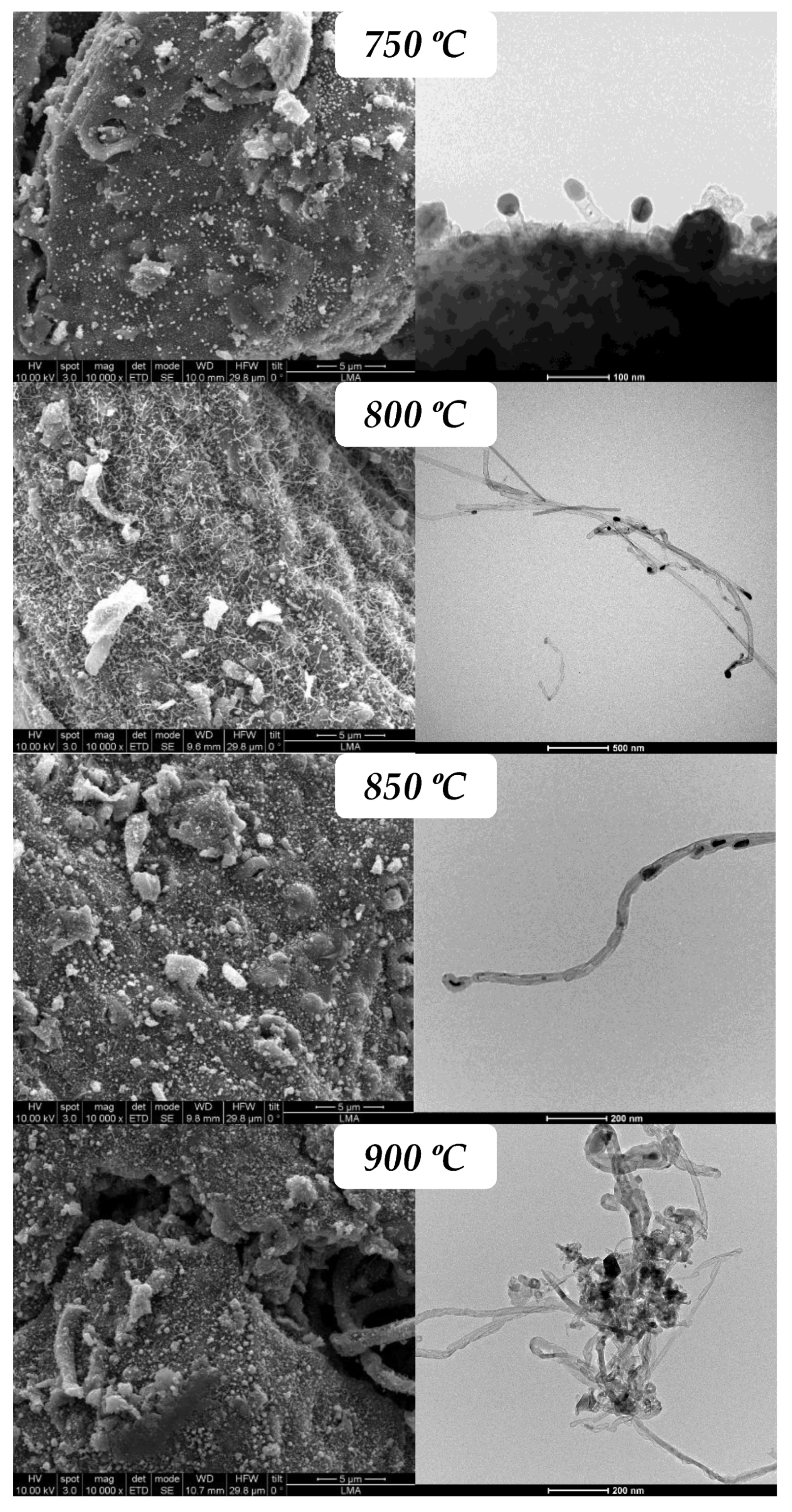



| Sample | BET Area (m2/g) | Pore Vol. 1 (cm3/g) | µpore Vol. 2 (cm3/g) | µpore Vol. (%) |
|---|---|---|---|---|
| Ni–Cu/ArDC | 404 | 0.168 | 0.138 | 82 |
| Co–Cu/ArDC | 433 | 0.182 | 0.164 | 90 |
| Ni–Cu/CDC [24] | 343 | 0.451 | 0.148 | 33 |
| Co–Cu/CDC [27] | 438 | 0.206 | 0.160 | 78 |
| Temperature (°C) | Ni–Cu/ArDC | Co–Cu/ArDC | ||||
|---|---|---|---|---|---|---|
| rC0 (gC/gmetal·min) | Carbon Product. (gC/gmetal·h) * | Carbon Product./rC0 | rC0 (gC/gmetal·min) | Carbon Product. (gC/gmetal·h) * | Carbon Product./rC0 | |
| 750 | 1.2 | 0.99 | 0.83 | 0.3 | 0.16 | 0.52 |
| 800 | 2.9 | 1.40 | 0.49 | 1.1 | 0.64 | 0.56 |
| 850 | 5.9 | 1.49 | 0.25 | 2.3 | 0.74 | 0.32 |
| 900 | 12.6 | 1.87 | 0.15 | 1.7 | 0.53 | 0.30 |
| 950 | 11.22 | 1.29 | 0.11 | 1.6 | 0.34 | 0.21 |
| CH4:H2 | Ni–Cu/ArDC | Co–Cu/ArDC | ||||
|---|---|---|---|---|---|---|
| rC0 (gC/gmetal·min) | Carbon Product. (gC/gmetal·h) * | Carbon Product./rC0 | rC0 (gC/gmetal·min) | Carbon Product. (gC/gmetal·h) * | Carbon Product./rC0 | |
| 0.5 | 0.8 | 0.65 | 0.83 | 0.2 | 0.20 | 0.83 |
| 1 | 2.0 | 1.16 | 0.57 | 0.6 | 0.40 | 0.67 |
| 2 | 2.9 | 1.40 | 0.49 | 1.1 | 0.64 | 0.56 |
| 3 | 4.1 | 1.67 | 0.41 | 1.6 | 0.71 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazaña, F.; Afailal, Z.; González-Martín, M.; Sánchez, J.L.; Latorre, N.; Romeo, E.; Arauzo, J.; Monzón, A. Hydrogen and CNT Production by Methane Cracking Using Ni–Cu and Co–Cu Catalysts Supported on Argan-Derived Carbon. ChemEngineering 2022, 6, 47. https://doi.org/10.3390/chemengineering6040047
Cazaña F, Afailal Z, González-Martín M, Sánchez JL, Latorre N, Romeo E, Arauzo J, Monzón A. Hydrogen and CNT Production by Methane Cracking Using Ni–Cu and Co–Cu Catalysts Supported on Argan-Derived Carbon. ChemEngineering. 2022; 6(4):47. https://doi.org/10.3390/chemengineering6040047
Chicago/Turabian StyleCazaña, Fernando, Zainab Afailal, Miguel González-Martín, José Luis Sánchez, Nieves Latorre, Eva Romeo, Jesús Arauzo, and Antonio Monzón. 2022. "Hydrogen and CNT Production by Methane Cracking Using Ni–Cu and Co–Cu Catalysts Supported on Argan-Derived Carbon" ChemEngineering 6, no. 4: 47. https://doi.org/10.3390/chemengineering6040047
APA StyleCazaña, F., Afailal, Z., González-Martín, M., Sánchez, J. L., Latorre, N., Romeo, E., Arauzo, J., & Monzón, A. (2022). Hydrogen and CNT Production by Methane Cracking Using Ni–Cu and Co–Cu Catalysts Supported on Argan-Derived Carbon. ChemEngineering, 6(4), 47. https://doi.org/10.3390/chemengineering6040047






