Abstract
Consumers’ interest in a high-quality healthy diet is creating a growing trend in the food industry, focusing on the design and development of new products rich in bioactive compounds. This work involves the formulation of a vegetable sauce obtained from a mixture of pumpkin and pepper, the study of the evolution of bioactive compounds, quality and sensory parameters during storage at 4 and 25 °C, the influence of the packaging materials (PVC, PE/PA, and PS), and the migration degree. Antioxidant activity, polyphenols, carotenoids, and brown pigments contents were studied at 25 °C. Overall migration of the containers and the evolution of the physicochemical parameters and sensory attributes of the sauce were analyzed. All plastic materials showed an overall migration lower than the limit of EU and Mercosur Regulations. PVC better preserved polyphenols, antioxidant activity, and carotenoids until 50, 10, and 30 days, respectively, and lower development of brown pigments was observed. Higher storage temperatures favored undesirable changes in sensory attributes before 50 days of storage. PVC can be used to achieve greater conservation of the sensory attributes of sauce, regardless of the storage temperature. It could be considered the best material to preserve the bioactive properties and sensory attributes of the sauce until 30 days.
1. Introduction
Currently, consumers are becoming increasingly aware of the benefits that high-quality healthy diets provide [1]. For this reason, the demand and consumption of functional compounds, such as antioxidants and dietary fiber, play an increasingly important role [2]. The market offers a wide spectrum of vegetables with these characteristics, which can be combined with other raw materials to attain the desired organoleptic properties and, additionally, to upgrade the bioactivity of the product [3].
Sauces and dressings are commonly consumed in everyday life. Ketchup, mustard, mayonnaise, soy sauce, fish sauce, and barbecue sauce are some of the most known products worldwide [4], which are formulated with additives such as thickeners, colorants, flavorings, and preservatives [5]. In this context, the formulation and development of a sauce, rich in bioactive compounds and obtained from fruits and vegetables, represents a great challenge.
Butternut squash (Cucurbita moschata, D.) and red pepper (Capsicum annuum, L.) are produced in large quantities in the north east of Argentina (NEA) and they have an appreciable content in bioactive compounds, mainly carotenoids, polyphenols, ascorbic acid, and fiber [6,7,8]. These raw materials are available throughout the year and they have a lot of potential to design and develop new products with high added value such as sauces which can be used to season meats, pastas, bread, and other culinary preparations.
It is important to highlight that the bioactive compounds as well as the organoleptic properties of the final product could be affected by the packaging material used and the storage conditions [9], as well as the perception of consumers. Thus, in order to understand the behavior of bioactive compounds during their storage in different materials and conditions, a study of the interactions between foodstuffs and packaging is very important.
There is a marked trend in the study and development of biodegradable packaging [10,11,12]. However, although much effort has been put into this packaging being made available for daily use, they have not been able to replace synthetic materials, mainly due to physical properties, especially mechanical properties and moisture barrier [13,14]. In contrast, the use of plastic food packaging has increased due to the increasing demand caused by population growth and market expansion [15], reaching a worldwide plastic production of 320 million tons in 2018 [16].
On the other hand, it is common knowledge that the plastic materials used for food packaging can produce the migration of compounds to the food which can affect consumers’ health [17]. In this context, one of the most important steps during the development of new products is the selection of the type of packaging to be used, the overall migration test being one of the most relevant parameters to be taken into account [14,18].
Polyvinyl chloride (PVC), polyamide/polyamide alloy (PE/PA), and polystyrene (PS) are widely used materials for many food packaging products such as meat, dairy, sauces, and baked goods, although PVC has a restricted use for non-fat foods [19]. It should be taken into account that the chemical components of these materials, such as phthalates in PVC [20], PA 6 (polycaprolactam) in PE/PA [21], and styrene monomer in PS [22], can migrate to food, affecting its final quality and nutritional value. Storage is mainly affected by the type of plastic materials, food composition, and storage conditions until consumption [22]. Food processing conditions also have a great impact on such migration; and although these components are not toxic, exposure to high doses can cause serious health and safety problems to consumers [23].
The European Regulation specifies a limit on the total mass of substance permitted to migrate from the packaging to the food called the overall migration limit (OML), which corresponds to the determination of the release of substances from the material or article either into food or into a food simulant [24]. This can be understood by using a food simulant to test a medium that imitates food behavior and the migration from food packaging [14]. There are five simulants described in the legislation for plastics: distilled water or water of equivalent quality, 3% acetic acid (m/V) in aqueous solution, 10% ethanol (by volume) in aqueous solution, rectified olive oil, and 50% ethanol (by volume) in aqueous solution [25,26]. These simulants mimic under worst case conditions aqueous foods, acidic foods, alcoholic foods, and fatty foods, respectively.
The purpose of the present research was to develop a sauce rich in bioactive compounds and to determine the effect of the packaging and storage conditions on the functional properties and sensory attributes, as well as knowing the degree of global migration of the components from containers.
2. Materials and Methods
2.1. Raw Material and Sauce Preparation
Butternut squash (Cucurbita moschata, D.) and red pepper (Capsicum annuum, L.) were bought on a local market located in Resistencia, Chaco (Argentina) and stored at 4 ± 1 °C until their processing. To prepare the sauce, the vegetables were tempered at 25 °C, washed, peeled, and cut into slices of approximately 1.5 ± 0.2 cm. The slices of butternut squash were washed, drained, and steamed for 20 min. Finally, the slices were mashed using a high-speed mill (model FW100, Huanghua, China) at 24,000 rpm for 6 s and sieved with a mesh. On the other hand, red pepper puree was prepared as follows: after washing, peppers were covered in aluminum foil and kept in an oven for 1 h at 180 °C. Peels and seeds were removed and the peppers were mashed using a high-speed mill (model FW100, Huanghua, China) at 24,000 rpm for 3 s. A diagram of the sauce preparation is shown in Figure 1.
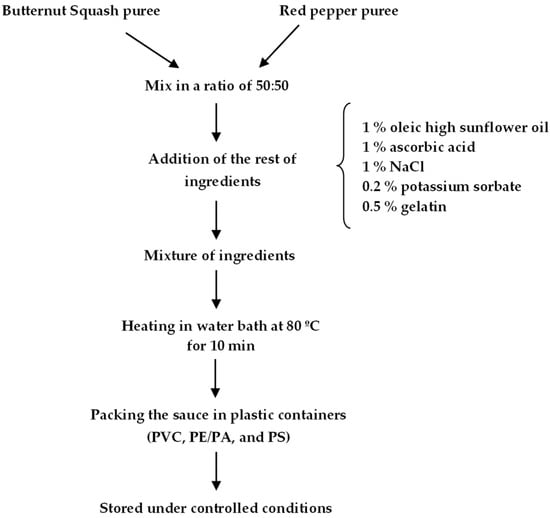
Figure 1.
Diagram of the sauce preparation.
According to previous results, purees were mixed in a ratio of 50:50 pumpkin/pepper [27] and other ingredients were added: 1% (w/w) commercial oleic high sunflower oil, 1% (w/w) ascorbic acid (Biopack®, Buenos Aires, Argentina), 1% (w/w) NaCl, 0.2% (w/w) potassium sorbate (Dalton®, Mendoza, Argentina), and 0.5% (w/w) of gelatin (200 bloom). All the ingredients were mixed and heated in a water bath at 80 °C for 10 min (HHS-S model). Finally, the sauces were aseptically packaged at room temperature, in three different sterile plastic containers: polyvinyl chloride (PVC) provided with pressure lid, polyamide/polyamide alloy (PE/PA) suitable for vacuum sealing, and polystyrene (PS) with threaded lid. In all cases, the presence of headspaces was avoided.
2.2. Experimental Procedure
The experimental plan was divided into the following stages. First, the overall migration test of plastic material was carried out using three different kinds of plastic containers at initial time, 5, 10, 15, and 20 days. Second, the sauces were packaged in each of the containers and in order to determine the behavior of the bioactive compounds in storage conditions, the content of polyphenols, carotenoids, brown pigments formation, and antioxidant activity was measured at initial time, 10, 20, 30, 40, and 50 days of storage at 25 °C. Moreover, pH and titratable acidity were determined for these times. Finally, a sensory analysis was carried out to determine a sensory description of the sauces and the impact of the packaging materials and the storage conditions.
2.3. Plastic Containers and Storage Assay
As described above, three different commercially available plastic food containers were used: PVC with a surface of 188 cm2, PE/PA (220 cm2), and PS with 87 cm2. All of them were bought in a commercial market located in Resistencia, Chaco (Argentina) and 200 g of sauce was placed in each one, leaving no head space. After filling, the containers were sealed at 25 °C and at atmospheric pressure. In the case of PE/PA, they were sealed at 30 °C in a vacuum packing machine (Ehrlich, Model EH-18, Buenos Aires, Argentina). Every 10 days of storage, samples were taken and the total polyphenols, antioxidant activity, carotenoids, and brown pigments contents were measured over a total period of 50 d.
2.4. Overall Migration Test of Plastic Material
Overall migration tests were carried out according to the European Regulation [24] which agrees with the Mercosur Technical Regulation on migration in plastic materials, packaging, and equipment intended to be in contact with food [25]. According to these regulations, the migration test was carried out using food simulants. Food simulants are simple liquids that allow the quantitative measurement of any non-volatile substance with relative ease that could migrate from the container under test conditions [28]. In this context, simulant B was used in order to simulate the same conditions of pH of our food system. This simulant consists of a solution of 3% acetic acid in water (w/v) and to carry out the migration assay, each plastic material was cut into squares of 30 cm2 and placed into a glass beaker which was filled with 15 mL of simulant.
The beakers were sealed with parafilm® M (Brand GmbH + CO KG, Wertheim, Germany) in order to avoid evaporation and incubated at 25 °C during 0, 5, 10, 15, and 20 days. The aforementioned Regulation indicates that the total period of the assay should be 10 d; however, 15 and 20 days were also analyzed. Three replicates were conducted plus one control sample (only simulant).
After the exposition of the simulant with the plastic food containers, the liquid phase was placed in porcelain capsules and evaporated to dryness in an oven at 105 ± 1 °C for around 30 min. The capsules containing the residue were kept in a desiccator for 1 h. The overall migration was determined gravimetrically by using the following Equation (1).
where M is the overall migration into the simulant (mgresidue/dm2sample); Ma is the mass of the residue from the test specimen after evaporation of the simulant which had filled the test specimen (g); Mb is the mass of residue from the blank simulant equal to the volume which had filled the test specimen (g), and S corresponds to the surface area of the test specimen which was in contact with the simulant during the exposure (dm2).
2.5. Analytical Methods
2.5.1. Determination of Total Polyphenol Content
Total polyphenol content (TPC) was determined using the Folin-Ciocalteu assay with modifications described by Tang et al. [29]. First, 30 g of the sauce was homogenized with 30 mL of methanol. The mixture was reposed for 20 min and after that, was centrifuged for 20 min. The supernatant was filtered and a methanolic extract of the sauce was obtained. Then, 1550 µL of distilled water and 150 µL of methanolic extract were added to 100 µL of the Folin-Ciocalteau reagent (Biopack®, Zárate, Buenos Aires, Argentina), allowed to react for 5 min, and then added 200 µL of Na2CO3 at 10% (w/V). The mixture was stirred and incubated for 1 h in the dark prior to measuring the absorbance at 760 nm using a UV/Vis Perkin Elmer spectrophotometer (LAMBDA 25, Walthman, MA, USA). Chlorogenic acid was used as reference standard and the results were expressed as mgCA/100gs, where the subscript CA corresponds to chlorogenic acid equivalent and s, sample [30,31].
2.5.2. Antioxidant Activity
Antioxidant activity was determined by spectrophotometry, measuring the loss of color of the radical chromogen DPPH [32]. A sample of 10 g was mixed with 25 mL of methanol and was filtered and diluted successively with methanol until complete discoloration of the residue. A 6 mL aliquot of methanolic DPPH solution (3 × 10−3 mM) was added to aliquots of 0.5 mL of the obtained methanol extract and left for 20 min in complete darkness to reach a stationary state. Then, an initial value of the methanolic DPPH solution was taken to monitor the decrease of its absorbance at 517 nm. DPPH radical is reduced in the presence of antioxidants, with a change in color in the solution over time. Trolox was used as reference standard, where the antioxidant capacity of the initial value of the sauce was expressed as μgTeq/gs, where the subscript Teq means Trolox equivalent and s, sample. Evolution during storage was expressed as the percentage of inhibition of DPPH radical.
2.5.3. Determination of Total Carotenoid Content
Total carotenoid content (TCC) was determined as follows: 10 g of the sample was homogenized with 25 mL of pre-cooled acetone, filtered under vacuum, and the residue was washed until its total discoloration. The filtrate was extracted with petroleum ether in a ratio of 1:1. The ether phase was separated and was measured as absorbance at 450 nm using a UV/Vis Perkin Elmer spectrophotometer (LAMBDA 25, Walthman, MA, USA), with petroleum ether as a blank. The results are expressed as μg β-carotene per g of sample (μgβC/gs) [33].
2.5.4. Determination of Brown Pigment Content
The determination of brown pigments was carried out according to the method described by Viña and Chávez [34]. First, 5 g of the sample was homogenized with 25 mL of absolute ethanol. The mixture was reposed for 15 min and it was filtered under vacuum for 7 min and centrifuged during 15 min at 2500 rpm (model 80-2B, Shanghai, China). Then, the volume of the alcoholic extract was measured and diluted to a final volume of 50 mL with ethanol. The absorbance was obtained at 320 nm by using a spectrophotometer Perkin Elmer LAMBDA 25 UV-visible (LAMBDA 25, Walthman, MA, USA) and using ethanol as a blank. Results were expressed as absorbance units (uA) per g of sample.
2.5.5. Physicochemical Determinations
The pH of the samples was measured with a pH-meter MP103 (MRC, Holon, Israel). Titratable acidity, expressed as citric acid in 100 mL of sauce, was analyzed following AOAC Method 934.06 [35], titrating an aliquot of the sauce with 0.1 N NaOH until it reached a pH of 8.1. Moisture was determined by drying in a vacuum oven DZF Model 6020 (Shangai, China) at 70 °C until constant weight was reached [35]. Physicochemical determinations were performed in triplicate.
2.6. Sensory Evaluation
2.6.1. Panel Training
The sensory assessment of sauces was performed following a quantitative descriptive analysis (QDA) by a trained panel consisting of 10 assessors (7 women and 3 males, in an age range of 23 to 33 years). The panelists were scientists, graduated chemical engineers, students, and grantees from Universidad Tecnológica Nacional-Facultad Regional Resistencia (UTN FRRe). Before analysis, panelists were trained in successive sessions, of short duration (maximum 1 h) to avoid exhaustion, to verify and discuss the vocabulary and to explain the scales being used.
Work was done on the development of the standardized vocabulary, on the selection of product descriptors and their definitions, and on the choice of references and scales. An exhaustive work was performed to standardize criteria on the intensity measure of the descriptors. The definition agreements of the descriptors were achieved by working with foods from the same category (commercial dressings and sauces acquired in a commercial market) and with the sauce under study, previously prepared. In the cases in which there was higher variability in the answers, a review and redefinition of the descriptors was carried out.
To describe the sauces packaged in different materials, the panel defined a set of 5 descriptors. Supplementary Table S1 shows the descriptors based on visual, manual, and oral perceptions, with the descriptions emitted by the panel, the references selected for scoring and the agreement of criteria for the measurement of intensity (scales extremes).
2.6.2. Sensory Evaluation of Sauces during Storage
QDA analysis was mainly used to evaluate the organoleptic changes of the sauce during storage. These changes are mainly affected by the temperature and time of storage. Sauces were prepared according to the protocol described above (Section 2.1), packaged in three different containers (PVC, PE/PA, and PS) and stored at 4 and 25 °C for 50 days. Every 10 days of storage at different temperatures, samples were extracted for their descriptive sensory evaluation. Changes in the color, aroma, spreadability, lumpiness, and flavor attributes at different stages of storage were evaluated.
Samples were served to the panelists at room temperature (2 ± 1 °C) in disposable trays in individual booths under white light and where each sample was given a random three-digit coded number. Drinking water was provided to the panelists to refresh the palate and at least one minute was required as a wait time before tasting the following sample. Unstructured graphic scales anchored at the ends were used. The intensity measurement of each of the descriptors was reflected using a 10-point scale ranging in the vertical marks that the evaluators made on the scale line. The corresponding quantification was carried out by measuring the distance from the left of the scale, representing the value 0 (zero), to the evaluators’ mark, to which a numerical value was assigned that was then statistically analyzed.
2.7. Statistical Analyses
The statistical analyses were performed by one-way ANOVA by using Minitab® 15.1.20 (Minitab Inc., State College, PA, USA) in order to determine significant differences between mean values of each measured parameter with 95% of confidence (p < 0.05).
3. Results and Discussion
Currently, numerous sauces are available on the market; however, information about pumpkin/pepper sauce and its storage stability is very limited. In this context, data on storage stability and quality deterioration would be useful for designing suitable storage conditions and to optimize their shelf-life [36]. The moisture obtained for the sauce was practically constant for all the packaging materials and for the whole studied period, reaching a value of 85.3 ± 0.3%.
Table 1 shows the physicochemical parameters (acidity and pH) of the sauce packaged in PVC, PE/PA, and PS during 50 days of storage. As a general trend, the acidity of the sauce packed in PVC, PE/PA did not show differences throughout the studied period, while PS showed little variation from day 20 of the assay. Regarding the different materials at the same time of storage, punctual variations were registered at day 10 for PS and at day 30 for PVC (p < 0.05). Although different acidity between the samples were registered, no specific trend between the different materials was observed. Sosa et al. [17] reported that acidity and pH were practically constant during a short-studied storage period at 4 ± 2 °C for pumpkin/pepper sauces packaged in PS material. For other similar food products, Baiano et al. [5] did not find changes in the pH during storage of tomato sauces at 5 °C, being around 4.2 for a sauce packaged in glass and about 4.0 for tomato sauce in polymeric containers.

Table 1.
Evolution of the physicochemical parameters of the sauce.
pH changes during storage were chosen as the primary quality index for determining the shelf-life of some sauces; for example, soybean paste seasoning packaged in semi-rigid PET bottles showed a pH close to 4.0 [36]. In this study, the pH of the sauces remained unchanged until day 30 of storage. From this time, an increase in this parameter was observed for all containers, PS being the material which showed the lowest variation. This effect could be due to the decomposition of some organic acids into smaller compounds or due to the reduction of oxygen pressure probably present in the containers. Baiano et al. also reported that PS material showed the lowest oxygen stability in tomato sauces compared to PVC, glass, and polypropylene [5]. These results are in accordance with the pH values obtained by Giovanelli and Lavelli [37] and Muzzaffar et al. [38] for commercial tomato pulp, purees, pastes, and fresh pumpkin.
When selecting the most suitable packaging for each type of food, one of the most important parameters to take into account is the overall migration of the plastic components to food. For example, PVC is one of the resins with the higher quantity of additives and the possibility of migration is always a concern when its intended use is food packaging [39].
In this context, overall migration assay of PVC, PE/PA, and PS was performed by using simulant B at 25 °C and for a total period of 20 days of storage.
As Figure 2 shows, no differences were obtained for PS material throughout the entire studied storage time (p > 0.05). Similarly, PVC and PE/PA do not show differences from the initial time until 10 days of storage; however, from 15 days the overall migration of these materials increase (8.5 ± 0.2 and 3.6 ± 1.1 mg/dm2 for PVC and PE/PA, respectively), remaining practically constant until the end of the assay.
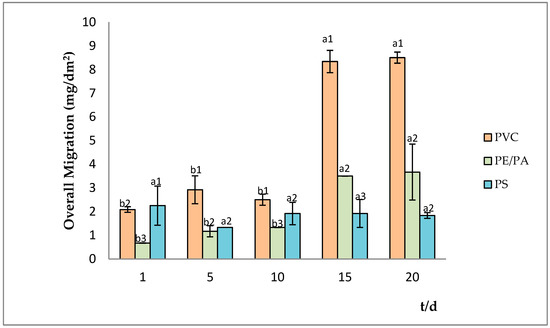
Figure 2.
Overall migration in simulant B at 25 °C. For a certain plastic material, bars with the same letter (a–b) indicate no significant differences (p > 0.05). Different numbers (1–3) indicate significant differences between different containers at the same time (p < 0.05). PVC = polyvinyl chloride, PE/PA = polyamide/polyamide alloy, PS = polystyrene.
On the other hand, significant differences (p < 0.05) were observed in the total migration in the packages studied at the different tested times; in addition, the highest values were obtained from PVC at day 5. It is important to highlight that although the overall migration of PVC to the simulant reached values of 8.5 ± 0.2 mg/dm2, this value does not exceed the maximum limit (10 mg/dm2) established by the Mercosur Technical Regulation [25] and the EU regulation [24].
As mentioned in the Section 2, once the overall migration was measured, the sauce developed by BIOTEC group (UTN FRRe) was stored in the different plastic containers (PVC, PE/PA, and PS) at room temperature for a total period of 50 days and the behavior of the bioactive compounds were measured during this time.
The initial values obtained for total polyphenols content of the sauce was 101.3 mgCA/100 gs. Figure 3a shows the evolution of TPC of the sauces during storage, expressed as mass fraction (kgP/kgT, where the subscripts P and T correspond to polyphenol and total, respectively). A TPC decrease for all types of containers used can be observed (58.23, 32.53, and 27.23% in PS, PE/PA, and PVC, respectively), reaching final values of 41.85 ± 1.34 mgCA/100 gs (for PS), 70.9 ± 0.7 mgCA/100 gs (for PE/PA), and 80.3 ± 1.6 mgCA/100 gs (for PVC), PVC being the material that best preserved these compounds during overall storage assay.

Figure 3.
(a) Mass fraction of total polyphenolic content (TPC). (b) Ratio of the percentage of inhibition of DPPH chromophoric radical. (c) Mass fraction of total carotenoid content (TCC) in the sauce during storage at 25 °C.
Bioactive compounds such as TPC are a critical factor for food quality, since these substances undergo degradation during storage. It is important to remark that phenolic stability is dependent on the storage conditions, especially the temperature [40], processing methods (peeling, grinding, heating, frying, or thermal treatments), and packaging materials [41]. Similar results were obtained by other researchers, who agreed that the TPC decreases over time during storage due to many different factors. In this sense, Radovanović et al. [42] monitored total phenol content in fruit extracts stored at 7 °C for 23 days and at room temperature (25 °C) for 90 days. All fruit extracts exhibited fluctuations in total phenol contents with an initial increase after 4 d, followed by a decrease at both storage temperatures (less than 20%). Zorić et al. [41] reported a retention of at least 50% of the initial polyphenol content in cherry samples stored at 20 °C packed in Pet 12 μm/PP 18 μm met/PE 100 μm (Pet/PPmet/PE) for 6 months of storage, whereas samples packed in PET/Al/PE (polyethylene terephthalate/aluminum/polyethylene) retained the same percentage for 3 months. Ranđelović et al. [43] studied the quality changes of dried apricot packed in different packaging materials during 12 months’ storage period at room temperature and results showed the highest polyphenol stability in polyester/aluminum/polyethylene (Pet/Al/PE) laminate. According to Henríquez et al. [44], in apple peel powder stored at 38 °C for 120 d, the loss of polyphenols was higher when packed in high density polyethylene than in metalized films of high barrier. Bakan and Eksi [45] reported a decrease in TPC in sour cherry nectar packaged in cartons and aluminum cans, and concluded that it could be due to the partial conversion of polyphenols to soluble polymers or due to their condensation during storage.
The main antioxidant compounds in pumpkin/pepper sauces are carotenoids, polyphenols, and ascorbic acid, which contribute to the total antioxidant activity of the product. Thus, total antioxidant activity is the result of the contribution of the individual compounds and to the synergistic and antagonistic effects between them and the food matrix [27].
The initial antioxidant activity value obtained for the sauce was 3.05 μgTeq/gs and in order to describe its evolution, data were expressed as a proportionality variable, which describes the relation between the % of DPPH chromophoric radical inhibition at time t and the % of DPPH chromophoric radical inhibition at the initial time (t = 0) [46]. As Figure 3b shows, all the studied sauces exhibited a decrease (p < 0.05) in the antioxidant capacity. In the case of PS and PE/PA packaging, a decrease in antioxidant capacity from the beginning of the assay was observed, whereas in the sauces in PVC, it remained stable until 10 days of storage. However, from this time and for the entire storage period (50 days), the antioxidant activity decreased (p < 0.05). At day 50, the reduction in PVC and PE/PA was 80%, while in PS it was 75%. Thus, for short storage periods, PVC could represent the best packaging option for pumpkin/pepper sauce, but for long periods, PS containers preserve the activity of these bioactive compounds in a better way, being the more suitable packaging. Bakan and Eksi [45] also observed a decrease trend in the antioxidant activity of sour cherry nectar packaged in different materials, the main responsible factors of this loss being the type of material used and the reduction of the TPC as was explained above.
It is important to note that the consumption of carotenoids in food shows important health benefits, such as pro-vitamin A activity, antioxidant properties, and prevention against certain types of cancer as well as degenerative and chronic diseases, and it also helps to improve the immune function [47]. The total carotenoid content in the sauce is due to the contribution of the carotenoids present in both pumpkin and red pepper. The main carotenoids present in red peppers are capsanthin, α and β-carotene, violaxanthin, cryptoxanthin, capsorubin, lutein, and zeaxanthin [27,48]; and in pumpkin, β-carotene, lycopene, lutein, zeaxanthin, and xanthophylls, which are positively related to several health benefits, such as the ability to treat the age-related macular degeneration [49]. On the other hand, due to the presence of double bonds in their chemical structure, these compounds are very susceptible to degradation reactions [50]. Figure 3c shows the TCC of the sauce (expressed as kgC/kgT, where C corresponds to carotenoid and T, total).
Regarding TCC of the sauces, the initial obtained value was 78.6 μgβC/gs, which was maintained up to 30 days of the assay for the sauces packaged in PVC. After this time, TCC decreased until 50 days (p < 0.05). However, for the sauces packaged in PS, the TCC decreased from the beginning of the storage, maintaining high values for the rest of the studied period, in comparison with the other materials. Oxidation could be considered the main cause of carotenoid degradation, due to the spontaneous reaction of free radical chains exposed to several factors such as light, the type of packaging, the storage conditions and/or oxygen concentration, metals, enzymes, and peroxides [51,52]. Castro-López et al. [53] observed a 25% decline of TCC on fruit juices stored at 4, 8, and 11 °C for 12 days, concluding that the oxygen present in the headspace of the containers is the main cause of the compound degradation.
Li et al. [54] demonstrated a decrease in the carotenoid content and color index in a tomato hot pot sauce packed in PET/PE and PET/Al/EAA/PE at high temperatures (25 and 37 °C) and an increase in hydroxymethylfurfural (HMF) content (browning indicator). In contrast, low storage temperatures and high oxygen resistance packaging reduced HMF accumulation and carotenoids reduction, slowing down the browning process, improving the color of the sauce.
In order to have a better comprehension of the degradation degree of the sauce during the storage in the different containers, the formation of brown pigments was also analyzed. It is important to highlight that browning reactions are some of the most important phenomena occurring in food during processing and storage, and have important implications in food stability as well as in nutrition and health, and they can proceed through different chemical pathways, enzymatic and non-enzymatic [55].
As can be appreciated in Figure 4, the sauce contained in PS developed the highest content of brown pigments, reaching a value of 4.72 ± 0.12 (at 50 days of storage), while PE/PA and PVC reached values of 2.47 ± 0.03 and 1.71 ± 0.14, respectively. Thus, the evolution of brown pigments was more stable in PVC than in other plastic materials. On the other hand, at 40 and 50 days, significant differences (p < 0.05) were observed in the formation of brown pigments among the three studied plastic containers. The development of brown pigments during storage could be related to the oxidative degradation of the sorbate used as antimicrobial agent, which produces an increase in the concentration of carboxyl groups which participate in the initiation of the Maillard reaction, affecting the color and the quality of food [56].
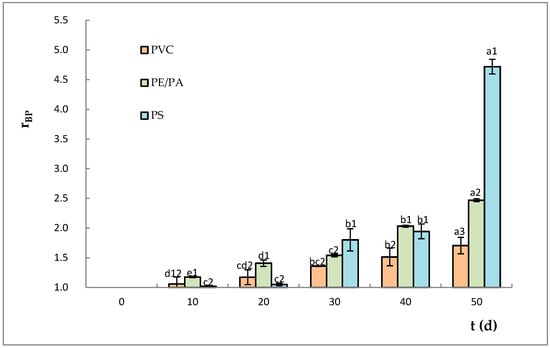
Figure 4.
Brown pigment generation in the sauces packed in PS, PVC, and PE/PA during storage (expressed as a proportionality variable). For a certain plastic material, bars with the same letter (a–e) indicate no significant differences (p > 0.05). Different numbers (1–3) indicate significant differences between the containers at the same time (p < 0.05). PVC = polyvinyl chloride, PE/PA = polyamide/polyamide alloy, PS = polystyrene.
On the other hand, it is important to highlight that ascorbic acid has been added to pumpkin/pepper sauces as an acidulant and antioxidant agent. However, the antioxidant effect of ascorbic acid depends on packaging material. Gliemmo et al. [57] reported a higher degradation rate of ascorbic acid in pumpkin (Cucurbita moschata, D.) puree, packaged in polyethylene material, indicating a pro-oxidant effect. Another research shows a significant impact of ascorbic acid degradation on brine browning in pickled vegetables packed in plastic pouches. This phenomenon was enhanced by the presence of both dissolved oxygen and sorbate which could act as a catalyst in the degradation reaction of ascorbic acid, which in turn accelerated the browning reaction [58]. Similar browning process have been reported by Kohan-nia et al. [59], who observed that the color of catsup sauce contained in PE, PP, and PET was altered after 180 days of storage at 22 °C, and Baiano et al. [5] noticed that a* (color parameter) decreased in a tomato sauce packed in glass, PET (PET containing oxygen scavengers), and PP, at 5 °C after 4 months.
Sensory Evaluation
The effect of storage temperature on the sensory attributes of sauces packaged in different materials was studied and results are shown in Figure 5.
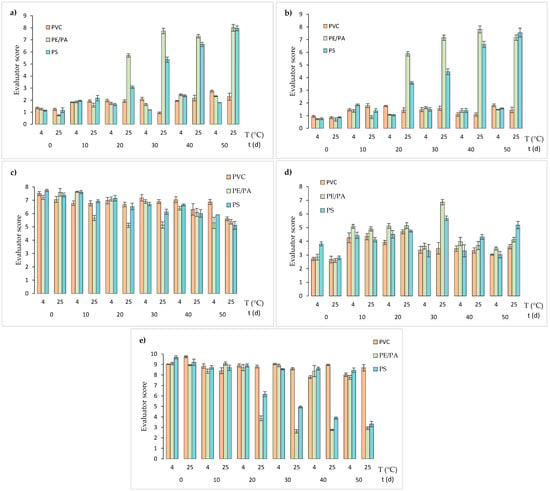
Figure 5.
QDA analysis of sauces packaged in PVC, PE/PA, and PS for 50 days at 4 and 25 °C, with (a) aroma, (b) color, (c) spreadability, (d) lumpiness, and (e) flavor attributes.
Aroma attribute of the sauces (Figure 5a) at 4 °C kept their characteristic “sweet pepper” aroma throughout the studied period for all the packaging materials, obtaining scores varying between 1.13 ± 0.04 and 2.74 ± 0.08. Although the differences in the scores obtained between day 0 and 50 for each packaging materials are statistically significant, it is important to highlight that all the materials retained the characteristic aroma of the sauce at 4 °C with scores close to the lower end of the scale. During storage at 25 °C, the sauces packaged in PE/PA and PS experienced a tendency to develop a “rancid/oxidized” aroma from 20 days of storage, according to the increase in the scores given by the evaluators (between 8.1 ± 0.3 and 7.9 ± 0.2) for both cases. Meanwhile, the sauces packaged in PVC presented a little variation of the same attribute, reaching a value of 2.3 ± 0.3 on 50 days of the trial, which means that PVC kept the characteristic aroma of the fresh sauce during the whole studied period (near to the lower end of the scale).
Regarding color parameters (Figure 5b), a similar trend of aroma attributes during storage at both temperatures was observed. At 25 °C, the higher scores were obtained for sauces packaged in PS and PE/PA, specifically from 20 days of the trial. This result means that the sauce changed its color from “intense orange” to “dark brown”, possibly due to the occurrence of a browning process. On the other hand, the sauces packaged in PVC did not exhibit color changes during the studied period, since the scores given by the panel of trained judges remained near the lower value of the scale (between 0.8 ± 0.1 and 1.5 ± 0.2). These results are also in agreement with the brown pigment generation in the sauces explained in Figure 4, where PS showed the higher formation of these compounds, which could be related to the rancid/oxidized aroma.
Concerning textural attributes of the product, sauce spreadability (Figure 5c) showed a slight trend to decrease at 25 °C for all studied sauces (“quite spreadable” to “something spreadable”). It is important to highlight that sauces showed a similar spreadability to commercial dressings such as ketchup or barbecue sauce, and null lumpiness, since the values obtained for both parameters were close to the upper end of the scale, corresponding to the reference “cream cheese” (according to the descriptive analysis). At 4 °C, as storage progressed, a decrease in the scores obtained for spreadability of all sauces analyzed was observed, indicating that they became “less spreadable”. This change was more pronounced in PE/PA and PS sauces, which after 40 days of storage, experienced a decrease of 1 point in the aforementioned attribute. The loss of spreadability coincided with the increase in lumpiness (Figure 5d), with the sauces going from being “very slightly lumpy” to “somewhat lumpy”. In this case, the sauces packaged in a PVC were the ones that experienced the least changes in lumpiness during the period studied at 4 °C. However, at 25 °C the lumpiness of PE/PA and PS increases during storage.
Finally, flavor attribute showed a decrease in the scores after 20 days of storage at 25 °C, mainly in sauces packaged in PE/PA (from 9.1 ± 0.2 to 2.6 ± 0.2) and PS (from 9.2 ± 0.3 to 3.3 ± 0.3), indicating a tendency to an “atypical/rusty” flavor, while sauces packaged in PVC maintained their scores from day 10 to 50 of storage (between 9.7 ± 0.1 and 8.4 ± 0.3).
On the other hand, samples stored at 4 °C and packaged in PE/PA and PS reached flavor scores close to the maximum of the scale (between 9.1 ± 0.1 and 9.7 ± 0.2) during the entire storage period, while for PVC, no differences from the beginning and until day 30 of storage were observed. In conclusion, low storage temperatures favor the preservation of the sensory attributes of vegetable sauces in PE/PA and PS containers, helping to maintain a stable flavor attribute during storage. For PVC materials, both storage temperatures could be used since there was no significant effect on the sensory attributes of the sauces.
4. Conclusions
A new product obtained from native vegetables from the NEA region (Argentina), with considerable functional properties, was designed and developed. A study of the sauce behavior packaged in different materials and storage conditions was performed. Valuable information was obtained that can be used in future innovations. The content of total polyphenols, antioxidant activity, and carotenoid compounds in the sauces indicated its excellent nutritional quality.
The use of simulants is useful to determine the migration from containers and the data were compared to the legislated maximum concentration. The results showed that the overall migration values for PVC, PE/PA, and PS plastic materials into simulant B (acid) were lower than the upper limit for migration during the recommended period established by EU and Mercosur Regulations.
The effect of different materials packaging on the functional compounds was deeply studied. Regarding total polyphenol content, PVC was the material that best preserved these compounds with a final value of 80.3 ± 1.58 mgCA/100 gs after 50 days of storage. On the other hand, sauces packaged in PVC showed the highest values of antioxidant activity and carotenoid contents until 10 and 30 days, respectively, compared to PE/PA and PS materials. Finally, lower development of brown pigments was observed in sauces stored in this material.
Quantitative descriptive sensory analysis is a very useful tool to evaluate the sensory quality of sauces during storage, at different temperatures and packaging materials, and can provide an objective basis for determining the sensory changes under the studied conditions. According to this study, higher storage temperatures (25 °C) produced undesirable changes in all sensory attributes before 50 days of storage. On the other hand, low temperature conditions favored keeping the sensory quality of the sauces because the evaluated attributes remained with little variation throughout the entire studied period. With regard to the packaging material, PVC can be used to achieve greater conservation of the sensory attributes of sauce, regardless of the storage temperature to be applied.
Taking into account the results of this research, PVC could be considered the most recommended material to preserve the bioactive properties and sensory attributes of the developed vegetable sauce until 30 days of storage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemengineering6030034/s1, Table S1: Definition of sensory attributes. Anchors for all the attributes are in the brackets at the end of each definition.
Author Contributions
Conceptualization, C.A.S.; formal analysis, C.G.G., M.V.T.-S. and C.A.S.; funding acquisition, S.C.S. and C.A.S.; investigation, C.G.G., M.V.T.-S., S.C.S. and C.A.S.; methodology, C.G.G., M.V.T.-S. and C.A.S.; project administration, C.A.S.; resources, S.C.S. and C.A.S.; supervision, M.V.T.-S. and C.A.S.; validation, C.G.G., M.V.T.-S. and C.A.S.; writing—original draft, C.G.G.; writing—review and editing, M.V.T.-S. and C.A.S.; visualization, M.V.T.-S. and C.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SCTyP-UTN and FRRe-UTN (Secretaría de Ciencia, Tecnología y Posgrado de la Universidad Tecnológica Nacional y Facultad Regional Resistencia de la Universidad Tecnológica Nacional) and PID UTN-FRRe (project ALUTIRE0004517TC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated in this work are presented in the manuscript. No data are withheld or available elsewhere.
Acknowledgments
This publication is dedicated to the memory of our dear co-worker Verónica Fátima Cerviño.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Traffano-Schiffo, M.V.; Castro-Giraldez, M.; Colom, R.J.; Fito, P.J. New spectrophotometric system to segregate tissues in Mandarin fruit. Food Bioprocess Technol. 2018, 11, 399–406. [Google Scholar] [CrossRef]
- Melgar, B.; Pereira, E.; Oliveira, M.B.P.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Sokovic, M.; Barros, L.; Ferreira, I.C.F.R. Extensive profiling of three varieties of Opuntia spp. fruit for innovative food ingredients. Food Res. Int. 2017, 101, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Nawirska-Olszańska, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A. Content of bioactive compounds and antioxidant capacity of pumpkin puree enriched with japanese quince, cornelian cherry, strawberry and apple. Acta Sci. Pol. Technol. Aliment. 2011, 10, 51–60, ISSN 1889-9594. [Google Scholar] [PubMed]
- García-Casal, M.N.; Peña-Rosas, J.P.; Malavé, H.G. Sauces, spices, and condiments: Definitions, potential benefits, consumption patterns, and global markets. Ann. N. Y. Acad. Sci. 2016, 1379, 3–16. [Google Scholar] [CrossRef]
- Baiano, A.; Tamagnone, P.; Marchitelli, V.; Nobile, M.A.D. Quality decay kinetics of semi-preserved sauce as affected by packaging. J. Food Sci. 2005, 70, E92–E97. [Google Scholar] [CrossRef]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2018, 274, 872–885. [Google Scholar] [CrossRef]
- Conti, S.; Villari, G.; Amico, E.; Caruso, G. Effects of production system and transplanting time on yield, quality and antioxidant content of organic winter squash (Cucurbita moschata Duch.). Sci. Hortic. 2015, 183, 136–143. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, J.; Diao, W.; Wang, C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohydr. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef]
- Tamarindo, S.; Pastore, C. Packaging Film Impact on Food Organoleptic Properties: An Experimental Study. J. Appl. Packag. Res. 2016, 8, 78–87. [Google Scholar]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and smart biodegradable packaging based on starch and natural extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef]
- Sapper, M.; Wilcaso, P.; Santamarina, M.P.; Roselló, J.; Chiralt, A. Antifungal and functional properties of starch-gellan films containing thyme (Thymus zygis) essential oil. Food Control 2018, 92, 505–515. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Vargas, M.; Atarés, L.; Chiralt, A. Thermoplastic cassava starch-chitosan bilayer films containing essential oils. Food Hydrocoll. 2018, 75, 107–115. [Google Scholar] [CrossRef]
- Chiralt, A.; González-Martínez, C.; Vargas, M.; Atarés, L. Edible films and coatings from proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2018; pp. 477–500. ISBN 9780081007297. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Warhurst, A.M. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2018, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic contamination of the food chain: A threat to human health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Fasano, E.; Bono-Blay, F.; Cirillo, T.; Montuori, P.; Lacorte, S. Migration of phthalates, alkylphenols, bisphenol A and di (2-ethylhexyl) adipate from food packaging. Food Control 2012, 27, 132–138. [Google Scholar] [CrossRef]
- Petersen, K.; Nielsen, P.V.; Bertelsen, G.; Lawther, M.; Olsen, M.B.; Nilsson, N.H.; Mortensen, G. Potential of biobased materials for food packaging. Trends Food Sci. Technol. 1999, 10, 52–68. [Google Scholar] [CrossRef]
- De Anda-Flores, Y.B.; Cordón-Cardona, B.A.; González-León, A.; Valenzuela-Quintanar, A.I.; Peralta, E.; Soto-Valdez, H. Effect of assay conditions on the migration of phthalates from polyvinyl chloride cling films used for food packaging in Mexico. Food Packag. Shelf 2021, 29, 100684. [Google Scholar] [CrossRef]
- Carlos, K.S.; Dejager, L.S.; Begley, T.H. Investigation of the primary plasticizers present un polyvinyl chloride (PVC) products currently authorized as food contact materials. Food Addit. Contam. 2018, 35, 1214–1222. [Google Scholar] [CrossRef]
- Borzi, F.; Torrieri, E.; Wrona, M.; Nerín, C. Polyamide modified with green tea extract for fresh minced meat active packaging applications. Food Chem. 2019, 300, 125242. [Google Scholar] [CrossRef]
- Pilevar, Z.; Bahrami, A.; Beikzadeh, S.; Hosseini, H.; Seid, M.J. Migration of styrene monomer from polystyrene packaging materials into foods. Characterization and safety evaluation. Trends Food Sci. Technol. 2019, 91, 248–261. [Google Scholar] [CrossRef]
- Gelbke, H.P.; Banton, M.; Block, C.; Dawkins, G.; Eisert, R.; Leibold, E.; Pemberton, I.M.P.; Sakoda, A.; Yasukawa, A. Risk assessment for migration of styrene oligomers into food from polystyrene food containers. Food Chem. Toxicol. 2019, 124, 151–167. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No. 10/2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- Common Market of the South (MERCOSUR); Resolutions of the Common Market Group. MERCOSUR/GMC/RES No. 20/21: Annex: General Provisions for Containers and Plastic Equipment in Contact with Food; GMC (Dec. CMC N° 20/02, Art. 6); Common Market of the South (MERCOSUR): Montevideo, Uruguay, 2021. [Google Scholar]
- Schmid, P.; Welle, F. Chemical migration from beverage packaging materials—A review. Beverages 2020, 6, 37. [Google Scholar] [CrossRef]
- Sosa, C.A.; Sgroppo, S.C.; Bevilacqua, A.E. Physicochemical changes on pumpkin/pepper sauces during refrigerated storage. J. Food Process. Preserv. 2012, 37, 262–268. [Google Scholar] [CrossRef]
- Watson, H.D.; Mead, M.N. Revisiones sobre ciencia y tecnología de los alimentos. In Volumen 2: Migración de Sustancias Químicas Desde el Envase al Alimento, 1st ed.; Acribia Ed: Zaragoza, Spain, 1995; ISBN 9788420007878. [Google Scholar]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef]
- Angelova, Y.; Petkova, S.; Zozikova, E.; Kotseva, E.; Iliev, L. Effects of kinetin and 4PU-30 on the growth and the content of polyphenols in tobacco callus tissue. Bulg. J. Plant Physiol. 2001, 27, 36–42. [Google Scholar]
- Ueda, Y.; Matsuda, Y.; Murata, T.; Hoshi, Y.; Kabata, K.; Ono, M.; Yasuda, S. Increased phenolic content and antioxidant capacity of the heated leaves of yacon (Smallanthus sonchifolius). Biosci. Biotechnol. Biochem. 2019, 83, 2288–2297. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. Lebensm-Wiss Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Davies, B.H.; Matthews, S.; Kirk, J.T.O. The nature and biosynthesis of the carotenoids of different colour varieties of Capsicum annuum. Phytochemistry 1970, 9, 797–805. [Google Scholar] [CrossRef]
- Viña, S.Z.; Chaves, A.R. Antioxidant responses in minimally processed celery during refrigerated storage. Food Chem. 2006, 94, 68–74. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis. In Volume I: Agricultural Chemicals, Contaminants, Dugs, 15th ed.; Association Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990; ISBN 0935584420. [Google Scholar]
- Yun, J.H.; Cha, Y.J.; Lee, D.S. Storage stability and shelf life characteristics of Korean savory sauce products. Prev. Nutr. Food Sci. 2007, 12, 242–250. [Google Scholar] [CrossRef][Green Version]
- Giovanelli, G.; Lavelli, V. Evaluation of heat and oxidative damage during storage of processed tomato products. I. Study of heat damage indices. J. Sci. Food Agric. 2002, 82, 1263–1267. [Google Scholar] [CrossRef]
- Muzzaffar, S.; Babas, W.N.; Nazir, N.; Masoodi, F.A.; Bhat, M.M.; Bazaz, R. Effect of storage on physicochemical, microbial and antioxidant properties of pumpkin (Cucurbita moschata) candy. Cogent Food Agric. 2016, 2, 1163650. [Google Scholar] [CrossRef]
- Coltro, L.; Pitta, J.B.; da Costa, P.A.; Fávaro Perez, M.A.; Aparecida de Araújo, V.; Rodrigues, R. Migration of conventional and new plasticizers from PVC films into food simulants: L A comparative study. Food Control 2014, 44, 118–129. [Google Scholar] [CrossRef]
- Noureddine, T.; Hayette, L.; Chaalal, M. Effect of Time and Temperature Storage on Orange Beverage Stability. EC Nutr. 2017, 11, 48–56. [Google Scholar]
- Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Ježek, D.; Dragović-Uzelac, V. Impact of packaging material and storage conditions on polyphenol stability, colour and sensory characteristics of freeze-dried sour cherry (prunus cerasus var. Marasca). J. Food Sci. Technol.-Mysore 2016, 53, 1247–1258. [Google Scholar] [CrossRef]
- Radovanović, B.; Radovanović, A.; Nikolić, V.; Manojlović, N.; Dimitrijević, J. Storage effect on phenolic content and antioxidant activity in selected fruit extracts. Bulg. Chem. Commun. 2017, 49, 879–883. [Google Scholar]
- Ranđelović, D.; Lazić, V.; Tepić, A.; Mošić, I. The influence of packaging materials protective properties and applying modified atmosphere on packed dried apricot quality changes. Hem. Ind. 2014, 68, 289–295. [Google Scholar] [CrossRef]
- Henríquez, C.; Córdova, A.; Lutz, M.; Saavedra, J. Storage stability test of apple peel powder using two packaging materials: High-density polyethylene and metalized films of high barrier. Ind. Crop. Prod. 2013, 45, 121–127. [Google Scholar] [CrossRef]
- Bakan, A.; Eksi, A. Effect of packaging materials and storage temperature on the quality of sour cherry nectar. Int. J. Food Sci. Technol. 2014, 49, 2425–2432. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Laghi, L.; Castro-Giraldez, M.; Tylewicz, U.; Rocculi, P.; Ragni, L.; Fito, P.J. Osmotic dehydration of organic kiwifruit pre-treated by pulsed electric fields and monitored by NMR. Food Chem. 2017, 236, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. J. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Kim, J.B.; Park, J.S.; Lee, S.W.; Cho, K.J. A comparison of the carotenoid accumulation in Capsicum varieties that show different ripening colours: Deletion of the capsanthin-capsorubin synthase gene is not a prerequisite for the formation of a yellow pepper. J. Exp. Bot. 2007, 58, 3135–3144. [Google Scholar] [CrossRef] [PubMed]
- Montesano, D.; Rocchetti, G.; Putnik, P.; Lucini, L. Bioactive profile of pumpkin: An overview on terpenoids and their health-promoting properties. Curr. Opin. Food Sci. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Provesi, J.G.; Dias, C.O.; Amante, E.R. Changes in carotenoids during processing and storage of pumpkin puree. Food Chem. 2011, 128, 195–202. [Google Scholar] [CrossRef]
- Provesi, J.G.; Amante, E.R. Carotenoids in pumpkin and impact of processing treatments and storage. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: London, UK, 2015; pp. 71–80. [Google Scholar] [CrossRef]
- Song, J.; Wei, Q.; Wang, X.; Li, D.; Liu, C.; Zhang, M.; Meng, L. Degradation of carotenoids in dehydrated pumpkins as affected by different storage conditions. Food Res. Int. 2018, 107, 130–136. [Google Scholar] [CrossRef]
- Castro-López, C.; Sánchez-Alejo, E.J.; Saucedo-Pompa, S.; Rojas, R.; Aranda-Ruiz, J.; Martínez-Avila, G.C.G. Fluctuations in phenolic content, ascorbic acid and total carotenoids and antioxidant activity of fruit beverages during storage. Heliyon 2016, 2, e00152. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Wang, Y.; Li, J.; Yang, Y.; Liu, X. The Effects of Storage Conditions on Lycopene Content and Color of Tomato Hot Pot Sauce. Int. J. Anal. Chem. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2000, 11, 340–346. [Google Scholar] [CrossRef]
- Gliemmo, M.F.; Campos, C.A.; Gerschenson, L.N. Interaction between potassium sorbate and aspartame in aqueous model sugar systems. J. Food Sci. 2001, 66, 428–431. [Google Scholar] [CrossRef]
- Gliemmo, M.F.; Latorre, M.E.; Gerschenson, L.N.; Campos, C.A. Color stability of pumpkin (Cucurbita moschata, Duchesne ex Poiret) puree during storage at room temperature: Effect of pH, potassium sorbate, ascorbic acid and packaging material. LWT 2009, 42, 196–201. [Google Scholar] [CrossRef]
- Sánchez, A.H.; Casado, F.J.; Beato, V.M.; de Castro, A.; Montano, A. Chemical and colour changes related to the use of sorbates and ascorbic acid in pickled cucumbers and caperberries during long-term storage. Int. J. Food Sci. Technol. 2013, 48, 179–186. [Google Scholar] [CrossRef]
- Kohan-nia, N.; Pakbin, B.; Mahmoudi, R.; Fakhri, O. Effect of packaging material on color properties of catsup tomato sauce. J. Appl. Packag. Res. 2016, 8, 10–16. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).