Chemical Model for Thermal Treatment of Sewage Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Numerical Model
2.2. Chemical Model Development
2.2.1. Selection of the Surrogate Species
2.2.2. Reaction Scheme of the Surrogate Species
2.2.3. Formulation of the Sewage Sludge Surrogates
3. Results and Discussion
3.1. Representation of Sewage Sludge
3.2. Thermogravimetric Analysis
3.3. Producer Gas Composition
3.4. Emission Release Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Directive 2008⁄98⁄EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives. Off. J. Eur. Union 2008, 51, 1–45. Available online: https://www.legislation.gov.uk/eudr/2008/98/contents# (accessed on 1 January 2022).
- Meng, X.; Huang, Q.; Xu, J.; Gao, H.; Yan, J. A review of phosphorus recovery from different thermal treatment products of sewage sludge. Waste Dispos. Sustain. Energy 2019, 1, 99–115. [Google Scholar] [CrossRef] [Green Version]
- Adar, E.; Ince, M.; Bilgili, M.S. Supercritical water gasification of sewage sludge by continuous flow tubular reactor: A pilot scale study. Chem. Eng. J. 2020, 391, 123499. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Stasta, P.; Boran, J.; Bebar, L.; Stehlik, P.; Oral, J. Thermal processing of sewage sludge. Appl. Therm. Eng. 2006, 26, 1420–1426. [Google Scholar] [CrossRef]
- Tsybina, A.; Wuensch, C. Analysis of Sewage Sludge Thermal Treatment Methods in the Context of Circular Economy. Detritus 2018, 2, 3–15. [Google Scholar] [CrossRef]
- Eriksson, E.; Christensen, N.; Ejbye Schmidt, J.; Ledin, A. Potential priority pollutants in sewage sludge. Desalination 2008, 226, 371–388. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, M.; Srivastava, S. Recent Advances in Wastewater Sludge Valorization. In Bio-valorization of Waste: Trends and Perspectives; Shah, S., Venkatramanan, V., Prasad, R., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2021; Chapter 10; pp. 225–247. [Google Scholar]
- Houillon, G.; Jolliet, O. Life cycle assessment of processes for the treatment of wastewater urban sludge: Energy and global warming analysis. J. Clean. Prod. 2005, 13, 287–299. [Google Scholar] [CrossRef]
- Freda, C.; Cornacchia, G.; Romanelli, A.; Valerio, V.; Grieco, M. Sewage sludge gasification in a bench scale rotary kiln. Fuel 2018, 212, 88–94. [Google Scholar] [CrossRef]
- Werle, S. Gasification of a Dried Sewage Sludge in a Laboratory Scale Fixed Bed Reactor. Energies 2015, 8, 8562–8572. [Google Scholar] [CrossRef] [Green Version]
- Uggetti, E.; Llorens, E.; Pedescoll, A.; Ferrer, I.; Castellnou, R.; García, J. Sludge dewatering and stabilization in drying reed beds: Characterization of three full-scale systems in Catalonia, Spain. Bioresour. Technol. 2009, 100, 3882–3890. [Google Scholar] [CrossRef]
- Channiwala, S.; Parikh, P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef] [Green Version]
- Sørum, L. Characterisation of MSW for Combustion Systems; SINTEF Energy Research: Trondheim, Norway, 2001. [Google Scholar]
- Speth, K.; Murer, M.; Spliethoff, H. Experimental Investigation of Nitrogen Species Distribution in Wood Combustion and Their Influence on NOx Reduction by Combining Air Staging and Ammonia Injection. Energy Fuels 2016, 30, 5816–5824. [Google Scholar] [CrossRef]
- Maffei, T.; Sommariva, S.; Ranzi, E.; Faravelli, T. A predictive kinetic model of sulfur release from coal. Fuel 2012, 91, 213–223. [Google Scholar] [CrossRef]
- Bøjer, M.; Jensen, P.; Dam-Johansen, K.; Madsen, O.; Lundtorp, K. Release of Corrosive Species above the Grate in a Waste Boiler and the Implication for Improved Electrical Efficiency. Energy Fuels 2010, 24, 5696–5707. [Google Scholar] [CrossRef] [Green Version]
- Seggiani, M.; Vitolo, S.; Puccini, M.; Bellini, A. Cogasification of sewage sludge in an updraft gasifier. Fuel 2012, 93, 486–491. [Google Scholar] [CrossRef]
- Kääntee, U.; Zevenhoven, R.; Backman, R.; Hupa, M. Cement manufacturing using alternative fuels and the advantages of process modelling. Fuel Process. Technol. 2004, 85, 293–301. [Google Scholar] [CrossRef]
- Zheng, J.; Jin, Y.Q.; Chi, Y.; Wen, J.M.G.; Jiang, X.G.; Ni, M.J. Pyrolysis characteristics of organic components of municipal solid waste at high heating rates. Waste Manag. 2009, 29, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Sørum, L.; Grønli, M.; Hustad, J. Pyrolysis characteristics and kinetics of municipal solid wastes. Fuel 2001, 80, 1217–1227. [Google Scholar] [CrossRef]
- Singh, S.; Wu, C.; Williams, P.T. Pyrolysis of waste materials using TGA-MS and TGA-FTIR as complementary characterisation techniques. J. Anal. Appl. Pyrolysis 2012, 94, 99–107. [Google Scholar] [CrossRef]
- Grammelis, P.; Basinas, P.; Malliopoulou, A.; Sakellaropoulos, G. Pyrolysis kinetics and combustion characteristics of waste recovered fuels. Fuel 2009, 88, 195–205. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, Z.; Fang, S.; Lin, Y.; Lin, Y.; Liao, Y.; Ma, X. Investigation on the co-combustion of oil shale and municipal solid waste by using thermogravimetric analysis. Energy Convers. Manag. 2016, 117, 367–374. [Google Scholar] [CrossRef]
- Fang, S.; Yu, Z.; Lin, Y.; Hu, S.; Liao, Y.; Ma, X. Thermogravimetric analysis of the co-pyrolysis of paper sludge and municipal solid waste. Energy Convers. Manag. 2015, 101, 626–631. [Google Scholar] [CrossRef]
- Font, R.; Marcilla, A.; García, A.; Caballero, J.; Conesa, J. Kinetic models for the thermal degradation of heterogeneous materials. J. Anal. Appl. Pyrolysis 1995, 32, 29–39. [Google Scholar] [CrossRef]
- Seo, M.W.; Kim, S.D.; Lee, S.H.; Lee, J.G. Pyrolysis characteristics of coal and RDF blends in non-isothermal and isothermal conditions. J. Anal. Appl. Pyrolysis 2010, 88, 160–167. [Google Scholar] [CrossRef]
- Yao, Z.; Yu, S.; Su, W.; Wu, W.; Tang, J.; Qi, W. Kinetic studies on the pyrolysis of plastic waste using a combination of model-fitting and model-free methods. Waste Manag. Res. 2020, 38, 77–85. [Google Scholar] [CrossRef]
- Alves, J.L.F.; Da Silva, J.C.G.; Di Domenico, M.; Galdino, W.V.D.A.; Andersen, S.L.F.; Alves, R.F.; De Sena, R.F. Exploring Açaí Seed (Euterpe oleracea) Pyrolysis Using Multi-component Kinetics and Thermodynamics Assessment Towards Its Bioenergy Potential. BioEnergy Res. 2021, 14, 209–225. [Google Scholar] [CrossRef]
- Netzer, C.; Li, T.; Løvås, T. Surrogate Reaction Mechanism for Waste Incineration and Pollutant Formation. Energy Fuels 2021, 35, 7030–7049. [Google Scholar] [CrossRef]
- Ranzi, E.; Pierucci, S.; Aliprandi, P.; Stringa, S. Comprehensive and Detailed Kinetic Model of a Traveling Grate Combustor of Biomass. Energy Fuels 2011, 25, 4195–4205. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Mehrabian-Bardar, R.; Scharler, R.; Obernberger, I. Kinetic scheme of biomass pyrolysis considering secondary charring reactions. Energy Convers. Manag. 2014, 87, 687–696. [Google Scholar] [CrossRef]
- Sommariva, S.; Maffei, T.; Migliavacca, G.; Faravelli, T.; Ranzi, E. A predictive multi-step kinetic model of coal devolatilization. Fuel 2010, 89, 318–328. [Google Scholar] [CrossRef]
- Ranzi, E.; Cuoci, A.; Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Pierucci, S.; Sommariva, S. Chemical Kinetics of Biomass Pyrolysis. Energy Fuels 2008, 22, 4292–4300. [Google Scholar] [CrossRef]
- Debiagi, P.; Pecchi, C.; Gentile, G.; Frassoldati, A.; Cuoci, A.; Faravelli, T.; Ranzi, E. Extractives Extend the Applicability of Multistep Kinetic Scheme of Biomass Pyrolysis. Energy Fuels 2015, 29, 6544–6555. [Google Scholar] [CrossRef]
- Debiagi, P.E.A.; Trinchera, M.; Frassoldati, A.; Faravelli, T.; Vinu, R.; Ranzi, E. Algae characterization and multistep pyrolysis mechanism. J. Anal. Appl. Pyrolysis 2017, 128, 423–436. [Google Scholar] [CrossRef]
- Debiagi, P.; Gentile, G.; Cuoci, A.; Frassoldati, A.; Ranzi, E.; Faravelli, T. A predictive model of biochar formation and characterization. J. Anal. Appl. Pyrolysis 2018, 134, 326–335. [Google Scholar] [CrossRef]
- Žnidarčič, A.; Katrašnik, T.; Zsély, I.; Nagy, T.; Seljak, T. Sewage sludge combustion model with reduced chemical kinetics mechanisms. Energy Convers. Manag. 2021, 236, 114073. [Google Scholar] [CrossRef]
- LOGEresearch v1.10, LOGE AB. 2018. Available online: http://www.logesoft.com (accessed on 1 December 2021).
- Weber, K.; Li, T.; Løvås, T.; Perlman, C.; Seidel, L.; Mauss, F. Stochastic reactor modeling of biomass pyrolysis and gasification. J. Anal. Appl. Pyrolysis 2017, 124, 592–601. [Google Scholar] [CrossRef] [Green Version]
- Netzer, C.; Li, T.; Seidel, L.; Mauß, F.; Løvås, T. Stochastic Reactor-Based Fuel Bed Model for Grate Furnaces. Energy Fuels 2020, 34, 16599–16612. [Google Scholar] [CrossRef]
- Francisca Gómez-Rico, M.; Font, R.; Fullana, A.; Martín-Gullón, I. Thermogravimetric study of different sewage sludges and their relationship with the nitrogen content. J. Anal. Appl. Pyrolysis 2005, 74, 421–428. [Google Scholar] [CrossRef]
- Shao, L.; Fan, S.; Zhang, H.; Yao, Q.; He, P. SO2 and NOx emissions from sludge combustion in a CO2/O2 atmosphere. Fuel 2013, 109, 178–183. [Google Scholar] [CrossRef]
- Van Doorn, J.; Bruyn, P.; Vermeij, P. Combined combustion of biomass, municipal sewage sludge and coal in an atmospheric fluidized bed installation. In Biomass for Energy and the Environment: Proceedings of the 9th European Bioenergy Conference; Chartier, P., Ed.; Elsevier: Oxford, UK, 1996; Volume 2, pp. 1007–1012. [Google Scholar]
- Van Daalen, W.; van Doorn, J. Brandstoffen uit Reststromen voor Circulerend Wervelbedvergassing; NV Afvalzorg Noord-Holland: Haarlem, The Netherlands, 1997; 36p. [Google Scholar]
- Font, R.; Fullana, A.; Conesa, J.; Llavador, F. Analysis of the pyrolysis and combustion of different sewage sludges by TG. J. Anal. Appl. Pyrolysis 2001, 58–59, 927–941. [Google Scholar] [CrossRef]
- Owczarek, C.; Krautz, H.J.; Griebe, S. Cycloid combustion—A new concept for industrial combustion with low-calorific fuels. In Proceedings of the 5th European Conference on Industrial Furnaces and Boilers, Espinho-Porto, Portugal, 11–14 April 2000; Volume 2, pp. 25–34. [Google Scholar]
- Storm, C.; Spliethoff, H.; Hein, K.R. Generation of a gaseous fuel by gasification or pyrolysis of biomass for use as reburn gas in coal-fired boilers. In Proceedings of the 5th European Conference on Industrial Furnaces and Boilers, Espinho-Porto, Portugal, 11–14 April 2000; Volume 1, pp. 689–699. [Google Scholar]
- Hein, K.R.G. Combined Combustion of Biomass/Sewage Sludge and Coals; Clean Coal Technology Programme 1992–1994, Stuttgart, IVD; Institute for Process Engineering and Power Plant Technology: Stuttgart, Germany, 1994; ISBN 3-928123-16-5. [Google Scholar]
- Inguanzo, M.; Menéndez, J.; Fuente, E.; Pis, J. Reactivity of pyrolyzed sewage sludge in air and CO2. J. Anal. Appl. Pyrolysis 2001, 58–59, 943–954. [Google Scholar] [CrossRef]
- Lumley, N.P.; Ramey, D.F.; Prieto, A.L.; Braun, R.J.; Cath, T.Y.; Porter, J.M. Techno-economic analysis of wastewater sludge gasification: A decentralized urban perspective. Bioresour. Technol. 2014, 161, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Alzueta, M.U.; Tena, A.; Bilbao, R. Pyridine conversion in a flow reactor and its interaction with nitric oxide. Combust. Sci. Technol. 2002, 174, 151–169. [Google Scholar] [CrossRef]
- Wu, L.; Tian, Z.; Weng, J.; Yu, D.; Liu, Y.; Tian, D.; Cao, C.; Zou, J.; Zhang, Y.; Yang, J. Experimental and kinetic study on the low-temperature oxidation of pyridine as a representative of fuel-N compounds. Combust. Flame 2019, 202, 394–404. [Google Scholar] [CrossRef]
- Cuoci, A.; Faravelli, T.; Frassoldati, A.; Granata, S.; Migliavacca, G.; Pierucci, S.; Ranzi, E.; Sommariva, S. A General Mathematical Model of Biomass Devolatilization. Note 2. Detailed Kinetics of Volatile Species. In Proceedings of the 30th Meeting of The Italian Section of The Combustion Institute, Naples, Italy, 20–23 June 2007; p. 8. [Google Scholar]
- Netzer, C.; Li, T.; Løvås, T. Nitrogen Oxide Prediction within a Woody Biomass Fuel Bed Using Detailed Chemistry. In Proceedings of the European Combustion Meeting, Naples, Italy, 14–15 April 2021; p. 171. [Google Scholar]
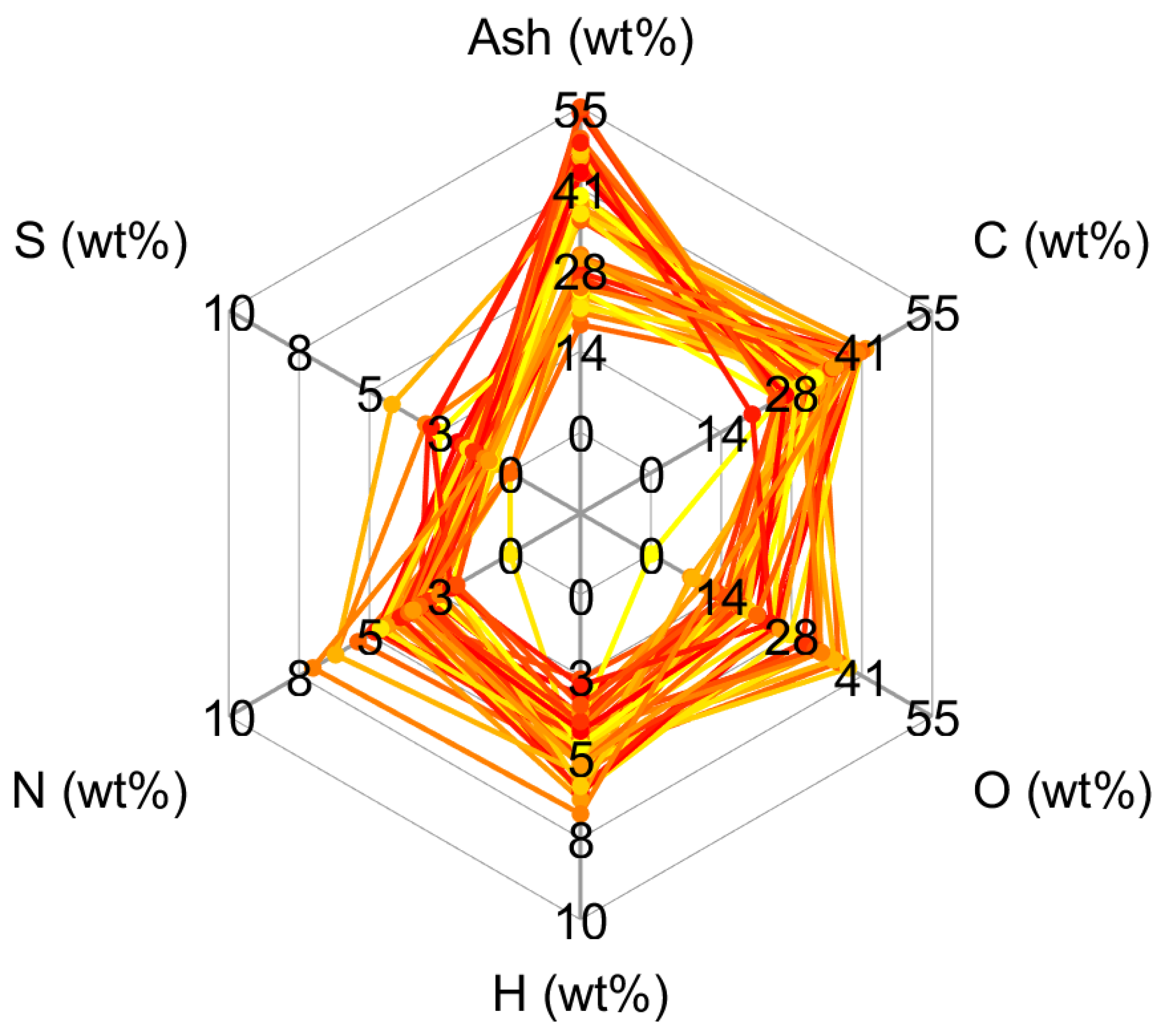


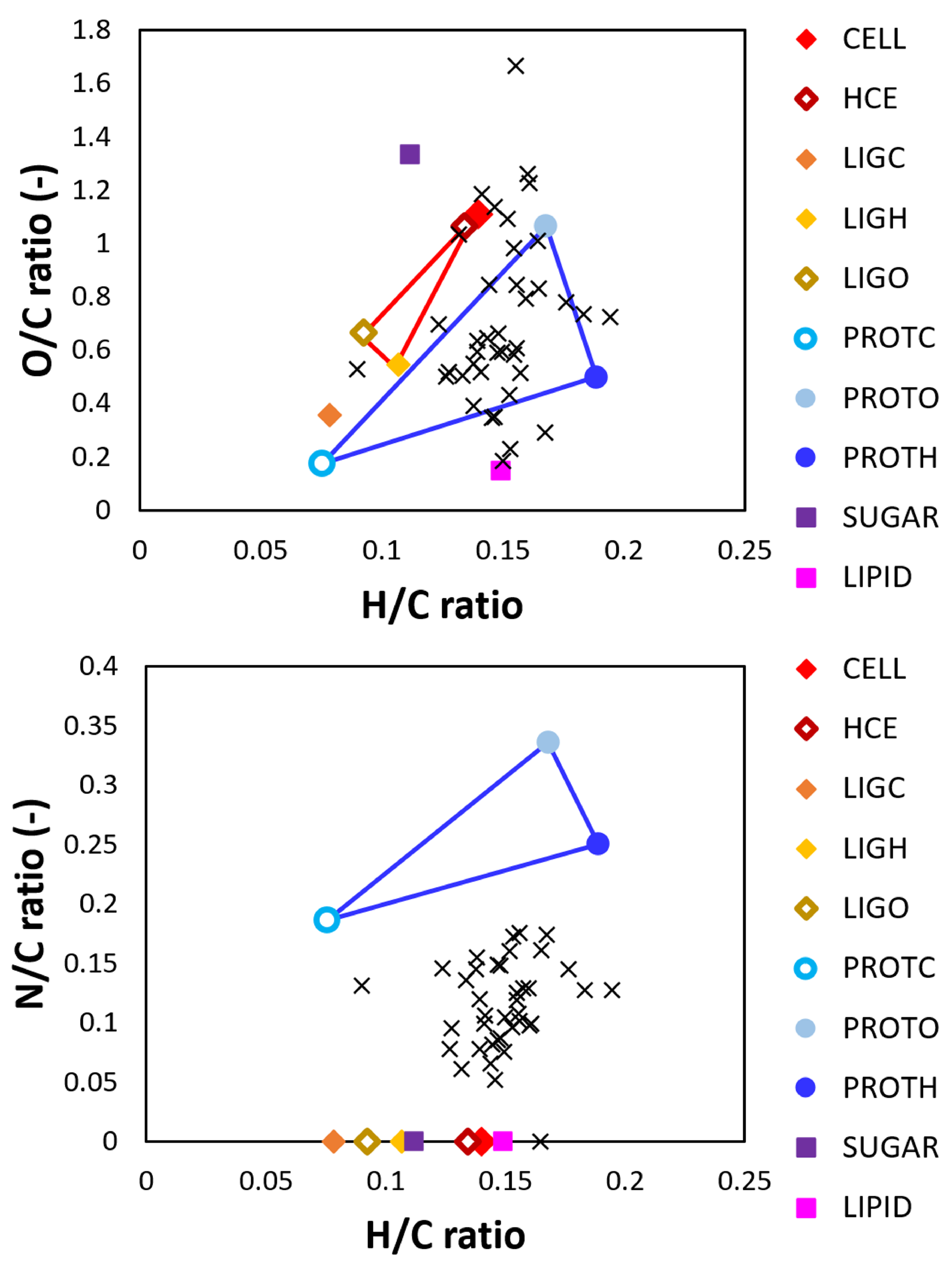



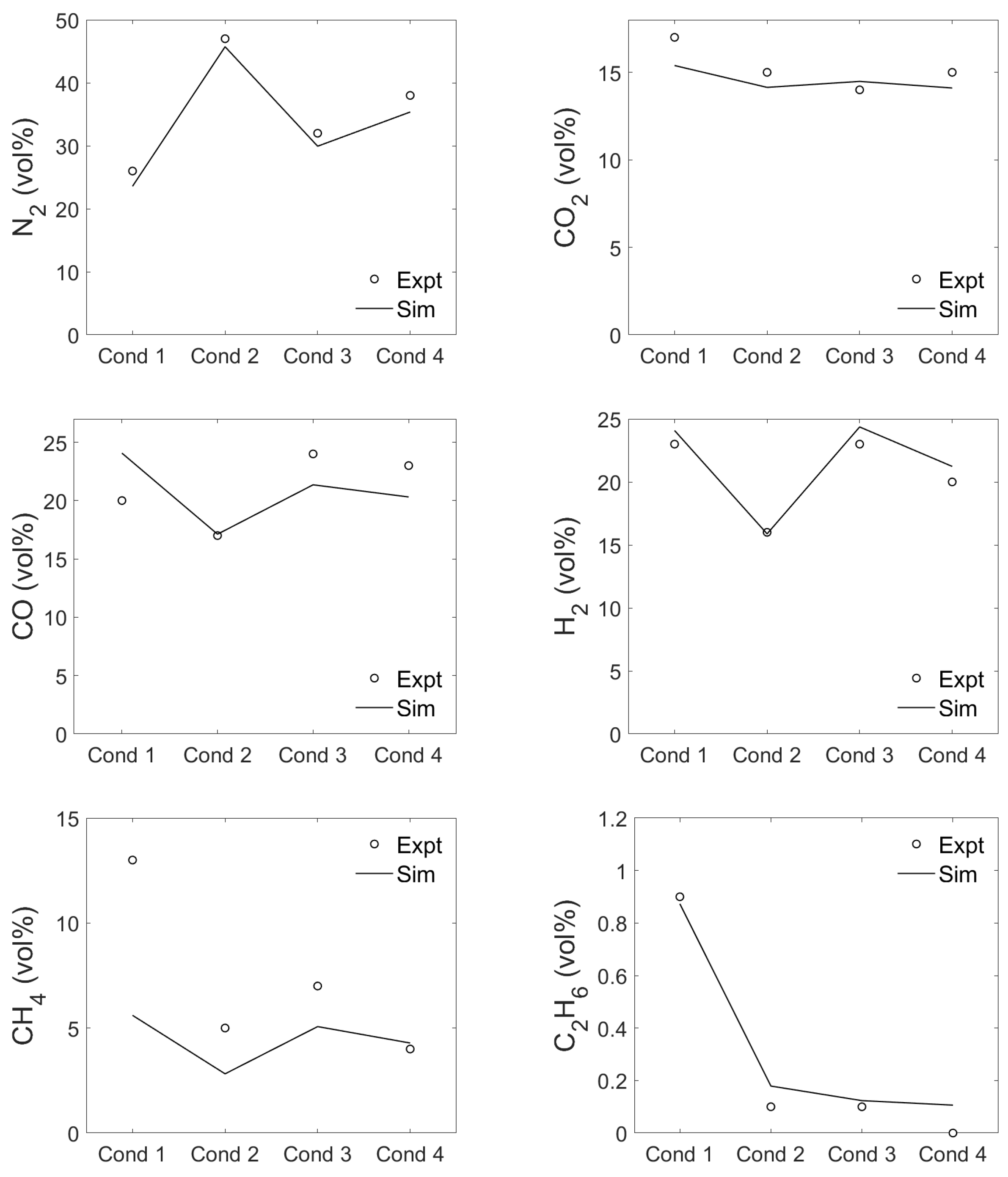


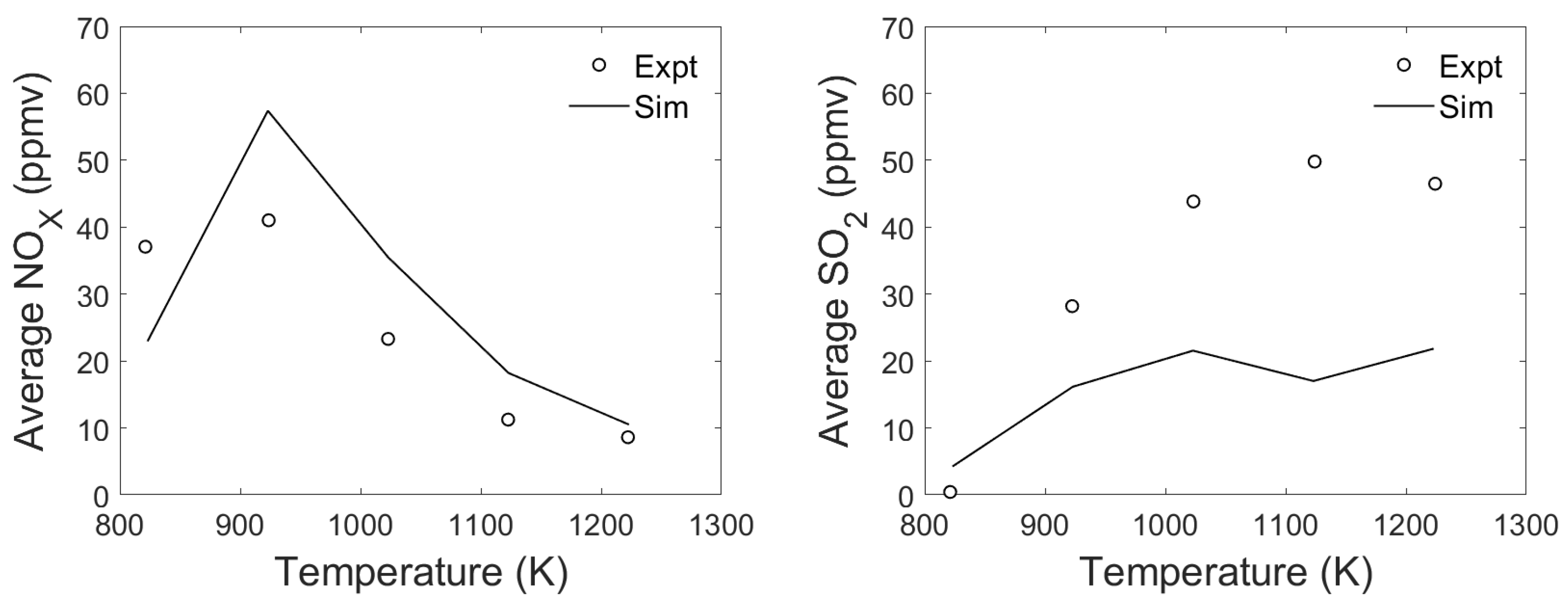
| Surrogate | Trivial Name | Elementary Composition | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Species | C | H | O | N | S | Si | ||
| CELL | cellulose | 6 | 10 | 5 | 0 | 0 | 0 | [32] |
| HCE | hemi-cellulose | 5 | 8 | 4 | 0 | 0 | 0 | [32] |
| LIG | lignin rich in C | 15 | 14 | 4 | 0 | 0 | 0 | [32] |
| LIG | lignin rich in H | 22 | 28 | 9 | 0 | 0 | 0 | [32] |
| LIG | lignin rich in O | 20 | 22 | 10 | 0 | 0 | 0 | [32] |
| SUGAR | sugar | 6 | 8 | 6 | 0 | 0 | 0 | [37], (*) |
| LIPID | lipid | 18 | 32 | 2 | 0 | 0 | 0 | [37], (*) |
| PROT | protein rich in H | 400 | 900 | 150 | 86 | 0 | 0 | [31,37] |
| PROT | protein rich in C | 500 | 450 | 65 | 80 | 0 | 0 | [37], (*) |
| PROT | protein rich in O | 250 | 500 | 200 | 72 | 0 | 0 | [37], (*) |
| NHI | product gas | 0 | 3 | 0 | 1 | 0 | 0 | [31] |
| inorganic nitrogen | ||||||||
| COI | product gas | 1 | 0 | 2 | 0 | 0 | 0 | [37] |
| inorganic carbon | ||||||||
| (HS SO COS)I | product gas | 1 | 2 | 3 | 0 | 3 | 0 | [31] |
| inorganic sulfur | ||||||||
| HO(S) | moisture content | 0 | 2 | 1 | 0 | 0 | 0 | [32] |
| ASH | ash | 0 | 0 | 0 | 0 | 0 | 1 | [32] |
| No. | Reaction | A (1/s) | n (-) | Ea (kcal/kmol) |
|---|---|---|---|---|
| 16 | PROT →2.5PROT + 0.6PROT + 25NH + 10NO + HCN + CHN + CHN + CHO + 12.75CH + 37.3CO + 7.7CO + 71HO | 1.00 × 10 | 0.0 | 15,500.0 |
| 17 | PROT →5PROT+ 27NH + 7.5HCN + 0.5NO + 0.5CHN + 0.5CHN + 0.5CHO + 20.75G{H} + 21.5CH + 70CH + 23.5HO | 1.00 × 10 | 0.0 | 15,500.0 |
| 18 | PROT →4.5PROT + 8HCN + 2NH + 0.5CHN + 0.5CHN + 0.5CHO + 29CO + 1.5NO + 23.5CH + 5CO + 10HO | 1.00 × 10 | 0.0 | 15,500.0 |
| 19 | PROT →27.5char + 2char + 0.5HCN + 0.5NO + 0.5CHN + 0.5CHN + 0.5CHO + 1.083333CH + 3G{NH} + 3G{HCN} + 1.5G{CO} + 22HO | 1.00 × 10 | 0.0 | 15,500.0 |
| 20 | PROT →42.75char + 2char + 0.5HCN + 0.5NO + 0.5CHN + 0.5CHN + 0.5CHO + 3.5CH + NH + 10G{HCN} + 3.375CH + 3CH + 1.5HO | 1.00 × 10 | 0.0 | 15,500.0 |
| 21 | SUGAR →0.47SUGAR + 0.53SUGAR | 8.00 × 10 | 0.0 | 26,000.0 |
| 22 | SUGAR →0.68CHO4 + 0.48HO + 1.2CO + 0.2CHO + 0.4G{CO} + 0.4HO | 1.50 × 10 | 0.0 | 16,000.0 |
| 23 | SUGAR →1.6char + 0.25G{CH} + 0.1G{CH} + 0.73G{COH} + 0.62G{CO} + 1.3G{CO} + 0.88HO + 0.26CHOH + 0.13CHOH + 0.39CHCOOH | 2.00 × 10 | 0.0 | 20,000.0 |
| 24 | LIPID →0.75CHCOOH + 3CH + 2.25CH + 0.75CH + 0.25HLIPID | 8.00 × 10 | 0.0 | 18,000.0 |
| 25 | HLIPID →9char + 2G{COH} + 6G{H} + 3G{CH} + G{CH} | 7.00 × 10 | 0.0 | 49,700.0 |
| 26 | NHI →NH | 2.50 × 10 | 0.0 | 27,800.0 |
| 27 | (HSSOCOS)I →(HSCOS)I + SO | 1.00 × 10 | 0.0 | 41,800 |
| 28 | (HSCOS)I →HS + COS | 1.00 × 10 | 0.0 | 25,100 |
| 29 | COI →CO | 1.00 × 10 | 0.0 | 38,000.0 |
| Sewage Sludge | Surrogate | Error in % | Absolut Deviation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | C | H | N | S | C | H | N | S | C | H | N | S | C | H | N | S |
| 1 | 41.2 | 3.6 | 6.1 | 0.83 | 40.8 | 3.7 | 5.7 | 0.80 | 1.1 | 2.8 | 5.8 | 3.35 | 0.4 | 0.1 | 0.4 | 0.03 |
| 2 | 25.1 | 2.7 | 3.9 | 2.50 | 25.1 | 2.7 | 3.9 | 2.50 | 0.2 | 0.0 | 0.0 | 0.08 | 0.0 | 0.0 | 0.0 | 0.00 |
| 3 | 40.8 | 3.1 | 6.1 | 0.71 | 40.3 | 3.3 | 5.5 | 0.66 | 1.3 | 5.6 | 10.2 | 6.72 | 0.5 | 0.2 | 0.6 | 0.05 |
| 4 | 38.5 | 3.9 | 6.0 | 1.20 | 38.2 | 4.0 | 5.6 | 1.17 | 0.8 | 2.5 | 5.9 | 2.26 | 0.3 | 0.1 | 0.4 | 0.03 |
| 5 | 35.3 | 2.9 | 5.1 | 0.90 | 34.9 | 3.0 | 4.8 | 0.88 | 1.2 | 2.9 | 5.8 | 2.20 | 0.4 | 0.1 | 0.3 | 0.02 |
| 6 | 31.1 | 1.9 | 4.1 | 0.71 | 30.8 | 1.9 | 4.1 | 0.71 | 1.0 | 0.1 | 0.2 | 0.28 | 0.3 | 0.0 | 0.0 | 0.00 |
| 7 | 37.3 | 3.6 | 5.7 | 1.30 | 36.9 | 3.7 | 5.3 | 1.27 | 1.0 | 2.8 | 6.6 | 1.95 | 0.4 | 0.1 | 0.4 | 0.03 |
| 8 | 29.3 | 2.5 | 4.3 | 0.56 | 29.2 | 2.5 | 4.3 | 0.56 | 0.3 | 0.0 | 0.1 | 0.12 | 0.1 | 0.0 | 0.0 | 0.00 |
| 9 | 42.0 | 4.4 | 6.3 | 1.50 | 41.4 | 4.4 | 5.7 | 1.32 | 1.5 | 1.1 | 9.0 | 11.71 | 0.6 | 0.0 | 0.6 | 0.18 |
| 10 | 35.9 | 6.2 | 5.5 | 4.20 | 35.7 | 6.2 | 5.4 | 4.17 | 0.6 | 0.2 | 1.7 | 0.73 | 0.2 | 0.0 | 0.1 | 0.03 |
| 11 | 40.5 | 2.1 | 5.9 | 0.52 | 40.2 | 2.2 | 5.5 | 0.49 | 0.8 | 5.1 | 6.4 | 5.10 | 0.3 | 0.1 | 0.4 | 0.03 |
| 12 | 28.5 | 4.6 | 4.7 | 1.50 | 28.4 | 4.6 | 4.7 | 1.50 | 0.2 | 0.0 | 0.0 | 0.04 | 0.1 | 0.0 | 0.0 | 0.00 |
| 13 | 32.0 | 2.1 | 4.6 | 0.69 | 31.7 | 2.2 | 4.4 | 0.68 | 0.8 | 2.4 | 3.7 | 2.13 | 0.3 | 0.1 | 0.2 | 0.01 |
| 14 | 26.3 | 3.4 | 4.2 | 1.20 | 26.2 | 3.4 | 4.2 | 1.20 | 0.2 | 0.0 | 0.0 | 0.13 | 0.1 | 0.0 | 0.0 | 0.00 |
| 15 | 19.8 | 2.1 | 2.8 | 2.80 | 19.7 | 2.1 | 2.8 | 2.80 | 0.7 | 0.1 | 0.1 | 0.04 | 0.1 | 0.0 | 0.0 | 0.00 |
| 16 | 24.8 | 3.2 | 3.9 | 1.30 | 24.7 | 3.2 | 3.9 | 1.30 | 0.4 | 0.1 | 0.1 | 0.04 | 0.1 | 0.0 | 0.0 | 0.00 |
| A | 41.2 | 5.2 | 3.2 | 0.00 | 40.9 | 5.2 | 3.2 | 0.00 | 0.7 | 0.2 | 0.0 | 0.00 | 0.3 | 0.0 | 0.0 | 0.00 |
| B | 40.3 | 6.8 | 7.0 | 0.90 | 39.9 | 5.2 | 6.4 | 0.91 | 1.0 | 22.6 | 8.5 | 1.0 | 0.4 | 1.5 | 0.6 | 0.01 |
| ID | Fuel Feed | Airflow Rate | Temperature | |
|---|---|---|---|---|
| (g/h) | Nl/min | (K) | (-) | |
| Cond 1 | 261 | 1 | 1023 | 0.05 |
| Cond 2 | 171 | 2 | 1073 | 0.15 |
| Cond 3 | 237 | 3 | 1123 | 0.16 |
| Cond 4 | 244 | 4.5 | 1123 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netzer, C.; Løvås, T. Chemical Model for Thermal Treatment of Sewage Sludge. ChemEngineering 2022, 6, 16. https://doi.org/10.3390/chemengineering6010016
Netzer C, Løvås T. Chemical Model for Thermal Treatment of Sewage Sludge. ChemEngineering. 2022; 6(1):16. https://doi.org/10.3390/chemengineering6010016
Chicago/Turabian StyleNetzer, Corinna, and Terese Løvås. 2022. "Chemical Model for Thermal Treatment of Sewage Sludge" ChemEngineering 6, no. 1: 16. https://doi.org/10.3390/chemengineering6010016
APA StyleNetzer, C., & Løvås, T. (2022). Chemical Model for Thermal Treatment of Sewage Sludge. ChemEngineering, 6(1), 16. https://doi.org/10.3390/chemengineering6010016







