Performance Evaluation of a Small-Scale Latent Heat Thermal Energy Storage Unit for Heating Applications Based on a Nanocomposite Organic PCM
Abstract
:1. Introduction
2. Experimental Approach
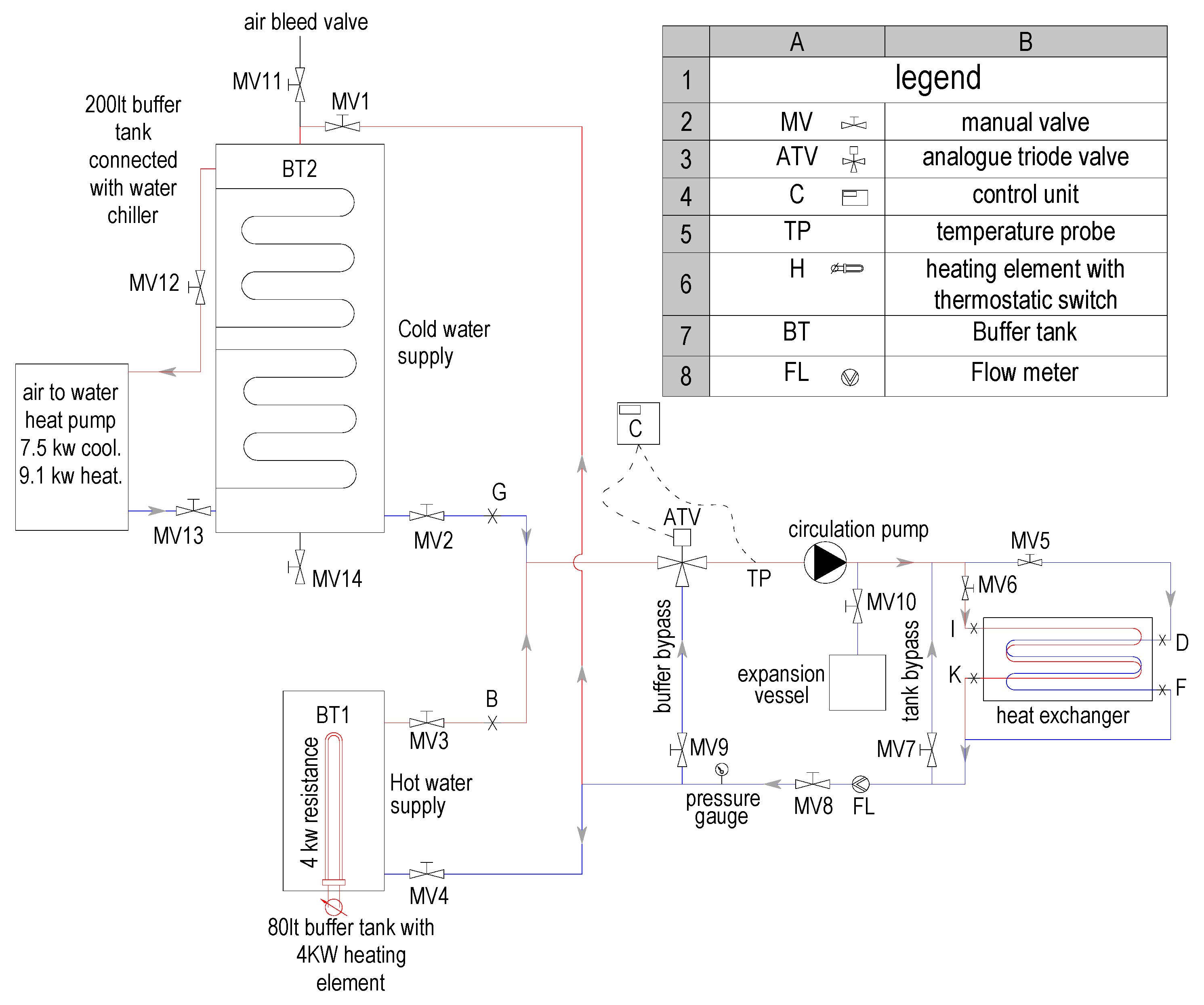
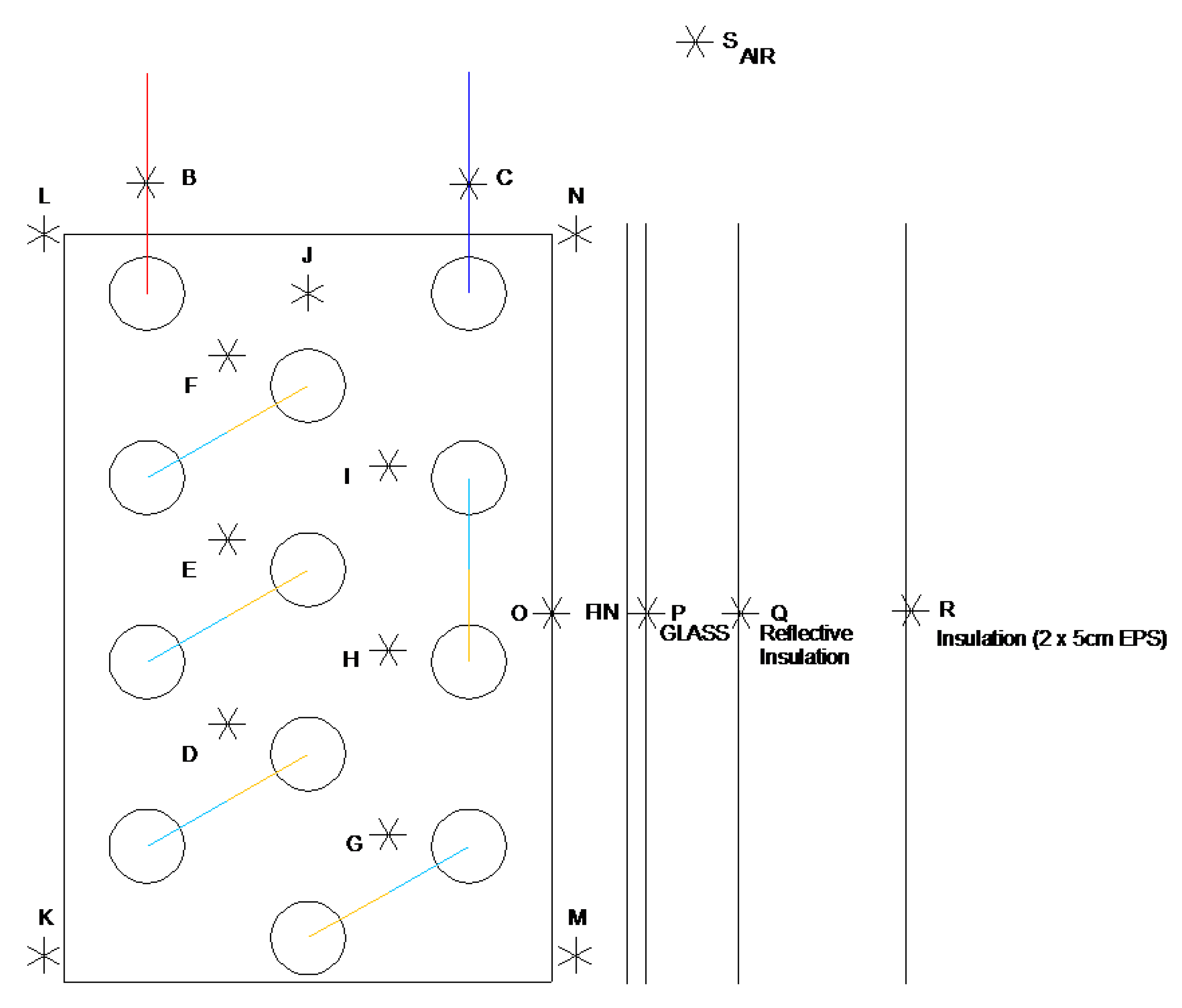
2.1. Description of the LHTES Unit
2.2. Organic PCM Properties, Experimental Protocol, and Conditions
2.3. Repeatability and Error Analysis
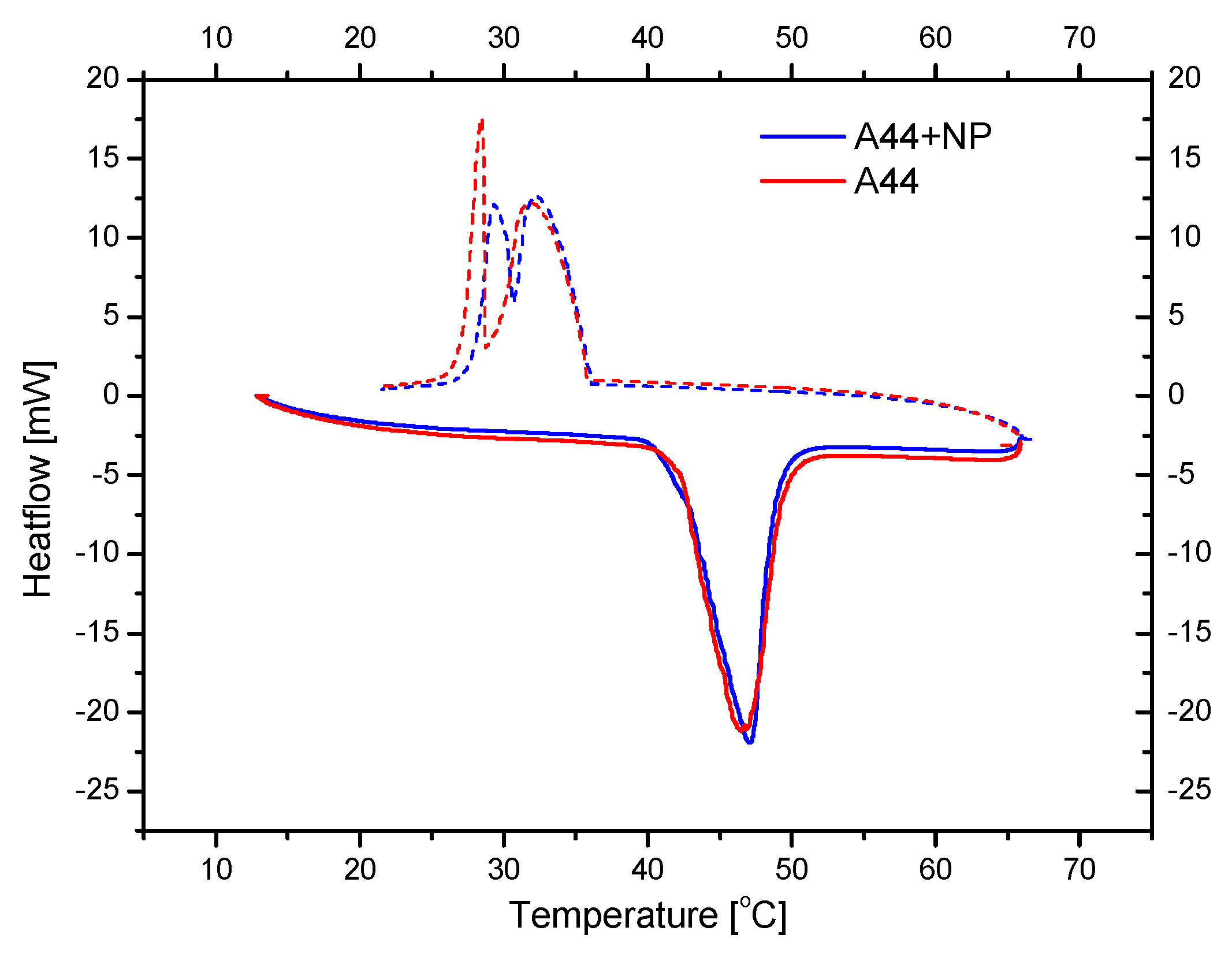
2.4. Nano-Enhancement of Organic Paraffin
3. Results and Discussion
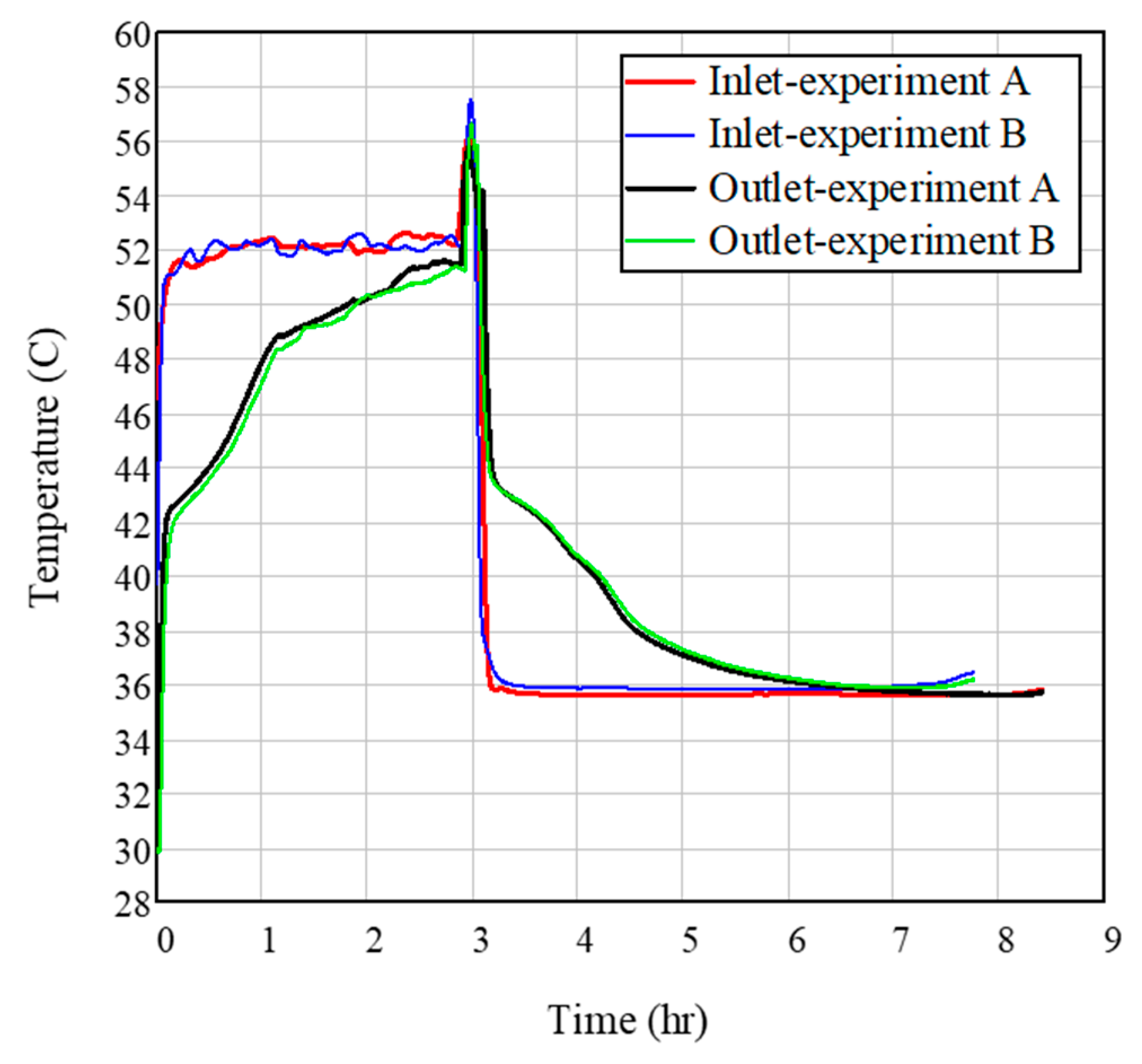
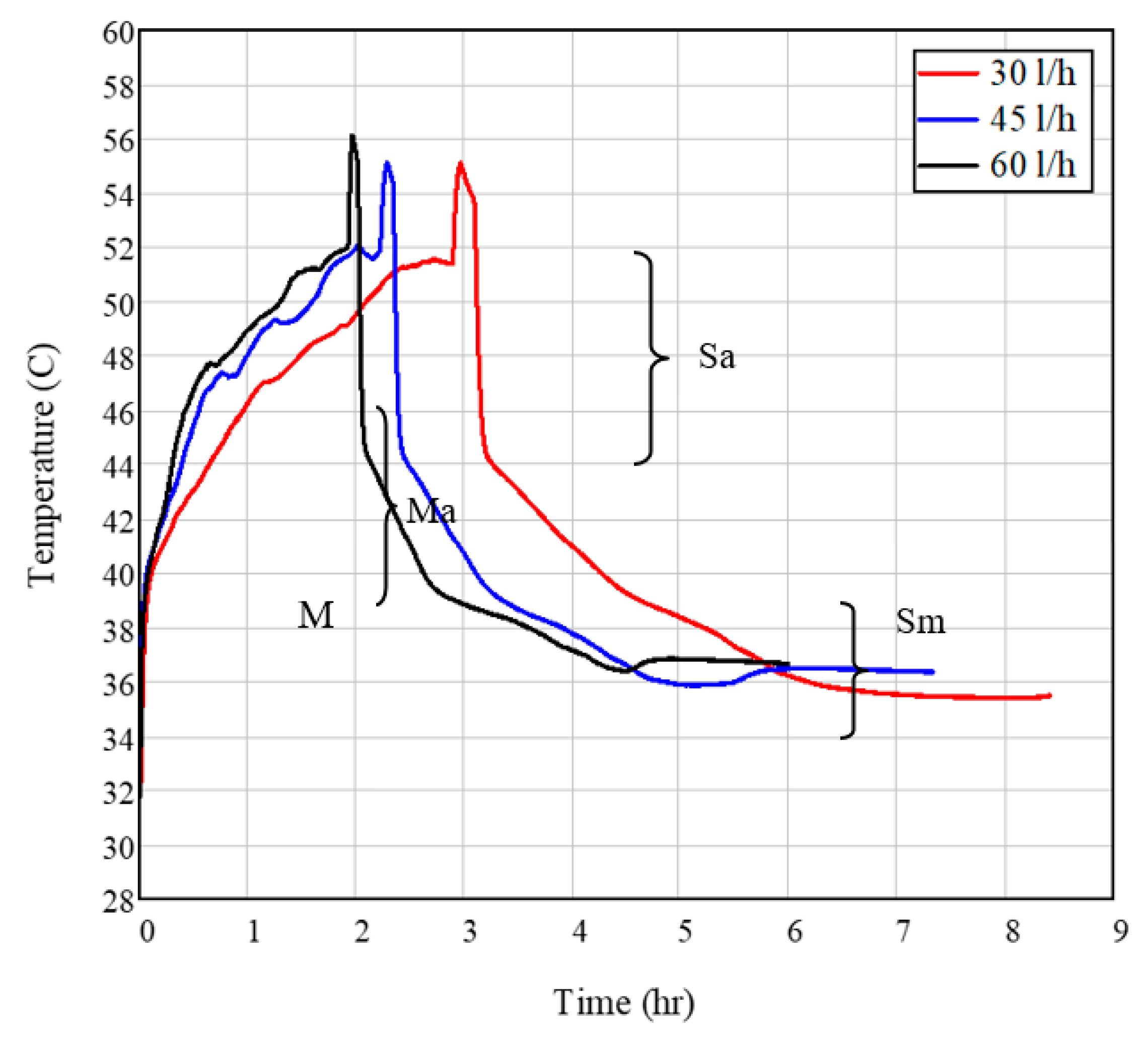
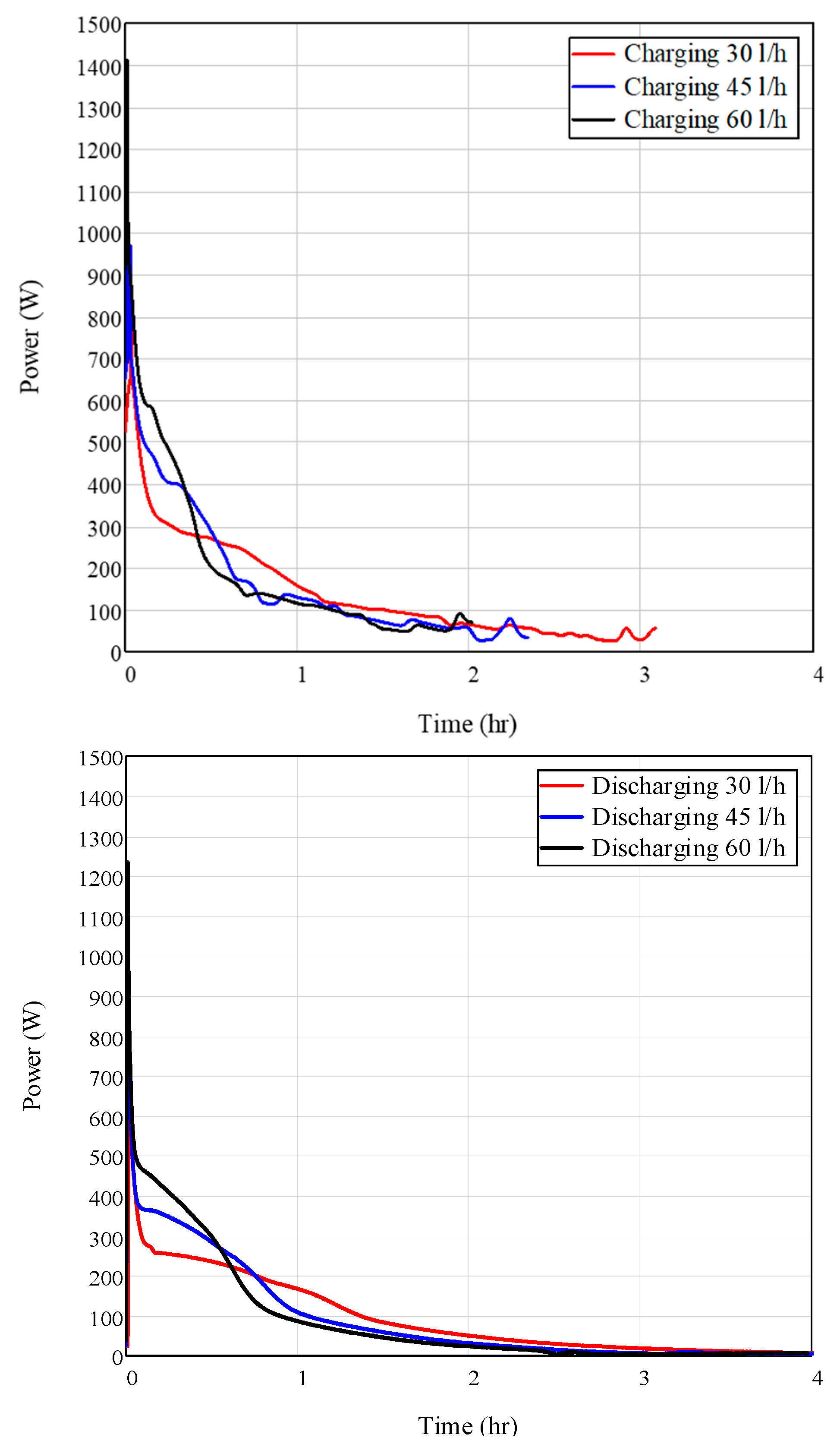
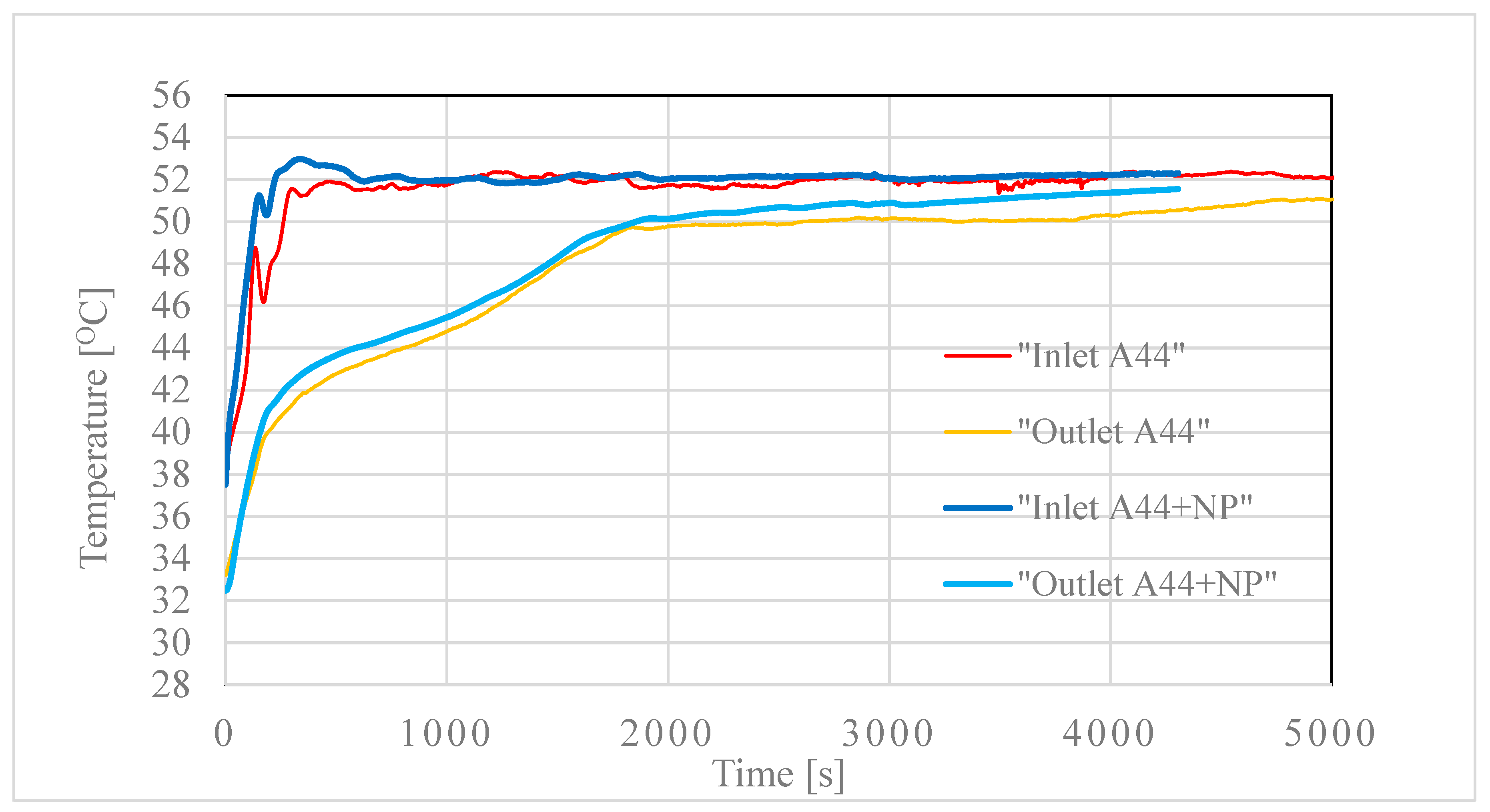
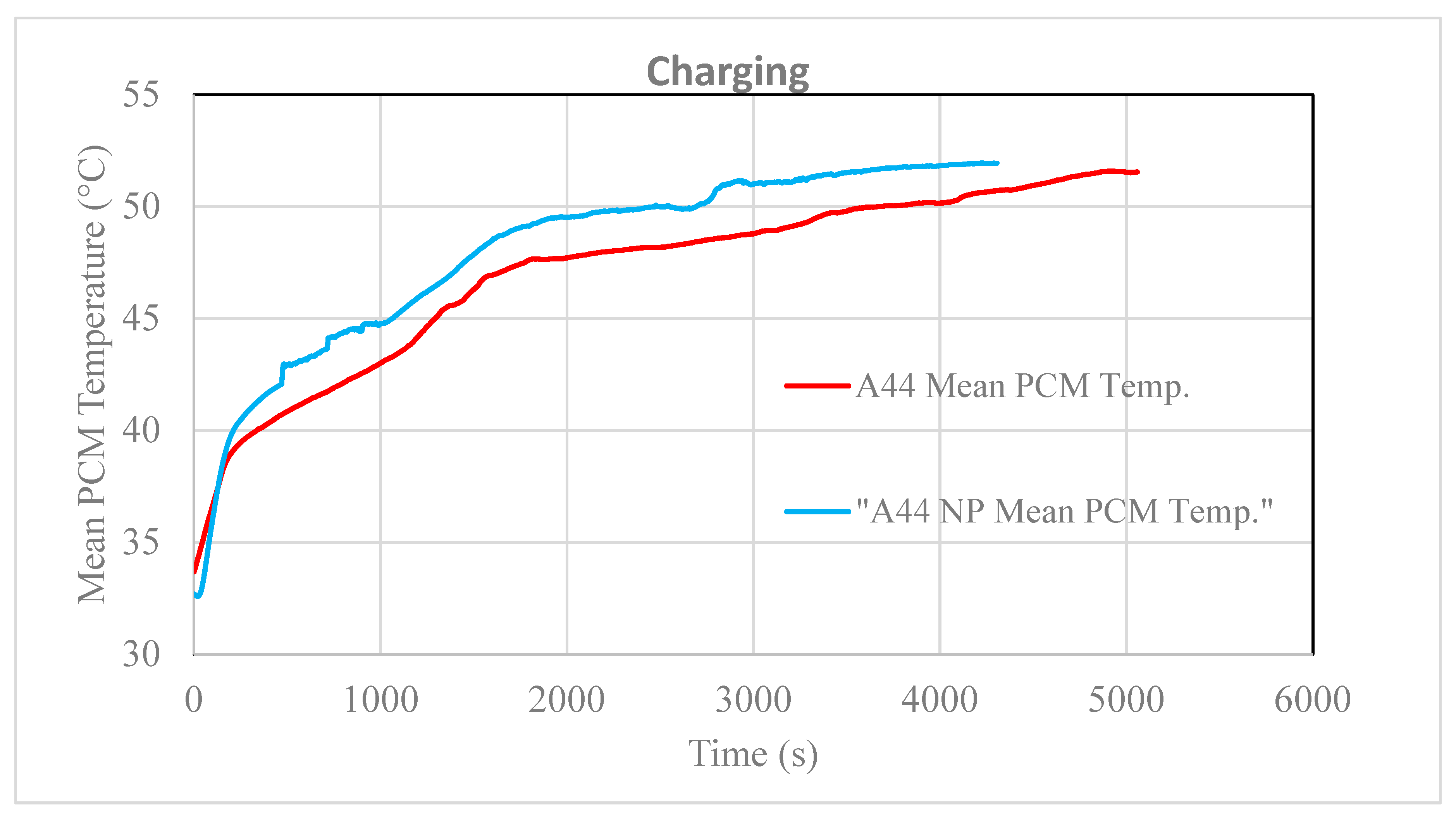
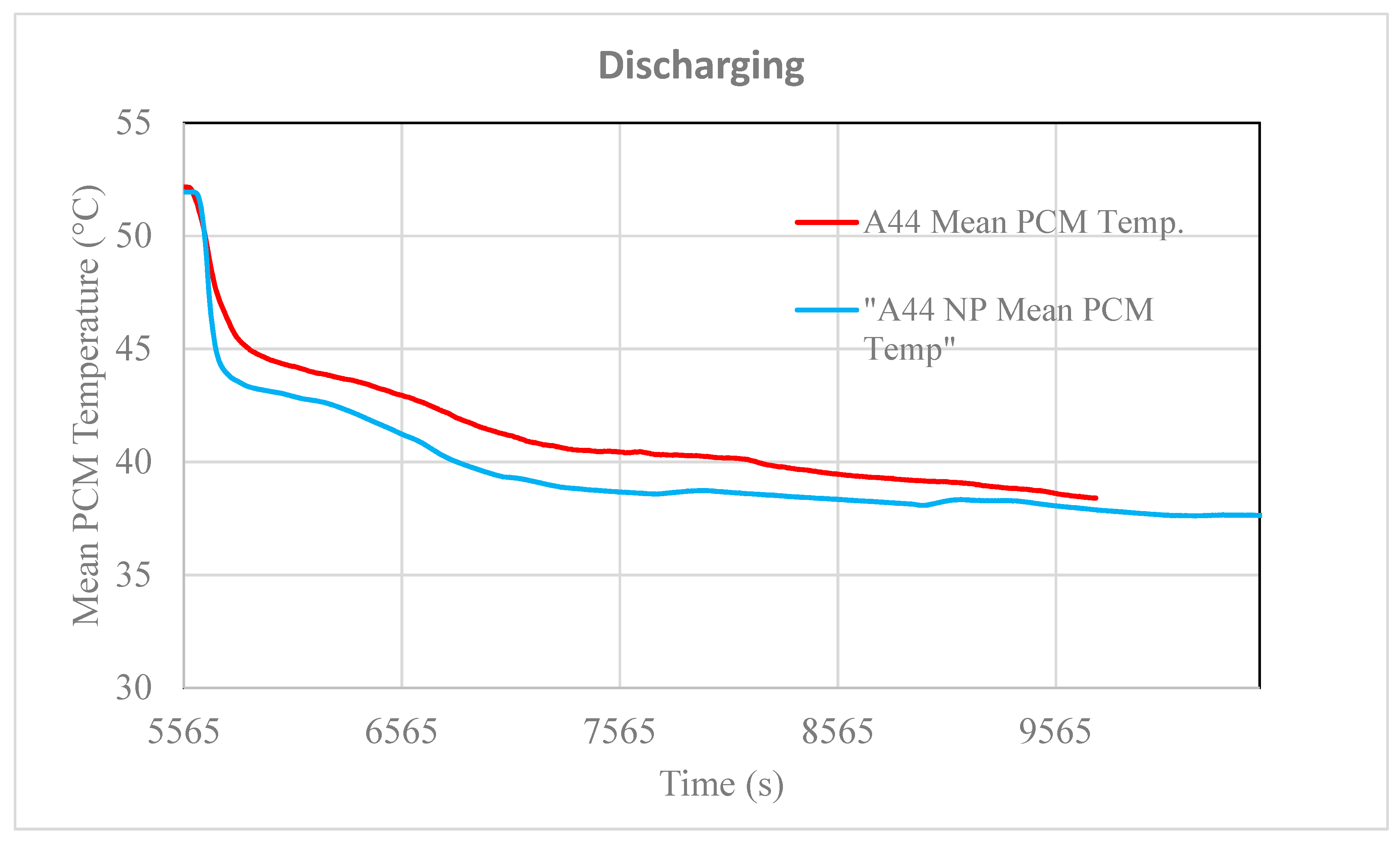
3.1. Effect of Flow Rate on HE Performance
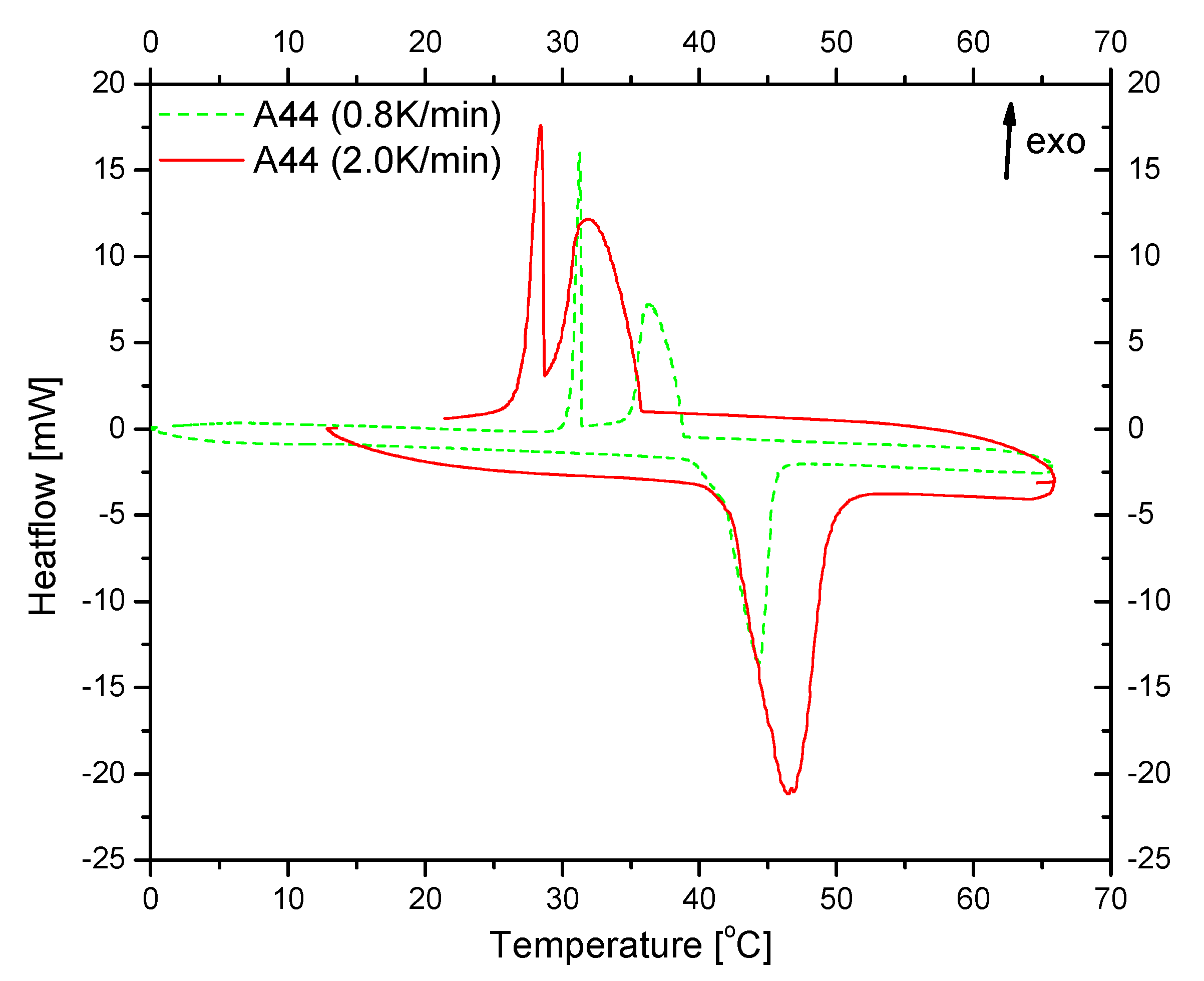
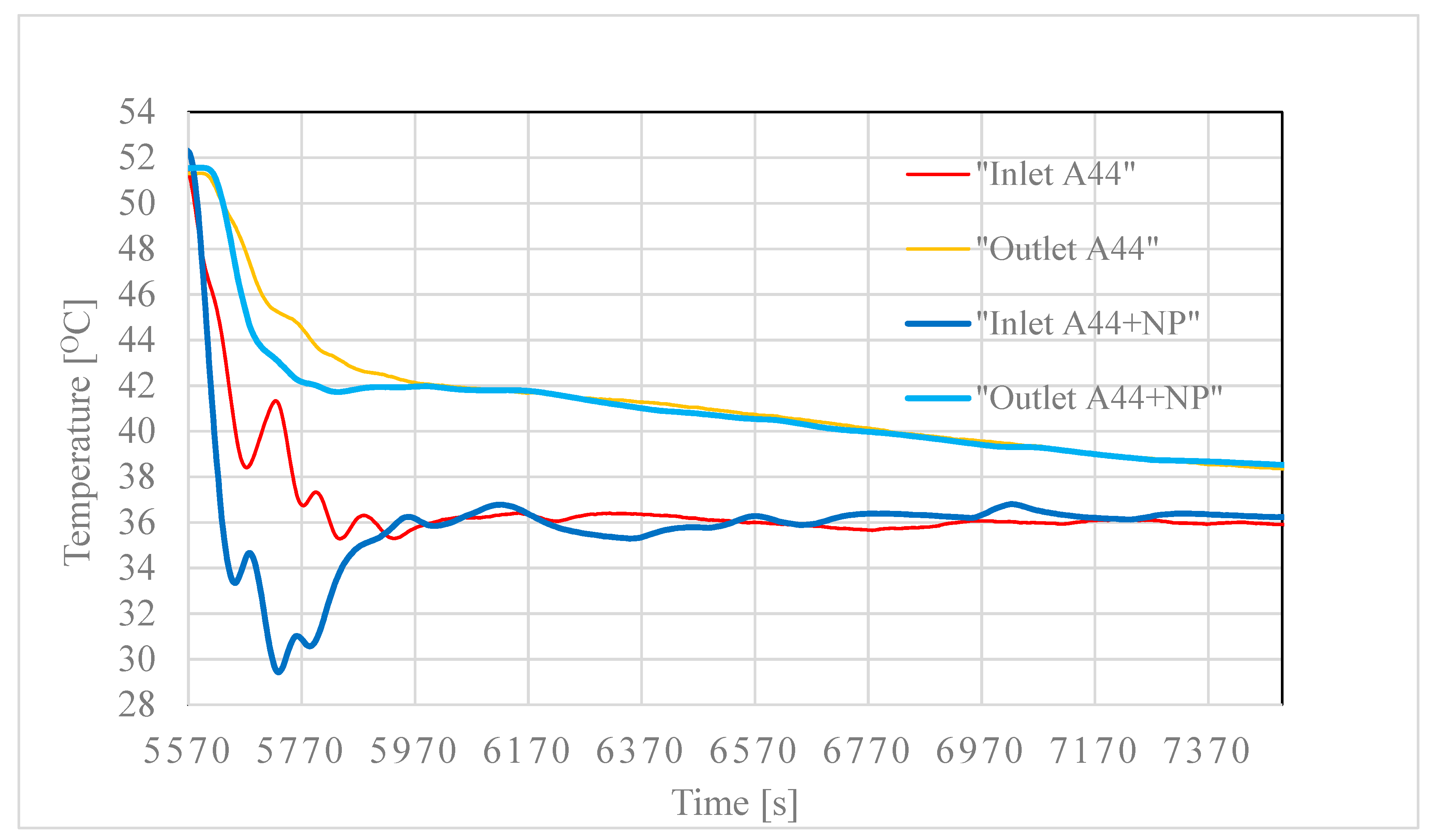
3.2. Testing of the Nancomposite Paraffin Thermal Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lizana, J.; Chargartegui, R.; Barrios-Padura, A.; Ortiz, C. Advanced low-carbon energy measures based on thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2018, 82, 3705–3749. [Google Scholar] [CrossRef]
- Nkwetta, D.N.; Haghighat, F. Thermal energy storage with phase change materials—A state-of-the art review. Sustain. Cities Soc. 2014, 10, 87–100. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L. Heat and Cold Storage with PCM—An up to Date Introduction into Basics and Applications; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Cunha, J.; Eames, P. Thermal Energy Storage for Low and Medium Temperature Applications Using Phase Change Materials—A Review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef]
- Palomba, V.; Frazzica, A. Comparative analysis of thermal energy storage technologies through the definition of suitable key performance indicators. Energy Build. 2019, 185, 88–102. [Google Scholar] [CrossRef]
- Tesse2b Project Deliverable 8.5: Training Material. Available online: http://www.tesse2b.eu/Content/images/tesse2b/Training_Material.pdf (accessed on 5 September 2019).
- Amini, A.; Miller, J.; Jouhara, H. An investigation into the use of the heat pipe technology in thermal energy storage heat exchangers. Energy 2017, 136, 163–172. [Google Scholar] [CrossRef]
- Alam, M.; Sanjayan, J.; Zou, P.X.W.; Ramakrishnan, S.; Wilson, J. A Comparative Study on the Effectiveness of Passive and Free Cooling Application Methods of Phase Change Materials for Energy Efficient Retrofitting in Residential Buildings. Procedia Eng. 2017, 180, 993–1002. [Google Scholar] [CrossRef]
- Elias, C.; Stathopoulos, V.N. A comprehensive review of recent advances in materials aspects of phase change materials in thermal energy storage. Energy Procedia 2019, 161, 385–394. [Google Scholar] [CrossRef]
- Chalkia, V.; Tachos, N.; Pandis, P.K.; Giannakas, A.; Koukou, M.K.; Vrachopoulos, M.Gr.; Coelho, L.; Ladavos, A.; Stathopoulos, V.N. Influence of organic phase change materials on the physical and mechanical properties of HDPE and PP polymer. RSC Adv. 2018, 8, 27438–27447. [Google Scholar] [CrossRef]
- Pandis, P.K.; Papaioannou, S.; Koukou, M.K.; Vrachopoulos, M.G.; Stathopoulos, V.N. Differential scanning calorimetry based evaluation of 3D printed PLA for phase change materials encapsulation or as container material of heat storage tanks. Energy Procedia 2019, 161, 429–437. [Google Scholar] [CrossRef]
- Languri, E.; Clifford, A.; Alvarado, J. Latent Thermal Energy Storage System Using Phase Change Material in Corrugated Enclosures. Appl. Therm. Eng. 2013, 50, 1008–1014. [Google Scholar] [CrossRef]
- Rathod, M.; Banerjee, J. Thermal Performance Enhancement of Shell and Tube Latent Storage Unit Using Longitudinal Fins. Appl. Therm. Eng. 2015, 75, 1084–1092. [Google Scholar] [CrossRef]
- Abdulateef, A.M.; Mat, S.; Sopian, K.; Abdulateef, J.; Gitan, A.A. Experimental and Computational Study of Melting Phase-Change Material in a Triplex Tube Heat Exchanger with Longitudinal/Triangular Fins. Sol. Energy 2017, 155, 142–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Faghri, A. Heat Transfer Enhancement in Latent Heat Thermal Energy Storage System by Using the Internally Finned Tube. Int. J. Heat Mass Transf. 1996, 39, 3165–3173. [Google Scholar] [CrossRef]
- Gasia, J.; Bourke, M.; Van Bael, J.; Cabeza, L. Comparative Study of the Thermal Performance of Four Different Shell-and-Tube Heat Exchangers Used as Latent Heat Thermal Energy Storage Systems. Renew. Energy 2017, 114, 934–944. [Google Scholar] [CrossRef]
- Jmal, I.; Baccar, M. Numerical Study of PCM Solidification in a Finned Tube Thermal Storage Including Natural Convection. Appl. Therm. Eng. 2015, 84, 320–330. [Google Scholar] [CrossRef]
- Koukou, M.K.; Vrachopoulos, M.G.; Tachos, N.S.; Dogkas, G.; Lymperis, K.; Stathopoulos, V. Experimental and Computational Investigation of a Latent Heat Energy Storage System with a Staggered Heat Exchanger for Various Phase Change Materials. Therm. Sci. Eng. Prog. 2018, 7, 87–98. [Google Scholar] [CrossRef]
- Taylor, C. Measurement of Finned-Tube Heat Exchanger Performance. Ph.D. Thesis, Georgia InstituTe of Technology, Atlanta, GA, USA, 2004. [Google Scholar]
- Rahimi, M.; Ranjbar, A.; Ganji, D.; Sedighi, K.; Hosseini, M.; Bahrampoury, R. Analysis of Geometrical and Operational Parameters of PCM in a Fin and Tube Heat Exchanger. Int. Commun. Heat Mass Transf. 2014, 53, 109–115. [Google Scholar] [CrossRef]
- Paria, S.; Baradaran, S.; Amiri, A.; Sarhan, A.; Kazi, A. Performance Evaluation of Latent Heat Energy Storage in Horizontal Shell-and-Finned Tube for Solar Application. J. Therm. Anal. Calorim. 2016, 123, 1371–1381. [Google Scholar] [CrossRef]
- Medrano, M.; Yilmaz, M.; Nogues, M.; Martorell, I.; Roca, J.; Cabeza, L. Experimental Evaluation of Commercial Heat Exchangers for Use as PCM Thermal Storage Systems. Appl. Energy 2009, 86, 2047–2055. [Google Scholar] [CrossRef]
- Li, M.; Mu, B. Effect of different dimensional carbon materials on the properties and application of phase change materials: A review. Appl. Energy 2019, 242, 695–715. [Google Scholar] [CrossRef]
- Shah, K.W. A review on enhancement of phase change materials—A nanomaterials perspective. Energy Build. 2018, 175, 57–68. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J. Thermal enhancement of paraffin/hydrophobic expanded perlite granular phase change composite using graphene nanoplatelets. Energy Build. 2018, 169, 206–215. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J. Effects of various carbon additives on the thermal storage performance of form-stable PCM integrated cementitious composites. Appl. Therm. Eng. 2019, 148, 491–501. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, D.; Zhang, X.; Huang, J. Preparation and melting/freezing characteristics of Cu/paraffin nanofluid as phase-change material (PCM). Energy Fuels 2010, 24, 1894–1898. [Google Scholar] [CrossRef]
- Sharma, G.; Kanwar Singh, M.; Sehgal, S.S.; Sandhu, H. Experimental Study of Thermal Properties of PCM with Addition of Nano Particles. Indian J. Sci. Technol. 2018, 11, 1–5. [Google Scholar]
- Shi, J.N.; Ger, M.D.; Liu, Y.M.; Fan, Y.C.; Wen, N.T.; Lin, C.K.; Pu, N.W. Improving the thermal conductivity and shape-stabilization of phase change materials using nanographite additives. Carbon 2013, 51, 365–372. [Google Scholar] [CrossRef]
- Yu, Z.T.; Fang, X.; Fan, L.W.; Wang, X.; Xiao, Y.Q.; Zeng, Y.; Xu, X.; Hu, Y.C.; Cen, K.F. Increased thermal conductivity of liquid paraffin-based suspensions in the presence of carbon nanoadditives of various sizes and shapes. Carbon 2013, 53, 277–285. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.A. Experimental and numerical investigations of nano-additives enhanced paraffin in a shell-and-tube heat exchanger: A comparative study. Appl. Therm. Eng. 2018, 143, 777–790. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, X. Study on paraffin/expanded graphite composite phase change thermal energy storage material. Energy Convers. Manag. 2013, 47, 303–310. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, N.; Peng, J.; Fang, X.; Gao, X.; Fang, Y. Preparation and thermal energy storage properties of paraffin/expanded graphite composite phase change material. Applied Energy 2012, 91, 426–431. [Google Scholar] [CrossRef]
- Drissi, S.; Ling, T.C.; Hung Mo, K. Thermal efficiency and durability performances of paraffinic phase change materials with enhanced thermal conductivity—A review. Thermochim. Acta 2019, 673, 198–210. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Leong, K.Y.; Abdul Rahman, M.R.; Gurunathan, B.A. Nano-composite phase change materials: A review of thermo-physical properties, applications and challenges. J. Energy Storage 2019, 21, 18–31. [Google Scholar] [CrossRef]
- Koukou, M.K.; Vrachopoulos, M.Gr.; Konstantaras, J.; Dogkas, G.; Coelho, L.; Rebola, A. Testing the performance of a prototype thermal energy storage tank working with organic phase change material for space heating application conditions. In Proceedings of the International Conference on Advances in Energy Systems and Environmental Engineering (ASEE19), Wroclaw, Poland, 9–12 June 2019. [Google Scholar]
- PCM Products. Available online: http://www.pcmproducts.net (accessed on 20 May 2019).
- American Association for Laboratory Accreditation. G104—Guide for Estimation of Measurement Uncertainty in Testing; American Association for Laboratory Accreditation: Frederick, MD, USA, 2014. [Google Scholar]
- Moffat, R.J. Describing the Uncertainties in Experimental Results. Exp. Therm. Fluid Sci. 1988, 1, 3–17. [Google Scholar] [CrossRef]


| Freezing Point (°C) | 45.5 |
| Melting point (°C) | 44 |
| Latent heat (kJ/kg) | 268 |
| Density solid (kg/m3) | 830 |
| Density liquid (kg/m3) | 775 |
| Thermal conductivity solid (W/m∙K) | 0.24 |
| Thermal conductivity liquid (W/m∙K) | 0.24 |
| Specific heat solid (kJ/kg∙K) | 2.4 |
| Specific heat liquid (kJ/kg∙K) | 1.8 |
| Accuracy | Temperature, °C | Volumetric Flow Rate, Lt/min |
|---|---|---|
| Sensor | ±(0.005∙Temp) | ±(0.04∙|Flow|) |
| Sensor reader | ±(0.15 + 0.002∙Temp) | - |
| A/D Converter | ±(0.002 Temp range) | - |
| Flow Rate (L/h) | Inlet Temperature | Outlet Temperature | Volumetric Flow Rate | Thermal Power | Accumulated Energy |
|---|---|---|---|---|---|
| 30 | 1.23% | 1.24% | 4.62% | 0.25% | 5.95% |
| 45 | 1.20% | 1.21% | 4.62% | 0.28% | 6.95% |
| 60 | 1.28% | 1.29% | 4.62% | 0.35% | 7.50% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukou, M.K.; Dogkas, G.; Vrachopoulos, M.G.; Konstantaras, J.; Pagkalos, C.; Lymperis, K.; Stathopoulos, V.; Evangelakis, G.; Prouskas, C.; Coelho, L.; et al. Performance Evaluation of a Small-Scale Latent Heat Thermal Energy Storage Unit for Heating Applications Based on a Nanocomposite Organic PCM. ChemEngineering 2019, 3, 88. https://doi.org/10.3390/chemengineering3040088
Koukou MK, Dogkas G, Vrachopoulos MG, Konstantaras J, Pagkalos C, Lymperis K, Stathopoulos V, Evangelakis G, Prouskas C, Coelho L, et al. Performance Evaluation of a Small-Scale Latent Heat Thermal Energy Storage Unit for Heating Applications Based on a Nanocomposite Organic PCM. ChemEngineering. 2019; 3(4):88. https://doi.org/10.3390/chemengineering3040088
Chicago/Turabian StyleKoukou, Maria K., George Dogkas, Michail Gr. Vrachopoulos, John Konstantaras, Christos Pagkalos, Kostas Lymperis, Vassilis Stathopoulos, George Evangelakis, Costas Prouskas, Luis Coelho, and et al. 2019. "Performance Evaluation of a Small-Scale Latent Heat Thermal Energy Storage Unit for Heating Applications Based on a Nanocomposite Organic PCM" ChemEngineering 3, no. 4: 88. https://doi.org/10.3390/chemengineering3040088
APA StyleKoukou, M. K., Dogkas, G., Vrachopoulos, M. G., Konstantaras, J., Pagkalos, C., Lymperis, K., Stathopoulos, V., Evangelakis, G., Prouskas, C., Coelho, L., & Rebola, A. (2019). Performance Evaluation of a Small-Scale Latent Heat Thermal Energy Storage Unit for Heating Applications Based on a Nanocomposite Organic PCM. ChemEngineering, 3(4), 88. https://doi.org/10.3390/chemengineering3040088








