Corrosion Protection of Monel Alloy Coated with Graphene Quantum Dots Starts with a Surge
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
3.1. Monel Polarization Studies
3.2. Monel Coated with Graphene Oxide
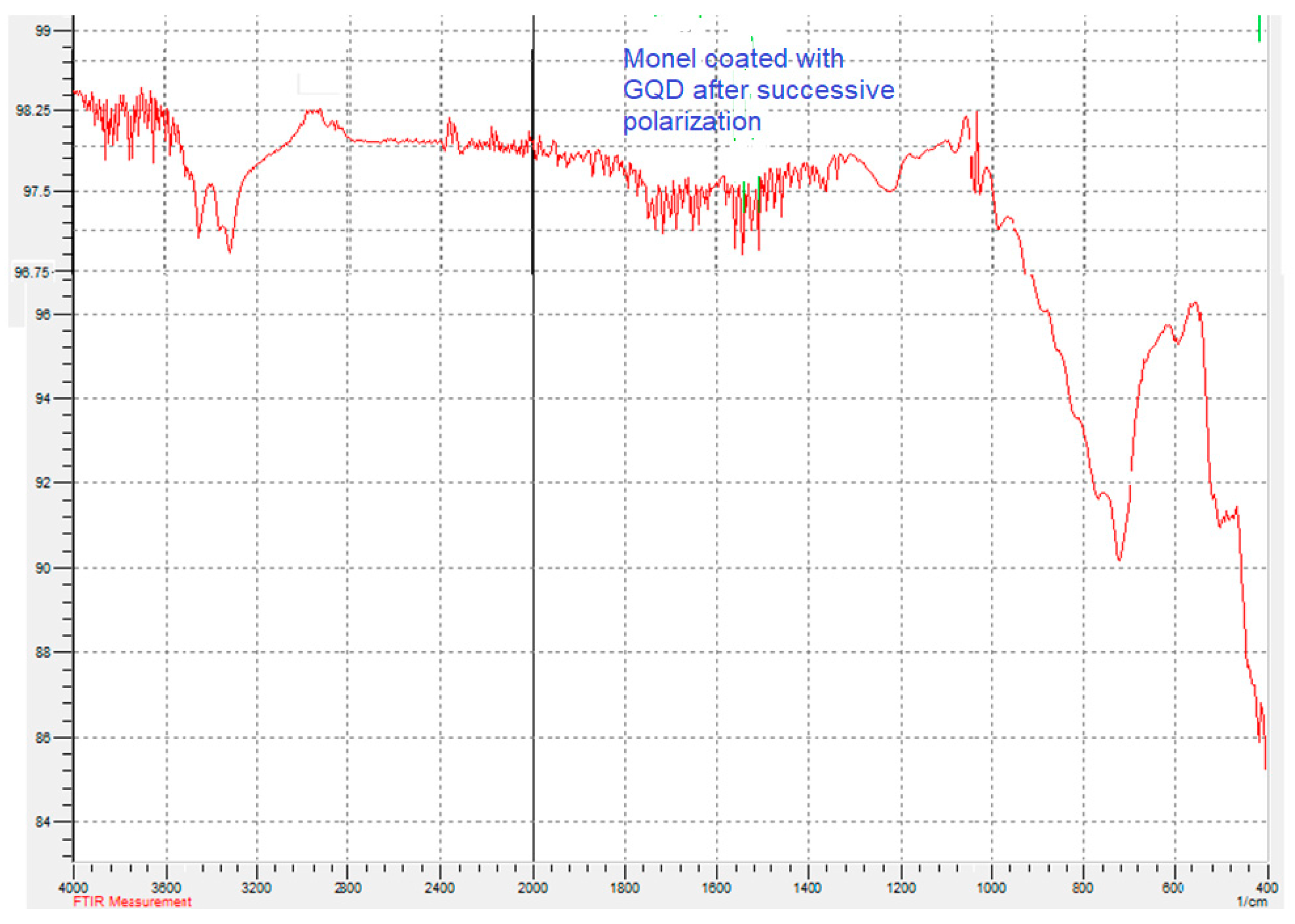
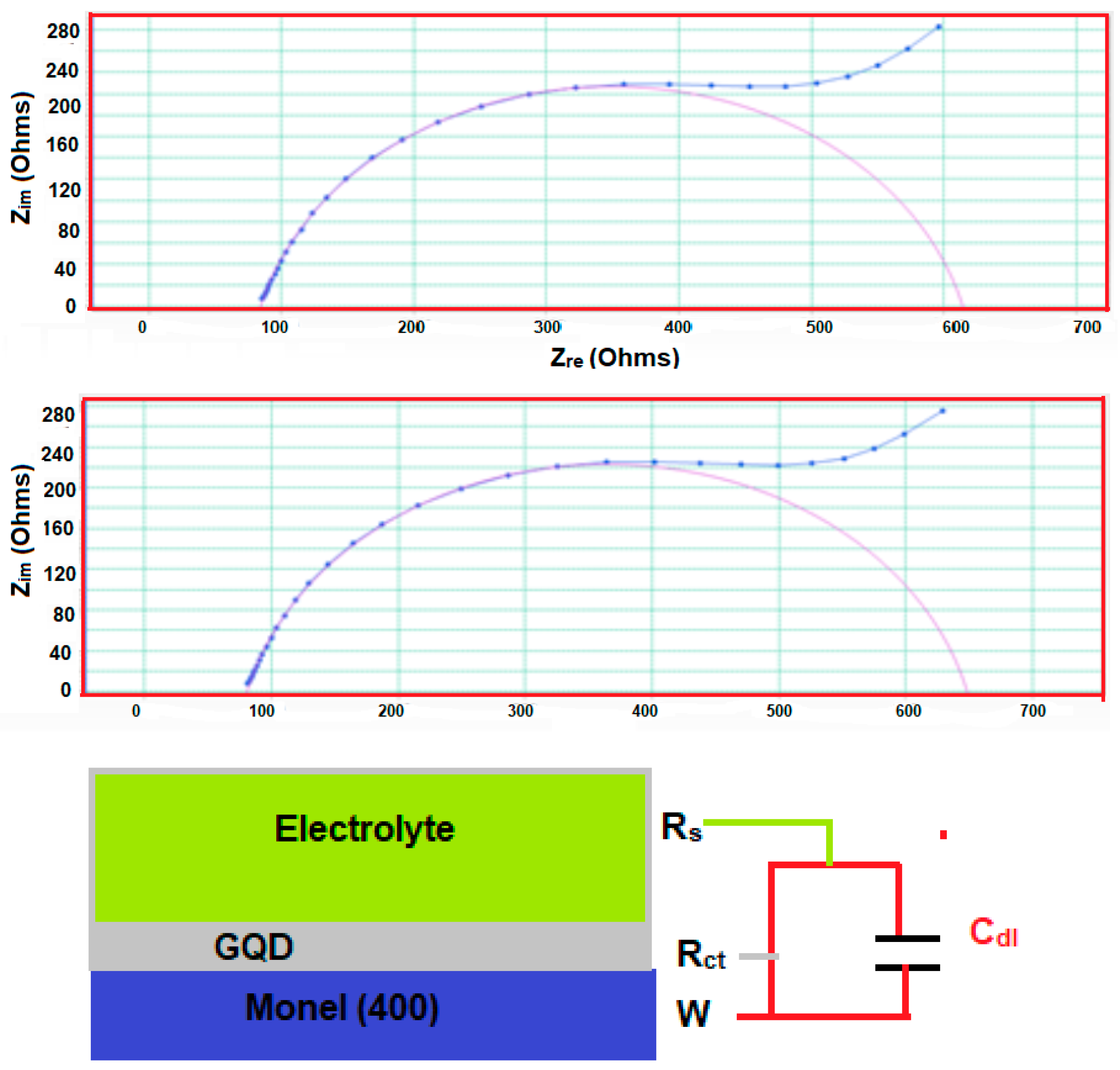
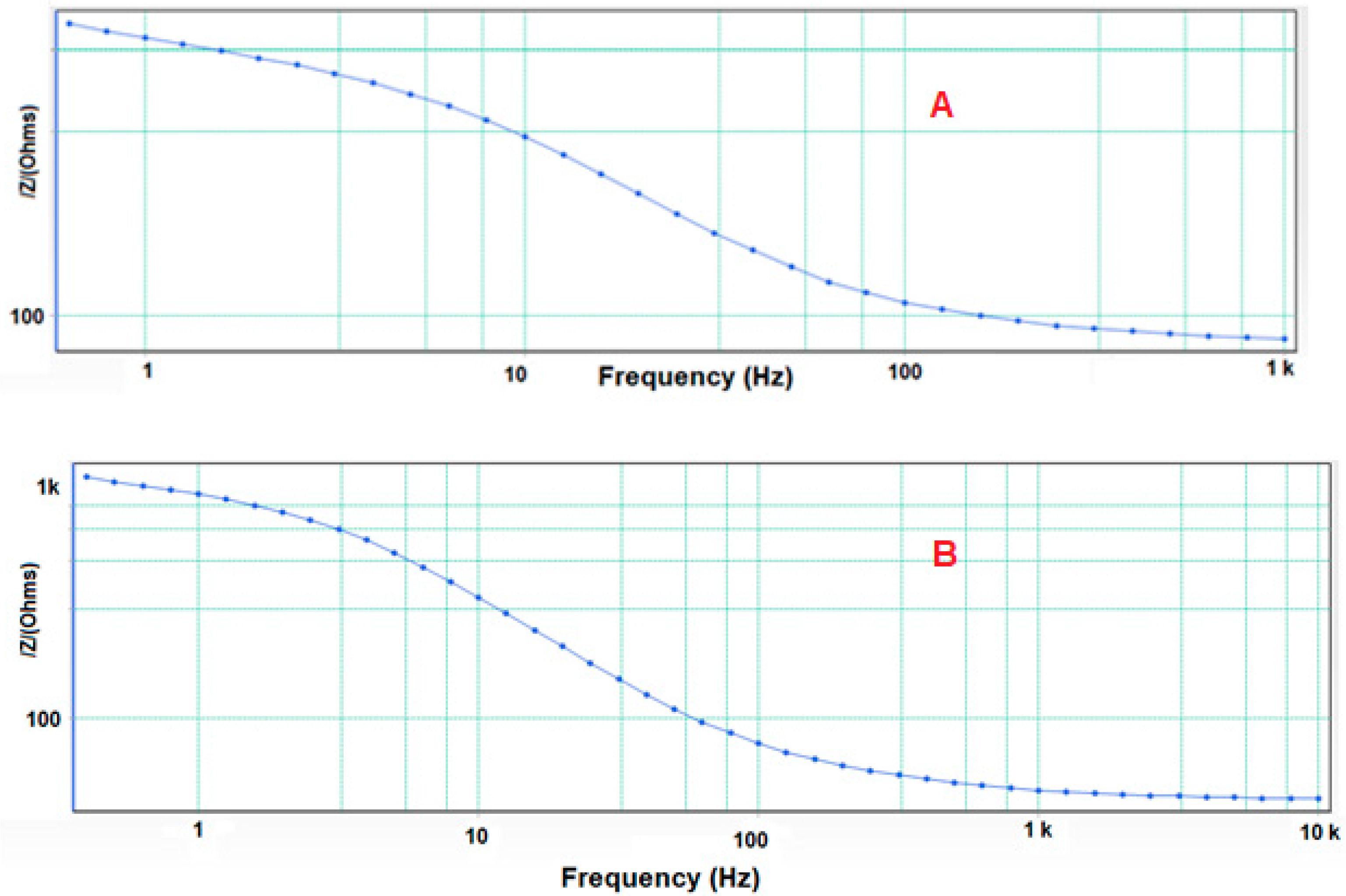
3.3. Electrochemical Impedance Spectroscopy
3.4. Mechanism of Corrosion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhaduri, A. Mechanical Properties and Working of Metals and Alloys; Springer: Singapore, 2018; ISBN 978-981-10-7209-3. [Google Scholar]
- Special Metals. Available online: http://www.specialmetals.com/assets/smc/documents/alloys/Monel/Monel-alloy-400.pdf (accessed on 20 March 2019).
- Ramkumar, K.D.; Joshi, V.; Pandit, S.; Agrawal, M.; Kumar, O.S.; Periwal, S.; Manikandan, M.; Arivazhagan, N. Investigations on the microstructure and mechanical properties of multi- pass pulsed current gas tungsten arc weldments of Monel 400 and Hastelloy C276. Mater. Des. 2014, 64, 775–782. [Google Scholar] [CrossRef]
- Nikitina, E.V.; Kazakovtseva, N.A.; Maikov, M.A.; Malkov, V.B.; Karfidov, E.A.; Chuikin, A.Y. Electrochemical Corrosion Behavior of Monel Alloy in Carbonate Melts. Russ. J. Electrochem. 2018, 54, 697–698. [Google Scholar] [CrossRef]
- Ma, F.Y.; Gao, F.; Zeng, Z. Improving the tribocorrosion resistance of Monel 400 alloy by aluminizing surface modification. J. Mater. Eng. Perform. 2018, 27, 3439–3440. [Google Scholar] [CrossRef]
- Zhan, J.Z.; Li, J.; Lu, Y.J.; Wu, L.Q.; Zhao, N.Y. Technics and performance analysis of Monel alloy coating prepared by high velocity arc spraying. Mater. Res. Innov. 2013, 17, s112–s114. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Jaberinia, F. Electrochemical study of Monel alloy corrosion in hydrochloric acid solution and pyrrolidine dithiocarboxylate self-assembled monolayers as its corrosion protector. J. Alloy. Compd. 2018, 750, 677679. [Google Scholar] [CrossRef]
- Aljinović, L.; Gudić, S.; Šmith, M. Inhibition of CuNi10Fe corrosion in seawater by sodium-diethyl-dithiocarbamate: An electrochemical and analytical study. J. Appl. Electrochem. 2000, 30, 973–979. [Google Scholar] [CrossRef]
- Mishra, A. Corrosion Study of Base Material and Welds of a Ni-Cr-Mo-W Alloy. Acta Metall. Sin. (Engl. Lett.) 2017, 30, 326–332. [Google Scholar] [CrossRef]
- Phillips, W.; Merwin, A.; Chidambaram, D. On the Corrosion Performance of Monel 400 in Molten LiCl-Li2O-Li at 923 K. Metall. Mater. Trans. A 2018, 49, 2384. [Google Scholar] [CrossRef]
- Kherraf, S.; Zouaoui, E.; Medjram, M.S. Corrosion inhibition of Monel 400 in hydrochloric solution by some green leaves. Anti Corros. Methods Mater. 2017, 64, 347. [Google Scholar] [CrossRef]
- Jun, C. Corrosion wear characteristics of TC4, 316 stainless steel, and Monel K500 in artificial Seawater. RSC Adv. 2017, 7, 23835–23845. [Google Scholar] [CrossRef]
- Ramkumar, K.D.; Arivazhagan, N.; Narayanan, S.; Mishra, D. Hot Corrosion Behavior of Monel 400 and AISI 304 Dissimilar Weldments Exposed in the Molten Salt Environment Containing Na2SO4 + 60% V2O5 at 600 °C. Mater. Res. 2014, 17, 1273–1284. [Google Scholar] [CrossRef]
- Sherif, E.M.; Almajid, A.A.; Bairamov, A.K.; Al-Zahrani, E. A comparative Study on the Corrosion of Monel-400 in Aerated and Deaerated Arabian Gulf Water and 3.5% Sodium Chloride Solutions. J. Electrochem. Sci. 2012, 7, 2796–2810. [Google Scholar]
- Alar, V.; Stojanović, I.; Židov, B.; Ivušić, F. Corrosion Resistance of Highly Alloyed Materials in 3.5% NaCl Solution at Elevated Temperature. Int. J. Electrochem. Sci. 2013, 8, 12477. [Google Scholar]
- Wang, F.; Zhang, J.; Zou, J.T.; Fan, Z.K.; Zhang, F.S. Effects of Al contents on microstructure and properties of monel alloys. Metal Mater. Eng. 2010, 39, 1933–1937. [Google Scholar]
- Kravets, V.G.; Jalil, R.; Kim, Y.-J.; Ansell, D.; Aznakayeva, D.E.; Thackray, B.; Britnell, L.; Belle, B.D.; Withers, F.; Radko, I.P.; et al. Graphene-protected copper and silver plasmonics. Sci. Rep. 2014, 4, 5517. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S. Graphene against corrosion. Nat. Nanotechnol. 2014, 9, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Hu, Y.; Liu, Z.; Cheng, G.J.; Zhao, K. Defects Mediated Corrosion in Graphene Coating Layer Defects Mediated Corrosion in Graphene Coating Layer. ACS Appl. Mater. Interfaces 2017, 9, 11902–11908. [Google Scholar] [CrossRef]
- Hu, J.; Ji, Y.; Shi, Y.; Hui, F.; Duan, H.; Lanza, M. A Review on the Use of Graphene as a Protective Coating against Corrosion. Ann. Mater. Sci. Eng. 2015, 1, 16. [Google Scholar]
- Shen, L.; Li, Y.; Zhao, W.; Miao, L.; Xie, W.; Lu, H.; Wang, K. Corrosion Protection of Graphene-Modified Zinc-Rich Epoxy Coatings in Dilute NaCl Solution. ACS Appl. Nano Mater. 2019, 2, 180–190. [Google Scholar] [CrossRef]
- Jayakumar, N.; Veedu, K.K.; Gopalan, N.K. Durable Hydrophobic Coating Based on Cerium Phosphate Nanorod Siliconized Epoxy for Corrosion Protection. ACS Appl. Nano Mater. 2019, 2, 2689–2696. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Zhao, H.; Wang, L. Synthesis of L-Histidine-Attached Graphene Nanomaterials and Their Application for Steel Protection. ACS Appl. Nano Mater. 2018, 1, 1385–1395. [Google Scholar] [CrossRef]
- Santhanam, K.S.V.; Kandlikar, S.; Valentina, M.; Yang, Y. Electrochemical Process for Producing Graphene, Graphene Oxide, Metal Composites and Coated Substrates. US Patent 9,840,782, 12 December 2017. [Google Scholar]
- Wong, P.; Santhanam, K.S.V.; Kandlikar, S. Cobalt Deposition from graphene quantum dot bath: Electrochemical and Spectroscopic Features-A Prospective Sensor Material. J. Electrochem. Soc. 2018, 165, B232. [Google Scholar] [CrossRef]
- Protich, Z.; Wong, P.; Santhanam, K.S.V. A new graphene composite with a high coulombic efficiency. J. Power Sources 2016, 332, 337–344. [Google Scholar] [CrossRef]
- Cusati, T.; Fiori, G.; Gahoi, A.; Passi, V.; Lemme, M.C.; Fortunelli, A.; Lannaccone, G. Electrical properties of graphene-metal contacts. Sci. Rep. 2017, 7, 5109. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Wasim, M. Performance evaluation of k0-instrumental neutron activation analysis and flame atomic absorption spectrophotometry in the characterization of various types of alloys. J. Radioanal. Nucl. Chem. 2013, 297, 153–159. [Google Scholar] [CrossRef]
- Uhlig, H.H.; Revie, R.W. Corrosion and Its Control; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Malar, L.M.; Piment, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in Graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Budipramana, Y.; Ersam, S.T.; Kurniawan, F. Synthesis nickel hydroxide by electrolysis at high voltage. ARPN J. Eng. Appl. Sci. 2014, 9, 2074. [Google Scholar]
- Khan, Y.; Durrani, S.K.; Mehmood, M.; Jan, A.; Abbasi, M.A. pH-dependant structural and morphology evolution of Ni(OH)2 nanostructures and their morphology retention upon thermal annealing to NiO. Mater. Chem. Phys. 2011, 130, 1169–1174. [Google Scholar] [CrossRef]
- Shearer, C.J.; Slattery, A.D.; Stapleton, A.J.; Shapter, J.G.; Gibson, C.T. Accurate thickness measurement of graphene. Nanotechnology 2016, 29, 125704. [Google Scholar] [CrossRef]
- Jennings, G.K.; Munro, J.C.; Yong, T.H.; Laibinis, P.E. Effect of Chain Length on the Protection of Copper by n-Alkanethiols. Langmuir 1998, 14, 6130–6139. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, Z.; Xie, D.; Fang, Y. Graphene: Fabrication, Characterizations, Properties and Applications; Academic Press: London, UK, 2018. [Google Scholar]
- Rossiter, P.L.; Bass, J. The Electrical Resistivity of Metals and Alloys. Phys. Today 1988, 41, 78. [Google Scholar] [CrossRef]
- Roberge, P.R. Corrosion Engineering: Principles and Practice; McGraw-Hill Education: Boston, MA, USA, 2008. [Google Scholar]
- Santhanam, K.S.V.; Press, R.; Miri, M.; Bailey, A.; Takacs, G. Introduction to Hydrogen Technology, 2nd ed.; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
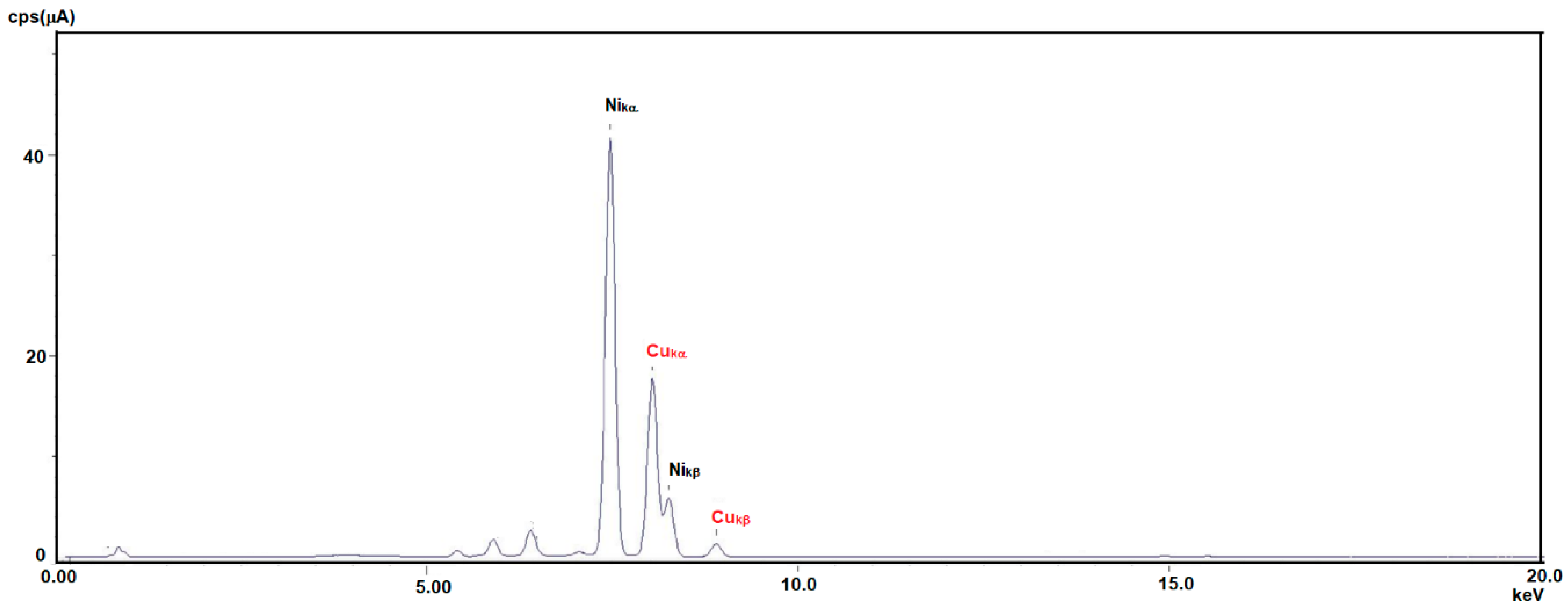
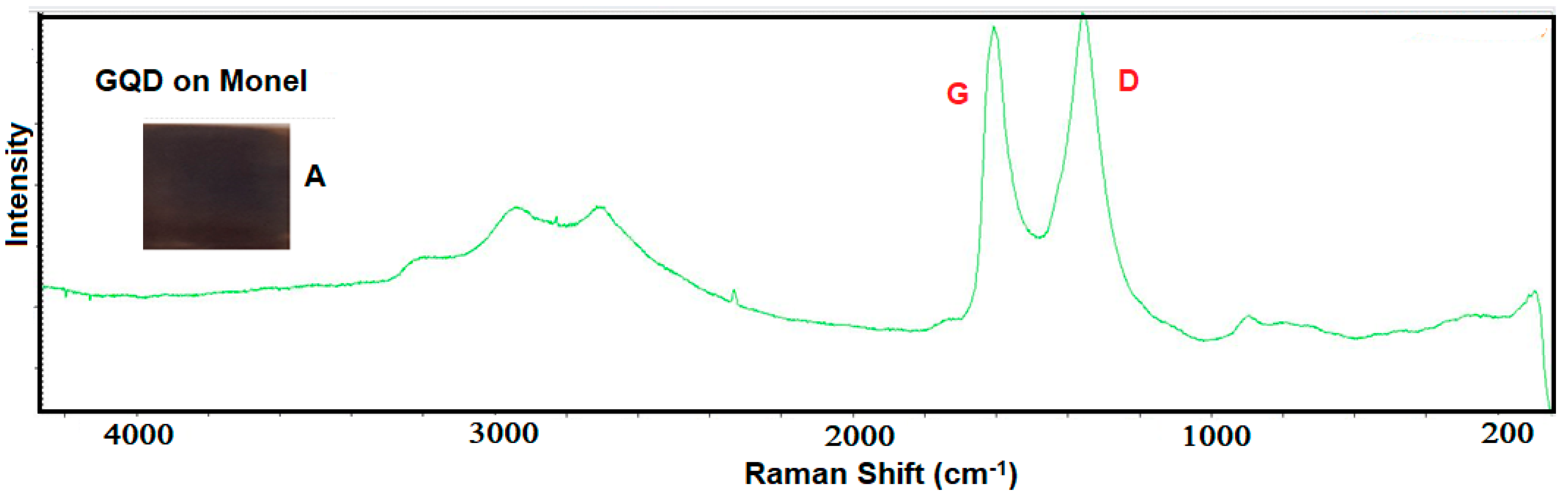
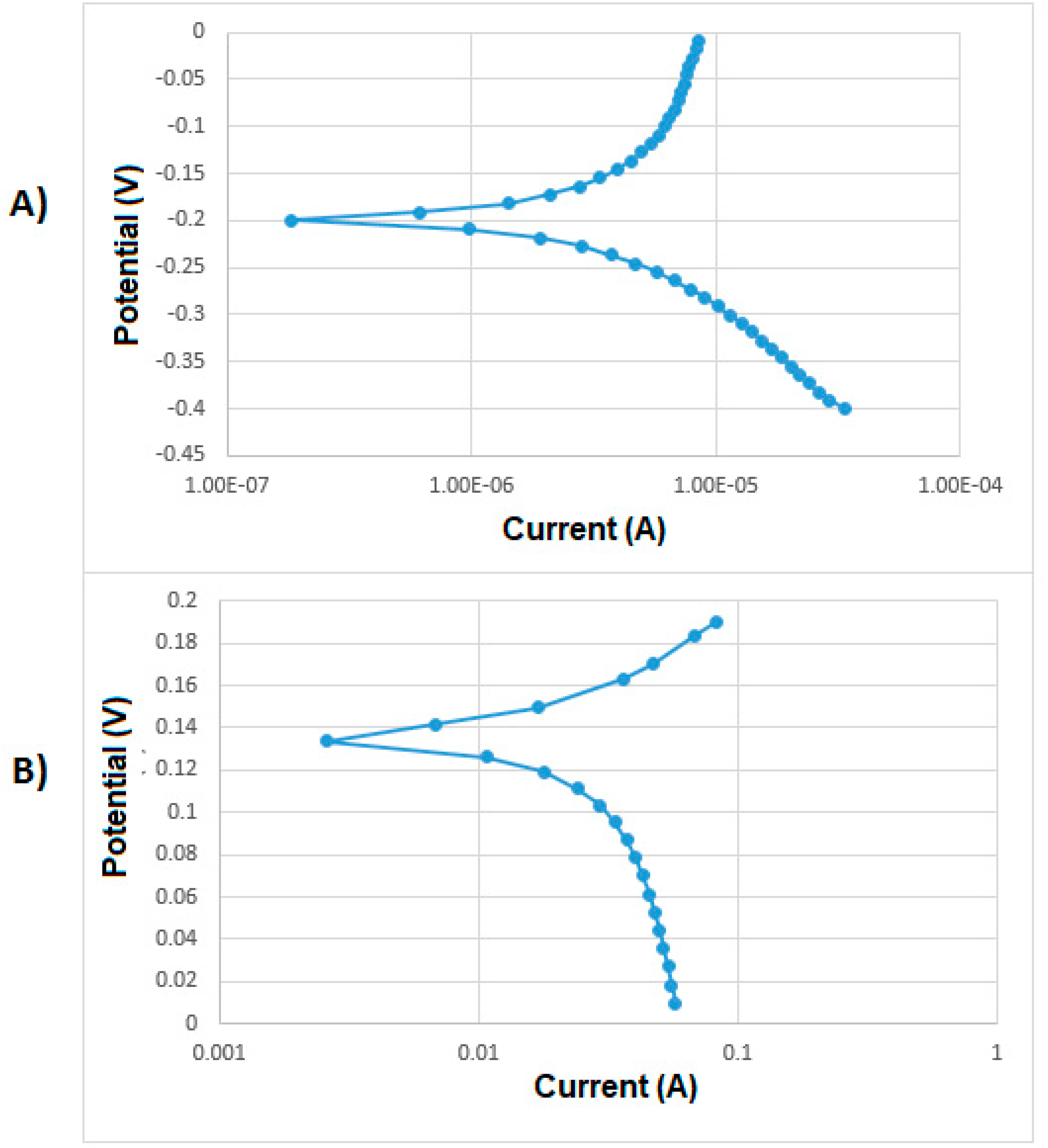
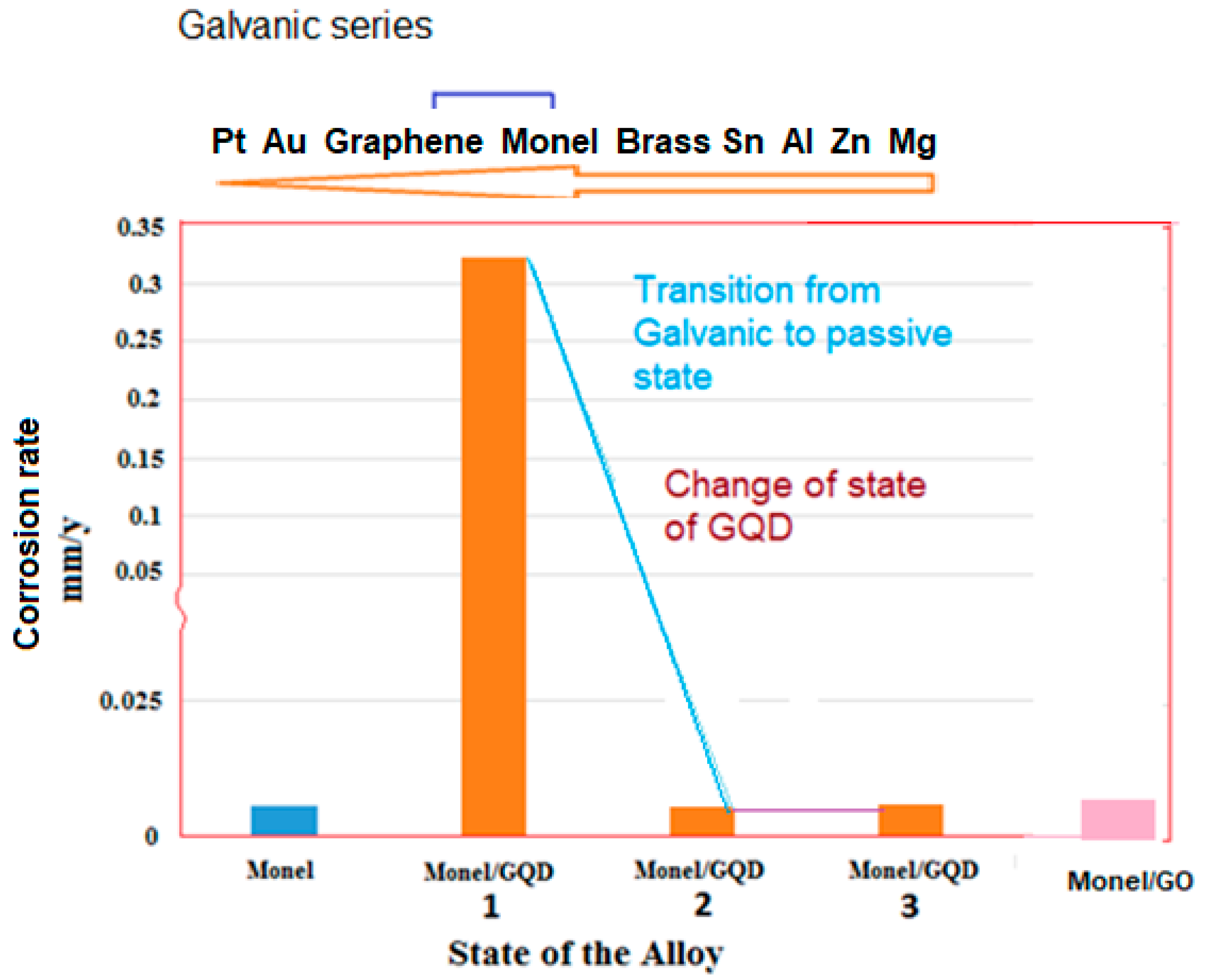
| Analyte | Percentage | Std. Dev |
|---|---|---|
| Ni | 67.053 | 0.085 |
| Cu | 29.424 | 0.047 |
| Fe | 1.4740 | 0.009 |
| Mn | 1.0360 | 0.008 |
| Cr | 0.0461 | 0.006 |
| Ca | 0.1440 | 0.009 |
| Si | 0.1100 | 0.012 |
| Treatment | Medium | Icorr (A/cm2) | Kcorr (mm/y) |
|---|---|---|---|
| None | 0.1 M NaCl | 3.11 × 10−6 | 0.035 |
| None | 3.5 wt % NaCl | 2.13 × 10−6 | 0.024 |
| None | 0.1 M Na2SO4 | 7.25 × 10−7 | 0.008 |
| 5 min | 0.1 M NaCl | 4.17 × 10−7 | 0.005 |
| 5 min | 3.5 wt % NaCl | 1.02 × 10−6 | 0.011 |
| 5 min | 0.1 M Na2SO4 | 5.78 × 10−7 | 0.006 |
| 10 min | 0.1 M NaCl | 1.29 × 10−6 | 0.014 |
| 10 min | 3.5 wt % NaCl | 1.60 × 10−6 | 0.018 |
| 10 min | 0.1 M Na2SO4 | 5.36 × 10−7 | 0.006 |
| 15 min | 0.1 M NaCl | 1.21 × 10−6 | 0.013 |
| 15 min | 3.5 wt % NaCl | 3.44 × 10−7 | 0.004 |
| 15 min | 0.1 M Na2SO4 | 1.84 × 10−6 | 0.021 |
| Treatment Duration | Polarization Number | Icorr (A/cm2) | kcorr (mm/y) |
|---|---|---|---|
| 1 h | 1 | 2.89 × 10−5 | 0.322 |
| 1 h | 2 | 3.24 × 10−6 | 0.036 |
| 1 h | 3 | 1.21 × 10−6 | 0.014 |
| 25 h | 1 | 6.06 × 10−3 | 67.68 |
| 25 h | 2 | 4.69 × 10−7 | 0.005 |
| 25 h | 3 | 3.78 × 10−7 | 0.004 |
| 25 h | 4 | 3.94 × 10−7 | 0.004 |
| 25 h | 5 | 4.80 × 10−7 | 0.005 |
| 25 h | 6 | 4.36 × 10−7 | 0.005 |
| Electrode | Area (cm2) | Ecorr (mV) | Icorr (µA) | Icorr (µA/cm2) | kcorr (mm/y) |
|---|---|---|---|---|---|
| Monel-Graphene oxide | 2.897 | −139.1 | 4.908 | 1.694 | 0.0378 |
| Monel-Graphene oxide | 2.897 | −141.5 | 4.348 | 1.501 | 0.0335 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bopp, C.; Santhanam, K. Corrosion Protection of Monel Alloy Coated with Graphene Quantum Dots Starts with a Surge. ChemEngineering 2019, 3, 80. https://doi.org/10.3390/chemengineering3040080
Bopp C, Santhanam K. Corrosion Protection of Monel Alloy Coated with Graphene Quantum Dots Starts with a Surge. ChemEngineering. 2019; 3(4):80. https://doi.org/10.3390/chemengineering3040080
Chicago/Turabian StyleBopp, Charles, and Kalathur Santhanam. 2019. "Corrosion Protection of Monel Alloy Coated with Graphene Quantum Dots Starts with a Surge" ChemEngineering 3, no. 4: 80. https://doi.org/10.3390/chemengineering3040080
APA StyleBopp, C., & Santhanam, K. (2019). Corrosion Protection of Monel Alloy Coated with Graphene Quantum Dots Starts with a Surge. ChemEngineering, 3(4), 80. https://doi.org/10.3390/chemengineering3040080






