The Effect of Cu and Ga Doped ZnIn2S4 under Visible Light on the High Generation of H2 Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Photocatalysts
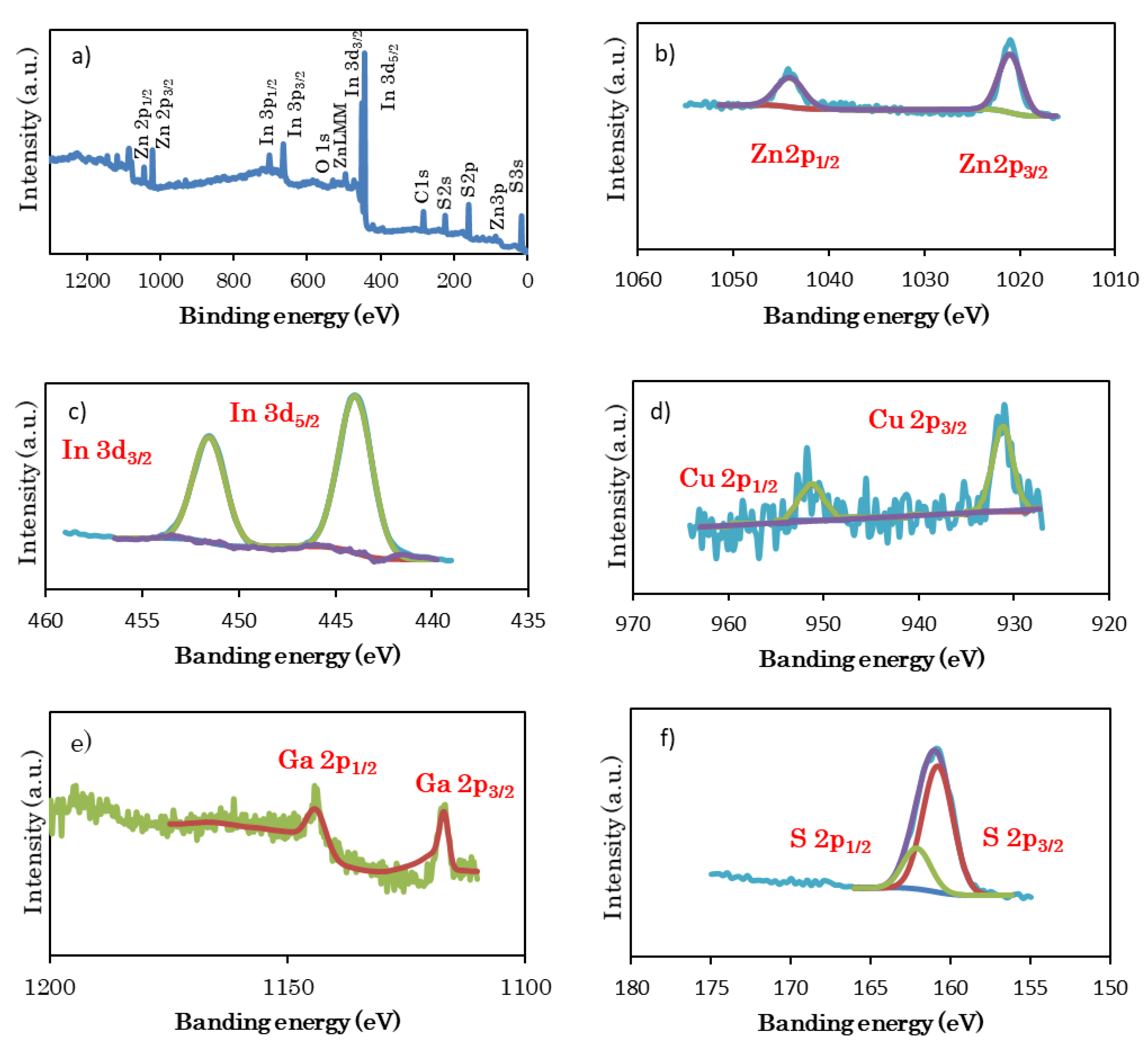
2.2. Characterization of Samples
2.3. Photocatalytic Hydrogen Generation
3. Results and Discussion
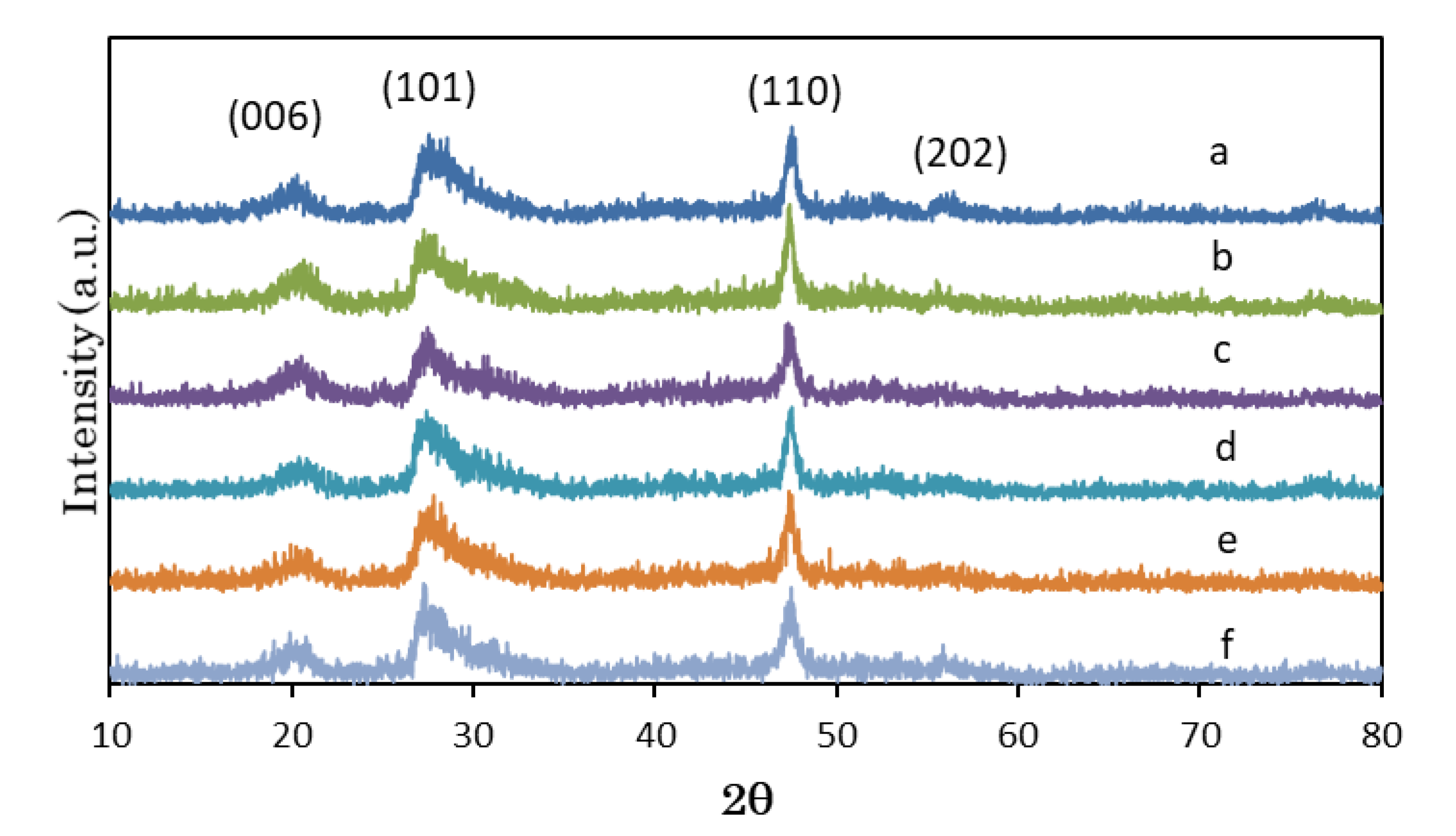
3.1. Structural Characterization
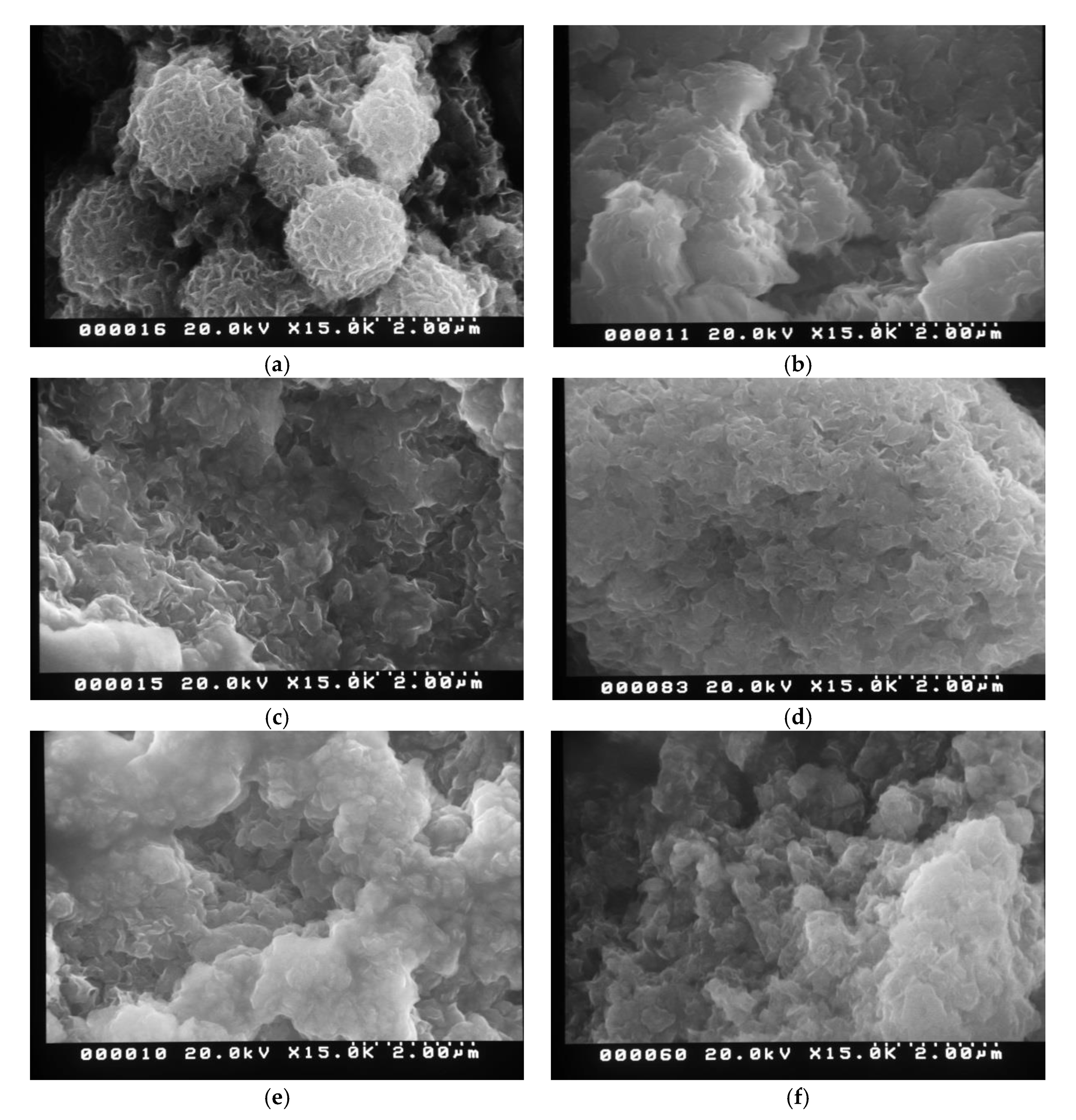
3.2. Morphological Analysis
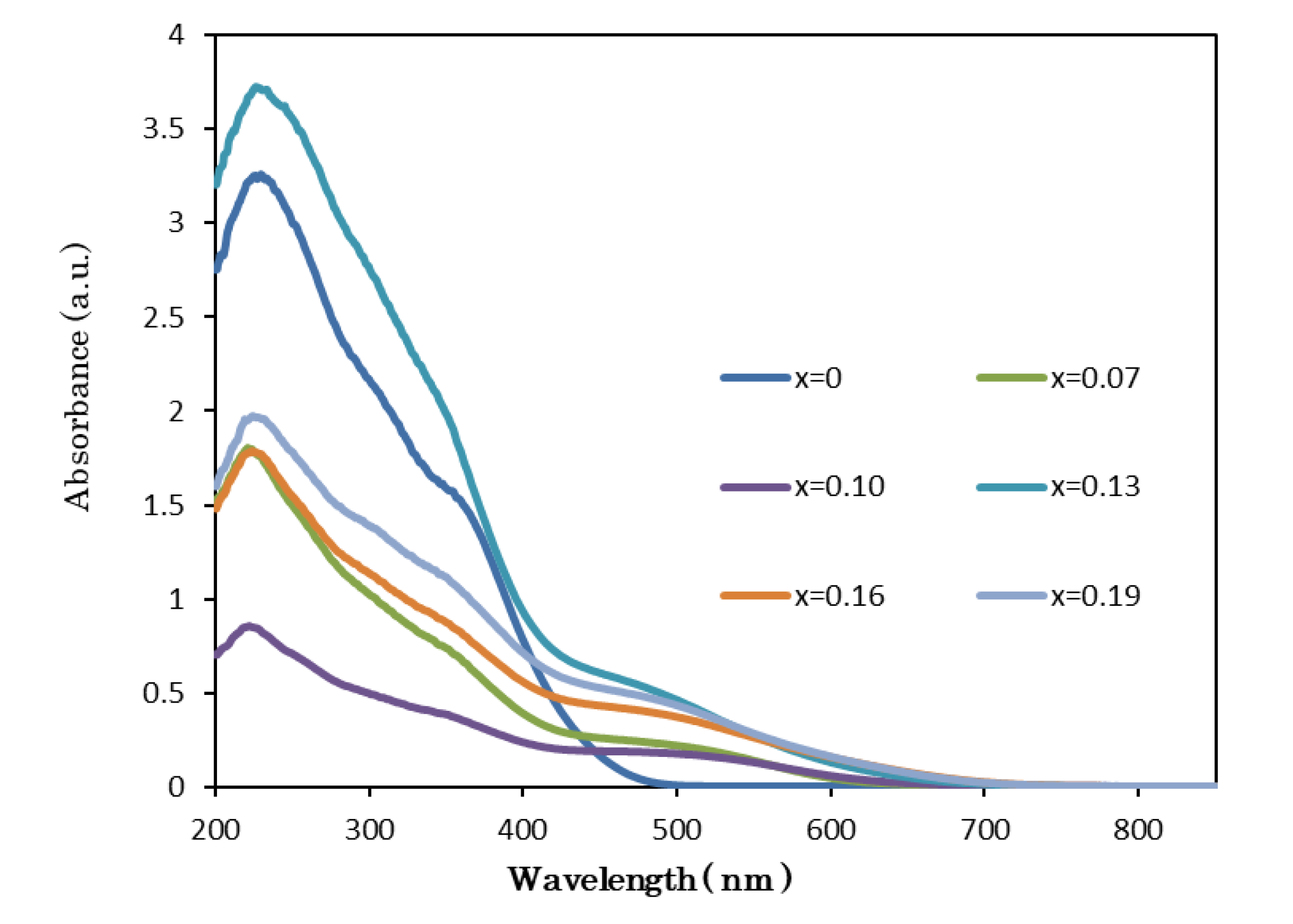
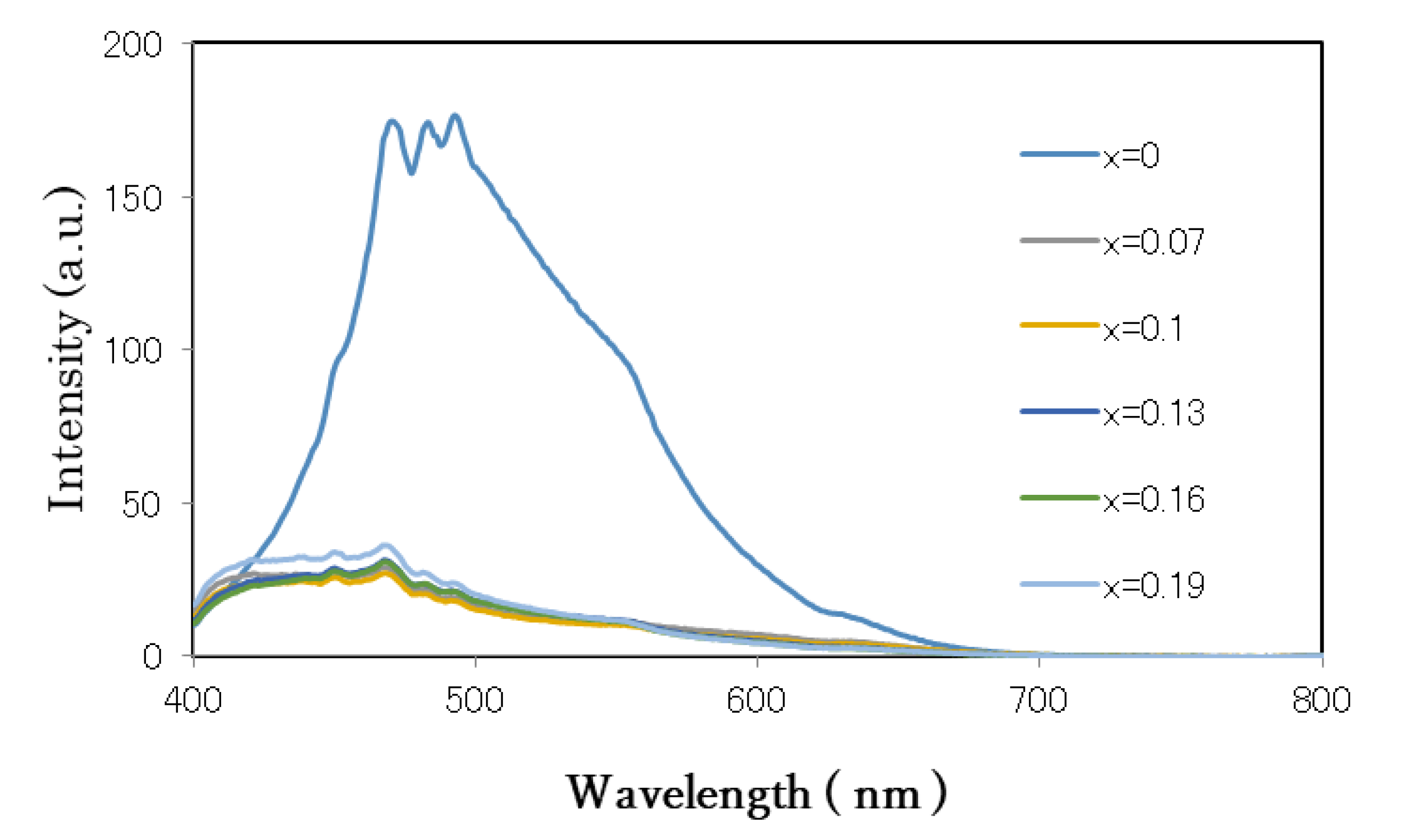
3.3. Optical Analysis
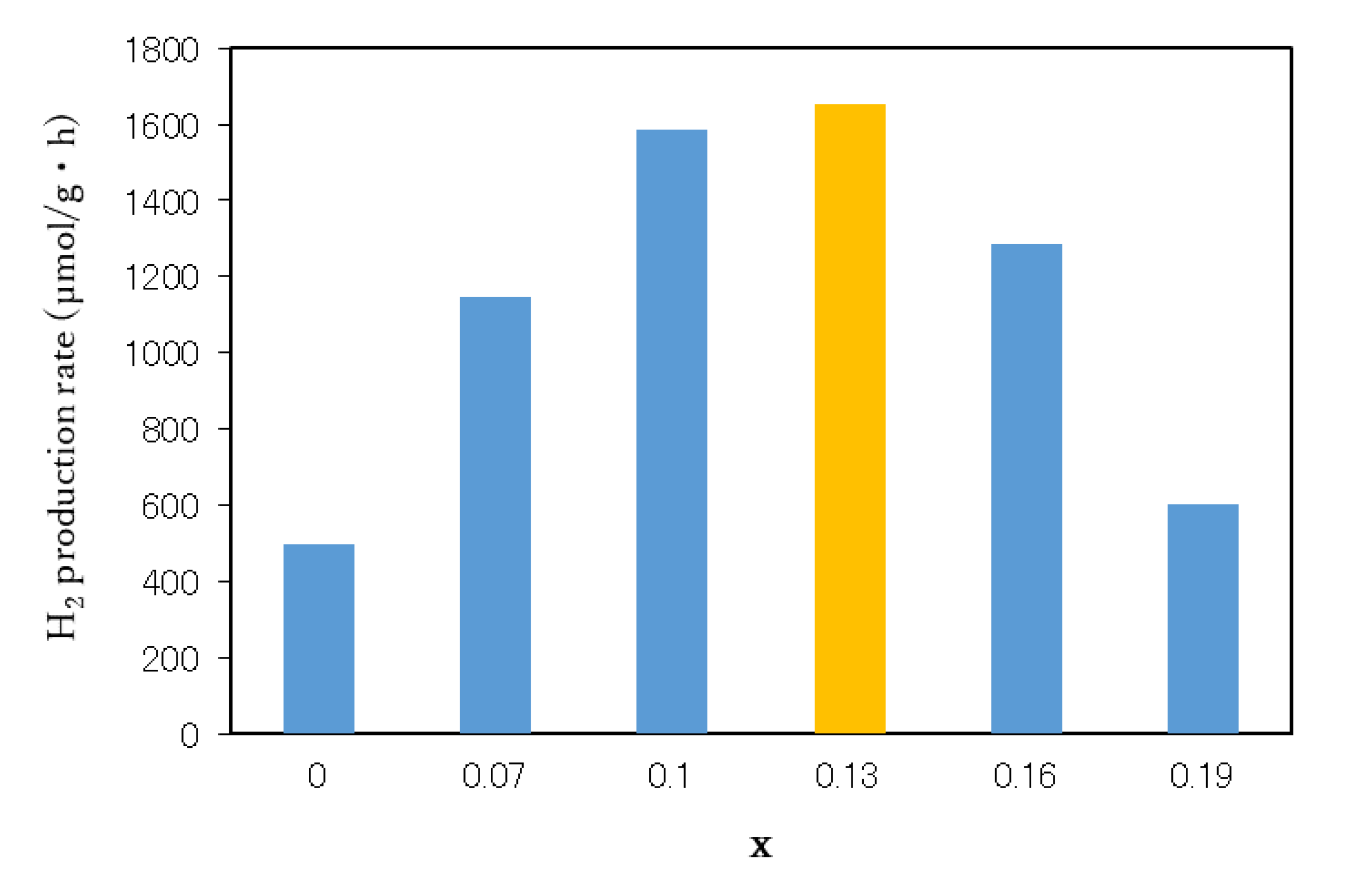
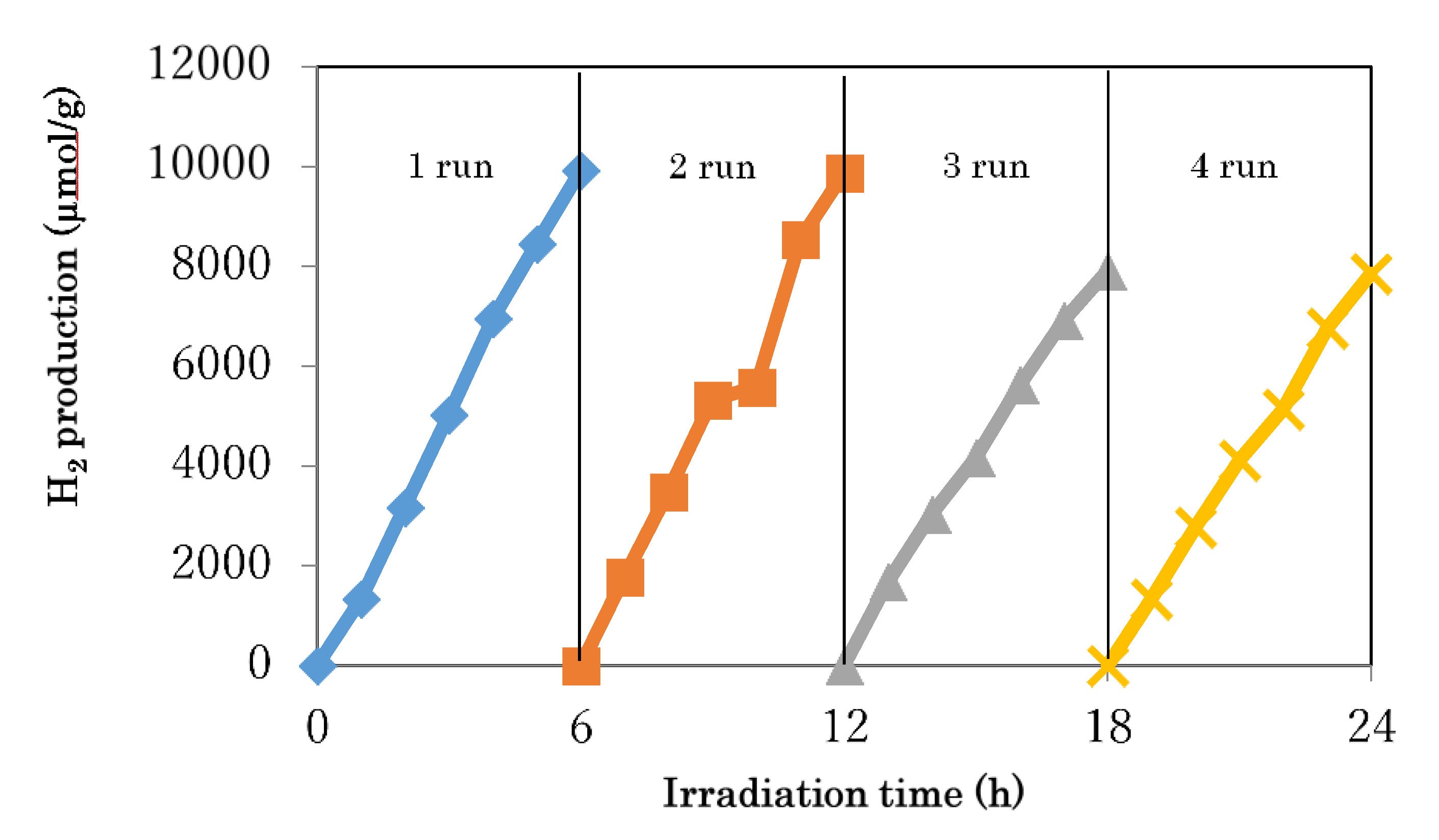
3.4. Photocatalytic Activity
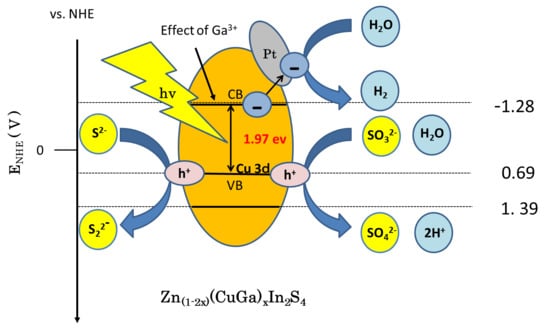
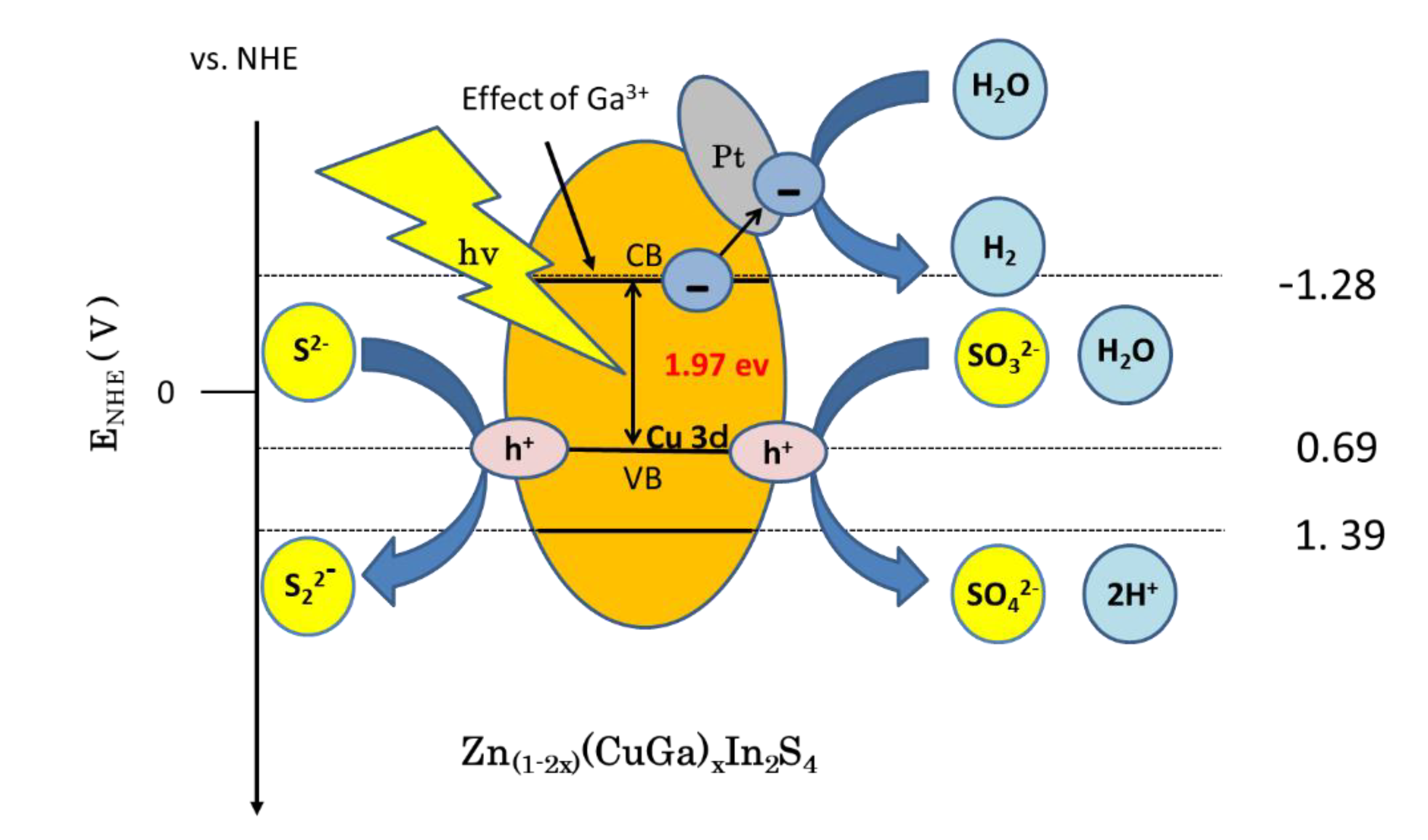
3.5. Proposed Hydrogenation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
Note
References
- Kudo, A. Development of photocatalyst materials for water splitting. Int. J. Hydrogen Energy 2006, 31, 197–202. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Photocatalytic water splitting into H2 and O2 over various tantalate photocatalysts. Catal. Today 2003, 78, 561–569. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Sorcar, S.; Hwang, Y.; Lee, J.; Kim, H.; Grimes, K.M.; Grimes, C.A.; Jung, J.-W.; Cho, C.-H.; Majima, T.; Hoffmann, M.R.; et al. CO2, water, and sunlight to hydrocarbon fuels: A sustained sunlight to fuel (Joule-to-Joule) photoconversion efficiency of 1%. Energy Environ. Sci. 2019, 12, 2685–2696. [Google Scholar] [CrossRef]
- Schreier, M.; Héroguel, F.; Steier, L.; Ahmad, S.; Luterbacher, J.S.; Mayer, M.T.; Luo, J.; Grätzel, M. Solar conversion of CO2 to CO using Earth-abundant electrocatalysts prepared by atomic layer modification of CuO. Nat. Energy 2017, 2, 17087. [Google Scholar] [CrossRef]
- Kumar, B.; Asadi, M.; Pisasale, D.; Ray, S.S.; Rosen, B.A.; Haasch, R.; Abiade, J.; Yarin, A.L.; Khojin, A.S. Renewable and metal-free carbon nanofiber catalysts for carbon dioxide reduction. Nat. Commun. 2013, 4, 2819. [Google Scholar] [CrossRef]
- Tsuji, I.; Kato, H.; Kobayashi, H.; Kudo, A. Photocatalytic H2 evolution reaction from aqueous solutions over band structure-controlled (Agln)xZn2(1−x)S2 solid solution photocatalysts with visible-light response and their surface nanostructures. J. Am. Chem. Soc. 2004, 126, 13406–13413. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhu, P.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S. Facile solution deposition of ZnIn2S4 nanosheet films on FTO substrates for photoelectric application. Nanoscale 2011, 3, 2602–2608. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Zhuang, C.; Peng, T.; Li, R.; Li, X. Highly Asymmetric Phthalocyanine as a Sensitizer of Graphitic Carbon Nitride for Extremely Efficient Photocatalytic H2 Production under Near-Infrared Light. ACS Catal. 2014, 4, 162–170. [Google Scholar] [CrossRef]
- Dai, W.W.; Zhao, Z.Y. DFT study on the interfacial properties of vertical and in-plane BiOI/BiOIO3 hetero-structures. Phys. Chem. Chem. Phys. 2017, 19, 9900–9911. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, V.; Iervolino, G.; Rizzo, L. Cu-doped ZnO as efficient photocatalyst for the oxidation of arsenite to arsenate under visible light. Appl. Catal. B 2018, 238, 471–479. [Google Scholar] [CrossRef]
- Shafaee, M.; Goharshadi, E.K.; Mashreghi, M.; Sadeghinia, M. TiO2 nanoparticles and TiO2@graphene quantum dots nancomposites as effective visible/solar light photocatalysts. J. Photochem. Photobiol. A Chem. 2018, 357, 90–102. [Google Scholar] [CrossRef]
- Chang, C.J.; Wang, C.W.; Wei, Y.H.; Chen, C.Y. Enhanced photocatalytic H2 production activity of Ag-doped Bi2WO6-graphene based photocatalysts. Int. J. Hydrogen Energy 2018, 43, 11345–11354. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, D.; Li, M.; Han, H.; Li, C. La and Cr Co-doped SrTiO3 as an H2 evolution photocatalyst for construction of a Z-scheme overall water splitting system. Chin. J. Catal. 2018, 39, 421–430. [Google Scholar] [CrossRef]
- Yan, X.; Xue, C.; Yang, B.; Yang, G. Novel three-dimensionally ordered macroporous Fe3+-doped TiO2 photocatalysts for H2 production and degradation applications. Appl. Surf. Sci. 2017, 394, 248–257. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Sharma, S.; Singh, R.C. Effect of tungsten doping on structural and optical properties of rutile TiO2 and band gap narrowing. Optik 2019, 182, 538–547. [Google Scholar] [CrossRef]
- Sharma, P.K.; Cortes, M.A.L.; Hamilton, J.W.; Han, Y.; Byrne, J.A.; Nolan, M. Surface modification of TiO2 with copper clusters for band gap narrowing. Catal. Today 2018, 321–322, 9–17. [Google Scholar] [CrossRef]
- Liu, C.; Chai, B.; Wang, C.; Yan, J.; Ren, Z. Solvothermal fabrication of MoS2 anchored on ZnIn2S4 microspheres with boosted photocatalytic hydrogen evolution activity. Int. J. Hydrogen Energy 2018, 43, 6977–6986. [Google Scholar] [CrossRef]
- Gao, P.; Li, A.; Sun, D.D.; Ng, W.J. Effects of various TiO2 nanostructures and graphene oxide on photocatalytic activity of TiO2. J. Hazard. Mater. 2014, 279, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liao, X.; Lee, M.-H.; Hyett, G.; Huang, C.C.; Hewak, D.W.; Mails, S.; Zhou, W.; Jiang, Z. Experimental and DFT insights of the Zn-doping effects on the visible-light photocatalytic water splitting and dye decomposition over Zn-doped BiOBr photocatalysts. Appl. Catal. B 2019, 243, 502–512. [Google Scholar] [CrossRef]
- Behzadifard, Z.; Shariatinia, Z.; Jourshabani, M. Novel visible light driven CuO/SmFeO3 nanocomposite photocatalysts with enhanced photocatalytic activities for degradation of organic pollutants. J. Mol. Liq. 2018, 262, 533–548. [Google Scholar] [CrossRef]
- Li, T.L.; Cai, C.D.; Yeh, T.F.; Teng, H. Capped CuInS2 quantum dots for H2 evolution from water under visible light illumination. J. Alloys Compd. 2013, 550, 326–330. [Google Scholar] [CrossRef]
- You, D.; Pan, B.; Jiang, F.; Zhou, Y.; Su, W. CdS nanoparticles/CeO2 nanorods composite with high-efficiency visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2016, 363, 154–160. [Google Scholar] [CrossRef]
- Jin, X.; Chen, F.; Jia, D.; Cao, Y.; Duan, H.; Long, M.; Yang, L. Influences of synthetic conditions on the photocatalytic performance of ZnS/graphene composites. J. Alloys Compd. 2019, 780, 299–305. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liu, C.; Luo, J.; Crittenden, J.; Liu, X.; Ca, T.; Yuan, J.; Pei, Y.; Liu, Y. Photocatalytic wastewater purification with simultaneous hydrogen production using MoS2 QD-decorated hierarchical assembly of ZnIn2S4 on reduced graphene oxide photocatalyst. Water Res. 2017, 121, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Chen, Z.H.; Liu, Q.; Zhang, Z.G.; Fang, X.M. One-pot hydrothermal synthesis of Ni-doped ZnIn2S4 nanostructured film photoelectrodes with enhanced photoelectrochemical performance. Appl. Surf. Sci. 2016, 370, 252–259. [Google Scholar] [CrossRef]
- Ding, Y.; Gao, Y.; Li, Z. Carbon quantum dots (CQDs) and Co(dmgH)2PyCl synergistically promote photocatalytic hydrogen evolution over hexagonal ZnIn2S4. Appl. Surf. Sci. 2018, 462, 255–262. [Google Scholar] [CrossRef]
- Zeng, C.; Huang, H.; Zhang, T.; Dong, F.; Zhang, Y.; Hu, Y. Fabrication of Heterogeneous-Phase Solid-Solution Promoting Band Structure and Charge Separation for Enhancing Photocatalytic CO2 Reduction: A Case of ZnXCa1–XIn2S4. ACS Appl. Mater. Interfaces 2017, 9, 27773–27783. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, X.; Zeng, Q.; Yan, T.; Ji, P.G.; Wu, T.T.; Wei, D.; Du, B. Facile synthesis of hierarchical ZnIn2S4/CdIn2S4 microspheres with enhanced visible light driven photocatalytic activity. Appl. Surf. Sci. 2017, 407, 328–336. [Google Scholar] [CrossRef]
- Liu, A.; Yu, C.; Lin, J.; Sun, G.; Xu, G.; Huang, Y.; Liu, Z.; Tang, C. Construction of CuInS2@ZIF-8 nanocomposites with enhanced photocatalytic activity and durability. Mater. Res. Bull. 2019, 112, 147–153. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Zhou, Z.; Guo, L. Enhanced Photocatalytic Hydrogen Evolution over Cu-Doped ZnIn2S4 under Visible Light Irradiation. J. Phys. Chem. C 2008, 112, 16148–16155. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; John, C.C.; Chen, Y.; Minakata, D.; Wang, P. Photocatalytic hydrogen production under visible-light irradiation on (CuAg)0.15In0.3Zn1.4S2 systhesized by precipitation and calcination. Chin. J. Catal. 2013, 34, 1926–1935. [Google Scholar] [CrossRef]
- Liu, X.; Khan, M.; Liu, W.; Xiang, W.; Guan, M.; Jiang, P.; Cao, W. Synthesis of nanocrystalline Ga–TiO2 powders by mild hydrothermal method and their visible light photoactivity. Ceram. Int. 2015, 41, 3075–3080. [Google Scholar] [CrossRef]
- Khallaf, H.; Chai, G.; Lupan, O.; Chow, C.; Park, S.; Schulte, A. Characterization of gallium-doped CdS thin films grown by chemical bath deposition. Appl. Surf. Sci. 2009, 255, 4129–4134. [Google Scholar] [CrossRef]
- Sola, A.C.; Gösser, M.B.; de la Piscina, P.R.; Homs, N. Promoter effect of Ga in Pt/Ga-TiO2 catalysts for the photo-production of H2 from aqueous solutions of ethanol. Catal. Today 2017, 287, 85–90. [Google Scholar] [CrossRef]
- Da Costa, R.C.; Rodrigues, A.D.; Pizani, P.S. Phase mixture, solid solution or composite: Raman scattering analyses of NixPb1-xTiO3 and (NiTiO3)xþ (PbTiO3)1−x. J. Alloys Compd. 2017, 697, 68–71. [Google Scholar] [CrossRef]
- Kaga, H.; Kudo, A. Cosubstituting effects of copper(I) and gallium(III) for ZnGa2S4 with defect chalcopyrite structure on photocatalytic activity for hydrogen evolution. J. Catal. 2014, 310, 31–36. [Google Scholar]
- Chai, B.; Liu, C.; Wang, C.; Yan, J.; Ren, Z. Photocatalytic hydrogen evolution activity over MoS2/ZnIn2S4 microspheres. Chin. J. Catal. 2017, 38, 2067–2075. [Google Scholar] [CrossRef]
- Cui, W.; Guo, D.; Liu, L.; Hu, J.; Rana, D.; Liang, Y. Preparation of ZnIn2S4/K2La2Ti3O10 composites and their photocatalytic H2 evolution from aqueous Na2S/Na2SO3 under visible light irradiation. Catal. Commun. 2014, 48, 55–59. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Q.; Lin, Z.; Yan, B.; Xia, C.; Yang, G. Half-unit-cell ZnIn2S4 monolayer with sulfur vacancies for photocatalytic hydrogen evolution. Appl. Catal. B 2019, 248, 193–201. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Lv, K.L.; Li, M. Heterojunction construction between TiO2 hollowsphere and ZnIn2S4 flower for photocatalysis application. Appl. Surf. Sci. 2017, 398, 81–88. [Google Scholar]
- Yue, W.; Han, S.; Peng, R.; Shen, W.; Geng, H.; Wu, F.; Tao, S.; Wang, M. CuInS2 quantum dots synthesized by a solvothermal route and their application as effective electron acceptors for hybrid solar cells. J. Mater. Chem. 2010, 20, 7570–7578. [Google Scholar] [CrossRef]
- Sudip, K.B.; Tian, L.; Venkatram, N.; Wei, J.; Vittal, J.J. Phase-selective synthesis of CuInS2 nanocrystals. J. Phys. Chem. C 2009, 113, 15037–15042. [Google Scholar]
- Zepeda, T.A.; Pawelec, B.; De Leon, J.D.; De los Reyes, J.A.; Olivas, A. Effect of gallium loading on the hydrodesulfurization activity of unsupported Ga2S3/WS2 catalysts. Appl. Catal. B 2012, 111–112, 10–19. [Google Scholar] [CrossRef]
- Chen, S.; Li, S.; Xiong, L.; Wang, G. In-situ growth of ZnIn2S4 decorated on electrospun TiO2 nanofibers with enhanced visible-light photocatalytic activity. Chem. Phys. Lett. 2018, 706, 68–75. [Google Scholar] [CrossRef]
| Photocatalyst | Zn(1−2x)(CuGa)xIn2S(4−1.5x) (x = 0, 0.07, 0.10, 0.13, 0.16, 0.19) Zn0.87Cu0.13In2S3.935, Zn0.87Ga0.13In2S4.065 |
|---|---|
| Cocatalyst | H2PtCl6 (1.0 wt%) |
| Medium | 0.25 M Na2SO3/0.35 M Na2S 40 mL, (pH 12) |
| Reactor | Pyrex glass vessel (volume: 123 mL) |
| Temperature | Room Temperature (25 °C) |
| Light source | Xenon lamp (λ ≧ 420 nm, 4500 µW/cm2) |
| Irradiation time | 6 h |
| Analysis | Gas chromatography (TCD) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tateishi, I.; Furukawa, M.; Katsumata, H.; Kaneco, S. The Effect of Cu and Ga Doped ZnIn2S4 under Visible Light on the High Generation of H2 Production. ChemEngineering 2019, 3, 79. https://doi.org/10.3390/chemengineering3040079
Tateishi I, Furukawa M, Katsumata H, Kaneco S. The Effect of Cu and Ga Doped ZnIn2S4 under Visible Light on the High Generation of H2 Production. ChemEngineering. 2019; 3(4):79. https://doi.org/10.3390/chemengineering3040079
Chicago/Turabian StyleTateishi, Ikki, Mai Furukawa, Hideyuki Katsumata, and Satoshi Kaneco. 2019. "The Effect of Cu and Ga Doped ZnIn2S4 under Visible Light on the High Generation of H2 Production" ChemEngineering 3, no. 4: 79. https://doi.org/10.3390/chemengineering3040079
APA StyleTateishi, I., Furukawa, M., Katsumata, H., & Kaneco, S. (2019). The Effect of Cu and Ga Doped ZnIn2S4 under Visible Light on the High Generation of H2 Production. ChemEngineering, 3(4), 79. https://doi.org/10.3390/chemengineering3040079