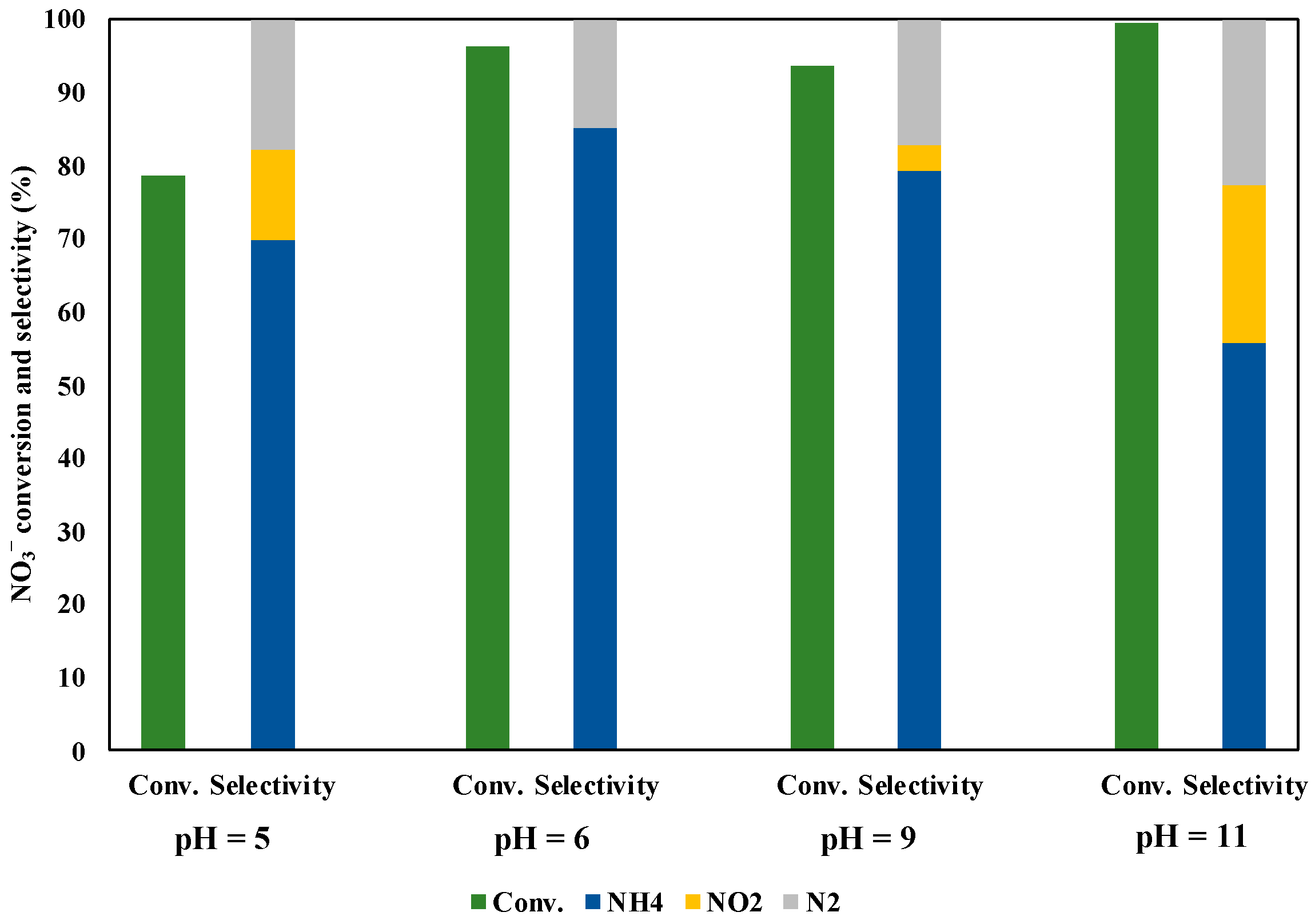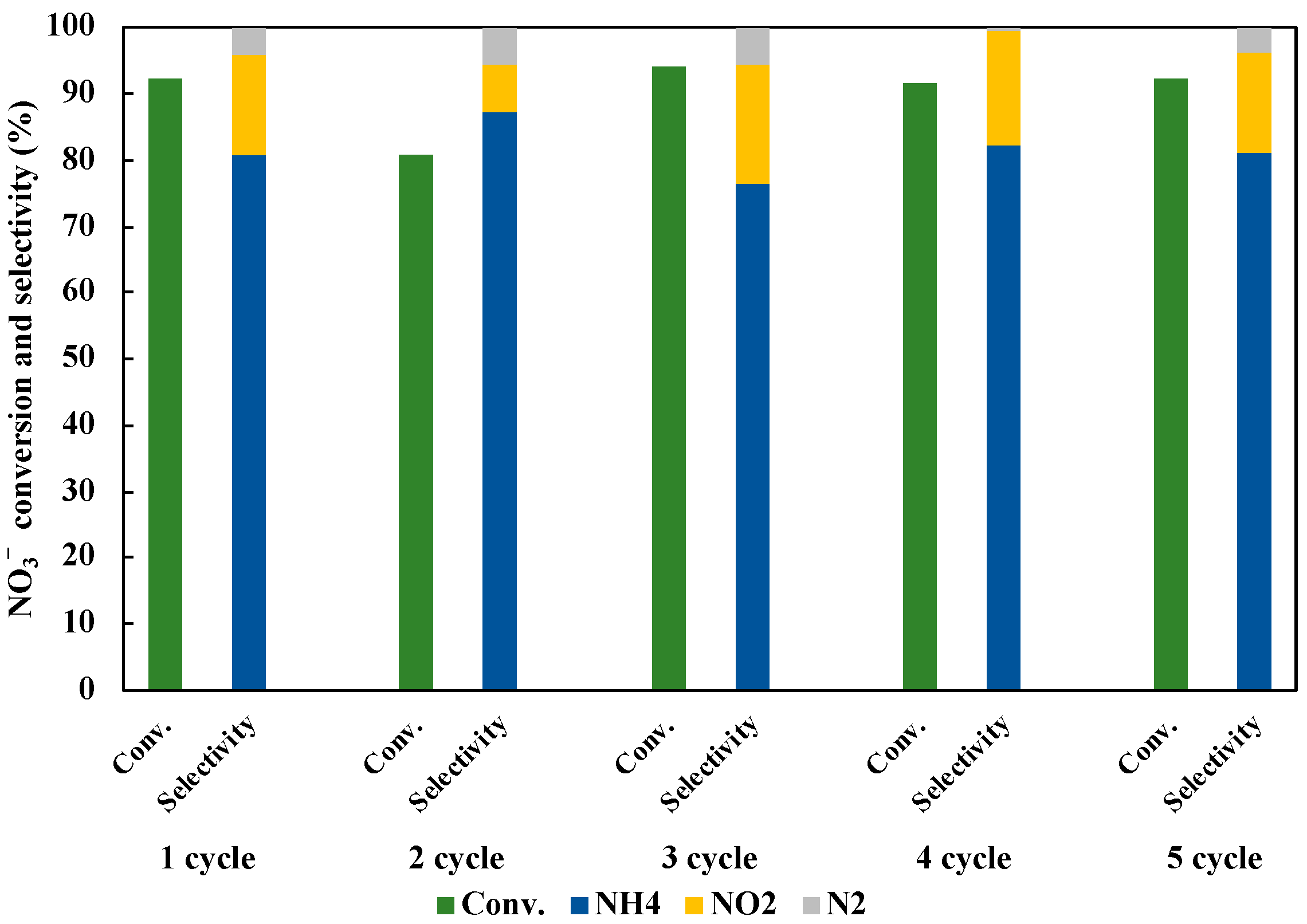Abstract
The highly effective reaction system was investigated for the photocatalytic ammonia synthesis from the reduction of nitrate ions by using the semiconductor photocatalyst, Cu and Ag doped on TiO2 (CuAg/TiO2) at room temperature under UV light irradiation (max. 352 nm). In this study, CuAg/TiO2 gave the high efficiency and the selectivity for the ammonia synthesis by the photoreduction of nitrate in the presence of methanol as a hole scavenger. For the evaluation of the photocatalytic activity over CuAg/TiO2, various TiO2 samples, such as standard TiO2, Cu/TiO2, and Ag/TiO2, were evaluated in the same procedure. The chemical properties were investigated by XRD, TEM, XPS, PL, and DRS. We examined the optimum conditions for the experimental factors and the important issues, including the effect of the molar ratio of Cu and Ag onto TiO2, the optimization of the CuAg amount loaded on TiO2, the influence of the loading amount of the catalyst on the reduction of nitrate ions, the exploration of the optimum hole scavenger, and the reusability of the optimum photocatalyst. The very efficient conversion of nitrate ions (95%) and the highest selectivity (86%) were achieved in the reaction with the optimum conditions. Here, we reported the process that nitrate ions can efficiently be reduced, and ammonia can be selectively synthesized over CuAg/TiO2.
1. Introduction
Ammonia (NH3) has recently been recognized as one of the most important chemical products in the present world [1]. It is used for the chemical fertilizers, dyes, medicines, and so on. Also, it is expected to utilize the energy careers with the hydrogen production and substitute to the CO2 free fuel [2]. Therefore, it clearly shows that the demand on the NH3 production expands, as the population increases in the future. However, there are some serious problems in the present NH3 production. In the current NH3 synthesis, NH3 is directly synthesized by N2 and H2 over an iron-based catalyst (Haber-Bosch process). However, this method needs the conditions at high temperature (400–500 °C) and high pressure (150–250 bar), which is not a sustainable process in the NH3 production for a long time. In addition to this disadvantage, a large amount of fossil fuels is consumed, and its production simultaneously releases a huge amount of CO2 [3]. Therefore, these issues may create significantly negative effects for the environment on the earth. Many studies were evaluated for the NH3 synthesis with the operation using the low energy without using fossil fuel [4]. As the method for the substitution of Haber Bosch process, various ammonia production processes were investigated such as following methods; the plasma catalysis [5], the electrochemical system [6], the production from biomass [7].
The nitrate ion (NO3−) reduction by using the photocatalysts should be noted as the method to synthesize NH3. Various photocatalysts were applied into the new approaches for the photocatalytic NH3 synthesis are as follows: M/TiO2 (M = Ru, Rh, Pd, Pt) [8], Ru/C [9], Au/SrTiO3 [10], and BiOCl [11] as the photocatalysts for synthesizing NH3 at a lower reaction temperature and pressure. For the application to the industrial process, it is suggested that NO3− is separated from wastewater by the ion exchange method, and the catalyst is added to this treated water including NO3−. After the photocatalytic reduction of NO3− in the treated water, ammonium ions are generated in the solution. This can be collected as ammonia by the ammonia stripping process. In this process, ammonia is released in the gas phase by adjusting to the basic pH condition. However, the above catalysts did not indicate the high reactivity in the reduction processes because of the poor photocatalytic activity.
Titanium dioxide (TiO2) is one of the well-known semiconductor photocatalysts, which absorb the UV light and can cause the photocatalytic reaction. Also, TiO2 has some advantages such as the very high stability in the solution with the various pH, the low cost, and the high practicality due to easy handling [12]. Therefore, the reaction system of the photocatalytic NO3− reduction to NH3 over metal-doped TiO2 may be significant as a green process for the photocatalytic NH3 synthesis by the reduction of NO3− in the lower energy without using fossil fuel. As the source of N2, NO3− has attracted attention by the recent studies because it is the very toxic pollutant in the waste water and gives the very harmful effect to human health [13]. Therefore, the NH3 synthesis by using NO3− as a raw material may enable to contribute to the improvement of the environmental issues by the decontamination of waste water including NO3− and the development of the excellent ecological approach for the NH3 production.
However, the reduction efficiency of NO3− and the selectivity of NH3 are still very poor in the photocatalytic reduction of NO3− [14]. Methanol (CH3OH) may be applied into the reduction of NO3− to improve this problem. Generally, CH3OH was used as a hole scavenger and it was oxidized to formaldehyde by holes. Also, the reduction simultaneously produces reactive hydrogen [15], which results in the more efficient reduction of NO3−. Thus, CH3OH may become very useful for the photocatalytic NO3− reduction.
In the present study, we used Cu and Ag as doping metals for the preparation of photocatalysts. Cu is one of the well-known metals and it is widely applied to the photocatalysts because it has several advantages of the high electro conductivity and the low cost. Noble metals, such as Au, Ag, and Pt show the localized surface plasmonic resonance (LSPR), which has the ability to absorb the visible light [16]. It can improve the photoabsorption by its light trapping effects [17]. Also, it is indicated that these metals nanoparticles can perform as an electron donor to promote the electron transfer from metal to semiconductor [18,19]. In addition to this function, these metals can suppress the recombination of surface electrons and holes [20,21]. In the photocatalytic reaction over these metals doped TiO2, Kamat et al. proposed that the excited electrons were transferred in these metals to TiO2. Therefore, the Cu and Ag doped TiO2 photocatalyst minimized the recombination of photoelectron-hole and gave the high photoabsorption and the proper bandgap for the photocatalytic reaction [22]. Here, we investigated the method for the selective NH3 synthesis by the photocatalytic reduction of NO3− over Cu and Ag doped TiO2 (CuAg/TiO2). The reaction was operated in the presence of 50 ppm of NO3− aqueous solution including 10 vol% CH3OH at room temperature under UV irradiation (λ: max. 352 nm). In the present work, we examined in detail the photocatalytic activity, the optimization of the experimental conditions, and the reusability of the optimum catalyst. This reaction system would give the excellent approach for the selective NH3 synthesis by photocatalytic NO3− reduction over the optimized photocatalyst.
2. Materials and Methods
2.1. Materials
Titanium dioxide (TiO2-P25, 99.50%) was purchased from Degussa Co. Cu 1000 ppm standard solution Cu(NO3)2 in 0.1 mol/L HNO3 was obtained from FUJIFILM Wako Pure Chemical Corp. Ag 1000 ppm standard solution (AgNO3 in 0.1 mol/L HNO3) and potassium nitrate (KNO3) were obtained from Nacalai Tesque Inc. All of the reagents and the materials used in this research were analytical grade and were used without further purification. KNO3 and distilled water were used for the preparation of the simulated waste water including NO3−. Methanol (CH3OH) was added to the simulated waste water as a hole scavenger. For the evaluation of the optimum hole scavenger, ethanol (C2H5OH), isopropyl alcohol ((CH3)2CHOH), and t-butyl alcohol ((CH3)3COH) were applied in this study.
2.2. Preparation of TiO2 Samples
Cu2+ 1000 ppm standard solution, Ag+ 1000 ppm standard solution and 300 mg of TiO2 were added in 10 vol% CH3OH aqueous solution. The total amount of metals (Cu and Ag) were 1 wt% for the amount of TiO2. The mixed solution was stirred under blacklight irradiation (λ: max 352 nm) for an hour. After the centrifugation, the supernatant was removed, and the photocatalyst was dried under vacuum overnight. The different TiO2 samples were obtained in this preparation method (Table S1). The obtained products were used for NH3 synthesis by NO3− reduction.
2.3. Characterization
X-ray diffraction (XRD) was performed by Ultima Ⅳ, RIGAKU with Cu-Kα radiation, equipped with the graphite monochromator on the diffracted beam. The morphology of the catalysts was operated by transmission electron microscope (TEM) using JEM-1011, HITACHI. X-ray photoelectron spectroscopy (XPS) was measured by PHI Quantera SXM, ULVAC-PHI. The C1s peak at 284.6 eV was used to calibrate all of the binding energy. The photoluminescence spectra (PL) were given by a fluorescence spectrophotometer (RF-5300PC, SHIMADZU) at an excitation wavelength of 330 nm using D2 lamp at ambient temperature. UV-Vis diffuse reflectance spectra (DRS) were operated on V-750 spectrophotometer, JASCO in the range of 200–800 nm using D2 lamp.
2.4. Photocatalytic Reduction of NO3− to NH3
The photocatalytic reduction of NO3− was performed in the Pyrex reactor. Firstly, 50 ppm of NO3− aqueous solution (45 mL) including 10 vol% CH3OH was prepared, and 30 mg of the photocatalyst was added to the solution. The suspension was stirred with a magnetic stirrer at room temperature under UV irradiation for 3 hours. As the light source, black light (max: 352 nm) was used for NO3− reduction. The measurements of the concentration of ions (NO3−, NO2−, and NH4+) in the solution after the reaction were performed by ion chromatography (Metrohm Compact IC 761).
3. Results and Discussion
3.1. Calculation of the NO3− Conversion and Selectivity
The NO3− conversion and the selectivity of the products were calculated by the following equations:
where [n]0 is the amount of material (mmol) at time = 0 and [n]t is the amount of material (mmol) at time = t.
3.2. Characterization of Photocatalysts
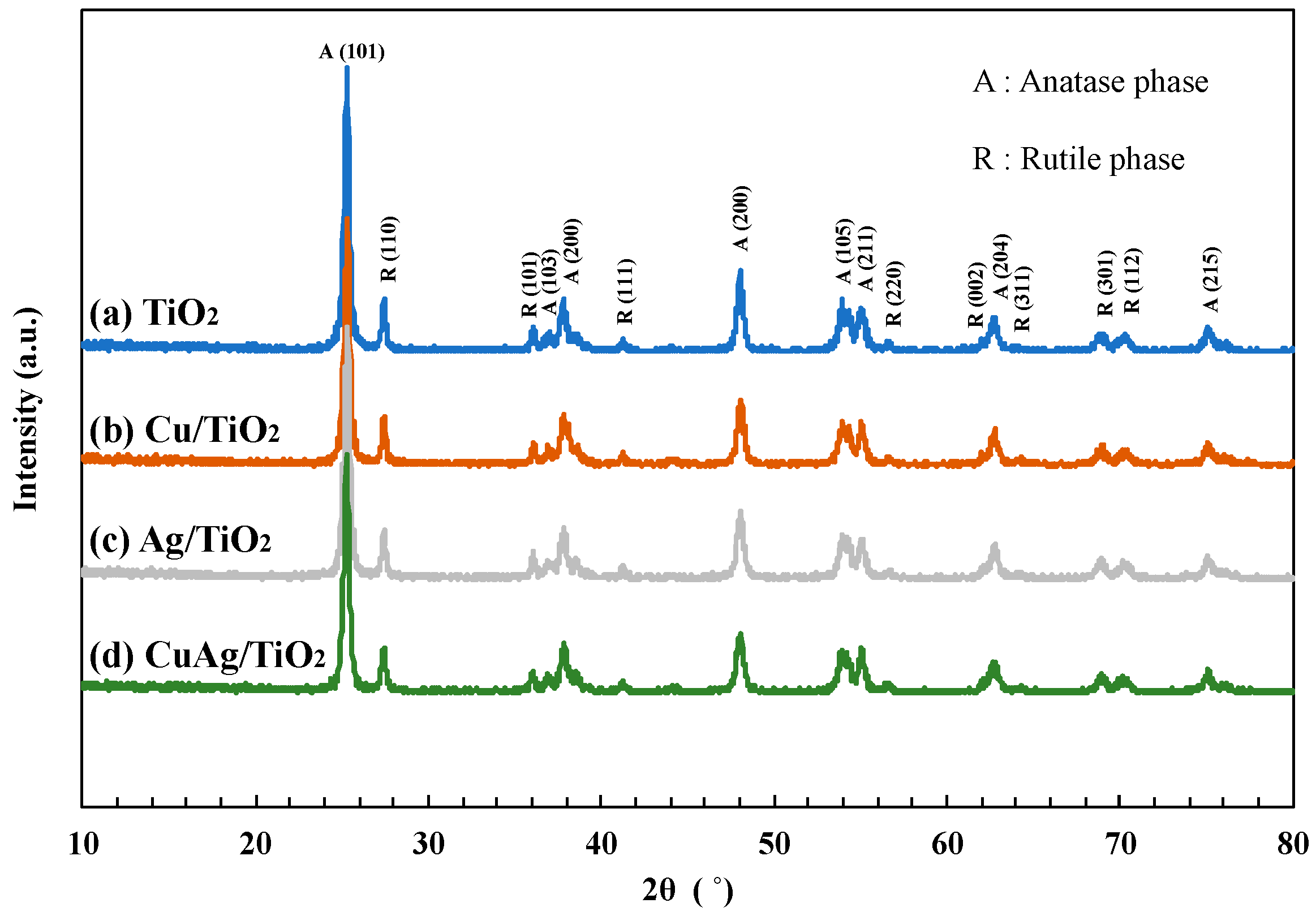
X-ray diffraction (XRD) spectra of various TiO2 samples were shown in Figure 1. The TiO2 P25 particle is consisted of anatase phase and rutile phase. The structure ratio of anatase phase and rutile phase are 80% and 20%, respectively. The characteristic patterns of anatase phase were clearly found at 2θ = 25.2° (101), 37.0° (103), 37.9° (200), 48.0° (200), 53.9° (105), 55.0° (211), 62.8° (204), and 75.1° (215). The typical patterns of the rutile phase were observed at 2θ = 27.5° (110), 36.0° (101), 41.3° (111), 56.5° (220), 62.0° (002), 64.2° (311), 69.0° (301), and 70.3° (112) [23]. These sharp peaks showed that the crystallinity of all TiO2 samples was very high. However, the peaks of Cu and Ag were not confirmed due to the relatively low amount of metals loaded on TiO2. Also, the XRD results of other TiO2 samples used in this study were summarized in Figure S1.
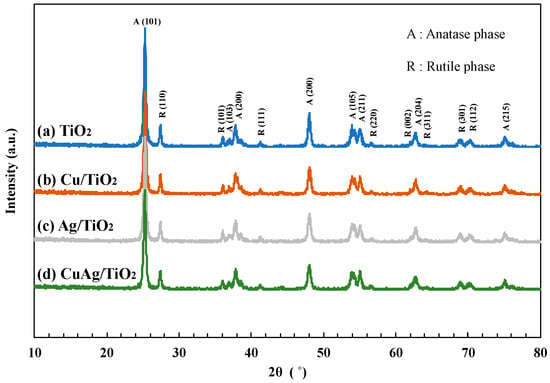
Figure 1.
XRD spectra of (a) TiO2 P25, (b) Cu/TiO2, (c) Ag/TiO2 and (d) CuAg/TiO2.
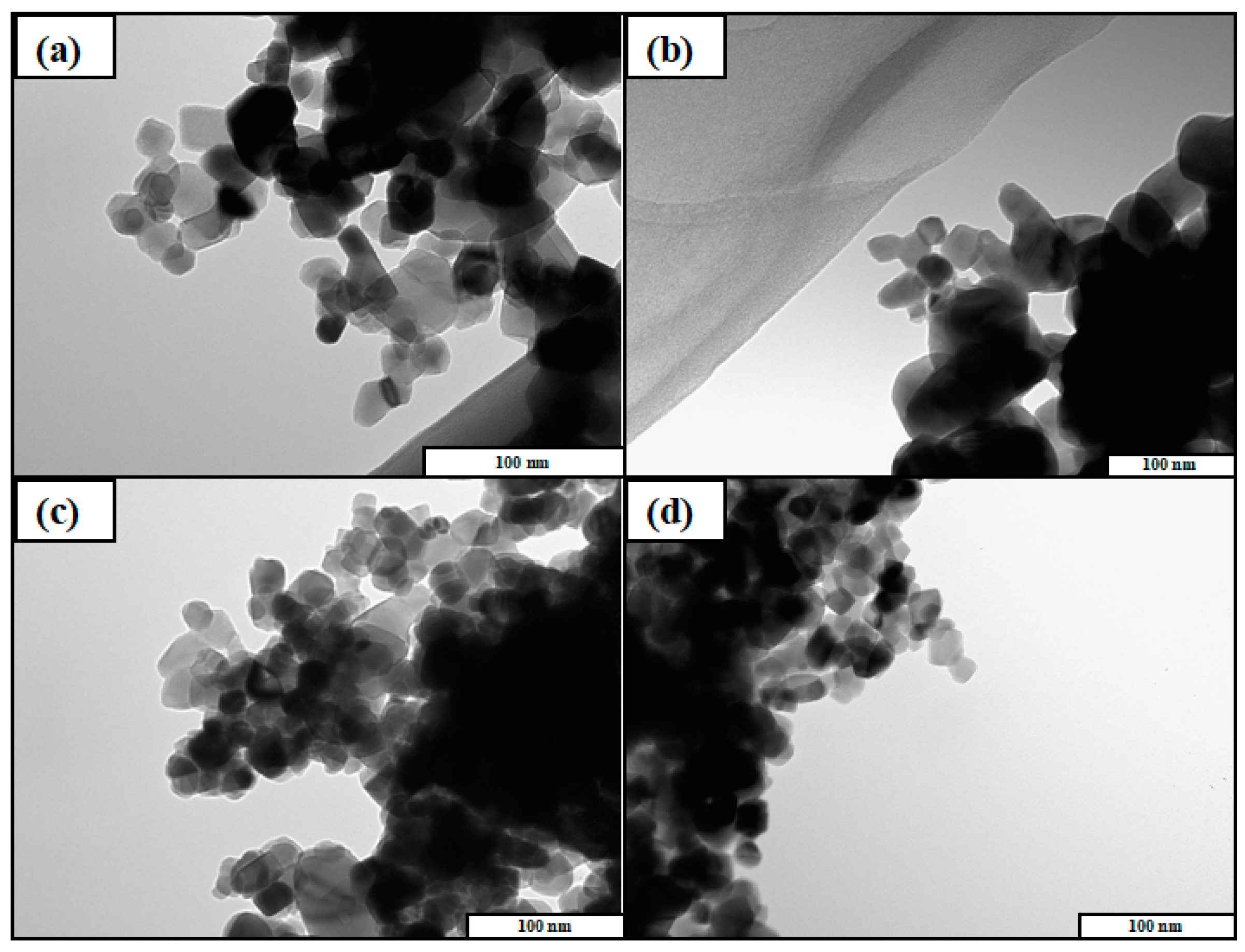
The TEM images of the photocatalysts were shown in Figure 2. As a result, the existence of Cu and Ag was not confirmed, as the amount of Cu and Ag was very low compared with the amount of TiO2. On the other hand, it was obviously confirmed that all of the TiO2 samples had the same morphology. Therefore, it was indicated that the morphology of photocatalyst could not be changed by doping Cu and Ag. TEM images of other TiO2 samples used in this study were shown in Figure S2. All of the TiO2 samples were nanoparticles and the size were roughly 20–30 nm. Cu and Ag particles were not observed because they are too small to be detected by TEM.
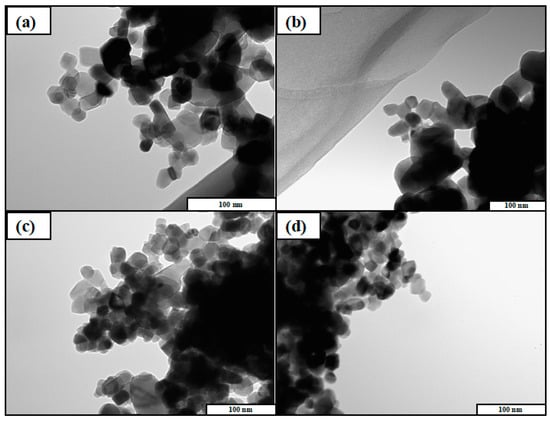
Figure 2.
Transmission electron microscope (TEM) images of (a) TiO2, (b) Cu/TiO2, (c) Ag/TiO2, and (d) Cu0.9Ag/TiO2.
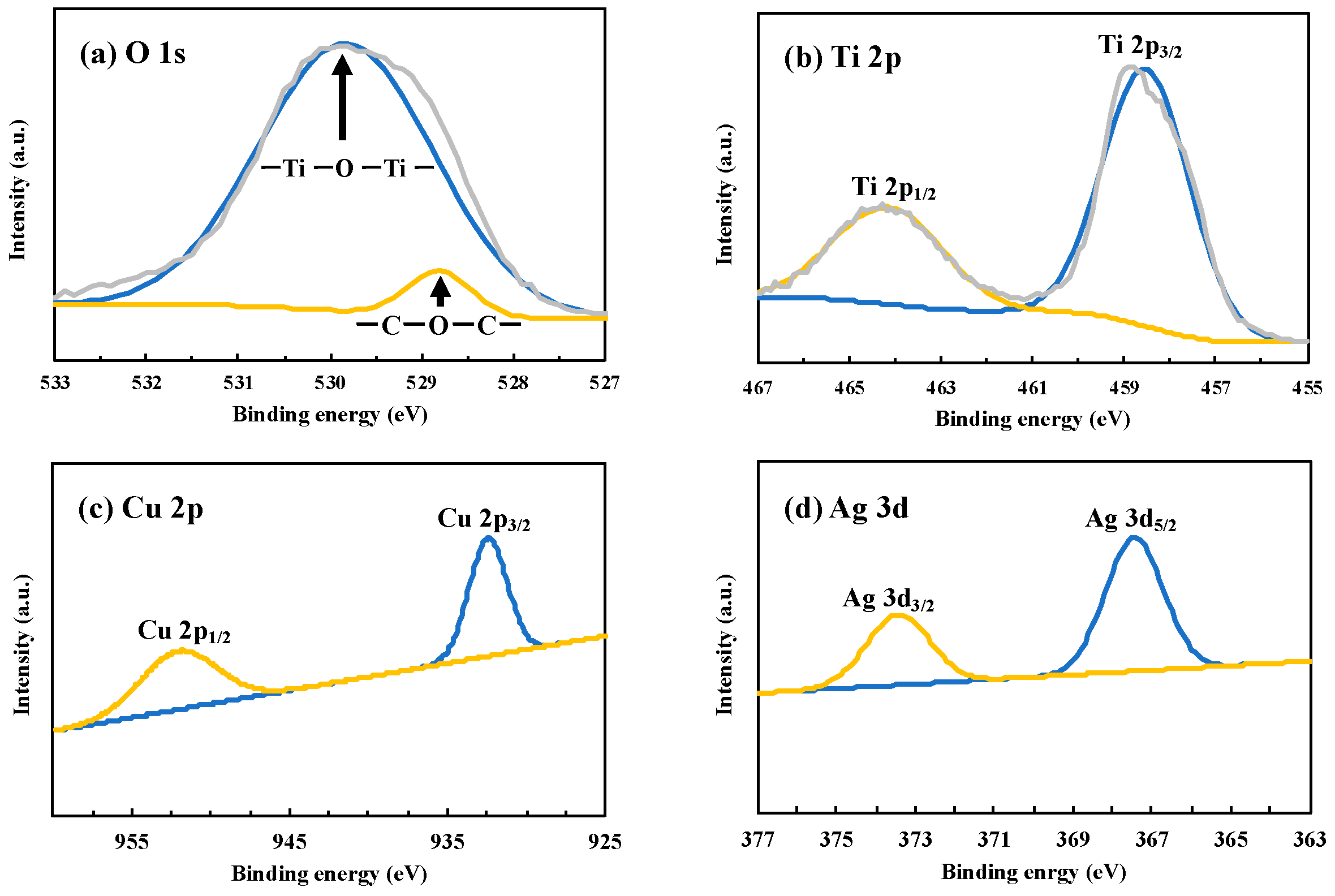
For the identification of the composition of Cu and Ag on TiO2, X-ray photoelectron spectroscopy (XPS) was applied to the Cu0.9Ag/TiO2 composite photocatalyst (Figure 3). The peak of C1s was used for the calibration, which was attributed to the contaminated carbon [24,25].
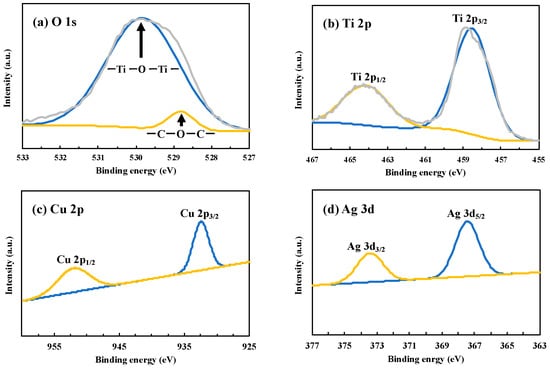
Figure 3.
XPS spectra of (a) O 1s, (b) Ti 2p, (c) Cu 2p, and (d) Ag 3d over CuAg/TiO2.
In the O 1s signals, the presence of Ti-O of titania at about 530 eV could be confirmed, and the peak at 528.9 eV was attributed to CO (Figure 3a) [26]. The spectrum region corresponding to Ti 2p revealed that two peaks at 464.1 eV and 458.6 eV were due to Ti 2p1/2 and Ti 2p3/2, respectively. As the results, we assigned it with 4+ oxidation number by analyzing the intensity of Ti 2p3/2 of this sample. These peaks were highly sharp and intense, so that it indicated that this photocatalyst consists only of Ti4+ species [27]. This result shows that Cu0.9Ag/TiO2 was properly prepared and its structure was not changed by the deposition of Cu and Ag under UV irradiation.
The narrow scan of Cu 2p of Cu0.9Ag/TiO2 showed two peaks at 950.9 eV and 931.9 eV and these peaks were attributed to Cu 2p1/2 and Cu 2p3/2, respectively (Figure 3c). However, it cannot easily be distinguished between Cu0 and Cu2+, because the difference of the binding energy between Cu0 and Cu2+ is only 0.1 eV. In addition, Ag 3d peaks at 373.2 eV and 367.1 eV were corresponding to Ag 3d3/2 and Ag 3d5/2, respectively (Figure 3d) [28]. These characteristic peaks indicated that it was existed as metal Ag [29].
As a result, the existence of Cu and Ag was confirmed in the samples, and all of elements including O, Ti, Cu, and Ag were observed. It clearly showed that Cu and Ag were successfully formed on TiO2. In addition to these results, XPS spectra of other TiO2 samples used in this study were shown in Figures S3–S6.
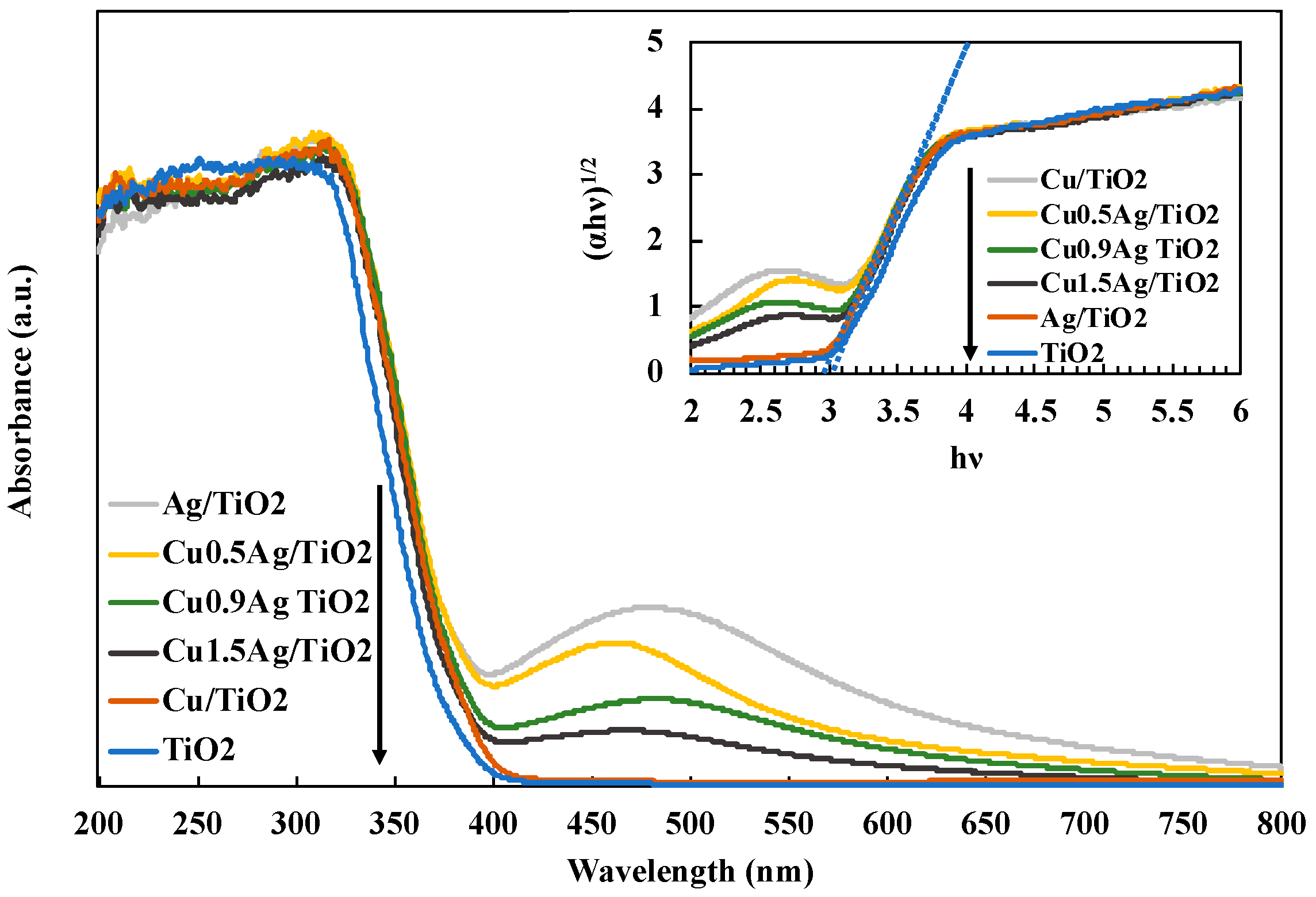
UV-Vis diffuse reflectance spectra (DRS) were evaluated to identify the optical absorption properties (Figure 4). The absorption edge of the standard TiO2 was about 380 nm. All of TiO2 samples were confirmed to show the light absorption in the UV region, and the intensity was enhanced by loading Cu and Ag doped on TiO2. In this research, the band-gap energy was calculated by following formula [30]:
where Eg refers to the band-gap energy and λg is the wavelength at the absorption edge [16]. The band-gap energies Eg of TiO2 and Cu0.9Ag/TiO2 were estimated to 3.26 eV and 2.92 eV, respectively.
Eg (eV) = 1240/λg (nm)
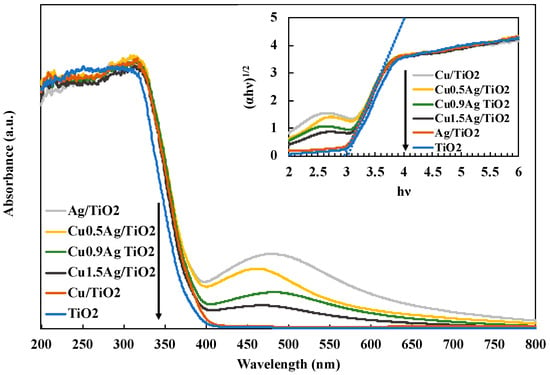
Figure 4.
DRS spectra of various TiO2 samples.
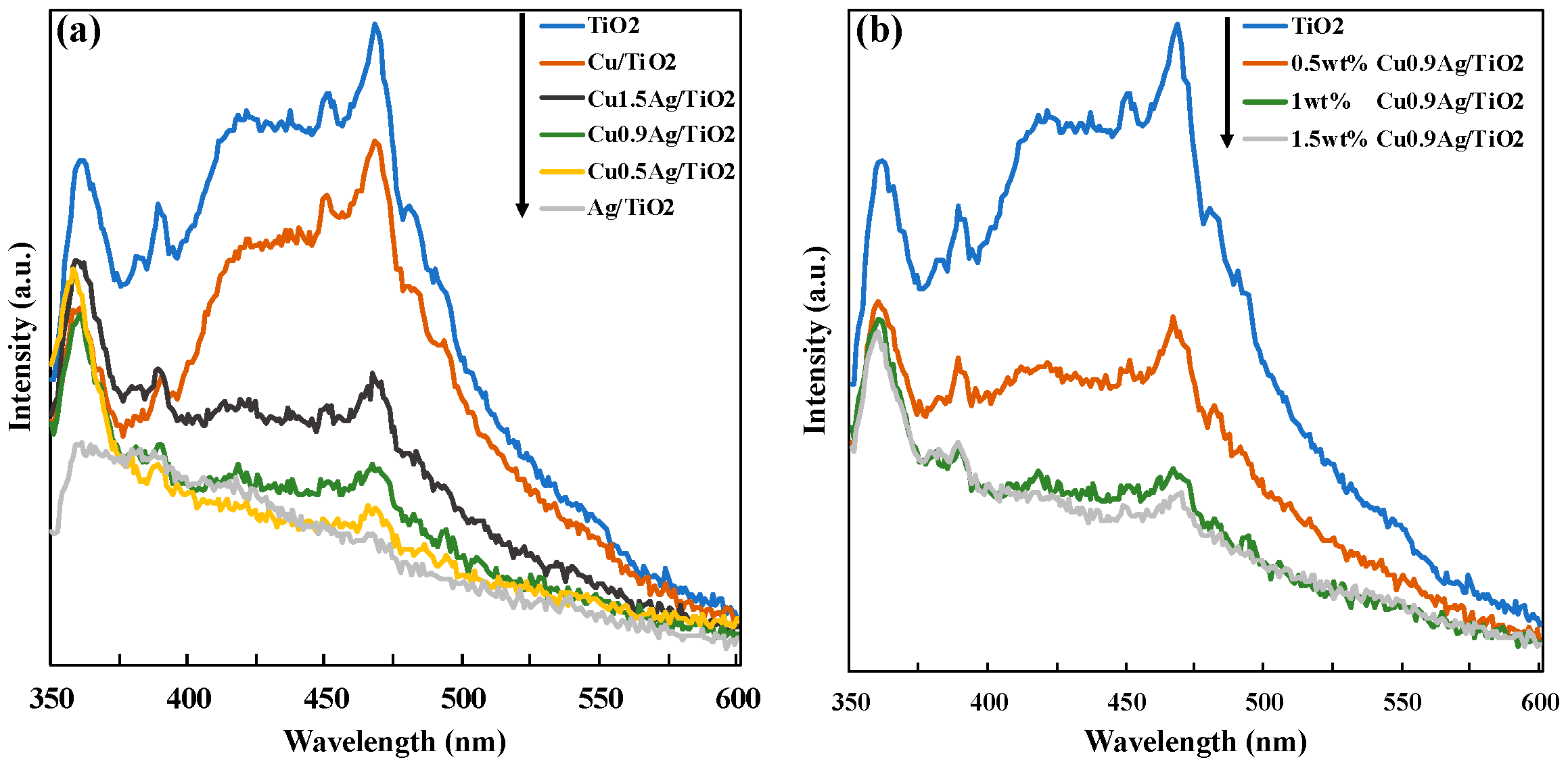
Photoluminescence (PL) spectra were measured to examine the recombination of electron-hole over the photocatalysts (Figure 5). All of the PL spectra were obtained in the range of 350–600 with an excitation wavelength at 330 nm. The comparison of TiO2 and metals-doped TiO2 samples was investigated in Figure 5a. Compared with standard TiO2, metal-doped TiO2 samples showed the low intensity. It revealed that loading metals gave the decrease of the PL intensity, and the recombination of photogenerated electron and hole was suppressed by doping metals on TiO2. This reason may be considered it that the electrons were efficiently trapped by CuAg bimetal. The PL spectra of the TiO2 samples with the different amount of the CuAg doped on TiO2 were shown in Figure 5b. As the amount of CuAg increased, the PL intensity decreased. Therefore, the doping of Cu and Ag gave the good effect on the recombination of charge careers, which showed the high photocatalytic activity.
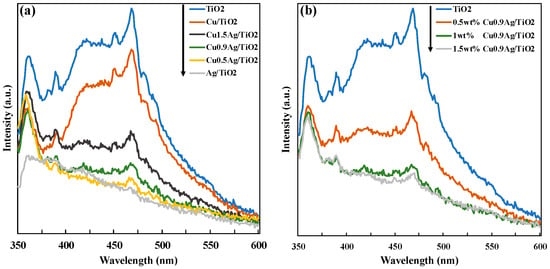
Figure 5.
(a) Comparison of PL spectra of the different TiO2 samples, and (b) effect of the loading amount of CuAg alloy onto TiO2.
3.3. Photocatalytic Activity in NO3− Reduction to NH3
Firstly, the evaluation for the photocatalytic activity in the reduction of NO3− to NH3 was performed over TiO2 alone, Cu/TiO2, Ag/TiO2, and CuAg/TiO2 in the presence of CH3OH under UV irradiation for three hours (Figure 6). The amount of photocatalyst was 30 mg in all of test cases. In the photocatalytic NO3− reduction, NH4+, NO2−, and N2 seem to be mainly generated as the products. In addition, CH3OH was oxidized to HCHO by a photocatalytic reaction as a hole scavenger and the reduction produced hydrogen, which played an important role in NO3− reduction by following the equations:
2H+ + 2e− → H2
NO3− + H2 → NO2− + H2O
NO3− + 4H2 → NH4+ + H2O + 2OH−
NO3− + 5/2H2 → 1/2N2 + 2H2O + OH−
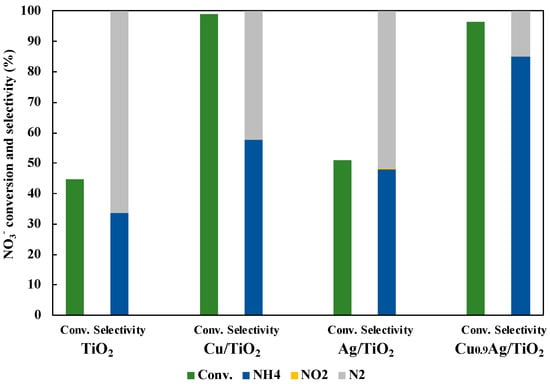
Figure 6.
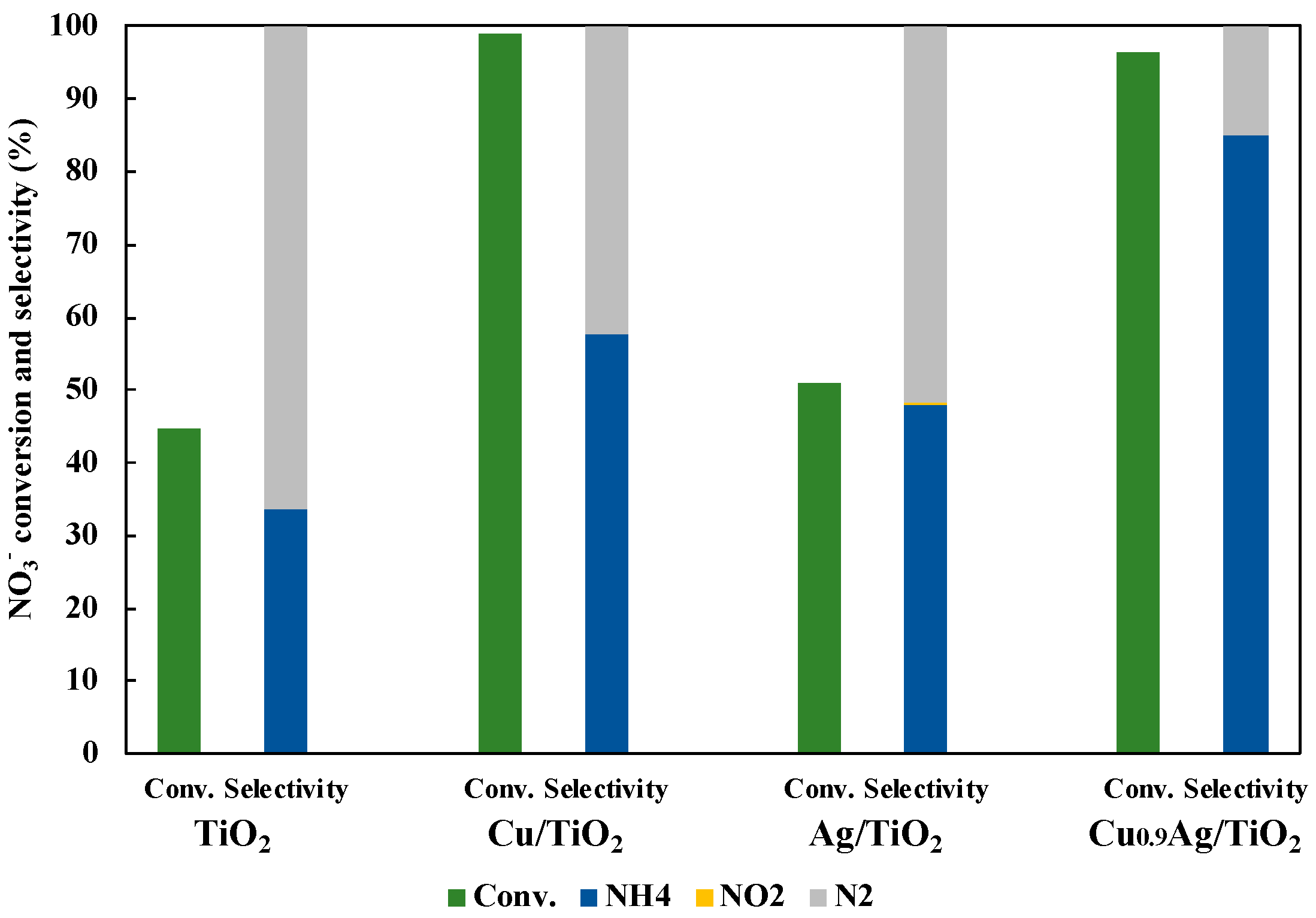
Comparison of photocatalytic activity over TiO2, Cu/TiO2, Ag/TiO2 and Cu0.9Ag/TiO2 in 45 mL of 50 ppm NO3− aqueous solution including 10 vol% CH3OH under UV irradiation for three hours. The loading amount of each samples was 30 mg. Left bar is NO3− conversion, right bars (blue, yellow, and gray) are product selectivity (NH4+, NO2−, and N2 product, respectively).
As a result, Cu0.9Ag/TiO2 gave the very efficient NO3− conversion (96%) and the highest NH3 selectivity (85%), while the NO3− conversion and the NH3 selectivity over TiO2 alone were only 44.9% and 33.6%, respectively. Also, the reaction had not occurred without the photocatalyst. In addition to these results, the comparison of the photocatalytic activity with other catalysts was performed (Table S2). Consequently, it clearly showed that loading the optimum amount of Cu and Ag onto TiO2 gave the excellent photocatalytic activity in the reduction of NO3− to NH3. Hence, CuAg/TiO2 was very useful to photocatalytic NO3− reduction rather than other TiO2 samples.
3.4. Optimization of Structure and Loading Amount of CuAg/TiO2
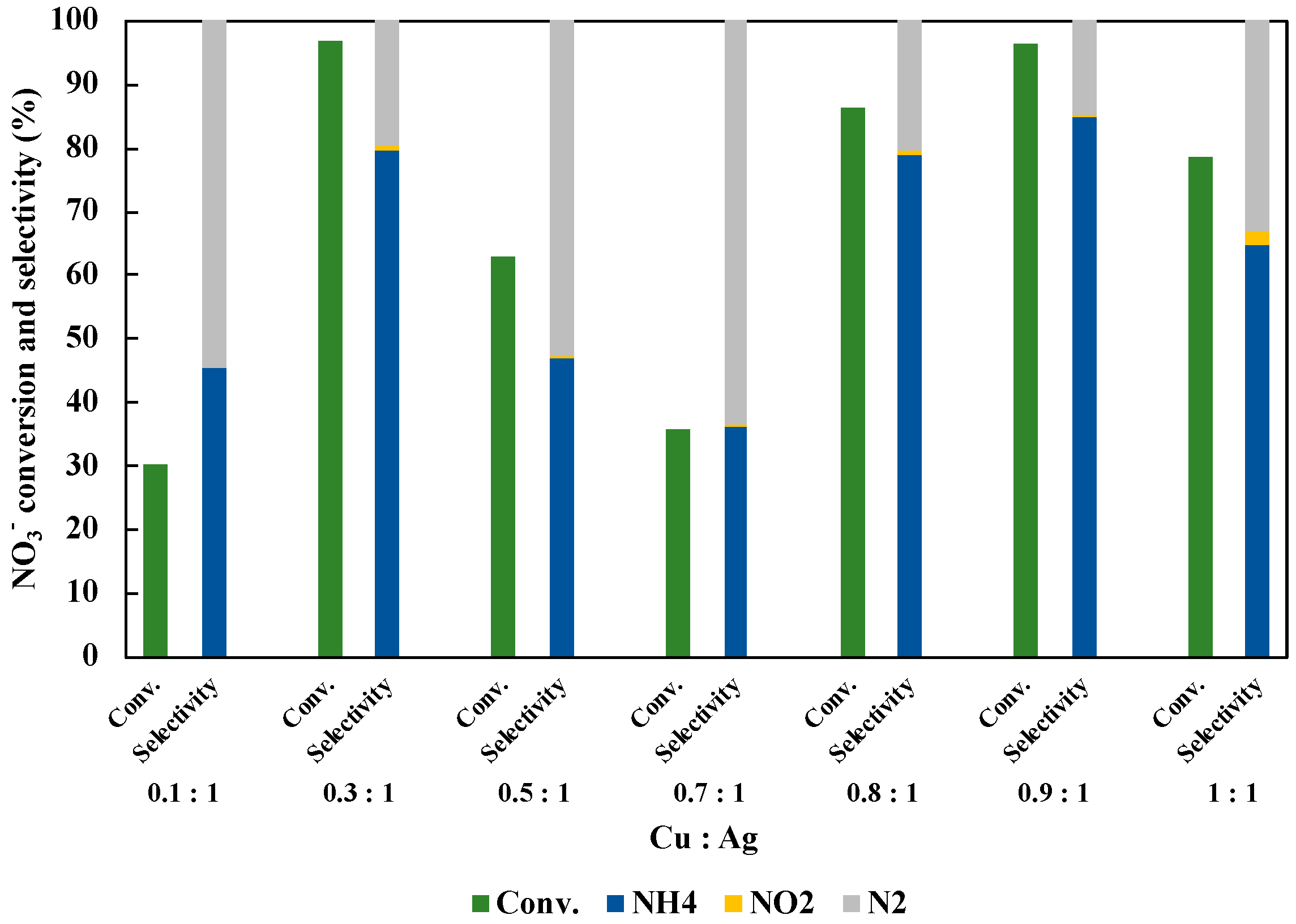
We optimized the molar ratio of Cu and Ag doped on TiO2 (Figure 7). The total amount of Cu and Ag was controlled to be 1 wt% of TiO2 amount. As shown in the result, the highest NO3− conversion and the NH3 selectivity were achieved over Cu0.9Ag/TiO2 (Cu:Ag = 0.9:1). Consequently, both the NO3− conversion and NH4+ selectivity was respectively almost same when Cu0.3Ag/TiO2 and Cu0.9Ag/TiO2 were used. Silver is one of the precious metals and it is so expensive for the application to the chemical industry. This means that the catalyst with the lower Ag amount (Cu:Ag = 0.9:1) is better than the catalyst with higher Ag amount (Cu:Ag = 0.3:1). Therefore, we selected Cu:Ag = 0.9:1 as an optimum molar ratio in this study.
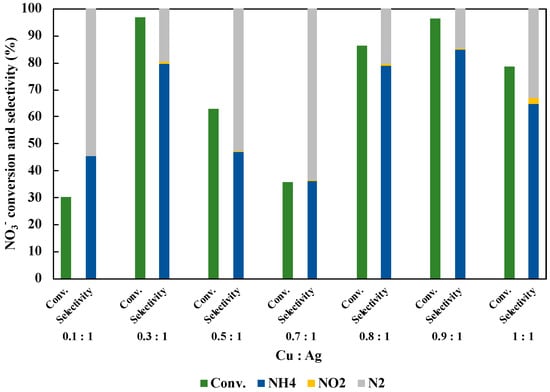
Figure 7.
Effect of molar ratio of Cu and Ag loaded on TiO2 in 45 mL of 50 ppm NO3− aqueous solution including 10 vol% CH3OH under UV irradiation for three hours. The loading amount of each samples was 30 mg.
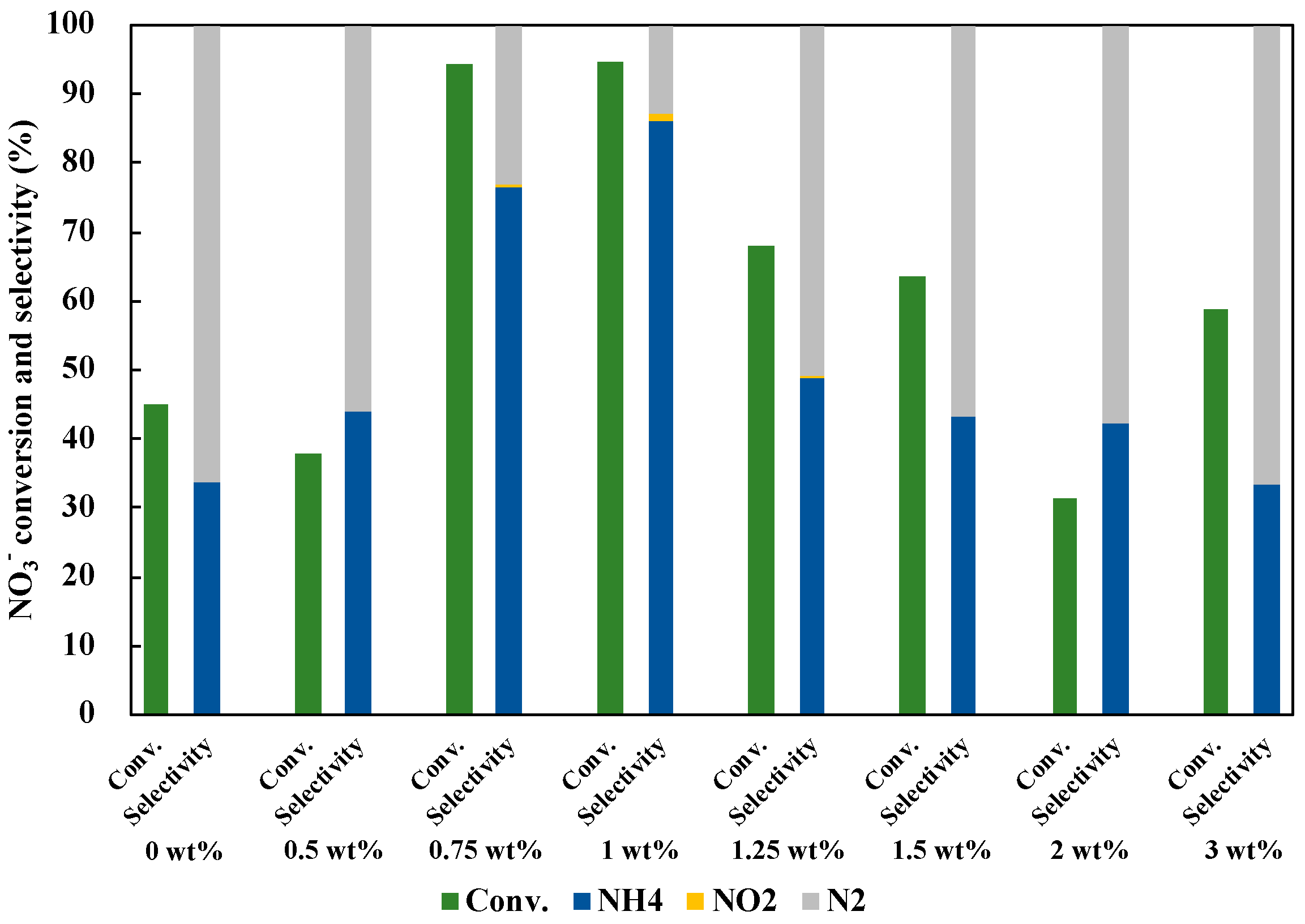
In addition to the optimization of the molar ratio, the loading amount of Cu0.9Ag on TiO2 was investigated (Figure 8). The NO3− conversion and the NH3 selectivity were improved as the loading amount of Cu0.9Ag increased. When the excess amount of the catalyst was used, the photocatalytic activity decreased. The highest NO3− conversion and the NH3 selectivity were given by 1 wt% of Cu0.9Ag doped on TiO2. Therefore, 1 wt% Cu0.9Ag/TiO2 was used as an optimum photocatalyst in this study.
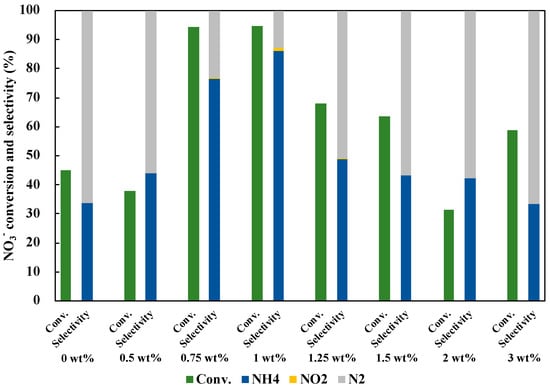
Figure 8.
Effect of loading amount of Cu0.9Ag on TiO2 in 45 mL of 50 ppm NO3− aqueous solution including 10 vol% CH3OH under UV irradiation for three hours. The loading amount of each samples was 30 mg.
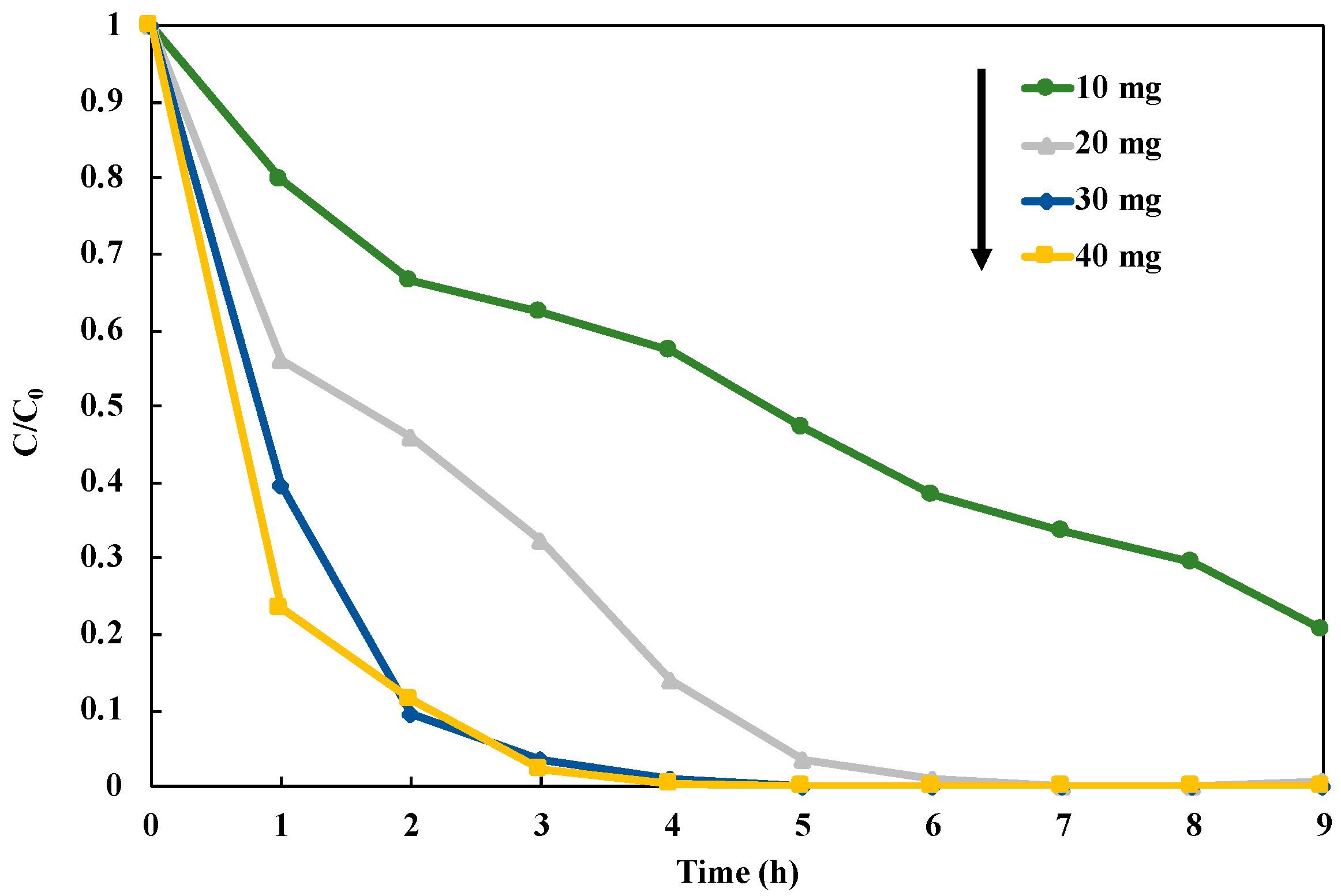
After the optimization to the structure of the photocatalyst, we investigated the optimum dosage of the photocatalyst in the reduction of NO3− to NH3 over 1 wt% Cu0.9AgTiO2 (Figure 9). This experiment was performed in the presence of CH3OH under UV irradiation for nine hours, and the photocatalysts in the range of 10–40 mg were used. Loading 30 mg and 40 mg of the catalyst gave the significantly high NO3− reduction rate (Figure S7). Thus, we selected 30 mg as an optimum dosage of the photocatalyst in the NO3− reduction, because it can perform the reduction of NO3− with the lower catalyst amount. In this study, we fixed 30 mg of 1 wt% Cu0.9Ag/TiO2 as the optimal conditions for the reaction system.
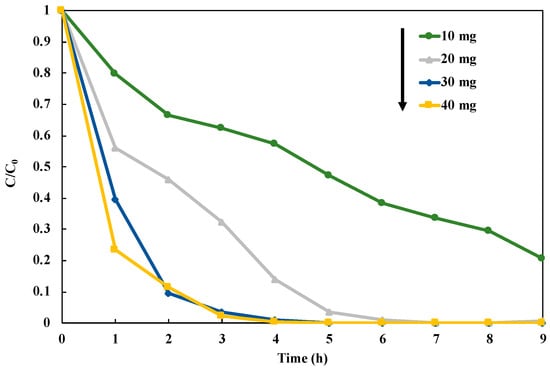
Figure 9.
Influence of loading catalyst amount on NO3− reduction in 45 mL of 50 ppm NO3− aqueous solution including 10 vol% CH3OH under UV irradiation for nine hours.
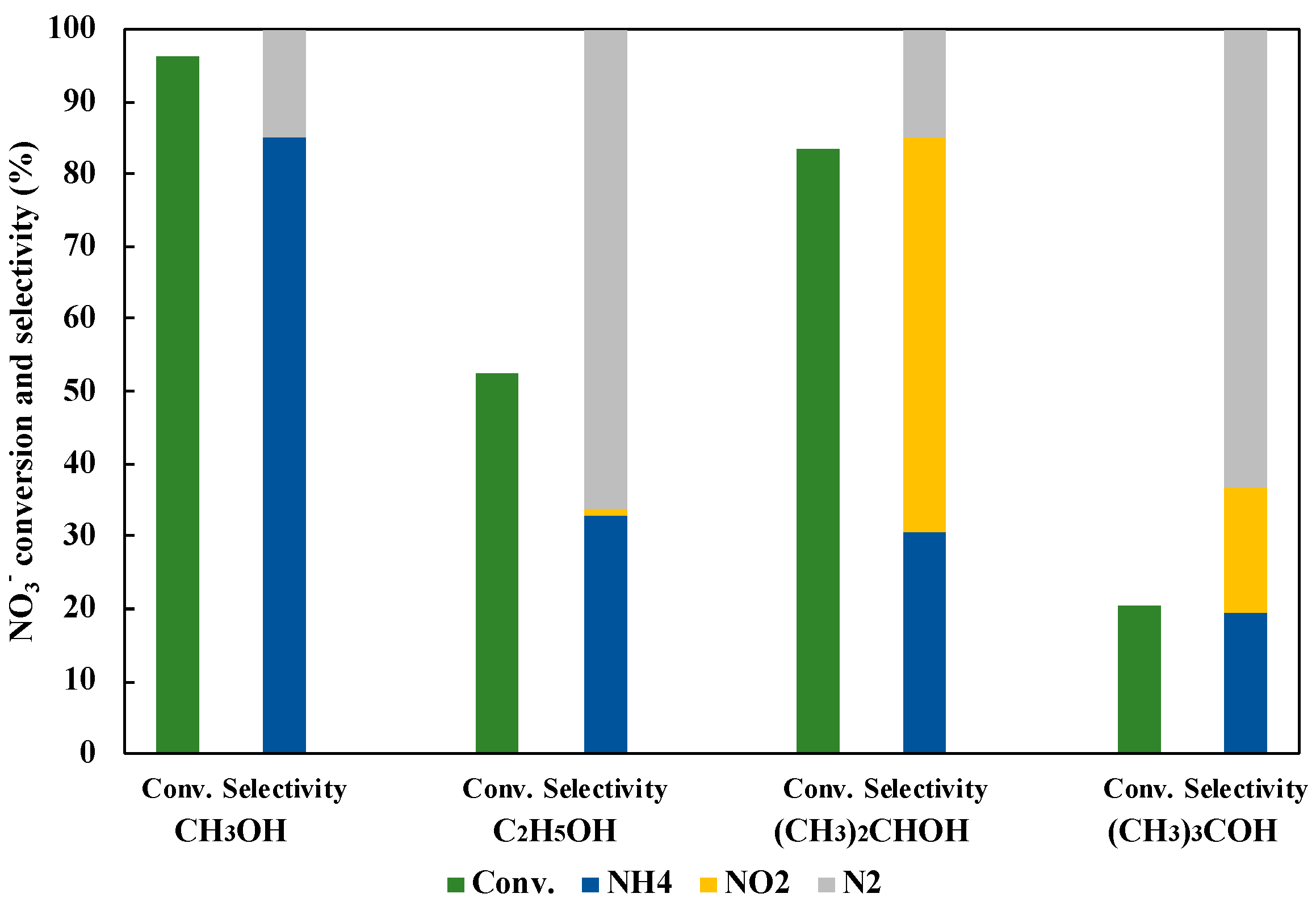
3.5. Effect of Hole Scavenger on NO3− Reduction to NH3
The hole scavenger was also the very important factor in the reaction with the photocatalyst. Figure 10 shows the effect of the hole scavenger on the reduction of NO3− to NH3. As a result, CH3OH indicated the most efficient NO3− conversion and the NH3 selectivity. In the photocatalytic NO3− reduction, CH3OH was turned to HCHO with h+, and then H2 was simultaneously released. Generated H2 on the photocatalyst surface was useful to reduce NO3− and NO2− (Equations (6)–(11)).
NO2− + 3H2 → NH4+ + 2OH−
2NO2− + 2H2 → N2 + 4OH−
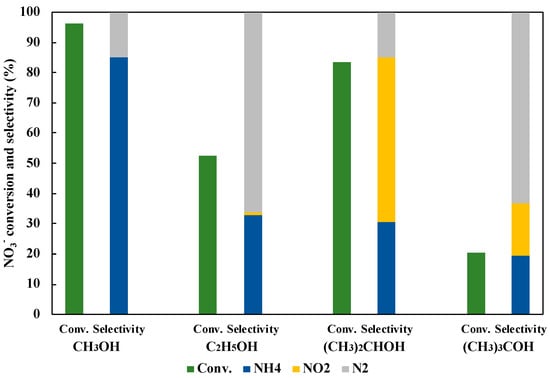
Figure 10.
Effect of hole scavengers on NO3− reduction to NH3 in 45 mL of 50 ppm NO3− aqueous solution including 10 vol% of hole scavenger under UV irradiation for three hours.
On the other hand, it is considered that C2H5OH, (CH3)2CHOH, and (CH3)3COH were not so reactive, because of the larger steric hindrance. Therefore, CH3OH could give more H2 than those with other hole scavengers, and CH3OH was selected as an optimal hole scavenger in this study.
3.6. Effect of pH
We checked the pH effect in the solution during the NO3− reduction under UV irradiation for three hours (Figure 11). The initial pH value was about six without the pH adjustment. The NO3− conversion and the selectivity of NH3 decreased under pH = 5. This reason may be owing to it that the protons in the solution were not sufficiently reacted with NO3− adsorbed on the catalyst surface, as the protons repelled to the positive charges on the photocatalyst surface. Under alkaline conditions, the NO3− conversion was kept high, while the NH3 selectivity decreased compared with the case of pH = 6. There are two proposed reasons; i) some •OH radicals were generated in the solution, which promoted the oxidation of methanol. Hence, the reduction rate of NO3− was simultaneously enhanced and more NO2− ions and N2 products were generated; ii) the H+ concentration in the solution decreased under alkaline conditions, which leads to inhibit further reductions of NO2− to NH4+. On the other hand, the high NO3− conversion and the NH3 selectivity were achieved without the pH adjustment (pH = 6). This result may be shown that it was given by the sufficiently high H+ concentration and the good adsorption of NO3− on the catalyst surface under pH = 6.
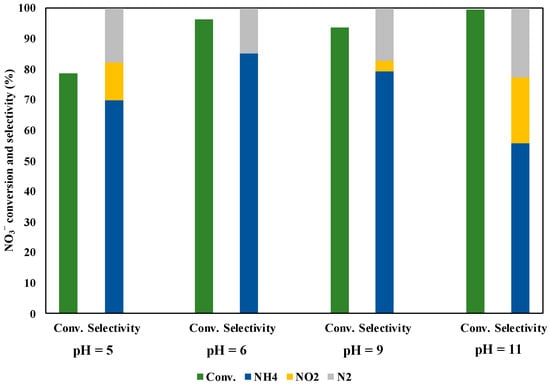
Figure 11.
pH effect in solution during NO3− reduction under UV irradiation for three hours.
3.7. Reusability of Photocatalyst in NO3− Reduction to NH3
We investigated the reusability of 1 wt% Cu0.9Ag/TiO2 photocatalyst in the reduction of NO3− to NH3 under UV irradiation for three hours. Five repeated experiments were operated to evaluate the stability of the optimum photocatalyst. As shown in Figure 12, the NO3− conversion and the NH3 selectivity were changed for each cycle. It may be owing to the loss of the catalyst amount in the operation for sample collection. Therefore, it is considered that the photocatalytic activity was not degraded by five cycles. It clearly shows that the stability of 1 wt% Cu0.9Ag/TiO2 in this research.
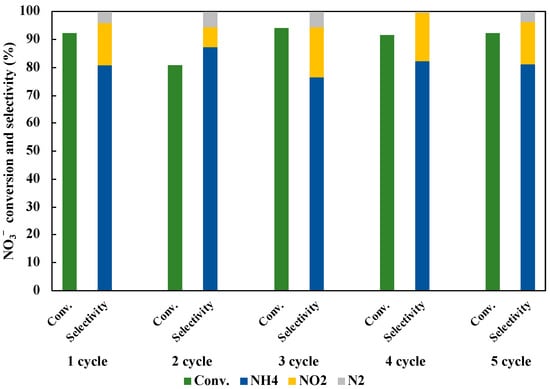
Figure 12.
Reusability of 1 wt% Cu0.9Ag/TiO2 in 45 mL of 50 ppm NO3− aqueous solution including 10 vol% of CH3OH under UV irradiation for three hours for five cycles.
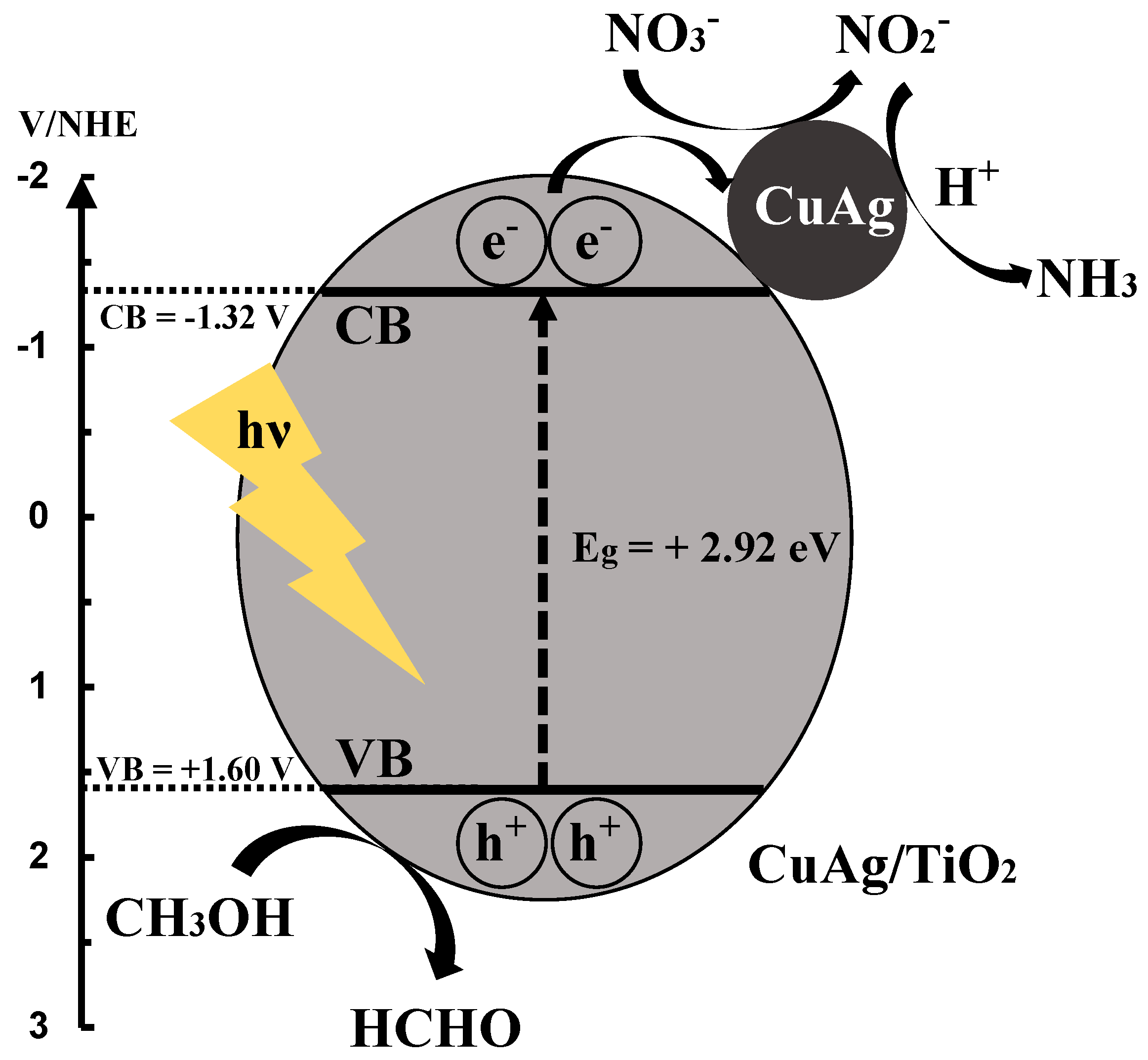
3.8. Reaction Mechanism of Photocatalytic NO3− Reduction to NH3
Scheme 1 shows the proposed reaction mechanism of CuAg/TiO2 in this study. First, CuAg/TiO2 is stimulated by UV light, and electrons and holes are generated. The photogenerated electrons in conduction band are transferred to valence band, and then are moved to CuAg alloy. NO3− is reduced to NO2− with the electrons, and NO2− is reduced to NH3 with the electrons and protons. The hole scavenger, CH3OH is oxidized to HCOOH with holes, and H2 was released, which gives further reduction of NO3−. The oxidation of methanol to formaldehyde was confirmed by the experiment shown in Figure S7.
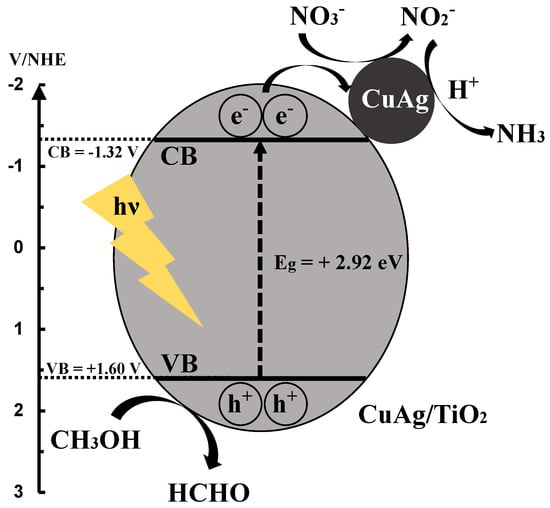
Scheme 1.
Reaction mechanism of CuAg/TiO2 in NO3− reduction to NH3.
The reaction processes can be expressed as following formulas:
NO3− + 2H+ +2e− → NO2− + H2O
NO3− + 10H+ +8e− → NH4+ + 3H2O
NO3− + 6H+ +5e− → 1/2N2 + 3H2O
4. Conclusions
The significantly effective photocatalyst CuAg/TiO2 was prepared for the highly selective NH3 synthesis by photocatalytic reduction of NO3−. Cu to Ag molar ratio of 0.9:1 and 1 wt% Cu0.9Ag doped TiO2 indicated the excellent NO3− conversion and the NH3 selectivity. These results were brought by the advantages of the CuAg alloy and the presence of CH3OH in the reduction of NO3−. Loading Cu and Ag onto TiO2 gave the effective separation of electron-hole pairs and enhanced the light absorption in UV region. CH3OH was used as the optimum hole scavenger for the source of H2. From the result of the cycle test, the stability of CuAg/TiO2 was indicated by five cycles.
Supplementary Materials
The following are available online at https://www.mdpi.com/2305-7084/3/2/49/s1, Table S1: Summary of TiO2 samples, Figure S1: XRD spectra of (a) TiO2, (b) Cu/TiO2, (c) Ag/TiO2, (d) Cu0.5Ag/TiO2, (e) Cu0.9Ag/TiO2, (f) Cu1.5Ag/TiO2, (g) 0.5 wt% Cu0.9Ag/TiO2, and (h) 1.5 wt% Cu0.9Ag/TiO2., Figure S2: TEM images of (a) TiO2, (b) Cu/TiO2, (c) Ag/TiO2, (d) Cu0.5Ag/TiO2, (e) Cu0.9Ag/TiO2, (f) Cu1.5Ag/TiO2, (g) 0.5 wt% Cu0.9Ag/TiO2, and (h) 1.5 wt% Cu0.9Ag/TiO2, Figure S3: O 1s core level spectra in TiO2 samples, Figure S4: Ti 2p core level spectra in TiO2 samples, Figure S5: Cu 2p core level spectra in TiO2 samples, Figure S6: Ag 3d core level spectra in TiO2 samples, and Figure S7: Oxidation of methanol to HCOOH during NO3− reduction under UV irradiation for 3 h.
Author Contributions
R.K. and S.K. conceived and designed the experiments. R.K. performed the experiments and wrote the paper. I.T., M.F. and H.K. analyzed the results and advised the project.
Funding
The present research was partly supported by Grant-in-Aid for Scientific Research (C) 15K00602 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Acknowledgments
All experiments were conducted at Mie University. Any opinions, findings, conclusions or recommendations expressed in this paper are those of the authors and do not necessarily reflect the view of the supporting organizations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gruber, N.; Galloway, J.N. An earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrogen Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- McEaney, J.M.; Singh, A.R.; Schwalbe, J.A.; Kibsgaard, J.; Lin, J.C.; Cargnello, M.; Jaramillo, T.F.; Nørscov, J.K. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy Environ. Sci. 2017, 10, 1621–1630. [Google Scholar] [CrossRef]
- Baltrusaitis, J. Sustainable ammonia production. ACS Sustain. Chem. Eng. 2017, 5, 9527. [Google Scholar] [CrossRef]
- Jungmi, H.; Steven, P.; Anthony, B.M. Plasma catalysis as an alternative route for ammonia production: status, mechanisms, and prospects for progress. ACS Sustain. Chem. Eng. 2018, 6, 15–31. [Google Scholar]
- Yusuf, B.; Ibrahim, D. Assessment of a sustainable electrochemical ammonia production system using photoelectrochemically produced hydrogen under concentrated sunlight. ACS Sustain. Chem. Eng. 2017, 5, 8035–8043. [Google Scholar]
- Pratham, A.; Andrew, F.A.H.; Sanjay, M.M.; Anuradda, G. Small-scale ammonia production from biomass: A techno-enviro-economic perspective. Ind. Eng. Chem. Res. 2016, 55, 6422–6434. [Google Scholar]
- Ranjit, K.T.; Varadarajan, T.K.; Viswanathan, B. Photocatalytic reduction of dinitrogen to ammonia over noble-metal-loaded TiO2. J. Photochem. Photobiol. A Chem. 1996, 96, 181–185. [Google Scholar] [CrossRef]
- Ozaki, A.; Aika, K. Catalytic activation of dinitrogen. Catal. Sci. Technol. 1981, 1, 87–158. [Google Scholar]
- Oshikiri, T.; Ueno, K.; Misawa, H. Plasmon-induced ammonia synthesis through nitrogen photofixation with visible light irradiation. Angew. Chem. Int. Ed. Engl. 2014, 53, 9802–9805. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shang, J.; Shi, J.; Zhao, K.; Zhang, L. Facet-dependent solar ammonia synthesis of BiOCl nanosheets via a proton-assisted electron transfer pathway. Nanoscale 2016, 8, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Koltsakidou, A.; Antonopoulou, M.; Evgenidou, E.; Konstantinou, I.; Gianakas, A.E.; Papadaki, M.; Bikiaris, D.; Lambropoulou, D.A. Photocatalytical removal of fluorouracil using TiO2-P25 and N/S doped TiO2 catalysts: A kinetic and mechanistic study. Sci. Total Environ. 2017, 578, 257–267. [Google Scholar] [CrossRef]
- Xue, Y.; Song, J.; Zhang, Y.; Kong, F.; Wen, M.; Zhang, G. Nitrate pollution and preliminary source identification of surface water in a semi-arid river basin, using isotopic and hydrochemical approaches. Water 2016, 8, 328. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. Photocatalytic reduction of nitrate ions over tantalate photocatalysts. Phys. Chem. Chem. Phys. 2002, 4, 2833–2838. [Google Scholar] [CrossRef]
- Kotesh, K.M.; Bhavani, K.; Naresh, G.; Srinivas, B.; Venugopal, A. Plasmonic resonance nature of Ag-Cu/TiO2 photocatalyst under solar and artificial light: Synthesis, characterization and evaluation of H2O splitting activity. Appl. Catal. B 2016, 199, 282–291. [Google Scholar]
- Xuming, Z.; Yu, L.C.; Ru-Shi, L.; Din, P.T. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401–046407. [Google Scholar]
- Stuart, H.R.; Hall, D.G. Island size effects in nanoparticle-enhanced photodetectors. Appl. Phys. Lett. 1998, 73, 3815–3817. [Google Scholar] [CrossRef]
- Yang, T.; Tetsu, T. Plasmon-induced photoelectrochemistry at metal nanoparticles supported on nanoporous TiO2. Chem. Commun. 2004, 16, 1810–1811. [Google Scholar]
- Syed, M.; Gerardo, H.; Daniel, M.; Joun, L.; Martin, M. Plasmonic photosensitization of a wide band gap semiconductor: Converting plasmons to charge carriers. Nano Lett. 2011, 11, 5548–5552. [Google Scholar]
- Nirmala, C.; Prashant, V.K. Improving the photoelectrochemical performance of nanostructured TiO2 Films by Adsorption of gold nanoparticles. J. Phys. Chem. B 2000, 104, 10851–10857. [Google Scholar]
- Azusa, T.; Prashant, V.K. Capture, store, and discharge. Shuttling photogenerated electrons across TiO2–silver interface. ACS Nano. 2011, 5, 7369–7376. [Google Scholar]
- Jian, L.; Fuyi, C. Plasmon enhanced photoelectrochemical activity of Ag-Cu nanoparticles on TiO2/Ti substrates. Int. J. Electrochem. Sci. 2012, 7, 9560–9572. [Google Scholar]
- Jhuang, Y.Y.; Cheng, W.T. Fabrication and characterization of silver/titanium dioxide composite nanoparticles in ethylene glycol with alkaline solution through sonochemical process. Ultrason. Sonochem. 2016, 28, 327–333. [Google Scholar] [CrossRef]
- Ning, S.; Ding, L.; Lin, Z.; Lin, Q.; Zhang, H.; Lin, H.; Long, J.; Wang, X. One-pot fabrication of Bi3O4Cl/BiOCl plate-on-plate heterojunction with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2016, 185, 203–212. [Google Scholar] [CrossRef]
- He, Z.; Shi, Y.; Gao, C.; Wen, L.; Chen, J.; Song, S. Fas-associated factor 1 plays a negative regulatory role in the antibacterial immunity of locusta migratoria. J. Phys. Chem. C 2013, 22, 389–398. [Google Scholar]
- Henrik, J.; Alexei, S.; Zheshen, L.; Erik, S. XPS and FTIR investigation of the surface properties of different prepared titania nano-powders. Appl. Surf. Sci. 2005, 246, 239–249. [Google Scholar]
- Lopez, T.; Cuevas, J.L.; Ilharco, L.; Ramírez, P.; Rodríguez-Reinoso, F.; Rodríguez-Castellón, E. XPS characterization and E. Coli DNA degradation using functionalized Cu/TiO2 nanobiocatalysts. Mol. Catal. 2018, 449, 62–71. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Kim, H. Synthesis of shape and size-dependent CuAg bimetallic dumbbell structures for organic pollutant hydrogenation. Phys. E Low-dimens. Syst. Nanostruct. 2018, 102, 44–49. [Google Scholar] [CrossRef]
- Méndezmedrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeaujustin, C.; Rodríguezlópez, J.L.; Remita, H. Surface modification of TiO2 with Ag nanoparticles and CuO nanoclusters for application in photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar]
- He, Y.; Zhu, Y.; Wu, N. Synthesis of nanosized NaTaO3 in low temperature and its photocatalytic performance. J. Solid State Chem. 2004, 177, 3868–3872. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).