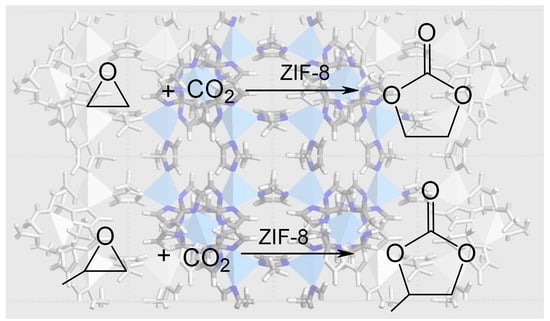
ZIF-8 as a Catalyst in Ethylene Oxide and Propylene Oxide Reaction with CO2 to Cyclic Organic Carbonates
Abstract
1. Introduction
2. Materials and Methods
3. Results
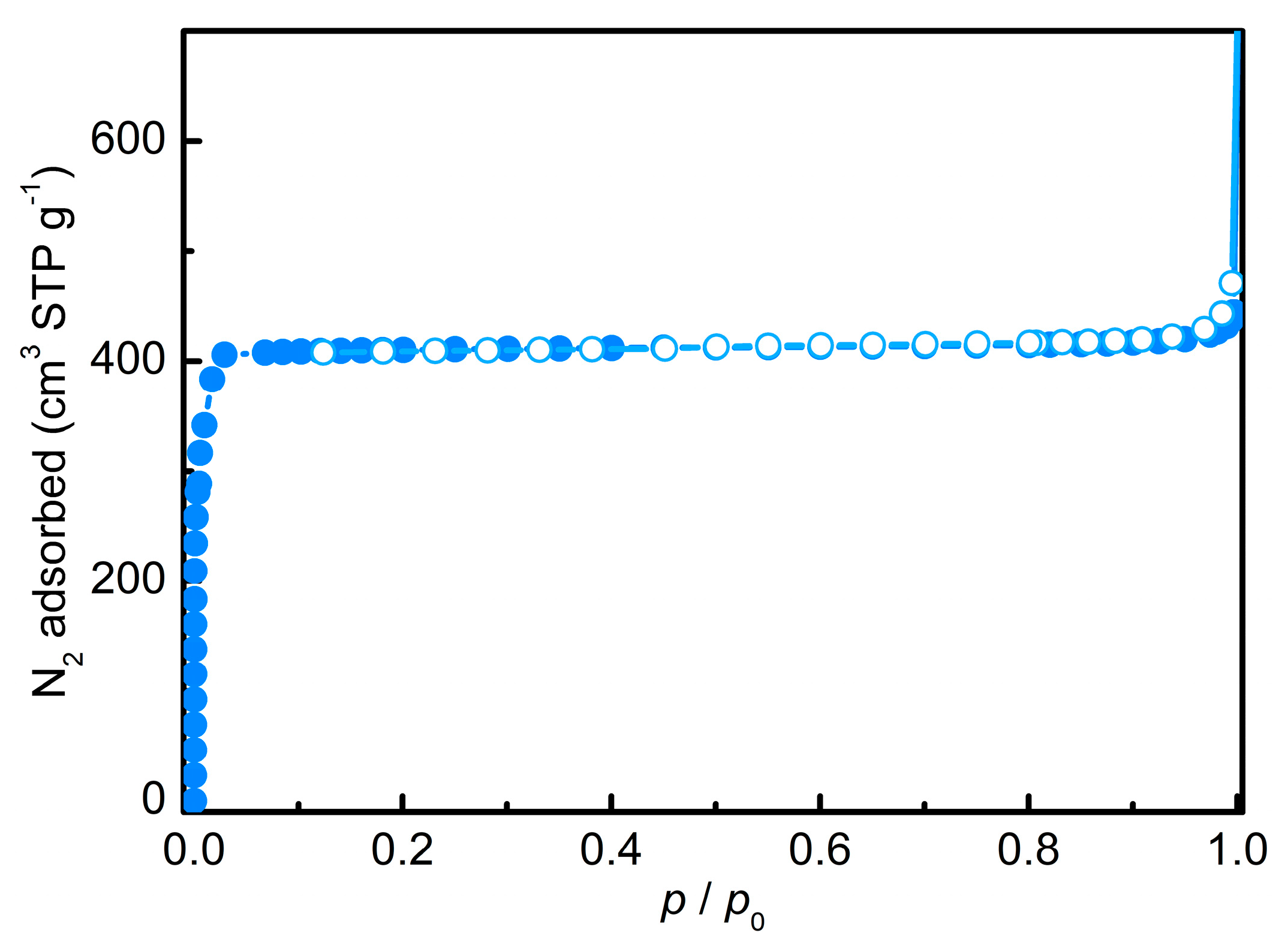
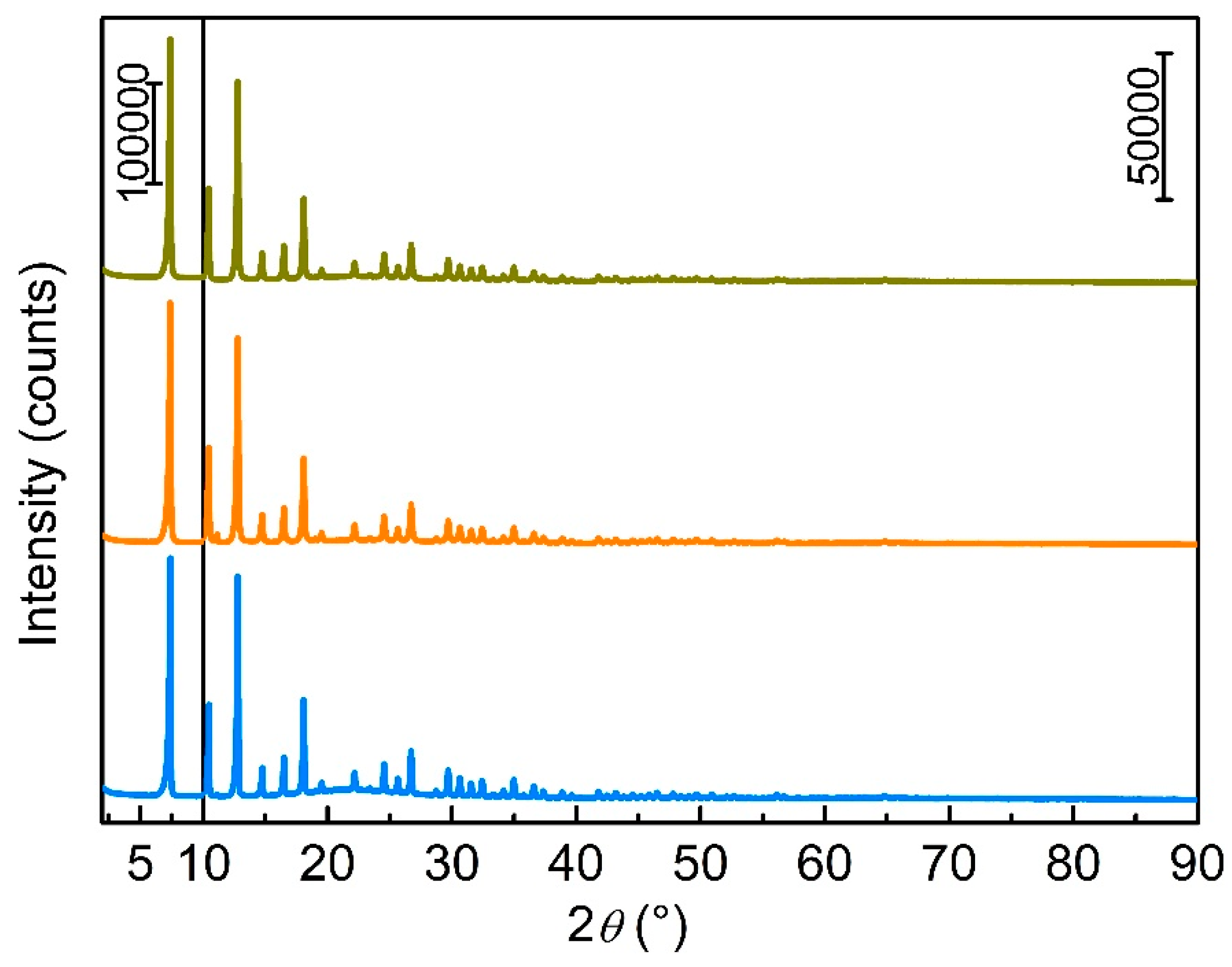
3.1. Zeolitic Imidazole Framework-8 (ZIF-8)
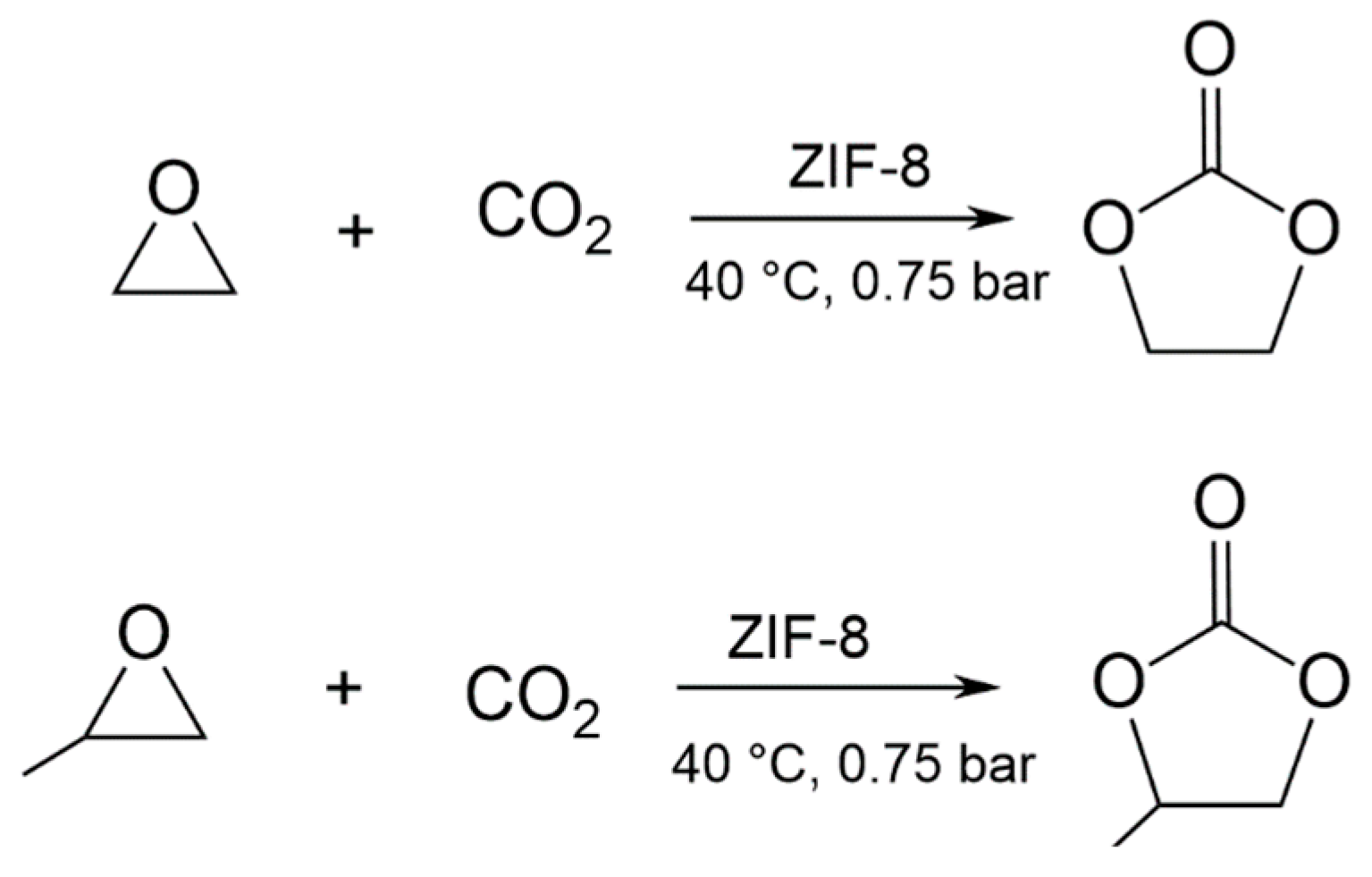
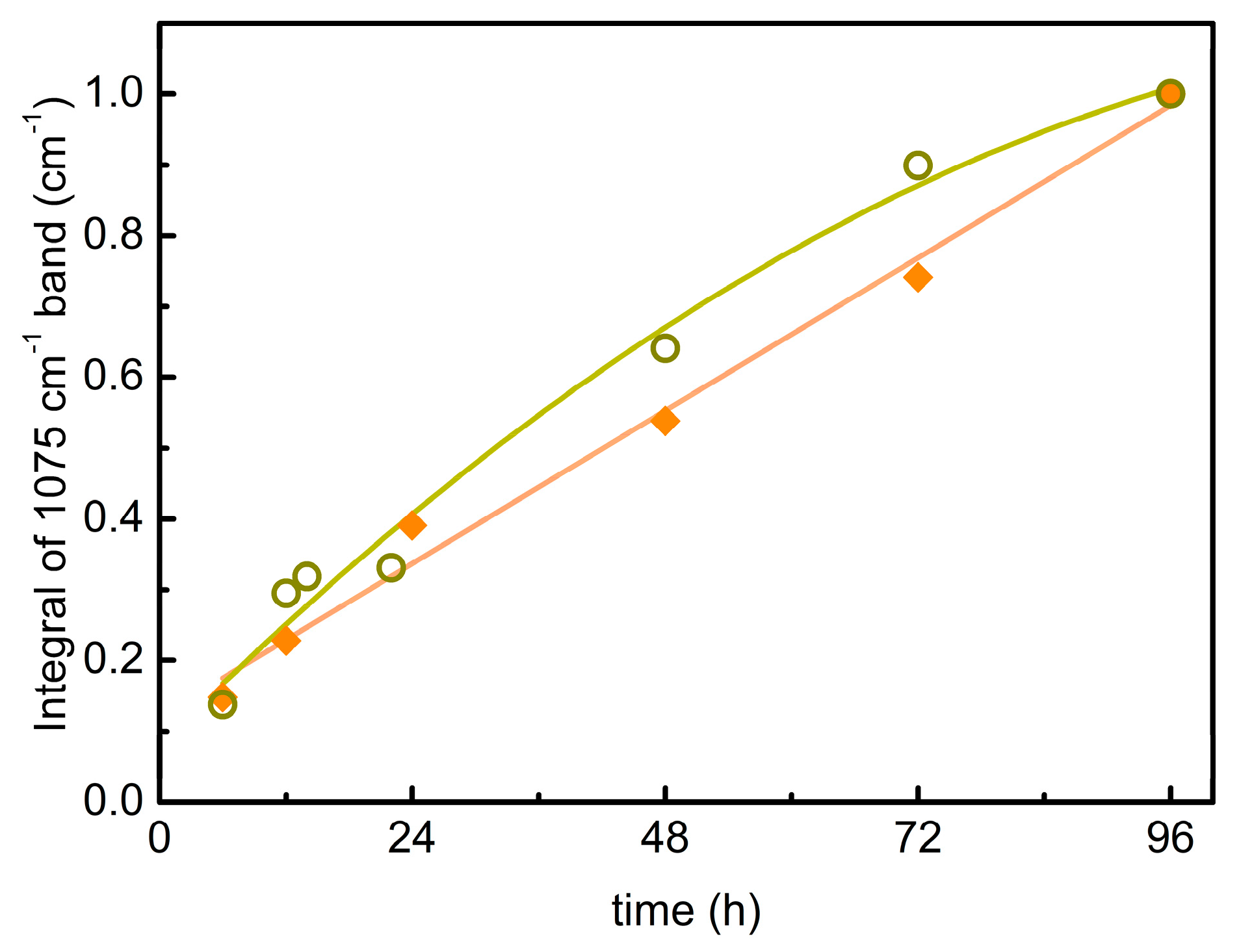
3.2. EtO-CO2/ZIF-8
3.3. PrO-CO2/ZIF-8
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A

Appendix B
| SBET 1 | SLangmuir 1 | Sext2 | Vmicro 2 |
|---|---|---|---|
| 1358 | 1791 | 11 | 0.66 |
Appendix C
| ΔEcreaction | ΔHcreaction | ΔGcreaction | |
|---|---|---|---|
| EtO +CO2 → EtC | −78.6 | −71.2 | −53.1 |
| PrO +CO2 → PrC | −77.2 | −69.8 | −49.6 |
References
- Olivier, J.G.J.; Janssens-Maenhout, G.; Muntean, M.; Peters, J.A.H.W. Trends in Global CO2 Emissions: 2016 Report; Netherlands Environmental Assessment Agency: Den Haag, The Netherlands, 2016. [Google Scholar]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem.-Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Quadrelli, E.A.; Centi, G.; Duplan, J.L.; Perathoner, S. Carbon dioxide recycling: Emerging large-scale technologies with industrial potential. ChemSusChem 2011, 4, 1194–1215. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.; Jorgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Aresta, M. Carbon dioxide reduction and uses as a chemical feedstock. In Activation of Small Molecules; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 1–41. [Google Scholar]
- Chen, C.; Kotyk, J.F.K.; Sheehan, S.W. Progress toward commercial application of electrochemical carbon dioxide reduction. Chem 2018, 4, 2571–2586. [Google Scholar] [CrossRef]
- Hall, J.F.B.; Bourne, R.A.; Han, X.; Earley, J.H.; Poliakoff, M.; George, M.W. Synthesis of antimalarial trioxanes via continuous photo-oxidation with 1O2 in supercritical CO2. Green Chem. 2013, 15, 177–180. [Google Scholar] [CrossRef]
- Joos, L.; Huck, J.M.; Van Speybroeck, V.; Smit, B. Cutting the cost of carbon capture: A case for carbon capture and utilization. Faraday Discuss. 2016, 192, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Vitillo, J.G. Magnesium-based systems for carbon dioxide capture, storage and recycling: From leaves to synthetic nanostructured materials. RSC Adv. 2015, 5, 36192–36239. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Groppo, E.; Bardaji, E.G.; Baricco, M.; Bordiga, S. Fast carbon dioxide recycling by reaction with g-mg(bh4)2. Phys. Chem. Chem. Phys. 2014, 16, 22482–22486. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.-A.G.; Sivaram, S. Organic carbonates. Chem. Rev. 1996, 96, 951–976. [Google Scholar] [CrossRef]
- Beyzavi, M.H.; Stephenson, C.J.; Liu, Y.; Karagiaridi, O.; Hupp, J.T.; Farha, O.K. Metal–organic framework-based catalysts: Chemical fixation of CO2 with epoxides leading to cyclic organic carbonates. Front. Energy Res. 2015, 2, 63. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Chung, W.-C.; Wang, K.; Zhou, H.-C. Sequestering CO2 for short-term storage in mofs: Copolymer synthesis with oxiranes. ACS Catal. 2014, 4, 1511–1515. [Google Scholar] [CrossRef]
- Kember, M.R.; Buchard, A.; Williams, C.K. Catalysts for CO(2)/epoxide copolymerisation. Chem. Commun. 2011, 47, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Grube, T.; Schiebahn, S.; Stolten, D. Closing the loop: Captured CO2 as a feedstock in the chemical industry. Energy Environ. Sci. 2015, 8, 3283–3297. [Google Scholar] [CrossRef]
- Jin, K.; Maalouf, J.H.; Lazouski, N.; Corbin, N.; Yang, D.; Manthiram, K. Epoxidation of cyclooctene using water as the oxygen atom source at manganese oxide electrocatalysts. J. Am. Chem. Soc. 2019, 141, 6413–6418. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, X.; Wang, F. Recent advances in carbon dioxide based copolymer. Prog. Chem. 2011, 23, 613–622. [Google Scholar] [CrossRef]
- Biswas, T.; Mahalingam, V. Efficient CO2 fixation under ambient pressure using poly(ionic liquid)-based heterogeneous catalysts. Sustain. Energy Fuels 2019, 3, 935–941. [Google Scholar] [CrossRef]
- Dani, A.; Groppo, E.; Barolo, C.; Vitillo, J.G.; Bordiga, S. Design of high surface area poly(ionic liquid)s to convert carbon dioxide into ethylene carbonate. J. Mater. Chem. A 2015, 3, 8508–8518. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Angelini, A.; Stufano, P. Use of carbon dioxide as feedstock for chemicals and fuels: Homogeneous and heterogeneous catalysis. J. Chem. Technol. Biotechnol. 2014, 89, 334–353. [Google Scholar] [CrossRef]
- Han, L.; Choi, H.-J.; Kim, D.-K.; Park, S.-W.; Liu, B.; Park, D.-W. Porous polymer bead-supported ionic liquids for the synthesis of cyclic carbonate from CO2 and epoxide. J. Mol. Catal. A Chem. 2011, 338, 58–64. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Luz, I.; Ortuño, M.A.; Oregui-Bengoechea, M.; Gandarias, I.; López, N.; Lail, M.A.; Soukri, M. Gas reactions under intrapore condensation regime within tailored metal–organic framework catalysts. Nat. Commun. 2019, 10, 2076. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of zif-8 by ftir spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef] [PubMed]
- Ethiraj, J.; Bonino, F.; Lamberti, C.; Bordiga, S. H2S interaction with hkust-1 and zif-8 mofs: A multitechnique study. Microporous Mesoporous Mater. 2015, 207, 90–94. [Google Scholar] [CrossRef]
- Yin, H.; Kim, H.; Choi, J.; Yip, A.C.K. Thermal stability of zif-8 under oxidative and inert environments: A practical perspective on using zif-8 as a catalyst support. Chem. Eng. J. 2015, 278, 293–300. [Google Scholar] [CrossRef]
- Lewis, D.W.; Ruiz-Salvador, A.R.; Gómez, A.; Rodriguez-Albelo, L.M.; Coudert, F.-X.; Slater, B.; Cheetham, A.K.; Mellot-Draznieks, C. Zeolitic imidazole frameworks: Structural and energetics trends compared with their zeolite analogues. CrystEngComm 2009, 11, 2272–2276. [Google Scholar] [CrossRef]
- Tran, U.P.N.; Le, K.K.A.; Phan, N.T.S. Expanding applications of metal-organic frameworks: Zeolite imidazolate framework zif-8 as an efficient heterogeneous catalyst for the knoevenagel reaction. ACS Catal. 2011, 1, 120–127. [Google Scholar] [CrossRef]
- Chizallet, C.l.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.-A.; Bats, N. Catalysis of transesterification by a nonfunctionalized metal−organic framework: Acido-basicity at the external surface of zif-8 probed by ftir and ab initio calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.L.; Le, K.K.A.; Phan, N.T.S. A zeolite imidazolate framework zif-8 catalyst for friedel-crafts acylation. Chin. J. Catal. 2012, 33, 688–696. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- McEwen, J.; Hayman, J.-D.; Ozgur Yazaydin, A. A comparative study of CO2, CH4 and N2 adsorption in zif-8, zeolite-13x and bpl activated carbon. Chem. Phys. 2013, 412, 72–76. [Google Scholar] [CrossRef]
- Miralda, C.M.; Macias, E.E.; Zhu, M.; Ratnasamy, P.; Carreon, M.A. Zeolitic imidazole framework-8 catalysts in the conversion of CO2 to chloropropene carbonate. ACS Catal. 2012, 2, 180–183. [Google Scholar] [CrossRef]
- Zhu, M.; Srinivas, D.; Bhogeswararao, S.; Ratnasamy, P.; Carreon, M.A. Catalytic activity of zif-8 in the synthesis of styrene carbonate from CO2 and styrene oxide. Catal. Commun. 2013, 32, 36–40. [Google Scholar] [CrossRef]
- Zalomaeva, O.V.; Chibiryaev, A.M.; Kovalenko, K.A.; Kholdeeva, O.A.; Balzhinimaev, B.S.; Fedin, V.P. Cyclic carbonates synthesis from epoxides and CO2 over metal–organic framework Cr-MIL-101. J. Catal. 2013, 298, 179–185. [Google Scholar] [CrossRef]
- Beyzavi, M.H.; Klet, R.C.; Tussupbayev, S.; Borycz, J.; Vermeulen, N.A.; Cramer, C.J.; Stoddart, J.F.; Hupp, J.T.; Farha, O.K. A hafnium-based metal–organic framework as an efficient and multifunctional catalyst for facile CO2 fixation and regioselective and enantioretentive epoxide activation. J. Am. Chem. Soc. 2014, 136, 15861–15864. [Google Scholar] [CrossRef] [PubMed]
- Chaemchuen, S.; Xiao, X.; Ghadamyari, M.; Mousavi, B.; Klomkliang, N.; Yuan, Y.; Verpoort, F. Robust and efficient catalyst derived from bimetallic zn/co zeolitic imidazolate frameworks for CO2 conversion. J. Catal. 2019, 370, 38–45. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press Inc.: London, UK, 1982. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Materials Studio; Version 6; Accelrys Software Inc.: San Diego, CA, USA, 2011.
- Becke, A.D. Density-functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; Revision B.05; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted gaussian-basis sets of triple zeta valence quality for atoms li to kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors Mol. Phys. 1970, 19, 553–566. [Google Scholar]
- Wee, L.H.; Lescouet, T.; Ethiraj, J.; Bonino, F.; Vidruk, R.; Garrier, E.; Packet, D.; Bordiga, S.; Farrusseng, D.; Herskowitz, M.; et al. Hierarchical zeolitic imidazolate framework-8 catalyst for monoglyceride synthesis. ChemCatChem 2013, 5, 3562–3566. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Savonnet, M.; Ricchiardi, G.; Bordiga, S. Tailoring metal-organic frameworks for CO2 capture: The amino effect. ChemSusChem 2011, 4, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.E. Infrared spectra. In Nist Chemistry Webbook, Nist Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Russell, B.A.; Migone, A.D. Low temperature adsorption study of CO2 in zif-8. Microporous Mesoporous Mater. 2017, 246, 178–185. [Google Scholar] [CrossRef]
- Autie-Castro, G.; Oliveira, D. Ch4 and CO2 adsorption study in zif-8 and al-bdc mofs. Biol. Chem. Res. 2017, 234–246. [Google Scholar]
- Gadipelli, S.; Travis, W.; Zhou, W.; Guo, Z. A thermally derived and optimized structure from zif-8 with giant enhancement in CO2 uptake. Energy Environ. Sci. 2014, 7, 2232–2238. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Atzori, C.; Civalleri, B.; Barbero, N.; Barolo, C.; Bonino, F. Design and characterization of mofs (metal organic frameworks) for innovative applications. In Hybrid Organic-Inorganic Interfaces. Towards Advanced Functional Materials; Delville, M.H., Taubert, A., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2017. [Google Scholar]
- Angell, C.L. The infra-red spectra and structure of ethylene carbonate. Trans. Faraday Soc. 1956, 52, 1178–1183. [Google Scholar] [CrossRef]
- Groppo, E.; Estephane, J.; Lamberti, C.; Spoto, G.; Zecchina, A. Ethylene, propylene and ethylene oxide in situ polymerization on the cr(ii)/sio2 system: A temperature- and pressure-dependent investigation. Catal. Today 2007, 126, 228–234. [Google Scholar] [CrossRef]
- Groppo, E.; Zecchina, A.; Barzan, C.; Vitillo, J.G. Low temperature activation and reactivity of CO2 over a crii-based heterogeneous catalyst: A spectroscopic study. Phys. Chem. Chem. Phys. 2012, 14, 6538–6543. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Ricchiardi, G. Effect of pore size, solvation, and defectivity on the perturbation of adsorbates in mofs: The paradigmatic MG2(dobpdc) case study. J. Phys.Chem. C 2017, 121, 22762–22772. [Google Scholar] [CrossRef]



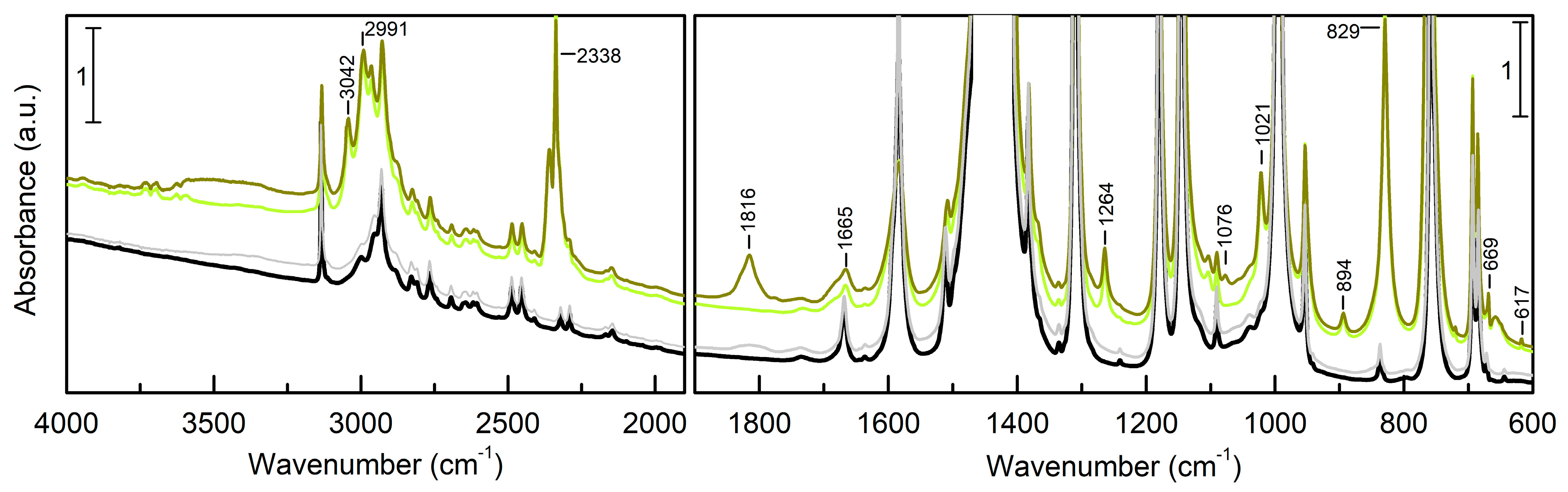
| ΔEc(ΔE) | ΔHc | ΔGc | 1 | |
|---|---|---|---|---|
| CO2 | −21.2 (−23.1) | −13.7 | 14.6 | −17 |
| EtO | −32.7 (−36.2) | −26.1 | 17.2 | 1 |
| PrO | −33.2 (−36.4) | −27.1 | 19.0 | 1 |
| EtC | −70.9 (−75.4) | −63.5 | −11.9 | −53 |
| PrC | −70.3 (−74.7) | −62.7 | −9.6 | −59 |
| CO2/EtO | −24.7 (−28.3) | −17.5 | 13.6 | −17/2 |
| CO2/PrO | −25.8 (−28.0) | −18.5 | 9.8 | −19/−1 |
| EtC | PrC | ||
|---|---|---|---|
| Time | I | Time | I |
| 6 | 1.8 | 6 | 0.5 |
| 12 | 2.8 | 12 | 1.1 |
| 24 | 4.8 | 14 | 1.2 |
| 48 | 6.6 | 22 | 1.3 |
| 72 | 9.1 | 48 | 2.5 |
| 96 | 12.3 | 72 | 3.5 |
| 96 | 3.8 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitillo, J.G.; Crocellà, V.; Bonino, F. ZIF-8 as a Catalyst in Ethylene Oxide and Propylene Oxide Reaction with CO2 to Cyclic Organic Carbonates. ChemEngineering 2019, 3, 60. https://doi.org/10.3390/chemengineering3030060
Vitillo JG, Crocellà V, Bonino F. ZIF-8 as a Catalyst in Ethylene Oxide and Propylene Oxide Reaction with CO2 to Cyclic Organic Carbonates. ChemEngineering. 2019; 3(3):60. https://doi.org/10.3390/chemengineering3030060
Chicago/Turabian StyleVitillo, Jenny G., Valentina Crocellà, and Francesca Bonino. 2019. "ZIF-8 as a Catalyst in Ethylene Oxide and Propylene Oxide Reaction with CO2 to Cyclic Organic Carbonates" ChemEngineering 3, no. 3: 60. https://doi.org/10.3390/chemengineering3030060
APA StyleVitillo, J. G., Crocellà, V., & Bonino, F. (2019). ZIF-8 as a Catalyst in Ethylene Oxide and Propylene Oxide Reaction with CO2 to Cyclic Organic Carbonates. ChemEngineering, 3(3), 60. https://doi.org/10.3390/chemengineering3030060