Investigations on Cationic Dye Degradation Using Iron-Doped Carbon Xerogel
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Catalysts Synthesis
2.3. Catalysts Characterization
2.4. Heterogeneous Fenton and Catalytic Wet Air Oxidation Processes
3. Results and Discussion
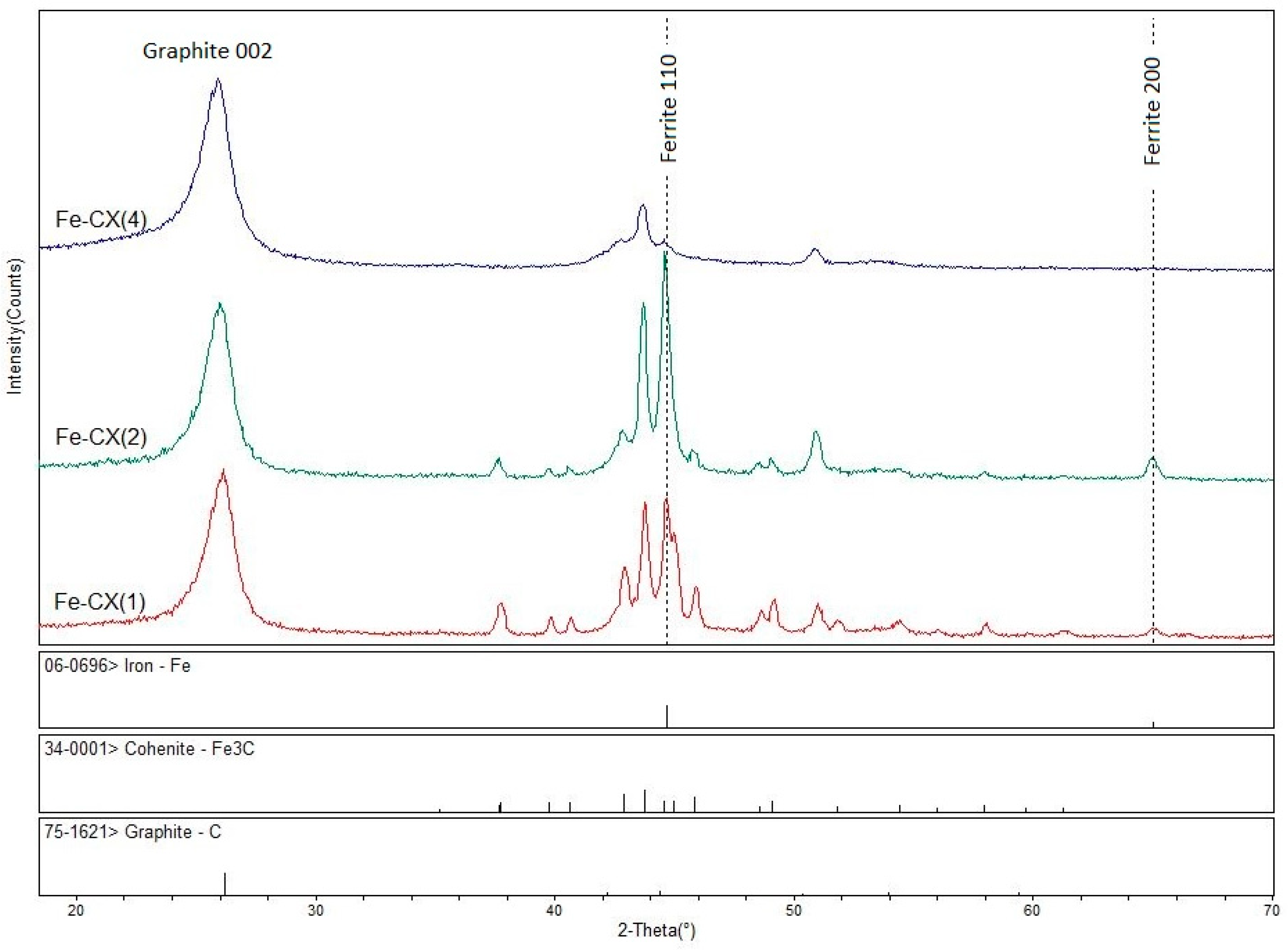
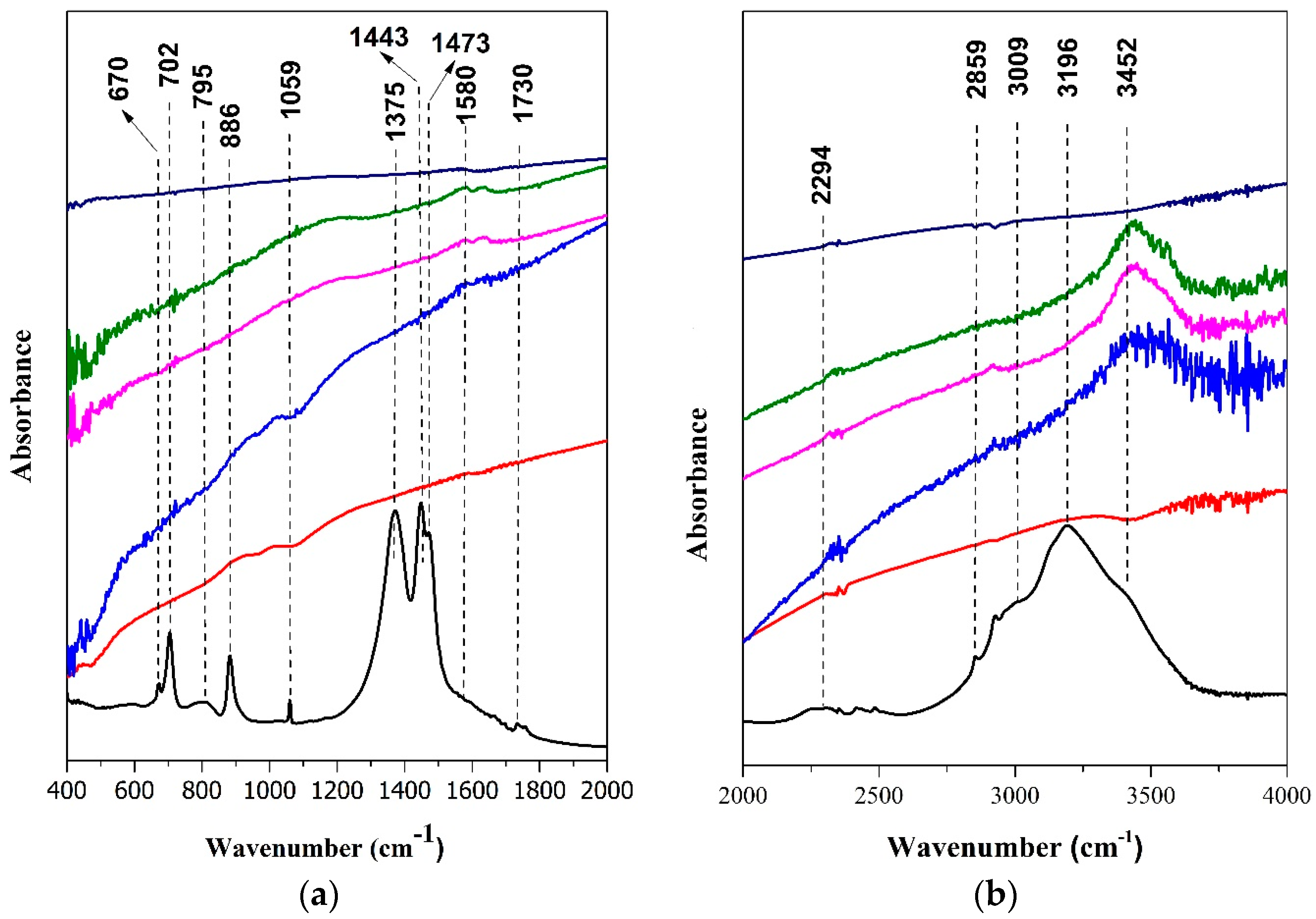
3.1. Catalysts Characterization
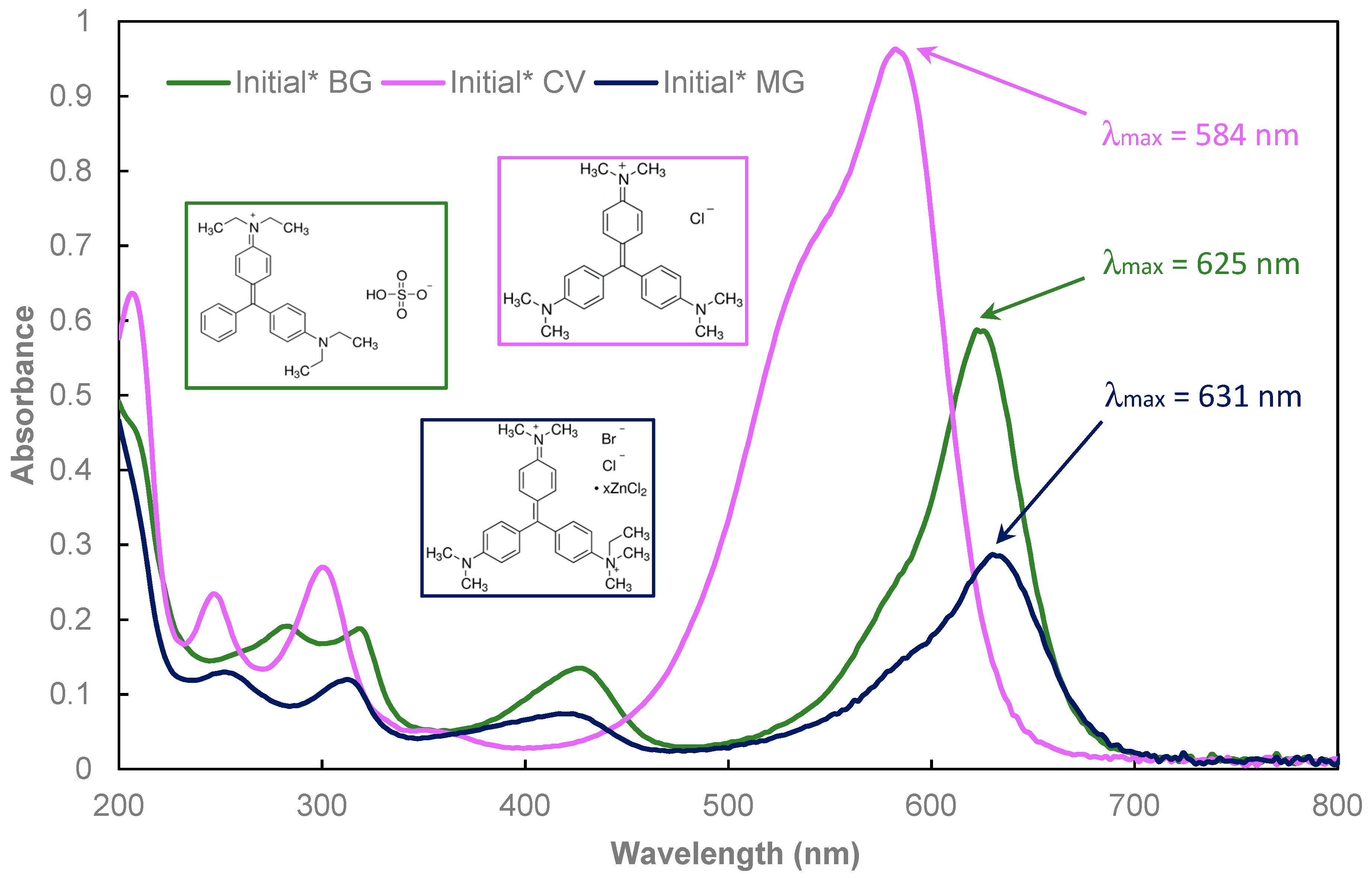
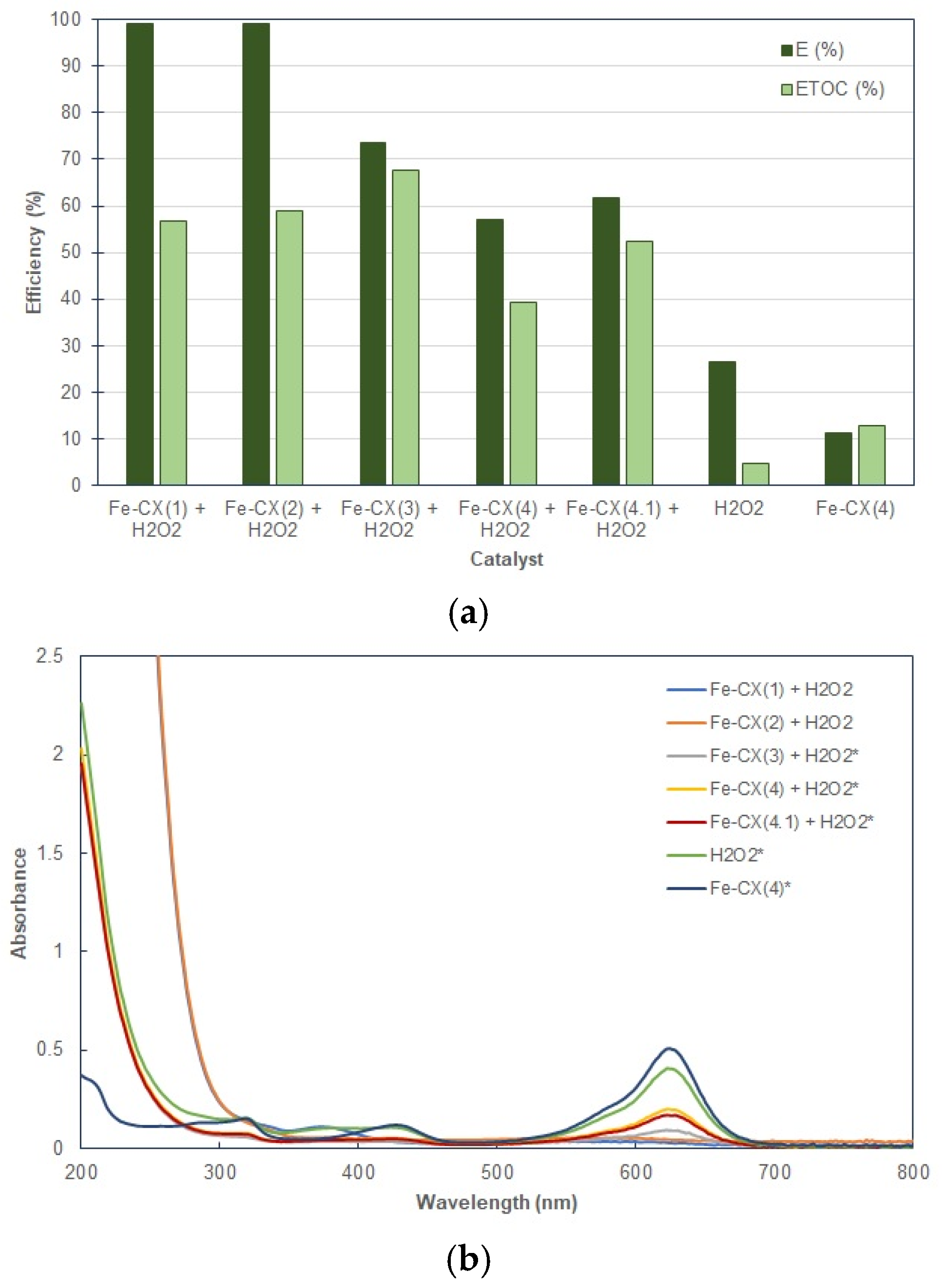
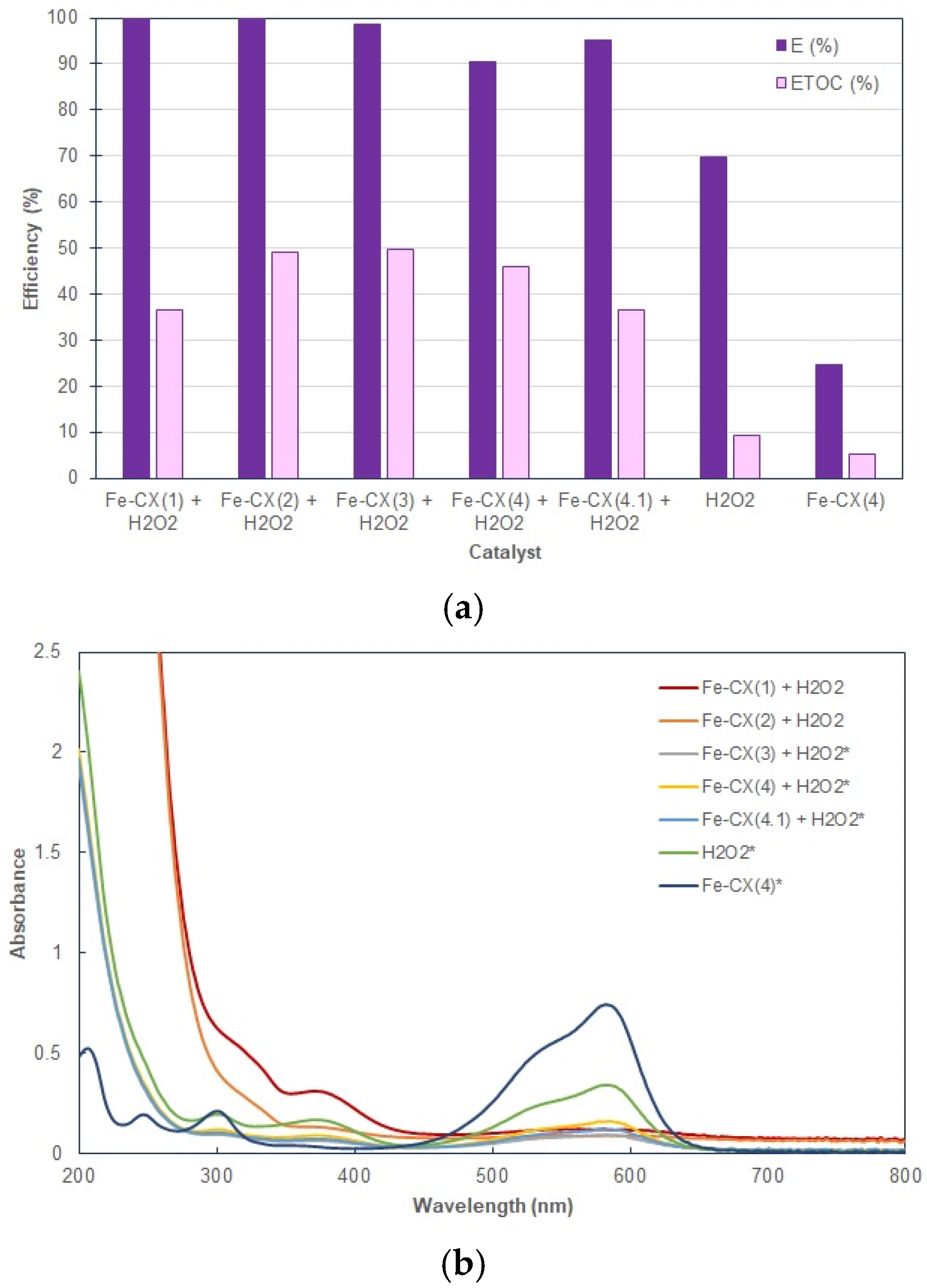
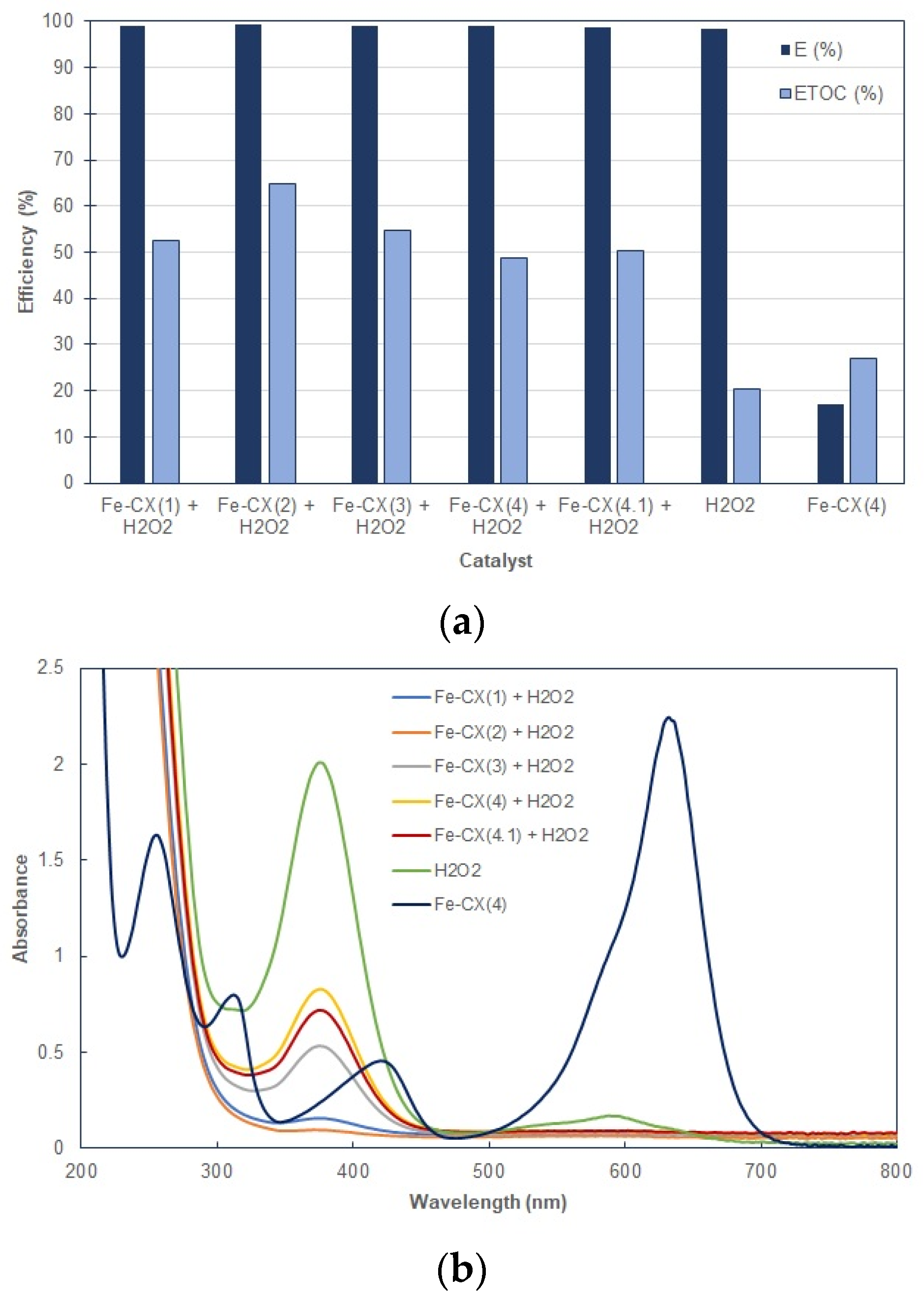
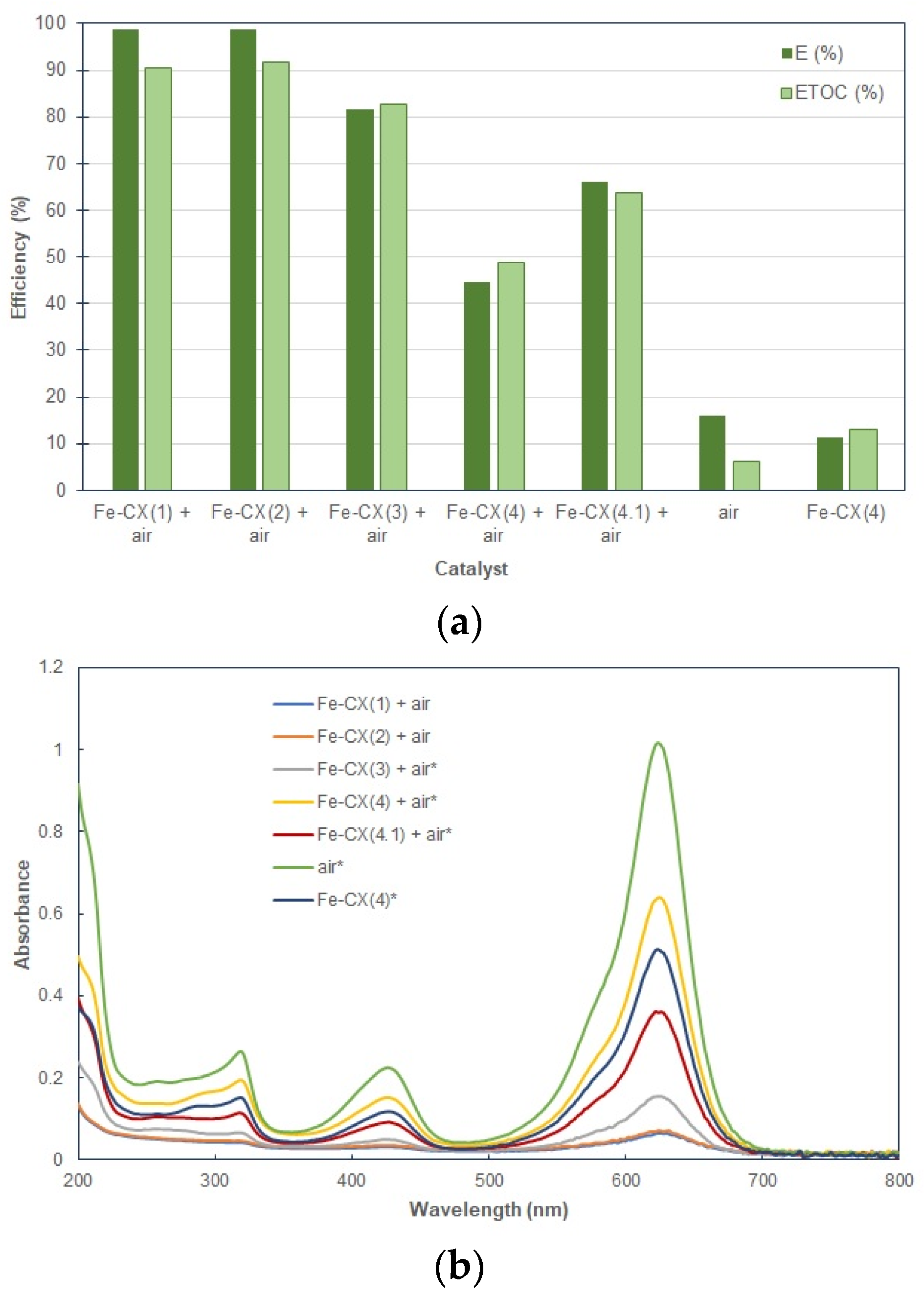
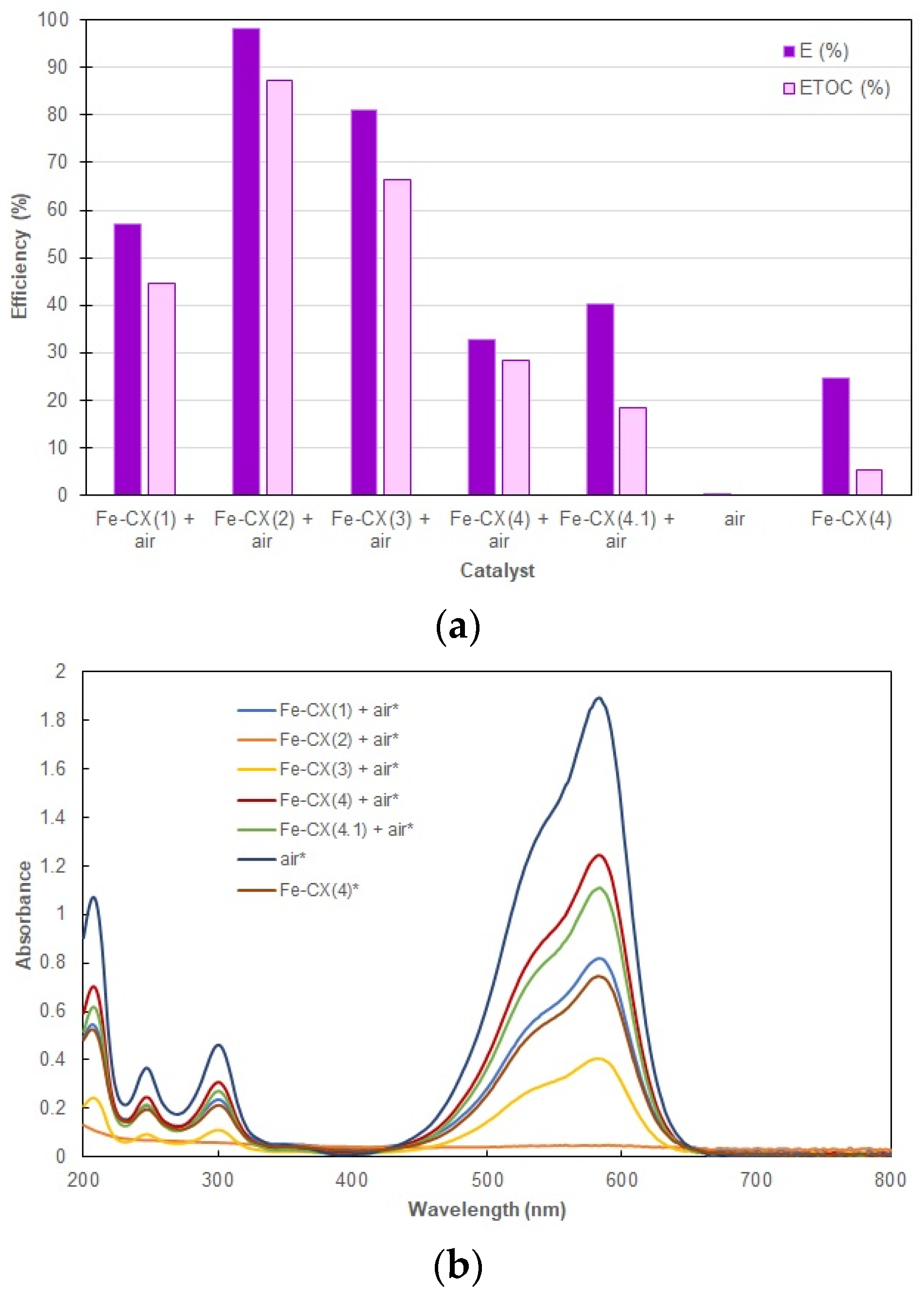
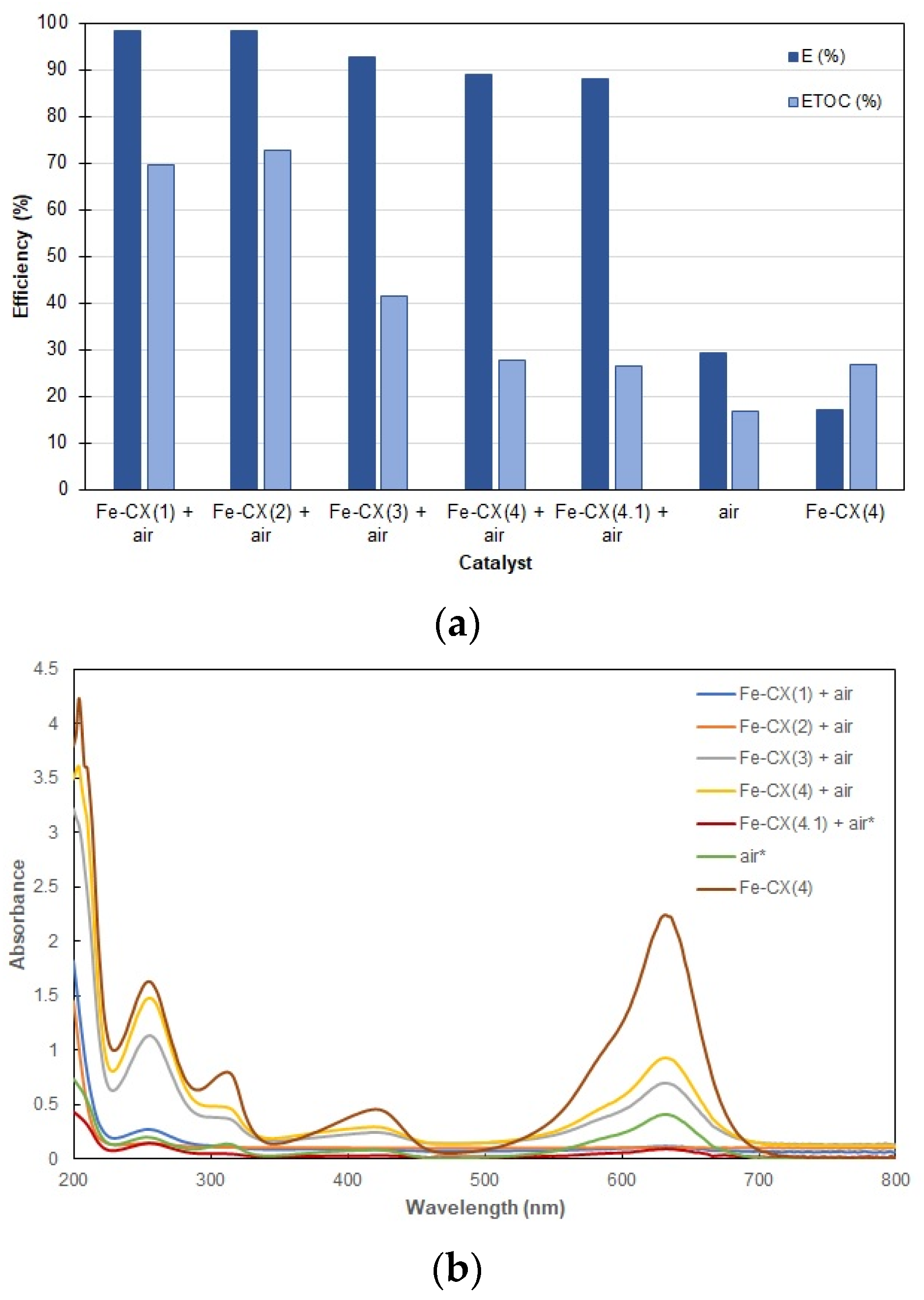
3.2. Dyes Oxidation Tests
3.3. Iron Leakage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tehrani-Bagha, A.R.; Balchi, T. Catalytic wet peroxide oxidation. In Advanced Oxidation Processes for Wastewater Treatment; Ameta, S.C., Ameta, R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 375–402. [Google Scholar]
- Fu, J.; Kyzas, G.Z. Wet air oxidation for the decolorization of dye wastewater: An overview of the last two decades. Chin. J. Catal. 2014, 35, 1–7. [Google Scholar] [CrossRef]
- Kolaczkowski, S.T.; Plucinski, P.; Beltran, F.J.; Rivas, F.J.; McLurgh, D.B. Wet air oxidation: A review of process technologies and aspects in reactor design. Chem. Eng. J. 1999, 73, 143–160. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, X.; Yue, P. Kinetics study on catalytic wet air oxidation of phenol. Chem. Eng. Sci. 2003, 58, 923–928. [Google Scholar] [CrossRef]
- Plesa Chicinas, R.; Gal, E.; Bedelean, H.; Darabantu, M.; Maicaneanu, A. Novel metal modified diatomite, zeolite and carbon xerogel catalysts for mild conditions wet air oxidation of phenol: Characterization, efficiency and reaction pathway. Sep. Purif. Technol. 2018, 197, 36–46. [Google Scholar] [CrossRef]
- Baldrian, P.; Merhautova, V.; Gabriel, J.; Nerud, F.; Stopka, P.; Hruby, M.; Benes, M.J. Decolorization of synthetic dyes by hydrogen peroxide with heterogeneous catalysis by mixed iron oxides. Appl. Catal. B Environ. 2006, 66, 258–264. [Google Scholar] [CrossRef]
- Unnu, B.A.; Gunduz, G.; Dukkanci, M. Heterogeneous Fenton-like oxidation of crystal violet using an iron loaded ZSM-5 zeolite. Desalin. Water Treat. 2015, 57, 1–15. [Google Scholar]
- Zhang, H.; Gao, H.; Cai, C.; Zhang, C.; Chen, L. Decolorization of crystal violet by ultrasound/heterogeneous Fenton process. Water Sci. Technol. 2013, 68, 2515–2520. [Google Scholar] [CrossRef]
- Ucoskia, G.M.; Machado, G.S.; Freitas Silva, G.; Nunes, F.S.; Wypych, F.; Nakagaki, S. Heterogeneous oxidation of the dye Brilliant Green with H2O2 catalyzed by supported manganese porphyrins. J. Molec. Catal. A Chem. 2015, 408, 123–131. [Google Scholar] [CrossRef]
- Rodriguez, A.; Ovejero, G.; Sotelo, J.L.; Mestanza, M.; Garcia, J. Heterogeneous Fenton catalyst supports screening for Mono Azo Dye degradation in contaminated wastewaters. Ind. Eng. Chem. Res. 2010, 49, 498–505. [Google Scholar] [CrossRef]
- Ovejero, G.; Rodriguez, A.; Vallet, A.; Willerich, S.; Garcia, J. Application of Ni supported over mixed Mg–Al oxides to crystal violet wet air oxidation: The role of the reaction conditions and the catalyst. Appl. Catal. B Environ. 2012, 111–112, 586–594. [Google Scholar] [CrossRef]
- Ovejero, G.; Rodriguez, A.; Vallet, A.; Garcia, J. Ni supported on Mg–Al oxides for continuous catalytic wet air oxidation of crystal violet. Appl. Catal. B Environ. 2012, 125, 166–171. [Google Scholar] [CrossRef]
- Ovejero, G.; Rodriguez, A.; Vallet, A.; Garcia, J. Intermediary products in the catalytic wet air oxidation of crystal violet with Ni/MgAlO as catalyst. Ind. Eng. Chem. Res. 2012, 51, 11367–11372. [Google Scholar] [CrossRef]
- Santos, A.; Yustos, P.; Rodriguez, S.; Garcia-Ochoa, F.; de Gracia, M. Decolorization of textile dyes by wet oxidation using activated carbon as catalyst. Ind. Eng. Chem. Res. 2007, 46, 2423–2427. [Google Scholar] [CrossRef]
- Rocha, R.P.; Soarez, O.S.G.P.; Goncalves, A.G.; Orfao, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Different methodologies for synthesis of nitrogen doped carbon nanotubes and their use in catalytic wet air oxidation. Appl. Catal. A Gen. 2017, 548, 62–70. [Google Scholar] [CrossRef]
- Cybulski, A.; Trawczynski, J. Catalytic wet air oxidation of phenol over platinum and ruthenium catalysts. Appl. Catal. B Environ. 2004, 47, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, D.Z. Development of Fe2O3-CeO2-TiO2/γ-Al2O3 as catalyst for catalytic wet air oxidation of methyl orange azo dye under room condition. Appl. Catal. B Environ. 2007, 72, 205–211. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.L.; Chen, Y.; Wang, X.H.; Wang, S.T. Catalytic wet air oxidation of dye pollutants by polyoxomolybdate nanotubes under room condition. Appl. Catal. B Environ. 2009, 86, 182–189. [Google Scholar] [CrossRef]
- Hua, L.; Ma, H.R.; Zhang, L. Degradation process analysis of the azo dyes by catalytic wet air oxidation with catalyst CuO/γ-Al2O3. Chemosphere 2013, 90, 143–149. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.H.; Rohit, G.S.; Holkar, C.R.; Pinjari, D.V.; Bhanvase, B.A.; Pandit, A.B. Investigation of TiO2 photocatalyst performance for decolorization in the presence of hydrodynamic cavitation as hybrid AOP. Ultrason. Sonochem. 2016, 28, 150–160. [Google Scholar] [CrossRef]
- Lozovskyi, O.; Gunduz, G.; Dukkancib, M.; Prihodkoa, R. Preparation and characterization of silver or copper-doped TiO2 catalysts and their catalytic activity in dye degradation. Color. Technol. 2015, 131, 245–254. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Sinha, A.S.K.; Kamsal, S.K. Efficient photocatalytic degradation of brilliant green using Sr-doped TiO2 nanoparticles. Ceram. Int. 2015, 41, 3533–3540. [Google Scholar] [CrossRef]
- Hadjltaief, H.B.; Omri, A.; Ben Zina, M.; Da Costa, P.; Galvez, M.E. Titanium dioxide supported on different porous materials as photocatalyst for the degradation of methyl green in wastewaters. Adv. Mater. Sci. Eng. 2015, 2015, 759853. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Shams-Ghahfarokhi, Z. Photodegradation of methyl green by Nickel-Dimethylglyoxime/ZSM-5 zeolite as a heterogeneous catalyst. J. Chem. 2013, 2013, 104093. [Google Scholar] [CrossRef]
- Yu, K.; Yang, S.; Liu, C.; Chen, H.; Li, H.; Sun, C.; Boyd, S.A. Degradation of organic dyes via bismuth silver oxide initiated direct oxidation coupled with sodium bismuthate based visible light photocatalysis. Environ. Sci. Technol. 2012, 46, 7318–7326. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Cai, J.; Yin, C.; Gao, L.; Zhou, J. Degradation performance of crystal violet over CuO@AC and CeO2-CuO@AC catalysts using microwave catalytic oxidation degradation method. J. Environ. Chem. Eng. 2016, 4, 958–964. [Google Scholar] [CrossRef]
- Ju, Y.; Wang, X.; Qiao, J.; Li, G.; Wu, Y.; Li, Y.; Zhang, X.; Xu, Z.; Qi, J.; Fang, J.; et al. Could microwave induced catalytic oxidation (MICO) process over CoFe2O4 effectively eliminate brilliant green in aqueous solution? J. Hazard. Mater. 2013, 263, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, M.; Guo, X. Studies on the treatment of brilliant green solution by combination microwave induced oxidation with CoFe2O4. Sep. Purif. Technol. 2008, 62, 458–463. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Guo, X.; Su, M.; Xu, T.; Song, X. Investigation on the degradation of brilliant green induced oxidation by NiFe2O4 under microwave irradiation. Chem. Eng. J. 2011, 173, 737–742. [Google Scholar] [CrossRef]
- Plesa Chicinas, R.; Contet, C.L.; Maicaneanu, A.; Vasilescu, M.; Vulpoi, A. Preparation, characterization, and testing of metal-doped carbon xerogels as catalyst for phenol CWAO. Environ. Sci. Pollut. Res. 2017, 24, 2980–2986. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Ribeiro, R.S.; Gomes, H.T.; Sotelo, J.L.; Garcia, J. Synthesis of carbon xerogels and their application in adsorption studies of caffeine and diclofenac as emerging contaminants. Chem. Eng. Res. Des. 2015, 95, 229–238. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Fathy, N.A.; Attia, A.A.; Silva, M.T.; Faria, J.L.; Gomes, H.T. Activated carbon xerogels for the removal of the anionic azo dyes Orange II and Chromotrope 2R by adsorption and catalytic wet peroxide oxidation. Chem. Eng. J. 2012, 195–196, 112–121. [Google Scholar] [CrossRef]
- Pinho, M.T.; Silva, A.M.T.; Hathy, N.A.; Attia, A.A.; Gomes, H.T.; Faria, J.L.; Pinho, M.T. Activated carbon xerogel–chitosan composite materials for 2 catalytic wet peroxide oxidation under intensified process conditions. J. Environ. Chem. Eng. 2015, 3, 1243–1251. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. The role of cobalt in bimetallic iron-cobalt magnetic carbon xerogels developed for catalytic wet peroxide oxidation. Catal. Today 2017, 296, 66–75. [Google Scholar] [CrossRef]
- Fathy, N.A.; Shouman, M.A.; Aboelenin, R.M.M. Nitrogen and phosphorous-doped porous carbon xerogels as metal-free catalysts for environmental catalytic peroxide oxidation of 4-nitrophenol. Asia Pac. J. Chem. Eng. 2016, 11, 836–845. [Google Scholar] [CrossRef]
- Fathy, N.A.; El-Khouly, S.M.; Hassan, N.A.; Awad, M.S. Free- and Ni-doped carbon xerogels catalysts for wet peroxide oxidation of methyl orange. J. Water Process Eng. 2017, 16, 21–27. [Google Scholar] [CrossRef]
- Rashwan, W.E.; Fathy, N.A.; Elkhouly, S.M. A novel catalyst of ceria-nanorods loaded on carbon xerogel for catalytic wet oxidation of methyl green dye. J. Taiwan Inst. Chem. Eng. 2018, 88, 234–242. [Google Scholar] [CrossRef]
- Sun, H.; Tian, H.; Hardjono, Y.; Buckley, C.E.; Wang, S. Preparation of cobalt/carbon-xerogel for heterogeneous oxidation of phenol. Catal. Today 2012, 186, 63–68. [Google Scholar] [CrossRef]
- Gomes, H.T.; Machado, B.F.; Ribeiro, A.; Moreira, I.; Rosario, A.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L. Catalytic properties of carbon materials for wet oxidation of aniline. J. Hazard. Mater. 2008, 159, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Apolinario, A.C.; Silva, A.M.T.; Macjado, B.F.; Gomes, H.T.; Araujo, P.P.; Figueiredo, J.L.; Faria, J.L. Wet air oxidation of nitro-aromatic compounds: Reactivity on single- and multi-component systems and surface chemistry studies with a carbon xerogel. Appl. Catal. B Environ. 2008, 84, 75–86. [Google Scholar] [CrossRef]
- De Moraes, N.C.; da Silva, M.L.C.P.; Rodriguez, L.A. Effect of metal doping in the photocatalytic properties of carbon xerogel-Nb2O5 composite towards visible light degradation of methylene blue. Mater. Lett. 2018, 228, 486–489. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Lei, L. Preparation, characterization and catalytic activity for ortho-dichlorobenzene ozonation of Fe-, Mn-Doped xerogels. Acta Phys. Chim. Sin. 2010, 26, 2443–2448. [Google Scholar]
- Osinska, M.; Krawczyk, P.; Rozmanowski, T.; Gurzeda, B. The electrochemical performance of carbon xerogels with the addition of graphite intercalation compound. Appl. Surf. Sci. 2009, 481, 545–553. [Google Scholar] [CrossRef]
- O’Rejlly, J.M.; Mosher, R.A. Functional groups in carbon black by FTIR spectroscopy. Carbon 1983, 21, 47–51. [Google Scholar]
- Selittiti, C.; Koenig, J.L.; Ishida, H. Surface characterization of graphitized carbon fibers by attenuated total reflection Fourier transform infrared spectroscopy. Carbon 1990, 28, 221–228. [Google Scholar] [CrossRef]
- Girgis, B.S.; Attia, A.A.; Fathy, N.A. Potential of nano-carbon xerogels in the remediation of dye-contaminated water discharges. Desalination 2011, 265, 169–176. [Google Scholar] [CrossRef]
 ), Fe-CX(1) (
), Fe-CX(1) ( ), Fe-CX(2) (
), Fe-CX(2) ( ), Fe-CX(3) (
), Fe-CX(3) ( ), Fe-CX(4) (
), Fe-CX(4) ( ), and Fe-CX(4.1) (
), and Fe-CX(4.1) ( ).
).
 ), Fe-CX(1) (
), Fe-CX(1) ( ), Fe-CX(2) (
), Fe-CX(2) ( ), Fe-CX(3) (
), Fe-CX(3) ( ), Fe-CX(4) (
), Fe-CX(4) ( ), and Fe-CX(4.1) (
), and Fe-CX(4.1) ( ).
).
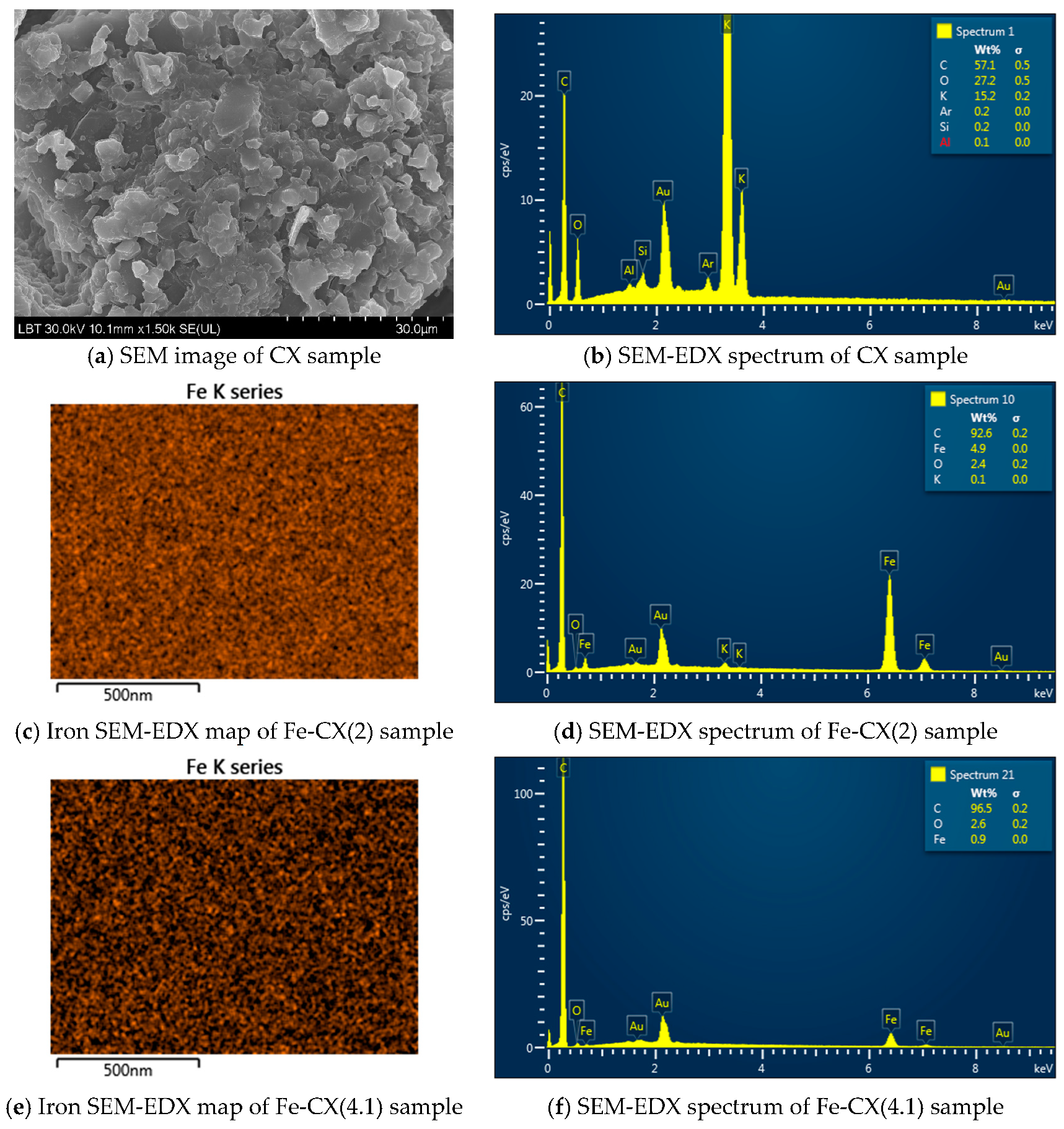
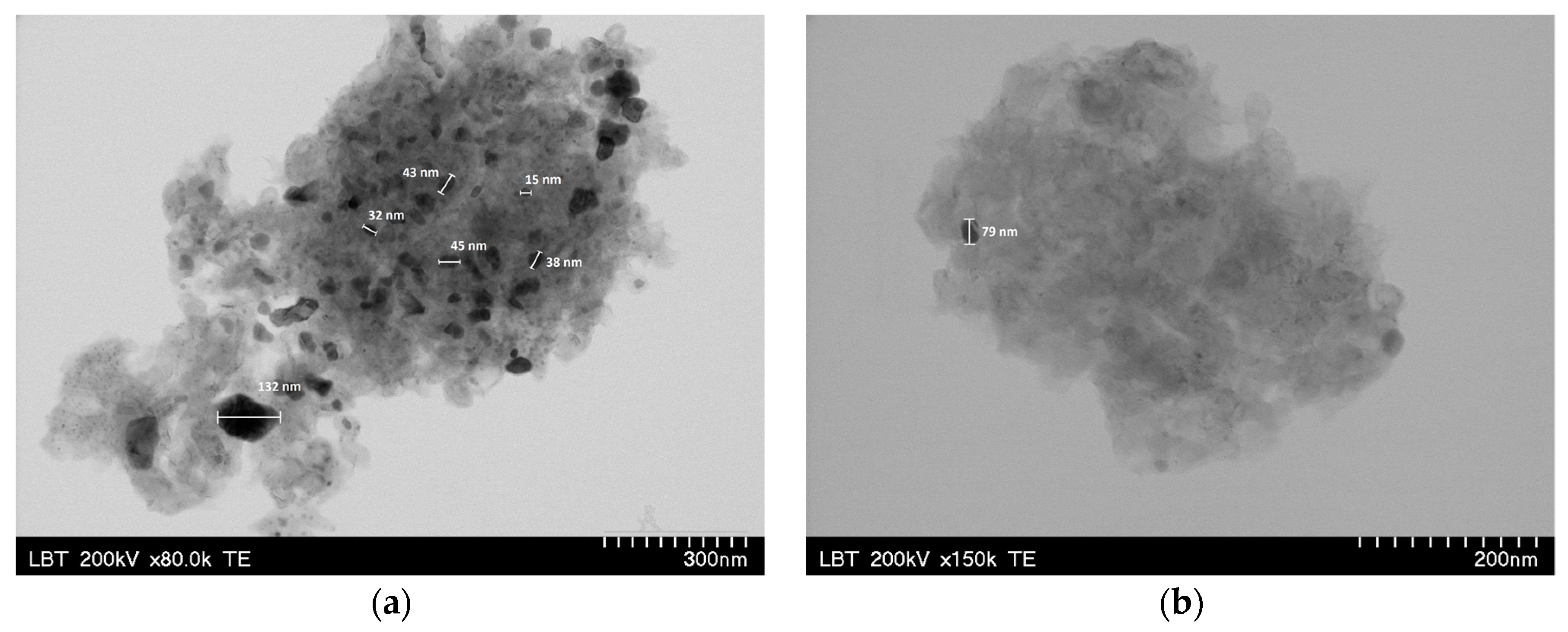
| Sample | Ethanol Wash | Water Wash 1 | Iron Exchange | Water Wash 2 |
|---|---|---|---|---|
| CX | N/A | N/A | N/A | N/A |
| Fe-CX(1) | 24 h | N/A | 72 h ** | N/A |
| Fe-CX(2) | 24 h | 72 h * | 72 h ** | N/A |
| Fe-CX(3) | 24 h | 72 h * | 72 h ** | 72 h * |
| Fe-CX(4) | 24 h | N/A | 72 h ** | 72 h * |
| Fe-CX(4.1) | 48 h | N/A | 72 h ** | 72 h * |
| CX | Fe-CX(1) | Fe-CX(2) | Fe-CX(3) | Fe-CX(4) | Fe-CX(4.1) | |
|---|---|---|---|---|---|---|
| C | 54.7 | 89.4 | 90.0 | 94.0 | 95.5 | 95.0 |
| Fe | - | 7.0 | 7.3 | 2.8 | 1.2 | 1.6 |
| O | 28.3 | 3.3 | 2.6 | 3.2 | 3.3 | 3.4 |
| K * | 16.8 | 0.2 | 0.1 | - | - | - |
| Ar, Si, Al | <0.2 | - | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maicaneanu, S.A.; McGhee, B.; Stefan, R.; Barbu-Tudoran, L.; Sedwick, C.; Lake, C.H. Investigations on Cationic Dye Degradation Using Iron-Doped Carbon Xerogel. ChemEngineering 2019, 3, 61. https://doi.org/10.3390/chemengineering3030061
Maicaneanu SA, McGhee B, Stefan R, Barbu-Tudoran L, Sedwick C, Lake CH. Investigations on Cationic Dye Degradation Using Iron-Doped Carbon Xerogel. ChemEngineering. 2019; 3(3):61. https://doi.org/10.3390/chemengineering3030061
Chicago/Turabian StyleMaicaneanu, S. Andrada, Breanna McGhee, Razvan Stefan, Lucian Barbu-Tudoran, Christopher Sedwick, and Charles H. Lake. 2019. "Investigations on Cationic Dye Degradation Using Iron-Doped Carbon Xerogel" ChemEngineering 3, no. 3: 61. https://doi.org/10.3390/chemengineering3030061
APA StyleMaicaneanu, S. A., McGhee, B., Stefan, R., Barbu-Tudoran, L., Sedwick, C., & Lake, C. H. (2019). Investigations on Cationic Dye Degradation Using Iron-Doped Carbon Xerogel. ChemEngineering, 3(3), 61. https://doi.org/10.3390/chemengineering3030061