2.2.1. Adsorption of the Anionic Dyes (Am and DGB)
Am and DGB adsorption tests were carried out with the inorganic LDH (MgAlCO3 and MgAlNO3) and the nanohybrids Mg2AlDBS and Mg3AlDBS for comparison purposes. The influence of pH on the adsorption process could have a relevant role and therefore this one was examined at an initial pH value between 4 and 10 for Am and DGB on MgAlNO3, MgAlCO3, Mg2AlDBS and Mg3AlDBS adsorbents. The final pH was measured after each adsorption experiment, and the maximum value obtained was between 7 and 9.5, which was consistent with the buffering properties of hydrotalcites. The pH influence results included in
Table S1 indicated that the maximum adsorption of Am for MgAlCO3 was at pH = 4, probably due to the elimination of the carbonate anion as CO
2 together with some dissolution of the LDH and possibly subsequent recrystallization with Am in the interlayer [
40]. However, the adsorption was not significantly affected by the initial pH = 4 and 7 for MgAlNO3 and MgxAlDBS (x = 2 and 3). This led us to adopt an initial pH ~ 6 corresponding to the pH of the Am solutions for all adsorption tests. The DGB adsorption was almost the same at pH 4 and 8, slightly higher at pH 8 for LDH containing inorganic anions, so the pH ~ 8 corresponding to the dye solutions, was used for all the adsorption tests. In general, with the increase of the solution pH, the percentage of the removal of Am and DGB decreases for MgAlCO3 and MgAlNO3 adsorbents due to a decrease in the positive charge on the external surface by deprotonation and competition between the anions (anion dyes and OH
−) for the positive charges of the LDH layer. For nanohybrid samples (MgxAlDBS, x = 2 and 3), the percentage of removal is lower than for the inorganic adsorbents and a decrease in adsorption was observed with the increase of pH solution, due to the repulsion of the negative charges.
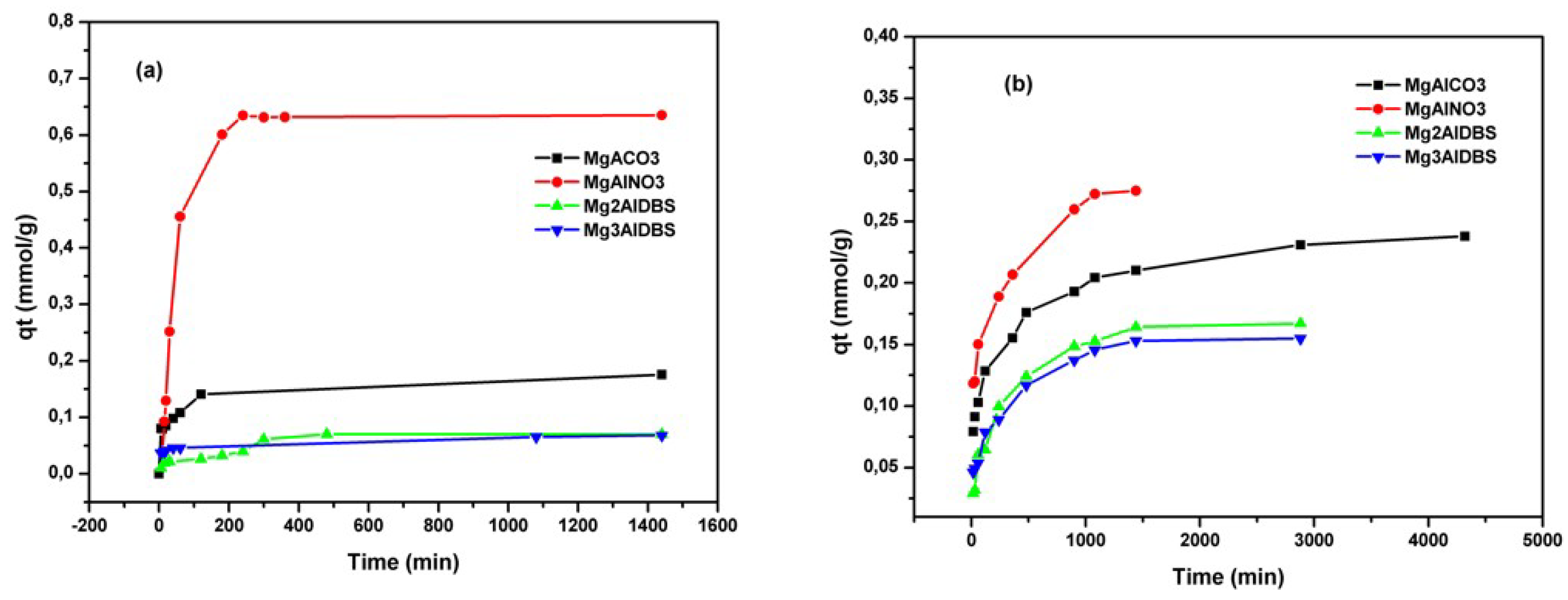
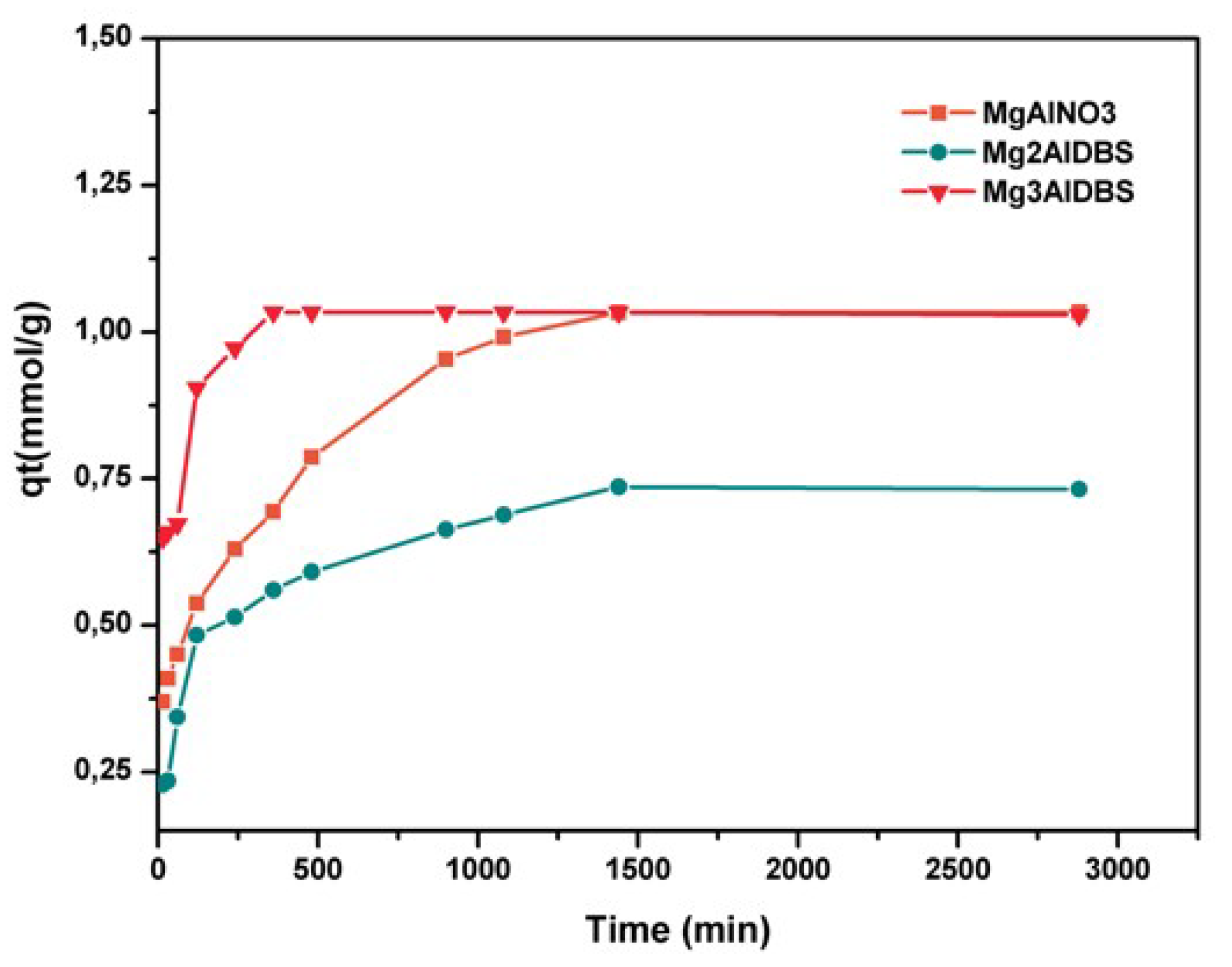
The kinetic results of Am and DGB adsorption on the prepared adsorbents are included in
Figure 4. The equilibrium for amaranth adsorption on inorganic LDH was reached at 180 min for MgAlCO3 and 360 min for MgAlNO3.
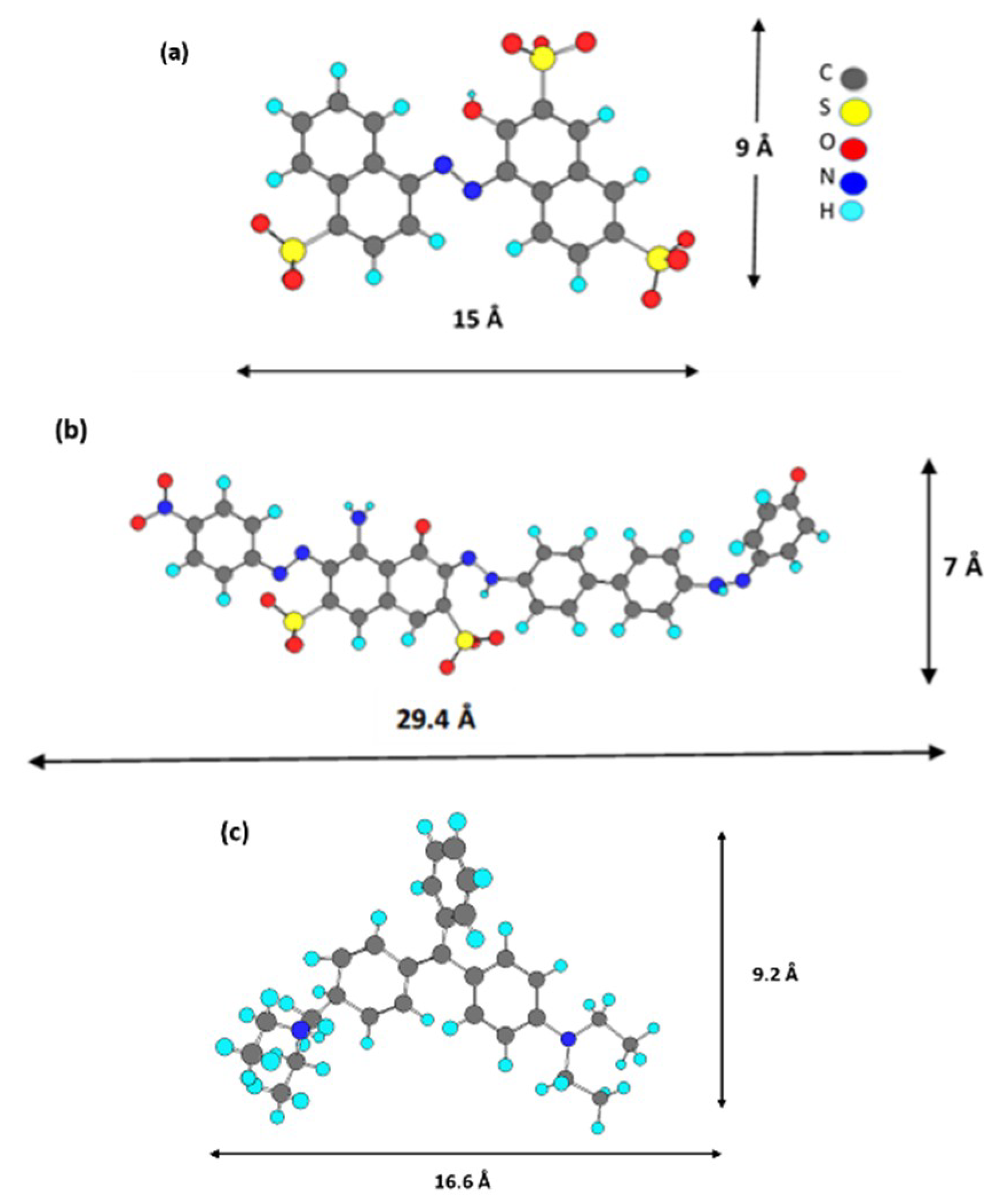
The adsorption of DGB was much slower than that of Am for all adsorbents. The larger size of DGB (29.4 Å × 7 Å for DGB vs. 15 Å × 9 Å for Am, from Chem Draw 3D 5.0 software package Program cause bigger steric hindrances for its adsorption on LDH. Kinetic modelling of the adsorption process provides a prediction of adsorption rates and allows the determination of suitable rate expressions characteristic of possible reaction mechanisms. In this study, the most frequently used models, the pseudo-first-order, Equation (1) [
41], the pseudo-second-order, Equation (2) [
42], and the intraparticle diffusion model, Equation (3) [
43] were tested:
where qe and qt (mmol/g) are the adsorption capacities of the adsorbate at equilibrium and at time t (min), respectively; k
1 (min
−1) and k
2 (g mmol
−1 min
−1) are the rate constants of pseudo-first-order and pseudo-second-order models; and k
ip (mmol g
−1 min
−1/2) is the intraparticle diffusion rate constant. If the intra-particle diffusion is involved in the process, the plot of qt vs. t
1/2 will be linear, and, if this line passes through the origin, the rate limiting process is only due to the intra-particle diffusion. Such plots may present a multilinearity indicating that two or more steps take place. The slowest of these steps determines the overall rate of the adsorption process [
44].
The kinetic parameters calculated for Am and DGB adsorption on the prepared samples are listed in
Table 2 and
Table 3. The pseudo-second-order model is the most suitable in describing the adsorption kinetics of dyes on LDH based on the correlation coefficient (R
2). The applicability of the pseudo-second order suggested that chemisorption might be the rate-limiting step that controls these adsorption processes. Furthermore, the theoretical qe calculated values are closer to the experimental qe values and explain the kinetics of most adsorption systems very well for a whole range of adsorption periods using different concentrations of dye and adsorbent dosages which present an advantage over pseudo-first order equation [
45].
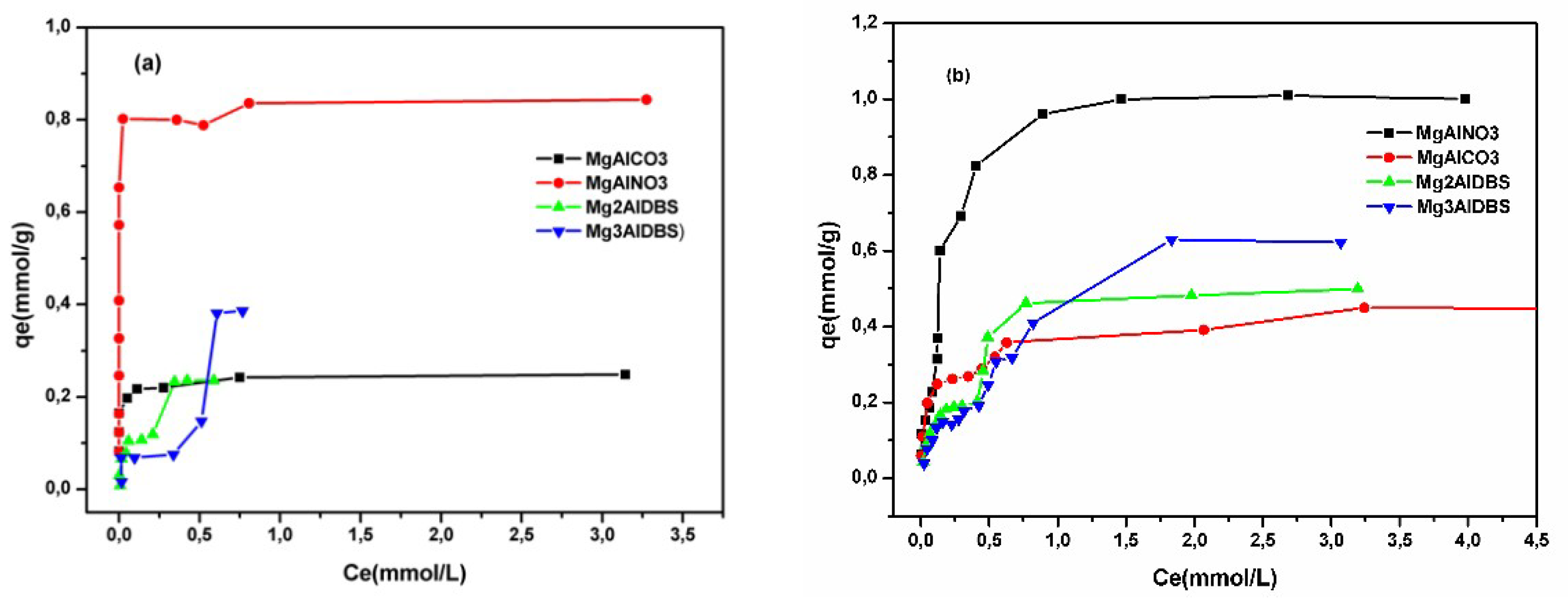
The adsorption isotherms of the two anionic dyes at room temperature and a constant initial pH of the dye solution are illustrated in
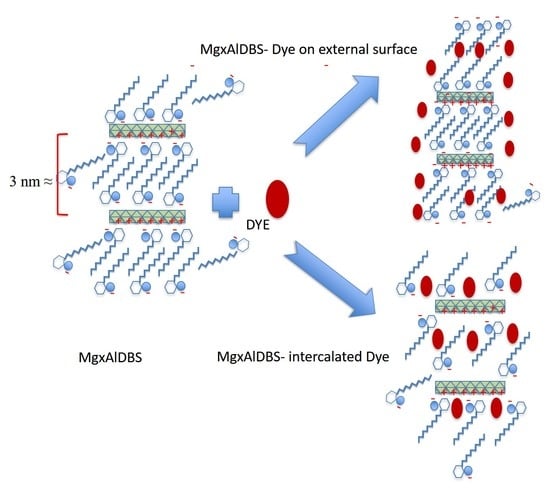
Figure 5. The adsorbed amount of the dyes was higher on MgAlNO3 than on MgAlCO3 because of the lower affinity of the nitrate anion for the hydroxide layer favoured the anionic exchange and produced a greater number of adsorption sites. The small amount adsorbed for MgAlCO3 probably occurred over the surface of the adsorbent. The adsorbed amount of both dyes was lower for hybrids than for MgAlNO3, since the external surface charge of the nanohybrid was negative (as suggested by the zeta potential values (
Figure S3) and suffers electrostatic repulsion with the sulfonate groups of the dye molecules. The experimental adsorption data were fitted to the Langmuir and Freundlich models, often used to describe adsorption processes and in which lineal forms are represented in Equations (4) and (5), respectively, and the model of Sips, nonlinear form, Equation (6):
where qe is the amount of dye adsorbed at equilibrium (mmol·g
−1); qm is the maximum monolayer sorption capacity (mmol·g
−1); Ce the equilibrium concentration of dye in solution (mmol·L
−1) and K
L (L·mmol
−1) is a constant related to the sorption energy:
where K
f and N
f are the Freundlich constants that are characteristic of the adsorbent–adsorbate systems [
46].
The Sips isotherm model [
47] is a combination of the Langmuir and Freundlich isotherms that is used to describe heterogeneous adsorption systems:
The Sips isotherm has the advantage of being reduced to the Freundlich isotherm at low concentrations, and at high concentrations approaches the capacity of the formation of a monolayer such as in the case of the Langmuir isotherm. When n is equal to 1, the Sips equation is reduced to the Langmuir equation and involves a homogeneous adsorption process.
The influence of the concentration (adsorption isotherms) of Am at room temperature was studied for the four adsorbents and included in
Figure 5a. The isotherm shape obtained for MgAlCO3 was of L-type [
48] which suggests a progressive saturation of the adsorbent. This type of isotherm is indicative of molecules adsorbed flat on the surface, or sometimes of vertically oriented adsorbed ions with a particularly strong intermolecular attraction. Moreover, the shape of the isotherms was of type H for dye adsorption on MgAlNO3 (
Figure 5a,b). The H-curve, whose initial section of the isotherm is vertical, arises in special cases of the L-curve, in which the solute has such a high affinity that it is completely adsorbed in dilute solutions. The adsorbate is strongly attracted to the adsorbent mainly by electrostatic interaction [
49].
In solid sorbent adsorption, multilayers often form and there may also be a change of orientation of the adsorbed molecules as they increase in concentration on the adsorbent surface. The adsorption isotherms of both dyes on nanohybrids were of the L-4 subtype, characteristic of the isotherms with two slopes (
Figure 5a,b). This isotherm type suggests the possibility of adsorption on different types of sites, successively. In both cases, the shape of the isotherms does not change with the increase in the amount of DBS in the interlayer of the hybrids, indicating that the type of interactions between the hybrids and dyes is not affected by the DBS amount.
Table 4 and
Table 5 include the parameters obtained from fitting the data to the different isotherm models discussed above. It was observed that the isotherms adjusted to the Sips model describe a heterogeneous adsorption, indicated by the R
2 values obtained. The application of the Freundlich model does not allow a satisfactory refinement of the results in all adsorbents.
The shape of the adsorption isotherms for the anionic dyes Am and DGB on nanohybrids MgxAlDBS (x = 2 and 3) can be related to the fact that the adsorption at low concentrations occurs through anion exchange mechanism, but, as the concentration of dye increases, the large size of the dyes anions impedes their intercalation and probably occurs on the surface through hydrophobic interactions.
2.2.2. Adsorption of the Cationic Dye BG
The adsorption process for BG was studied on MgAlNO3 and the nanohybrid MgxAlDBS, x = 2 and 3. The results of the adsorption kinetics of the BG cationic dye on the MgAlNO3, Mg2AlDBS and Mg3AlDBS samples shown in
Figure 6 indicate that the adsorption equilibrium was reached faster for Mg3AlDBS (in 6 h) and slower for Mg2AlDBS (in 24 h) similar to MgAlNO3.
The kinetic parameters obtained from the application of the different models are shown in
Table 6. The pseudo-second-order model is the most suitable in describing the adsorption kinetics of BG in all cases, based on the correlation coefficient (R
2) and the agreement between the experimental and calculated values of qe.
The results of the adsorption isotherms of the cationic dye BG presented in
Figure 7 are of H type [
48], suggesting a very high affinity between the adsorbent and the adsorbate.
According to the results shown in
Table 7, for the three adsorbents, the Sips model fits the experimental data of adsorption equilibrium better than the other models and this is used to describe heterogeneous adsorption systems. The
n value is near to 1 for MgAlNO3, therefore adsorption can be represented more appropriately by a monolayer adsorption. However, the nanohybrids MgxAlDBS (x = 2 and 3) show a value of
n much lower than 1; therefore, the adsorption is not represented by a homogeneous process.
The high amount of BG adsorbed on inorganic LDH could be due to the hydrotalcite buffer properties, i.e., initial pH solution increases during experiments, which provokes the precipitation of this dye. Nevertheless, for MgxAlDBS (x = 2 and 3) adsorbents, DBS incorporation changes the electrical properties of the particles making the surface potential negative (according to the zeta potential shown in
Figure S3) and favours the adsorption of cationic dye. Surprisingly, a minor amount of BG was adsorbed on the Mg2AlDBS regarding the Mg3AlDBS sample, which has a higher permanent charge due to the isomorphic substitution of divalent for trivalent metal, and, consequently, a higher DBS content. It could be because the higher DBS loading on Mg2AlDBS provokes steric hindrances for BG intercalation and, in the case of Mg3AlDBS, there is probably some cationic dye co-intercalated with DBS in the interlayer space as will be suggested below by the PXRD pattern of adsorption product (
Figure S6c).
2.2.3. Characterization of the Adsorption Products
The solids obtained after the adsorption experiments were analyzed by the PXRD technique in order to ascertain how the dye is located in the adsorbent.
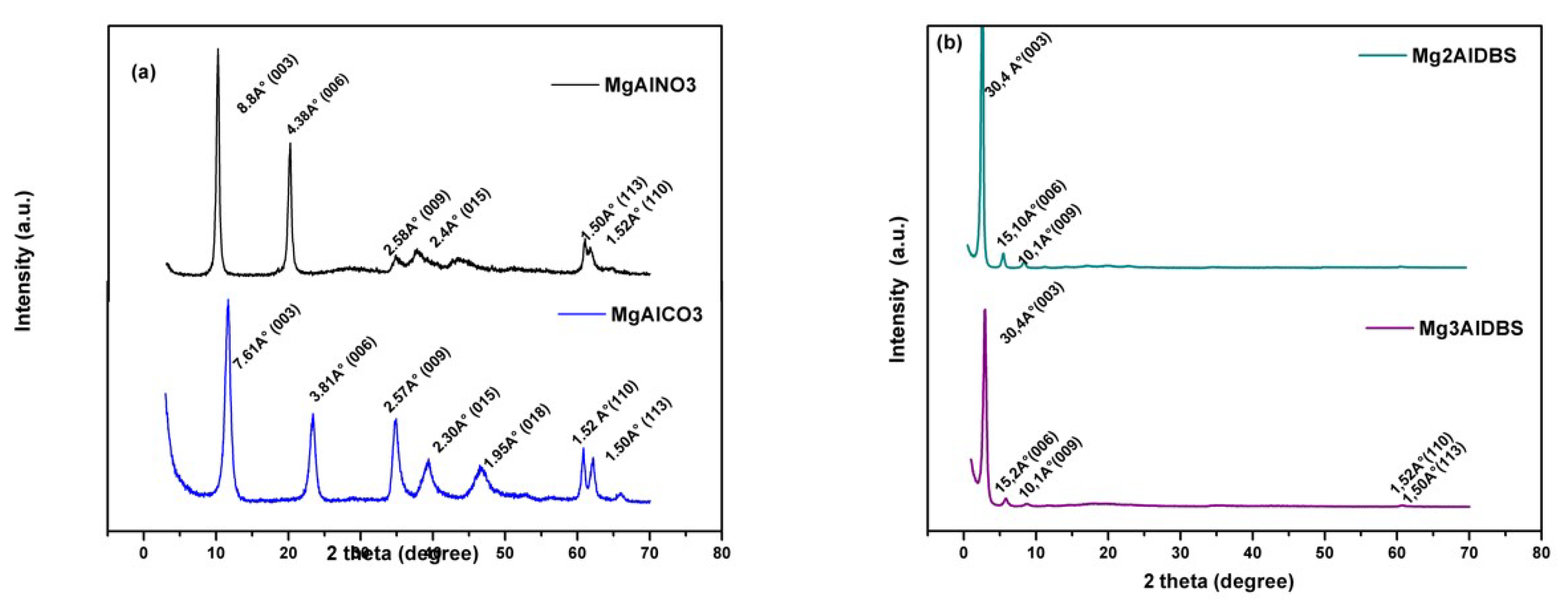
Figures S4 and S5 show the PXRD patterns of the products of Am and DGB adsorption, respectively, together with the patterns of the adsorbents and of the dyes. The results indicated that the positions of the diffraction lines of the MgAlCO3 sample were not modified after the Am and DGB adsorption process, possibly due to the fact that the small amounts of dye were adsorbed on the external surface of the adsorbent. However, for the Am adsorption product obtained with MgAlNO3, the PXRD pattern included in
Figure S4a shows a shift in the position of the reflection lines (
00l) to the lower angle (2θ) with a change in the d
(003) value from 8.9 Å to 17.7 Å. These lines were very sharp, indicating that the adsorption product retained the crystallinity of the adsorbent according to an anionic exchange process [
49]. Similar results were obtained for the DGB adsorption product. However, in this case, the DGB intercalation process is strongly dependent on the concentration of the dye. The PXRD pattern included in
Figure S5a shows a shift of the position of reflection line corresponding to d
(003) = 8.9 Å to 15.3 Å at low DGB concentration (0.06 mmol/L) according to the intercalation of the anion in the interlayer space. However, at a high DGB concentration (3.7 mmol/L), the interlayer space does not increase any further, indicating that the adsorption takes place on the external surface of the particles, which can be attributed to the large size and elongated shape of the dye anion. The adsorption of dye occurs provided the interlayer spacing of the LDH is greater than the size of the molecule; otherwise, the adsorbent can not accommodate the pollutant [
1]. At low concentrations of dye, it is possible to intercalate the DGB ions in a parallel manner or with a small inclination with respects to the sheets of LDH, giving rise to an interlayer space close to that observed in
Figure S5a (15.3 Å). This disposition of the ions would allow the cancellation of the layer charge by means of the electrostatic interaction with the sulfonate groups. However, as the concentration of the dye increases, the arrangement of bulky ions becomes more difficult, thus impeding their intercalation and favouring the adsorption on the external surface of the LDH particles.
The PXRD patterns of the Am adsorption products on nanohybrids adsorbents (
Figure S4c,d) showed no change in the position of the planes (
00l), so it can be deduced that the adsorption takes place on the external surface of the particles in the adsorbents studied. Similar results were obtained for the DGB adsorption products on nanohybrids (
Figure S5b).
The PXRD patterns of the BG adsorption products are shown in
Figure S6. It is interesting to note that there are no changes between the adsorbent Mg2AlDBS and the adsorption product Mg2 AlDBS-BG
Figure S6a,b. However, in the case of Mg3AlDBS, a decrease in the basal spacing of the adsorption product Mg3AlDS-BG compared with the pristine adsorbent, from d
003 = 30 Å to d
003 = 27.3 Å
Figure S6c, is observed. The reason for this decrease could be the change of orientation of the DBS chains as a result of some co-intercalation of BG between them [
50,
51].
Table 8 shows the maximum adsorption capacity of some adsorbents compared to the double layered hydroxides prepared in this study. It was observed that the LDH reach the highest adsorption values due to their structural characteristics, anion exchange capacity, the intercalation of anion and/or the ability to modify the hydrophilic nature of the interlaminar space.