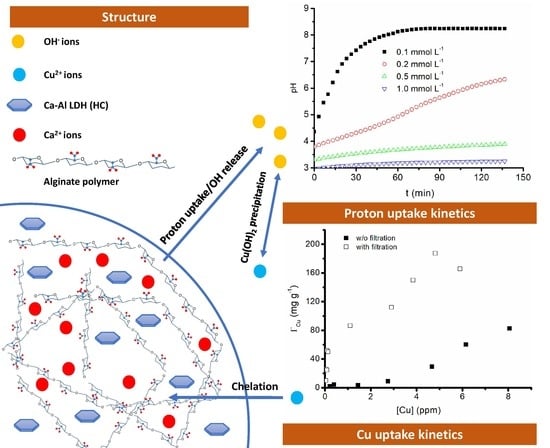
Reactivity and Heavy Metal Removal Capacity of Calcium Alginate Beads Loaded with Ca–Al Layered Double Hydroxides
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Ca–Al Layered Double Hydroxides (HC)
2.2. Calcium Alginate Beads (CaAlg and CaAlg/HC) Preparation
2.3. Structural Characterization
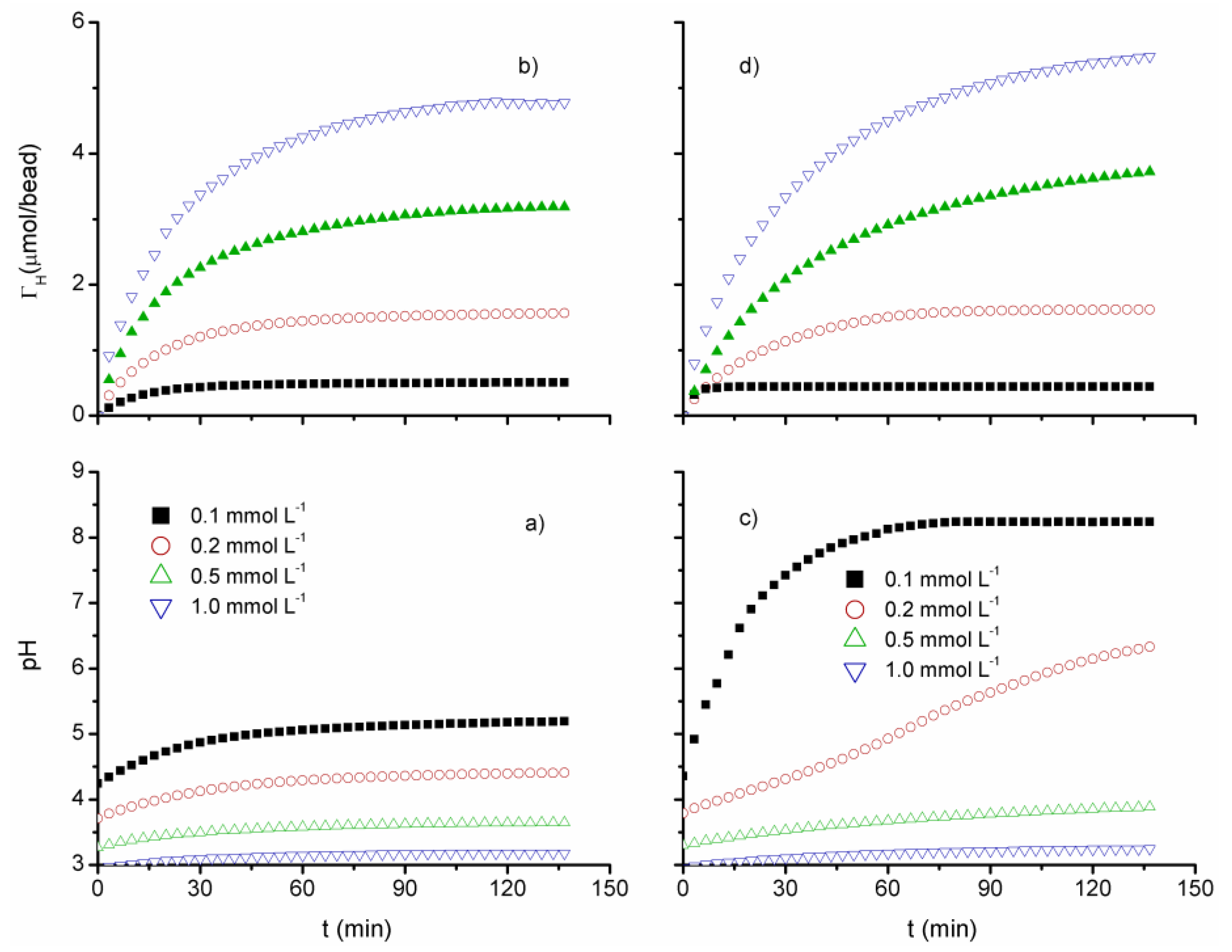
2.4. Proton Uptake Kinetics
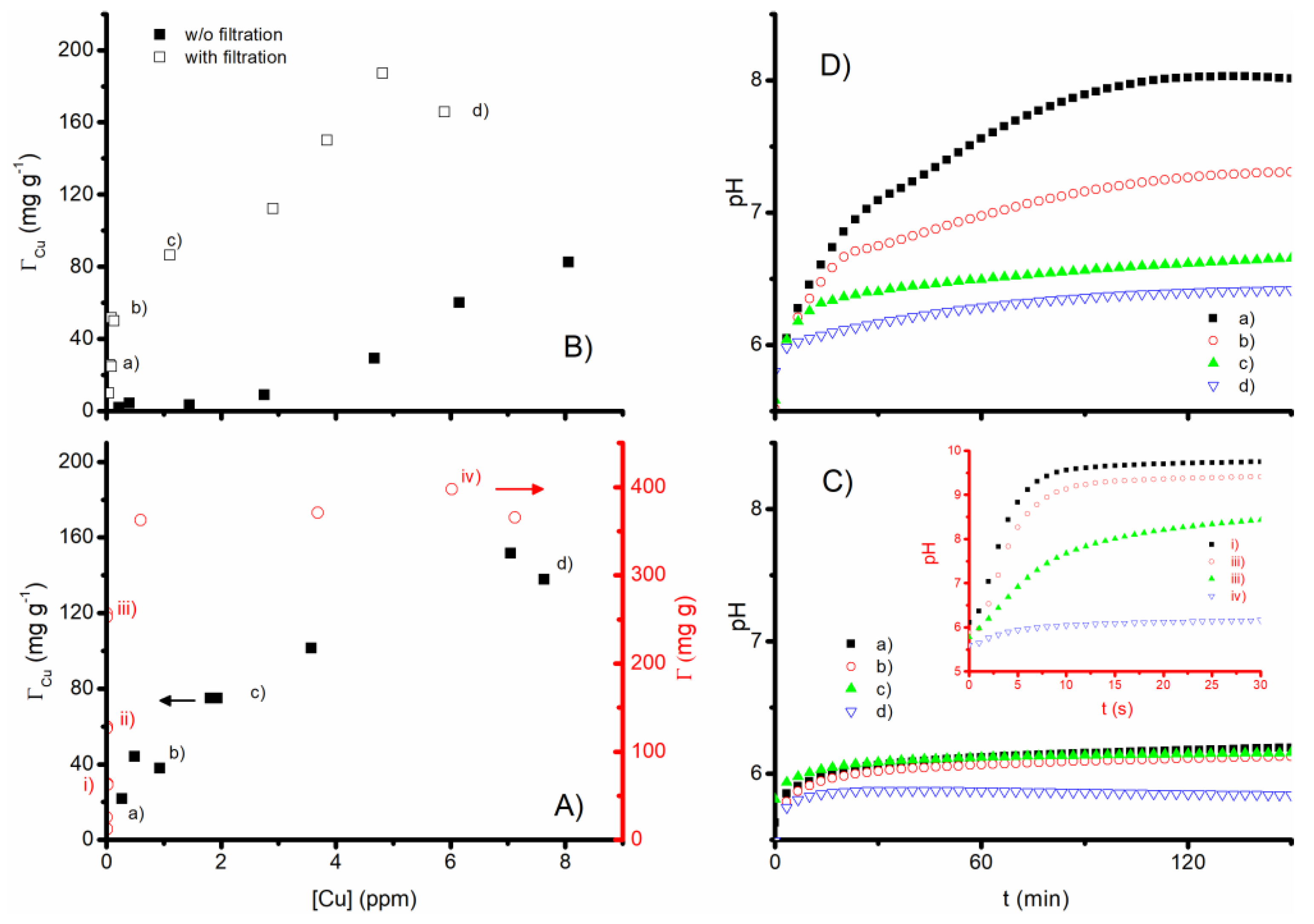
2.5. Cu2+ Removal Tests
3. Results
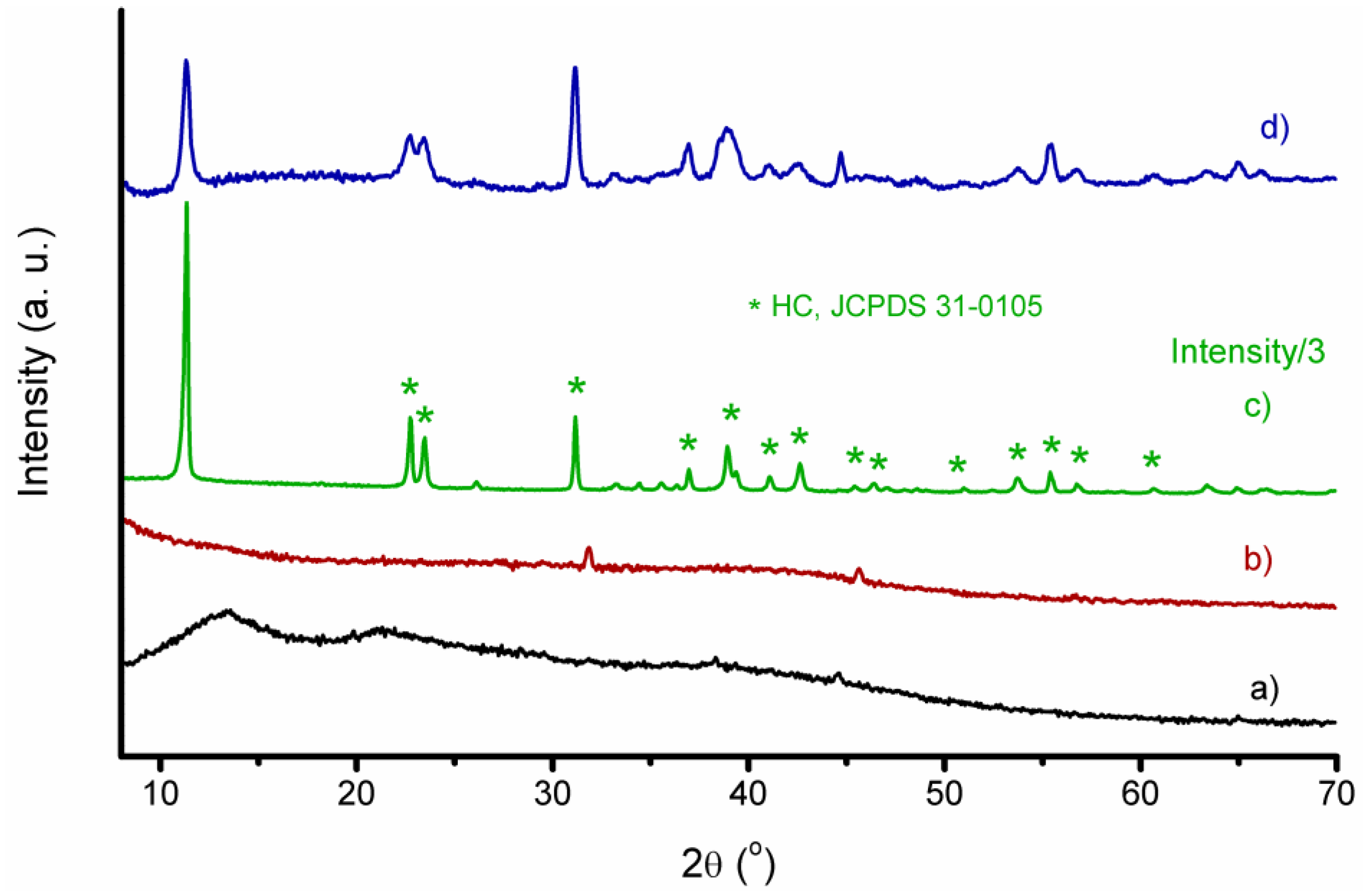
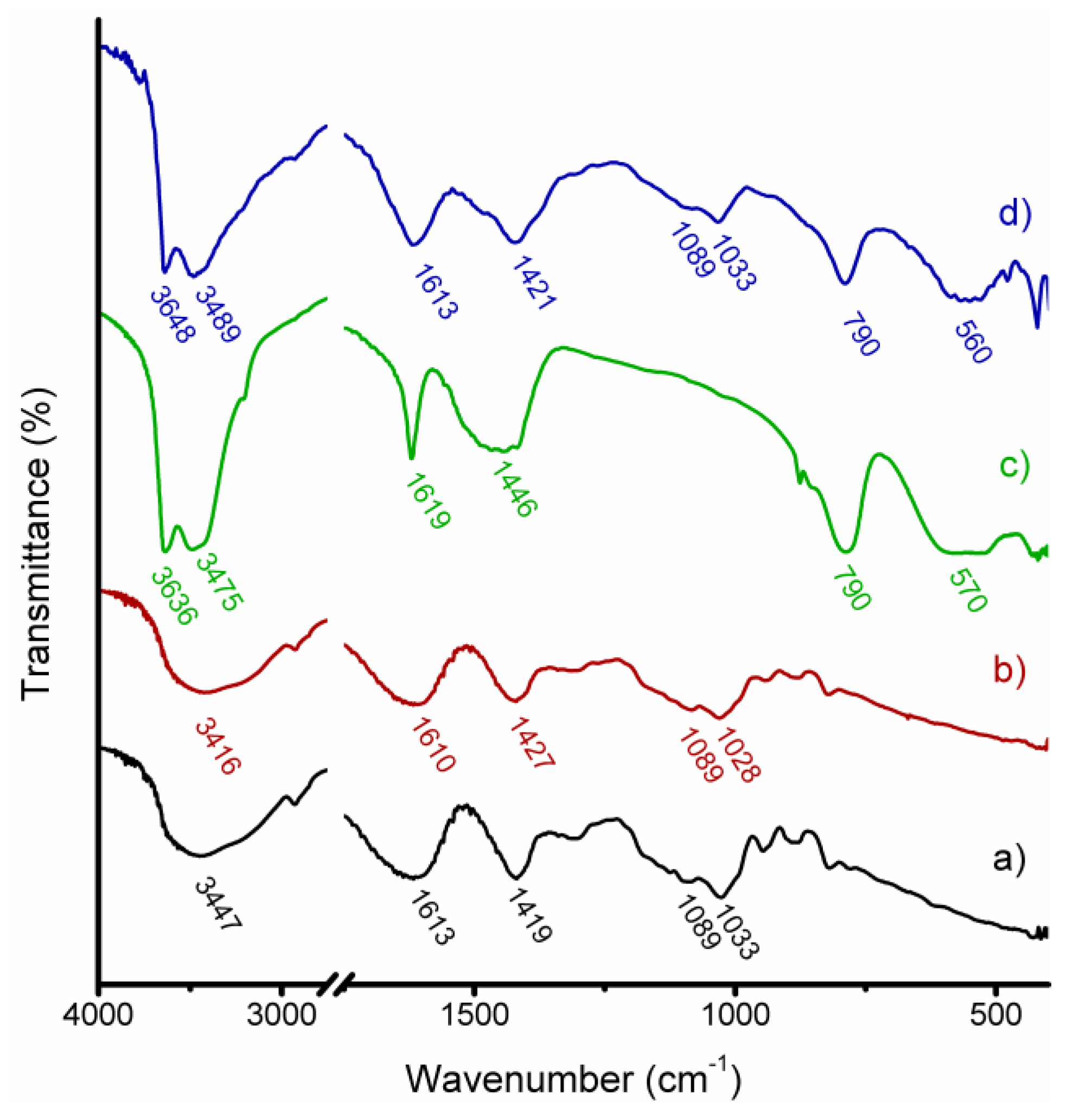
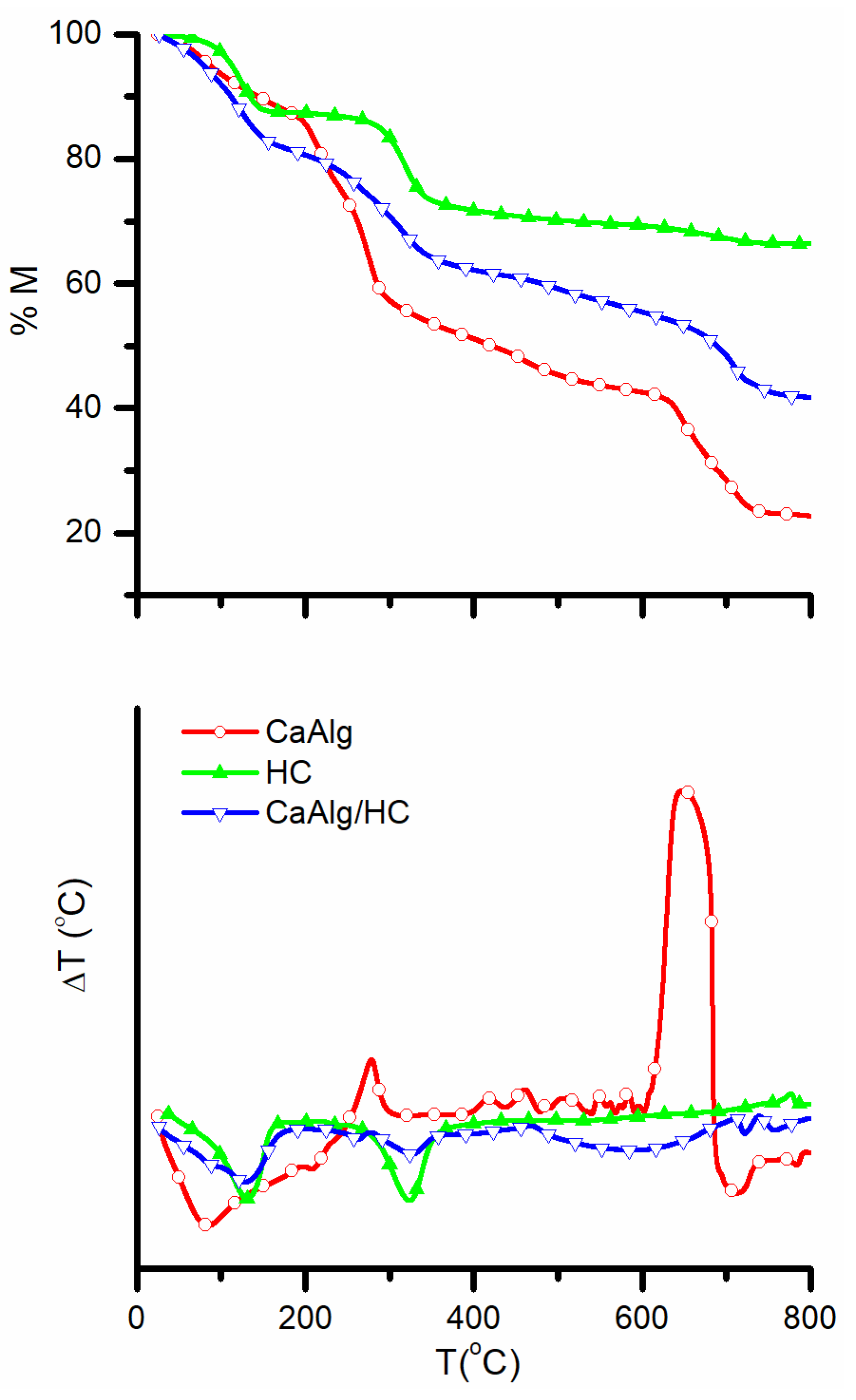
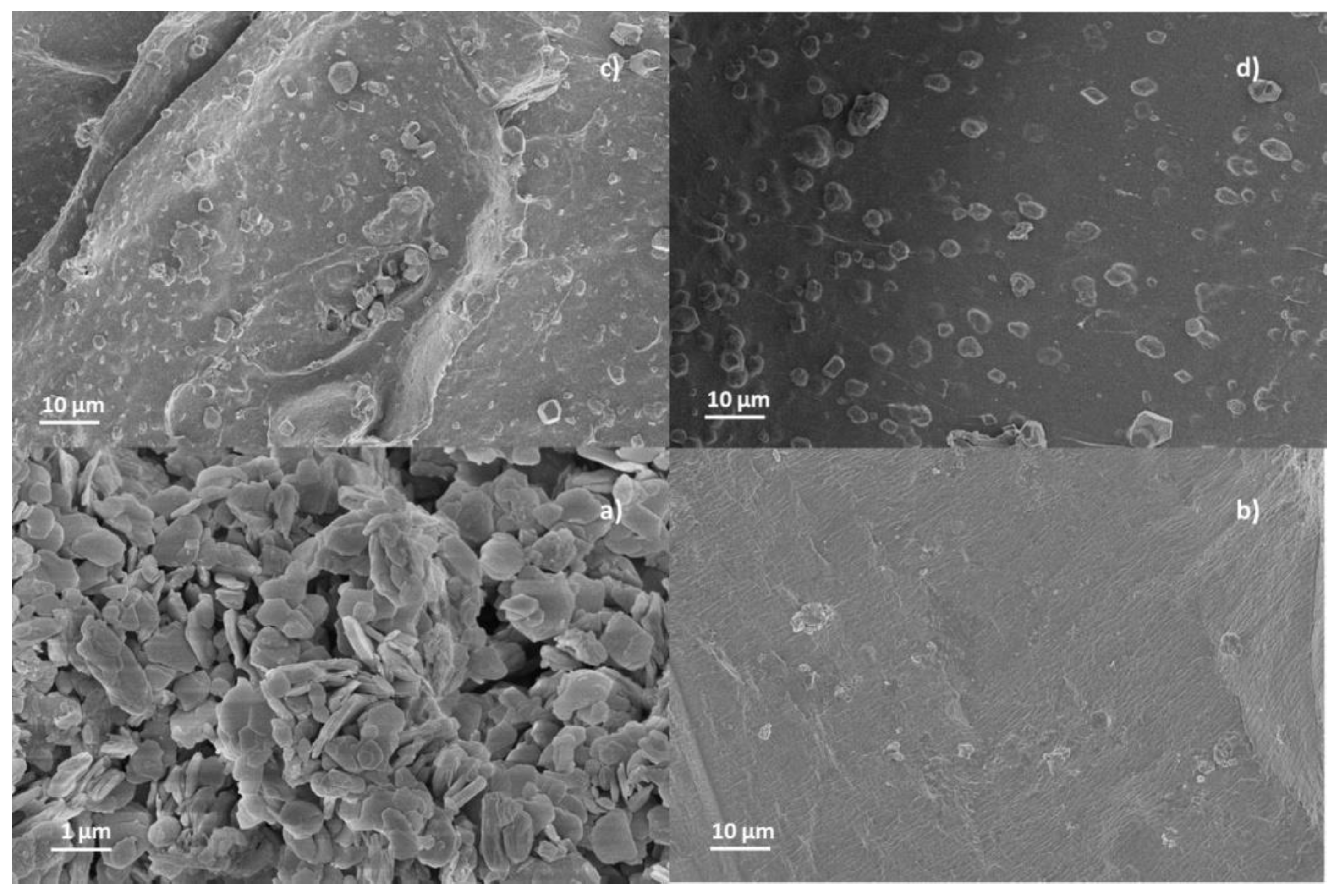
3.1. Structural and Morphological Characterization of CaAlg and CaAlg/HC
3.2. Proton Uptake Kinetics
3.3. Cu2+ Removal Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Chen, Y.; Yu, H.; Yan, L.; Du, B.; Pei, Z. Removal of Cu2+, Cd2+ and Pb2+ from aqueous solutions by magnetic alginate microsphere based on Fe3O4/MgAl-layered double hydroxide. J. Colloid Interface Sci. 2018, 532, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, A.C.S.; Aranda, P.; Darder, M.; Ruiz-Hitzky, E. Bionanocomposites based on alginate-zein/layered double hydroxide materials as drug delivery systems. J. Mater. Chem. 2010, 20, 9495–9504. [Google Scholar] [CrossRef]
- El Rouby, W.M.A. Efficient water decontamination using layered double hydroxide beads nanocomposites. Environ. Sci. Pollut. Res. 2018. Available online: https://doi.org/10.1007/s11356-018-3257-7 (accessed on 27 February 2019).
- Kim Phuong, N.T. Entrapment of Mg–Al layered double hydroxide into alginate/polyvinyl alcohol beads for water remediation. J. Environ. Chem. Eng. 2014, 2, 1082–1087. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Rocher, V.; Siaugue, J.-M.; Cabuil, V.; Bee, A. Removal of organic dyes by magnetic alginate beads. Water Res. 2008, 42, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Benettayeb, A.; Guibal, E.; Morsli, A.; Kessas, R. Chemical modification of alginate for enhanced sorption of Cd(II), Cu(II) and Pb(II). Chem. Eng. J. 2017, 316, 704–714. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Li, X.Y. Selective removals of heavy metals (Pb2+, Cu2+, and Cd2+) from wastewater by gelation with alginate for effective metal recovery. J. Hazard. Mater. 2016, 308, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R. Copper, lead and cadmium removal by Ca Al layered double hydroxides. Appl. Clay Sci. 2014, 87, 254–259. [Google Scholar] [CrossRef]
- Rojas, R. Effect of particle size on copper removal by layered double hydroxides. Chem. Eng. J. 2016, 303, 331–337. [Google Scholar] [CrossRef]
- Pérez, M.R.; Pavlovic, I.; Barriga, C.; Cornejo, J.; Hermosín, M.C.; Ulibarri, M.A. Uptake of Cu2+, Cd2+ and Pb2+ on Zn–Al layered double hydroxide intercalated with edta. Appl. Clay Sci. 2006, 32, 245–251. [Google Scholar] [CrossRef]
- Pavlovic, I.; Pérez, M.; Barriga, C.; Ulibarri, M.A. Adsorption of Cu2+, Cd2+ and Pb2+ ions by layered double hydroxides intercalated with the chelating agents diethylenetriaminepentaacetate and meso-2,3-dimercaptosuccinate. Appl. Clay Sci. 2009, 43, 125–129. [Google Scholar] [CrossRef]
- Kameda, T.; Hoshi, K.; Yoshioka, T. Uptake of Sc3+ and La3+ from aqueous solution using ethylenediaminetetraacetate-intercalated Cu–Al layered double hydroxide reconstructed from Cu–Al oxide. Solid State Sci. 2011, 13, 366–371. [Google Scholar] [CrossRef]
- Parello, M.L.; Rojas, R.; Giacomelli, C.E. Dissolution kinetics and mechanism of Mg-Al layered double hydroxides: a simple approach to describe drug release in acid media. J. Colloid Interf. Sci. 2010, 351, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of metal cations on layered double hydroxides. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, H.; Cao, H.; Li, H.; Li, Z. Removal of Cd2+ from water by Friedel’s salt (FS: 3CaO·A12O3·CaCl2·10H2O): Sorption characteristics and mechanisms. J. Environ. Sci. (China) 2013, 25, 1719–1725. [Google Scholar] [CrossRef]
- Milagres, J.L.; Bellato, C.R.; Vieira, R.S.; Ferreira, S.O.; Reis, C. Preparation and evaluation of the Ca-Al layered double hydroxide for removal of copper(II), nickel(II), zinc(II), chromium(VI) and phosphate from aqueous solutions. J. Environ. Chem. Eng. 2017, 5, 5469–5480. [Google Scholar] [CrossRef]
- Guo, Q.; Tian, J. Removal of fluoride and arsenate from aqueous solution by hydrocalumite via precipitation and anion exchange. Chem. Eng. J. 2013, 231, 121–131. [Google Scholar] [CrossRef]
- Li, D.; Guo, X.; Tian, Q.; Xu, R.; Xu, Z.; Zhang, J. Dearsenization of caustic solution by synthetic hydrocalumite. Hydrometallurgy 2016, 161, 1–6. [Google Scholar] [CrossRef]
- Rojas, R.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Giacomelli, C.E. Release kinetics from LDH-drug hybrids: Effect of layers stacking and drug solubility and polarity. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 463, 37–43. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic acid-based leaching system: A sustainable process for recovery of valuable metals from spent Li-ion batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Le Deit, H.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Lalhmunsiama; Pawar, R.R.; Hong, S.M.; Jin, K.J.; Lee, S.M. Iron-oxide modified sericite alginate beads: A sustainable adsorbent for the removal of As(V) and Pb(II) from aqueous solutions. J. Mol. Liq. 2017, 240, 497–503. [Google Scholar] [CrossRef]
- Etcheverry, M.; Cappa, V.; Trelles, J.; Zanini, G. Montmorillonite-alginate beads: Natural mineral and biopolymers based sorbent of paraquat herbicides. J. Environ. Chem. Eng. 2017, 5, 5868–5875. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Infrared and Raman Spectroscopic Studies of Layered Double Hydroxides (LDHs). In Layered Double Hydroxides: Present and Future; Rives, V., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2001; pp. 139–192. [Google Scholar]
- Jia, Y.; Wang, H.; Zhao, X.; Liu, X.; Wang, Y.; Fan, Q.; Zhou, J. Kinetics, isotherms and multiple mechanisms of the removal for phosphate by Cl-hydrocalumite. Appl. Clay Sci. 2016, 129, 116–121. [Google Scholar] [CrossRef]
- Zheng, L.; Xia, S.; Lu, X.; Hou, Z. Transesterification of glycerol with dimethyl carbonate over calcined Ca-Al hydrocalumite. Cuihua Xuebao/Chinese J. Catal. 2015, 36, 1759–1765. [Google Scholar] [CrossRef]
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb(II) removal using sodium alginate-carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr. Polym. 2016, 137, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Veglio, F.; Esposito, A.; Reverberi, A.P. Copper adsorption on calcium alginate beads: Equilibrium pH-related models. Hydrometallurgy 2002, 65, 43–57. [Google Scholar] [CrossRef]
- Felderhof, B.U.; Deutch, J.M. Concentration dependence of the rate of diffusion-controlled reactions. J. Chem. Phys. 1976, 64, 4551–4558. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency Lead and Copper Rule (LCR), 56 FR. 1991; pp. 26460–26564. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=60001N8P.txt (accessed on 27 February 2019).
| Beads | AlgCa | AlgCa/HC | ||||||
|---|---|---|---|---|---|---|---|---|
| [HCl] a | ΓH, 150 b | ΓH, 0 b | kD d | R2 | ΓH, 150 b | ΓH, 0b | kD d | R2 |
| 0.1 | 0.5 | −0.07 | 0.10 | 0.997 | 0.5 | −0.07 | 0.21 | 0.997 |
| 0.2 | 1.6 | −0.16 | 0.26 | 0.999 | 1.6 | −0.19 | 0.24 | 0.999 |
| 0.5 | 3.2 | −0.30 | 0.49 | 0.997 | 3.9 | −0.33 | 0.44 | 0.997 |
| 1.0 | 4.8 | −0.38 | 0.70 | 0.998 | 5.6 | −0.39 | 0.67 | 0.999 |
| Sample | ΓCu, max (mg/g) | KL (ppm−1) | R2 |
|---|---|---|---|
| CaAlg | 191 | 0.24 | 0.941 |
| HC | 382 | 16.48 | 0.998 |
| CaAlg/HC | 190 | 0.99 | 0.946 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgiallo, A.; Rojas, R. Reactivity and Heavy Metal Removal Capacity of Calcium Alginate Beads Loaded with Ca–Al Layered Double Hydroxides. ChemEngineering 2019, 3, 22. https://doi.org/10.3390/chemengineering3010022
Borgiallo A, Rojas R. Reactivity and Heavy Metal Removal Capacity of Calcium Alginate Beads Loaded with Ca–Al Layered Double Hydroxides. ChemEngineering. 2019; 3(1):22. https://doi.org/10.3390/chemengineering3010022
Chicago/Turabian StyleBorgiallo, Andres, and Ricardo Rojas. 2019. "Reactivity and Heavy Metal Removal Capacity of Calcium Alginate Beads Loaded with Ca–Al Layered Double Hydroxides" ChemEngineering 3, no. 1: 22. https://doi.org/10.3390/chemengineering3010022
APA StyleBorgiallo, A., & Rojas, R. (2019). Reactivity and Heavy Metal Removal Capacity of Calcium Alginate Beads Loaded with Ca–Al Layered Double Hydroxides. ChemEngineering, 3(1), 22. https://doi.org/10.3390/chemengineering3010022