3.1. Materials Characterization
As displayed in
Table 1, the LDH content in the different LDH/rGO nanocomposites determined by TG analysis is close to the nominal values demonstrating the complete coprecipitation of the magnesium and aluminium nitrate salts in presence of GO.
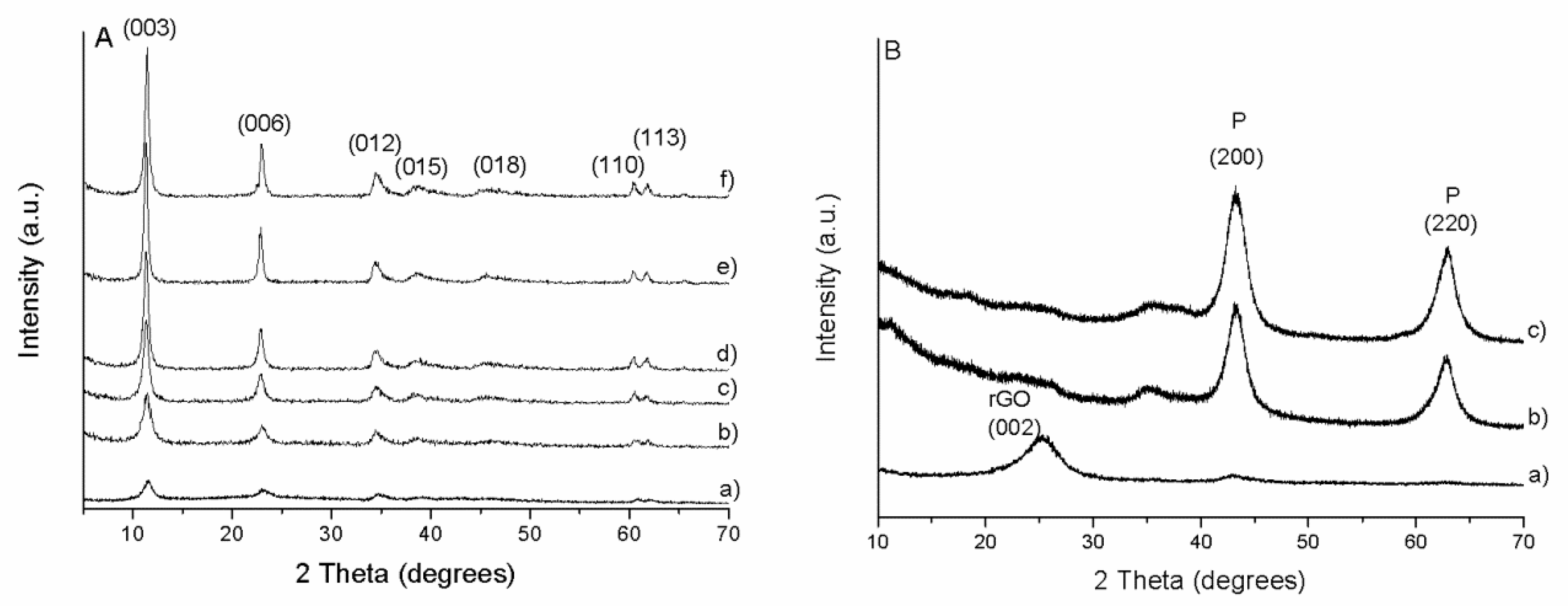
PXRD patterns of the prepared LDH/rGO hybrids are presented in
Figure 1A. They display the typical reflections corresponding to 2D LDH materials with particularly the most intense harmonic (003) and (006) reflections in the 2Ɵ range below 30°. The interlayer distance (d
003) of 0.772 nm is consistent with the presence of carbonate anions into the interlayer space provided by the alkaline synthesis solution (NaOH + Na
2CO
3) [
22].
As could be expected, the intensity and the shape of the LDH peaks depend on the LDH:rGO mass ratio. The intensity of the peaks decreases and the breadth increases with the decrease of the LDH content (
Table 1), indicating an apparent decrease of crystallinity. This is in accordance with the behaviour previously reported by Garcia-Gallastegui et al. [
23] and assigned not only to the increased GO contribution but mainly to the electrostatic interactions between GO and LDH during synthesis. This interaction influences the nucleation and then the crystallite size of the LDH particles. The absence of the basal reflection at 2θ = 11.3°, typical of GO, indicates its reduction into rGO upon hydrothermal treatment. This was further confirmed through analysis of bare GO submitted to the same treatment (figure not shown), where a shift is observed from the intense peak at 2θ ~ 12° for GO to a broad peak at 25–30° assigned to rGO. Moreover, the absence of a marked background in the 2θ range 25–35° in the nanocomposites indicates that the LDH nanoplatelets prevent the restacking of the rGO sheets. This suggests that the two components are intimately in contact and highly dispersed [
24]. The well-defined (110) and (113) peaks of the LDH structure observed in the high angle region reveal that the structure of the layers is preserved in the nanocomposites. A deeper analysis of these peaks also reveals an increase in the breath with the increase in the rGO content, as previously seen with (003) basal peak, indicating a decrease in crystallinity not only along the xz plane, but also in the xy plane. Crystallite size was calculated by applying the Scherrer equation with (003) basal plane or (110) plane, confirming this assumption (
Table 1). That is, the LDH crystallites in the nanocomposite are smaller and less stacked than in bare LDH. In all samples the lattice
a parameter of 0.306 nm calculated from the position of the (110) peak (a = 2 d
110) is in agreement with a Mg/Al molar ratio of 3 [
25].
After calcination at 450 °C the LDH/rGO composite materials lost the hydrotalcite-like layered structure.
Figure 1B shows the PXRD patterns of the calcined composites which exhibit different shapes in function of the LDH content as illustrated by the representative LDH/rGO-0.5, LDH/rGO-5 and LDH/rGO-10 materials. Calcined LDH/rGO-5 and LDH/rGO-10 samples show reflections at 2θ ~ 43° and 63°, corresponding to a periclase-like structure (JCPDS 87-0653) typical of a Mg(Al)O mixed oxide obtained by thermal decomposition of Mg/Al LDH. The calcined LDH/rGO-0.5 material presents a broad reflection at 2θ ~ 25° corresponding to rGO sheets. The absence of other peaks corresponding to periclase-like structure is due to both the low LDH content in the parent sample and to its high dispersion on the rGO surface.
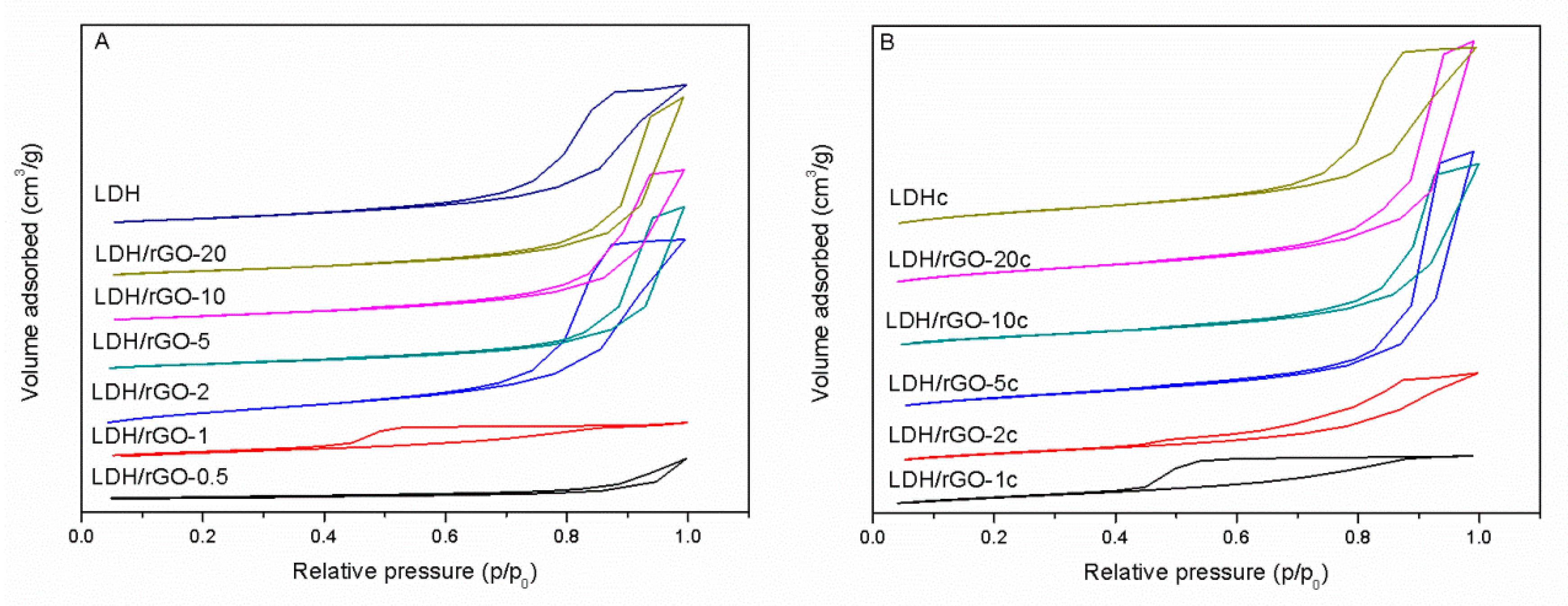
Textural and structural data of the nanocomposites are reported in
Table 1. All nanocomposite samples, as well as the bulk LDH show a type IV adsorption isotherm typical of mesoporous materials with a H4 hysteresis loop (except LDH/rGO-1) corresponding to slit-shaped mesopores which is normally observed in materials with aggregates of plate-like particles. Instead, sample LDH/rGO-1 displays a hysteresis loop of type H
2, which is typical of either disordered materials or bottleneck constrictions (
Figure 2). Remarkably, the specific surface areas varied slightly between 133 and 148 m
2·g
−1 for the nanocomposites with LDH:rGO ratios ranging from 20:1 to 1:1 and are rather similar to that of the bare LDH (140 m
2·g
−1). Due to the low surface area presented by rGO, as generally reported [
23], a decrease of specific surface area could be expected in the LDH/rGO composite materials with the decrease of LDH content. The rather similar specific surface areas observed suggest, on the contrary, the formation of homogeneous structure in materials with LDH:rGO ≥ 1. Therefore, as the LDH content increases, dispersion of the LDH platelets on the rGO surface may be improved and/or the GO restacking is reduced by the alternative LDH/GO stacking interactions as previously suggested [
10,
23]. The specific surface area decreases to 25 m
2·g
−1 at low LDH content for the LDH/rGO-0.5 composite, well below the specific surface area of the bare LDH, accounting for high aggregation of the LDH particles which, in addition, do not hinder the restacking of the GO layers.
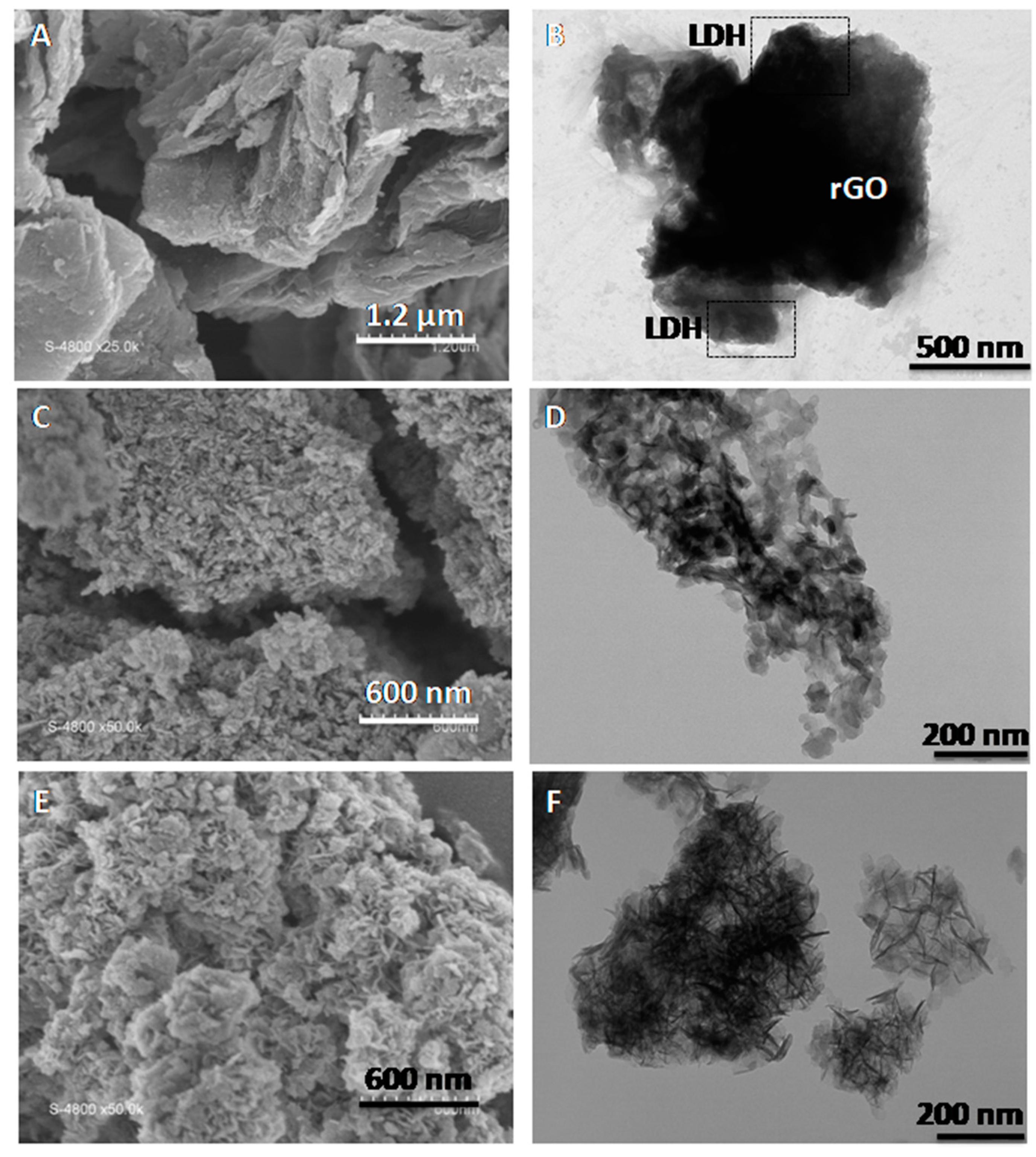
The behaviour observed for the nanocomposites with LDH:rGO ≥ 1 has been also confirmed by SEM and TEM analysis (
Figure 3). The samples are indeed constituted by GO or rGO sheets and relatively well dispersed LDH aggregates in contact displaying a house-of-cards-type structure which suggests the formation of mesopores. This is observed especially in those samples with higher LDH content, as previously reported in recent studies [
10], while at low LDH content, sample LDH/rGO-1 yet presented a more stacked structure. At very low LDH content, LDH/rGO-0.5 has the lowest specific surface area in the series (25 m
2·g
−1) suggesting the formation of a poorly porous material with highly packed LDH and rGO sheets (
Figure 4). SEM and TEM analyses indeed confirms such highly packed structure where rGO and LDH phases are in intimate contact, although the existence of completely separated LDH and rGO domains is also clearly seen. The high homogeneity and dispersion of the LDH platelets in contact with rGO in samples with higher LDH content merits to be highlighted.
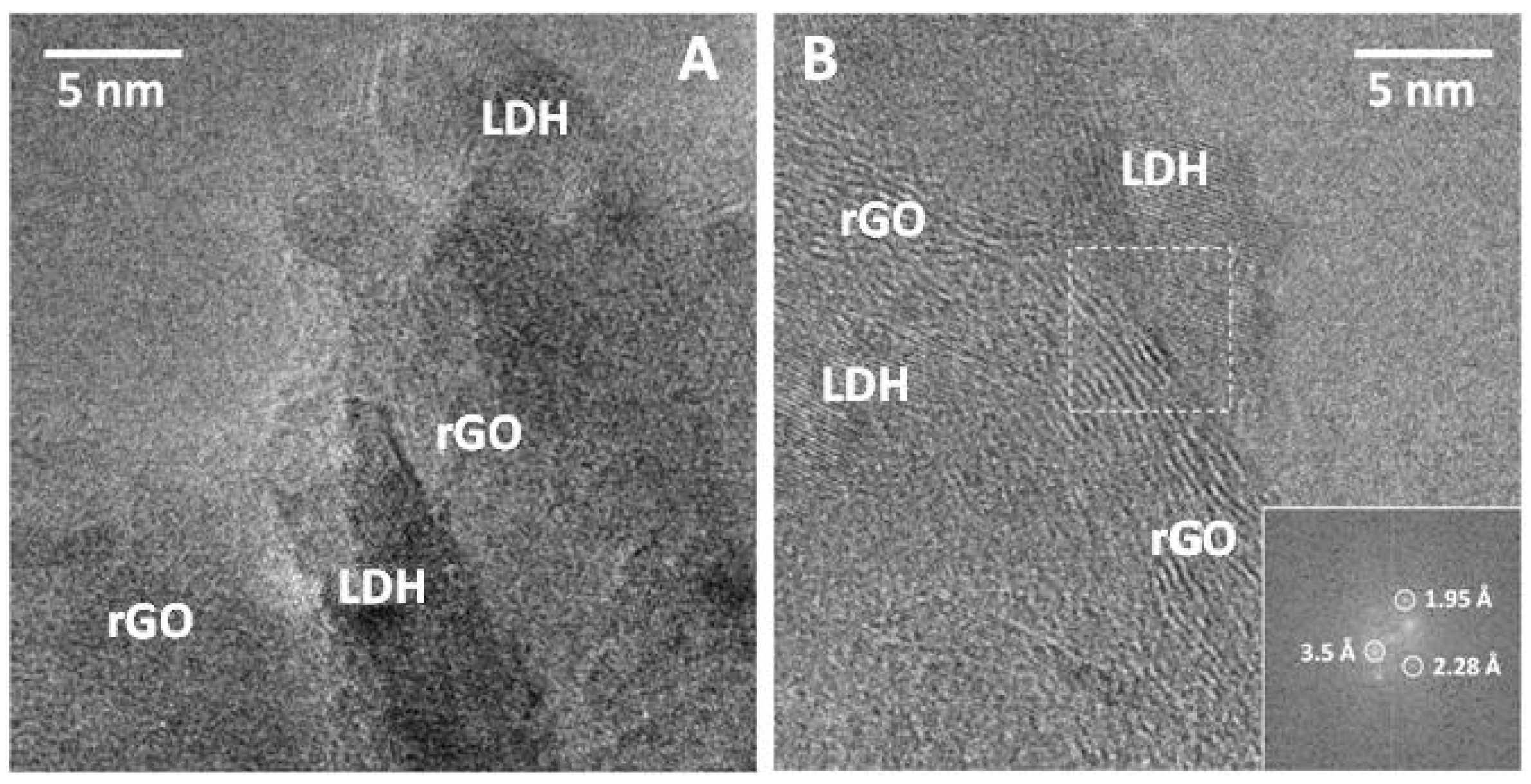
Figure 5 shows HRTEM analysis of sample LDH/rGO-10 where LHD crystals appeared intimately assembled into the rGO. Besides, the lattice-fringe image shows the different phases occurrence. Lattice fringes at 3.5 Å correspond to the (002) crystallographic planes of graphene, whereas straight planes at 2.28 and 1.95 Å corresponds to LDH.
After calcination at 450 °C, the values of the specific surface areas increase up to 1.4 and 2.2 times at low (LDH/rGO-1) and high (LDH/rGO-20) LDH content, respectively, in comparison to the non-calcined nanocomposites. This is in agreement with the structural transformation of the Mg-Al LDH component into Mg(Al)O mixed oxide of higher surface area as confirmed with the bare LDH whose specific surface area increases from 140 to 280 m2·g−1.
This suggests that the steadiness of the surface area in non-calcined hybrids may be related (in different extent) to both intrinsic porosity generated by incorporation of LDH in the hybrids as well as interporosity due to the lower stacking degree of both components (i.e., rGO and LDH) forming the nanocomposite. Whereas, the increase in surface area in calcined materials is due to the pore generation produced by gas release during the LDH decomposition upon thermal treatment confirmed by XRD (
Figure 1).
Volumetric adsorption of CO
2 at low pressure was applied to determine the number of basic sites on the LDH/rGO nanocomposites activated by in situ calcination at 450 °C. The amount of CO
2 adsorbed and then the number of basic sites significantly increases from 52 to 265 μmol·g
−1 with the LDH content in the nanocomposites (
Table 2). This is in accordance with earlier studies on basicity characterization of LDH/rGO nanocomposites [
10,
23,
26].
In the analysis conditions of our experiments, the CO
2 adsorption capacity of the bare rGO was found negligible. It is notable that CO
2 adsorption capacity of sample LDH/rGO-0.5 was around two times lower than that reached by the activated bare LDH and LDH/rGO-1 samples. This is consistent with the morphology and texture of LDH/rGO-0.5 giving rise to low accessibility of the probe to the basic sites. On the other hand, the CO
2 adsorption capacity of the samples having the highest LDH content, i.e., LDH/rGO-10 and LDH/rGO-20 is two-fold higher than that reached by the calcined LDH of similar specific surface area. One can suggest that, due to the high dispersion of the LDH layers in the parent nanocomposites, both the number of coordinatively unsaturated O
2− sites at the edge of the mixed oxide platelets and their accessibility are enhanced after activation. When the CO
2 adsorption of nanocomposite materials is normalized per mass of LDH, it results in values significantly higher for all of them than for the bulk LDH, similarly to what has been reported for LDHs supported on CF [
3], CNF [
5,
6] or MWCNT [
27].
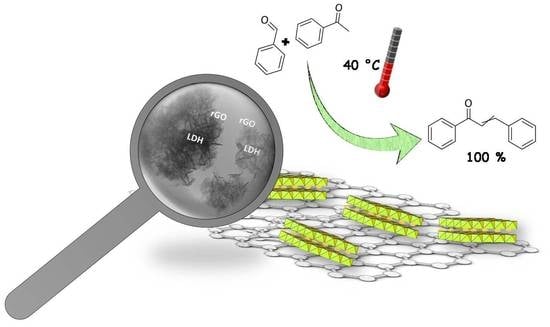
3.2. Claisen–Schmidt Reaction
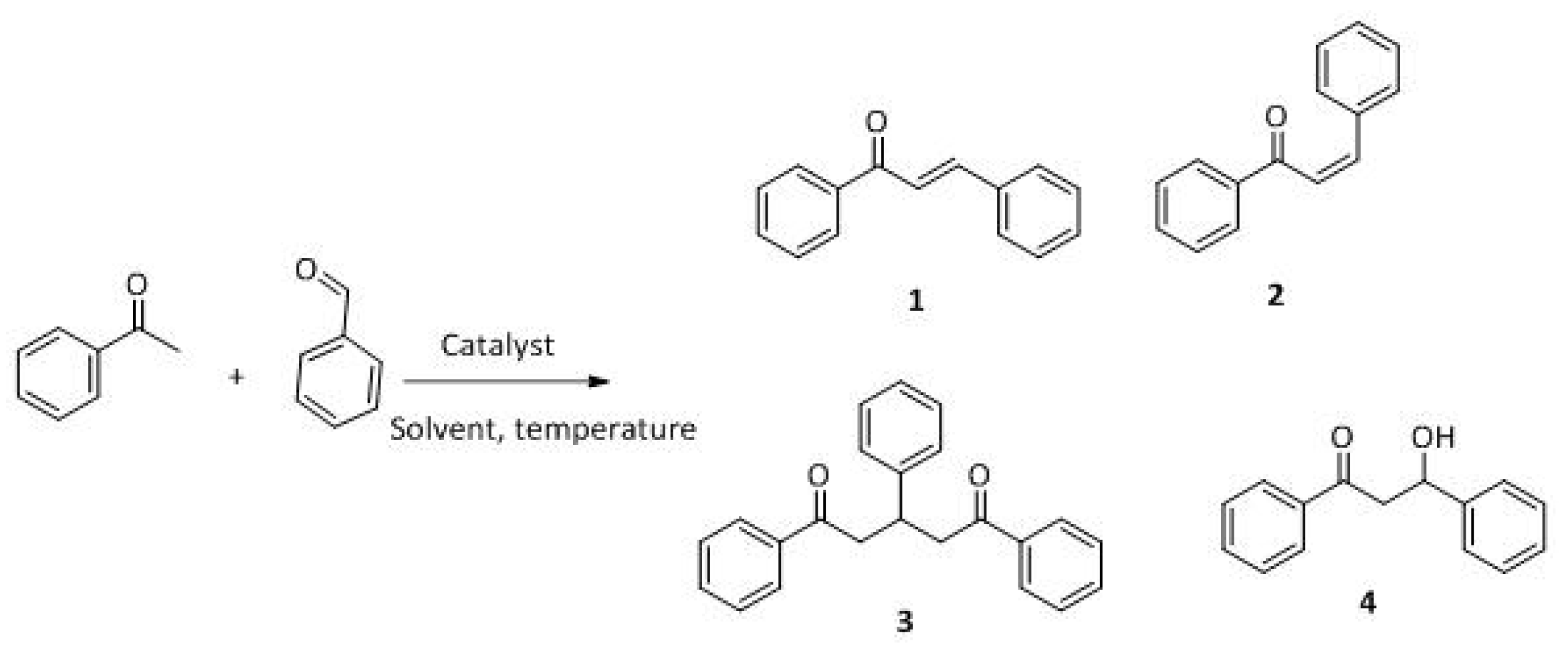
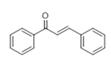
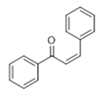
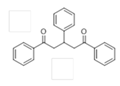
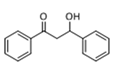
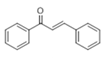
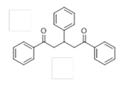
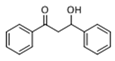
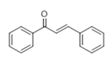
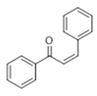
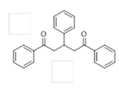
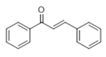
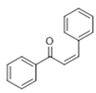
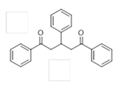
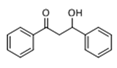
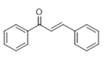
The Claisen–Schmidt condensation reaction between acetophenone and benzaldehyde in basic conditions can lead to different products: the chalcone isomers, i.e., trans-chalcone (1) and cis-chalcone (2), the Michael addition product (1,3,5-triphenylpentan-1-5-dione) (3) and the aldol addition product (4), as can be seen from
Scheme 1.
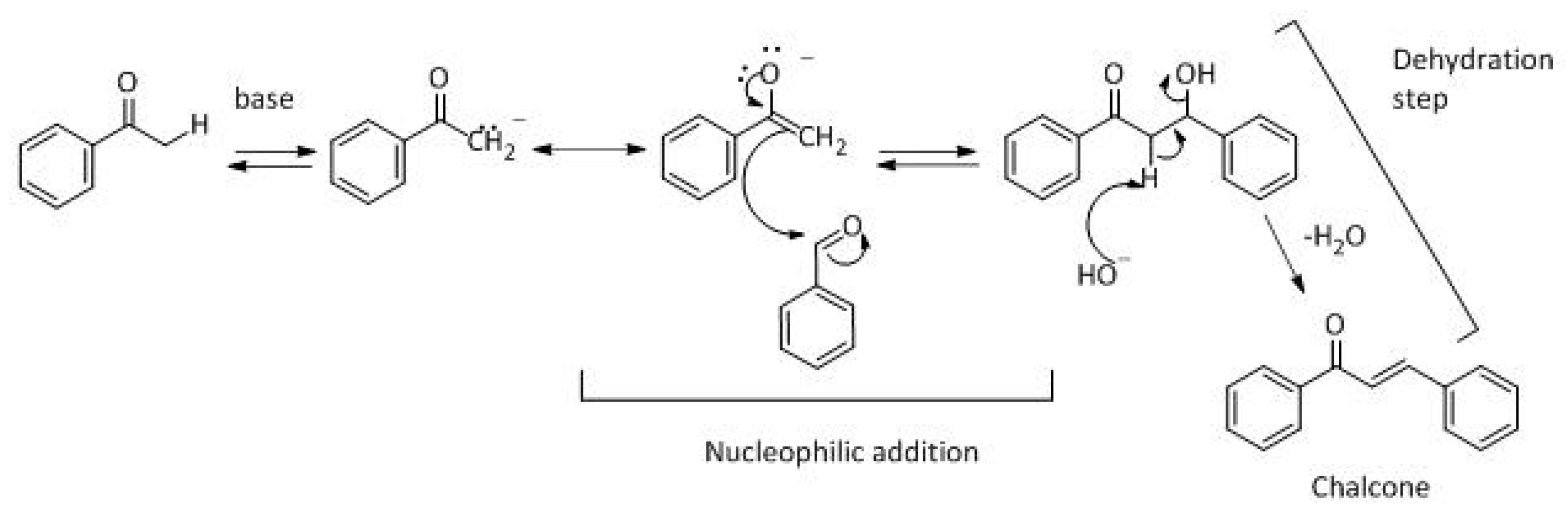
The generally accepted base-catalysed mechanism of Claisen–Schmidt condensation reaction starts with the abstraction of the acidic α-hydrogen of the ketone molecule by a base; the formed species undergoes a keto-enol tautomerism. The rate determining step of the entire reaction is the nucleophilic addition of the enolate, the anion of acetophenone, to the carbonyl carbon of the benzaldehyde, yielding the intermediate β-hydroxyl ketone, followed by dehydration to give chalcone (
Scheme 2).
The solvent where a chemical reaction takes place is rarely a non-inert medium. Its complex interactions with solute molecules and possible intermediates can result in a modification of the reaction pathway. In our study we have chosen three different solvents, i.e. polar methanol (MeOH), polar aprotic acetonitrile (ACN) and non-polar toluene whose physical constants are depicted in
Table 3.
The different products yields can allow more finely defining the influence of the textural properties and the basicity of the catalysts, particularly when one considers that formation of Michael addition product accounts for the presence of strong basic sites and/or for diffusional limitations favouring consecutive reactions. The production of Michael addition product also enhances with the temperature [
15,
28].
The reaction carried out at 40 °C in the different solvents and in absence of solvent with the activated LDH/rGO nanocatalysts allows reaching 100% conversion after 4 h in all cases while the conversion with the calcined LDH reaches values rather close to 100% after 8 h. Such differences of activity between the LDH/rGO nanocatalysts and the calcined LDH are in agreement with those previously reported for the acetone self-condensation [
10]. For the chalcone synthesis Climent et al. reported conversions of 4% and 82% after 1 h at 50 °C without solvent with the calcined and the calcined-rehydrated LDH sample, respectively, while Lopez et al. reported conversions of 4.6% and 70%, respectively, with the same type of catalysts after 3 h at 150 °C in DMF [
15,
18]. Although the reaction was performed in different conditions (temperature, catalyst/reactants mass ratio, reaction time), it is noteworthy that the LDH/rGO nanocomposites and the calcined-rehydrated LDHs are both more active than the calcined LDH, i.e. the Mg(Al)O mixed oxide. In the case of rehydrated LDHs, this enhancement in activity has been attributed to the presence of intercalated OH
− anions acting as Brönsted basic sites known to be the most active in aldol-type reactions. These reactions are indeed usually performed with NaOH or KOH in homogeneous catalysis. In our case, the increase in activity with the LDH/rGO nanocomposites respect to the calcined LDH likely accounts for an increase of the number and availability of the Lewis-type basic sites. rGO and LDH/rGO-0.5, the nanocomposite containing the lower amount of LDH, were not active in the condensation reaction of acetophenone and benzaldehyde, in accordance with the behaviour previously reported for acetone self-condensation reaction [
10]. Thus, in the following we will only consider the results from the nanocomposites with LDH:rGO ≥ 1.
The selectivities obtained after 8 h with the bare LDH calcined at 450 °C reveal the influence of the presence of solvent and of its nature (
Table 4). Without solvent
trans-chalcone (
1) and Michael addition product (
3) were obtained accounting for the presence in the catalyst of strong basic sites able to achieve the consecutive reaction between acetophenone and chalcone. The differences of selectivities observed with ACN and methanol cannot be related to their dielectric constant and are more likely related to their respective acidities. ACN of weak acidity poisons the stronger basic sites thus slightly decreasing the formation of the Michael addition product in comparison to the reaction without solvent. Moreover, as ACN is a polar aprotic solvent, hydrogen bonds with the anionic adducts, i.e. the enolate anion, are hindered during the reaction. The interaction between the non-solvated anion and the surface of the catalysts is thus favoured and consequently improves the formation of chalcone.
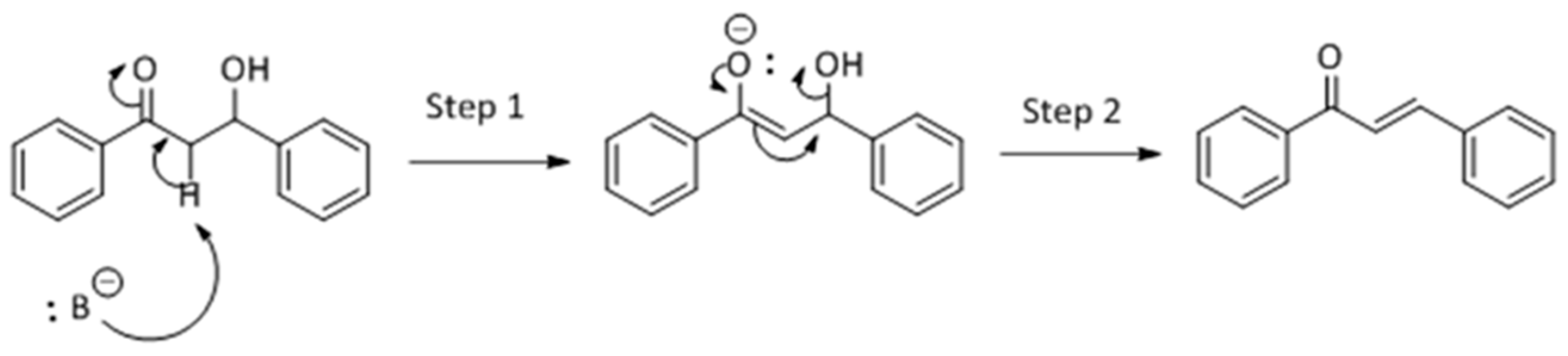
Methanol, a polar solvent of higher acidity than ACN, also poisons the basic sites of medium strength. The formation of aldol (
4) as only side-product suggests that the dehydration step of the Claisen–Schmidt condensation is inhibited. This reaction proceeds by an E1cB mechanism (
Scheme 3), in two steps. In the first step the basic site abstracts a proton from the α-C resulting in a carbanion which, in the second step, forms a π-bond and push the leaving group producing chalcone.
This E1cB mechanism is strongly favored in presence of a strong base, but the use of a slightly acidic solvent might poison the basic centers. Likewise, the anion formed in the first step of reaction (see
Scheme 3) may abstract a proton from MeOH thus inhibiting the reactivity towards the elimination step. Therefore, the selectivity towards chalcone decreases and that towards aldol product increases as the poisoning of the basic sites by methanol increases. Consequently, chalcone is produced in lower yield than with ACN and at the expense of the aldol product (
4), while formation of the Michael addition product (
3) is totally inhibited.
Toluene behaves similarly to ACN leading to chalcone (1 and 2) and Michael addition product (3). This accounts also for its very weak acidity and, probably, for differences of adsorption of the reactants with this non polar solvent.
A similar general behaviour is observed with the LDH/rGO catalysts than with the calcined LDH with differences depending on the composition of the nanocomposites (
Table 5,
Table 6 and
Table 7). Chalcone is the main product observed without solvent as well as with ACN and toluene as solvents.
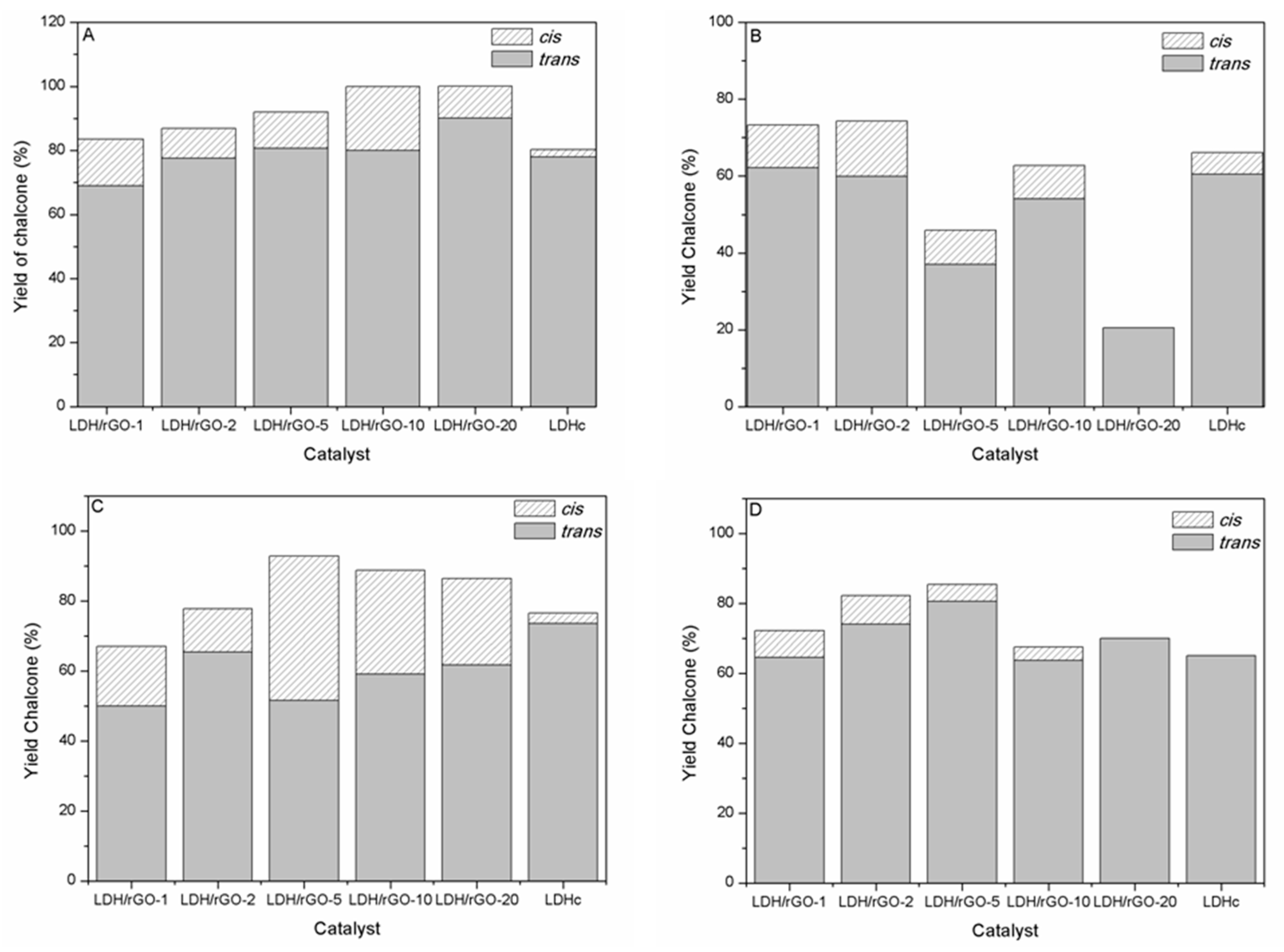
The higher selectivity towards chalcone and particularly
trans-chalcone (
1) is obtained with ACN (
Figure 6A and
Table 5). It is noticeable that the selectivity towards
cis- and
trans-chalcone ((
1) + (
2)) increases from 83 to 100% at the expense to that of the Michael addition product (
3) which decreases from 17 to 8% when going from LDH/rGO-1 to LDH/rGO-5. This by-product is not formed with LDH/rGO-10 and LDH/rGO-20 as catalysts. This suggests the presence of a larger amount of stronger basic sites poisoned by ACN in these latter samples thus inhibiting the consecutive condensation of acetophenone and chalcone. LDH-rGO-20 and LDH-rGO-10 lead to the higher yields of
trans-chalcone (
1) and
cis-chalcone (
2), respectively and, most importantly, are totally selective for the formation of chalcone isomers. However, LDH-rGO-10 with a
cis-chalcone (
2) yield of 20% exhibits a peculiar behaviour among the series of catalysts. No aldol product (
4) was obtained with ACN as solvent, no matter the composition of the nanocatalysts. It is also remarkable that the LDH/rGO nanocatalysts exhibit higher activity and selectivity towards
trans- and
cis-chalcone (
1,
2) than the calcined LDH (
Table 5). Since this latter is the catalytically active phase in the nanocomposites, this proves the synergistic effect produced by the hybridization of LDH and rGO. This synergistic effect is assigned to the smaller size and the higher dispersion of the LDH crystallites on the rGO surface as showed by TEM.
When methanol is used as solvent
cis-chalcone (
2),
trans-chalcone (
1) and aldol (
4) are only obtained while the Michael addition does not occur (
Figure 6B and
Table 6). There is a general trend towards a decrease of the chalcone yield ((
1) + (
2)) at the expense of the aldol yield (
4) as the LDH content in the LDH/rGO nanocatalysts increases, except for LDH/rGO-10 again exhibiting a peculiar behaviour as already noted with ACN as solvent (
Table 6 and
Figure 6B). Moreover, the 1,4 addition of acetophenone on chalcone leading to the Michael addition product (
3) is inhibited. LDH/rGO-1 and LDH/rGO-2 lead to the higher chalcone yields and exhibit similar behaviour than the calcined LDH in agreement with their closer basic properties.
When toluene is used as solvent,
trans-chalcone (
1),
cis-chalcone (
2) and Michael addition product (
3) were formed (
Figure 6C and
Table 7). A remarkable feature is that toluene gives rise to the higher
cis-chalcone and Michael addition product yields among all the solvents studied, despite
cis-chalcone being less thermodynamically stable than
trans-chalcone due to the strong steric effects between the carbonyl group and the aryl ring.
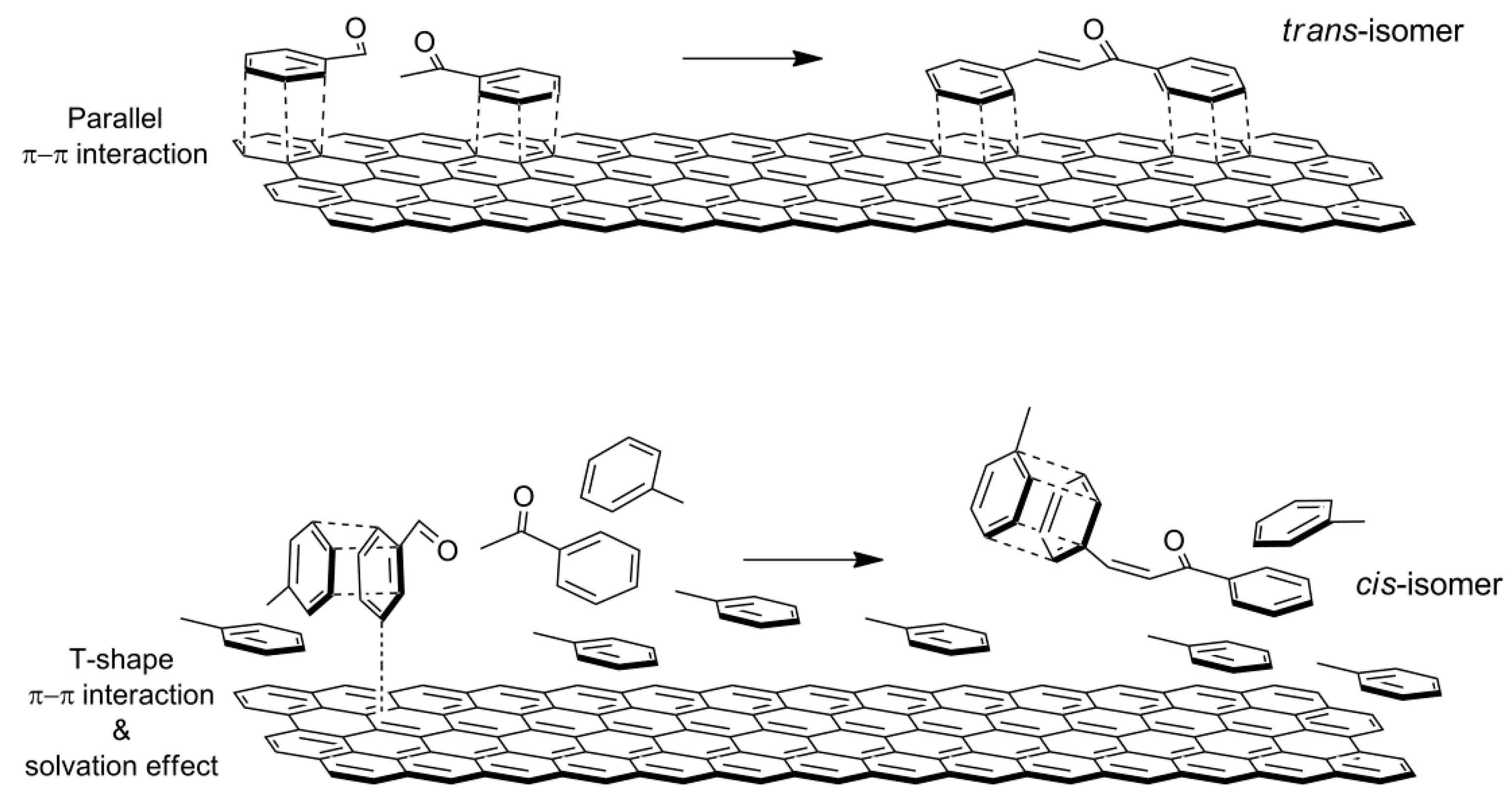
Our catalytic results show that different chalcone isomer ratios are observed accounting for modifications of the interaction among reactants and the solvent, and depending on the composition of the catalysts whose polarity is related to the LDH:rGO weight ratio. Aromatic rings interact with the surface of rGO by competitive π-π interactions. In the cases of ACN and methanol as solvents, the π-π interaction between the reactants and the surface of the catalyst more likely occurs via a parallel orientation of the aromatic rings to the surface, thus favouring the formation of trans-chalcone. In the case of toluene as solvent, the interaction with both the surface of catalyst and the reactants is favoured due to their similar geometry and polarity. Moreover, reactants solvated by toluene are in less close proximity to the surface. This allows a T-shape π-π interaction between aromatic rings of reactants and the surface of the catalyst increasing the formation of
cis-chalcone (
Scheme 4).
Cleaner synthetic routes, easier to handle, involving few or absence of solvent are currently desired in order to develop “green” processes. For this purpose and also to more finely define the LDH/rGO nanocatalysts interaction with the reagents, a series of experiments were conducted without solvent (
Figure 6D and
Table 8). Chalcone, with a larger selectivity towards
trans-chalcone (
1) is the main product of reaction. Michael addition product (
3) is the only by-product formed according to the high basicity of the samples. Aldol (
4) is formed in the case of LDH/rGO-1 due the lower basicity of this sample in the series of nanocomposites.
It must be noted that higher Michael addition product yield is obtained without solvent than with ACN and toluene confirming the poisoning effect of these weakly acidic solvents on the strong basic sites. As expected, the Michael addition product yield also increases with the LDH content in the nanocomposites according to their increasing basicity. It reaches a maximum for the LDH/rGO-10 catalyst, which confirms its different behaviour compared to the other samples in the series. The increase of LDH content also decreased the selectivity towards the cis-chalcone, demonstrating that the presence of this product (thermodynamically unfavoured compared to trans-chalcone) in all the other experiments might be influenced by the presence of the solvent, which can act as stabilizer, especially in the case of toluene.
The results above reported show that the LDH/rGO nanocomposites are efficient base catalysts for the Claisen–Schmidt condensation reaction between acetophenone and benzaldehyde. Due to the strong basicity of the catalysts, consecutive reaction leading to the Michael addition product occurs when the reaction is performed without solvent. However, performing the reaction in a slightly acidic solvent such as ACN allows obtaining total selectivity towards chalcone with the most basic LDH/rGO-10 and LDH/rGO-20 catalysts. The LDH/rGO nanocatalysts are by far more active than the calcined LDH, and total conversion is obtained after 4 h at 40 °C (against 8 h for bare LDH). This shows that the dispersion of the active Mg(Al)O mixed oxide phase in the activated nanocomposites creates strong and accessible O
2− basic sites which results in improved Lewis basicity compared to bare calcined LDH. The Brønsted-type basic sites in the calcined-rehydrated LDHs yet exhibit better efficiency than the Lewis-type basic sites in the LDH/rGO nanocomposites as reported in previous studies [
15,
18]. However, since the superior activity of the LDH/rGO catalysts with respect to bare calcined LDH has been proved, LDH/rGO materials are expected to boost their activity after proper rehydration/reconstruction. It must be point out that the objective of this work was to demonstrate that calcined LDH/rGO hybrids have fine properties which make them active heterogeneous base catalysts in different reactions of interest. The nature of sites was not investigated. So, catalysis with rehydrated hybrids was not included in this manuscript. The rehydration procedure (i.e. gas or liquid phase), time, temperature and even drying mode are parameters known to strongly affect the final catalyst properties. Furthermore, rehydration depends on the characteristics of the hybrid itself (i.e. loading of LDH, type of preparation and drying...) and could be kinetically limited. So that efforts are still needed to make a protocol that allows obtaining rehydrated samples accurately and in a reproducible way.