Abstract
Regenerative medicine uses the biological and medical knowledge on how the cells and tissue regenerate and evolve in order to develop novel therapies. Health conditions such as ageing, obesity and cancer lead to an impaired regeneration ability. Exercise, diet choices and sleeping pattern have significant impacts on regeneration biology via diverse pathways including reducing the inflammatory and oxidative components. Thus, exercise, diet and sleeping management can be optimized towards therapeutic applications in regenerative medicine. It could allow to prevent degeneration, optimize the biological regeneration and also provide adjuvants for regenerative medicine.
1. Regeneration and Medicine
Regeneration can be defined as the biological processes allowing the cells, organs and tissues to renew and proliferate. These processes also allow normal growth and development, maintenance of a healthy body [1] as well as the recovery from injuries [2] or from other external perturbations [3]. It involves various underlying pathways such as cytoprotective mechanisms induction [2], cellular plasticity [4], tissue remodeling [5] and biophysical aspects [6]. Impaired regeneration can have pathological impacts such as degenerative diseases in different tissues [7,8,9,10]. These diseases result from the loss of the regenerative ability leading to a status where cellular loss is superior to cellular regeneration. Regeneration processes might also be impaired or disturbed in various status including degenerative diseases [11,12,13], cancer [14], obesity [15], ageing [16,17], diabetes mellitus [18] and cholestatic liver [19]. Regenerative medicine comes as a branch that aims to understand the regenerative pathways and degenerative processes, both in biology and physiology, to develop methodologies and approaches aiming to correct regeneration-related health challenges and the impaired functions [20] or at least limit the impacts of the variables that can impair regeneration. The regenerative medicine is a medical field based on regeneration, used biomaterials [21], biochemistry [22], stem cells [23,24] and tissue engineering [25,26] and applies them in surgery [27], transplantation [28], ophthalmology [29] and cancer [23] among diverse applications [30,31,32,33,34,35].
Regenerative medicine, based on regenerative biology [36], aims to elucidate the mechanistic pathways underlying cellular and tissular regeneration along with the endogenous and exogenous factors that influence the regenerative processes and use that knowledge to develop novel therapeutic options. Such therapies target the correction or the optimization of an impaired regeneration resulting from a disease, a physiological adaptation or even therapeutic side effects. Regenerative medicine research involve multiple areas from stem cells [31], gene editing [37], nuclear transfer [38], proteomics, pharmacology, nanotechnology [39], tissue, engineering and three-dimensional printing [40]. Beside the various adjuvant used in regenerative medicine, mainly pharmacological (regenerative pharmacology) [41,42,43] or bioengineering [28,44], we aim to highlight the importance of lifestyle and how it impacts regeneration. In this piece of writing, we would like to provide illustrative examples on how lifestyle patterns—specifically exercise, diet and sleeping—influence regeneration and the related biological processes. We also present potential clinical and biomedical applications.
2. Exercise, Diet, Sleeping and Regeneration
The three main lifestyle pillars (exercise, diet and sleeping) represent key factors in regeneration and, thus, in regenerative medicine as we illustrate below. The facts that they impact regeneration and also influence the statuses (obesity, ageing, etc.) in which regeneration patterns change, support that exercise, diet and sleeping as key factors worth exploring to optimize regeneration medicine applications. In addition, exercise, diet and sleeping are also involved in different physiological changes and pathological prognoses.
Exercise, is known for numerous health benefits including metabolic enhancement [45,46,47] and regenerative induction. Indeed, exercise represents a cardiomyocyte regeneration inducer [48,49], a therapeutic cartilage regeneration adjuvant [50], a skeletal muscle regeneration enhancer [51], and a cardiac remodeling inducer [52]. Exercise might/can also slow down [53] or reverse muscle atrophy [54], improve the post-injury skeletal muscle regeneration [55], prevent stem cells senescence [56], promote peripheral nerve regeneration [57], and rejuvenate muscle stem cells [58,59]. Within the context of the mechanisms underlying the exercise-induced regenerative benefits, secreted protein acidic and rich in cysteine (SPARC) is at the center of a key theory. SPARC is both induced by exercise and has been hypothesized as a regeneration factor [60,61,62]. Such implication in regeneration enhancement would be explained by the various properties and roles it has [63] including anti-inflammatory [64], antitumor [61,65,66], extracellular matrix structure [67] and metabolism [68,69] in addition to studies linking SPARC to regeneration [70,71] as well as potential applications in personalized medicine [72]. This suggested that at least a part of the benefits induced by exercise are mediated by SPARC. Indeed, we have already presented data suggesting that that exercise-induced muscle phenotype changes are SPARC-dependent [73] which is in accordance with the theory linking myokines to the physical activity effects [66,74]. The positive impacts of exercise on regeneration could be explained by the properties that have been associated to exercise, such as anti-inflammatory [75], antioxidant [76], anticancer [77] and anti-ageing [78] properties, that lead to suitable outcomes for regeneration and bio-homeostasis in general.
Diet, an important determinant of health, has been studied in a variety of contexts including obesity, metabolism and cancer [79]. However, and although diet and nutrition have been exploited for tissue regeneration [80], many details of the molecular mechanistic pathways seem still emerging to light. The choice of diet quality as well as fasting (calorie restriction) [81] do impact regeneration. Diverse examples illustrate how dietary choices could impact regeneration. Supplemented nutrition diet affects regeneration in liver [82], high-fat and high-glucose microenvironment inhibits bone regeneration [83], proliferation and migration of human gingival fibroblasts is impaired by high glucose-induced oxidative stress [84] but following lidocaine-induced injury normal glucose enhances neuronal regeneration [85]. Such links between glucose and regeneration could be behind parts of the regeneration patterns seen during diabetes [86].
Another illustration in the same context is that fasting promotes stem cell-based regeneration [87], promotes intestinal regeneration [88,89], promote hematopoietic-stem-cell-based regeneration [90] and β-cell regeneration [91,92]. Such fasting benefits on regeneration would involve metabolic and body composition changes [87,93], anti-inflammatory effects [89], stem cell number increase [88], oxidative stress decrease and ageing delaying [94,95]. The dietary choices represent an important player as well. The rationale behind the dietary choice is to generate a biological microenvironment that can promote regeneration. This could be achieved, for instance, by reducing the intake of the high-fat diet since high-fat diet leads to inflammation [96,97] and cancer progress [96,98]. The other way to improve regeneration environment via diet is to create biological conditions that would optimize the regenerative abilities. This can be achieved with diets that provide properties such as antioxidant [99,100], anti-inflammatory [101,102], omega-3 fatty acids [103], protein intake [104] and microbiota composition change [105,106]. For instance, fasting-mimicking diet promotes intestinal regeneration [88], reduces intestinal inflammation [89] and reduce inflammatory bowel disease pathology [88].
The other pillar of lifestyle is represented by the sleeping wish is neuroprotective [107]. Impact of sleep on stem cell regenerative capacity is shown by the correlation between circadian rhythm and an improved stem cells proliferation microenvironment [108] leading to stem cell maintenance and division control [109,110]. This fits with the melatonin anti-inflammatory, antioxidant and neuroprotective properties [111,112] along with its free radical scavenger function [113], among others, that would be behind its role in regeneration of tissues [111,113]. In addition, protein and pre-sleep are also contributors to regeneration [114,115]. Following the same line of thoughts, sleep deprivation impairs muscle regeneration [116] and delays healing process [117] which supports the importance of sleeping for the regenerative processes.
These examples of the implications of exercise, diet and sleeping at various levels in regeneration and its variables clearly show their importance within any intervention aiming to stimulate or modulate regeneration.
3. Obesity and Ageing as Selected Illustrations
Beside the known diseases related to regeneration changes, obesity [118] and ageing [119] represent topics of concern and are health conditions worth exploring in the context of regeneration. They both share common biological and pathological features [120,121] including regeneration-related [122]. The focus on obesity and ageing, that have common patterns [120,123], derives from their globally increasing epidemiological profile along with the diseases and health problems related to them, including those impacting regeneration homeostasis.
Obesity, as a neuroendocrine reprogramming [124], represents a status of a broken energy balance [125] that has been classified as a disease [126,127,128]. It has been associated with various health problems and diseases [129]. In the ongoing COVID-19 pandemic, it is worth pointing that obesity both increases vulnerability to COVID-19 (vicious cycle [130]) and reduces the immunity [131,132]. Ageing, on the other hand, can be defined as the time-dependent biological and functional declines of living entities. It represents a risk factor for various diseases too [133,134]. Ageing has a genetic component [135] and is due—at least in part—to hormonal and metabolic changes [136]. At the molecular levels, epigenetic changes such as DNA methylation [137,138] are involved in age-related changes. Whereas obesity is a status in which regeneration is impaired [15,139], ageing is also accompanied by a decline in regeneration [59,140,141,142]. Studies and hypotheses have pointed various age-related underlying mechanism such as the loss of biological plasticity [143] and the changes in the regenerative environment [141].
The prescription of exercise for both obese [144,145] and elderly population [146,147,148] is well documented. Such prescription is based on the numerous benefits exercise has; among which we cite glycemic control [149,150], weight management [145], antioxidant [76], anti-inflammatory [151,152], cardiovascular risks improvement [153], immune system regulation [154] and anti-inflammatory milieu promotion [49]. All these benefits improve the negative consequences induced by ageing and obesity and, importantly, improve regeneration bioenvironment.
Exercise is prescribed in obesity and ageing for reasons initially independent from regeneration (weight and adiposity loss, muscle function improvement, etc.). However, the above examples clearly reflect how exercise would be important for regeneration, including in the contexts of obesity and ageing. Exercise would both improve regeneration and reduce the negative impacts that obesity and ageing have on regeneration. The dietary choices and sleeping patterns described above would also contribute to reduce the impacts of obesity and ageing as well. Therefore, indirectly improve the biological regeneration ability. Importantly, targeting regeneration-related pathways in both obesity and ageing represents an additional shared pattern between obesity and ageing.
4. Perspectives
The above illustrative examples point to the importance of exercise, diet and sleeping within the regenerative context and points the important of combining all these factors to reach the optimum regenerative outcome. This would have two key implications (Figure 1). First, developing an unhealthy lifestyle could lead to both regenerative problems and a possible therapeutic failure of the regenerative medical therapies. The second implication is the importance of introducing medically supervised choices for exercise, diet and sleeping patterns as a regenerative adjuvants either during regenerative therapies or for individuals suffering from conditions impacting the regenerative abilities such as obese and elderly patients, knowing the shared features between ageing and obesity [123].
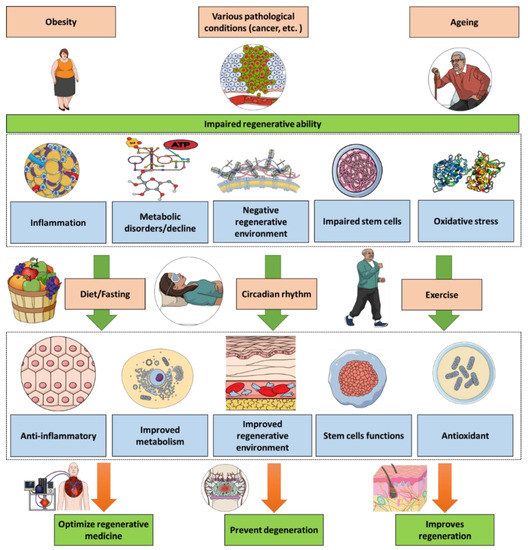
Figure 1.
Health conditions such as ageing, obesity and cancer lead to an impaired regenerative ability. Exercise, diet and sleeping have significant impacts on regeneration biology via diverse pathways including reducing the inflammatory and oxidative components. Thus, exercise, diet and sleeping management can be optimized towards therapeutic applications in regenerative medicine.
However, further studies are required in order to identify the quality and the quantity of each of these three elements and their combination for each specific case. Indeed, the choice of exercise patterns (types of exercise, duration, timing [150], etc.), diet (quantity, composition and timing) as well as sleeping (duration, timing and tissue-specific impacts [155]) are parameters for which additional optimization could improve the use and application of exercise, diet and sleeping as therapeutic adjuvants or even as stimulators for regeneration. This is encouraging considering the recent advances in biology, such as the possible regenerative ability of the adult heart [156]. Molecular tools such as functional genomics [157,158,159,160,161,162,163] and metabolics would allow the characterization of diverse genes, proteins and other molecular and biochemical changes related to exercise, diet and sleeping patterns, along with their implications in regeneration as well understanding regeneration via proteomics [164]. This would elucidate the molecular links and, thus, identify potential novel pharmacological targets based on advances in signaling in regeneration [165]. These targets are of a specific importance since they would allow, for instance, to mimic exercise effects via pharmacological agents without the need of performing exercise. Such an approach is important for individuals who have a limited ability to complete the prescribed physical activities, such as elderly patients and those with physical disabilities.
Overall, the effects of a healthy lifestyle (exercise, diet and sleeping) all contribute towards an improved regeneration ability, which is required to improve healthy ageing, especially with regard to the increased human lifespan [166], in addition to all the known benefits of a healthy lifestyle for a limitless number of health problems including diabetes [167], cancer [168], mental health [169], pediatric asthma [170] and reproductive health [171]. Although we have focused on the impacts exercise, diet and sleeping would have on the biology of regeneration in vivo, we can also extrapolate the concept towards possible in vitro applications. Within this context, the cytokines and factors induced by exercise (ex. SPARC or use the in vitro model of exercise [172]) and sleeping (ex. melatonin) as well as the diet chemical components (ex. antioxidant) could be supplemented during the bioengineering of cellular and tissular cultures (adding to the cells and tissues medium) to enhance the growth and optimize the regenerative abilities (ex. stem cells therapy) prior of their introduction in the organism as a regenerative medical therapy.
Author Contributions
A.G. designed the manuscript structure and wrote it. A.G., M.Y. and J.S.-A. discussed the content and exchanged ideas and suggestions (concepts to add, Figure 1, references selection, etc.) throughout the writing process, edited and critically revised the paper. J.S.-A. gave the final approval for the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Acknowledgments
A.G. received a scholarship under the Merit Scholarship Program for foreign students from the Ministry of Education and Higher Education of Quebec, Canada. The Fonds de recherche du Québec—Nature et technologies (FRQNT) is responsible for managing the program (Bourses d’excellence pour étudiants étrangers du Ministère de l’Éducation et de l’Enseignement supérieur du Québec, Le Fonds de recherche du Québec—Nature et technologies (FRQNT) est responsable de la gestion du programme). A.G. received the scholarship « Bourse Tremplin -Stage en milieu de pratique» (Internship scholarship) from the Fonds de recherche du Québec-Santé (FRQS), Quebec, Canada. Figure 1 was created using images from https://mindthegraph.com/ (accessed on 8 November 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gray, N.; Le Bot, N.; Heemels, M.T. Regeneration. Nature 2018, 557, 321. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M. Liver regeneration. Front. Biosci. 2002, 7, e301–e314. [Google Scholar] [CrossRef]
- Lin, A.; Makushok, T.; Diaz, U.; Marshall, W.F. Methods for the Study of Regeneration in Stentor. J. Vis. Exp. 2018, 136, 57759. [Google Scholar]
- Odelberg, S.J. Cellular plasticity in vertebrate regeneration. Anat. Rec. B New Anat. 2005, 287, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Galliot, B.; Crescenzi, M.; Jacinto, A.; Tajbakhsh, S. Trends in tissue repair and regeneration. Development 2017, 144, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Wooff, Y.; Man, S.M.; Aggio-Bruce, R.; Natoli, R.; Fernando, N. IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases. Front. Immunol. 2019, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Vajkoczy, P.; Weidner, N. Degenerative spinal diseases. Der Nervenarzt 2018, 89, 619. [Google Scholar]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.L.; Harasymowicz, N.S.; Klimak, M.A.; Collins, K.H.; Guilak, F. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthr. Cartil. 2020, 28, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Koepke, L.S.; Lopez, M.T.; Tong, X.; Ambrosi, T.H.; Gulati, G.S.; Marecic, O.; Wang, Y.; Ransom, R.C.; Hoover, M.Y.; et al. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 2020, 26, 1583–1592. [Google Scholar] [CrossRef]
- Jeon, S.; Oh, I.H. Regeneration of the retina: Toward stem cell therapy for degenerative retinal diseases. BMB Rep. 2015, 48, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, X.; Chen, H. Organoid models in lung regeneration and cancer. Cancer Lett. 2020, 475, 129–135. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Regeneration during Obesity: An Impaired Homeostasis. Animals 2020, 10, 2344. [Google Scholar] [CrossRef]
- Roberts, S.; Colombier, P.; Sowman, A.; Mennan, C.; Rölfing, J.H.; Guicheux, J.; Edwards, J.R. Ageing in the musculoskeletal system. Acta Orthop. 2016, 87, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakawa, H.; Kusumoto, D.; Hashimoto, H.; Yuasa, S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int. J. Mol. Sci. 2020, 21, 1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, J.M.; Zochodne, D.W. Impaired peripheral nerve regeneration in diabetes mellitus. J. Peripher. Nerv. Syst. 2005, 10, 144–157. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nagino, M.; Nimura, Y. Mechanism of impaired hepatic regeneration in cholestatic liver. J. Hepatobiliary Pancreat Surg. 2007, 14, 159–166. [Google Scholar] [CrossRef]
- Greenwood, H.L.; Singer, P.A.; Downey, G.P.; Martin, D.K.; Thorsteinsdóttir, H.; Daar, A.S. Regenerative medicine and the developing world. PLoS Med. 2006, 3, e381. [Google Scholar] [CrossRef] [PubMed]
- Brokesh, A.M.; Gaharwar, A.K. Inorganic Biomaterials for Regenerative Medicine. ACS Appl. Mater. Interfaces 2020, 12, 5319–5344. [Google Scholar] [CrossRef] [PubMed]
- Makarevich, P.I.; Efimenko, A.Y.; Tkachuk, V.A. Biochemical Regulation of Regenerative Processes by Growth Factors and Cytokines: Basic Mechanisms and Relevance for Regenerative Medicine. Biochemistry 2020, 85, 11–26. [Google Scholar]
- Cedar, S.H. The function of stem cells and their future roles in healthcare. Br. J. Nurs. 2006, 15, 104–107. [Google Scholar] [CrossRef]
- Forbes, S.J. Recent advances in stem cells and regenerative medicine. Qjm 2014, 107, 251–252. [Google Scholar] [PubMed] [Green Version]
- Raspa, A.; Pugliese, R.; Maleki, M.; Gelain, F. Recent therapeutic approaches for spinal cord injury. Biotechnol. Bioeng. 2016, 113, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Oliveira, J.M.; Martins, A.; Teixeira, F.G.; Silva, N.A.; Neves, N.M.; Sousa, N.; Reis, R.L. Tissue engineering and regenerative medicine: Past, present, and future. Int. Rev. NeuroBiol. 2013, 108, 1–33. [Google Scholar] [PubMed]
- McPhail, M.J.; Janus, J.R.; Lott, D.G. Advances in regenerative medicine for otolaryngology/head and neck surgery. BMJ 2020, 369, m718. [Google Scholar] [CrossRef]
- Edgar, L.; Pu, T.; Porter, B.; Aziz, J.M.; La Pointe, C.; Asthana, A.; Orlando, G. Regenerative medicine, organ bioengineering and transplantation. Br. J. Surg. 2020, 107, 793–800. [Google Scholar]
- Sahle, F.F.; Kim, S.; Niloy, K.K.; Tahia, F.; Fili, C.V.; Cooper, E.; Hamilton, D.J.; Lowe, T.L. Nanotechnology in regenerative ophthalmology. Adv. Drug Deliv. Rev. 2019, 148, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Jahangirian, H.; Azizi, S.; Rafiee-Moghaddam, R.; Baratvand, B.; Webster, T.J. Status of Plant Protein-Based Green Scaffolds for Regenerative Medicine Applications. Biomolecules 2019, 9, 619. [Google Scholar] [CrossRef] [Green Version]
- Cable, J.; Fuchs, E.; Weissman, I.; Jasper, H.; Glass, D.; Rando, T.A.; Blau, H.; Debnath, S.; Oliva, A.; Park, S.; et al. Adult stem cells and regenerative medicine-a symposium report. Ann. N. Y. Acad. Sci. 2020, 1462, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Taghiyar, L.; Safari, F.; Baghaban Eslaminejad, M. Regenerative Medicine Applications of Mesenchymal Stem Cells. Adv. Exp. Med. Biol. 2018, 1089, 115–141. [Google Scholar] [PubMed]
- Khan, A.; Wang, B.; Ni, Y. Chitosan-Nanocellulose Composites for Regenerative Medicine Applications. Curr. Med. Chem. 2020, 27, 4584–4592. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Domingues, A.; Ratajczak, J.; Ratajczak, M.Z. Potential Clinical Applications of Stem Cells in Regenerative Medicine. Adv. Exp. Med. Biol. 2019, 1201, 1–22. [Google Scholar]
- Nilforoushzadeh, M.A.; Sisakht, M.M.; Seifalian, A.M.; Amirkhani, M.A.; Banafshe, H.R.; Verdi, J.; Sharifzad, F.; Taghiabadi, E. Regenerative Medicine Applications in Wound Care. Curr. Stem Cell Res. Ther. 2017, 12, 658–674. [Google Scholar] [CrossRef]
- Stocum, D.L. Regenerative biology and medicine. J. Musculoskelet. Neuronal. Interact. 2002, 2, 270–273. [Google Scholar]
- Hsu, M.N.; Chang, Y.H.; Truong, V.A.; Lai, P.L.; Nguyen, T.K.N.; Hu, Y.C. CRISPR technologies for stem cell engineering and regenerative medicine. Biotechnol. Adv. 2019, 37, 107447. [Google Scholar] [CrossRef]
- Gouveia, C.; Huyser, C.; Egli, D.; Pepper, M.S. Lessons Learned from Somatic Cell Nuclear Transfer. Int. J. Mol. Sci. 2020, 21, 2314. [Google Scholar] [CrossRef]
- Lowe, T.L.; Agrahari, V.; Kannan, R.M.; Kannan, S. Nanotechnology enabled regenerative medicine for neurological disorders. Adv. Drug Deliv. Rev. 2019, 148, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Fricain, J.C.; De Olivera, H.; Devillard, R.; Kalisky, J.; Remy, M.; Kériquel, V.; Le Nihounen, D.; Grémare, A.; Guduric, V.; Plaud, A.; et al. 3D bioprinting in regenerative medicine and tissue engineering. Med. Sci. 2017, 33, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Christ, G.J.; Saul, J.M.; Furth, M.E.; Andersson, K.E. The pharmacology of regenerative medicine. Pharmacol. Rev. 2013, 65, 1091–1133. [Google Scholar] [CrossRef] [Green Version]
- Andersson, K.E.; Christ, G.J. Regenerative pharmacology: The future is now. Mol. Interv. 2007, 7, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.K.; Andersson, K.E. Regenerative pharmacology: Recent developments and future perspectives. Regen. Med. 2016, 11, 859–870. [Google Scholar] [CrossRef]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Mashayekhan, S.; Baradaran-Rafii, A.; Djalilian, A.R. Bioengineering Approaches for Corneal Regenerative Medicine. Tissue Eng. Regen. Med. 2020, 17, 567–593. [Google Scholar] [CrossRef]
- Craig, D.M.; Ashcroft, S.P.; Belew, M.Y.; Stocks, B.; Currell, K.; Baar, K.; Philp, A. Utilizing small nutrient compounds as enhancers of exercise-induced mitochondrial biogenesis. Front. Physiol. 2015, 6, 296. [Google Scholar] [CrossRef] [Green Version]
- Booth, F.W.; Ruegsegger, G.N.; Toedebusch, R.G.; Yan, Z. Endurance Exercise and the Regulation of Skeletal Muscle Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 129–151. [Google Scholar]
- McGee, S.L.; Hargreaves, M. Exercise and skeletal muscle glucose transporter 4 expression: Molecular mechanisms. Clin. Exp. Pharmacol. Physiol. 2006, 33, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Bo, B.; Zhou, Y.; Zheng, Q.; Wang, G.; Zhou, K.; Wei, J. The Molecular Mechanisms Associated with Aerobic Exercise-Induced Cardiac Regeneration. Biomolecules 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Smith, J.K. Exercise as an Adjuvant to Cartilage Regeneration Therapy. Int. J. Mol. Sci. 2020, 21, 9471. [Google Scholar] [CrossRef]
- Saito, Y.; Chikenji, T.S.; Matsumura, T.; Nakano, M.; Fujimiya, M. Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors. Nat. Commun. 2020, 11, 889. [Google Scholar] [CrossRef] [PubMed]
- Schüttler, D.; Clauss, S.; Weckbach, L.T.; Brunner, S. Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise. Cells 2019, 8, 1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, N.; Ye, H. Exercise and Muscle Atrophy. Adv. Exp. Med. Biol. 2020, 1228, 255–267. [Google Scholar] [PubMed]
- Nalbandian, A.; Nguyen, C.; Katheria, V.; Llewellyn, K.J.; Badadani, M.; Caiozzo, V.; Kimonis, V.E. Exercise training reverses skeletal muscle atrophy in an experimental model of VCP disease. PLoS ONE 2013, 8, e76187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perandini, L.A.; Chimin, P.; Lutkemeyer, D.D.S.; Câmara, N.O.S. Chronic inflammation in skeletal muscle impairs satellite cells function during regeneration: Can physical exercise restore the satellite cell niche? FEBS J. 2018, 285, 1973–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, M.T.; Dalle Carbonare, L.; Dorelli, G.; Mottes, M. Effects of physical exercise on the prevention of stem cells senescence. Stem Cell Rev. Rep. 2020, 16, 33–40. [Google Scholar] [CrossRef]
- Gordon, T.; English, A.W. Strategies to promote peripheral nerve regeneration: Electrical stimulation and/or exercise. Eur. J. Neurosci. 2016, 43, 336–350. [Google Scholar] [CrossRef] [Green Version]
- Larrick, J.W.; Mendelsohn, A.R. Exercise Partially Rejuvenates Muscle Stem Cells. Rejuvenation Res. 2020, 23, 262–265. [Google Scholar] [CrossRef]
- Brett, J.O.; Arjona, M.; Ikeda, M.; Quarta, M.; de Morrée, A.; Egner, I.M.; Perandini, L.A.; Ishak, H.D.; Goshayeshi, A.; Benjamin, D.I.; et al. Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1. Nat. Metab. 2020, 2, 307–317. [Google Scholar] [CrossRef]
- Son, J.S.; Chae, S.A.; Testroet, E.D.; Du, M.; Jun, H.P. Exercise-induced myokines: A brief review of controversial issues of this decade. Expert. Rev. Endocrinol. Metab. 2018, 13, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Hsiao, M. Exercise-induced SPARC prevents tumorigenesis of colon cancer. Gut 2013, 62, 810–811. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine as A Regeneration Factor: Beyond the Tissue Repair. Life 2021, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine as a Molecular Physiological and Pathological Biomarker. Biomolecules 2021, 11, 1689. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and inflammation: Another homeostatic property? Cytokine 2020, 133, 155179. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and cancer: A homeostatic hormone? Cytokine 2020, 127, 154996. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takagi, T.; Tanimura, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Hang, L.P.; Mizushima, K.; Hirai, Y.; et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 2013, 62, 882–889. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted Protein Acidic and Rich in Cysteine: Metabolic and Homeostatic Properties beyond the Extracellular Matrix Structure. Appl. Sci. 2020, 10, 2388. [Google Scholar] [CrossRef] [Green Version]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine and bioenergetics: Extracellular matrix, adipocytes remodeling and skeletal muscle metabolism. Int. J. BioChem. Cell Biol. 2019, 117, 105627. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Hirano, N.; Lassiter, D.G.; Björnholm, M.; Chibalin, A.V.; Sakuma, K.; Tanimura, Y.; Mizushima, K.; Takagi, T.; Naito, Y.; et al. Secreted protein acidic and rich in cysteine (SPARC) improves glucose tolerance via AMP-activated protein kinase activation. FASEB J. 2019, 33, 10551–10562. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, N.; Sousa, S.R.; Brekken, R.A.; Monteiro, F.J. Role of SPARC in bone remodeling and cancer-related bone metastasis. J. Cell BioChem. 2014, 115, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Chimal-Monroy, J.; Bravo-Ruíz, T.; Furuzawa-Carballeda, G.J.; Lira, J.M.; de la Cruz, J.C.; Almazán, A.; Krötzsch-Gómez, F.E.; Arrellín, G.; Díaz de León, L. Collagen-PVP accelerates new bone formation of experimentally induced bone defects in rat skull and promotes the expression of osteopontin and SPARC during bone repair of rat femora fractures. Ann. N. Y. Acad. Sci. 1998, 857, 232–236. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Measuring Exercise-Induced Secreted Protein Acidic and Rich in Cysteine Expression as a Molecular Tool to Optimize Personalized Medicine. Genes 2021, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise Training of Secreted Protein Acidic and Rich in Cysteine (Sparc) KO Mice Suggests That Exercise-Induced Muscle Phenotype Changes Are SPARC-Dependent. Appl. Sci. 2020, 10, 9108. [Google Scholar] [CrossRef]
- Kwon, J.H.; Moon, K.M.; Min, K.W. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef]
- Nilsson, M.I.; Bourgeois, J.M.; Nederveen, J.P.; Leite, M.R.; Hettinga, B.P.; Bujak, A.L.; May, L.; Lin, E.; Crozier, M.; Rusiecki, D.R.; et al. Lifelong aerobic exercise protects against inflammaging and cancer. PLoS ONE 2019, 14, e0210863. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Diet Impact on Obesity beyond Calories and Trefoil Factor Family 2 (TFF2) as an Illustration: Metabolic Implications and Potential Applications. Biomolecules 2021, 11, 1830. [Google Scholar] [CrossRef]
- Cheng, C.W.; Yilmaz, Ö.H. 100 Years of Exploiting Diet and Nutrition for Tissue Regeneration. Cell Stem Cell 2021, 28, 370–373. [Google Scholar] [CrossRef]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer 2018, 18, 707–719. [Google Scholar] [CrossRef]
- Cornide-Petronio, M.E.; Álvarez-Mercado, A.I.; Jiménez-Castro, M.B.; Peralta, C. Current Knowledge about the Effect of Nutritional Status, Supplemented Nutrition Diet, and Gut Microbiota on Hepatic Ischemia-Reperfusion and Regeneration in Liver Surgery. Nutrients 2020, 12, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Zhang, Y.; Xing, Y.; Zhao, B.; Zhou, C.; Wen, Y.; Xu, X. High-fat and high-glucose microenvironment decreases Runx2 and TAZ expression and inhibits bone regeneration in the mouse. J. Orthop. Surg. Res. 2019, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Pawaputanon Na Mahasarakham, C.; Kido, D.; Takeda, K.; Izumi, Y. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS ONE 2018, 13, e0201855. [Google Scholar]
- Abdel Nazeer, A.; Saito, S.; Sayed, S.; Hassan, L.; Askar, F.; Al-Jahdari, W.; Seki, T.; Hideaki, O. Normal glucose enhances neuronal regeneration after lidocaine-induced injury. Br. J. Anaesth. 2010, 104, 482–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargo, W.A.; de Vries, R.; van Luijk, J.; Hoekstra, J.W.; Bronkhorst, E.M.; Jansen, J.A.; van den Beucken, J. Diabetes Mellitus and Bone Regeneration: A Systematic Review and Meta-Analysis of Animal Studies. Tissue Eng. Part B Rev. 2017, 23, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Rangan, P.; Choi, I.; Wei, M.; Navarrete, G.; Guen, E.; Brandhorst, S.; Enyati, N.; Pasia, G.; Maesincee, D.; Ocon, V.; et al. Fasting-Mimicking Diet Modulates Microbiota and Promotes Intestinal Regeneration to Reduce Inflammatory Bowel Disease Pathology. Cell Rep. 2019, 26, 2704–2719.e2706. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Bai, M.; Ling, Z.; Lin, Y.; Wang, S.; Chen, Y. Intermittent administration of a fasting-mimicking diet reduces intestinal inflammation and promotes repair to ameliorate inflammatory bowel disease in mice. J. Nutr. Biochem. 2021, 96, 108785. [Google Scholar] [CrossRef]
- Cheng, C.W.; Adams, G.B.; Perin, L.; Wei, M.; Zhou, X.; Lam, B.S.; Da Sacco, S.; Mirisola, M.; Quinn, D.I.; Dorff, T.B.; et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 2014, 14, 810–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.W.; Villani, V.; Buono, R.; Wei, M.; Kumar, S.; Yilmaz, O.H.; Cohen, P.; Sneddon, J.B.; Perin, L.; Longo, V.D. Fasting-Mimicking Diet Promotes Ngn3-Driven β-Cell Regeneration to Reverse Diabetes. Cell 2017, 168, 775–788.e712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, C.A. Diabetes: Fasting and β cell regeneration. Nat. Rev. Endocrinol. 2017, 13, 252. [Google Scholar] [CrossRef]
- Arciero, P.J.; Edmonds, R.; He, F.; Ward, E.; Gumpricht, E.; Mohr, A.; Ormsbee, M.J.; Astrup, A. Protein-Pacing Caloric-Restriction Enhances Body Composition Similarly in Obese Men and Women during Weight Loss and Sustains Efficacy during Long-Term Weight Maintenance. Nutrients 2016, 8, 476. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Sun, S.; Geng, L.; Song, M.; Wang, W.; Ye, Y.; Ji, Q.; Zou, Z.; Wang, S.; He, X.; et al. Caloric Restriction Reprograms the Single-Cell Transcriptional Landscape of Rattus Norvegicus Aging. Cell 2020, 180, 984–1001.e1022. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zuo, L.; Ward, E.; Arciero, P.J. Serum Polychlorinated Biphenyls Increase and Oxidative Stress Decreases with a Protein-Pacing Caloric Restriction Diet in Obese Men and Women. Int. J. Environ. Res. Public Health 2017, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Fujita, K.; Nojima, S.; Hayashi, Y.; Nakano, K.; Ishizuya, Y.; Wang, C.; Yamamoto, Y.; Kinouchi, T.; Matsuzaki, K.; et al. High-Fat Diet-Induced Inflammation Accelerates Prostate Cancer Growth via IL6 Signaling. Clin. Cancer Res. 2018, 24, 4309–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.D.; Yoon, N.A.; Jin, S.; Diano, S. Microglial UCP2 Mediates Inflammation and Obesity Induced by High-Fat Feeding. Cell Metab. 2019, 30, 952–962.e955. [Google Scholar] [CrossRef]
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, Inflammation, and Prostate Cancer. J. Clin. Med. 2019, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef]
- Benzie, I.F.; Choi, S.W. Antioxidants in food: Content, measurement, significance, action, cautions, caveats, and research needs. Adv. Food Nutr. Res. 2014, 71, 1–53. [Google Scholar]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Mohd Sahardi, N.F.N.; Makpol, S. Ginger (Zingiber officinale Roscoe) in the Prevention of Ageing and Degenerative Diseases: Review of Current Evidence. Evid.-Based Complement. Alternat. Med. 2019, 2019, 5054395. [Google Scholar] [CrossRef] [Green Version]
- Marsman, H.A.; de Graaf, W.; Heger, M.; van Golen, R.F.; Ten Kate, F.J.; Bennink, R.; van Gulik, T.M. Hepatic regeneration and functional recovery following partial liver resection in an experimental model of hepatic steatosis treated with omega-3 fatty acids. Br. J. Surg. 2013, 100, 674–683. [Google Scholar] [CrossRef]
- Sharples, A.P.; Hughes, D.C.; Deane, C.S.; Saini, A.; Selman, C.; Stewart, C.E. Longevity and skeletal muscle mass: The role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 2015, 14, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Shavandi, A.; Saeedi, P.; Gérard, P.; Jalalvandi, E.; Cannella, D.; Bekhit, A.E. The role of microbiota in tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Eugene, A.R.; Masiak, J. The Neuroprotective Aspects of Sleep. MEDtube Sci. 2015, 3, 35–40. [Google Scholar] [PubMed]
- Elkhenany, H.; AlOkda, A.; El-Badawy, A.; El-Badri, N. Tissue regeneration: Impact of sleep on stem cell regenerative capacity. Life Sci. 2018, 214, 51–61. [Google Scholar] [CrossRef]
- Dierickx, P.; Van Laake, L.W.; Geijsen, N. Circadian clocks: From stem cells to tissue homeostasis and regeneration. EMBO Rep. 2018, 19, 18–28. [Google Scholar] [CrossRef]
- Brown, S.A. Circadian clock-mediated control of stem cell division and differentiation: Beyond night and day. Development 2014, 141, 3105–3111. [Google Scholar] [CrossRef] [Green Version]
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Mohebbi, I.; Rastegar, M.; Kaviani, M.; Darband, S.G.; Jahanban-Esfahlan, R.; Nabavi, S.M.; Yousefi, B. The multiple functions of melatonin in regenerative medicine. Ageing Res. Rev. 2018, 45, 33–52. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; You, W.; Shan, T. The regulatory role of melatonin in skeletal muscle. J. Muscle Res. Cell Motil. 2020, 41, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Pourabbas, M.; Bagheri, R.; Hooshmand Moghadam, B.; Willoughby, D.S.; Candow, D.G.; Elliott, B.T.; Forbes, S.C.; Ashtary-Larky, D.; Eskandari, M.; Wong, A.; et al. Strategic Ingestion of High-Protein Dairy Milk during a Resistance Training Program Increases Lean Mass, Strength, and Power in Trained Young Males. Nutrients 2021, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- Saracino, P.G.; Saylor, H.E.; Hanna, B.R.; Hickner, R.C.; Kim, J.S.; Ormsbee, M.J. Effects of Pre-Sleep Whey vs. Plant-Based Protein Consumption on Muscle Recovery Following Damaging Morning Exercise. Nutrients 2020, 12, 2049. [Google Scholar] [CrossRef] [PubMed]
- Mônico-Neto, M.; Dáttilo, M.; Ribeiro, D.A.; Lee, K.S.; de Mello, M.T.; Tufik, S.; Antunes, H.K.M. REM sleep deprivation impairs muscle regeneration in rats. Growth Factors 2017, 35, 12–18. [Google Scholar] [CrossRef]
- Chen, P.; Yao, H.; Su, W.; He, Y.; Cheng, K.; Wang, Y.; Peng, W.; Li, P. Sleep deprivation worsened oral ulcers and delayed healing process in an experimental rat model. Life Sci. 2019, 232, 116594. [Google Scholar] [CrossRef]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar]
- Steptoe, A.; Deaton, A.; Stone, A.A. Subjective wellbeing, health, and ageing. Lancet 2015, 385, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Tam, B.T.; Morais, J.A.; Santosa, S. Obesity and ageing: Two sides of the same coin. Obes. Rev. 2020, 21, e12991. [Google Scholar] [CrossRef]
- Ghosh, S.; Sinha, J.K.; Raghunath, M. ‘Obesageing’: Linking obesity & ageing. Indian J. Med. Res. 2019, 149, 610–615. [Google Scholar]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schürmann, A.; et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 2017, 20, 771–784.e776. [Google Scholar] [CrossRef] [Green Version]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Ageing and Obesity Shared Patterns: From Molecular Pathogenesis to Epigenetics. Diseases 2021, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Obesity as a Neuroendocrine Reprogramming. Medicina 2021, 57, 66. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Broken Energy Homeostasis and Obesity Pathogenesis: The Surrounding Concepts. J. Clin. Med. 2018, 7, 453. [Google Scholar] [CrossRef] [Green Version]
- Ghanemi, A.; St-Amand, J. Redefining obesity toward classifying as a disease. Eur. J. Intern. Med. 2018, 55, 20–22. [Google Scholar] [CrossRef]
- Conway, B.; Rene, A. Obesity as a disease: No lightweight matter. Obes. Rev. 2004, 5, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S. Recognizing obesity as a disease. J. Am. Assoc. Nurse Pract. 2020, 32, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Obese Animals as Models for Numerous Diseases: Advantages and Applications. Medicina 2021, 57, 399. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Will an obesity pandemic replace the coronavirus disease-2019 (COVID-19) pandemic? Med. Hypotheses 2020, 144, 110042. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Coronavirus Disease 2019 (COVID-19) Crisis: Losing Our Immunity When We Need It the Most. Biology 2021, 10, 545. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Impact of Adiposity and Fat Distribution, Rather Than Obesity, on Antibodies as an Illustration of Weight-Loss-Independent Exercise Benefits. Medicines 2021, 8, 57. [Google Scholar] [CrossRef]
- Aunan, J.R.; Watson, M.M.; Hagland, H.R.; Søreide, K. Molecular and biological hallmarks of ageing. Br. J. Surg. 2016, 103, e29–e46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Pilling, L.C.; Ferrucci, L. The genetics of human ageing. Nat. Rev. Genet. 2020, 21, 88–101. [Google Scholar] [CrossRef]
- van den Beld, A.W.; Kaufman, J.M.; Zillikens, M.C.; Lamberts, S.W.J.; Egan, J.M.; van der Lely, A.J. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 2018, 6, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Bacalini, M.G.; D’Aquila, P.; Marasco, E.; Nardini, C.; Montesanto, A.; Franceschi, C.; Passarino, G.; Garagnani, P.; Bellizzi, D. The methylation of nuclear and mitochondrial DNA in ageing phenotypes and longevity. Mech. Ageing Dev. 2017, 165, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.E.; Daughtry, M.R.; Yen, C.N.; Kirkpatrick, L.T.; Shi, H.; Gerrard, D.E. Dual effects of obesity on satellite cells and muscle regeneration. Physiol. Rep. 2020, 8, e14511. [Google Scholar] [CrossRef]
- Conboy, I.M.; Rando, T.A. Aging, stem cells and tissue regeneration: Lessons from muscle. Cell Cycle 2005, 4, 407–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, M.H. Changes in Regenerative Capacity through Lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blau, H.M.; Cosgrove, B.D.; Ho, A.T. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 2015, 21, 854–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, S.K.; Schneider, S.E.; Amundson, L.A.; Neu, C.P.; Henak, C.R. Maturity-dependent cartilage cell plasticity and sensitivity to external perturbation. J. Mech. Behav. BioMed. Mater. 2020, 106, 103732. [Google Scholar] [CrossRef] [PubMed]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metabolism 2019, 92, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Which type of exercise keeps you young? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 167–173. [Google Scholar] [CrossRef]
- Galloza, J.; Castillo, B.; Micheo, W. Benefits of Exercise in the Older Population. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 659–669. [Google Scholar] [CrossRef]
- Mora, J.C.; Valencia, W.M. Exercise and Older Adults. Clin. Geriatr. Med. 2018, 34, 145–162. [Google Scholar] [CrossRef]
- Reddy, R.; Wittenberg, A.; Castle, J.R.; El Youssef, J.; Winters-Stone, K.; Gillingham, M.; Jacobs, P.G. Effect of Aerobic and Resistance Exercise on Glycemic Control in Adults With Type 1 Diabetes. Can. J. Diabetes 2019, 43, 406–414.e401. [Google Scholar] [CrossRef]
- Savikj, M.; Gabriel, B.M.; Alm, P.S.; Smith, J.; Caidahl, K.; Björnholm, M.; Fritz, T.; Krook, A.; Zierath, J.R.; Wallberg-Henriksson, H. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: A randomised crossover trial. Diabetologia 2019, 62, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Collao, N.; Rada, I.; Francaux, M.; Deldicque, L.; Zbinden-Foncea, H. Anti-Inflammatory Effect of Exercise Mediated by Toll-Like Receptor Regulation in Innate Immune Cells—A Review. Int. Rev. Immunol. 2020, 39, 39–52. [Google Scholar] [CrossRef]
- Ho, S.S.; Dhaliwal, S.S.; Hills, A.P.; Pal, S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health 2012, 12, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liu, S.; Li, G.; Xiao, J. Exercise Regulates the Immune System. Adv. Exp. Med. Biol. 2020, 1228, 395–408. [Google Scholar]
- Plikus, M.V.; Van Spyk, E.N.; Pham, K.; Geyfman, M.; Kumar, V.; Takahashi, J.S.; Andersen, B. The circadian clock in skin: Implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J. Biol. Rhythm. 2015, 30, 163–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Mignone, J.; MacLellan, W.R. Cardiac Regeneration and Stem Cells. Physiol. Rev. 2015, 95, 1189–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Exercise and High-Fat Diet in Obesity: Functional Genomics Perspectives of Two Energy Homeostasis Pillars. Genes 2020, 11, 875. [Google Scholar] [CrossRef]
- Melouane, A.; Ghanemi, A.; Aubé, S.; Yoshioka, M.; St-Amand, J. Differential gene expression analysis in ageing muscle and drug discovery perspectives. Ageing Res. Rev. 2018, 41, 53–63. [Google Scholar] [CrossRef]
- Melouane, A.; Ghanemi, A.; Yoshioka, M.; St-Amand, J. Functional genomics applications and therapeutic implications in sarcopenia. Mutat. Res. Rev. Mutat. Res. 2019, 781, 175–185. [Google Scholar] [CrossRef]
- Mucunguzi, O.; Melouane, A.; Ghanemi, A.; Yoshioka, M.; Boivin, A.; Calvo, E.L.; St-Amand, J. Identification of the principal transcriptional regulators for low-fat and high-fat meal responsive genes in small intestine. Nutr. Metab. 2017, 14, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swindell, W.R. Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genom. 2009, 10, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linford, N.J.; Beyer, R.P.; Gollahon, K.; Krajcik, R.A.; Malloy, V.L.; Demas, V.; Burmer, G.C.; Rabinovitch, P.S. Transcriptional response to aging and caloric restriction in heart and adipose tissue. Aging Cell 2007, 6, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Wanders, D.; Ghosh, S.; Stone, K.P.; Van, N.T.; Gettys, T.W. Transcriptional impact of dietary methionine restriction on systemic inflammation: Relevance to biomarkers of metabolic disease during aging. Biofactors 2014, 40, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Franco, C.; Soares, R.; Pires, E.; Koci, K.; Almeida, A.M.; Santos, R.; Coelho, A.V. Understanding regeneration through proteomics. Proteomics 2013, 13, 686–709. [Google Scholar] [CrossRef] [PubMed]
- Stoick-Cooper, C.L.; Moon, R.T.; Weidinger, G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007, 21, 1292–1315. [Google Scholar] [CrossRef] [Green Version]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Lifestyle Management: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S38–S50. [CrossRef] [Green Version]
- El-Sherif, A.; El-Sherif, S.; Taylor, A.H.; Ayakannu, T. Ovarian Cancer: Lifestyle, Diet and Nutrition. Nutr. Cancer 2021, 73, 1092–1107. [Google Scholar] [CrossRef]
- Walsh, R. Lifestyle and mental health. Am. Psychol. 2011, 66, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.D.; Forno, E. Exercise and lifestyle changes in pediatric asthma. Curr. Opin. Pulm. Med. 2020, 26, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melouane, A.; Yoshioka, M.; Kanzaki, M.; St-Amand, J. Sparc, an EPS-induced gene, modulates the extracellular matrix and mitochondrial function via ILK/AMPK pathways in C2C12 cells. Life Sci. 2019, 229, 277–287. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).