Knockdown of Gene Expression in Macrophages by microRNA Mimic-Containing Poly (Lactic-co-glycolic Acid) Microparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Encapsulation of miRNA in PLGA-Based Microparticles
2.2. Microparticle Size Characterisation
2.3. Determination of Zeta Potential of Microparticles
2.4. miRNA Encapsulation Efficiency
2.5. Differentiation of U937 Monocytes into Macrophages
2.6. Transfection of PMA-Differentiated U937 Cells with PLGA Microparticles
2.7. Gene Expression Analysis by Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
2.8. TaqMan microRNA Assays
2.9. Statistical Analysis
3. Results
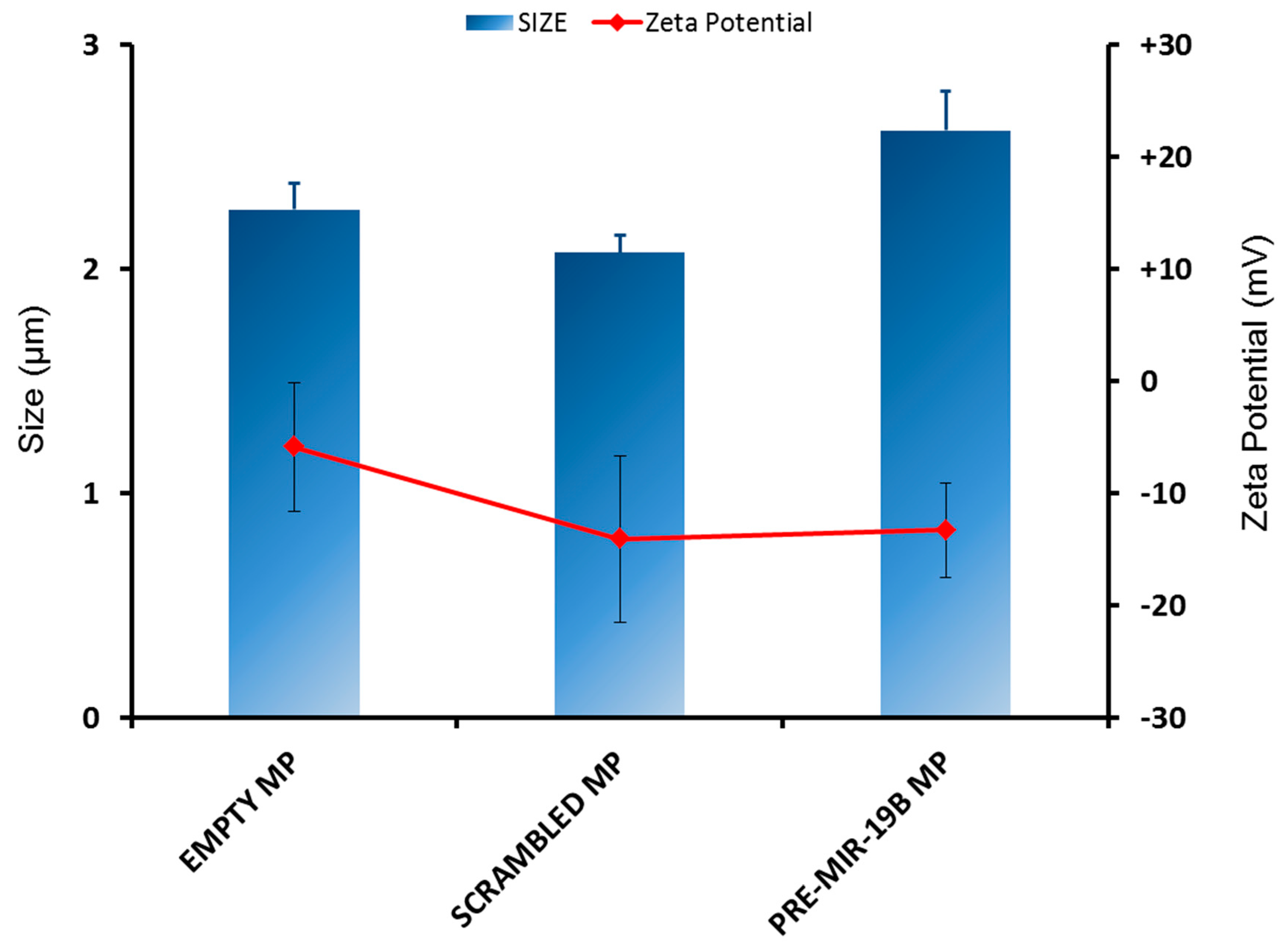
3.1. Characterisation of PLGA Microparticles
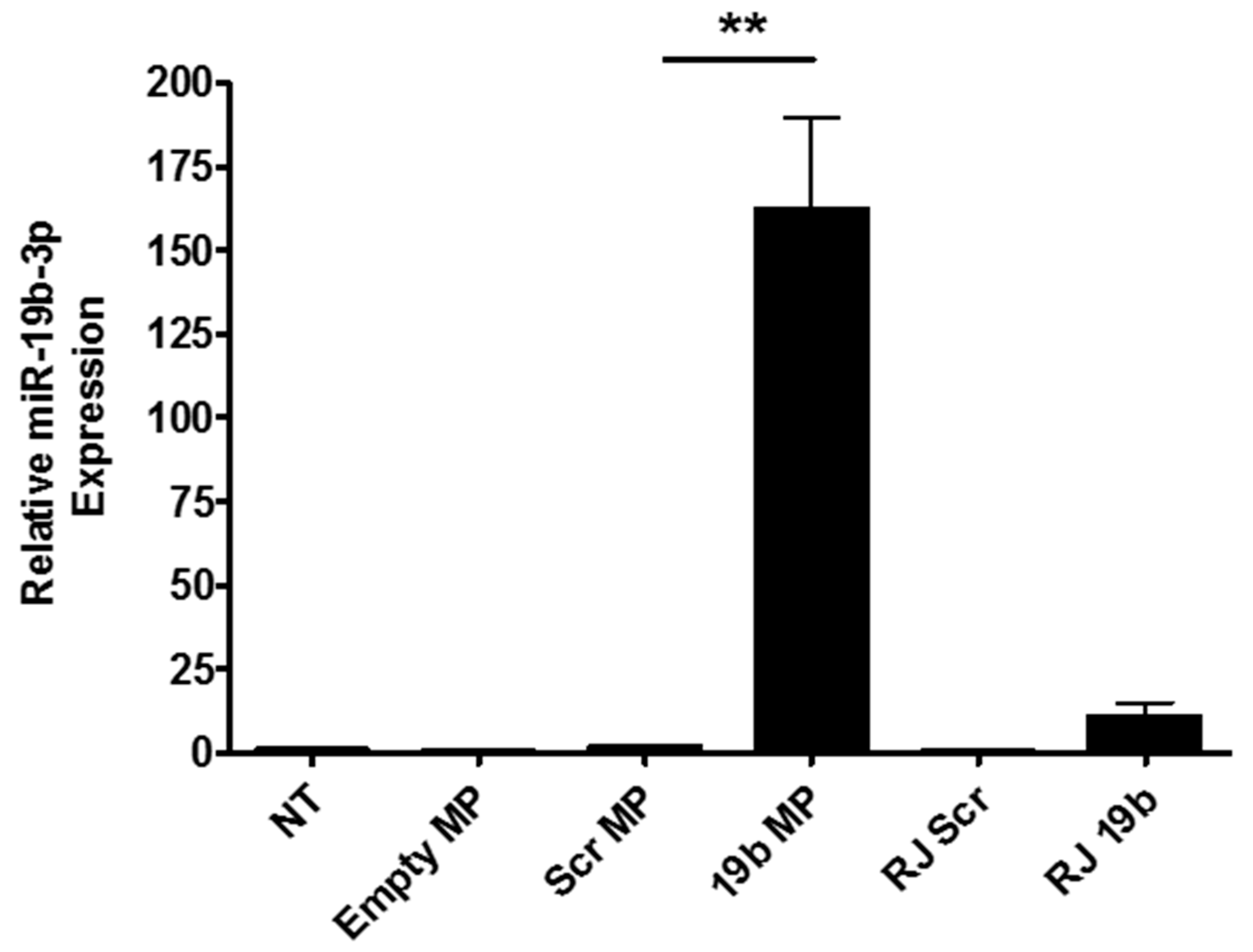
3.2. Internalisation and Intracellular Pre-miR-19b-3p Delivery to Macrophages Using Microparticles
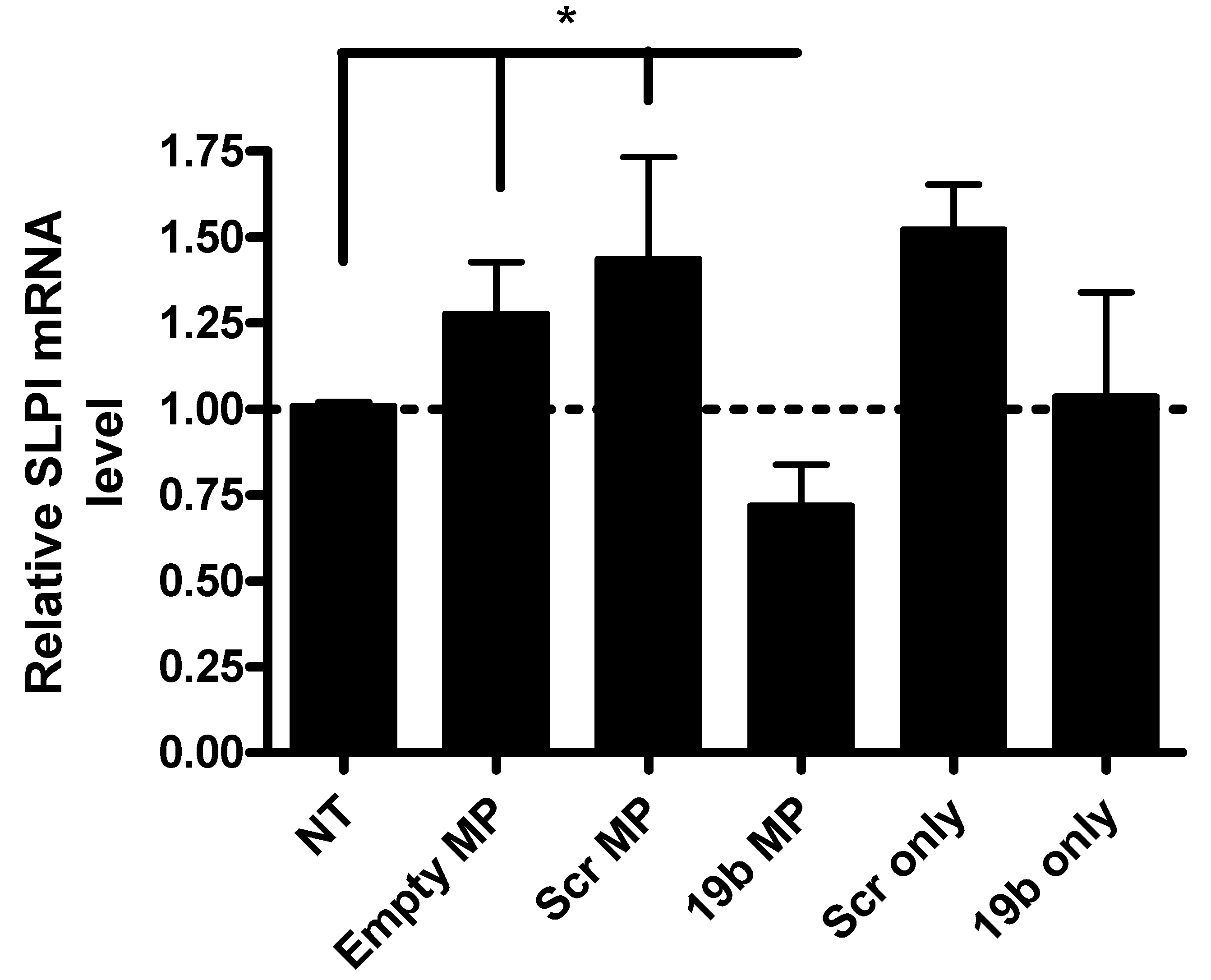
3.3. Functional Assessment of Pre-miR19b-Loaded PLGA Microparticles on the Expression of SLPI in PMA-Differentiated U937 Macrophages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Knowles, M.R.; Durie, P.R. What Is Cystic Fibrosis? N. Engl. J. Med. 2002, 347, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.B. Cystic Fibrosis Since 1938. Am. J. Respir. Crit. Care Med. 2006, 173, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; Gaggar, A.; Bruscia, E.; Hector, A.; Marcos, V.; Jung, A.; Greene, C.; McElvaney, G.; Mall, M.; Döring, G. Innate immunity in cystic fibrosis lung disease. J. Cyst. Fibros. 2012, 11, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Gharib, S.A.; Vaisar, T.; Aitken, M.L.; Park, D.R.; Heinecke, J.W.; Fu, X. Mapping the lung proteome in cystic fibrosis. J. Proteome Res. 2009, 8, 3020–3028. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Hilliard, K.A.; Norvell, T.M.; Berger, M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am. J. Respir. Crit. Care Med. 1994, 150, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.Z.; Wagener, J.; Bost, T.; Martinez, J.; Accurso, F.J.; Riches, D.W. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1995, 151, 1075–1082. [Google Scholar] [CrossRef]
- Greene, C.M.; McElvaney, N.G. Proteases and antiproteases in chronic neutrophilic lung disease—Relevance to drug discovery. Br. J. Pharmacol. 2009, 158, 1048–1058. [Google Scholar] [CrossRef]
- Vogelmeier, C.; Gillissen, A.; Buhl, R. Use of secretory leukoprotease inhibitor to augment lung antineutrophil elastase activity. Chest 1996, 110, 261S–266S. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Aninos, D.; Mikou, P.; Kanavaros, P.; Karameris, A.; Joardanoglou, J.; Rasidakis, A.; Veslemes, M.; Ozanne, B.; Spandidos, D.A. Expression of EGF, TGF-alpha and EGFR in squamous cell lung carcinomas. Anticancer Res. 1992, 12, 1183–1187. [Google Scholar]
- Jin, F.Y.; Nathan, C.; Radzioch, D.; Ding, A. Secretory leukocyte protease inhibitor: A macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell 1997, 88, 417–426. [Google Scholar] [CrossRef]
- Ding, A.; Thieblemont, N.; Zhu, J.; Jin, F.; Zhangm, J.; Wright, S. Secretory Leukocyte Protease Inhibitor Interferes with Uptake of Lipopolysaccharide by Macrophages. Infect. Immun. 1999, 67, 4485–4489. [Google Scholar] [PubMed]
- Weldon, S.; Taggart, C.C. Innate host defense functions of secretory leucoprotease inhibitor. Exp. Lung Res. 2007, 33, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Taggart, C.C.; Cryan, S.A.; Weldon, S.; Gibbons, A.; Greene, C.M.; Kelly, E.; Low, T.B.; O’Neill, S.J.; McElvaney, N.G. Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J. Exp. Med. 2005, 202, 1659–1668. [Google Scholar] [CrossRef]
- Nakamura, A.; Mori, Y.; Hagiwara, K.; Suzuki, T.; Sakakibara, T.; Kikuchi, T.; Igarashi, T.; Ebina, M.; Abe, T.; Miyazaki, J.; et al. Increased susceptibility to LPS-induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)-deficient mice. J. Exp. Med. 2003, 197, 669–674. [Google Scholar] [CrossRef]
- McElvaney, N.G.; Nakamura, H.; Birrer, P.; Hébert, C.; Wong, W.L.; Alphonso, M.; Baker, J.B.; Catalano, M.A.; Crystal, R.G. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J. Clin. Investig. 1992, 90, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, N.G.; Doujaiji, B.; Moan, M.J.; Burnham, M.R.; Wu, M.C.; Crystal, R.G. Pharmacokinetics of recombinant secretory leukoprotease inhibitor aerosolized to normals and individuals with cystic fibrosis. Am. Rev. Respir. Dis. 1993, 148, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Bergenfeldt, M.; Bjork, P.; Ohlsson, K. The elimination of secretory leukocyte protease inhibitor (SLPI) after intravenous injection in dog and man. Scand. J. Clin. Lab. Investig. 1990, 50, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Stolk, J.; Camps, J.; Feitsma, H.I.; Hermans, J.; Dijkman, J.H.; Pauwels, E.K. Pulmonary deposition and disappearance of aerosolised secretory leucocyte protease inhibitor. Thorax 1995, 50, 645–650. [Google Scholar] [CrossRef]
- Rana, T.M. Illuminating the silence: Understanding the structure and function of small RNAs. Nat. Rev. Mol. Cell Biol. 2007, 8, 23–36. [Google Scholar] [CrossRef]
- Griffiths-Jones, S. The microRNA Registry. Nucleic Acids Res. 2004, 32, D109–D111. [Google Scholar] [CrossRef] [PubMed]
- Oglesby, I.K.; Bray, I.M.; Chotirmall, S.H.; Stallings, R.L.; O’Neill, S.J.; McElvaney, N.G.; Greene, C.M. miR-126 Is Downregulated in Cystic Fibrosis Airway Epithelial Cells and Regulates TOM1 Expression. J. Immunol. 2010, 184, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.C.; Azevedo-Pouly, A.C.; Schmittgen, T.D. MicroRNA replacement therapy for cancer. Pharm Res. 2011, 28, 3030–3042. [Google Scholar] [CrossRef] [PubMed]
- Elmen, J.; Lindow, M.; Schütz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjärn, M.; Hansen, H.F.; Berger, U.; et al. LNA-mediated microRNA silencing in non-human primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Krutzfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 2005, 438. [Google Scholar] [CrossRef] [PubMed]
- Howard, K.A. Delivery of RNA interference therapeutics using polycation-based nanoparticles. Adv. Drug Deliv. Rev. 2009, 61, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Gemeinhart, R.A. Progress in microRNA delivery. J. Control. Release 2013, 172, 962–974. [Google Scholar] [CrossRef]
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef]
- Scott, A.; Weldon, S.; Taggart, C.C. SLPI and elafin: Multifunctional antiproteases of the WFDC family. Biochem. Soc. Trans. 2011, 39, 1437–1440. [Google Scholar] [CrossRef]
- Burke, B.; Sumner, S.; Maitland, N.; Lewis, C.E. Macrophages in gene therapy: Cellular delivery vehicles and in vivo targets. J. Leukocyte Biol. 2002, 72, 417–428. [Google Scholar] [PubMed]
- Kisich, K.O.; Malone, R.W.; Feldstein, P.A.; Erickson, K.L. Specific Inhibition of Macrophage TNF-α Expression by In Vivo Ribozyme Treatment. J. Immunol. 1999, 163, 2008–2016. [Google Scholar] [PubMed]
- Kusumawati, A.; Commes, T.; Liautard, J.P.; Widada, J.S. Transfection of Myelomonocytic Cell Lines: Cellular Response to a Lipid-Based Reagent and Electroporation. Anal. Biochem. 1999, 269, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, C.; O’Sullivan, M.P.; Sivadas, N.; O’Leary, S.; Gallagher, P.J.; Keane, J.; Cryan, S.A. The Application of High-Content Analysis in the Study of Targeted Particulate Delivery Systems for Intracellular Drug Delivery to Alveolar Macrophages. Mol. Pharm. 2011, 8, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; Yadav, A.B.; Lawlor, C.; Nolan, K.; O’Dwyer, J.; Greene, C.M.; McElvaney, N.G.; Sivadas, N.; Ramsey, J.M.; Cryan, S.A. Therapeutic Aerosol Bioengineering of siRNA for the Treatment of Inflammatory Lung Disease by TNFα Gene Silencing in Macrophages. Mol. Pharm. 2014, 11, 4270–4279. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Yeung, B.Z.; Wientjes, M.G.; Cole, D.J.; Au, J.L. Tumor priming enhances siRNA delivery and transfection in intraperitoneal tumors. J. Control. Release 2014, 178, 79–85. [Google Scholar] [CrossRef]
- Wang, G.; Pan, L.; Zhang, Y.; Wang, Y.; Zhang, Z.; Lü, J.; Zhou, P.; Fang, Y.; Jiang, S. Intranasal Delivery of Cationic PLGA Nano/Microparticles- Loaded FMDV DNA Vaccine Encoding IL-6 Elicited Protective Immunity against FMDV Challenge. PLoS ONE 2011, 6, e27605. [Google Scholar] [CrossRef]
- Pawar, D.; Goyal, A.K.; Mangal, S.; Mishra, N.; Vaidya, B.; Tiwari, S.; Jain, A.K.; Vyas, S.P. Evaluation of Mucoadhesive PLGA Microparticles for Nasal Immunization. AAPS J. 2010, 12, 130–137. [Google Scholar] [CrossRef]
- Ungaro, F.; d’Angelo, I.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Engineered PLGA nano- and micro-carriers for pulmonary delivery: Challenges and promises. J. Pharm. Pharmacol. 2012, 64, 1217–1235. [Google Scholar] [CrossRef]
- Rytting, E.; Nguyen, J.; Wang, X.; Kissel, T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Exp. Opin. Drug Deliv. 2008, 5, 629–639. [Google Scholar] [CrossRef]
- Gvili, K.; Benny, O.; Danino, D.; Machluf, M. Poly(d,l-lactide-co-glycolide acid) nanoparticles for DNA delivery: Waiving preparation complexity and increasing efficiency. Biopolymers 2007, 85, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Zhou, W.Z.; Prabha, S.; Sahoo, S.K.; Labhasetwar, V. Rapid endo-lysosomal escape of poly(dl-lactide-co-glycolide) nanoparticles: Implications for drug and gene delivery. FASEB J. 2002, 16, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.K.; Jensen, L.B.; Koocheki, S.; Bengtson, L.; Cun, D.; Nielsen, H.M.; Foged, C. Design of an inhalable dry powder formulation of DOTAP-modified PLGA nanoparticles loaded with siRNA. J. Control. Release 2012, 157, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.B.; Sawyer, A.J.; Saucier-Sawyer, J.; Saltzman, W.M.; Kyriakides, T.R. Nanoparticle delivery of miR-223 to attenuate macrophage fusion. Biomaterials 2016, 89, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C. Development of Nano- and Microparticle Technologies for Targeted Gene Silencing through RNA Interference: Manipulation of the Immune Response in Inflammatory Lung Disease. Ph.D. Thesis, National University of Ireland, Royal College of Surgeons in Ireland, Dublin, Ireland, 2011; p. 290. [Google Scholar]
- Wu, S.Y.; Putral, L.N.; Liang, M.; Chang, H.I.; Davies, N.M.; McMillan, N.A. Development of a novel method for formulating stable siRNA-loaded lipid particles for in vivo use. Pharm. Res. 2009, 26, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.J.; Yue, S.T.; Cheung, C.Y.; Singer, V.L. RNA quantitation by fluorescence-based solution assay: RiboGreen reagent characterization. Anal. Biochem. 1998, 265, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gaul, R.; Ramsey, J.M.; Heise, A.; Cryan, S.A.; Greene, C.M. Nanotechnology approaches to pulmonary drug delivery: Targeted delivery of small molecule and gene-based therapeutics to the lung. In Design of Nanostructures for Versatile Therapeutic Applications; Grumezescu, A.M., Ed.; Elsevier, William Andrew Applied Science Publishers: Oxford, UK, 2018; pp. 221–253. ISBN 978-0-12-813667-6. [Google Scholar]
- Soifer, H.S.; Rossi, J.J.; Saetrom, P. MicroRNAs in disease and potential therapeutic applications. Mol. Ther. 2007, 15, 2070–2079. [Google Scholar] [CrossRef]
- Fernández Fernández, E.; Santos-Carballal, B.; de Santi, C.; Ramsey, J.M.; MacLoughlin, R.; Cryan, S.A.; Greene, C.M. Biopolymer-based nanoparticles for cystic fibrosis lung gene therapy studies. Materials (Basel) 2018, 11, 122. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Nihant, N.; Schugens, C.; Grandfils, C.; Jérôme, R.; Teyssié, P. Polylactide microparticles prepared by double emulsion/evaporation technique. I. Effect of primary emulsion stability. Pharm. Res. 1994, 11, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Nihant, N.; Schugens, C.; Grandfils, C.; Jerome, R.; Teyssie, P. Polylactide Microparticles Prepared by Double Emulsion-Evaporation: II. Effect of the Poly(Lactide-co-Glycolide) Composition on the Stability of the Primary and Secondary Emulsions. J. Colloid Interface Sci. 1995, 173, 55–65. [Google Scholar] [CrossRef]
- Yeo, Y.; Park, K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch. Pharm. Res. 2004, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Coombes, A.G.; Yeh, M.K.; Lavelle, E.C.; Davis, S.S. The control of protein release from poly(dl-lactide co-glycolide) microparticles by variation of the external aqueous phase surfactant in the water-in oil-in water method. J. Control. Release 1998, 52, 311–320. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (d,l-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- McKiernan, P.J.; Smith, S.G.; Durham, A.L.; Adcock, I.M.; McElvaney, N.G.; Greene, C.M. The estrogen-induced miR-19 downregulates Secretory Leucoprotease Inhibitor expression in monocytes. J. Innate Immunity 2018. under review. [Google Scholar]
- Hassan, T.; Smith, S.G.; Gaughan, K.; Oglesby, I.K.; O’Neill, S.; McElvaney, N.G.; Greene, C.M. Isolation and identification of cell-specific microRNAs targeting a messenger RNA using a biotinylated anti-sense oligonucleotide capture affinity technique. Nucleic Acids Res. 2013, 41, e71. [Google Scholar] [CrossRef] [PubMed]
| mRNA | Primers (5’-3’) | Annealing Temperature (°C) | Product Size (bp) |
|---|---|---|---|
| GAPDH | (F)- CATGAGAAGTATGACAACAGCCT (R)- AGTCCTTCCACGATACCAAAGT | 57 | 113 |
| SLPI | (F)- AATGCCTGGATCCTGTTGAC (R)- AAAGGACCTGGACCACACAG | 57 | 243 |
| Microparticle Formulation | Encapsulation Efficiency (%) |
|---|---|
| Unloaded MP | N/A |
| pre-miR-19b-MP | 37.6 ± 13.37 |
| scrambled mimic-MP | 24.53 ± 9.65 |
| Dy547-pre-miR-MP | 23.2 ± 8.96 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKiernan, P.J.; Lynch, P.; Ramsey, J.M.; Cryan, S.A.; Greene, C.M. Knockdown of Gene Expression in Macrophages by microRNA Mimic-Containing Poly (Lactic-co-glycolic Acid) Microparticles. Medicines 2018, 5, 133. https://doi.org/10.3390/medicines5040133
McKiernan PJ, Lynch P, Ramsey JM, Cryan SA, Greene CM. Knockdown of Gene Expression in Macrophages by microRNA Mimic-Containing Poly (Lactic-co-glycolic Acid) Microparticles. Medicines. 2018; 5(4):133. https://doi.org/10.3390/medicines5040133
Chicago/Turabian StyleMcKiernan, Paul J., Patrick Lynch, Joanne M. Ramsey, Sally Ann Cryan, and Catherine M. Greene. 2018. "Knockdown of Gene Expression in Macrophages by microRNA Mimic-Containing Poly (Lactic-co-glycolic Acid) Microparticles" Medicines 5, no. 4: 133. https://doi.org/10.3390/medicines5040133
APA StyleMcKiernan, P. J., Lynch, P., Ramsey, J. M., Cryan, S. A., & Greene, C. M. (2018). Knockdown of Gene Expression in Macrophages by microRNA Mimic-Containing Poly (Lactic-co-glycolic Acid) Microparticles. Medicines, 5(4), 133. https://doi.org/10.3390/medicines5040133






