Traditional Uses of Cannabinoids and New Perspectives in the Treatment of Multiple Sclerosis
Abstract
1. Introduction
2. Medicinal Cannabinoids
2.1. Dronabinol
2.2. Nabilone
2.3. Nabiximols
3. Endocannabinoid System Modulators
3.1. CB1R and CB2R Ligands
3.2. Inhibitors of Metabolic Enzymes of ECs
4. Conclusions
Funding
Conflicts of Interest
References
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Koch-Henriksen, N.; Sørensen, P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Thygesen, L.C.; Stenager, E.; Laursen, B.; Magyari, M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology 2018, 90, e1954–e1963. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.E.; Lee, H.; Pavri, F.R.; Zhang, M.A. Sex-Based Differences in Multiple Sclerosis (Part I): Biology of Disease Incidence. Curr. Top. Behav. Neurosci. 2015, 26, 29–56. [Google Scholar] [PubMed]
- Dunn, S.E.; Gunde, E.; Lee, H. Sex-Based Differences in Multiple Sclerosis (MS): Part II: Rising Incidence of Multiple Sclerosis in Women and the Vulnerability of Men to Progression of this Disease. Curr. Top. Behav. Neurosci. 2015, 26, 57–86. [Google Scholar] [PubMed]
- Hafler, D.A.; Compston, A.; Sawcer, S.; Lander, E.S.; Daly, M.J.; De Jager, P.L.; de Bakker, P.I.; Gabriel, S.B.; Mirel, D.B.; Ivinson, A.J.; et al. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 2007, 357, 851–862. [Google Scholar] [PubMed]
- Huynh, J.L.; Casaccia, P. Epigenetic mechanisms in multiple sclerosis: Implications for pathogenesis and treatment. Lancet Neurol. 2013, 12, 195–206. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Nave, K.A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Soelberg, P.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014, 15, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Stys, P.K.; Zamponi, G.W.; van Minnen, J.; Geurts, J.J. Will the real multiple sclerosis please stand up? Nat. Rev. Neurosci. 2012, 137, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Trapp, B.D. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog. Neurobiol. 2011, 93, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, M.; Magliozzi, R.; Ciccarelli, O.; Geurts, J.J.; Reynolds, R.; Martin, R. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 2015, 16, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- Peferoen, L.; Kipp, M.; van der Valk, P.; van Noort, J.M.; Amor, S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 2014, 141, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Cambron, M.; D’Haeseleer, M.; Laureys, G.; Clinckers, R.; Debruyne, J.; De Keyser, J. White-matter astrocytes, axonal energy metabolism, and axonal degeneration in multiple sclerosis. J. Cereb. Blood Flow Metab. 2012, 323, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 692, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Berkovich, R. Treatment of Acute Relapses in Multiple Sclerosis. Neurotherapeutics 2013, 10, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Berkovich, R.; Agius, M.A. Mechanisms of action of ACTH in the management of relapsing forms of multiple sclerosis. Ther. Adv. Neurol. Disord. 2014, 7, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Broadley, S.A.; Barnett, M.H.; Boggild, M.; Brew, B.J.; Butzkueven, H.; Heard, R.; Hodgkinson, S.; Kermode, A.G.; Lechner-Scott, J.; Macdonell, R.A.; et al. Therapeutic approaches to disease modifying therapy for multiple sclerosis in adults: an Australian and New Zealand perspective: part 3 treatment practicalities and recommendations. MS Neurology Group of the Australian and New Zealand Association of Neurologists. J. Clin. Neurosci. 2014, 21, 1857–1865. [Google Scholar] [PubMed]
- Broadley, S.A.; Barnett, M.H.; Boggild, M.; Brew, B.J.; Butzkueven, H.; Heard, R.; Hodgkinson, S.; Kermode, A.G.; Lechner-Scott, J.; Macdonell, R.A.; et al. Therapeutic approaches to disease modifying therapy for multiple sclerosis in adults: an Australian and New Zealand perspective: Part 1 historical and established therapies. MS Neurology Group of the Australian and New Zealand Association of Neurologists. J. Clin. Neurosci. 2014, 21, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Broadley, S.A.; Barnett, M.H.; Boggild, M.; Brew, B.J.; Butzkueven, H.; Heard, R.; Hodgkinson, S.; Kermode, A.G.; Lechner-Scott, J.; Macdonell, R.A.; et al. Therapeutic approaches to disease modifying therapy for multiple sclerosis in adults: an Australian and New Zealand perspective: part 2 new and emerging therapies and their efficacy. MS Neurology Group of the Australian and New Zealand Association of Neurologists. J. Clin. Neurosci. 2014, 21, 1847–1856. [Google Scholar] [PubMed]
- Comi, G.; Cook, S.D.; Giovannoni, G.; Rammohan, K.; Peter Rieckmann, P.; Soelberg Sørensen, P.; Vermersch, P.; Hamlett, A.C.; Viglietta, V.; Greenberg, S.J. MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study. J. Neurol. 2013, 260, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Current strategies in the treatment of multiple sclerosis. Am. J. Manag. Care. 2018.
- Soelberg Sørensen, P. New management algorithms in multiple sclerosis. Curr. Opin. Neurol. 2014, 27, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.D.; Peacock, W.J. The surgical treatment of spasticity. Muscle Nerve 2000, 23, 153–163. [Google Scholar] [CrossRef]
- Otero-Romero, S.; Sastre-Garriga, J.; Comi, G.; Hartung, H.P.; Soelberg Sørensen, P.; Thompson, A.J.; Vermersch, P.; Gold, R.; Montalban, X. Pharmacological management of spasticity in multiple sclerosis: Systematic review and consensus paper. Mult. Scler. 2016, 22, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R.S.; Friede, T.; Hollis, S.; Young, C.A. Anticholinergics for urinary symptoms in multiple sclerosis. Cochrane Database Syst. Rev. 2009, 1, CD004193. [Google Scholar]
- Gajewski, J.B.; Awad, S.A. Oxybutynin versus propantheline in patients with multiple sclerosis and detrusor hyperreflexia. J. Urol. 1986, 5, 966–968. [Google Scholar] [CrossRef]
- Beard, S.; Hunn, A.; Wight, J. Treatments for spasticity and pain in multiple sclerosis: A systematic review. Health Technol. Assess. 2003, 7, 1–111. [Google Scholar] [CrossRef]
- Shakespeare, D.T.; Boggild, M.; Young, C. Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst. Rev. 2003, 4, CD001332. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B. Spasticity treatment with botulinum toxins. J. Neural Transm. 2008, 115, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, S.; Kalyon, T.A.; Dursun, H.; Mohur, H.; Bilgic, F. Peripheral nerve block with phenol to treat spasticity in spinal cord injured patients. Paraplegia 1992, 30, 808–811. [Google Scholar] [PubMed]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Cannabinoid signaling and neuroinflammatory diseases: A melting pot for the regulation of brain immune responses. J. Neuroimmune Pharmacol. 2015, 10, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; van der Stelt, M.; Centonze, D.; Maccarrone, M. The endocannabinoid system and its therapeutic exploitation in multiple sclerosis: Clues for other neuroinflammatory diseases. Prog. Neurobiol. 2018, 160, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. Targeting the endocannabinoid system: To enhance or reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.P.; Carpenter, D.; Leslie, F.M.; Freund, T.F.; Katona, I.; Sensi, S.L.; Kathuria, S.; Piomelli, D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA. 2002, 99, 10819–10824. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Ware, M.A.; Yazer, E.; Murray, T.J.; Lynch, M.E. Patterns of cannabis use among patients with multiple sclerosis. Neurology 2004, 62, 2098–2100. [Google Scholar] [CrossRef] [PubMed]
- Fife, T.D.; Moawad, H.; Moschonas, C.; Shepard, K.; Hammond, N. Clinical perspectives on medical marijuana (cannabis) for neurologic disorders. Neurol. Clin. Pract. 2015, 5, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C. Shared Care Guideline: Nabilone in the Management of Chronic Neuropathic Pain that Has Failed to Respond to Other First and Second Line Treatments; NHS Lincolnshire in Association with United Lincolnshire Hospitals Trust: Lincolnshire, UK, 2013. [Google Scholar]
- Gloss, D.S.; Maa, E.H. Medical marijuana. Between a plant and a hard place. Neurol. Clin. Pract. 2015, 5, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Killestein, J.; Hoogervorst, E.L.; Reif, M.; Kalkers, N.F.; Van Loenen, A.C.; Staats, P.G.; Gorter, R.W.; Uitdehaag, B.M.; Polman, C.H. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002, 58, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.; Fox, P.; Sanders, H.; Wright, D.; Vickery, J.; Nunn, A.; Thompson, A. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): Multicentre randomized placebo-controlled trial. Lancet 2003, 362, 1517–1526. [Google Scholar] [CrossRef]
- Clifford, D.B. Tetrahydrocannabinol for tremor in multiple sclerosis. Ann. Neurol. 1983, 13, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Killestein, J.; Hoogervorst, E.L.; Reif, M.; Blauw, B.; Smits, M.; Uitdehaag, B.M.; Nagelkerken, L.; Polman, C.H. Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. J. Neuroimmunol. 2003, 137, 140–143. [Google Scholar] [CrossRef]
- Svendsen, K.B.; Jensen, T.S.; Bach, F.W. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomized double blind placebo controlled crossover trial. BMJ 2004, 329, 253. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.P.; Sanders, H.P.; Wright, D.E.; Vickery, P.J.; Ingram, W.M.; Reilly, S.M.; Nunn, A.J.; Teare, L.J.; Fox, P.J.; Thompson, A.J. Cannabinoids in multiple sclerosis (CAMS) study: Safety and efficacy data for 12 months follow up. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Petro, D.J.; Ellenberger, C. Treatment of human spasticity with delta-9-tetrahydrocannabinol. J. Clin. Pharmacol. 1981, 21, 413S–416S. [Google Scholar] [CrossRef] [PubMed]
- Ungerleider, J.T.; Andrysiak, T.; Fairbanks, L.; Ellison, G.W.; Myers, L.W. D-9-THC in the treatment of spasticity associated with multiple sclerosis. Adv. Alcohol Subst. Abuse 1987, 7, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.M.; Adekanmi, O.; Waterfield, M.R.; Wright, D.; Zajicek, J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: A multicenter, randomized placebo-controlled trial (CAMS-LUTS). Int. Urogynecol. J. Pelvic Floor Dysfunct. 2006, 17, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.; Ball, S.; Wright, D.; Vickery, J.; Nunn, A.; Miller, D.; Cano, M.G.; McManus, D.; Mallik, S.; Hobart, J. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): A randomized, placebo-controlled trial. CUPID investigator group. Lancet Neurol. 2013, 12, 857–865. [Google Scholar] [CrossRef]
- Martyn, C.N.; Illis, L.S.; Thom, J. Nabilone in the treatment of multiple sclerosis. Lancet 1995, 345, 579. [Google Scholar] [CrossRef]
- Wissel, J.; Haydn, T.; Muller, J.; Brenneis, C.; Berger, T.; Poewe, W.; Schelosky, L.D. Low dose treatment with the synthetic cannabinoid nabilone significantly reduces spasticity-related pain: A double-blind placebo-controlled cross-over trial. J. Neurol. 2006, 253, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, D.; Doupe, M.; Torabi, M.; Gomori, A.; Ethans, K.; Esfahani, F.; Galloway, K.; Namaka, M. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: A randomized controlled trial. Pain Med. 2015, 16, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.T.; Makela, P.; Robson, P.; House, H.; Bateman, C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult. Scler. 2004, 10, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.; Ehler, E.; Waberzinek, G.; Alsindi, Z.; Davies, P.; Powell, K.; Notcutt, W.; O’Leary, C.; Ratcliffe, S.; Nováková, I.; et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol. Res. 2010, 32, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Centonze, D.; Mori, F.; Kock, G.; Buttari, F.; Codecà, C.; Rossi, S.; Cencioni, M.T.; Bari, M.; Fiore, S.; Bernardi, G.; et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol. Sci. 2009, 30, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C.A. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005, 65, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.T.; Makela, P.; House, H.; Bateman, C.; Robson, P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult. Scler. 2006, 12, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Bettolo, C.M.; Onesti, E.; Frasca, V.; Iacovelli, E.; Gilio, F.; Giacomelli, E.; Gabriele, M.; Aragona, M.; Tomassini, V.; et al. Cannabinoid-induced effects on the nociceptive system: A neurophysiological study in patients with secondary progressive multiple sclerosis. Eur. J. Pain 2009, 13, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Rog, D.J.; Nurmikko, T.J.; Young, C.A. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: An uncontrolled, open label, 2-year extension trial. Clin. Ther. 2007, 29, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Langford, R.M.; Mares, J.; Novotna, A.; Vachova, M.; Novakova, I.; Notcutt, W.; Ratcliffe, S. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J. Neurol. 2013, 260, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.; Davies, P.; Mutiboko, I.K.; Ratcliffe, S. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur. J. Neurol. 2007, 14, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Leocani, L.; Nuara, A.; Houdayer, E.; Del Carro, U.; Straffi, L.; Martinelli, V.; Rossi, P.; I Schiavetti, I.; Amadio, S.; Sormani, M.P.; Comi, G. Effect of THC-CBD oromucosal spray (Sativex) on measures of spasticity in multiple sclerosis: A double-blind, placebo-controlled, crossover study. Mult. Scler. 2014, 20, 498. [Google Scholar]
- Kavia, R.B.; De Ridder, D.; Constantinescu, C.S.; Stott, C.G.; Fowler, C.J. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult. Scler. 2010, 16, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Ben Amar, M. Cannabinoids in medicine: a review of their therapeutic potential. J. Ethnopharmacol. 2006, 105, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Zhornitsky, S.; Potvin, S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals 2012, 5, 529–552. [Google Scholar] [CrossRef] [PubMed]
- Koppel, B.S.; Brust, J.C.M.; Fife, T.; Bronstein, J.; Youssof, S.; Gronseth, G.; Gloss, D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014, 82, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Karst, M.; Wippermann, S.; Ahrens, J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drug 2010, 70, 2409–2438. [Google Scholar] [CrossRef] [PubMed]
- Jawahar, R.; Oh, U.; Yang, S.; Lapane, K.L. A systematic review of pharmacological pain management in multiple sclerosis. Drugs 2013, 73, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Yap, L.; Young, C.A. Treatment for ataxia in multiple sclerosis. Cochrane Database Syst. Rev. 2007, 1, CD005029. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, K.; Barbano, R.; Mink, J. Cannabinoids in the treatment of movement disorders: A systematic review of case series and clinical trials. Basal Ganglia 2016, 6, 173–181. [Google Scholar] [CrossRef]
- Wang, T.; Collet, J.P.; Shapiro, S.; Ware, M.A. Adverse effects of medical cannabinoids: A systematic review. Can. Med. Assoc. J. 2008, 178, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Germanos, R.; Weier, M.; Pollard, J.; Degenhardt, L.; Hall, W.; Buckley, N.; Farrell, M. The Use of Cannabis and Cannabinoids in Treating Symptoms of Multiple Sclerosis: A Systematic Review of Reviews. Curr. Neurol. Neurosci. Rep. 2018, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Bramanti, P.; Mazzon, E. Sativex in the management of multiple sclerosis-related spasticity: An overview of the last decade of clinical evaluation. Mult. Scler. Relat. Disord. 2017, 17, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Rowland, M. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurol. 2009, 9, ArtID 59. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Delta-9-Tetrahydrocannabinol/Cannabidiol Oromucosal Spray (Sativex®): A Review in Multiple Sclerosis-Related Spasticity. Drugs 2017, 77, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 2006, 66, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Novotna, A.; Mares, J.; Ratcliffe, S.; Novakova, I.; Vachova, M.; Zapletalova, O.; Gasperini, C.; Pozzilli, C.; Cefaro, L.; Comi, G.; et al. A randomized, doubleblind, placebo-controlled, parallel-group, enriched-design study of nabiximols (Sativex), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur. J. Neurol. 2011, 18, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, J.; Romero, J.; Ramos, J.A. Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease and others. Handb. Exp. Pharmacol. 2015, 231, 233–259. [Google Scholar] [PubMed]
- Galve-Roperh, I.; Chiurchiù, V.; Díaz-Alonso, J.; Bari, M.; Guzmán, M.; Maccarrone, M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res. 2013, 52, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Klegeris, A.; Bissonnette, C.J.; McGeer, P.L. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br. J. Pharmacol. 2003, 139, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Atwood, B.K.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Battistini, L.; Maccarrone, M. Endocannabinoid signaling in innate and adaptive immunity. Immunology 2015, 144, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Docagne, F.; Muñetón, V.; Clemente, D.; Ali, C.; Loría, F.; Correa, F.; Hernangómez, M.; Mestre, L.; Vivien, D.; Guaza, C. Excitotoxicity in a chronic model of multiple sclerosis: neuroprotective effects of cannabinoids through CB1 and CB2 receptor activation. Mol Cell Neurosci. 2007, 34, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Musella, A.; Sepman, H.; Mandolesi, G.; Gentile, A.; Fresegna, D.; Haji, N.; Conrad, A.; Lutz, B.; Maccarrone, M.; Centonze, D. Pre- and postsynaptic type-1 cannabinoid receptors control the alterations of glutamate transmission in experimental autoimmune encephalomyelitis. Neuropharm 2014, 79, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, J.; García, C.; Sagredo, O.; Gómez-Ruiz, M.; de Lago, E. The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Ther. Targets 2010, 14, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Carrillo-Salinas, F.J.; Feliú, A.; Mestre, L.; Guaza, C. Microglia activation states and cannabinoid system: Therapeutic implications. Pharmacol. Ther. 2016, 166, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Di Marzo, V. Cannabinoid receptors and endocannabinoids: Role in neuroinflammatory and neurodegenerative disorders. CNS & Neuro. Disorders Drug Targets 2010, 9, 564–573. [Google Scholar]
- McCarthy, D.P.; Richards, M.H.; Miller, S.D. Mouse models of multiple sclerosis: Experimental autoimmune encephalomyelitis and Theiler’s virus-induced demyelinating disease. Methods Mol Biol. 2012, 900, 381–401. [Google Scholar] [PubMed]
- Heremans, H.; Dillen, C.; Groenen, M.; Martens, E.; Billiau, A. Chronic relapsing experimental autoimmune encephalomyelitis (CREAE) in mice: enhancement by monoclonal antibodies against interferon-gamma. Eur. J. Immunol. 1996, 26, 2393–2398. [Google Scholar] [CrossRef] [PubMed]
- Clatch, R.J.; Miller, S.D.; Metzner, R.; Dal Canto, M.C.; Lipton, H.L. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler’s murine encephalomyelitis virus (TMEV). Virology 1990, 176, 244–254. [Google Scholar] [CrossRef]
- Lassmann, H.; Bradl, M. Multiple sclerosis: Experimental models and reality. Acta Neuropathol. 2017, 133, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Lyman, W.D.; Sonett, J.R.; Brosnan, C.F.; Elkin, R.; Bornstein, M.B. Δ9-Tetrahydrocannabinol: A novel treatment for experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1989, 23, 73–81. [Google Scholar] [CrossRef]
- Wirguin, I.; Mechoulam, R.; Breuer, A.; Schezen, E.; Weidenfeld, J.; Brenner, T. Suppression of experimental autoimmune encephalomyelitis by cannabinoids. Immunopharmacology 1994, 28, 209–214. [Google Scholar] [CrossRef]
- Baker, D.; Pryce, G.; Croxford, J.L.; Brown, P.; Pertwee, R.G.; Huffman, J.W.; Layward, L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 2000, 404, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Faizi, M.; Talebi, F.; Noorbakhsh, F.; Kahrizi, F.; Naderi, N. Interaction between the protective effects of cannabidiol and palmitoylethanolamide in experimental model of multiple sclerosis in C57BL/6 mice. Neuroscience 2015, 290, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.K.; Downer, E.J. Toll-like receptor signalling as a cannabinoid target in Multiple Sclerosis. Neuropharmacology 2017, 113, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Martin, A.; Vela, J.M.; Molina-Holgado, E.; Borrell, J.; Guaza, C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J. Neurosci. 2003, 23, 2511–2516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Martin, B.R.; Adler, M.W.; Razdan, R.J.; Kong, W.; Ganea, D.; Tuma, R.F. Modulation of Cannabinoid Receptor Activation as a Neuroprotective Strategy for EAE and Stroke. J. Neuroimmune Pharmacol. 2009, 4, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Li, H.; Tuma, R.F.; Ganea, D. Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cell Immunol. 2014, 287, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Taylor, B.K. Activation of cannabinoid CB2 receptors reduces hyperalgesia in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Neurosci. Lett. 2015, 595, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, F.F.; Qian, H.Y.; Chen, L.L.; Pu, J.B.; Xie, X.; Chen, J.Z. Development of Quinoline-2,4(1H,3H)-diones as Potent and Selective Ligands of the Cannabinoid Type 2 Receptor. J. Med. Chem. 2015, 58, 5751–5769. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Gomez-Canas, M.; Navarro, G.; Hurst, D.P.; Carrillo-Salinas, F.J.; Lagartera, L.; Pazos, R.; Goya, P.; Reggio, P.H.; Guaza, C.; et al. Chromenopyrazole, a versatile cannabinoid scaffold with in vivo activity in a model of multiple sclerosis. J. Med. Chem. 2016, 59, 6753–6771. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Carrillo-Salinas, F.J.; Mestre, L.; Feliu, A.; Guaza, C. Viral Models of Multiple Sclerosis: Neurodegeneration and Demyelination in Mice Infected with Theiler’s Virus. Prog. Neurobiol. 2013, 101–102, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Duan, Y.H.; Ji, Y.Y.; Wang, Z.L.; Wu, Y.R.; Gunosewoyo, H.; Xie, X.Y.; Chen, J.Z.; Yang, F.; Li, J.; et al. Amidoalkylindoles as Potent and Selective Cannabinoid Type 2 Receptor Agonists with in Vivo Efficacy in a Mouse Model of Multiple Sclerosis. J. Med. Chem. 2017, 60, 7067–7083. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, C.; Carrillo-Salinas, F.; Palomares, B.; Mecha, M.; Jiménez-Jiménez, C.; Mestre, L.; Feliú, A.; Bellido, M.L.; Fiebich, B.L.; Appendino, G.; et al. Hypoxia mimetic activity of VCE-004.8, a cannabidiol quinone derivative: implications for multiple sclerosis therapy. J. Neuroinflammation. 2018, 15, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Kamal, M.A.; Ojha, S. Polypharmacological properties and therapeutic potential of -caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef] [PubMed]
- Alberti, T.B.; Barbosa, W.L.; Vieira, J.L.; Raposo, N.R.; Dutra, R.C. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Romero, J.P.; Tolón, R.M.; Clemente, D.; Docagne, F.; Hillard, C.J.; Guaza, C.; Romero, J. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J. Neurosci. 2007, 27, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Cencioni, M.T.; Bisicchia, E.; De Bardi, M.; Gasperini, C.; Borsellino, G.; Centonze, D.; Battistini, L.; Maccarrone, M. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann. Neurol. 2013, 73, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Mestre, L.; Correa, F.; Arévalo-Martín, A.; Molina-Holgado, E.; Valenti, M.; Ortar, G.; Di Marzo, V.; Guaza, C. Pharmacological modulation of the endocannabinoid system in a viral model of multiple sclerosis. J. Neurochem. 2005, 92, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gutiérrez, S.; Molina-Holgado, E.; Arévalo-Martín, A.; Correa, F.; Viso, A.; López-Rodríguez, M.L.; Di Marzo, V.; Guaza, C. Activation of the endocannabinoid system as therapeutic approach in a murine model of multiple sclerosis. FASEB J. 2005, 19, 1338–1340. [Google Scholar] [CrossRef] [PubMed]
- Webb, M.; Luo, L.; Ma, J.Y.; Tham, C.S. Genetic deletion of Fatty Acid Amide Hydrolase results in improved long-term outcome in chronic autoimmune encephalitis. Neurosci. Lett. 2008, 439, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Lourbopoulos, A.; Grigoriadis, N.; Lagoudaki, R.; Touloumi, O.; Polyzoidou, E.; Mavromatis, I.; Tascos, N.; Breuer, A.; Ovadia, H.; Karussis, D.; et al. Administration of 2-arachidonoylglycerol ameliorates both acute and chronic experimental autoimmune encephalomyelitis. Brain Res. 2011, 1390, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Schlosburg, J.E.; Blankman, J.L.; Long, J.Z.; Nomura, D.K.; Pan, B.; Kinsey, S.G.; Nguyen, P.T.; Ramesh, D.; Booker, L.; Burston, J.J.; et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 2010, 13, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Chico, A.; Canedo, M.; Manterola, A.; Victoria Sánchez-Gómez, M.; Pérez-Samartín, A.; Rodríguez-Puertas, R.; Matute, C.; Mato, S. Blockade of monoacylglycerol lipase inhibits oligodendrocyte excitotoxicity and prevents demyelination in vivo. Glia 2015, 63, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Brindisi, M.; Maramai, S.; Gemma, S.; Brogi, S.; Grillo, A.; Di Cesare Mannelli, L.; Gabellieri, E.; Lamponi, S.; Saponara, S.; Gorelli, B.; et al. Development and pharmacological characterization of selective blockers of 2-arachidonoyl glycerol degradation with efficacy in rodent models of multiple sclerosis and pain. J. Med. Chem. 2016, 59, 2612–2632. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Torres, G.; Cipriano, M.; Hedén, E.; Björklund, E.; Canales, Á.; Zian, D.; Feliú, A.; Mecha, M.; Guaza, C.; Fowler, C.J.; et al. A reversible and selective inhibitor of monoacylglycerol lipase ameliorates multiple sclerosis. Angew. Chem. Int. Ed. Engl. 2014, 53, 13765–13770. [Google Scholar] [CrossRef] [PubMed]
- Pryce, G.; Cabranes, A.; Fernández-Ruiz, J.; Bisogno, T.; Di Marzo, V.; Long, J.Z.; Cravatt, B.F.; Giovannoni, G.; Baker, D. Control of experimental spasticity by targeting the degradation of endocannabinoids using selective fatty acid amide hydrolase inhibitors. Mult. Scler. 2013, 19, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; Cascio, M.G.; Pryce, G.; Kulasegram, S.; Beletskaya, I.; De Petrocellis, L.; Saha, B.; Mahadevan, A.; Visintin, C.; Wiley, J.L.; et al. New potent and selective inhibitors of anandamide reuptake with antispastic activity in a mouse model of multiple sclerosis. Br. J. Pharmacol. 2006, 147, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chicca, A.; Arena, C.; Bertini, S.; Gado, F.; Ciaglia, E.; Abate, M.; Digiacomo, M.; Lapillo, M.; Poli, G.; Bifulco, M.; et al. Polypharmacological profile of 1,2-dihydro-2-oxo-pyridine-3-carboxamides in the endocannabinoid system. Eur. J. Med. Chem. 2018, 154, 155–171. [Google Scholar] [CrossRef] [PubMed]
| Dronabinol (Synthetic∆9-THC) | Nabilone (Synthetic Analogue of ∆9-THC) | Nabiximols (∆9-THC: Cannabidiol~1:1 (w/w)) | |
|---|---|---|---|
| Structure(s) | 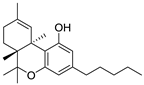 | 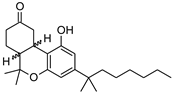 | 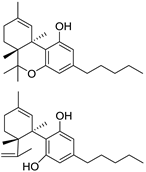 |
| Formulation | Soft gelatin capsules (2.5, 5, 10 mg) | Capsules (0.25, 0.5, 1 mg) | Oro-mucosal spray (27 mg of ∆9-THC and 25 mg of cannabidiol/1.0 mL) |
| Disability and disease progression | No evident changes | No studies | No evident changes |
| Pain | Positive effects | Positive effects | Mixed findings (mostly positive effects) |
| Spasticity | Mixed findings | Positive effects | Mixed findings (mostly positive effects) |
| Bladder function | Mixed findings | Positive effects | Mixed findings |
| Ataxia and tremor | No evident changes | No studies | No evident changes |
| Sleep | Mixed findings (mostly positive effects) | No studies | Positive effects |
| Quality of life | Mixed findings | Mixed findings (moslty positive effects) | Mixed findings |
| Adverse effects | Mild to moderate. Principally dizziness, euphoria, dry mouth, fatigue and drowsiness. | Moderate sedation, dizziness and moderate weakness in the legs. | Mild to moderate. Principally drowsiness, dizziness, headache, fatigue, impaired balance and disturbance in attention. |
| Number of studies | 10 | 3 | 11 |
| Number of reviews | 11 | 5 | 12 |
| Studies (references) | [45,46,47,48,49,50,51,52,53,54] | [55,56,57] | [58,59,60,61,62,63,64,65,66,67,68] |
| Reviews (references) | [33,69,70,71,72,73,74,75,76,77,78] | [69,70,72,73,78] | [69,70,72,73,74,75,76,77,78,79,80,81] |
| Structure and Name | Origin and Activity | Animal Model and Effects |
|---|---|---|
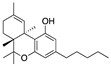 ∆9-THC | Phytocannabinoid CB1R partial agonist | In EAE rats: amelioration of EAE progression [100]. In CREAE mice: amelioration of tremor and spasticity [102]. |
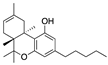 ∆8-THC | Phytocannabinoid CB1R ligand | In EAE rats: amelioration of the clinical manifestations of EAE [101]. |
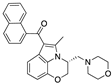 WIN-55212 | Synthetic cannabinoid CB2R agonist | In CREAE mice: amelioration of tremor and spasticity [102]. In TMEV-infected mice: improvement of motor function on established neurological symptomatology; stimulation of the remyelination; reduction of microglial activation and of the number of CD4+ infiltrated T cells [105]. |
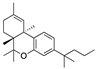 JWH-133 | Synthetic cannabinoid CB2R agonist | In CREAE mice: amelioration of tremor and spasticity [102]. Intrathecal administration in EAE mice: reduction, dose-dependently, of both mechanical and cold hypersensitivity without any signs of ataxia or sedation [108]. |
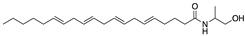 Methanadamide | Endocannabinoid CB1R/CB2R agonist | In CREAE mice: amelioration of tremor and spasticity [102]. |
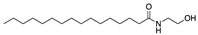 Palmitoylethanolamide (PEA) | Endocannabinoid CB1R/CB2R agonist | In CREAE mice: transient inhibition of spasticity [102]. |
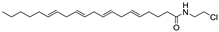 Arachidonyl-2-chloroethylamide (ACEA) | Synthetic cannabinoid CB1R agonist | In TMEV-infected mice: improvement of motor function on established neurological symptomatology; stimulation of the remyelination; reduction of microglial activation and of the number of CD4+ infiltrated T cells [105]. |
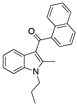 JWH-015 | Synthetic cannabinoid CB2R agonist | In TMEV-infected mice: improvement of motor function on established neurological symptomatology; stimulation of the remyelination; reduction of microglial activation and of the number of CD4+ infiltrated T cells [105]. |
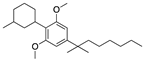 O-1966 | Synthetic cannabinoid CB2R agonist | In the chronic EAE model: improved motor function; reduction of rolling and adhesion of endogenous leukocytes to pial microvasculature [106]. |
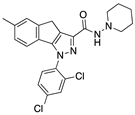 Gp-1a | Synthetic cannabinoid CB2R agonist | In EAE mice: reduction of clinical scores; amelioration of the recovery [107]. |
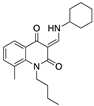 compound 21 | Synthetic cannabinoid CB2R agonist | In EAE mice: reduction of the clinical scores and symptoms; decrease of leukocyte infiltration in the spinal cord and demyelination in white matter [109]. |
 PM-226 | Synthetic cannabinoid CB2R agonist | In TMEV-infected mice: dampening of neuroinflammation; reduction of microglial activation [110,111]. |
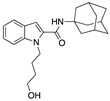 compound 57 | Synthetic cannabinoid CB2R agonist | In EAE mice: alleviation of the clinical symptoms of EAE; protection of the murine central nervous system from immune damage; reduction of leukocyte infiltration and demyelination [112]. |
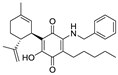 VCE-004.8 | Synthetic cannabinoid CB2R agonist | In EAE and TMEV mice: immunomodulatory activity; inhibition of inflammatory chemokines, chemokines receptors, and cytokines; inhibition of the expression of adhesion molecules (VCAM and ICAM-1); induction of the expression of the hypoxia-inducible factor (HIF) [113]. |
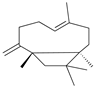 β-caryophyllene (BCP) | Phytocannabinoid CB2R agonist | In EAE mice: reduction of mechanical hyperalgesia, inflammation and pain [115]. |
| Structure and Name | Activity | Animal Model Effects |
|---|---|---|
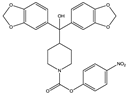 JZL 184 | Irreversible MAGL inhibitor | In EAE mice: reduction of myelin loss; reduction of inflammation on spinal cord white matter [123] |
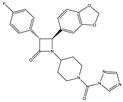 Compound 4a | Irreversible MAGL inhibitor | In EAE mice: analgesic effect [124] |
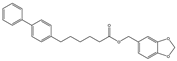 Compound 21 | Reversible MAGL inhibitor | In EAE mice: decrease of tissue damage in the spinal cords [125] |
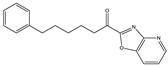 CAY 10402 | Irreversible FAAH inhibitor | In Biozzi ABH mice: inhibition of spasticity [126] |
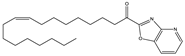 CAY 10400 | Irreversible FAAH inhibitor | In Biozzi ABH mice: inhibition of spasticity [126] |
 URB597 | Irreversible FAAH inhibitor | In Biozzi ABH mice: inhibition of spasticity [126] |
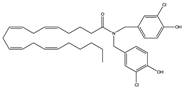 O-3246 | AEA reuptake inhibitor | In CREAE mice: inhibition of spasticity [127] |
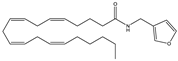 UCM707 | AEA reuptake inhibitor | In TMEV-IDD mice: improvement of motor function; reduction of microglial activation; decrease of cellular infiltrates in the spinal cord [119] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gado, F.; Digiacomo, M.; Macchia, M.; Bertini, S.; Manera, C. Traditional Uses of Cannabinoids and New Perspectives in the Treatment of Multiple Sclerosis. Medicines 2018, 5, 91. https://doi.org/10.3390/medicines5030091
Gado F, Digiacomo M, Macchia M, Bertini S, Manera C. Traditional Uses of Cannabinoids and New Perspectives in the Treatment of Multiple Sclerosis. Medicines. 2018; 5(3):91. https://doi.org/10.3390/medicines5030091
Chicago/Turabian StyleGado, Francesca, Maria Digiacomo, Marco Macchia, Simone Bertini, and Clementina Manera. 2018. "Traditional Uses of Cannabinoids and New Perspectives in the Treatment of Multiple Sclerosis" Medicines 5, no. 3: 91. https://doi.org/10.3390/medicines5030091
APA StyleGado, F., Digiacomo, M., Macchia, M., Bertini, S., & Manera, C. (2018). Traditional Uses of Cannabinoids and New Perspectives in the Treatment of Multiple Sclerosis. Medicines, 5(3), 91. https://doi.org/10.3390/medicines5030091





