Bioactive Compounds and Extracts from Traditional Herbs and Their Potential Anti-Inflammatory Health Effects
Abstract
1. Introduction
2. Natural Extracts and Compounds with Anti-Inflammatory Properties
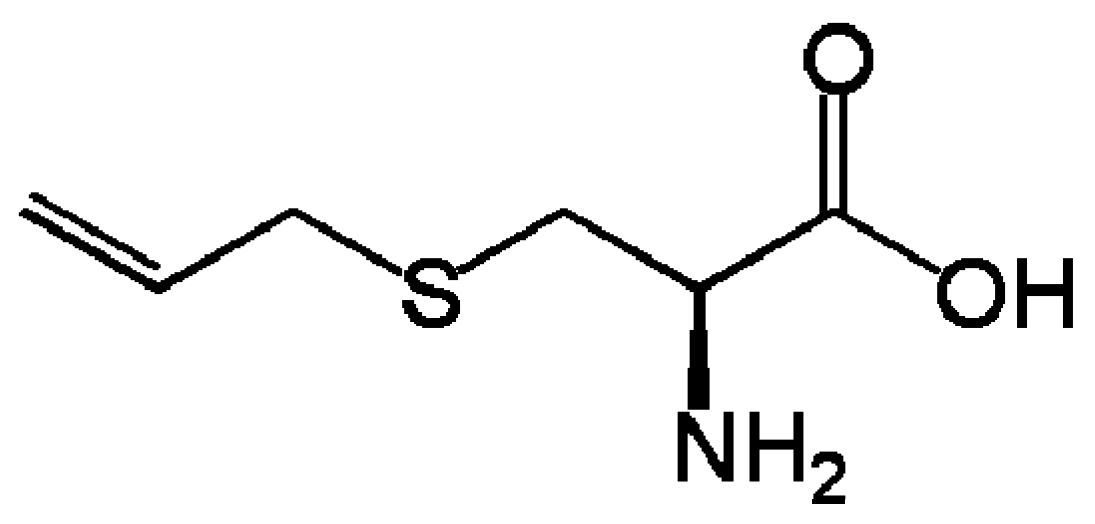
2.1. Allium nigrum (Black Garlic)
2.2. Uncaria tomentosa (“Cat’s Claw”)
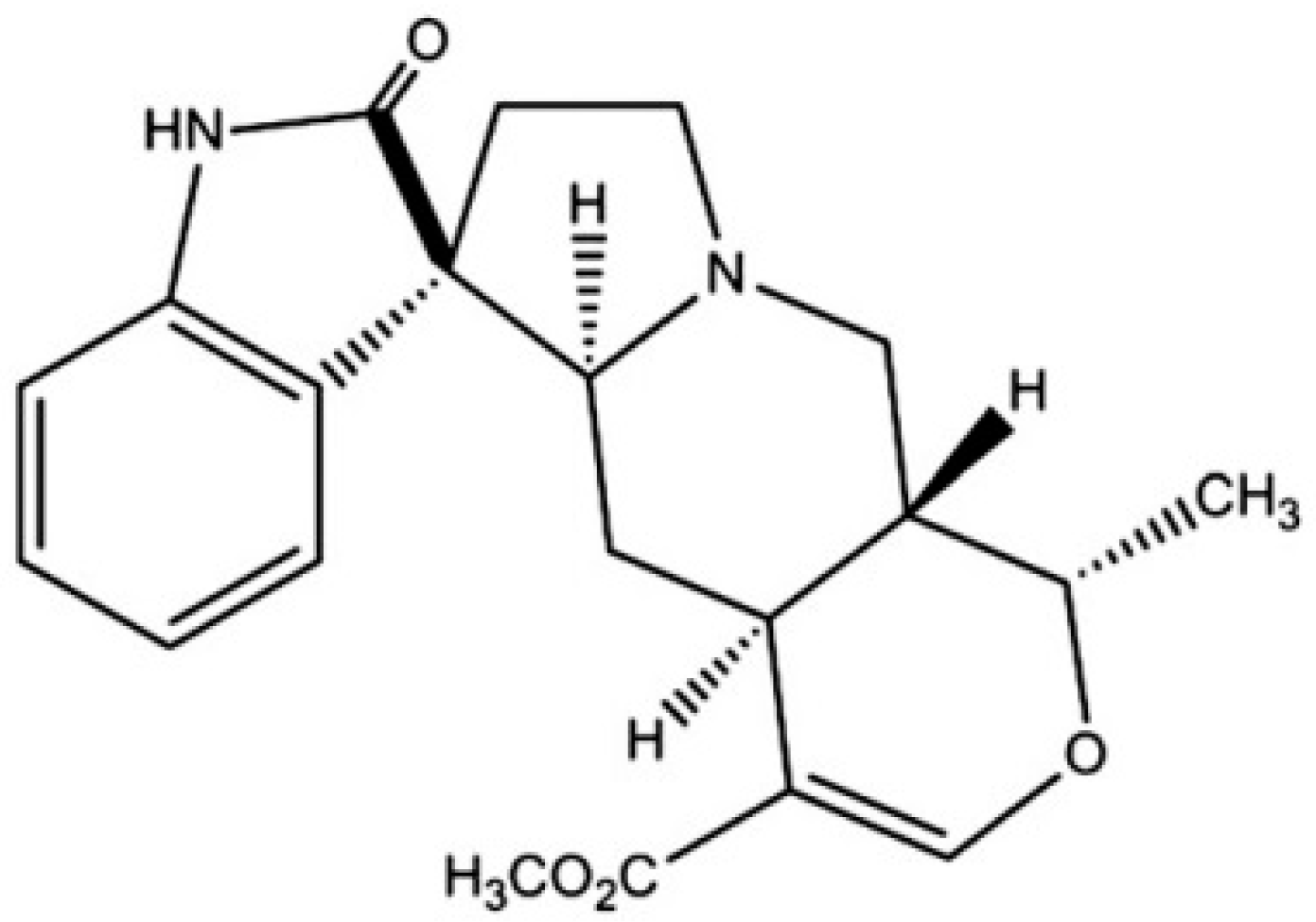
2.3. Harpagophytum procumbens (Devil’s Claw)
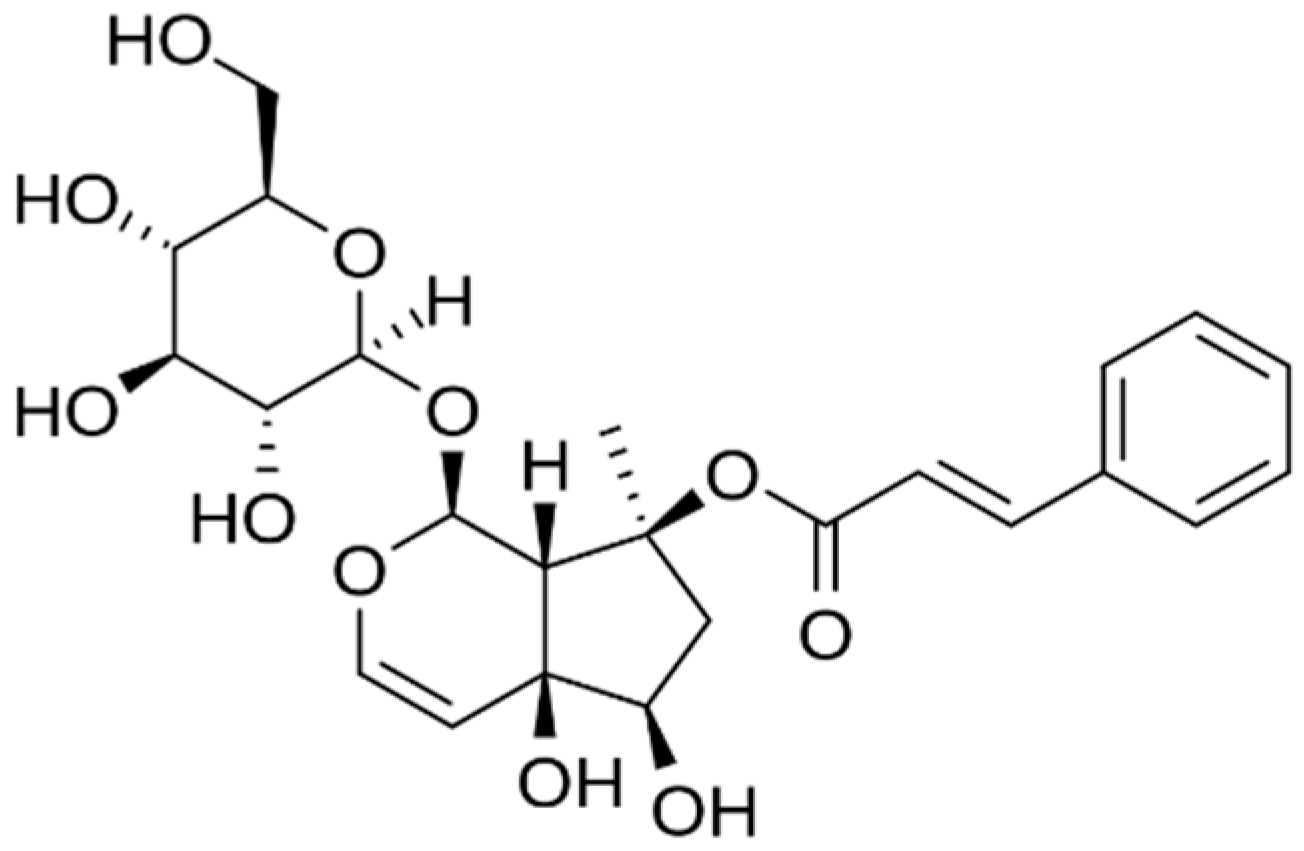
2.4. Myrciaria dubia (Camu Camu)
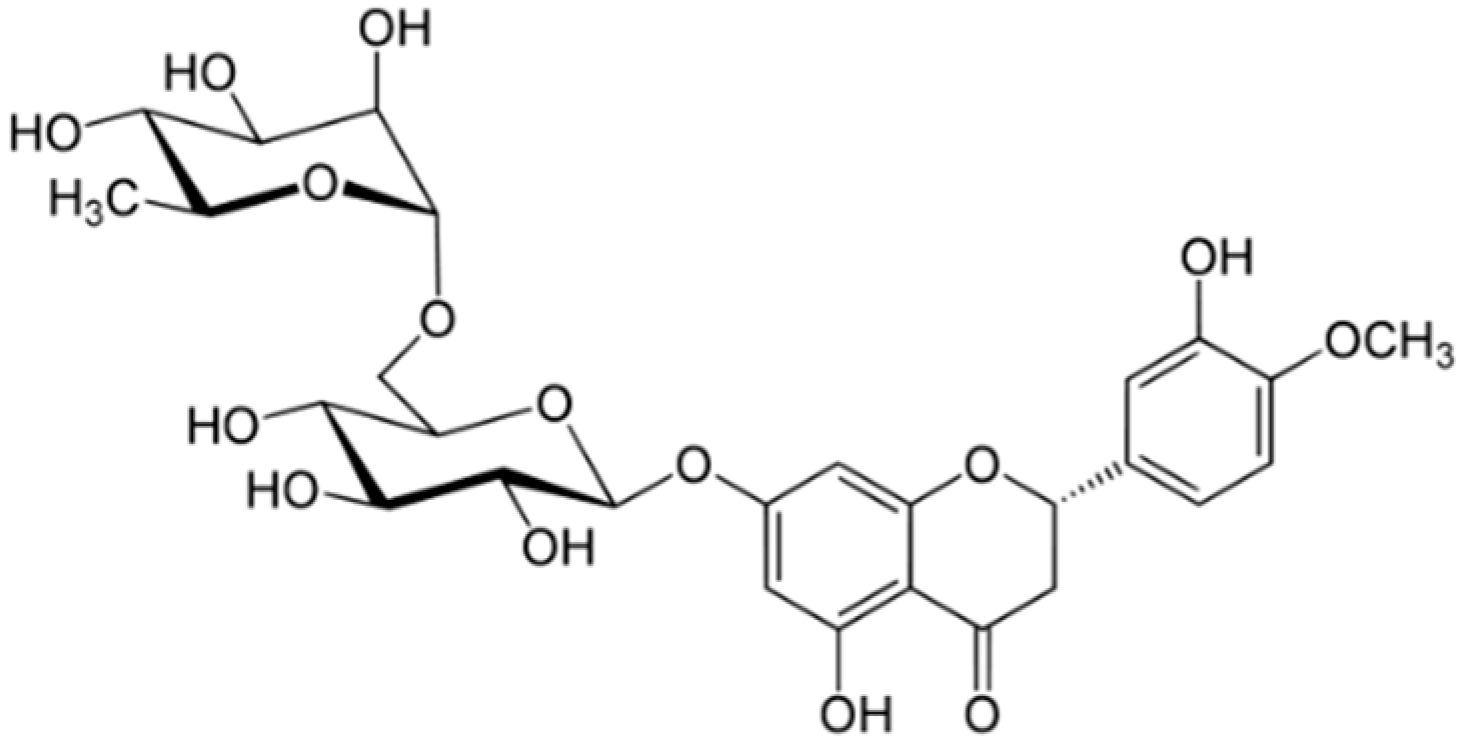
2.5. Citrus Fruits Rich in Hesperidin
2.6. Ribes nigrum (Blackcurrant)
3. Comparing Emergent Extracts with Classics
4. Conclusions
Funding
Conflicts of Interest
References
- Wang, M.; Honn, K.; Nie, D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007, 26, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.; Vinoy, S.; Russell, W.; Baka, A.; Roche, H.; Tuohy, K.; Teeling, J.; Blaak, E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Block, E.; Naganathan, S.; Putman, D.; Zhao, S. Allium chemistry: HPLC analysis of thiosulfinates from onion, garlic, wild garlic (ramsoms), leek, scallion, shallot, elephant (great-headed) garlic, chive, and Chinese chive. Uniquely high allyl to methyl ratios in some garlic samples. J. Agric. Food Chem. 1992, 40, 2418–2430. [Google Scholar] [CrossRef]
- Horita, C.; Farías-Campomanes, A.; Barbosa, T.; Esmerino, E.; da Cruz, A.; Bolini, H.; Meireles, M.; Pollonio, M. The antimicrobial, antioxidant and sensory properties of garlic and its derivatives in Brazilian low-sodium frankfurters along shelf-life. Food Res. Int. 2016, 84, 1–8. [Google Scholar] [CrossRef]
- Kimura, S.; Tung, Y.; Pan, M.; Su, N.; Lai, Y.; Cheng, K. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An analysis of the changes on intermediate products during the thermal processing of black garlic. Food Chem. 2018, 239, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Casas, L.; Lage-Yusty, M.; López-Hernández, J. Changes in the Aromatic Profile, Sugars, and Bioactive Compounds When Purple Garlic Is Transformed into Black Garlic. J. Agric. Food Chem. 2017, 65, 10804–10811. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kang, D. Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules 2017, 22, 919. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yoo, Y.; Kim, H.; Shin, S.; Sohn, E.; Min, A.; Sung, N.; Kim, M. Aged Black Garlic Exerts Anti-Inflammatory Effects by Decreasing NO and Proinflammatory Cytokine Production with Less Cytoxicity in LPS-Stimulated RAW 264.7 Macrophages and LPS-Induced Septicemia Mice. J. Med. Food 2014, 17, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kang, M.; Hong, S.; Choi, Y.; Shin, J. Anti-inflammatory Effects of Functionally Active Compounds Isolated from Aged Black Garlic. Phytother. Res. 2016, 31, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Cha, H.; Lee, Y. Physicochemical and Antioxidant Properties of Black Garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef] [PubMed]
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, phytochemistry and pharmacology of (Rubiaceae). Phytochemistry 2005, 66, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Navarro Hoyos, M.; Sánchez-Patán, F.; Murillo Masis, R.; Martín-Álvarez, P.; Zamora Ramirez, W.; Monagas, M.; Bartolomé, B. Phenolic Assesment of Uncaria tomentosa L. (Cat’s Claw): Leaves, Stem, Bark and Wood Extracts. Molecules 2015, 20, 22703–22717. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Duran, R.; González-Aspajo, G.; Ruiz-Martel, C.; Bourdy, G.; Doroteo-Ortega, V.; Alban-Castillo, J.; Robert, G.; Auberger, P.; Deharo, E. Anti-inflammatory activity of Mitraphylline isolated from Uncaria tomentosa bark. J. Ethnopharmacol. 2012, 143, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, R.; Filip, B.; Wietrzyk, J.; Kuraś, M.; Gulewicz, K. Anticancer activity of the Uncaria tomentosa (Willd.) DC. preparations with different oxindole alkaloid composition. Phytomedicine 2010, 17, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Bors, M.; Michałowicz, J.; Pilarski, R.; Sicińska, P.; Gulewicz, K.; Bukowska, B. Studies of biological properties of Uncaria tomentosa extracts on human blood mononuclear cells. J. Ethnopharmacol. 2012, 142, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.; Dinis, T.; Batista, M. Antioxidant properties of proanthocyanidins of bark decoction: A mechanism for anti-inflammatory activity. Phytochemistry 2005, 66, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Allen-Hall, L.; Cano, P.; Arnason, J.; Rojas, R.; Lock, O.; Lafrenie, R. Treatment of THP-1 cells with Uncaria tomentosa extracts differentially regulates the expression if IL-1β and TNF-α. J. Ethnopharmacol. 2007, 109, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Domingues, A.; Sartori, A.; Valente, L.; Golim, M.; Siani, A.; Viero, R. Uncaria tomentosa Aqueous-ethanol Extract Triggers an Immunomodulation toward a Th2 Cytokine Profile. Phytother. Res. 2011, 25, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Gurrola-Díaz, C.; García-López, P.; Gulewicz, K.; Pilarski, R.; Dihlmann, S. Inhibitory mechanisms of two Uncaria tomentosa extracts affecting the Wnt-signaling pathway. Phytomedicine 2011, 18, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Mncwangi, N.; Chen, W.; Vermaak, I.; Viljoen, A.; Gericke, N. Devil’s Claw—A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharmacol. 2012, 143, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Manon, L.; Béatrice, B.; Thierry, O.; Jocelyne, P.; Fathi, M.; Evelyne, O.; Alain, B. Antimutagenic potential of harpagoside and Harpagophytum procumbens against 1-nitropyrene. Pharmacogn. Mag. 2015, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Fiebich, B.; Muñoz, E.; Rose, T.; Weiss, G.; McGregor, G. Molecular Targets of the Anti-inflammatory Harpagophytum procumbens (Devil’s claw): Inhibition of TNFα and COX-2 Gene Expression by Preventing Activation of AP-1. Phytother. Res. 2011, 26, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Hostanska, K.; Melzer, J.; Rostock, M.; Suter, A.; Saller, R. Alteration of anti-inflammatory activity of Harpagophytum procumbens(devil’s claw) extract after external metabolic activation with S9 mix. J. Pharm. Pharmacol. 2014, 66, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, L.; Peroza, L.; Boligon, A.; Athayde, M.; Alves, S.; Fachinetto, R.; Wagner, C. Harpagophytum procumbens Prevents Oxidative Stress and Loss of Cell Viability In Vitro. Neurochem. Res. 2013, 38, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Alipieva, K.; Orhan, I. Cholinesterases Inhibitory and Antioxidant Activities of Harpagophytum procumbens from In Vitro Systems. Phytother. Res. 2011, 26, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Oh, S.; Eun, J.; Ahmed, M. Nutritional compositions and health promoting phytochemicals of camu-camu (Myrciaria dubia) fruit: A review. Food Res. Int. 2011, 44, 1728–1732. [Google Scholar] [CrossRef]
- Inoue, T.; Komoda, H.; Uchida, T.; Node, K. Tropical fruit camu-camu (Myrciaria dubia) has anti-oxidative and anti-inflammatory properties. J. Cardiol. 2008, 52, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Fracassetti, D.; Costa, C.; Moulay, L.; Tomás-Barberán, F. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chem. 2013, 139, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Kaneshima, T.; Myoda, T.; Nakata, M.; Fujimori, T.; Toeda, K.; Nishizawa, M. Antioxidant activity of C-Glycosidic ellagitannins from the seeds and peel of camu-camu (Myrciaria dubia). LWT Food Sci. Technol. 2016, 69, 76–81. [Google Scholar] [CrossRef]
- Da Silva, F.; Arruda, A.; Ledel, A.; Dauth, C.; Romão, N.; Viana, R.; de Barros Falcão Ferraz, A.; Picada, J.; Pereira, P. Antigenotoxic effect of acute, subacute and chronic treatments with Amazonian camu–camu (Myrciaria dubia) juice on mice blood cells. Food Chem. Toxicol. 2012, 50, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Garg, S.; Zaneveld, L.; Singla, A. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.; Rajavel, T.; Nabavi, S.; Setzer, W.; Ahmadi, A.; Mansouri, K.; Nabavi, S. Hesperidin: A promising anticancer agent from nature. Ind. Crops Prod. 2015, 76, 582–589. [Google Scholar] [CrossRef]
- Saiprasad, G.; Chitra, P.; Manikandan, R.; Sudhandiran, G. Hesperidin alleviates oxidative stress and downregulates the expressions of proliferative and inflammatory markers in azoxymethane-induced experimental colon carcinogenesis in mice. Inflamm. Res. 2013, 62, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Parmar, H. Evaluation of antioxidative and anti-inflammatory potential of hesperidin and naringin on the rat air pouch model of inflammation. Inflamm. Res. 2010, 60, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Vaibhav, K.; Ahmed, M.; Khan, A.; Tabassum, R.; Islam, F.; Safhi, M.; Islam, F. Effect of hesperidin on neurobehavioral, neuroinflammation, oxidative stress and lipid alteration in intracerebroventricular streptozotocin induced cognitive impairment in mice. J. Neurol. Sci. 2015, 348, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Rotimi, S.; Bankole, G.; Adelani, I.; Rotimi, O. Hesperidin prevents lipopolysaccharide-induced endotoxicity in rats. Immunopharmacol. Immunotoxicol. 2016, 38, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chu, H.; Lin, Y.; Han, F.; Li, Y.; Wang, A.; Wang, F.; Chen, D.; Wang, J. Hesperidin alleviates rat postoperative ileus through anti-inflammation and stimulation of Ca2+-dependent myosin phosphorylation. Acta Pharmacol. Sin. 2016, 37, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Kumar, A.; Sajad, M.; Zargan, J.; Ansari, M.; Ahmad, S.; Katiyar, C.; Khan, H. Hesperidin inhibits collagen-induced arthritis possibly through suppression of free radical load and reduction in neutrophil activation and infiltration. Rheumatol. Int. 2013, 33, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Heybar, H.; Jalali, M.; Ahmadi Engali, K.; Helli, B.; Shirbeigi, E. Hesperidin Supplementation Modulates Inflammatory Responses Following Myocardial Infarction. J. Am. Coll. Nutr. 2015, 34, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Tits, M. Prodelphinidins from Ribes nigrum. Phytochemistry 1992, 31, 971–973. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Polyphenolic constituents of blackcurrant seed residue. Food Chem. 2003, 80, 71–76. [Google Scholar] [CrossRef]
- Olejnik, A.; Kowalska, K.; Olkowicz, M.; Juzwa, W.; Dembczyński, R.; Schmidt, M. A Gastrointestinally Digested Ribes nigrum L. Fruit Extract Inhibits Inflammatory Response in a Co-culture Model of Intestinal Caco-2 Cells and RAW264.7 Macrophages. J. Agric. Food Chem. 2016, 64, 7710–7721. [Google Scholar] [CrossRef] [PubMed]
- Huebbe, P.; Giller, K.; de Pascual-Teresa, S.; Arkenau, A.; Adolphi, B.; Portius, S.; Arkenau, C.; Rimbach, G. Effects of blackcurrant-based juice on atherosclerosis-related biomarkers in cultured macrophages and in human subjects after consumption of a high-energy meal. Br. J. Nutr. 2011, 108, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, B.; Yang, Y.; Pham, T.; Park, Y.; Manatou, J.; Koo, S.; Chun, O.; Lee, J. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.; McGhie, T.; Cooney, J.; Jensen, D.; Gould, E.; Lyall, K.; Hurst, R. Blackcurrant proanthocyanidins augment IFN-γ-induced suppression of IL-4 stimulated CCL26 secretion in alveolar epithelial cells. Mol. Nutr. Food Res. 2010, 54, S159–S170. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, J.; Tanabe, S.; Bergeron, C.; Gafner, S.; Grenier, D. Anthocyanin-Rich Black Currant Extract and Cyanidin-3-O-Glucoside Have Cytoprotective and Anti-Inflammatory Properties. J. Med. Food 2012, 15, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Garbacki, N.; Tits, M.; Angenot, L.; Damas, J. Inhibitory effects of proanthocyanidins from Ribes nigrum leaves on carrageenin acute inflammatory reactions induced in rats. BMC Pharmacol. 2004, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Mbimba, T.; Thoppil, R.; Háznagy-Radnai, E.; Sipos, P.; Darvesh, A.; Folkesson, H.; Hohmann, J. Anthocyanin-rich black currant (Ribes nigrum L.) extract affords chemoprevention against diethylnitrosamine-induced hepatocellular carcinogenesis in rats. J. Nutr. Biochem. 2011, 22, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Ahmad, M.; Aslam, H.; Tahir, M.; Aftab, M.; Bibi, N.; Ahmad, S. Green tea phytocompounds as anticancer: A review. Asian Pac. J. Trop. Dis. 2016, 6, 330–336. [Google Scholar] [CrossRef]
- Chen, L.; Mo, H.; Zhao, L.; Gao, W.; Wang, S.; Cromie, M.; Lu, C.; Wang, J.; Shen, C. Therapeutic properties of green tea against environmental insults. J. Nutr. Biochem. 2017, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Baeza, G.; Amigo-Benavent, M.; Sarriá, B.; Goya, L.; Mateos, R.; Bravo, L. Green coffee hydroxycinnamic acids but not caffeine protect human HepG2 cells against oxidative stress. Food Res. Int. 2014, 62, 1038–1046. [Google Scholar] [CrossRef]
- Shin, H.; Satsu, H.; Bae, M.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Strat, K.; Rowley, T.; Smithson, A.; Tessem, J.; Hulver, M.; Liu, D.; Davy, B.; Davy, K.; Neilson, A. Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. J. Nutr. Biochem. 2016, 35, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Giribabu, N.; Karim, K.; Kilari, E.; Kassim, N.; Salleh, N. Anti-Inflammatory, Antiapoptotic and Proproliferative Effects of Vitis vinifera Seed Ethanolic Extract in the Liver of Streptozotocin-Nicotinamide-Induced Type 2 Diabetes in Male Rats. Can. J. Diabetes 2018, 42, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Handoussa, H.; Hanafi, R.; Eddiasty, I.; El-Gendy, M.; El Khatib, A.; Linscheid, M.; Mahran, L.; Ayoub, N. Anti-inflammatory and cytotoxic activities of dietary phenolics isolated from Corchorus olitorius and Vitis vinifera. J. Funct. Foods 2013, 5, 1204–1216. [Google Scholar] [CrossRef]
- Pawlus, A.; Cantos-Villar, E.; Richard, T.; Bisson, J.; Poupard, P.; Papastamoulis, Y.; Monti, J.; Teissedre, P.; Waffo-Téguo, P.; Mérillon, J. Chemical dereplication of wine stilbenoids using high performance liquid chromatography–nuclear magnetic resonance spectroscopy. J. Chromatogr. A 2013, 1289, 19–26. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, A.; Ros, G.; Nieto, G. Bioactive Compounds and Extracts from Traditional Herbs and Their Potential Anti-Inflammatory Health Effects. Medicines 2018, 5, 76. https://doi.org/10.3390/medicines5030076
Serrano A, Ros G, Nieto G. Bioactive Compounds and Extracts from Traditional Herbs and Their Potential Anti-Inflammatory Health Effects. Medicines. 2018; 5(3):76. https://doi.org/10.3390/medicines5030076
Chicago/Turabian StyleSerrano, Antonio, Gaspar Ros, and Gema Nieto. 2018. "Bioactive Compounds and Extracts from Traditional Herbs and Their Potential Anti-Inflammatory Health Effects" Medicines 5, no. 3: 76. https://doi.org/10.3390/medicines5030076
APA StyleSerrano, A., Ros, G., & Nieto, G. (2018). Bioactive Compounds and Extracts from Traditional Herbs and Their Potential Anti-Inflammatory Health Effects. Medicines, 5(3), 76. https://doi.org/10.3390/medicines5030076






