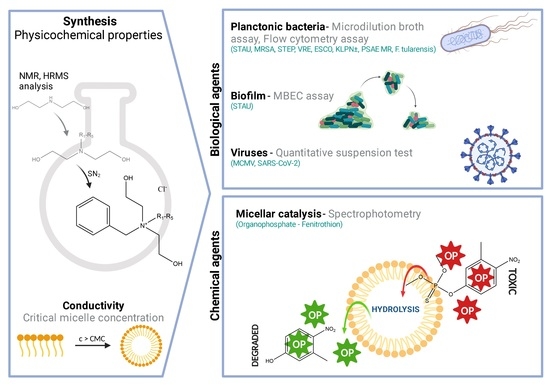
Synthesis and Decontamination Effect on Chemical and Biological Agents of Benzoxonium-Like Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Analysis
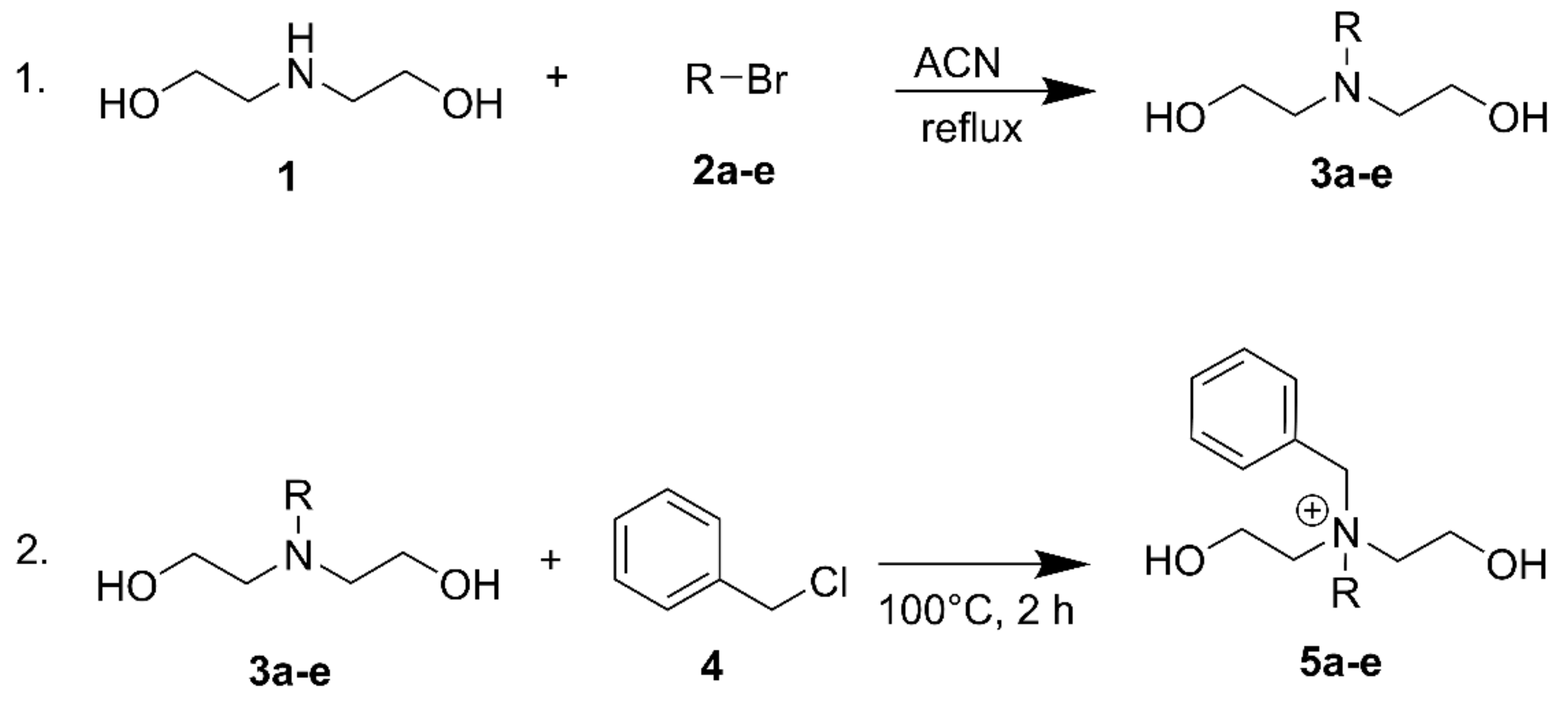
2.1.1. General Procedure for Synthesis of N,N-bis(2-hydroxyethyl)-N-alkylamines (3a-e)
2.1.2. General Procedures for Synthesis of N-benzyl-N,N-bis(2-hydroxyethyl)alkane-1-aminium Chloride (5a-e)
2.1.3. NMR and HRMS Analysis
2.2. Conductivity
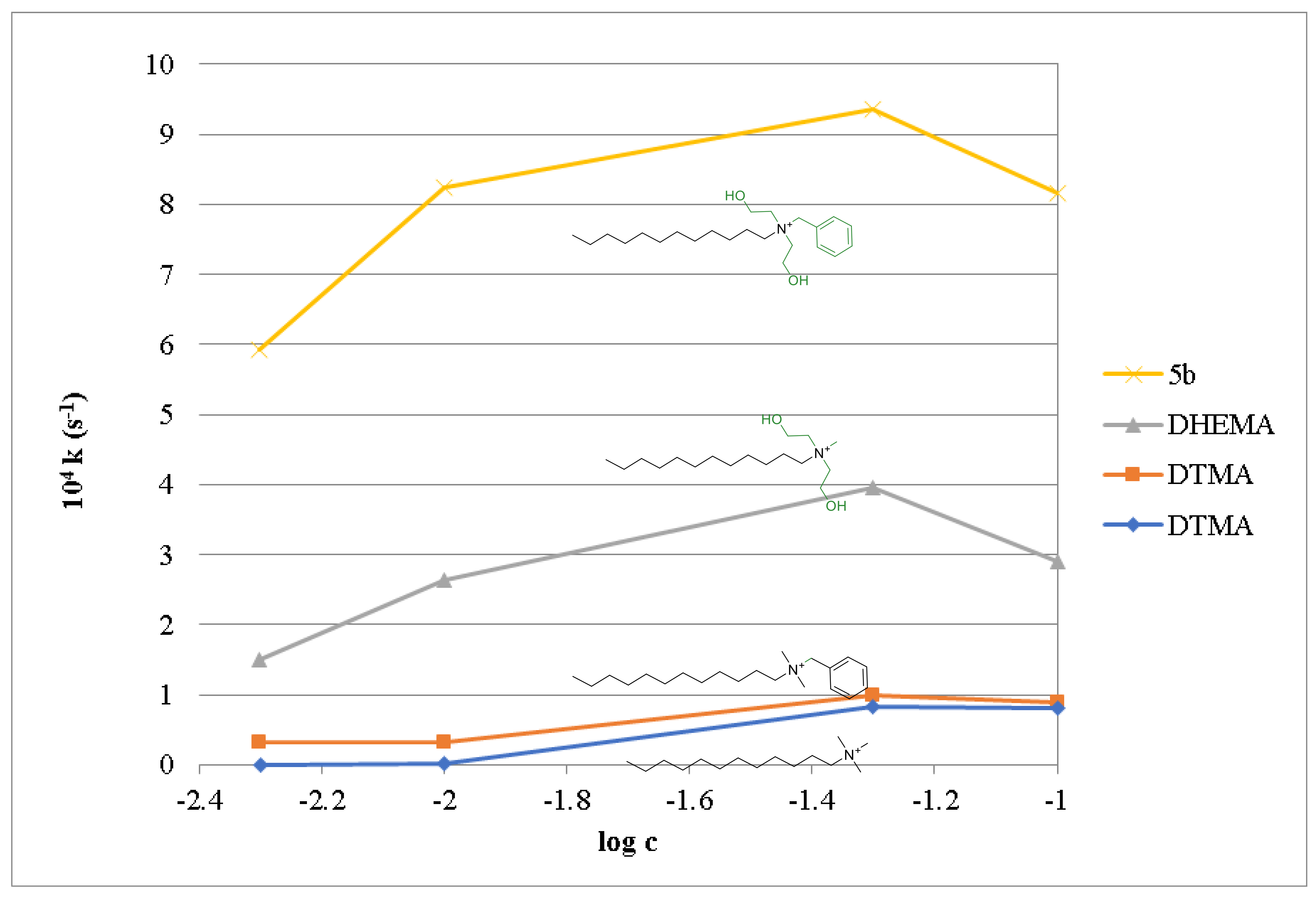
2.3. Micellar Catalysis
2.4. Bacterial Strains
2.5. Biofilm Cultivation
2.6. Antimicrobial Activity Evaluation
2.6.1. Broth Microdilution Method
2.6.2. Flow Cytometry Assay
2.6.3. MBEC Assay
2.7. Antiviral Activity Evaluation
2.7.1. Viruses and Cell Cultures
2.7.2. Virucidal Activity Testing
2.8. In Vitro Cytotoxicity and Selectivity Index
3. Results and Discussion
3.1. Synthesis
3.2. Conductometry
3.3. Micellar Catalysis
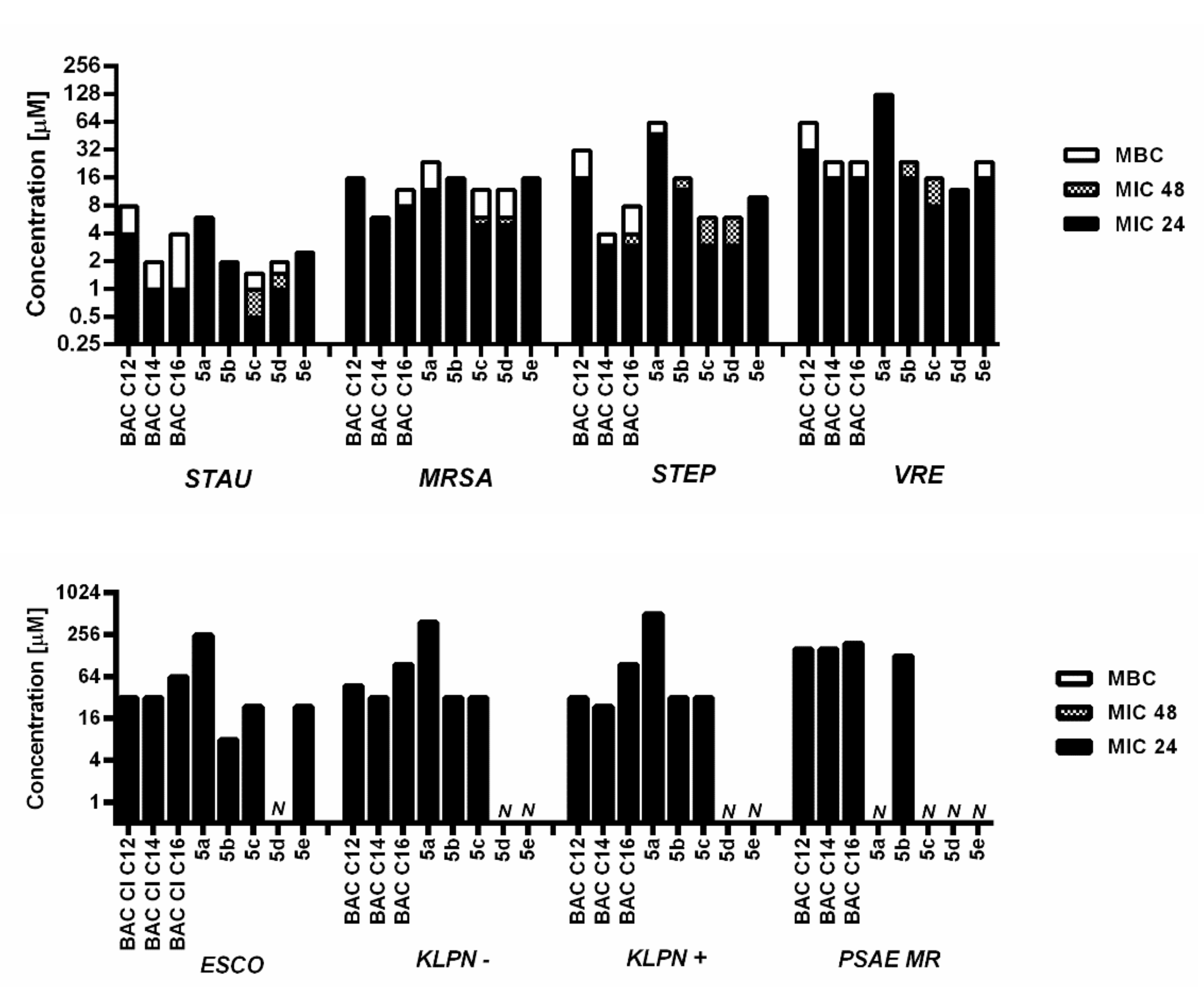
3.4. Antimicrobial Activity
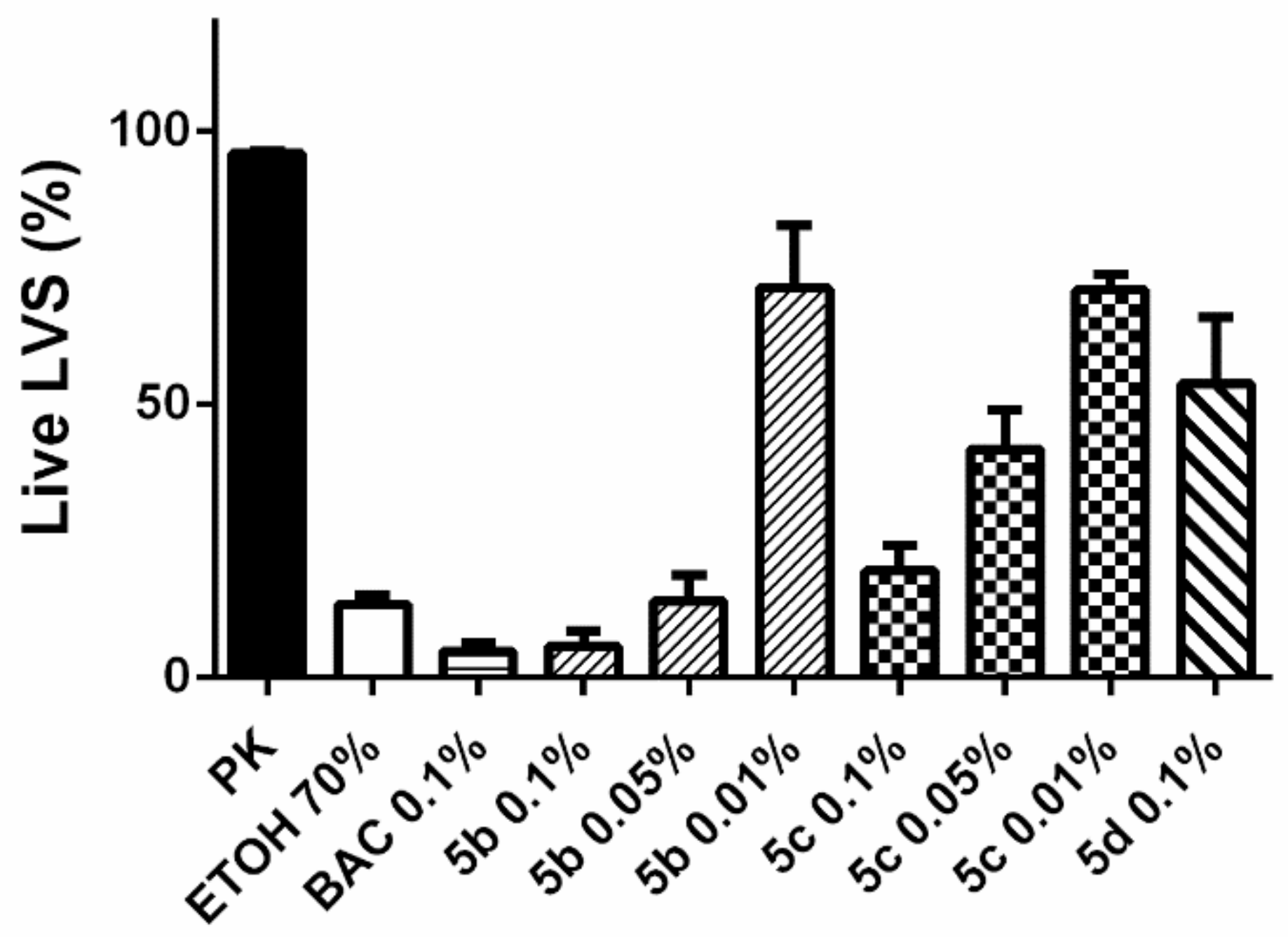
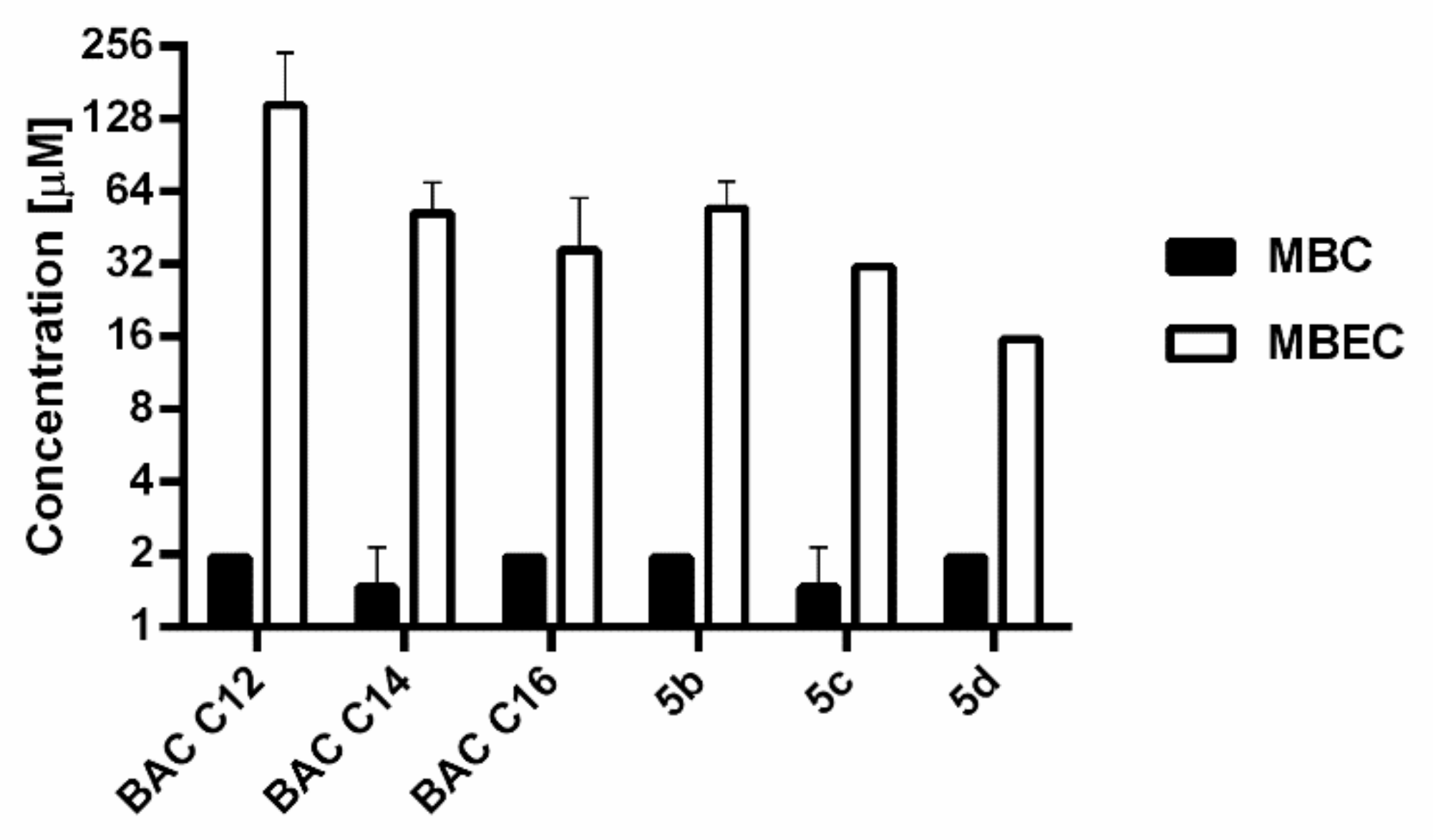
3.5. Antibiofilm Activity and Comparison in Effectiveness against S. aureus in Planktonic and Biofilm Form
3.6. Virucidal Activity against Enveloped Viruses
3.7. Cell Viability Evaluation and Selectivity Index
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EP3061864A1—Textiles Having Antimicrobial Properties—Google Patents. Available online: https://patents.google.com/patent/EP3061864A1/en (accessed on 18 September 2020).
- Weibel, M.A.; Cortat, M.; Lebek, G.; LeCotonnec, J.Y.; Kitler, M.E.; Barcherini, G. An Approach of the in Vivo Antibacterial Activity of Benzoxonium Chloride and Comparison with Other Buccopharyngeal Disinfectants. Arzneimittelforschung 1987, 37, 467–471. [Google Scholar] [PubMed]
- Benzoxonium Chloride. Available online: https://www.drugs.com/international/benzoxonium-chloride.html (accessed on 18 September 2020).
- Ponzielli, G.; Taidelli-Palmizi, G. Dodecyl-di-beta-oxyethyl-benzylammonium chloride in the topical therapy of burns. Clin. Ter. 1979, 90, 251–259. [Google Scholar] [PubMed]
- Firestone, A.R.; Schmid, R.; Mühlemann, H.R. Topical Effects of a Quaternary Ammonium Compound on Caries Incidence and Bacterial Agglomerate Formation in the Rat. Caries Res. 1981, 15, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Daie Parizi, M.H.; Karvar, M.; Sharifi, I.; Bahrampour, A.; Heshmat Khah, A.; Rahnama, Z.; Baziar, Z.; Amiri, R. The Topical Treatment of Anthroponotic Cutaneous Leishmaniasis with the Tincture of Thioxolone plus Benzoxonium Chloride (Thio-Ben) along with Cryotherapy: A Single-Blind Randomized Clinical Trial. Dermatol. Ther. 2015, 28, 140–146. [Google Scholar] [CrossRef]
- Hakimi Parizi, M.; Pardakhty, A.; sharifi, I.; Farajzadeh, S.; Daie Parizi, M.H.; Sharifi, H.; Keyhani, A.R.; Mostafavi, M.; Bamorovat, M.; Ghaffari, D. Antileishmanial Activity and Immune Modulatory Effects of Benzoxonium Chloride and Its Entrapped Forms in Niosome on Leishmania Tropica. J. Parasit. Dis. 2019, 43, 406–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kis, G.L. Antimicrobial Compositions. CA2025728C, 26 February 2002. Available online: https://worldwide.espacenet.com/patent/search/family/004256126/publication/CA2025728C?q=CA2025728C (accessed on 18 September 2020).
- Szekacs, A. Mechanism-Related Teratogenic, Hormone Modulant and Other Toxicological Effects of Veterinary and Agricultural Surfactants. Insights Vet. Sci. 2017, 1, 24–31. [Google Scholar] [CrossRef]
- Labranche, L.-P.; Dumont, S.N.; Levesque, S.; Carrier, A. Rapid Determination of Total Benzalkonium Chloride Content in Ophthalmic Formulation. J. Pharm. Biomed. Anal. 2007, 43, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Horn, G. Method and Composition Which Reduces Stimulation of Muscles Which Dilate the Eye. U.S. Patent 20060211753A1, 21 September 2006. [Google Scholar]
- Ioannou, C.J.; Hanlon, G.W.; Denyer, S.P. Action of Disinfectant Quaternary Ammonium Compounds against Staphylococcus Aureus. Antimicrob. Agents Chemother. 2007, 51, 296–306. [Google Scholar] [CrossRef] [Green Version]
- Minbiole, K.P.C.; Jennings, M.C.; Ator, L.E.; Black, J.W.; Grenier, M.C.; LaDow, J.E.; Caran, K.L.; Seifert, K.; Wuest, W.M. From Antimicrobial Activity to Mechanism of Resistance: The Multifaceted Role of Simple Quaternary Ammonium Compounds in Bacterial Eradication. Tetrahedron 2016, 72, 3559–3566. [Google Scholar] [CrossRef] [Green Version]
- Dolezal, R.; Soukup, O.; Malinak, D.; Savedra, R.M.L.; Marek, J.; Dolezalova, M.; Pasdiorova, M.; Salajkova, S.; Korabecny, J.; Honegr, J.; et al. Towards Understanding the Mechanism of Action of Antibacterial N-Alkyl-3-Hydroxypyridinium Salts: Biological Activities, Molecular Modeling and QSAR Studies. Eur. J. Med. Chem. 2016, 121, 699–711. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef]
- Tiwari, S.; Ghosh, K.; Marek, J.; Kuca, K. Cationic Micellar-Catalyzed Hydrolysis of Pesticide Fenitrothion Using α-Nucleophiles. Lett. Drug Des. Discov. 2010, 7, 194–199. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, B.; Yadav, T.; Sinha, S.; Sahu, A.K.; Karpichev, Y.; Gathergood, N.; Marek, J.; Kuca, K.; Ghosh, K.K. Degradation of Organophosphate Pesticides Using Pyridinium Based Functional Surfactants. ACS Sustain. Chem. Eng. 2016, 4, 6962–6973. [Google Scholar] [CrossRef]
- Singh, N.; Karpichev, Y.; Gupta, B.; Satnami, M.L.; Marek, J.; Kuca, K.; Ghosh, K.K. Physicochemical Properties and Supernucleophilicity of Oxime-Functionalized Surfactants: Hydrolytic Catalysts toward Dephosphorylation of Di- and Triphosphate Esters. J. Phys. Chem. B 2013, 117, 3806–3817. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ghosh, K.K.; Marek, J.; Kuca, K. Hydrolysis of Carboxylate and Phosphate Esters Using Monopyridinium Oximes in Cationic Micellar Media. Int. J. Chem. Kinet. 2011, 43, 569–578. [Google Scholar] [CrossRef]
- Banjare, M.K.; Kurrey, R.; Yadav, T.; Sinha, S.; Satnami, M.L.; Ghosh, K.K. A Comparative Study on the Effect of Imidazolium-Based Ionic Liquid on Self-Aggregation of Cationic, Anionic and Nonionic Surfactants Studied by Surface Tension, Conductivity, Fluorescence and FTIR Spectroscopy. J. Mol. Liq. 2017, 241, 622–632. [Google Scholar] [CrossRef]
- Domagk, G. A Method for Disinfection and Preservation. DE680599C, 1 September 1939. [Google Scholar]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 2020, 37. Available online: https://jcm.asm.org/content/37/6/1771/figures-only (accessed on 8 April 2021).
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guggenheim, E.A. XLVI. On the Determination of the Velocity Constant of a Unimolecular Reaction. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1926, 2, 538–543. [Google Scholar] [CrossRef]
- Zajicek, M.; Radl, Z. Katalyticky Vliv Kationaktivniho Tenzidu Na Hydrolyzu Fosfonatu. In Sbornik Vyzkumneho Ustavu 070; Ministry of Defense: Brno, Czech Republic, 1979; pp. 115–129. (In Czech) [Google Scholar]
- Cabal, J.; Kuča, K.; Míčová, J. Kinetics of Decompositition of Organophosphate Fenitrothion by Decontaminating Foam-Making Blends. J. Appl. Biomed. 2007, 5, 167–170. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute (Ed.) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A10; Approved Standard, 10th ed.; Documents/Clinical and Laboratory Standards Institute; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2015. [Google Scholar]
- Marek, J.; Malinak, D.; Dolezal, R.; Soukup, O.; Pasdiorova, M.; Dolezal, M.; Kuca, K. Synthesis and Disinfection Effect of the Pyridine-4-Aldoxime Based Salts. Molecules 2015, 20, 3681–3696. [Google Scholar] [CrossRef] [PubMed]
- Malinak, D.; Dolezal, R.; Marek, J.; Salajkova, S.; Soukup, O.; Vejsova, M.; Korabecny, J.; Honegr, J.; Penhaker, M.; Musilek, K.; et al. 6-Hydroxyquinolinium Salts Differing in the Length of Alkyl Side-Chain: Synthesis and Antimicrobial Activity. Bioorg. Med. Chem. Lett. 2014, 24, 5238–5241. [Google Scholar] [CrossRef]
- Soukup, O.; Benkova, M.; Dolezal, R.; Sleha, R.; Malinak, D.; Salajkova, S.; Markova, A.; Hympanova, M.; Prchal, L.; Ryskova, L.; et al. The Wide-Spectrum Antimicrobial Effect of Novel N-Alkyl Monoquaternary Ammonium Salts and Their Mixtures; the QSAR Study against Bacteria. Eur. J. Med. Chem. 2020, 206, 112584. [Google Scholar] [CrossRef]
- Spearman, C. The Method of ‘Right and Wrong Cases’ (‘Constant Stimuli’) Without Gauss’s Formulae. Br. J. Psychol. 1908, 2, 227–242. [Google Scholar] [CrossRef]
- BS EN 14476:2013+A2:2019—Chemical Disinfectants and Antiseptics. Quantitative Suspension Test for the Evaluation of Virucidal Activity in the Medical Area. Test Method and Requirements (Phase 2/Step 1). 31 August 2019. Available online: https://shop.bsigroup.com/ProductDetail?pid=000000000030401479 (accessed on 8 April 2021).
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture: Methods and Protocols; Cree, I.A., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar] [CrossRef]
- Limanov, V.E.; Épshtein, A.E.; Skvortsova, E.K.; Aref’eva, L.I.; Gleiberman, S.E.; Volkova, A.P. Synthesis and Antibacterial Action of Surface-Active Quaternary Ammonium Salts Containing Hydroxyethyl Radicals. Pharm. Chem. J. 1976, 10, 55–58. [Google Scholar] [CrossRef]
- Chernyavskaya, M.A.; Stefanovich, V.V.; Sergeeva, I.A.; Belova, A.S. Antimicrobial and Surface-Active Properties of Cationic Surfactants Based on Chloroalkanes and Alkylbenzenes. Pharm. Chem. J. 1984, 18, 784–787. [Google Scholar] [CrossRef]
- Stefanović, G.; Ćirić, J. Synthese und bacterizide Wirkung einiger quaternärer, höherer Alkyl- und Alkenyl-bis-(2-oxyäthyl)-Ammoniumsalze. Recl. Trav. Chim. Pays-Bas 1954, 73, 401–409. [Google Scholar] [CrossRef]
- Benkova, M.; Soukup, O.; Prchal, L.; Sleha, R.; Eleršek, T.; Novak, M.; Sepčić, K.; Gunde-Cimerman, N.; Dolezal, R.; Bostik, V.; et al. Synthesis, Antimicrobial Effect and Lipophilicity-Activity Dependence of Three Series of Dichained N -Alkylammonium Salts. ChemistrySelect 2019, 4, 12076–12084. [Google Scholar] [CrossRef]
- Traube, I. Über Die Kapillaritätskonstanten Organischer Stoffe in Wässriger Lösung. Annu. Chem. 1891, 265, 27–55. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Balakrishnan, V.K.; VanLoon, G.W.; Buncel, E. Degradation of the Pesticide Fenitrothion as Mediated by Cationic Surfactants and Alpha-Nucleophilic Reagents. Langmuir ACS J. Surf. Colloids 2006, 22, 9009–9017. [Google Scholar] [CrossRef]
- Balakrishnan, V.K.; Han, X.; VanLoon, G.W.; Dust, J.M.; Toullec, J.; Buncel, E. Acceleration of Nucleophilic Attack on an Organophosphorothioate Neurotoxin, Fenitrothion, by Reactive Counterion Cationic Micelles. Regioselectivity as a Probe of Substrate Orientation within the Micelle. Langmuir 2004, 20, 6586–6593. [Google Scholar] [CrossRef]
- Bunton, C.A. Micellar Catalysis and Inhibition. Prog. Solid State Chem. 1973, 8, 239–281. [Google Scholar] [CrossRef]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary Ammonium Salts and Their Antimicrobial Potential: Targets or Nonspecific Interactions? ChemMedChem 2012, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Shtyrlin, N.V.; Sapozhnikov, S.V.; Galiullina, A.S.; Kayumov, A.R.; Bondar, O.V.; Mirchink, E.P.; Isakova, E.B.; Firsov, A.A.; Balakin, K.V.; Shtyrlin, Y.G. Synthesis and Antibacterial Activity of Quaternary Ammonium 4-Deoxypyridoxine Derivatives. BioMed Res. Int. 2016, 2016, 3864193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC/Bioterrorism Agents/Diseases (by category)/Emergency Preparedness & Response. Available online: https://emergency.cdc.gov/agent/agentlist-category.asp (accessed on 9 April 2021).
- Kuca, K.; Marek, J.; Stodulka, P.; Musilek, K.; Hanusova, P.; Hrabinova, M.; Jun, D. Preparation of Benzalkonium Salts Differing in the Length of a Side Alkyl Chain. Mol. J. Synth. Chem. Nat. Prod. Chem. 2007, 12, 2341–2347. [Google Scholar] [CrossRef] [Green Version]


| Compound | R | Yield (%) | m.p. (°C) | Purity (%) | Clog P |
|---|---|---|---|---|---|
| 5a | C10H21 | 79 | 114.0–114.6 | 97 | −1.907 |
| 5b | C12H25 | 42 | 118.7–119.0 | 95 | −1.018 |
| 5c | C14H29 | 67 | 120.6–120.9 | 95 | −0.129 |
| 5d | C16H33 | 69 | 121.6–121.9 | 98 | 0.76 |
| 5e | C18H37 | 7 | 120.6–122.3 | 95 | 1.649 |
| C Length (Compound) | CMC [mol/L] | log CMC |
|---|---|---|
| 10 (5a) | 4.836 × 10−2 | −1.316 |
| 12 (5b) | 8.710 × 10−3 | −2.060 |
| 14 (5c) | 3.003 × 10−3 | −2.522 |
| 16 (5d) | 1.159 × 10−3 | −2.936 |
| 18 (5e) | 4.707 × 10−4 | −3.332E |
| Compound | c (mM) | pH 11, t 37 °C | pH 10, t 37 °C | ||
|---|---|---|---|---|---|
| 104 k (s−1) | T1/2 (min) | 104 k (s−1) | T1/2 (min) | ||
| 5a | 500 | 6.20 | 18.6 | 2.30 | 50.3 |
| 100 | 12.82 | 9.0 | 3.97 | 29.2 | |
| 50 | 12.68 | 9.1 | 3.77 | 30.7 | |
| 10 | 0.50 | 230.8 | 0.18 | 606.3 | |
| 5 | 0.35 | 323.2 | - | - | |
| 5b | 100 | 8.17 | 14.1 | 1.67 | 69.1 |
| 50 | 9.35 | 12.4 | 2.35 | 49.3 | |
| 10 | 8.23 | 14.0 | 2.65 | 43.5 | |
| 5 | 5.93 | 19.5 | 1.15 | 100.8 | |
| 1 | 1.80 | 64.5 | - | - | |
| 5c | 10 | 24.62 | 4.7 | 6.77 | 17.1 |
| 5 | 23.62 | 4.9 | 5.82 | 19.9 | |
| 1 | 11.28 | 10.2 | 2.02 | 57.5 | |
| 0.5 | 3.80 | 30.5 | 1.12 | 103 | |
| 0.1 | 1.17 | 98.8 | 0.50 | 230.8 | |
| 5d | 10 | 50.73 | 2.3 | 11.56 | 10.0 |
| 5 | 49.06 | 2.4 | 11.14 | 10.4 | |
| 1 | 12.32 | 9.4 | 3.97 | 29.2 | |
| 0.5 | 6.47 | 17.9 | 2.30 | 50.3 | |
| 0.1 | 1.77 | 65.3 | 1.10 | 105.2 | |
| 5e | 5 | 23.30 | 5.0 | 6.98 | 16.5 |
| 1 | 13.95 | 8.3 | 4.37 | 26.4 | |
| 0.5 | 7.82 | 14.8 | 2.97 | 38.9 | |
| 0.1 | 1.87 | 62.0 | 0.82 | 142.5 | |
| 0.05 | 0.83 | 138.4 | 0.28 | 404.1 | |
| SH | 0.14 | 808.4 | - | - | |
| Compounds | Conc. (mM) | ΔlogTCID50 (MCMV) | ΔlogTCID50 (SARS-CoV-2) |
|---|---|---|---|
| 5b | 0.1% (2.690) | >3.34 a | >3.5 1a |
| 0.01% (0.269) | 5.33 | 0.47 | |
| 5c | 0.1% (2.340) | >2.34 a | >3.51 a |
| 0.01% (0.234) | 3.34 | 0.64 | |
| 5d | 0.1% (2.190) | >3.34 a | 1.00 |
| 0.01% (0.219) | −0.50 | 0.84 |
| Compound | IC50 ± SEM (µM) | Clog P |
|---|---|---|
| 5a | 128.40 ± 12.7 | −1.91 |
| 5b | 36.09 ± 0.5 | −1.02 |
| 5c | 27.34 ± 1.1 | −0.13 |
| 5d | 19.58 ± 0.5 | 0.76 |
| 5e | 19.14 ± 1.3 | 1.64 |
| BAC 12 a | 19.54 ± 1.2 | 2.63 |
| BAC 14 a | 15.04 ± 0.1 | 3.52 |
| BAC 16 a | 12.85 ± 1.4 | 4.41 |
| SI (IC50/MIC) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | STAU | MRSA | STEP | VRE | ESCO | KLPN- | KLPN+ | PSAE MR |
| 5a | 21.986 | 10.956 | 2.739 | 1.027 | 0.514 | 0.342 | 0.257 | <0.257 |
| 5b | 18.508 | 2.309 | 3.079 | 2.309 | 4.621 | 1.155 | 1.155 | 0.289 |
| 5c | 55.796 | 5.602 | 9.331 | 3.501 | 1.166 | 0.875 | 0.875 | <0.437 |
| 5d | 19.980 | 4.012 | 6.683 | 1.671 | <0.313 | <0.313 | <0.313 | <0.313 |
| 5e | 8.700 | 1.225 | 1.959 | 1.225 | 0.817 | <0.612 | <0.612 | <0.612 |
| BAC 12 | 3.750 | 0.625 | 0.625 | 0.375 | 0.313 | 0.156 | 0.094 | 0.039 |
| BAC 14 | 13.193 | 5.127 | 3.847 | 2.310 | 0.722 | 0.577 | 0.241 | 0.030 |
| BAC 16 | 19.668 | 0.987 | 4.936 | 3.946 | 1.234 | 0.822 | 0.822 | <0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markova, A.; Hympanova, M.; Matula, M.; Prchal, L.; Sleha, R.; Benkova, M.; Pulkrabkova, L.; Soukup, O.; Krocova, Z.; Jun, D.; et al. Synthesis and Decontamination Effect on Chemical and Biological Agents of Benzoxonium-Like Salts. Toxics 2021, 9, 222. https://doi.org/10.3390/toxics9090222
Markova A, Hympanova M, Matula M, Prchal L, Sleha R, Benkova M, Pulkrabkova L, Soukup O, Krocova Z, Jun D, et al. Synthesis and Decontamination Effect on Chemical and Biological Agents of Benzoxonium-Like Salts. Toxics. 2021; 9(9):222. https://doi.org/10.3390/toxics9090222
Chicago/Turabian StyleMarkova, Aneta, Michaela Hympanova, Marek Matula, Lukas Prchal, Radek Sleha, Marketa Benkova, Lenka Pulkrabkova, Ondrej Soukup, Zuzana Krocova, Daniel Jun, and et al. 2021. "Synthesis and Decontamination Effect on Chemical and Biological Agents of Benzoxonium-Like Salts" Toxics 9, no. 9: 222. https://doi.org/10.3390/toxics9090222
APA StyleMarkova, A., Hympanova, M., Matula, M., Prchal, L., Sleha, R., Benkova, M., Pulkrabkova, L., Soukup, O., Krocova, Z., Jun, D., & Marek, J. (2021). Synthesis and Decontamination Effect on Chemical and Biological Agents of Benzoxonium-Like Salts. Toxics, 9(9), 222. https://doi.org/10.3390/toxics9090222