The Bladder Is a Novel Target of Developmental Polychlorinated Biphenyl Exposure Linked to Increased Inflammatory Cells in the Bladder of Young Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Developmental PCB Exposures
2.3. Immunohistochemistry
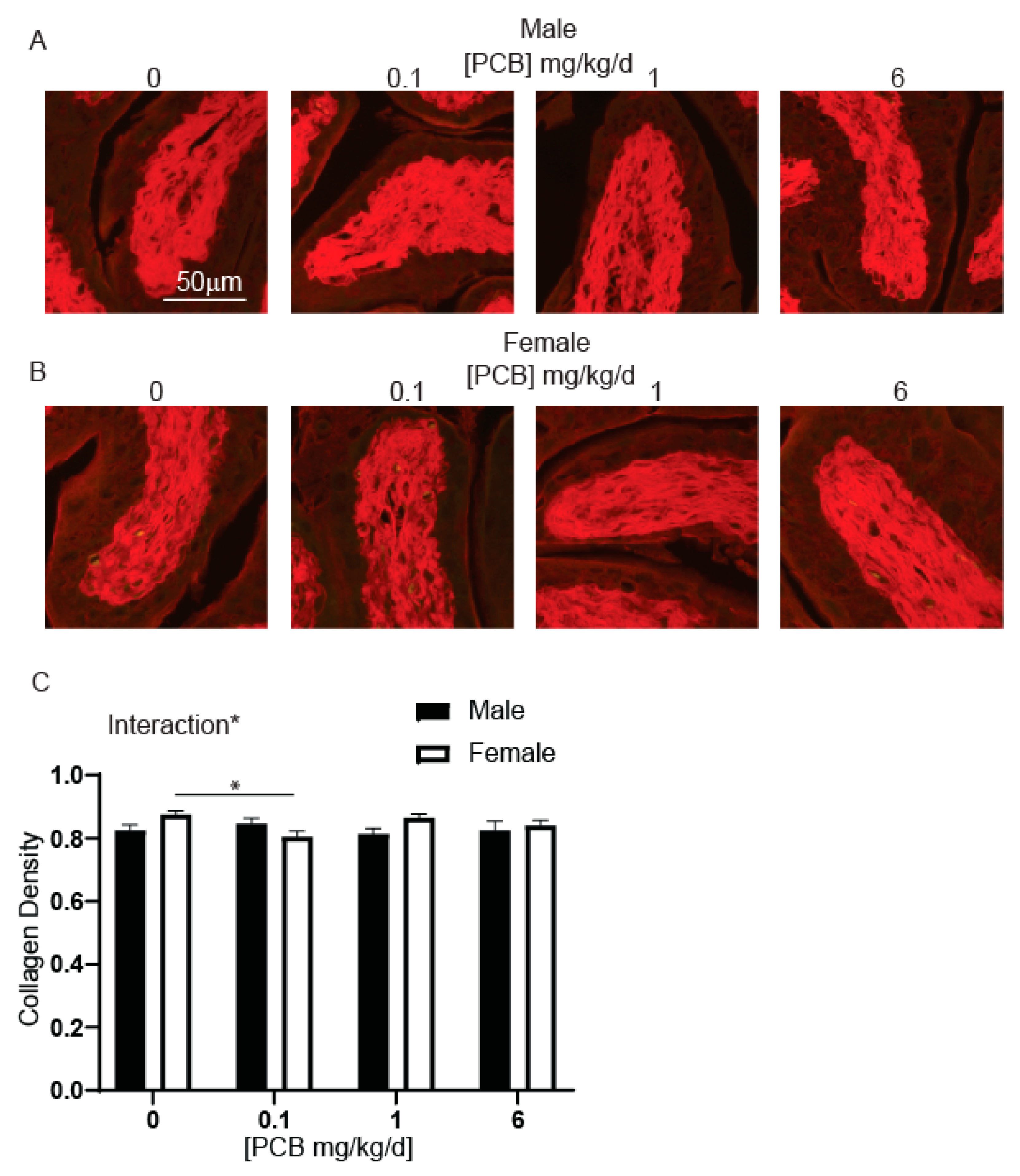
2.4. Collagen Quantification
2.5. Statistics
3. Results
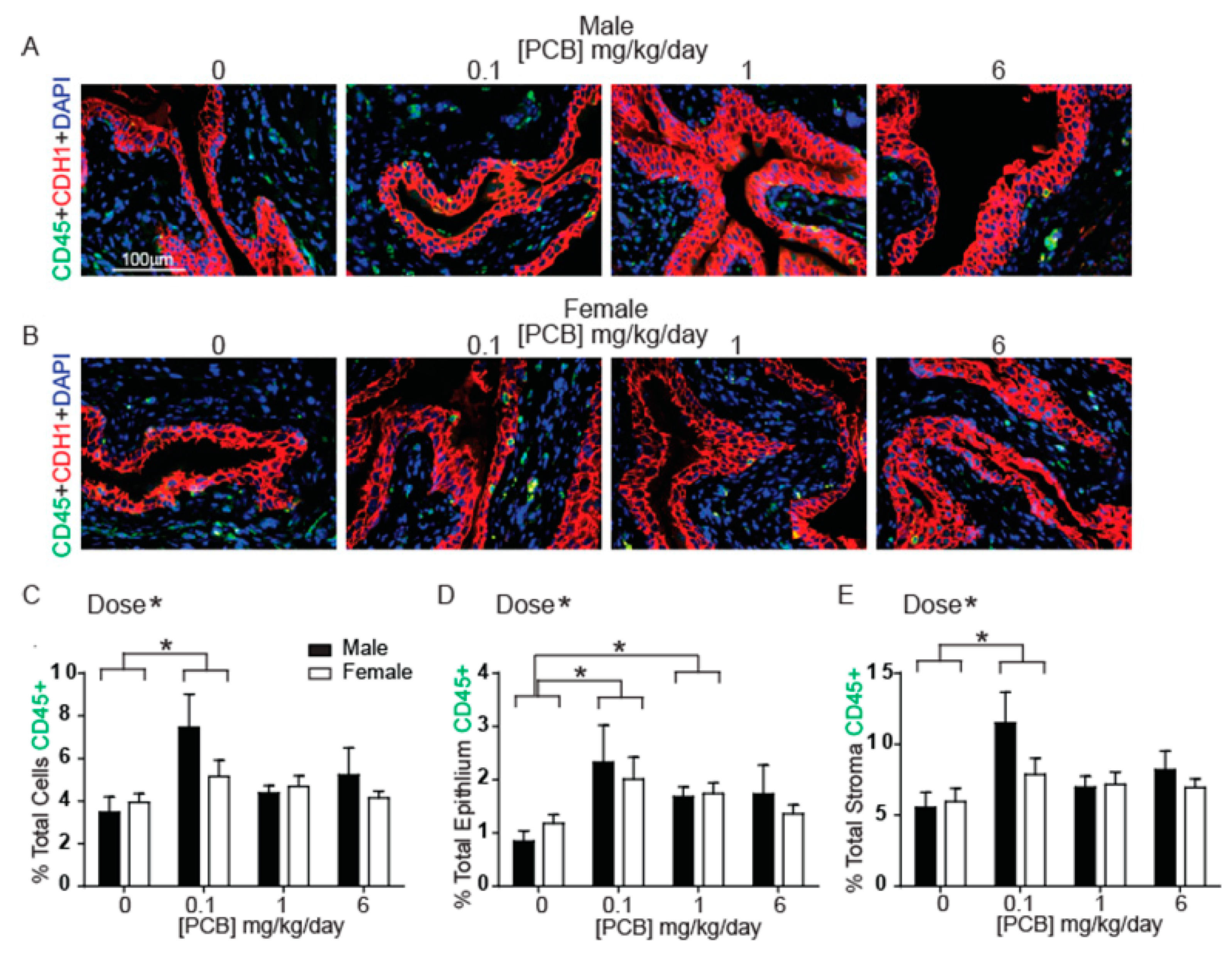
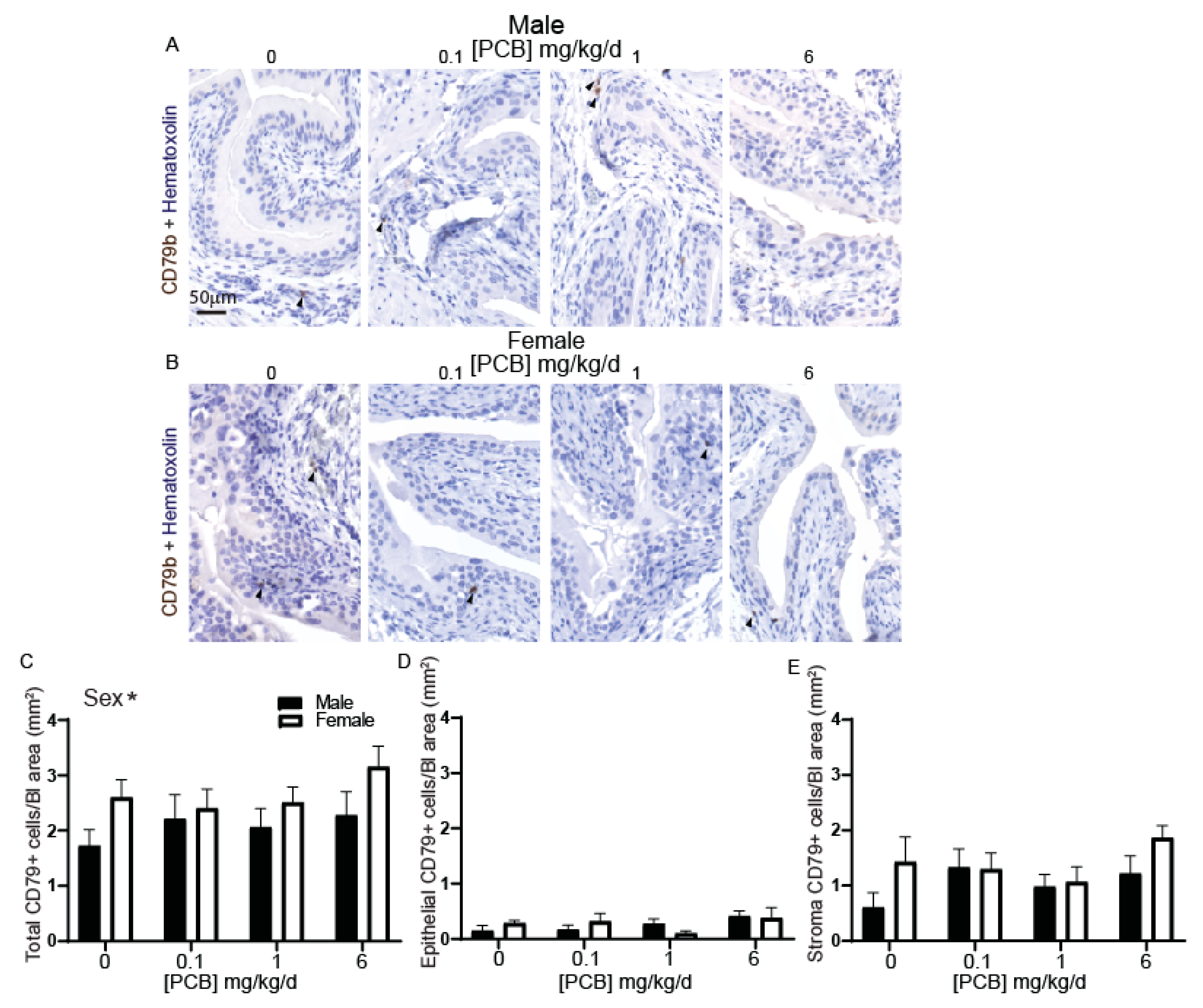
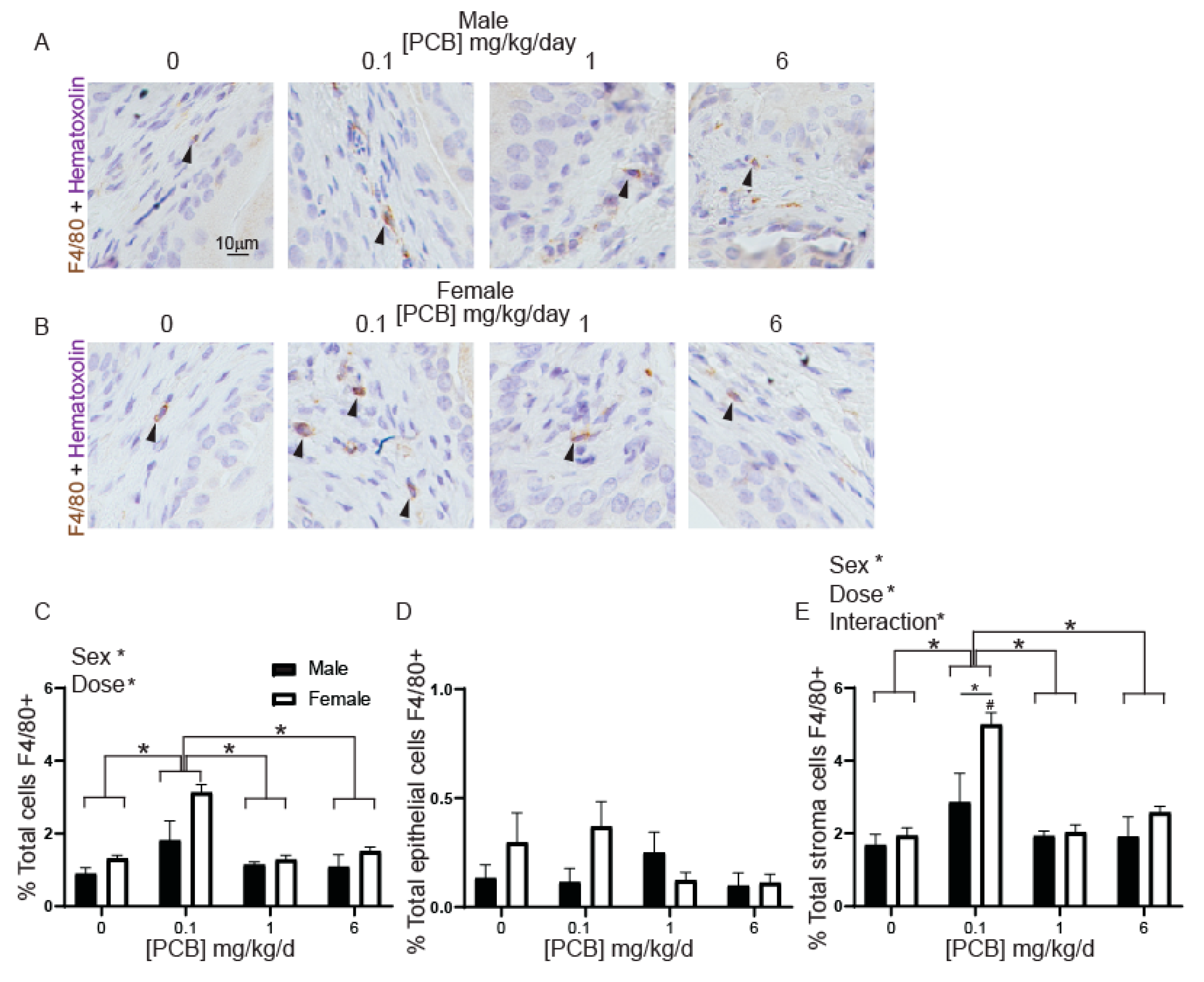
3.1. Developmental PCB Exposure Increases Inflammatory Cells in the Bladder
3.2. Developmental PCB Exposure Decreases Collagen Density within Female Bladder Stroma
3.3. Developmental PCB Exposure Increases Proliferation in a Sex- and Dose-Specific Manner
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubin, E.B.; Buehler, A.E.; Halpern, S.D. States worse than death among hospitalized patients with serious illnesses. JAMA Intern. Med. 2016, 176, 1557–1559. [Google Scholar] [CrossRef] [PubMed]
- Onukwugha, E.; Zuckerman, I.H.; McNally, D.; Coyne, K.S.; Vats, V.; Mullins, C.D. The total economic burden of overactive bladder in the united states: A disease-specific approach. Am. J. Manag. Care 2009, 15, S90–S97. [Google Scholar]
- Grover, S.; Srivastava, A.; Lee, R.; Tewari, A.K.; Te, A.E. Role of inflammation in bladder function and interstitial cystitis. Ther. Adv. Urol. 2011, 3, 19–33. [Google Scholar] [CrossRef]
- Warren, J.W.; Brown, J.; Tracy, J.K.; Langenberg, P.; Wesselmann, U.; Greenberg, P. Evidence-based criteria for pain of interstitial cystitis/painful bladder syndrome in women. Urology 2008, 71, 444–448. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.M.; Shin, J.H.; Yu, H.Y.; Ju, H.; Kim, S.; Lim, J.; Heo, J.; Lee, S.; Shin, D.M.; Choo, M.S. N-acetylcysteine prevents bladder tissue fibrosis in a lipopolysaccharide-induced cystitis rat model. Sci. Rep. 2019, 9, 8134. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Augé, C.; Gamé, X.; Vergnolle, N.; Lluel, P.; Chabot, S. Characterization and validation of a chronic model of cyclophosphamide-induced interstitial cystitis/bladder pain syndrome in rats. Front. Pharmacol. 2020, 11, 1305. [Google Scholar] [CrossRef] [PubMed]
- Panesar, H.K.; Kennedy, C.L.; Keil Stietz, K.P.; Lein, P.J. Polychlorinated biphenyls (pcbs): Risk factors for autism spectrum disorder? Toxics 2020, 8, 70. [Google Scholar] [CrossRef]
- Lyall, K.; Croen, L.A.; Sjödin, A.; Yoshida, C.K.; Zerbo, O.; Kharrazi, M.; Windham, G.C. Polychlorinated biphenyl and organochlorine pesticide concentrations in maternal mid-pregnancy serum samples: Association with autism spectrum disorder and intellectual disability. Environ. Health. Perspect. 2017, 125, 474–480. [Google Scholar] [CrossRef] [Green Version]
- Eubig, P.A.; Aguiar, A.; Schantz, S.L. Lead and pcbs as risk factors for attention deficit/hyperactivity disorder. Environ. Health Perspect. 2010, 118, 1654–1667. [Google Scholar] [CrossRef]
- Schantz, S.L.; Widholm, J.J.; Rice, D.C. Effects of pcb exposure on neuropsychological function in children. Environ. Health Perspect. 2003, 111, 357–576. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.S.; Chen, M.H.; Lin, C.C.; Ng, S.; Hsieh, C.J.; Liu, C.Y.; Hsieh, W.S.; Chen, P.C. Children’s environmental health based on birth cohort studies of asia. Sci. Total. Environ. 2017, 609, 396–409. [Google Scholar] [CrossRef]
- Neblett, M.F.; Curtis, S.W.; Gerkowicz, S.A.; Spencer, J.B.; Terrell, M.L.; Jiang, V.S.; Marder, M.E.; Barr, D.B.; Marcus, M.; Smith, A.K. Examining reproductive health outcomes in females exposed to polychlorinated biphenyl and polybrominated biphenyl. Sci. Rep. 2020, 10, 3314. [Google Scholar] [CrossRef]
- Meeker, J.D.; Hauser, R. Exposure to polychlorinated biphenyls (pcbs) and male reproduction. Syst. Biol. Reprod. Med. 2010, 56, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leijs, M.M.; Koppe, J.G.; Olie, K.; van Aalderen, W.M.; de Voogt, P.; ten Tusscher, G.W. Effects of dioxins, pcbs, and pbdes on immunology and hematology in adolescents. Environ. Sci. Technol. 2009, 43, 7946–7951. [Google Scholar] [CrossRef]
- Heilmann, C.; Grandjean, P.; Weihe, P.; Nielsen, F.; Budtz-Jørgensen, E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006, 3, e311. [Google Scholar] [CrossRef] [Green Version]
- Schæbel, L.K.; Bonefeld-Jørgensen, E.C.; Vestergaard, H.; Andersen, S. The influence of persistent organic pollutants in the traditional inuit diet on markers of inflammation. PLoS ONE 2017, 12, e0177781. [Google Scholar] [CrossRef] [Green Version]
- Granillo, L.; Sethi, S.; Keil, K.P.; Lin, Y.; Ozonoff, S.; Iosif, A.M.; Puschner, B.; Schmidt, R.J. Polychlorinated biphenyls influence on autism spectrum disorder risk in the marbles cohort. Environ. Res. 2019, 171, 177–184. [Google Scholar] [CrossRef]
- Schantz, S.L.; Gardiner, J.C.; Aguiar, A.; Tang, X.; Gasior, D.M.; Sweeney, A.M.; Peck, J.D.; Gillard, D.; Kostyniak, P.J. Contaminant profiles in southeast asian immigrants consuming fish from polluted waters in northeastern wisconsin. Environ. Res. 2010, 110, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Chen, X.; Kass, P.H.; Puschner, B. Polychlorinated biphenyl and polybrominated diphenyl ether profiles in serum from cattle, sheep, and goats across california. Chemosphere 2017, 181, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Lin, Y.; Dang, K.; Puschner, B. Quantification of polychlorinated biphenyls and polybrominated diphenyl ethers in commercial cows’ milk from california by gas chromatography-triple quadruple mass spectrometry. PLoS ONE 2017, 12, e0170129. [Google Scholar] [CrossRef] [Green Version]
- Heiger-Bernays, W.J.; Tomsho, K.S.; Basra, K.; Petropoulos, Z.E.; Crawford, K.; Martinez, A.; Hornbuckle, K.C.; Scammell, M.K. Human health risks due to airborne polychlorinated biphenyls are highest in new bedford harbor communities living closest to the harbor. Sci. Total Environ. 2020, 710, 135576. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Andersen, Z.J.; Frederiksen, M.; Specht, I.O.; Hougaard, K.S.; Ebbehøj, N.; Bailey, J.; Giwercman, A.; Steenland, K.; Longnecker, M.P.; et al. Health effects of pcbs in residences and schools (hesperus): Pcb-health cohort profile. Sci. Rep. 2016, 6, 24571. [Google Scholar] [CrossRef] [Green Version]
- Howell, N.L.; Suarez, M.P.; Rifai, H.S.; Koenig, L. Concentrations of polychlorinated biphenyls (pcbs) in water, sediment, and aquatic biota in the houston ship channel, texas. Chemosphere 2008, 70, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Hornbuckle, K.C. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 2010, 44, 2822–2827. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, J.C.; Hornbuckle, K.C. Pcb emissions from paint colorants. Environ. Sci. Technol. 2019, 53, 5187–5194. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Keil, K.P.; Chen, H.; Hayakawa, K.; Li, X.; Lin, Y.; Lehmler, H.J.; Puschner, B.; Lein, P.J. Detection of 3,3’-dichlorobiphenyl in human maternal plasma and its effects on axonal and dendritic growth in primary rat neurons. Toxicol. Sci. 2017, 158, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Morgan, R.K.; Feng, W.; Lin, Y.; Li, X.; Luna, C.; Koch, M.; Bansal, R.; Duffel, M.W.; Puschner, B.; et al. Comparative analyses of the 12 most abundant pcb congeners detected in human maternal serum for activity at the thyroid hormone receptor and ryanodine receptor. Environ. Sci. Technol. 2019, 53, 3948–3958. [Google Scholar] [CrossRef] [Green Version]
- Klocke, C.; Sethi, S.; Lein, P.J. The developmental neurotoxicity of legacy vs. Contemporary polychlorinated biphenyls (pcbs): Similarities and differences. Environ. Sci. Pollut. Res. Int. 2020, 27, 8885–8896. [Google Scholar] [CrossRef]
- Kodavanti, P.R.; Kannan, N.; Yamashita, N.; Derr-Yellin, E.C.; Ward, T.R.; Burgin, D.E.; Tilson, H.A.; Birnbaum, L.S. Differential effects of two lots of aroclor 1254: Congener-specific analysis and neurochemical end points. Environ. Health Perspect. 2001, 109, 1153–1161. [Google Scholar] [CrossRef]
- Hertz-Picciotto, I.; Schmidt, R.J.; Walker, C.K.; Bennett, D.H.; Oliver, M.; Shedd-Wise, K.M.; LaSalle, J.M.; Giulivi, C.; Puschner, B.; Thomas, J.; et al. A prospective study of environmental exposures and early biomarkers in autism spectrum disorder: Design, protocols, and preliminary data from the marbles study. Environ. Health. Perspect. 2018, 126, 117004. [Google Scholar] [CrossRef] [Green Version]
- Keil Stietz, K.P.; Kennedy, C.L.; Sethi, S.; Valenzuela, A.; Nunez, A.; Wang, K.; Wang, Z.; Wang, P.; Spiegelhoff, A.; Puschner, B.; et al. In utero and lactational pcb exposure drives anatomic changes in the juvenile mouse bladder. Curr. Res. Toxicol. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Lü, Y.C.; Wu, Y.C. Clinical findings and immunological abnormalities in yu-cheng patients. Environ. Health Perspect. 1985, 59, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Svensson, B.G.; Hallberg, T.; Nilsson, A.; Schütz, A.; Hagmar, L. Parameters of immunological competence in subjects with high consumption of fish contaminated with persistent organochlorine compounds. Int. Arch. Occup. Environ. Health 1994, 65, 351–358. [Google Scholar] [CrossRef]
- Dietert, R.R. Developmental immunotoxicity, perinatal programming, and noncommunicable diseases: Focus on human studies. Adv. Med. 2014, 2014, 867805. [Google Scholar] [CrossRef] [Green Version]
- Stølevik, S.B.; Nygaard, U.C.; Namork, E.; Haugen, M.; Meltzer, H.M.; Alexander, J.; Knutsen, H.K.; Aaberge, I.; Vainio, K.; van Loveren, H.; et al. Prenatal exposure to polychlorinated biphenyls and dioxins from the maternal diet may be associated with immunosuppressive effects that persist into early childhood. Food Chem. Toxicol. 2013, 51, 165–172. [Google Scholar] [CrossRef]
- Heilmann, C.; Budtz-Jørgensen, E.; Nielsen, F.; Heinzow, B.; Weihe, P.; Grandjean, P. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ. Health Perspect. 2010, 118, 1434–1438. [Google Scholar] [CrossRef] [Green Version]
- Peinado, F.M.; Artacho-Cordón, F.; Barrios-Rodríguez, R.; Arrebola, J.P. Influence of polychlorinated biphenyls and organochlorine pesticides on the inflammatory milieu. A systematic review of in vitro, in vivo and epidemiological studies. Environ. Res. 2020, 186, 109561. [Google Scholar] [CrossRef] [PubMed]
- Imbeault, P.; Findlay, C.S.; Robidoux, M.A.; Haman, F.; Blais, J.M.; Tremblay, A.; Springthorpe, S.; Pal, S.; Seabert, T.; Krümmel, E.M.; et al. Dysregulation of cytokine response in canadian first nations communities: Is there an association with persistent organic pollutant levels? PLoS ONE 2012, 7, e39931. [Google Scholar] [CrossRef]
- Kuwatsuka, Y.; Shimizu, K.; Akiyama, Y.; Koike, Y.; Ogawa, F.; Furue, M.; Utani, A. Yusho patients show increased serum il-17, il-23, il-1β, and tnfα levels more than 40 years after accidental polychlorinated biphenyl poisoning. J. Immunotoxicol. 2014, 11, 246–249. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Guo, X.; Li, N.; Pan, Q.; Ma, Y.Z. Effects of quercetin on aroclor 1254-induced expression of cyp. J. Immunotoxicol. 2019, 16, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, R.G.; El-Gareib, A.W.; Shaker, H.M. Gestational 3,3’,4,4’,5-pentachlorobiphenyl (pcb 126) exposure disrupts fetoplacental unit: Fetal thyroid-cytokines dysfunction. Life. Sci. 2018, 192, 213–220. [Google Scholar] [CrossRef]
- Bell, M.R.; Dryden, A.; Will, R.; Gore, A.C. Sex differences in effects of gestational polychlorinated biphenyl exposure on hypothalamic neuroimmune and neuromodulator systems in neonatal rats. Toxicol. Appl. pharmacol. 2018, 353, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Rude, K.M.; Pusceddu, M.M.; Keogh, C.E.; Sladek, J.A.; Rabasa, G.; Miller, E.N.; Sethi, S.; Keil, K.P.; Pessah, I.N.; Lein, P.J.; et al. Developmental exposure to polychlorinated biphenyls (pcbs) in the maternal diet causes host-microbe defects in weanling offspring mice. Environ. Pollut. 2019, 253, 708–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matelski, L.; Keil Stietz, K.P.; Sethi, S.; Taylor, S.L.; Van de Water, J.; Lein, P.J. The influence of sex, genotype, and dose on serum and hippocampal cytokine levels in juvenile mice developmentally exposed to a human-relevant mixture of polychlorinated biphenyls. Curr. Res. Toxicol. 2020, 1, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Klocke, C.; Lein, P.J. Evidence implicating non-dioxin-like congeners as the key mediators of polychlorinated biphenyl (pcb) developmental neurotoxicity. Int. J. Mol. Sci. 2020, 21, 1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abler, L.L.; Keil, K.P.; Mehta, V.; Joshi, P.S.; Schmitz, C.T.; Vezina, C.M. A high-resolution molecular atlas of the fetal mouse lower urogenital tract. Dev. Dyn. 2011, 240, 2364–2377. [Google Scholar] [CrossRef] [Green Version]
- Wegner, K.A.; Keikhosravi, A.; Eliceiri, K.W.; Vezina, C.M. Fluorescence of picrosirius red multiplexed with immunohistochemistry for the quantitative assessment of collagen in tissue sections. J. Histochem. Cytochem. 2017, 65, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Petriello, M.C.; Brandon, J.A.; Hoffman, J.; Wang, C.; Tripathi, H.; Abdel-Latif, A.; Ye, X.; Li, X.; Yang, L.; Lee, E.; et al. Dioxin-like pcb 126 increases systemic inflammation and accelerates atherosclerosis in lean ldl receptor-deficient mice. Toxicol. Sci. 2018, 162, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Hennig, B.; Meerarani, P.; Slim, R.; Toborek, M.; Daugherty, A.; Silverstone, A.E.; Robertson, L.W. Proinflammatory properties of coplanar pcbs: In vitro and in vivo evidence. Toxicol. Appl. Pharmacol. 2002, 181, 174–183. [Google Scholar] [CrossRef]
- Akiyama, Y.; Yao, J.R.; Kreder, K.J.; O’Donnell, M.A.; Lutgendorf, S.K.; Lyu, D.; Maeda, D.; Kume, H.; Homma, Y.; Luo, Y. Autoimmunity to urothelial antigen causes bladder inflammation, pelvic pain, and voiding dysfunction: A novel animal model for hunner-type interstitial cystitis. Am. J. Physiol. Renal. Physiol. 2021, 320, F174–F182. [Google Scholar] [CrossRef]
- Horváthová, M.; Jahnová, E.; Palkovičová, L.; Trnovec, T.; Hertz-Picciotto, I. Dynamics of lymphocyte subsets in children living in an area polluted by polychlorinated biphenyls. J. Immunotoxicol. 2011, 8, 333–345. [Google Scholar] [CrossRef]
- Wang, C.; Petriello, M.C.; Zhu, B.; Hennig, B. Pcb 126 induces monocyte/macrophage polarization and inflammation through ahr and nf-kappab pathways. Toxicol. Appl. Pharmacol. 2019, 367, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, R.; Franklin, L.; Tikkinen, K.A.O.; Kalliala, I.; Miotla, P.; Rechberger, T.; Offiah, I.; McMahon, S.; O’Reilly, B.; Lince, S.; et al. Genome wide association study identifies two novel loci associated with female stress and urgency urinary incontinence. J. Urol. 2021, 101097JU0000000000001822. [Google Scholar]
- Ferrante, M.C.; Mattace Raso, G.; Esposito, E.; Bianco, G.; Iacono, A.; Clausi, M.T.; Amero, P.; Santoro, A.; Simeoli, R.; Autore, G.; et al. Effects of non-dioxin-like polychlorinated biphenyl congeners (pcb 101, pcb 153 and pcb 180) alone or mixed on j774a.1 macrophage cell line: Modification of apoptotic pathway. Toxicol. Lett. 2011, 202, 61–68. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Qin, Q.; Xia, X.; Liu, Z.; Song, E.; Song, Y. Polychlorinated biphenyl quinone promotes macrophage-derived foam cell formation. Chem. Res. Toxicol. 2019, 32, 2422–2432. [Google Scholar] [CrossRef]
- Osei, E.T.; Mostaço-Guidolin, L.B.; Hsieh, A.; Warner, S.M.; Al-Fouadi, M.; Wang, M.; Cole, D.J.; Maksym, G.N.; Hallstrand, T.S.; Timens, W.; et al. Epithelial-interleukin-1 inhibits collagen formation by airway fibroblasts: Implications for asthma. Sci. Rep. 2020, 10, 8721. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wahlang, B.; Shi, H.; Hardesty, J.E.; Falkner, K.C.; Head, K.Z.; Srivastava, S.; Merchant, M.L.; Rai, S.N.; Cave, M.C.; et al. Dioxin-like and non-dioxin-like pcbs differentially regulate the hepatic proteome and modify diet-induced nonalcoholic fatty liver disease severity. Med. Chem. Res. 2020, 29, 1247–1263. [Google Scholar] [CrossRef] [PubMed]
- Ramajayam, G.; Sridhar, M.; Karthikeyan, S.; Lavanya, R.; Veni, S.; Vignesh, R.C.; Ilangovan, R.; Djody, S.S.; Gopalakrishnan, V.; Arunakaran, J.; et al. Effects of aroclor 1254 on femoral bone metabolism in adult male wistar rats. Toxicology 2007, 241, 99–105. [Google Scholar] [CrossRef]
- Lind, P.M.; Larsson, S.; Oxlund, H.; Hâkansson, H.; Nyberg, K.; Eklund, T.; Orberg, J. Change of bone tissue composition and impaired bone strength in rats exposed to 3,3’,4,4’,5-pentachlorobiphenyl (pcb126). Toxicology 2000, 150, 41–51. [Google Scholar] [CrossRef]
- Kullmann, F.A.; Clayton, D.R.; Ruiz, W.G.; Wolf-Johnston, A.; Gauthier, C.; Kanai, A.; Birder, L.A.; Apodaca, G. Urothelial proliferation and regeneration after spinal cord injury. Am. J. Physiol. Renal. Physiol. 2017, 313, F85–F102. [Google Scholar] [CrossRef] [PubMed]
- Golubeva, A.V.; Zhdanov, A.V.; Mallel, G.; Dinan, T.G.; Cryan, J.F. The mouse cyclophosphamide model of bladder pain syndrome: Tissue characterization, immune profiling, and relationship to metabotropic glutamate receptors. Physiol. Rep. 2014, 2, e00260. [Google Scholar] [CrossRef]
- Turco, A.E.; Oakes, S.R.; Stietz, K.P.K.; Dunham, C.L.; Joseph, D.B.; Chathurvedula, T.S.; Girardi, N.M.; Schneider, A.J.; Gawdzik, J.; Sheftel, C.M.; et al. A neuroanatomical mechanism linking perinatal tcdd exposure to lower urinary tract dysfunction in adulthood. Dis. Model. Mech. 2021, 14, dmm049068. [Google Scholar] [CrossRef]
- Turco, A.E.; Thomas, S.; Crawford, L.K.; Tang, W.; Peterson, R.E.; Li, L.; Ricke, W.A.; Vezina, C.M. In utero and lactational 2,3,7,8-tetrachlorodibenzo- p-dioxin (tcdd) exposure exacerbates urinary dysfunction in hormone-treated c57bl/6j mice through a non-malignant mechanism involving proteomic changes in the prostate that differ from those elicited by testosterone and estradiol. Am. J. Clin. Exp. Urol. 2020, 8, 59–72. [Google Scholar] [PubMed]
- Ricke, W.A.; Lee, C.W.; Clapper, T.R.; Schneider, A.J.; Moore, R.W.; Keil, K.P.; Abler, L.L.; Wynder, J.L.; López Alvarado, A.; Beaubrun, I.; et al. In utero and lactational tcdd exposure increases susceptibility to lower urinary tract dysfunction in adulthood. Toxicol. Sci. 2016, 150, 429–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arima, A.; Kato, H.; Ise, R.; Ooshima, Y.; Inoue, A.; Muneoka, A.; Kamimura, S.; Fukusato, T.; Kubota, S.; Sumida, H.; et al. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) induces disruption of glands of the prostate and fibrosis in rhesus monkeys. Reprod. Toxicol. 2010, 29, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef]
- Yang, D.; Kim, K.H.; Phimister, A.; Bachstetter, A.D.; Ward, T.R.; Stackman, R.W.; Mervis, R.F.; Wisniewski, A.B.; Klein, S.L.; Kodavanti, P.R.; et al. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ. Health Perspect. 2009, 117, 426–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wayman, G.A.; Bose, D.D.; Yang, D.; Lesiak, A.; Bruun, D.; Impey, S.; Ledoux, V.; Pessah, I.N.; Lein, P.J. Pcb-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth. Environ. Health Perspect. 2012, 120, 1003–1009. [Google Scholar] [CrossRef] [Green Version]
- Wahlang, B.; Song, M.; Beier, J.I.; Cameron Falkner, K.; Al-Eryani, L.; Clair, H.B.; Prough, R.A.; Osborne, T.S.; Malarkey, D.E.; States, J.C.; et al. Evaluation of aroclor 1260 exposure in a mouse model of diet-induced obesity and non-alcoholic fatty liver disease. Toxicol. Appl. Pharmacol. 2014, 279, 380–390. [Google Scholar] [CrossRef] [Green Version]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Rathcke, C.N.; Vestergaard, H. Ykl-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm. Res. 2006, 55, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Barcena, M.L.; Jeuthe, S.; Niehues, M.H.; Pozdniakova, S.; Haritonow, N.; Kühl, A.A.; Messroghli, D.R.; Regitz-Zagrosek, V. Sex-specific differences of the inflammatory state in experimental autoimmune myocarditis. Front. Immunol. 2021, 12, 686384. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.; Jobin, K.; Kurts, C. Urinary tract infection: Recent insight into the evolutionary arms race between uropathogenic escherichia coli and our immune system. Nephrol. Dial. Transplant. 2017, 32, 1977–1983. [Google Scholar] [CrossRef] [Green Version]
- Lacerda Mariano, L.; Ingersoll, M.A. Bladder resident macrophages: Mucosal sentinels. Cell Immunol. 2018, 330, 136–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacerda Mariano, L.; Rousseau, M.; Varet, H.; Legendre, R.; Gentek, R.; Saenz Coronilla, J.; Bajenoff, M.; Gomez Perdiguero, E.; Ingersoll, M.A. Functionally distinct resident macrophage subsets differentially shape responses to infection in the bladder. Sci. Adv. 2020, 6, eabc5739. [Google Scholar] [CrossRef]
- Kim, J.; De Hoedt, A.; Wiggins, E.; Haywood, K.; Jin, P.; Greenwood, B.; Narain, N.R.; Tolstikov, V.; Bussberg, V.; Barbour, K.E.; et al. Diagnostic utility of serum and urinary metabolite analysis in patients with interstitial cystitis/painful bladder syndrome. Urology 2021. [Google Scholar] [CrossRef]


| Primary Antibodies | Catalog # | Company | Source | Dilution | Pairing |
|---|---|---|---|---|---|
| CD79b | ab134147 | Abcam | Rabbit | 1:250 | |
| CD3 | ab11089 | Abcam | Rat | 1:250 | |
| CD45 (PTPRC) | ab10558 | Abcam | Rabbit | 1:750 | |
| E-Cadherin (CDH1) | 610181 | BD Transduction Labs (via Fisher) | Mouse | 1:250 | |
| F4/80 (EMR1; Adgre1) | 123102 | Biolegend | Rat | 1:50 | |
| Ki67 | ab15580 | Abcam | Rabbit | 1:200 | |
| Keratin 5 | 905901 | Biologend | Chicken | 1:500 | |
| Secondary Antibodies/Detection Kits | |||||
| Anti-Mouse Alexa Fluor 594 | 715-545-150 | Jackson ImmunoResearch | Donkey | 1:250 | Cdh1 |
| Anti-Rabbit Alexa Fluor 488 | 711-545-152 | Jackson ImmunoResearch | Donkey | 1:250 | CD45 |
| Anti-Rabbit Alexa Fluor 594 | 111-585-144 | Jackson ImmunoResearch | Goat | 1:250 | Ki67 |
| Anti-Rat Biotinylated | 112-066-003 | Jackson ImmunoResearch | Goat | 1:250 | CD3, F4/80 |
| Anti-Chicken Alexa 488 | 703-546-155 | Jackson ImmunoResearch | Donkey | 1:250 | Krt5 |
| ImmPRESS® HRO Horse Anti-Rabbit IgG Polymer Detection Kit, Peroxidase | MP-7401 | Vector Laboratories | Horse | Per manufacturer’s instructions | CD79b |
| VECTASTAIN ELITE HRP ABC KIT paired with DAB substrate Kit, Peroxidase | PK-6100, SK-4100 | Vector Laboratories | Per manufacturer’s instructions | CD3, F4/80 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, C.L.; Spiegelhoff, A.; Wang, K.; Lavery, T.; Nunez, A.; Manuel, R.; Hillers-Ziemer, L.; Arendt, L.M.; Stietz, K.P.K. The Bladder Is a Novel Target of Developmental Polychlorinated Biphenyl Exposure Linked to Increased Inflammatory Cells in the Bladder of Young Mice. Toxics 2021, 9, 214. https://doi.org/10.3390/toxics9090214
Kennedy CL, Spiegelhoff A, Wang K, Lavery T, Nunez A, Manuel R, Hillers-Ziemer L, Arendt LM, Stietz KPK. The Bladder Is a Novel Target of Developmental Polychlorinated Biphenyl Exposure Linked to Increased Inflammatory Cells in the Bladder of Young Mice. Toxics. 2021; 9(9):214. https://doi.org/10.3390/toxics9090214
Chicago/Turabian StyleKennedy, Conner L., Audrey Spiegelhoff, Kathy Wang, Thomas Lavery, Alexandra Nunez, Robbie Manuel, Lauren Hillers-Ziemer, Lisa M. Arendt, and Kimberly P. Keil Stietz. 2021. "The Bladder Is a Novel Target of Developmental Polychlorinated Biphenyl Exposure Linked to Increased Inflammatory Cells in the Bladder of Young Mice" Toxics 9, no. 9: 214. https://doi.org/10.3390/toxics9090214
APA StyleKennedy, C. L., Spiegelhoff, A., Wang, K., Lavery, T., Nunez, A., Manuel, R., Hillers-Ziemer, L., Arendt, L. M., & Stietz, K. P. K. (2021). The Bladder Is a Novel Target of Developmental Polychlorinated Biphenyl Exposure Linked to Increased Inflammatory Cells in the Bladder of Young Mice. Toxics, 9(9), 214. https://doi.org/10.3390/toxics9090214







