A Preconception Paternal Fish Oil Diet Prevents Toxicant-Driven New Bronchopulmonary Dysplasia in Neonatal Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. TCDD Exposure and Mating Scheme
2.4. Diet and Mating Scheme for the F1 Generation
2.5. Formula Feeding
2.6. Euthanasia and Collection of Tissue
2.7. Hematoxylin and Eosin (H and E) Staining
2.8. Alveolus Diameter and Radial Alveoli Count
2.9. Histological Determination of BPD
2.10. qRT-PCR
2.11. Immunoblot Assays
2.12. Statistical Analysis
3. Results
3.1. A Paternal Fish Oil Diet Preconception Improves Postnatal Growth in Pups with a History of TCDD Exposure
3.2. A Paternal Fish Oil Diet Mitigates Delayed Lung Development in Pups with a History of TCDD Exposure
3.3. A Paternal Fish Oil Diet Reduces the Incidence of New BPD in Pups with a History of TCDD Exposure
3.4. Diet and History of TCDD Exposure Influences Pup Pulmonary Beta-Catenin and E-Cadherin Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J. Epidemiological evidence for the developmental origins of health and disease: Effects of prenatal undernutrition in humans. J. Endocrinol. 2019, 242, T135–T144. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Lawler, C.; Gluckman, P.D.; Hanson, M.A. Developmental Origins of Health and Disease: Role of exposure to environmental chemicals in developmental origins of health and disease. Endocrinology 2015, 156, 3416–3421. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-N.; Tain, Y.-L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Codagnone, M.G.; Spichak, S.; O’Mahony, S.M.; O’Leary, O.F.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Programming Bugs: Microbiota and the Developmental Origins of Brain Health and Disease. Biol. Psychiatry 2019, 85, 150–163. [Google Scholar] [CrossRef]
- Moretti, E.; Capitani, S.; Figura, N.; Pammolli, A.; Federico, M.G.; Giannerini, V.; Collodel, G. The presence of bacteria species in semen and sperm quality. J. Assist. Reprod. Genet. 2009, 26, 47–56. [Google Scholar] [CrossRef]
- Singer, R.; Sagiv, M.; Barnet, M.; Levinsky, H. Semen volume and fructose content of human semen. Survey of the years 1980–1989. Acta Eur. Fertil. 1990, 21, 205–206. [Google Scholar]
- Singer, R.; Landau, B.; Joshua, H.; Zukerman, Z.; Pick, I.; Sigienriech, E.; Chowers, I. Protein content of human seminal plasma and spermatozoa in relation to sperm counts. Acta Eur. Fertil. 1976, 7, 281–284. [Google Scholar]
- Schjenken, J.E.; Sharkey, D.J.; Green, E.S.; Chan, H.Y.; Matias, R.A.; Moldenhauer, L.M.; Robertson, S.A. Sperm modulate uterine immune parameters relevant to embryo implantation and reproductive success in mice. Commun. Biol. 2021, 4, 572. [Google Scholar] [CrossRef]
- Ding, T.; Mokshagundam, S.; Rinaudo, P.F.; Osteen, K.G.; Bruner-Tran, K.L. Paternal developmental toxicant exposure is associated with epigenetic modulation of sperm and placental Pgr and Igf2 in a mouse model. Biol. Reprod. 2018, 99, 864–876. [Google Scholar] [CrossRef]
- Di Mascio, D.; Saccone, G.; Bellussi, F.; Vitagliano, A.; Berghella, V. Type of paternal sperm exposure before pregnancy and the risk of preeclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 251, 246–253. [Google Scholar] [CrossRef]
- Robertson, S.A.; Sharkey, D.J. Seminal fluid and fertility in women. Fertil. Steril. 2016, 106, 511–519. [Google Scholar] [CrossRef]
- Bromfield, J.J. Seminal fluid and reproduction: Much more than previously thought. J. Assist. Reprod. Genet. 2014, 31, 627–636. [Google Scholar] [CrossRef]
- Wang, X.; Miller, D.C.; Harman, R.; Antczak, D.F.; Clark, A.G. Paternally expressed genes predominate in the placenta. Proc. Natl. Acad. Sci. USA 2013, 110, 10705–10710. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; McConaha, M.; Boyd, K.L.; Osteen, K.G.; Bruner-Tran, K.L. Developmental dioxin exposure of either parent is associated with an increased risk of preterm birth in adult mice. Reprod. Toxicol. 2011, 31, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Osteen, K.G. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod. Toxicol. 2011, 31, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Schagdarsurengin, U.; Steger, K. Epigenetics in male reproduction: Effect of paternal diet on sperm quality and offspring health. Nat. Rev. Urol. 2016, 13, 584–595. [Google Scholar] [CrossRef]
- McConaha, M.E.; Ding, T.; Lucas, J.A.; Arosh, J.A.; Osteen, K.G.; Bruner-Tran, K.L. Preconception omega-3 fatty acid supplementation of adult male mice with a history of developmental 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure prevents preterm birth in unexposed female partners. Reproduction 2011, 142, 235–241. [Google Scholar] [CrossRef]
- Mestan, K.K.; Steinhorn, R.H. Fetal origins of neonatal lung disease: Understanding the pathogenesis of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L858–L859. [Google Scholar] [CrossRef]
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers 2019, 5, 78. [Google Scholar] [CrossRef]
- Young, K.; Sosenko, I.; Claure, N. Placental dysfunction and impaired fetal growth: A relationship with bronchopulmonary dysplasia and pulmonary hypertension. Thorax 2021. [CrossRef]
- Torchin, H.; Ancel, P.-Y.; Goffinet, F.; Hascoët, J.-M.; Truffert, P.; Tran, D.; Lebeaux, C.; Jarreau, P.-H. Placental Complications and Bronchopulmonary Dysplasia: EPIPAGE-2 Cohort Study. Pediatrics 2016, 137, e20152163. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.N.; Chalak, L.F.; Brown, L.S.; Johnson-Welch, S.; Heyne, R.; Rosenfeld, C.R.; Kapadia, V.S. Impact of multiple placental pathologies on neonatal death, bronchopulmonary dysplasia, and neurodevelopmental impairment in preterm infants. Pediatr. Res. 2020, 87, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Redline, R.W.; Wilson-Costello, D.; Hack, M. Placental and Other Perinatal Risk Factors for Chronic Lung Disease in Very Low Birth Weight Infants. Pediatr. Res. 2002, 52, 713–719. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rice, J.L.; McGrath-Morrow, S.A.; Collaco, J.M. Indoor Air Pollution Sources and Respiratory Symptoms in Bronchopulmonary Dysplasia. J. Pediatr. 2020, 222, 85–90.e82. [Google Scholar] [CrossRef]
- Collaco, J.M.; Morrow, M.; Rice, J.L.; McGrath-Morrow, S.A. Impact of road proximity on infants and children with bronchopulmonary dysplasia. Pediatr. Pulmonol. 2020, 55, 369–375. [Google Scholar] [CrossRef]
- Collaco, J.M.; Aoyama, B.C.; Rice, J.L.; McGrath-Morrow, S.A. Influences of environmental exposures on preterm lung disease. Expert Rev. Respir. Med. 2021, 15, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Latzin, P.; Röösli, M.; Huss, A.; Kuehni, C.E.; Frey, U. Air pollution during pregnancy and lung function in newborns: A birth cohort study. Eur. Respir. J. 2009, 33, 594–603. [Google Scholar] [CrossRef]
- Mokshagundam, S.; Ding, T.; Rumph, J.T.; Dallas, M.; Stephens, V.R.; Osteen, K.G.; Bruner-Tran, K.L. Developmental 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure of either parent enhances the risk of necrotizing enterocolitis in neonatal mice. Birth Defects Res. 2020, 112, 1209–1223. [Google Scholar] [CrossRef]
- Yang, H.; Fu, J.; Xue, X.; Yao, L.; Qiao, L.; Hou, A.; Jin, L.; Xing, Y. Epithelial-mesenchymal transitions in bronchopulmonary dysplasia of newborn rats. Pediatr. Pulmonol. 2014, 49, 1112–1123. [Google Scholar] [CrossRef]
- Bartis, D.; Mise, N.; Mahida, R.Y.; Eickelberg, O.; Thickett, D.R. Epithelial–mesenchymal transition in lung development and disease: Does it exist and is it important? Thorax 2014, 69, 760–765. [Google Scholar] [CrossRef]
- Sung, N.J.; Kim, N.H.; Bae, N.Y.; Jo, H.S.; Park, S.-A. DHA inhibits Gremlin-1-induced epithelial-to-mesenchymal transition via ERK suppression in human breast cancer cells. Biosci. Rep. 2020, 40, BSR20200164. [Google Scholar] [CrossRef]
- Gao, Z.; Bu, Y.; Liu, X.; Wang, X.; Zhang, G.; Wang, E.; Ding, S.; Liu, Y.; Shi, R.; Li, Q.; et al. TCDD promoted EMT of hFPECs via AhR, which involved the activation of EGFR/ERK signaling. Toxicol. Appl. Pharm. 2016, 298, 48–55. [Google Scholar] [CrossRef]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Villamor, E. Mother’s Own Milk and Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2019, 7, 224. [Google Scholar] [CrossRef]
- Schlosser-Brandenburg, J.; Ebner, F.; Klopfleisch, R.; Kühl, A.A.; Zentek, J.; Pieper, R.; Hartmann, S. Influence of Nutrition and Maternal Bonding on Postnatal Lung Development in the Newborn Pig. Front. Immunol. 2021, 12, 734153. [Google Scholar] [CrossRef] [PubMed]
- Alapati, D.; Rong, M.; Chen, S.; Hehre, D.; Hummler, S.C.; Wu, S. Inhibition of β-catenin signaling improves alveolarization and reduces pulmonary hypertension in experimental bronchopulmonary dysplasia. Am. J. Respir. Cell Mol. Biol. 2014, 51, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Lecarpentier, Y.; Gourrier, E.; Gobert, V.; Vallée, A. Bronchopulmonary Dysplasia: Crosstalk Between PPARγ, WNT/β-Catenin and TGF-β Pathways; The Potential Therapeutic Role of PPARγ Agonists. Front. Pediatr. 2019, 7, 176. [Google Scholar] [CrossRef]
- Sucre, J.M.; Vijayaraj, P.; Aros, C.J.; Wilkinson, D.; Paul, M.; Dunn, B.; Guttentag, S.H.; Gomperts, B.N. Posttranslational modification of β-catenin is associated with pathogenic fibroblastic changes in bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L186–L195. [Google Scholar] [CrossRef] [PubMed]
- Sucre, J.M.S.; Deutsch, G.H.; Jetter, C.S.; Ambalavanan, N.; Benjamin, J.T.; Gleaves, L.A.; Millis, B.A.; Young, L.R.; Blackwell, T.S.; Kropski, J.A.; et al. A Shared Pattern of β-Catenin Activation in Bronchopulmonary Dysplasia and Idiopathic Pulmonary Fibrosis. Am. J. Pathol. 2018, 188, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Zhao, Y.; Wong, P.; Young, N.F.; Matsumura, F. The use of c-src knockout mice for the identification of the main toxic signaling pathway of TCDD to induce wasting syndrome. J. Biochem. Mol. Toxicol. 2003, 17, 305–315. [Google Scholar] [CrossRef]
- Davenport, M.L.; Sherrill, T.P.; Blackwell, T.S.; Edmonds, M.D. Perfusion and Inflation of the Mouse Lung for Tumor Histology. J. Vis. Exp. 2020, 162, e60605. [Google Scholar] [CrossRef] [PubMed]
- Karasutani, K.; Baskoro, H.; Sato, T.; Arano, N.; Suzuki, Y.; Mitsui, A.; Shimada, N.; Kodama, Y.; Seyama, K.; Fukuchi, Y.; et al. Lung Fixation under Constant Pressure for Evaluation of Emphysema in Mice. J. Vis. Exp. 2019, 151, e58197. [Google Scholar] [CrossRef]
- Cooney, T.P.; Thurlbeck, W.M. The radial alveolar count method of Emery and Mithal: A reappraisal 1-postnatal lung growth. Thorax 1982, 37, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Dechelotte, P.; Labbé, A.; Caux, O.; Vanlieferinghen, P.; Raynaud, E.J. Defect in pulmonary growth. Comparative study of 3 diagnostic criteria. Arch. Fr. Pediatr. 1987, 44, 255–261. [Google Scholar] [PubMed]
- Klein, A.W.; Becker, R.F.; Bryson, M.R. A method for estimating the distribution of alveolar sizes from histological lung sections. Trans. Am. Microsc. Soc. 1972, 91, 195–208. [Google Scholar] [CrossRef]
- Crowley, G.; Kwon, S.; Caraher, E.J.; Haider, S.H.; Lam, R.; Batra, P.; Melles, D.; Liu, M.; Nolan, A. Quantitative lung morphology: Semi-automated measurement of mean linear intercept. BMC Pulm. Med. 2019, 19, 206. [Google Scholar] [CrossRef]
- Baker, C.D.; Alvira, C.M. Disrupted lung development and bronchopulmonary dysplasia: Opportunities for lung repair and regeneration. Curr. Opin. Pediatr. 2014, 26, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Tóth, S.; Pingorová, S.; Jonecová, Z.; Morochovic, R.; Pomfy, M.; Veselá, J. Adult Respiratory Distress Syndrome and alveolar epithelium apoptosis: An histopathological and immunohistochemical study. Folia Histochem. Cytobiol. 2009, 47, 431–434. [Google Scholar] [CrossRef][Green Version]
- Go, H.; Ohto, H.; Nollet, K.E.; Sato, K.; Ichikawa, H.; Kume, Y.; Kanai, Y.; Maeda, H.; Kashiwabara, N.; Ogasawara, K.; et al. Red cell distribution width as a predictor for bronchopulmonary dysplasia in premature infants. Sci. Rep. 2021, 11, 7221. [Google Scholar] [CrossRef]
- Janz, D.R.; Ware, L.B. The role of red blood cells and cell-free hemoglobin in the pathogenesis of ARDS. J. Intensive Care 2015, 3, 20. [Google Scholar] [CrossRef]
- Hansmann, G.; Sallmon, H.; Roehr, C.C.; Kourembanas, S.; Austin, E.D.; Koestenberger, M. Pulmonary hypertension in bronchopulmonary dysplasia. Pediatr. Res. 2021, 89, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Bos, A.P.; Hussain, S.M.; Hazebroek, F.W.; Tibboel, D.; Meradji, M.; Molenaar, J.C. Radiographic evidence of bronchopulmonary dysplasia in high-risk congenital diaphragmatic hernia survivors. Pediatr. Pulmonol. 1993, 15, 231–234. [Google Scholar] [CrossRef]
- Singer, L.T.; Davillier, M.; Preuss, L.; Szekely, L.; Hawkins, S.; Yamashita, T.; Baley, J. Feeding interactions in infants with very low birth weight and bronchopulmonary dysplasia. J. Dev. Behav. Pediatr. JDBP 1996, 17, 69–76. [Google Scholar] [CrossRef]
- Dassios, T.; Williams, E.E.; Hickey, A.; Bunce, C.; Greenough, A. Bronchopulmonary dysplasia and postnatal growth following extremely preterm birth. Arch. Dis. Child. Fet. Neonatal Ed. 2021, 106, 386–391. [Google Scholar] [CrossRef]
- Laberge, J.-M.; Puligandla, P. Chapter 64-Congenital Malformations of the Lungs and Airways. In Pediatric Respiratory Medicine, 2nd ed.; Taussig, L.M., Landau, L.I., Eds.; Mosby: Philadelphia, PA, USA, 2008; pp. 907–941. [Google Scholar] [CrossRef]
- Askenazi, S.S.; Perlman, M. Pulmonary hypoplasia: Lung weight and radial alveolar count as criteria of diagnosis. Arch. Dis. Child. 1979, 54, 614–618. [Google Scholar] [CrossRef]
- D’Angio, C.T.; Maniscalco, W.M. Bronchopulmonary dysplasia in preterm infants: Pathophysiology and management strategies. Paediatr. Drugs 2004, 6, 303–330. [Google Scholar] [CrossRef]
- Pereira, G.R.; Baumgart, S.; Bennett, M.J.; Stallings, V.A.; Georgieff, M.K.; Hamosh, M.; Ellis, L. Use of high-fat formula for premature infants with bronchopulmonary dysplasia: Metabolic, pulmonary, and nutritional studies. J. Pediatr. 1994, 124, 605–611. [Google Scholar] [CrossRef]
- Young, T.E. Nutritional support and bronchopulmonary dysplasia. J. Perinatol. 2007, 27, S75–S78. [Google Scholar] [CrossRef][Green Version]
- Delgado-Peña, Y.P.; Torrent-Vernetta, A.; Sacoto, G.; de Mir-Messa, I.; Rovira-Amigo, S.; Gartner, S.; Moreno-Galdó, A.; Molino-Gahete, J.A.; Castillo-Salinas, F. Pulmonary hypoplasia: An analysis of cases over a 20-year period. Pediatria 2016, 85, 70–76. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Tang, J.; Shi, J.; Qu, Y.; Xiong, T.; Mu, D. Human milk as a protective factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fet. Neonatal Ed. 2019, 104, F128–F136. [Google Scholar] [CrossRef]
- Smukowska-Gorynia, A.; Tomaszewska, I.; Malaczynska-Rajpold, K.; Marcinkowska, J.; Komosa, A.; Janus, M.; Olasinska-Wisniewska, A.; Slawek, S.; Araszkiewicz, A.; Jankiewicz, S.; et al. Red Blood Cells Distribution Width as a Potential Prognostic Biomarker in Patients With Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension. Heart Lung Circ. 2018, 27, 842–848. [Google Scholar] [CrossRef]
- Berkelhamer, S.K.; Mestan, K.K.; Steinhorn, R.H. Pulmonary hypertension in bronchopulmonary dysplasia. Semin. Perinatol. 2013, 37, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ramani, M.; Bradley, W.E.; Dell’Italia, L.J.; Ambalavanan, N. Early exposure to hyperoxia or hypoxia adversely impacts cardiopulmonary development. Am. J. Respir. Cell Mol. Biol. 2015, 52, 594–602. [Google Scholar] [CrossRef]
- Harris, W.S.; Baack, M.L. Beyond building better brains: Bridging the docosahexaenoic acid (DHA) gap of prematurity. J. Perinatol. 2015, 35, 1–7. [Google Scholar] [CrossRef]
- Voynow, J.A.; Auten, R. Environmental Pollution and the Developing Lung. Clin. Pulm. Med. 2015, 22, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, T.W.; Stern, D.A.; Morgan, W.J.; Martinez, F.D.; Wright, A.L. Effect of breastfeeding on lung function in childhood and modulation by maternal asthma and atopy. Am. J. Respir. Crit. Care Med. 2007, 176, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, J.; Xie, H.; Wang, J.; Meng, X.; Wang, R. HIF-1α regulates EMT via the Snail and β-catenin pathways in paraquat poisoning-induced early pulmonary fibrosis. J. Cell Mol. Med. 2016, 20, 688–697. [Google Scholar] [CrossRef]
- Zhu, G.J.; Song, P.P.; Zhou, H.; Shen, X.H.; Wang, J.G.; Ma, X.F.; Gu, Y.J.; Liu, D.D.; Feng, A.N.; Qian, X.Y.; et al. Role of epithelial-mesenchymal transition markers E-cadherin, N-cadherin, β-catenin and ZEB2 in laryngeal squamous cell carcinoma. Oncol. Lett. 2018, 15, 3472–3481. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.H.; Zheng, G. E-Cadherin/β-Catenin Complex and the Epithelial Barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef] [PubMed]
- Conquer, J.A.; Martin, J.B.; Tummon, I.; Watson, L.; Tekpetey, F. Effect of DHA supplementation on DHA status and sperm motility in asthenozoospermic males. Lipids 2000, 35, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P. Effect of placental function on fatty acid requirements during pregnancy. Eur. J. Clin. Nutr. 2004, 58, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Rouse, C.A. Docosahexaenoic acid and the preterm infant. Matern. Health Neonatol. Perinatol. 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed]
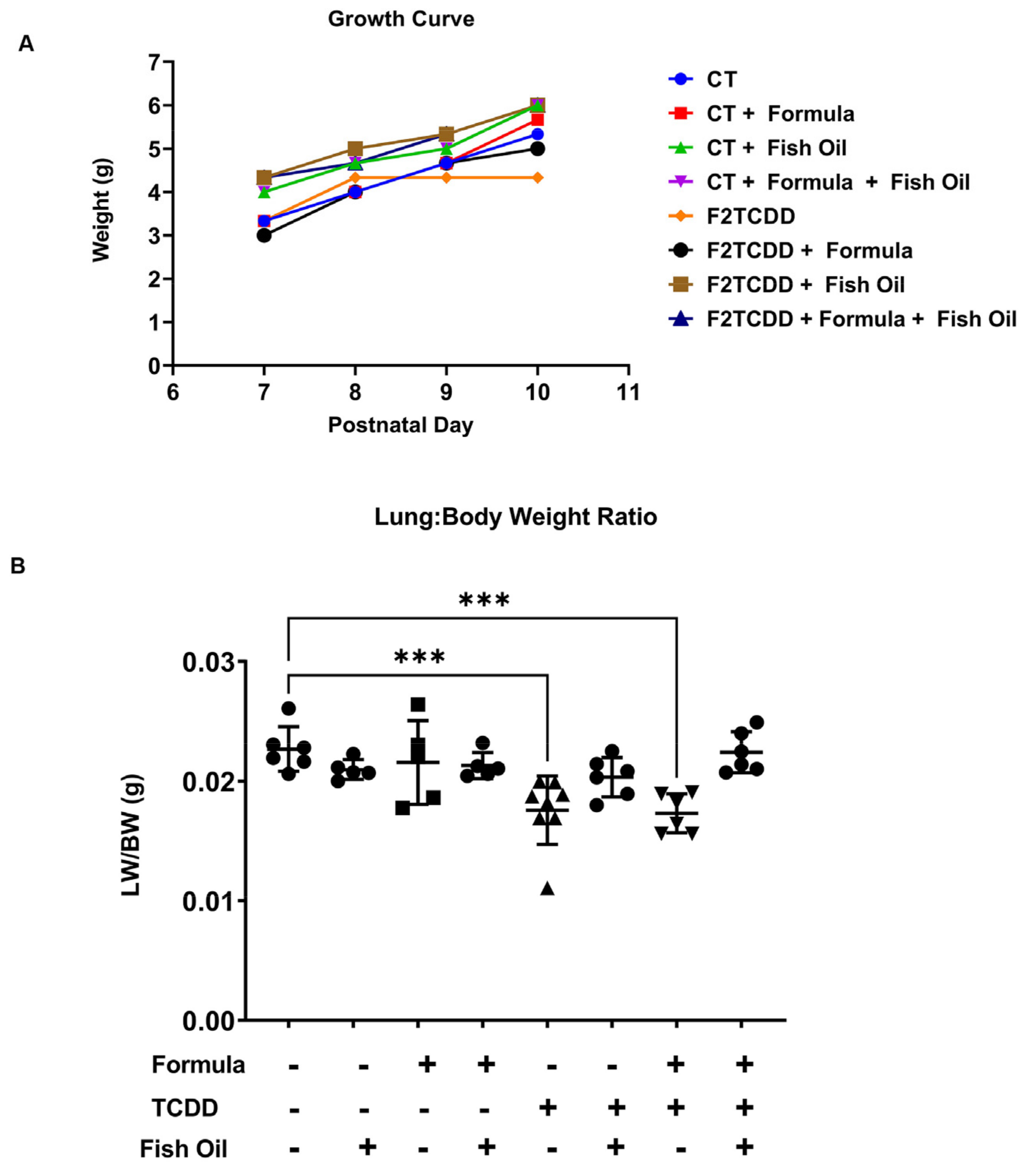

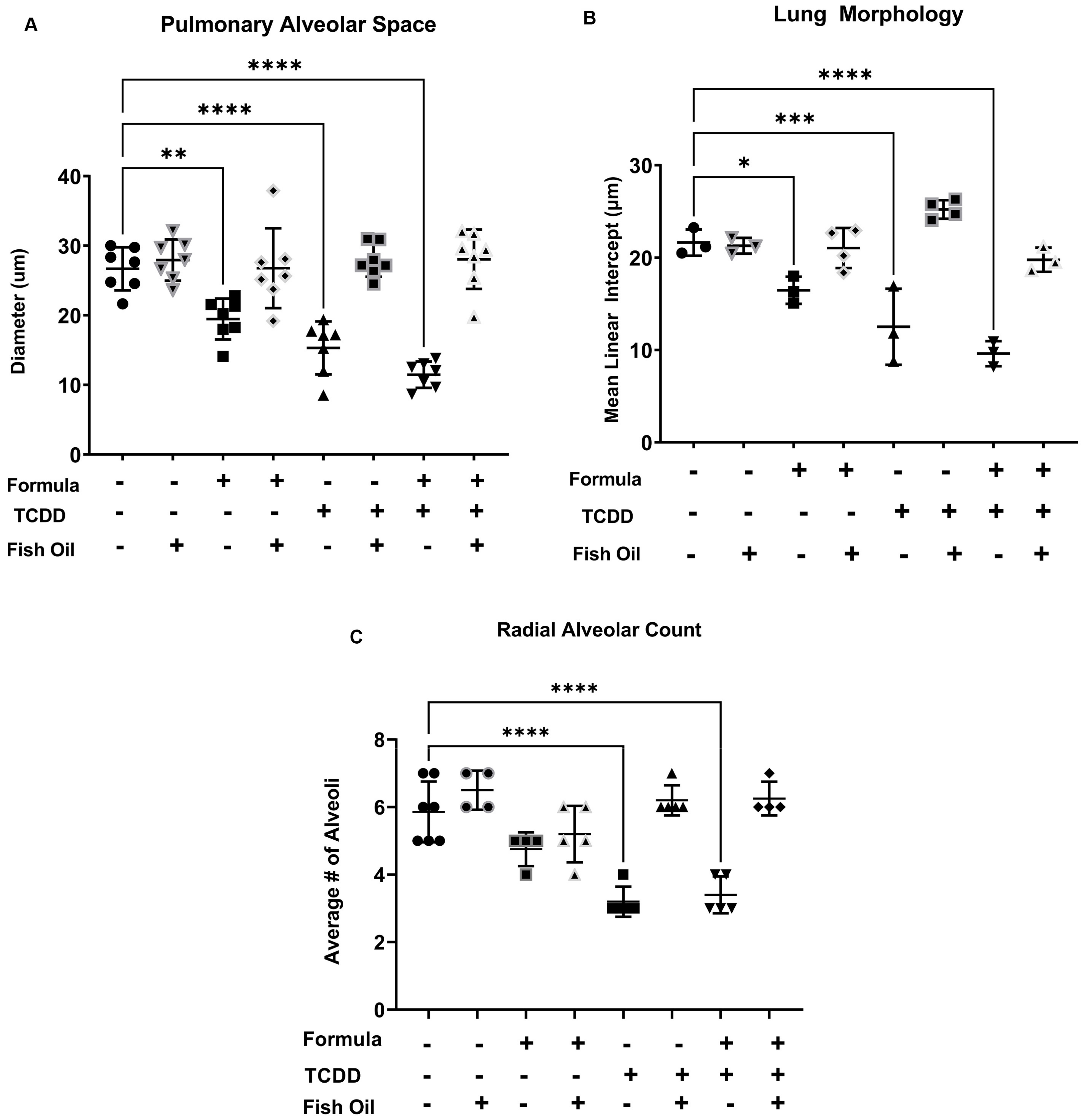
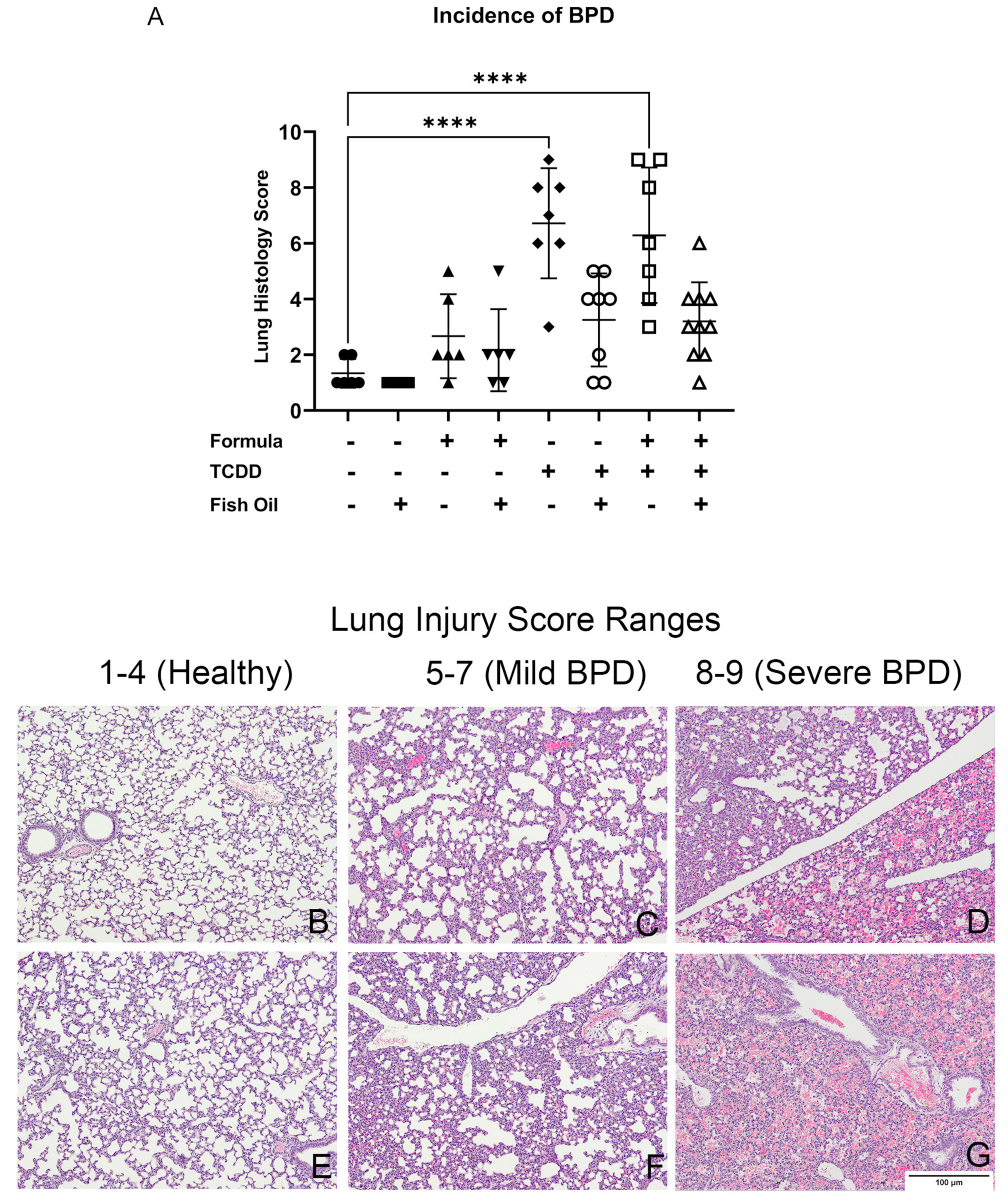
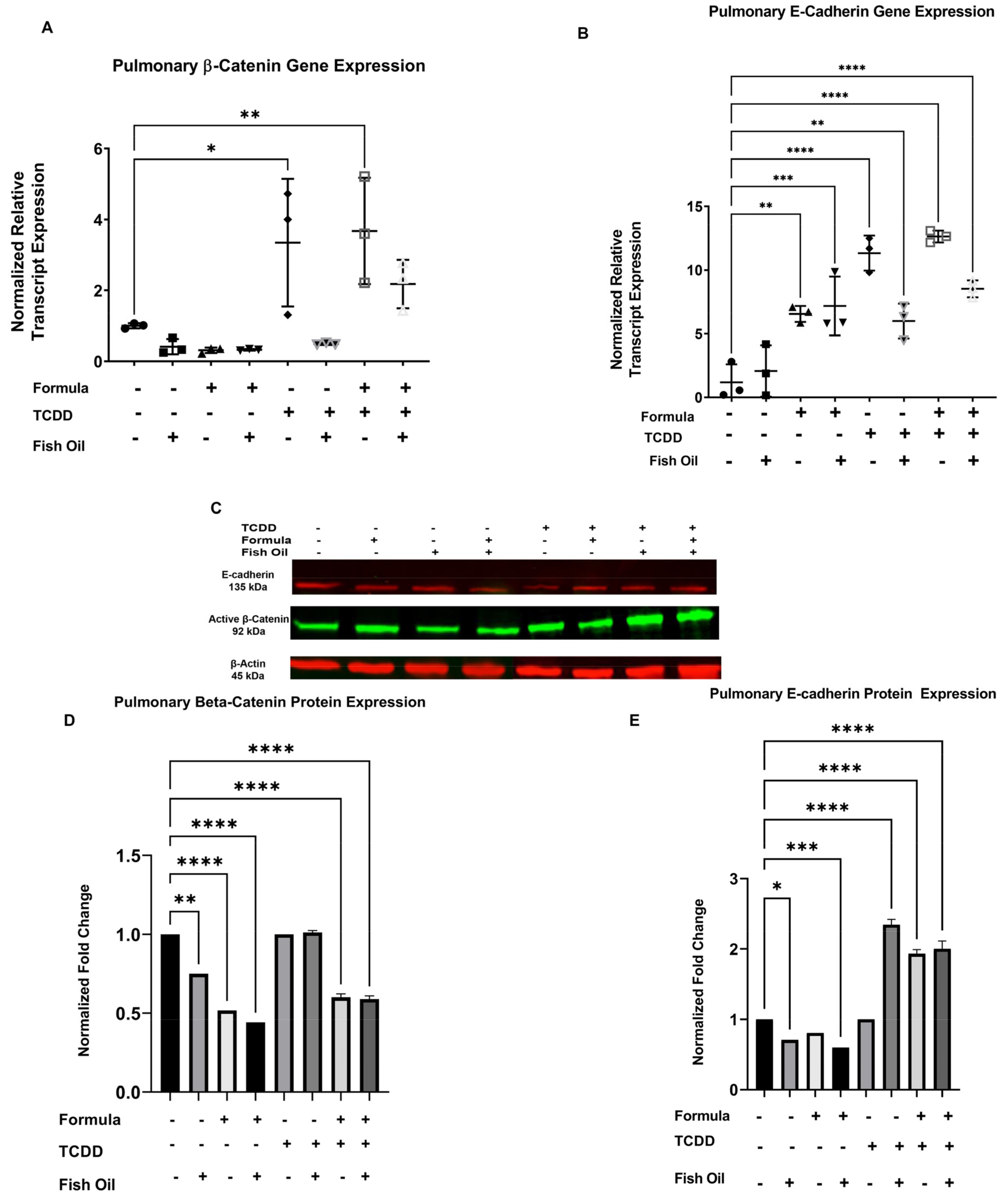
| Exposure Group | Incidence of New BPD | Average Lung Injury Score |
|---|---|---|
| CT | 0/7 = 0% | 1 |
| CT + FO | 0/7 = 0% | 1 |
| CT + FORMULA | 1/16 = 16% | 3 |
| CT + FO + FORMULA | 1/6 = 16% | 2 |
| F2TCDD | 6/7 = 85% | 6 |
| F2TCDD + FO | 2/8 = 25% | 3 |
| F2TCDD + FORMULA | 5/7 = 71% | 7 |
| F2TCDD + FO + FORMULA | 1/10 = 10% | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumph, J.T.; Rayford, K.J.; Stephens, V.R.; Ameli, S.; Nde, P.N.; Osteen, K.G.; Bruner-Tran, K.L. A Preconception Paternal Fish Oil Diet Prevents Toxicant-Driven New Bronchopulmonary Dysplasia in Neonatal Mice. Toxics 2022, 10, 7. https://doi.org/10.3390/toxics10010007
Rumph JT, Rayford KJ, Stephens VR, Ameli S, Nde PN, Osteen KG, Bruner-Tran KL. A Preconception Paternal Fish Oil Diet Prevents Toxicant-Driven New Bronchopulmonary Dysplasia in Neonatal Mice. Toxics. 2022; 10(1):7. https://doi.org/10.3390/toxics10010007
Chicago/Turabian StyleRumph, Jelonia T., Kayla J. Rayford, Victoria R. Stephens, Sharareh Ameli, Pius N. Nde, Kevin G. Osteen, and Kaylon L. Bruner-Tran. 2022. "A Preconception Paternal Fish Oil Diet Prevents Toxicant-Driven New Bronchopulmonary Dysplasia in Neonatal Mice" Toxics 10, no. 1: 7. https://doi.org/10.3390/toxics10010007
APA StyleRumph, J. T., Rayford, K. J., Stephens, V. R., Ameli, S., Nde, P. N., Osteen, K. G., & Bruner-Tran, K. L. (2022). A Preconception Paternal Fish Oil Diet Prevents Toxicant-Driven New Bronchopulmonary Dysplasia in Neonatal Mice. Toxics, 10(1), 7. https://doi.org/10.3390/toxics10010007






