Removal of Antibiotics and Nutrients by Vetiver Grass (Chrysopogon zizanioides) from a Plug Flow Reactor Based Constructed Wetland Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Wastewater Collection
2.3. Experimental Design
2.4. Constructed Wetland Setup
2.5. Analytical Methods
2.5.1. Physico-Chemical Parameters
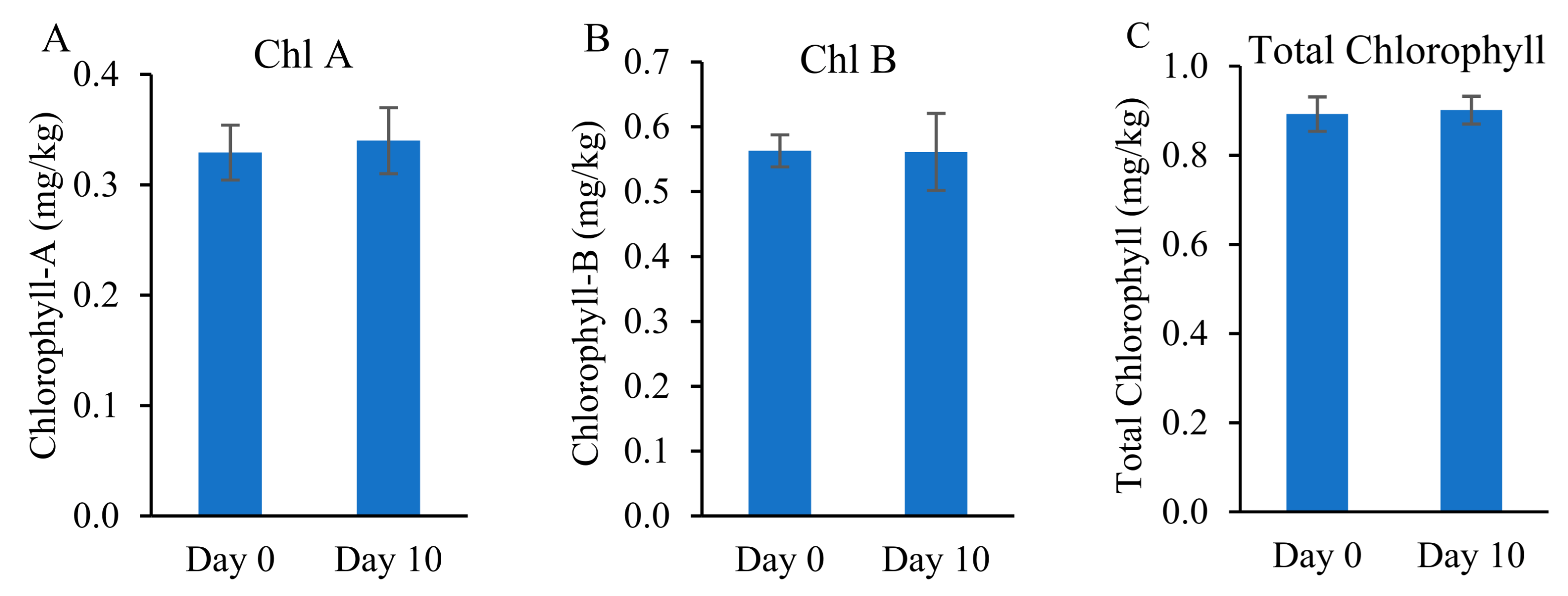
2.5.2. Chlorophyll Content
2.5.3. Antibiotics
2.5.4. Total Nitrogen, Total Phosphorus, and Chemical Oxygen Demand
2.5.5. Statistical Analysis
3. Results
3.1. Wastewater Characterization
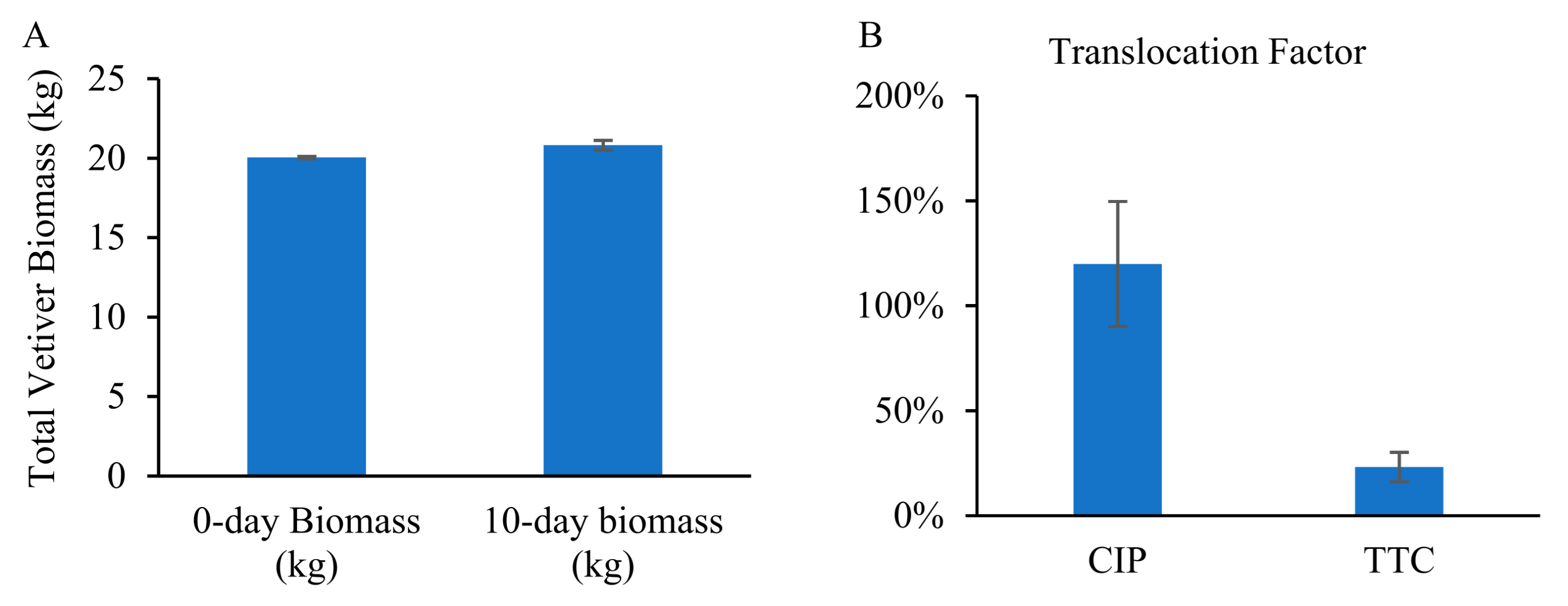
3.2. Constructed Wetland Experiment
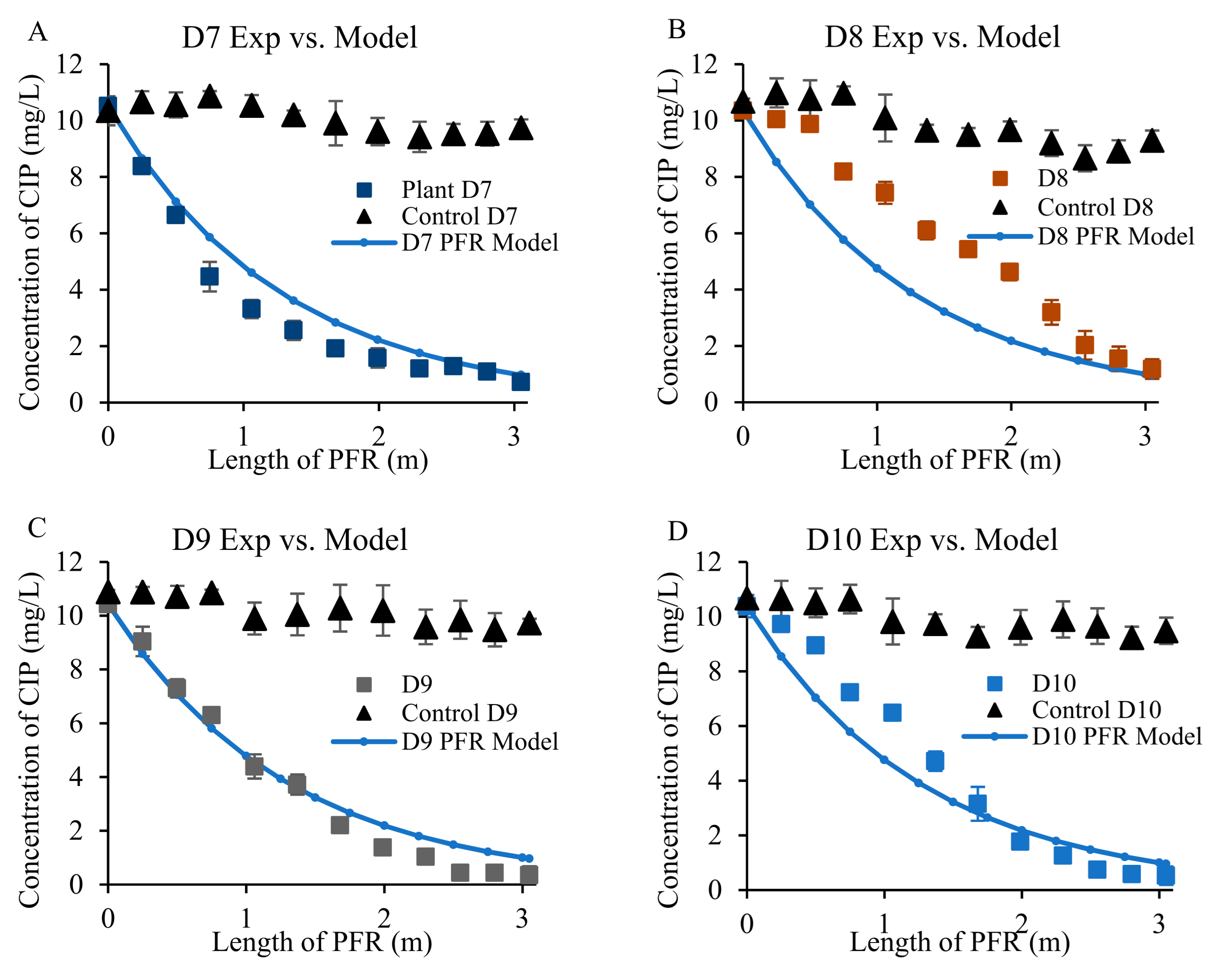
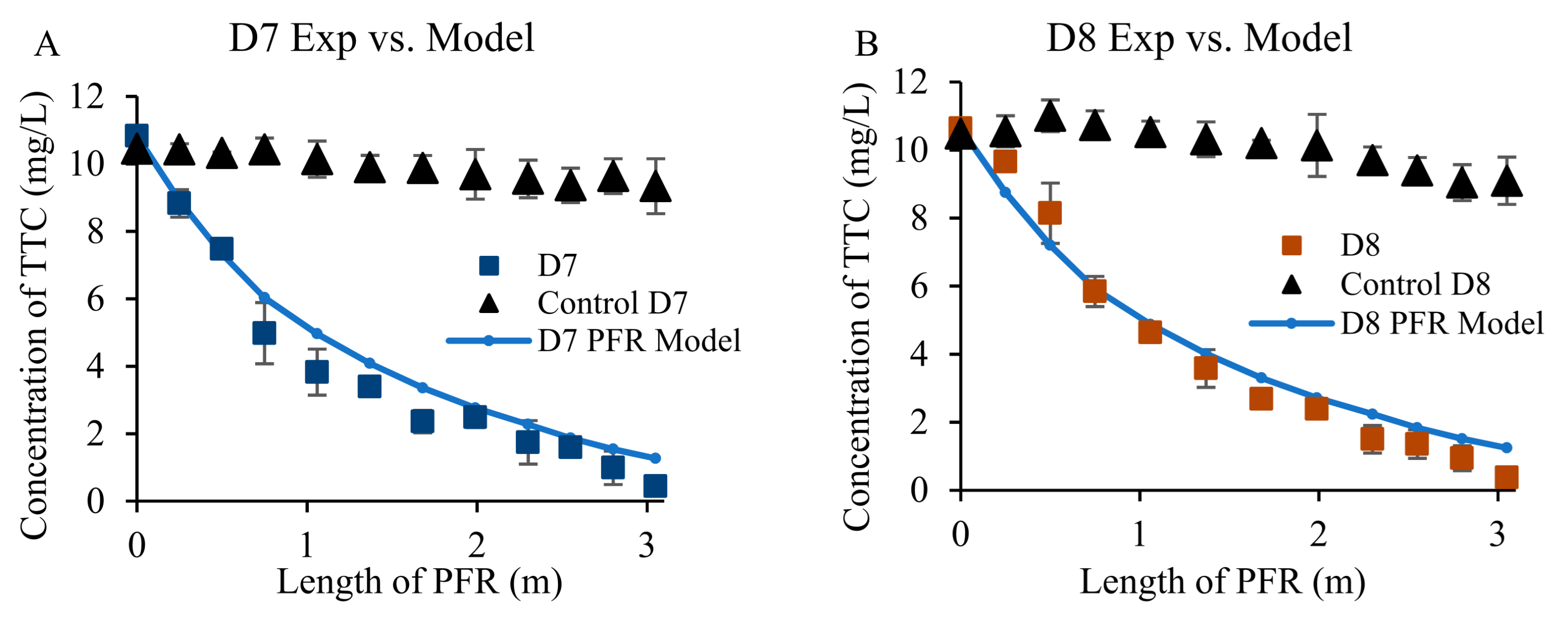
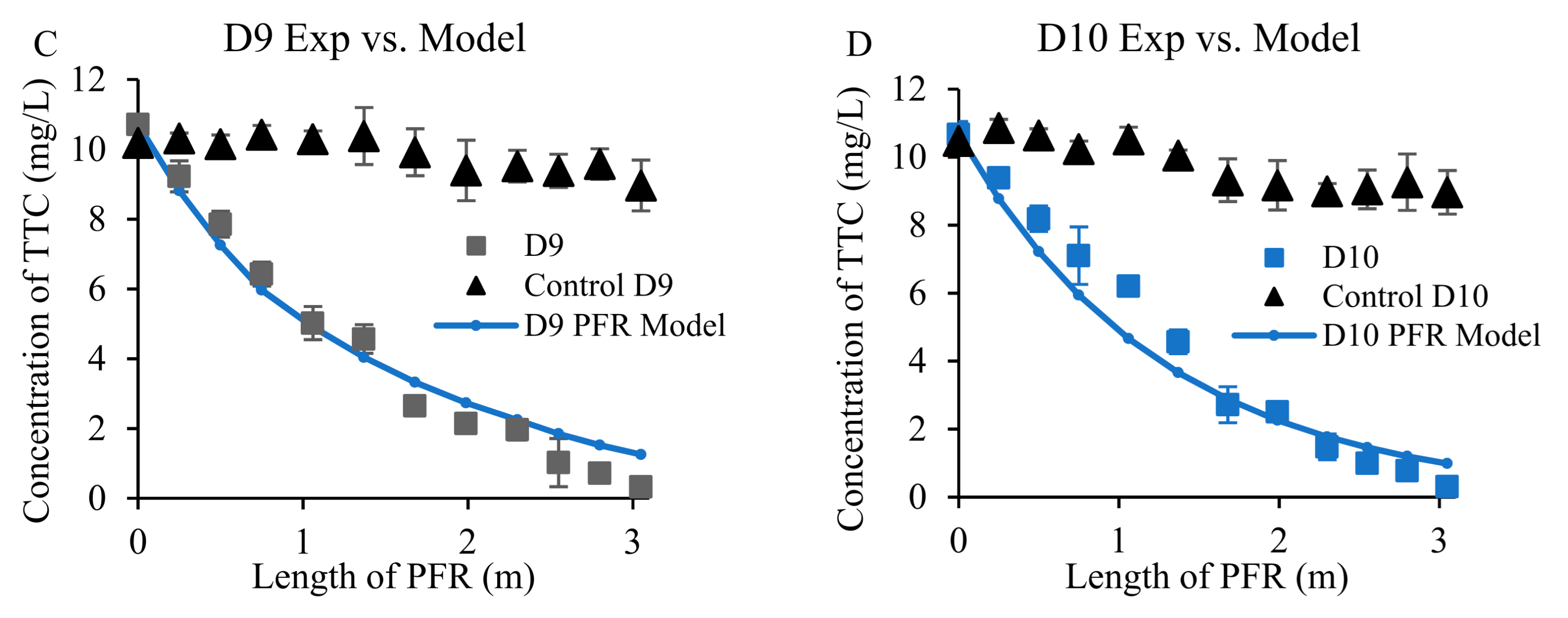
3.3. Removal of Antibiotics from Wastewater
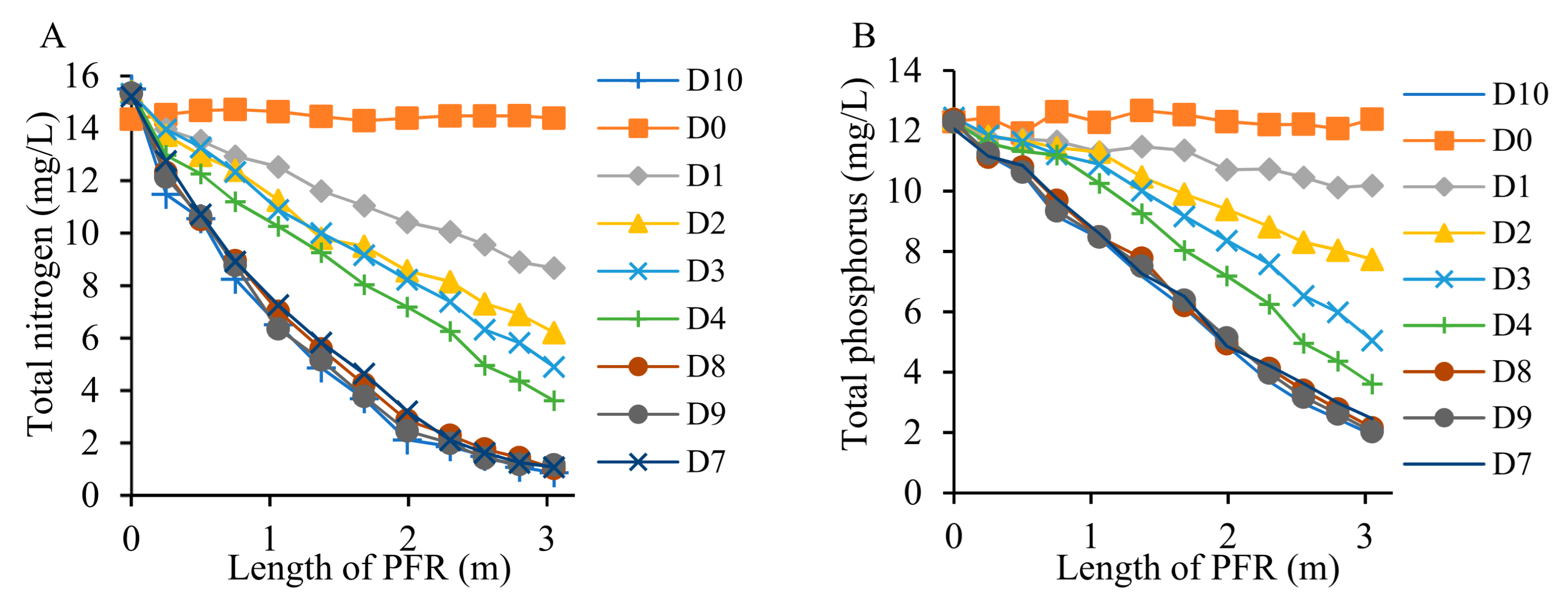
3.4. Removal of Nutrients from Wastewater
3.5. Removal of COD from Wastewater
3.6. Chlorophyll Content and Biomass Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Casado, J.; Brigden, K.; Santillo, D.; Johnston, P. Screening of pesticides and veterinary drugs in small streams in the European Union by liquid chromatography high resolution mass spectrometry. Sci. Total Environ. 2019, 670, 1204–1225. [Google Scholar] [CrossRef] [PubMed]
- Breton, R.; Boxall, A. Pharmaceuticals and personal care products in the environment: Regulatory drivers and research needs. Qsar Comb. Sci. 2003, 22, 399–409. [Google Scholar] [CrossRef]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Removal of pharmaceuticals and personal care products in aquatic plant-based systems: A review. Environ. Pollut. 2014, 184, 620–639. [Google Scholar] [CrossRef]
- Hartley, D.L.; Jones, K.R.; Tobian, J.; LeBlanc, D.J.; Macrina, F. Disseminated tetracycline resistance in oral streptococci: Implication of a conjugative transposon. Infect. Immun. 1984, 45, 13–17. [Google Scholar] [CrossRef]
- Chopra, I.; Hawkey, P.; Hinton, M. Tetracyclines, molecular and clinical aspects. J. Antimicrob. Chemother. 1992, 29, 245–277. [Google Scholar] [CrossRef]
- Roberts, M. Tetracycline resistance determinants: Mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol. Rev. 1996, 19, 1–24. [Google Scholar] [CrossRef]
- Hawker, D.W.; Cropp, R.; Boonsaner, M. Uptake of zwitterionic antibiotics by rice (Oryza sativa L.) in contaminated soil. J. Hazard. Mater. 2013, 263, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, A.; Carrupt, P.-A.; Caron, G.; Gaillard, P.; Testa, B. Lipophilicity profiles of ampholytes. Chem. Rev. 1997, 97, 3385–3400. [Google Scholar] [CrossRef]
- Qiang, Z.; Adams, C. Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics. Water Res. 2004, 38, 2874–2890. [Google Scholar] [CrossRef]
- Ding, S.; Han, C.; Wang, Y.; Yao, L.; Wang, Y.; Xu, D.; Sun, Q.; Williams, P.N.; Zhang, C. In situ, high-resolution imaging of labile phosphorus in sediments of a large eutrophic lake. Water Res. 2015, 74, 100–109. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Liu, Y.; Chen, F.; Duan, Y.; Wei, G.; Zheng, X.; Li, M. The missing nitrogen pieces: A critical review on the distribution, transformation, and budget of nitrogen in the vadose zone-groundwater system. Water Res. 2019, 165, 114977. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef]
- Feng, Y.; He, W.; Liu, J.; Wang, X.; Qu, Y.; Ren, N. A horizontal plug flow and stackable pilot microbial fuel cell for municipal wastewater treatment. Bioresour. Technol. 2014, 156, 132–138. [Google Scholar] [CrossRef]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. TRENDS Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- De Witte, B.; Dewulf, J.; Demeestere, K.; Van Langenhove, H. Ozonation and advanced oxidation by the peroxone process of ciprofloxacin in water. J. Hazard. Mater. 2009, 161, 701–708. [Google Scholar] [CrossRef]
- Ji, Y.; Ferronato, C.; Salvador, A.; Yang, X.; Chovelon, J.-M. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: Implications for remediation of groundwater contaminated by antibiotics. Sci. Total Environ. 2014, 472, 800–808. [Google Scholar] [CrossRef]
- Vasconcelos, T.G.; Henriques, D.M.; König, A.; Martins, A.F.; Kümmerer, K. Photo-degradation of the antimicrobial ciprofloxacin at high pH: Identification and biodegradability assessment of the primary by-products. Chemosphere 2009, 76, 487–493. [Google Scholar] [CrossRef]
- Fu, H.; Yang, L.; Wan, Y.; Xu, Z.; Zhu, D. Adsorption of pharmaceuticals to microporous activated carbon treated with potassium hydroxide, carbon dioxide, and steam. J. Environ. Qual. 2011, 40, 1886–1894. [Google Scholar] [CrossRef]
- Rakshit, S.; Sarkar, D.; Elzinga, E.J.; Punamiya, P.; Datta, R. Mechanisms of ciprofloxacin removal by nano-sized magnetite. J. Hazard. Mater. 2013, 246, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Gersberci, R.; Man, G. Role of aquatic plants in wastewater treatment by artificial wetlands. Water Res. 1986. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Eapen, S.; Singh, S.; D’souza, S. Advances in development of transgenic plants for remediation of xenobiotic pollutants. Biotechnol. Adv. 2007, 25, 442–451. [Google Scholar] [CrossRef]
- Gan, S.; Lau, E.; Ng, H. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Y.; Zhang, J.; Luo, L.; Yang, Y.; Huang, H.; Peng, H.; Tang, L.; Mu, Y. Insight into electro-Fenton and photo-Fenton for the degradation of antibiotics: Mechanism study and research gaps. Chem. Eng. J. 2018, 347, 379–397. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, J.; Sharp, R. Phytoremediation in engineered wetlands: Mechanisms and applications. Procedia Environ. Sci. 2010, 2, 1315–1325. [Google Scholar] [CrossRef]
- Susarla, S.; Medina, V.F.; McCutcheon, S.C. Phytoremediation: An ecological solution to organic chemical contamination. Ecol. Eng. 2002, 18, 647–658. [Google Scholar] [CrossRef]
- Haberl, R.; Grego, S.; Langergraber, G.; Kadlec, R.H.; Cicalini, A.-R.; Dias, S.M.; Novais, J.M.; Aubert, S.; Gerth, A.; Thomas, H. Constructed wetlands for the treatment of organic pollutants. J. Soils Sediments 2003, 3, 109. [Google Scholar] [CrossRef]
- Imfeld, G.; Braeckevelt, M.; Kuschk, P.; Richnow, H.H. Monitoring and assessing processes of organic chemicals removal in constructed wetlands. Chemosphere 2009, 74, 349–362. [Google Scholar] [CrossRef]
- Olette, R.; Couderchet, M.; Biagianti, S.; Eullaffroy, P. Toxicity and removal of pesticides by selected aquatic plants. Chemosphere 2008, 70, 1414–1421. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L. Removal of organics in constructed wetlands with horizontal sub-surface flow: A review of the field experience. Sci. Total Environ. 2009, 407, 3911–3922. [Google Scholar] [CrossRef]
- Truong, P.N.; Foong, Y.K.; Guthrie, M.; Hung, Y.-T. Phytoremediation of heavy metal contaminated soils and water using vetiver grass. In Environmental Bioengineering; Humana Press: Totowa, NJ, USA, 2010; pp. 233–275. [Google Scholar]
- Truong, P.; Van, T.; Pinners, E. Vetiver system for prevention and treatment of contaminated water and land. In The Vetiver System for Improving Water Quality the Prevention and Treatment of Contaminated Water and Land; The Vetiver Network International: San Antonio, TX, USA, 2008; Volume 1, p. 33. Available online: http://www.vetiver.org/TVN_Water_quality%202%20ed.pdf (accessed on 10 March 2021).
- Diyabalanage, S.; Samarakoon, K.; Adikari, S.; Hewawasam, T. Impact of soil and water conservation measures on soil erosion rate and sediment yields in a tropical watershed in the Central Highlands of Sri Lanka. Appl. Geogr. 2017, 79, 103–114. [Google Scholar] [CrossRef]
- Donjadee, S.; Tingsanchali, T. Soil and water conservation on steep slopes by mulching using rice straw and vetiver grass clippings. Agric. Nat. Resour. 2016, 50, 75–79. [Google Scholar] [CrossRef]
- Chowdhury, M.E.; Hossain, A.; Muktadir, H. Evaluation of the river training work of Padma river through a mathematical approach. J. Mod. Sci. Technol. 2018, 6, 52–62. [Google Scholar]
- Tang, V.T.; Fu, D.; Ngoc Binh, T.; Rene, E.R.; Sang, T.T.T.; Singh, R.P. An Investigation on performance and structure of ecological revetment in a sub-tropical area: A case study on cuatien river, vinh city, Vietnam. Water 2018, 10, 636. [Google Scholar] [CrossRef]
- Zaman, M.W.; Asik, T.Z.; Rumi, M.Y.; Shahin, H. Geotechnical hazard analysis of river embankment of bangladesh and its protectability. Int. J. 2016, 10, 2050–2057. [Google Scholar] [CrossRef]
- Andra, S.S.; Datta, R.; Sarkar, D.; Makris, K.C.; Mullens, C.P.; Sahi, S.V.; Bach, S.B. Induction of lead-binding phytochelatins in vetiver grass [Vetiveria zizanioides (L.)]. J. Environ. Qual. 2009, 38, 868–877. [Google Scholar] [CrossRef]
- Attinti, R.; Barrett, K.R.; Datta, R.; Sarkar, D. Ethylenediaminedisuccinic acid (EDDS) enhances phytoextraction of lead by vetiver grass from contaminated residential soils in a panel study in the field. Environ. Pollut. 2017, 225, 524–533. [Google Scholar] [CrossRef]
- Pidatala, V.R.; Li, K.; Sarkar, D.; Ramakrishna, W.; Datta, R. Identification of biochemical pathways associated with lead tolerance and detoxification in Chrysopogon zizanioides L. Nash (Vetiver) by metabolic profiling. Environ. Sci. Technol. 2016, 50, 2530–2537. [Google Scholar] [CrossRef]
- Punamiya, P.; Datta, R.; Sarkar, D.; Barber, S.; Patel, M.; Das, P. Symbiotic role of Glomus mosseae in phytoextraction of lead in vetiver grass [Chrysopogon zizanioides (L.)]. J. Hazard. Mater. 2010, 177, 465–474. [Google Scholar] [CrossRef]
- Das, P.; Datta, R.; Makris, K.C.; Sarkar, D. Vetiver grass is capable of removing TNT from soil in the presence of urea. Environ. Pollut. 2010, 158, 1980–1983. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Sarkar, D.; Datta, R. Remediation of acid mine drainage-impacted water. Curr. Pollut. Rep. 2015, 1, 131–141. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Datta, R.; Sarkar, D. Heavy Metal Pollution and Remediation. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–373. [Google Scholar]
- Makris, K.C.; Shakya, K.M.; Datta, R.; Sarkar, D.; Pachanoor, D. High uptake of 2, 4, 6-trinitrotoluene by vetiver grass–Potential for phytoremediation? Environ. Pollut. 2007, 146, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Sarkar, D.; Makris, K.C.; Datta, R. Urea-facilitated uptake and nitroreductase-mediated transformation of 2, 4, 6-trinitrotoluene in soil using vetiver grass. J. Environ. Chem. Eng. 2015, 3, 445–452. [Google Scholar] [CrossRef]
- Panja, S.; Sarkar, D.; Datta, R. Vetiver grass (Chrysopogon zizanioides) is capable of removing insensitive high explosives from munition industry wastewater. Chemosphere 2018, 209, 920–927. [Google Scholar] [CrossRef] [PubMed]
- RoyChowdhury, A.; Mukherjee, P.; Panja, S.; Datta, R.; Christodoulatos, C.; Sarkar, D. Evidence for Phytoremediation and Phytoexcretion of NTO from Industrial Wastewater by Vetiver Grass. Molecules 2021, 26, 74. [Google Scholar] [CrossRef]
- Percy, I.; Truong, P. Landfill leachate disposal with irrigated vetiver grass. In Proceedings of the Third International Conference on Vetiver, Guangzhou, China, 6–9 October 2003. [Google Scholar]
- Roongtanakiat, N.; Osotsapar, Y.; Yindiram, C. Effects of soil amendment on growth and heavy metals content in vetiver grown on iron ore tailings. Kasetsart J. 2008, 42, 397–406. [Google Scholar]
- Datta, R.; Das, P.; Smith, S.; Punamiya, P.; Ramanathan, D.M.; Reddy, R.; Sarkar, D. Phytoremediation potential of vetiver grass [Chrysopogon zizanioides (L.)] for tetracycline. Int. J. Phytoremed. 2013, 15, 343–351. [Google Scholar] [CrossRef]
- Sengupta, A.; Sarkar, D.; Das, P.; Panja, S.; Parikh, C.; Ramanathan, D.; Bagley, S.; Datta, R. Tetracycline uptake and metabolism by vetiver grass (Chrysopogon zizanioides L. Nash). Environ. Sci. Pollut. Res. 2016, 23, 24880–24889. [Google Scholar] [CrossRef]
- Panja, S.; Das, P.; Sarkar, D.; Deng, Y.; Datta, R. Potential of vetiver grass to remove oxytetracycline and ciprofloxacin from aquatic media: Preliminary results from a hydroponic study. In Proceedings of the Geological Society of America Abstracts with Program, Vancouver, MD, Canada, 19–22 October 2014. [Google Scholar]
- Worku, A.; Tefera, N.; Kloos, H.; Benor, S. Bioremediation of brewery wastewater using hydroponics planted with vetiver grass in Addis Ababa, Ethiopia. Bioresour. Bioprocess. 2018, 5, 39. [Google Scholar] [CrossRef]
- Panja, S.; Sarkar, D.; Datta, R. Removal of antibiotics and nutrients by Vetiver grass (Chrysopogon zizanioides) from secondary wastewater effluent. Int. J. Phytoremed. 2020, 22, 764–773. [Google Scholar] [CrossRef]
- Panja, S.; Sarkar, D.; Datta, R. Removal of tetracycline and ciprofloxacin from wastewater by vetiver grass (Chrysopogon zizanioides (L.) Roberty) as a function of nutrient concentrations. Environ. Sci. Pollut. Res. 2020, 27, 34951–34965. [Google Scholar] [CrossRef]
- Panja, S.; Sarkar, D.; Li, K.; Datta, R. Uptake and transformation of ciprofloxacin by vetiver grass (Chrysopogon zizanioides). Int. Biodeterior. Biodegrad. 2019, 142, 200–210. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A review on removing pharmaceutical contaminants from wastewater by constructed wetlands: Design, performance and mechanism. Sci. Total Environ. 2014, 468–469, 908–932. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.-h.; Liu, C.-x.; Wang, Z.; Dong, J.; Zhu, G.-f.; Huang, X. Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions. Ecol. Eng. 2013, 53, 138–143. [Google Scholar] [CrossRef]
- Arnon, D. Estimation of total chlorophyll. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, X.; Shi, C.; Yan, W.; Zhang, L.; Wang, G. Ecotoxicological effects and accumulation of ciprofloxacin in Eichhornia crassipes under hydroponic conditions. Environ. Sci. Pollut. Res. 2019, 26, 30348–30355. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, M.; Zhong, H.; Li, P.; Zhang, C.; Wei, D.; Zhao, T. Responses of the growth and physiological characteristics of Myriophyllum aquaticum to coexisting tetracyclines and copper in constructed wetland microcosms. Environ. Pollut. 2020, 261, 114204. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Sharma, P.; Arya, S.L.; Panwar, P. Acacia nilotica-based silvipastoral systems for resource conservation and improved productivity from degraded lands of the Lower Himalayas. Agrofor. Syst. 2014, 88, 851–863. [Google Scholar] [CrossRef]
- Xu, L. Vetiver research and development: A decade experience from China. In Proceedings of the Second International Vetiver Conference, ORDPB, Bangkok, Thailand, 18–22 January 2002; pp. 311–322. [Google Scholar]
- Tambunan, J.A.M.; Effendi, H.; Krisanti, M. Phytoremediating batik wastewater using vetiver Chrysopogon zizanioides (L). Polish J. Environ. Stud. 2018, 27, 1281–1288. [Google Scholar] [CrossRef]
- Rydzyński, D.; Piotrowicz-Cieślak, A.I.; Grajek, H.; Michalczyk, D.J. Instability of chlorophyll in yellow lupin seedlings grown in soil contaminated with ciprofloxacin and tetracycline. Chemosphere 2017, 184, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Golet, E.M.; Xifra, I.; Siegrist, H.; Alder, A.C.; Giger, W. Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environ. Sci. Technol. 2003, 37, 3243–3249. [Google Scholar] [CrossRef]
- Lou, Y.; Ye, Z.-L.; Chen, S.; Ye, X.; Deng, Y.; Zhang, J. Sorption behavior of tetracyclines on suspended organic matters originating from swine wastewater. J. Environ. Sci. 2018, 65, 144–152. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Kalavrouziotis, I.K.; Koukoulakis, P.H.; Vasquez, M. The risks associated with wastewater reuse and xenobiotics in the agroecological environment. Sci. Total Environ. 2011, 409, 3555–3563. [Google Scholar] [CrossRef]
- Gao, P.; Munir, M.; Xagoraraki, I. Correlation of tetracycline and sulfonamide antibiotics with corresponding resistance genes and resistant bacteria in a conventional municipal wastewater treatment plant. Sci. Total Environ. 2012, 421, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ojoawo, S.O.; Udayakumar, G.; Naik, P. Phytoremediation of phosphorus and nitrogen with Canna x generalis reeds in domestic wastewater through NMAMIT constructed wetland. Aquat. Procedia 2015, 4, 349–356. [Google Scholar] [CrossRef]
- Mohd Nizam, N.U.; Mohd Hanafiah, M.; Mohd Noor, I.; Abd Karim, H.I. Efficiency of five selected aquatic plants in phytoremediation of aquaculture wastewater. Appl. Sci. 2020, 10, 2712. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Peng, L.; Yang, Y.-S.; Chen, W.-R. Phytoremediation of urban wastewater by model wetlands with ornamental hydrophytes. J. Environ. Sci. 2007, 19, 902–909. [Google Scholar] [CrossRef]
- Song, H.; Li, X.; Wang, X.; Lu, X. Enhancing nitrogen removal performance of vegetated floating-bed by adding Hyriopsiscumingii Lea and an artificial medium. Fresenius Environ. Bull. 2011, 20, 2435–2441. [Google Scholar]
- Angassa, K.; Leta, S.; Mulat, W.; Kloos, H.; Meers, E. Effect of hydraulic loading on bioremediation of municipal wastewater using constructed wetland planted with vetiver grass, Addis Ababa, Ethiopia. Nanotechnol. Environ. Eng. 2019, 4, 6. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Sample, D.J. Assessment of the nutrient removal effectiveness of floating treatment wetlands applied to urban retention ponds. J. Environ. Manag. 2014, 137, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.S.; Chan, D.J.C. Wastewater phytoremediation by Salvinia molesta. J. Water Process. Eng. 2017, 15, 107–115. [Google Scholar] [CrossRef]
- Tanjung, R.; Fahruddin, F.; Samawi, M. Phytoremediation relationship of lead (Pb) by Eichhornia crassipes on pH, BOD and COD in groundwater. J. Phys. Conf. Ser. 2019, 022020. [Google Scholar] [CrossRef]
- Mahajan, P.; Kaushal, J.; Upmanyu, A.; Bhatti, J. Assessment of phytoremediation potential of Chara vulgaris to treat toxic pollutants of textile effluent. J. Toxicol. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]



| Wastewater Parameters | Values | Units |
|---|---|---|
| pH | 6.8 | |
| Electrical conductivity | 801 | μS/cm |
| Turbidity | 13.4 | NTU * |
| Dissolved oxygen | 7.3 | mg/L |
| Chemical oxygen demand (COD) | 62 | mg/L |
| Total nitrogen (TN) | 14.4 | mg/L |
| Total phosphate (TP) | 12.3 | mg/L |
| Total organic carbon (TOC) | 21 | mg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panja, S.; Sarkar, D.; Zhang, Z.; Datta, R. Removal of Antibiotics and Nutrients by Vetiver Grass (Chrysopogon zizanioides) from a Plug Flow Reactor Based Constructed Wetland Model. Toxics 2021, 9, 84. https://doi.org/10.3390/toxics9040084
Panja S, Sarkar D, Zhang Z, Datta R. Removal of Antibiotics and Nutrients by Vetiver Grass (Chrysopogon zizanioides) from a Plug Flow Reactor Based Constructed Wetland Model. Toxics. 2021; 9(4):84. https://doi.org/10.3390/toxics9040084
Chicago/Turabian StylePanja, Saumik, Dibyendu Sarkar, Zhiming Zhang, and Rupali Datta. 2021. "Removal of Antibiotics and Nutrients by Vetiver Grass (Chrysopogon zizanioides) from a Plug Flow Reactor Based Constructed Wetland Model" Toxics 9, no. 4: 84. https://doi.org/10.3390/toxics9040084
APA StylePanja, S., Sarkar, D., Zhang, Z., & Datta, R. (2021). Removal of Antibiotics and Nutrients by Vetiver Grass (Chrysopogon zizanioides) from a Plug Flow Reactor Based Constructed Wetland Model. Toxics, 9(4), 84. https://doi.org/10.3390/toxics9040084









