Manganese-Induced Neurotoxicity through Impairment of Cross-Talk Pathways in Human Neuroblastoma Cell Line SH-SY5Y Differentiated with Retinoic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Manganese Species
2.2. Human Neuroblastoma SH-SY5Y Cell Line Experimental Setup
2.3. Cell Viability Assay
2.4. Transcriptomics
2.4.1. RNA Extraction and Purification
2.4.2. Microarray Assays
2.4.3. Validation of Toxicogenomics Results through Real-Time Reverse Transcription-PCR (qRT-PCR)
2.5. Bioinformatics and Data Analysis
2.6. Prediction of Protein–Protein Interaction (PPI) and Gene Ontology (GO) Analysis
3. Results
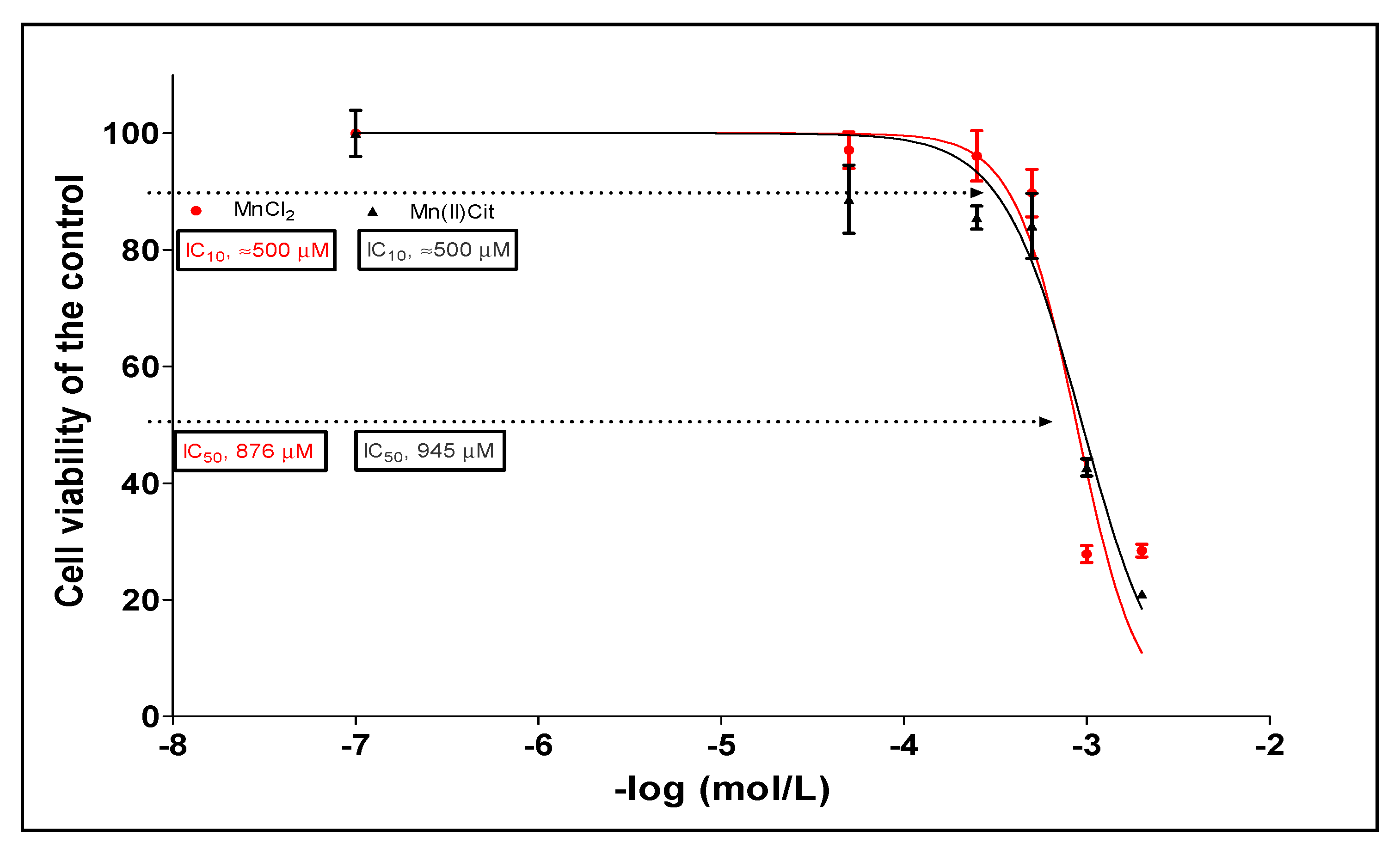
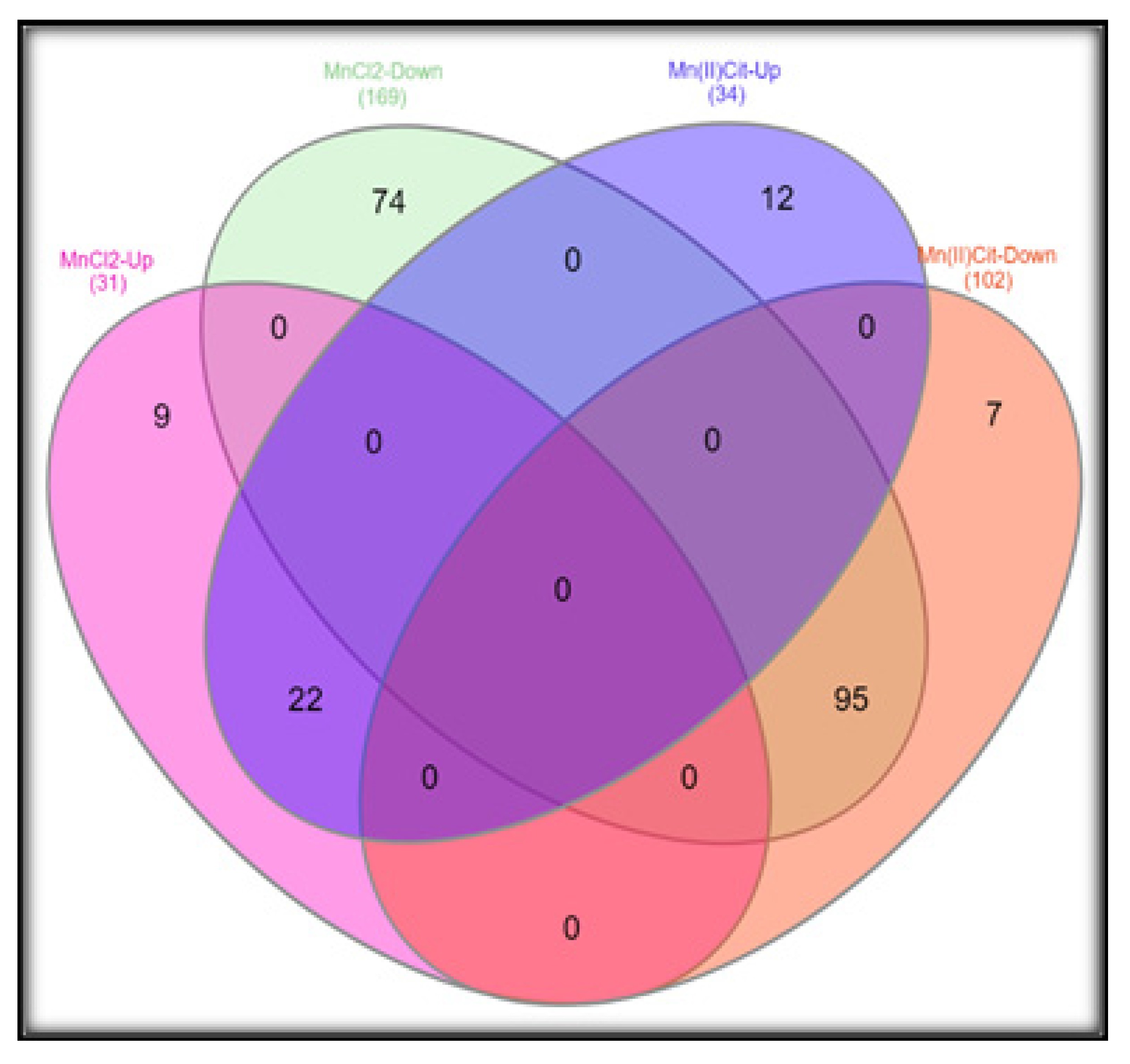
3.1. Manganese-Induced Toxicity and Differential Gene Expression in the RA-Differentiated SH-SY5Y-DAergic Cell Model
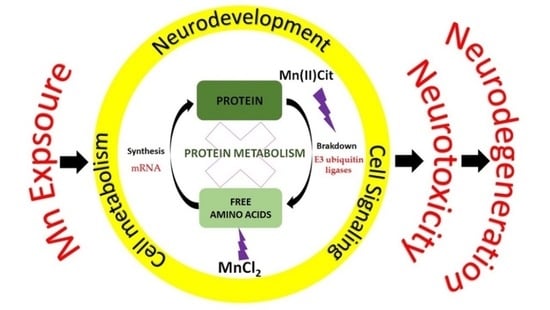
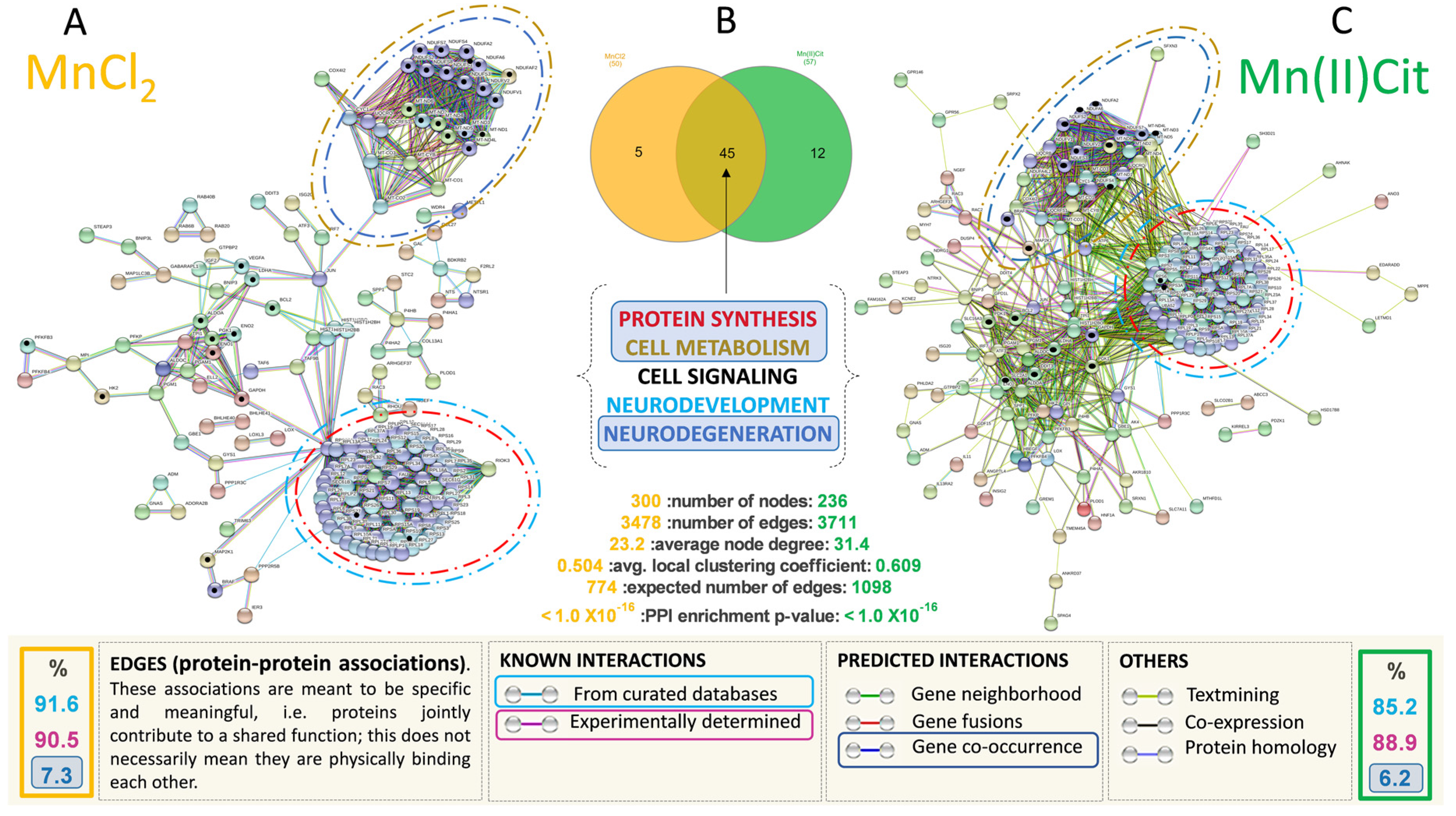
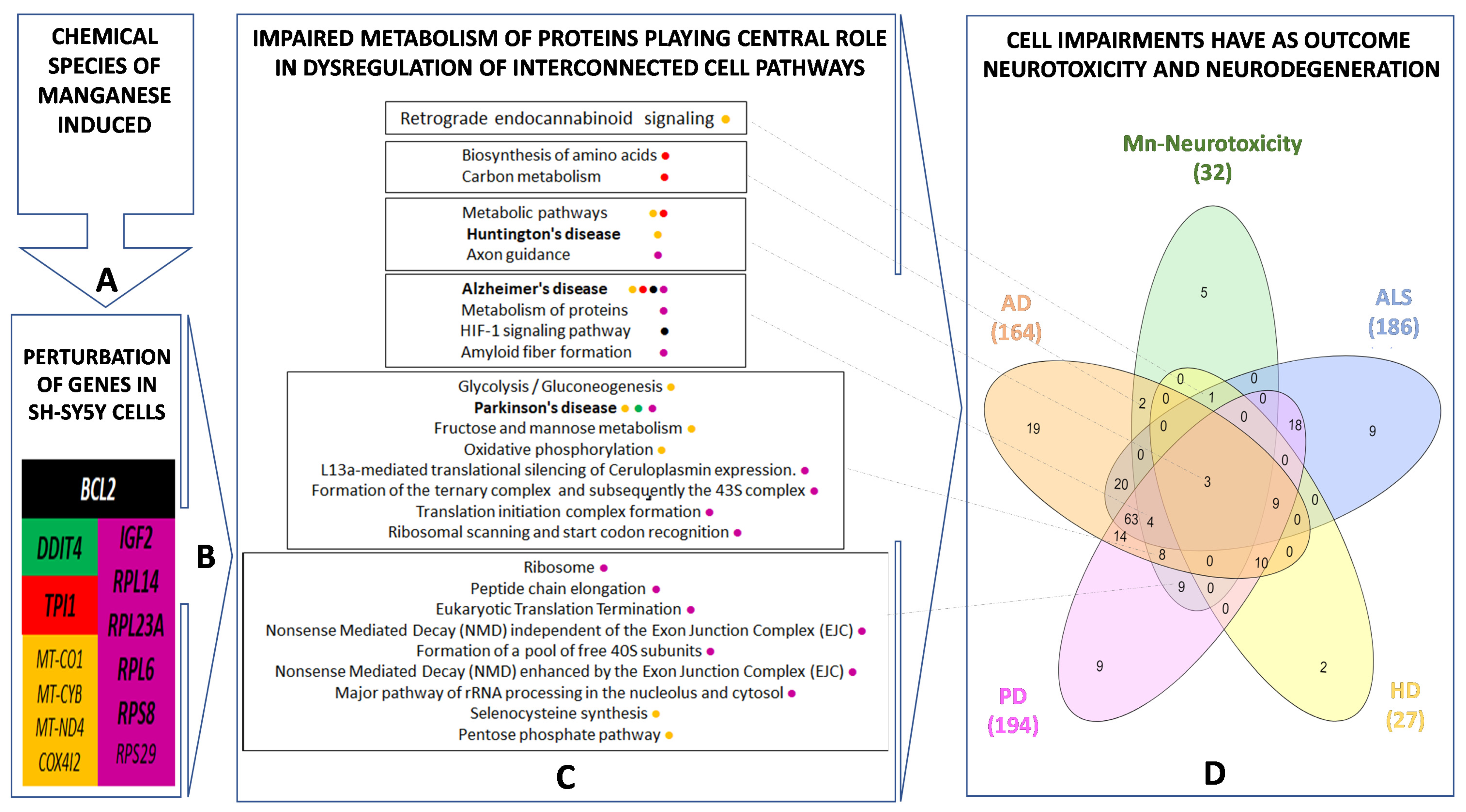
3.2. Manganese-Induced Cross-Impairment of Several Pathways Associated with Neurotoxicity and Neurodegeneration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howe, P.D.; Malcolm, H.M.; Dobson, S. Manganese and Its Compounds: Environmental Aspects; World Health Organization & International Programme on Chemical Safety: Geneva, Switzerland, 2004. [Google Scholar]
- Luo, X.G.; Li, S.F.; Lu, L.; Liu, B.; Kuang, X.; Shao, G.Z.; Yu, S.X. Gene Expression of Manganese-Containing Superoxide Dismutase as a Biomarker of Manganese Bioavailability for Manganese Sources in Broilers. Poult. Sci. 2007, 86, 888–894. [Google Scholar] [CrossRef]
- Kwakye, G.; Paoliello, M.; Mukhopadhyay, S.; Bowman, A.; Aschner, M. Manganese-Induced Parkinsonism and Parkinson’s Disease: Shared and Distinguishable Features. Int. J. Environ. Res. Public Health 2015, 12, 7519–7540. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.B.; Kwakye, G.F.; Herrero, E.; Aschner, M. Journal of Trace Elements in Medicine and Biology Role of Manganese in Neurodegenerative Diseases. J. Trace Elem. Med. Biol. 2011, 25, 191–203. [Google Scholar] [CrossRef]
- Šarić, M.; Lucchini, R. Handbook on the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Academic Press Inc.: Burlington, MA, USA, 2007; pp. 645–674. [Google Scholar]
- Hafeman, D.; Factor-Litvak, P.; Cheng, Z.; van Geen, A.; Ahsan, H. Association between Manganese Exposure through Drinking Water and Infant Mortality in Bangladesh. Environ. Health Perspect. 2007, 115, 1107–1112. [Google Scholar] [CrossRef]
- Ljung, K.; Vahter, M. Time to Re-Evaluate the Guideline Value for Manganese in Drinking Water? Environ. Health Perspect. 2007, 115, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Jordão, C.P.; Pereira, J.L.; Jham, G.N.; Bellato, C.R. Distribution of Heavy Metals in Environmental Samples Near Smelters and Mining Areas in Brazil. Environ. Technol. 1999, 20, 489–498. [Google Scholar] [CrossRef]
- Avnimelech, Y.; Kochba, M. Evaluation of Nitrogen Uptake and Excretion by Tilapia in Bio Floc Tanks, Using 15N Tracing. Aquaculture 2009, 287, 163–168. [Google Scholar] [CrossRef]
- Bonne Hernández, R.; Oliveira, E.; Espósito, B.P. Distribution and Behavior of Manganese in the Alto Do Paranapanema Basin. J. Environ. Monit. 2009, 11, 1236. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; van Leeuwen, H.P.; Lobinski, R. Guidelines for Terms Related to Chemical Speciation and Fractionation of Elements. Definitions, Structural Aspects, and Methodological Approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Stueben, B.L.; Cantrelle, B.; Sneddon, J.; Beck, J.N. Manganese K-Edge XANES Studies of Mn Speciation in Lac Des Allemands as a Function of Depth. Microchem. J. 2004, 76, 113–120. [Google Scholar] [CrossRef]
- Kenneth Klewicki, J.; Morgan, J.J. Kinetic Behavior of Mn(III) Complexes of Pyrophosphate, EDTA, and Citrate. Environ. Sci. Technol. 1998, 32, 2916–2922. [Google Scholar] [CrossRef]
- Hernández, R.B.; Farina, M.; Espósito, B.P.; Souza-Pinto, N.C.; Barbosa, F.; Suñol, C. Mechanisms of Manganese-Induced Neurotoxicity in Primary Neuronal Cultures: The Role of Manganese Speciation and Cell Type. Toxicol. Sci. 2011, 124, 414–423. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Ying, S.C.; Abernathy, M.; Barcellos, D.; Gabriel, F.A.; Otero, X.L.; Nóbrega, G.N.; Bernardino, A.F.; Ferreira, T.O. Manganese: The Overlooked Contaminant in the World Largest Mine Tailings Dam Collapse. Environ. Int. 2021, 146, 106284. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.B.; Nishita, M.I.; Espósito, B.P.; Scholz, S.; Michalke, B. The Role of Chemical Speciation, Chemical Fractionation and Calcium Disruption in Manganese-Induced Developmental Toxicity in Zebrafish (Danio Rerio) Embryos. J. Trace Elem. Med. Biol. 2015, 32, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Mehrifar, Y.; Bahrami, M.; Sidabadi, E.; Pirami, H. The Effects of Occupational Exposure to Manganese Fume on Neurobehavioral and Neurocognitive Functions: An Analytical Cross-Sectional Study among Welders. EXCLI J. 2020, 19, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Neves-Silva, P.; Heller, L. Rompimento Da Barragem Em Brumadinho e o Acesso à Água Das Comunidades Atingidas: Um Caso de Direitos Humanos. Ciência Cult. 2020, 72, 47–50. [Google Scholar] [CrossRef]
- De Azevedo, D.C.B.; de Araujo Toledo, G.; Cohen, S.C.; Kligerman, D.C.; de Oliveira Cardoso, T.A. Desastre de Brumadinho: Contribuições Para Políticas Públicas e Gestão Do Saneamento Em Períodos Emergenciais. Saúde Debate 2020, 44, 221–233. [Google Scholar] [CrossRef]
- De Freitas, C.M.; Barcellos, C.; Asmus, C.I.R.F.; da Silva, M.A.; Xavier, D.R. Da Samarco Em Mariana à Vale Em Brumadinho: Desastres Em Barragens de Mineração e Saúde Coletiva. Cad. Saúde Pública 2019, 35, 1–7. [Google Scholar] [CrossRef]
- Mitchell, E.J.; Frisbie, S.H.; Roudeau, S.; Carmona, A.; Ortega, R. How Much Manganese Is Safe for Infants? A Review of the Scientific Basis of Intake Guidelines and Regulations Relevant to the Manganese Content of Infant Formulas. J. Trace Elem. Med. Biol. 2021, 65, 126710. [Google Scholar] [CrossRef]
- Williams, B.B.; Kwakye, G.F.; Wegrzynowicz, M.; Li, D.; Aschner, M.; Erikson, K.M.; Bowman, A.B. Altered Manganese Homeostasis and Manganese Toxicity in a Huntington’s Disease Striatal Cell Model Are Not Explained by Defects in the Iron Transport System. Toxicol. Sci. 2010, 117, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Pfalzer, A.C.; Wilcox, J.M.; Codreanu, S.G.; Totten, M.; Bichell, T.J.V.; Halbesma, T.; Umashanker, P.; Yang, K.L.; Parmalee, N.L.; Sherrod, S.D.; et al. Huntington’s Disease Genotype Suppresses Global Manganese-Responsive Processes in Pre-Manifest and Manifest YAC128 Mice. Metallomics 2020, 12, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Morcillo, P.; Ijomone, O.M.; Venkataramani, V.; Harrison, F.E.; Lee, E.; Bowman, A.B.; Aschner, M. New Insights on the Role of Manganese in Alzheimer’s Disease and Parkinson’s Disease. Int. J. Environ. Res. Public Health 2019, 16, 3546. [Google Scholar] [CrossRef]
- Hernández, R.B.; Carrascal, M.; Abian, J.; Michalke, B.; Farina, M.; Gonzalez, Y.; Iyirhiaro, G.; Moteshareie, H.; Burnside, D.; Golshani, A.; et al. Manganese-Induced Neurotoxicity in Cerebellar Granule Neurons Due to Perturbation of Cell Network Pathways with Potential Implications for Neurodegenerative Disorders. Metallomics 2020, 12, 1656–1678. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, D.; Sivanesan, S.; Kannan, K. Manganese-Induced Neurotoxicity and Alterations in Gene Expression in Human Neuroblastoma SH-SY5Y Cells. Biol. Trace Elem. Res. 2018, 183, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Chandler, J.D.; Liu, K.H.; Uppal, K.; Hao, L.; Hu, X.; Go, Y.-M.; Jones, D.P. Metabolomic Responses to Manganese Dose in SH-SY5Y Human Neuroblastoma Cells. Toxicol. Sci. 2019, 169, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Chandler, J.D.; Lili, L.N.; Uppal, K.; Hu, X.; Hao, L.; Go, Y.-M.; Jones, D.P. Transcriptome Analysis Reveals Distinct Responses to Physiologic versus Toxic Manganese Exposure in Human Neuroblastoma Cells. Front. Genet. 2019, 10, 676. [Google Scholar] [CrossRef] [PubMed]
- Kovalevich, J.; Langford, D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef]
- Altamirano, M.; Coates, C.W.; Grundfest, H. Mechanisms of direct and neural excitability in electroplaques of electric eel. J. Gen. Physiol. 1955, 38, 319–360. [Google Scholar] [CrossRef] [PubMed]
- Korecka, J.A.; van Kesteren, R.E.; Blaas, E.; Spitzer, S.O.; Kamstra, J.H.; Smit, A.B.; Swaab, D.F.; Verhaagen, J.; Bossers, K. Phenotypic Characterization of Retinoic Acid Differentiated SH-SY5Y Cells by Transcriptional Profiling. PLoS ONE 2013, 8, e63862. [Google Scholar] [CrossRef]
- Schlachetzki, J.C.M.; Saliba, S.W.; de Oliveira, A.C.P. Studying Neurodegenerative Diseases in Culture Models. Rev. Bras. Psiquiatr. 2013, 35 (Suppl. 2), S92–S100. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y Cell Line in Parkinson’s Disease Research: A Systematic Review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Hernández, R.B.; Moteshareie, H.; Burnside, D.; McKay, B.; Golshani, A. Manganese-Induced Cellular Disturbance in the Baker’s Yeast, Saccharomyces Cerevisiae with Putative Implications in Neuronal Dysfunction. Sci. Rep. 2019, 9, 6563. [Google Scholar] [CrossRef] [PubMed]
- Bonne Hernández, R.; Moteshareie, H.; Golshani, A. Manganese-Induced Disruption of Cross-Talking Pathways in Danio Rerio (Zebrafish) Is Potentially Linked to Toxicity and Neurodegeneration. EC Pharmacol. Toxicol. 2019, 7, 175–187. [Google Scholar]
- Warner, J.R. The Economics of Ribosome Biosynthesis in Yeast. Trends Biochem. Sci. 1999, 24, 437–440. [Google Scholar] [CrossRef]
- Thomson, E.; Ferreira-Cerca, S.; Hurt, E. Eukaryotic Ribosome Biogenesis at a Glance. J. Cell Sci. 2013, 126, 4815–4821. [Google Scholar] [CrossRef]
- Liu, C.; Yan, D.; Wang, C.; Ma, Z.; Deng, Y.; Liu, W.; Xu, B. Manganese Activates Autophagy to Alleviate Endoplasmic Reticulum Stress–Induced Apoptosis via PERK Pathway. J. Cell. Mol. Med. 2020, 24, 328–341. [Google Scholar] [CrossRef]
- Higashi, Y.; Asanuma, M.; Miyazaki, I.; Hattori, N.; Mizuno, Y.; Ogawa, N. Parkin Attenuates Manganese-Induced Dopaminergic Cell Death. J. Neurochem. 2004, 89, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wen, Q.; Huang, J.; Luo, M.; Xiao, Y.; Mo, R.; Wang, J. Manganese (II) Chloride Leads to Dopaminergic Neurotoxicity by Promoting Mitophagy through BNIP3-Mediated Oxidative Stress in SH-SY5Y Cells. Cell. Mol. Biol. Lett. 2021, 26, 23. [Google Scholar] [CrossRef]
- Rabin, O.; Hegedus, L.; Bourre, J.-M.; Smith, Q.R. Rapid Brain Uptake of Manganese(II) Across the Blood-Brain Barrier. J. Neurochem. 2006, 61, 509–517. [Google Scholar] [CrossRef]
- Yokel, R.A. Manganese Flux Across the Blood–Brain Barrier. NeuroMol. Med. 2009, 11, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.P.; Schneider, J.A.; Nelson, B.C.; Atha, D.H.; Jain, A.; Soliman, K.F.A.; Aschner, M.; Mazzio, E.; Reams, R.R. Manganese-Induced Oxidative DNA Damage in Neuronal SH-SY5Y Cells: Attenuation of Thymine Base Lesions by Glutathione and N-Acetylcysteine. Toxicol. Lett. 2013, 218, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Traverso, M.; Malnati, M.; Minetti, C.; Regis, S.; Tedeschi, S.; Pedemonte, M.; Bruno, C.; Biassoni, R.; Zara, F. Multiplex Real-Time PCR for Detection of Deletions and Duplications in Dystrophin Gene. Biochem. Biophys. Res. Commun. 2006, 339, 145–150. [Google Scholar] [CrossRef]
- Jouannic, J.; Stieltjes, N.; Costa, J.; Girodon, E. Quantitative Real-Time PCR Assay for Rapid Identification of Deletion Carriers in Hemophilia. Clin. Chem. 2004, 50, 1269–1270. [Google Scholar] [CrossRef][Green Version]
- Samanfar, B.; Shostak, K.; Moteshareie, H.; Hajikarimlou, M.; Shaikho, S.; Omidi, K.; Hooshyar, M.; Burnside, D.; Márquez, I.G.; Kazmirchuk, T.; et al. The Sensitivity of the Yeast, Saccharomyces Cerevisiae, to Acetic Acid Is Influenced by DOM34 and RPL36A. PeerJ 2017, 2017, e4037. [Google Scholar] [CrossRef][Green Version]
- Alonso, R.; Salavert, F.; Garcia-Garcia, F.; Carbonell-Caballero, J.; Bleda, M.; Garcia-Alonso, L.; Sanchis-Juan, A.; Perez-Gil, D.; Marin-Garcia, P.; Sanchez, R.; et al. Babelomics 5.0: Functional Interpretation for New Generations of Genomic Data. Nucleic Acids Res. 2015, 43, W117–W121. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING Database in 2017: Quality-Controlled Protein-Protein Association Networks, Made Broadly Accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; King, B.L.; McMorran, R.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. The Comparative Toxicogenomics Database: Update 2017. Nucleic Acids Res. 2017, 45, D972–D978. [Google Scholar] [CrossRef]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway Enrichment Analysis and Visualization of Omics Data Using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef] [PubMed]
- Tarale, P.; Daiwile, A.P.; Sivanesan, S.; Stöger, R.; Bafana, A.; Naoghare, P.K.; Parmar, D.; Chakrabarti, T.; Krishnamurthi, K. Manganese Exposure: Linking down-Regulation of MiRNA-7 and MiRNA-433 with α-Synuclein Overexpression and Risk of Idiopathic Parkinson’s Disease. Toxicol. In Vitro 2018, 46, 94–101. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Paoliello, M.M.B.; Mazilina, A.N.; Skalny, A.V.; Martins, A.C.; Voskresenskaya, O.N.; Aaseth, J.; Santamaria, A.; Notova, S.V.; Tsatsakis, A.; et al. Molecular Targets of Manganese-Induced Neurotoxicity: A Five-Year Update. Int. J. Mol. Sci. 2021, 22, 4646. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Vergilio, C.; Lacerda, D.; de Oliveira, B.C.V.; Sartori, E.; Campos, G.M.; de Souza Pereira, A.L.; de Aguiar, D.B.; da Silva Souza, T.; de Almeida, M.G.; Thompson, F.; et al. Metal Concentrations and Biological Effects from One of the Largest Mining Disasters in the World (Brumadinho, Minas Gerais, Brazil). Sci. Rep. 2020, 10, 5936. [Google Scholar] [CrossRef]
- Thompson, F.; de Oliveira, B.C.; Cordeiro, M.C.; Masi, B.P.; Rangel, T.P.; Paz, P.; Freitas, T.; Lopes, G.; Silva, B.S.; Cabral, A.S.; et al. Severe Impacts of the Brumadinho Dam Failure (Minas Gerais, Brazil) on the Water Quality of the Paraopeba River. Sci. Total Environ. 2020, 705, 135914. [Google Scholar] [CrossRef]
- Maddirala, Y.; Tobwala, S.; Ercal, N. N-Acetylcysteineamide Protects against Manganese-Induced Toxicity in SHSY5Y Cell Line. Brain Res. 2015, 1608, 157–166. [Google Scholar] [CrossRef]
- Kemsheh, M.; Oblitey, R. Manganese Toxicity on Cultured SH-SY5Y Cells. In All Zyzzogeton Presentations; Augsburg University: Minneapolis, MN, USA, 2020; Volume 15. [Google Scholar]
- Lehmkuhl, E.M.; Zarnescu, D.C. Lost in Translation: Evidence for Protein Synthesis Deficits in ALS/FTD and Related Neurodegenerative Diseases. Adv Neurobiol. 2018, 283–301. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Cai, T.; Zhang, Y.; Lv, S.; Wang, Y.; Ye, L. α-Synuclein Overexpression during Manganese-Induced Apoptosis in SH-SY5Y Neuroblastoma Cells. Brain Res. Bull. 2010, 81, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Younts, T.J.; Monday, H.R.; Dudok, B.; Klein, M.E.; Jordan, B.A.; Katona, I.; Castillo, P.E. Presynaptic Protein Synthesis Is Required for Long-Term Plasticity of GABA Release. Neuron 2016, 92, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Kano, M. Control of Synaptic Function by Endocannabinoid-Mediated Retrograde Signaling. Proc. Jpn. Acad. Ser. B 2014, 90, 235–250. [Google Scholar] [CrossRef]
- Stoeckli, E.T. Understanding Axon Guidance: Are We Nearly There Yet? Development 2018, 145, dev151415. [Google Scholar] [CrossRef]
- Liu, X.-A.; Rizzo, V.; Puthanveettil, S. Pathologies of Axonal Transport in Neurodegenerative Diseases. Transl. Neurosci. 2012, 3, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shin, J.E.; Ewan, E.E.; Oh, Y.M.; Pita-Thomas, W.; Cavalli, V. Activating Injury-Responsive Genes with Hypoxia Enhances Axon Regeneration through Neuronal HIF-1α. Neuron 2015, 88, 720–734. [Google Scholar] [CrossRef]
- Hou, C.; Wang, Y.; Liu, J.; Wang, C.; Long, J. Neurodegenerative Disease Related Proteins Have Negative Effects on SNARE-Mediated Membrane Fusion in Pathological Confirmation. Front. Mol. Neurosci. 2017, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Corgnali, M.; Piconi, L.; Ihnat, M.; Ceriello, A. Evaluation of Gliclazide Ability to Attenuate the Hyperglycaemic ‘Memory’ Induced by High Glucose in Isolated Human Endothelial Cells. Diabetes Metab. Res. Rev. 2008, 24, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Ethell, D.W. Maneb Potentiates Paraquat Neurotoxicity by Inducing Key Bcl-2 Family Members. J. Neurochem. 2008, 105, 2091–2097. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Mishra, S.; Ali, W.; Shukla, Y. Protective Effects of Lupeol against Mancozeb-Induced Genotoxicity in Cultured Human Lymphocytes. Phytomedicine 2016, 23, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jung, H. Local Protein Synthesis in Neuronal Axons: Why and How We Study. BMB Rep. 2015, 48, 139–146. [Google Scholar] [CrossRef]
- Hu, X.P.; Yang, Y.; Ma, B.G. Amino Acid Flux from Metabolic Network Benefits Protein Translation: The Role of Resource Availability. Sci. Rep. 2015, 5, 11113. [Google Scholar] [CrossRef]
- Seo, Y.A.; Li, Y.; Wessling-Resnick, M. Iron Depletion Increases Manganese Uptake and Potentiates Apoptosis through ER Stress. NeuroToxicol. 2013, 38, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Putrament, A.; Baranowska, H.; Ejchart, A.; Prazmo, W. Manganese Mutagenesis in Yeast. A Practical Application of Manganese for the Induction of Mitochondrial Antibiotic-Resistant Mutations. J. Gen. Microbiol. 1975, 90, 265–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Putrament, A.; Baranowska, H.; Ejchart, A.; Jachymczyk, W. Manganese Mutagenesis in Yeast-VI. Mn2+ Uptake, MitDNA Replication and ER Induction. Comparison with Other Divalent Cations. MGG Mol. Gen. Genet. 1977, 151, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.G.; Fox, O.F.; Kishore, G.S.; Carubelli, R. Effect of Manganese Ions on the Interaction between Ribosomes and Endoplasmic Reticulum Membranes Isolated from Rat Liver. Biosci. Rep. 1981, 1, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Dambach, M.; Sandoval, M.; Updegrove, T.B.; Anantharaman, V.; Aravind, L.; Waters, L.S.; Storz, G. The Ubiquitous YybP-YkoY Riboswitch Is a Manganese-Responsive Regulatory Element. Mol. Cell 2015, 57, 1099–1109. [Google Scholar] [CrossRef]
- Bray, M.S.; Lenz, T.K.; Haynes, J.W.; Bowman, J.C.; Petrov, A.S.; Reddi, A.R.; Hud, N.V.; Williams, L.D.; Glass, J.B. Multiple Prebiotic Metals Mediate Translation. Proc. Natl. Acad. Sci. USA 2018, 115, 12164–12169. [Google Scholar] [CrossRef]
- Lee, C.-D.; Tu, B.P. Metabolic Influences on RNA Biology and Translation. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 176–184. [Google Scholar] [CrossRef]
- Cioni, J.-M.; Lin, J.Q.; Holtermann, A.V.; Koppers, M.; Jakobs, M.A.H.; Azizi, A.; Turner-Bridger, B.; Shigeoka, T.; Franze, K.; Harris, W.A.; et al. Late Endosomes Act as MRNA Translation Platforms and Sustain Mitochondria in Axons. Cell 2019, 176, 56–72. [Google Scholar] [CrossRef]
- Giasson, B.I.; Lee, V.M.-Y. Are Ubiquitination Pathways Central to Parkinson’s Disease? Cell 2003, 114, 1–8. [Google Scholar] [CrossRef]
- Agbas, A. Trends of Protein Aggregation in Neurodegenerative Diseases. In Neurochemistry; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Sidoryk-Wȩgrzynowicz, M.; Lee, E.-S.; Ni, M.; Aschner, M. Manganese-Induced Downregulation of Astroglial Glutamine Transporter SNAT3 Involves Ubiquitin-Mediated Proteolytic System. Glia 2010, 58, 1905–1912. [Google Scholar] [CrossRef]
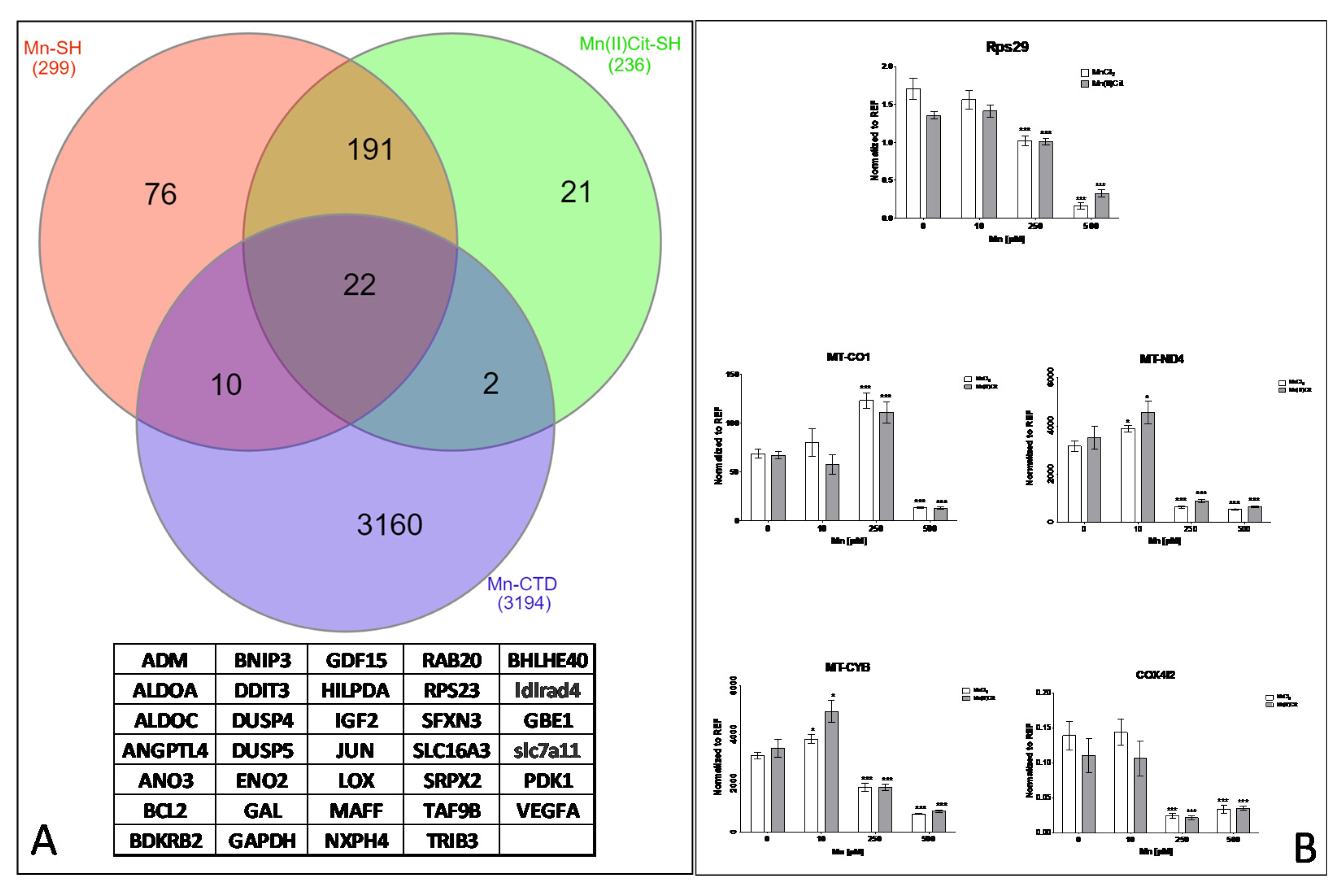
| Gene Symbol | Gene Name | PL | Sequence (5′→3′) | TS | Length | Start | Stop | Tm | GC% | Self C. | Self 3′ C. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RPS29 | RPS29 ribosomal protein S29 | 135 | FP | ACACTGGCGGCACATATTGA | Plus | 20 | 49,585,986 | 49,586,005 | 60.04 | 50 | 4 | 2 |
| RP | GGTAGTAGCCGTCTGAGTGC | Minus | 20 | 49,586,120 | 49,586,101 | 59.9 | 60 | 4 | 3 | |||
| MT-CO1 | mitochondrially encoded cytochrome c oxidase I | 107 | FP | CCCCGATGCATACACCACAT | Plus | 20 | 7232 | 7251 | 60.18 | 55 | 6 | 2 |
| RP | TCGAAGCGAAGGCTTCTCAA | Minus | 20 | 7338 | 7319 | 59.68 | 50 | 7 | 3 | |||
| COX4I2 | cytochrome c oxidase subunit 4I2 | 118 | FP | GATGAACCGTCGCTCCAATG | Plus | 20 | 30,135,127 | 30,135,146 | 59.35 | 55 | 5 | 3 |
| RP | GATGAGGTGTTGCCACTCAC | Minus | 20 | 30,135,244 | 30,135,225 | 58.84 | 55 | 4 | 3 | |||
| MT-CYB | mitochondrially encoded cytochrome b | 134 | FP | ACCCCCTAGGAATCACCTCC | Plus | 20 | 15,366 | 15,385 | 60.03 | 60 | 6 | 1 |
| RP | GCCTAGGAGGTCTGGTGAGA | Minus | 20 | 15,499 | 15,480 | 60.03 | 60 | 6 | 2 | |||
| MT-ND4 | mitochondrially encoded NADH dehydrogenase 4 | 296 | FP | CCCCATCGCTGGGTCAATAG | Plus | 20 | 11,428 | 11,447 | 60.25 | 60 | 8 | 2 |
| RP | TAAGCCCGTGGGCGATTATG | Minus | 20 | 11,723 | 11,704 | 60.25 | 55 | 6 | 1 | |||
| Gapdh | glyceraldehyde-3-phosphate dehydrogenase | 117 | FP | AAAGGGCCCTGACAACTCTTT | Plus | 21 | 6,538,069 | 6,538,089 | 59.78 | 47.62 | 8 | 3 |
| RP | GGTGGTCCAGGGGTCTTACT | Minus | 20 | 6,538,185 | 6,538,166 | 60.55 | 60 | 5 | 1 | |||
| Cell Pathways | Term Description | Observed | Background | FDR |
|---|---|---|---|---|
| Gene Count | Gene Count | |||
| Protein Synthesis | Peptide chain elongation | 75 | 86 | 4.37 × 10−92 |
| Viral mRNA Translation | 75 | 86 | 4.37 × 10−92 | |
| SRP-dependent cotranslational protein targeting to membrane | 78 | 109 | 1.15 × 10−91 | |
| Eukaryotic Translation Termination | 75 | 90 | 1.22 × 10−91 | |
| Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) | 75 | 92 | 2.25 × 10−91 | |
| Formation of a pool of free 40S subunits | 75 | 98 | 5.96 × 10−90 | |
| L13a-mediated translational silencing of Ceruloplasmin expression | 75 | 107 | 5.93 × 10−88 | |
| GTP hydrolysis and joining of the 60S ribosomal subunit | 75 | 108 | 9.04 × 10−88 | |
| Ribosome | 76 | 130 | 3.15 × 10−84 | |
| Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) | 75 | 112 | 6.29 × 10−87 | |
| Major pathway of rRNA processing in the nucleolus and cytosol | 76 | 179 | 1.10 × 10−76 | |
| Metabolism of amino acids and derivatives | 77 | 354 | 2.98 × 10−59 | |
| Metabolism of RNA | 78 | 652 | 7.72 × 10−43 | |
| Formation of the ternary complex and subsequently the 43S complex | 31 | 49 | 1.27 × 10−34 | |
| Translation initiation complex formation | 31 | 55 | 1.85 × 10−33 | |
| Ribosomal scanning and start codon recognition | 31 | 55 | 1.85 × 10−33 | |
| Metabolism of proteins | 102 | 1948 | 1.12 × 10−27 | |
| Biosynthesis of amino acids | 9 | 72 | 5.98 × 10−05 | |
| Cell Metabolism | Selenocysteine synthesis | 75 | 90 | 1.22 × 10−91 |
| Selenoamino acid metabolism | 76 | 112 | 2.37 × 10−88 | |
| Metabolism | 137 | 2032 | 6.11 × 10−52 | |
| Oxidative phosphorylation | 26 | 131 | 9.48 × 10−18 | |
| The citric acid (TCA) cycle and respiratory electron transport | 28 | 173 | 7.83 × 10−18 | |
| Thermogenesis | 29 | 228 | 1.68 × 10−15 | |
| Complex I biogenesis | 17 | 55 | 8.42 × 10−15 | |
| Metabolic pathways | 50 | 1250 | 2.11 × 10−08 | |
| Glycolysis/Gluconeogenesis | 12 | 68 | 5.27 × 10−08 | |
| Fructose and mannose metabolism | 9 | 33 | 1.85 × 10−07 | |
| Metabolism of carbohydrates | 20 | 266 | 2.71 × 10−07 | |
| Carbon metabolism | 10 | 116 | 2.90 × 10−04 | |
| Cell Signaling | Retrograde endocannabinoid signaling | 18 | 148 | 2.25 × 10−09 |
| HIF-1 signaling pathway | 14 | 98 | 3.22 × 10−08 | |
| Negative regulation of MAPK pathway | 6 | 40 | 1.00 × 10−03 | |
| Neurodevelopment | Axon guidance | 79 | 541 | 3.00 × 10−49 |
| Neurodegeneration | Parkinson’s disease | 26 | 142 | 3.69 × 10−17 |
| Alzheimer’s disease | 20 | 168 | 3.22 × 10−10 | |
| Huntington’s disease | 19 | 193 | 1.57 × 10−08 |
| Cell Pathways | Term Description | Observed | Background | FDR |
|---|---|---|---|---|
| Gene Count | Gene Count | |||
| Protein Synthesis | Peptide chain elongation | 76 | 86 | 7.69 × 10−103 |
| Viral mRNA Translation | 76 | 86 | 7.69 × 10−103 | |
| Eukaryotic Translation Termination | 76 | 90 | 2.96 × 10−102 | |
| Nonsense Mediated Decay (NMD) independent of the Exon Junction Complex (EJC) | 76 | 92 | 4.89 × 10−102 | |
| Formation of a pool of free 40S subunits | 76 | 98 | 1.34 × 10−100 | |
| L13a-mediated translational silencing of Ceruloplasmin expression | 76 | 107 | 1.58 × 10−98 | |
| GTP hydrolysis and joining of the 60S ribosomal subunit | 76 | 108 | 2.37 × 10−98 | |
| SRP-dependent cotranslational protein targeting to membrane | 76 | 109 | 3.59 × 10−98 | |
| Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) | 76 | 112 | 1.52 × 10−97 | |
| Ribosome | 77 | 130 | 6.14 × 10−95 | |
| Formation of the ternary complex and subsequently the 43S complex | 31 | 49 | 5.38 × 10−38 | |
| Translation initiation complex formation | 31 | 55 | 7.97 × 10−37 | |
| Ribosomal scanning and start codon recognition | 31 | 55 | 7.97 × 10−37 | |
| Metabolism of proteins | 88 | 1948 | 2.38 × 10−27 | |
| Biosynthesis of amino acids | 8 | 72 | 6.26 × 10−05 | |
| Senescence-Associated Secretory Phenotype (SASP) | 6 | 78 | 6.50 × 10−03 | |
| Amyloid fiber formation | 6 | 78 | 6.50 × 10−03 | |
| E3 ubiquitin ligases ubiquitinate target proteins | 5 | 53 | 7.80 × 10−03 | |
| Cell Metabolism | Selenocysteine synthesis | 76 | 90 | 2.96 × 10−102 |
| Oxidative phosphorylation | 26 | 131 | 2.08 × 10−20 | |
| Thermogenesis | 28 | 228 | 2.41 × 10−17 | |
| Metabolic pathways | 45 | 1250 | 1.71 × 10−09 | |
| Glycolysis/Gluconeogenesis | 12 | 68 | 3.66 × 10−09 | |
| Carbon metabolism | 10 | 116 | 3.67 × 10−05 | |
| Pentose phosphate pathway | 5 | 30 | 6.60 × 10−04 | |
| Starch and sucrose metabolism | 5 | 33 | 9.30 × 10−04 | |
| Galactose metabolism | 4 | 31 | 8.10 × 10−03 | |
| Endocrine resistance | 6 | 95 | 1.26 × 10−02 | |
| Cell Signaling | Retrograde endocannabinoid signaling | 16 | 148 | 2.85 × 10−09 |
| HIF-1 signaling pathway | 12 | 98 | 1.42 × 10−07 | |
| Negative regulation of MAPK pathway | 5 | 40 | 2.50 × 10−03 | |
| Neurodevelopment | Axon guidance | 78 | 541 | 5.39 × 10−57 |
| Neurodegeneration | Parkinson’s disease | 26 | 142 | 8.36 × 10−20 |
| Alzheimer’s disease | 20 | 168 | 3.71 × 10−12 | |
| Huntington’s disease | 19 | 193 | 2.88 × 10−10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, R.B.; de Souza-Pinto, N.C.; Kleinjans, J.; van Herwijnen, M.; Piepers, J.; Moteshareie, H.; Burnside, D.; Golshani, A. Manganese-Induced Neurotoxicity through Impairment of Cross-Talk Pathways in Human Neuroblastoma Cell Line SH-SY5Y Differentiated with Retinoic Acid. Toxics 2021, 9, 348. https://doi.org/10.3390/toxics9120348
Hernández RB, de Souza-Pinto NC, Kleinjans J, van Herwijnen M, Piepers J, Moteshareie H, Burnside D, Golshani A. Manganese-Induced Neurotoxicity through Impairment of Cross-Talk Pathways in Human Neuroblastoma Cell Line SH-SY5Y Differentiated with Retinoic Acid. Toxics. 2021; 9(12):348. https://doi.org/10.3390/toxics9120348
Chicago/Turabian StyleHernández, Raúl Bonne, Nadja C. de Souza-Pinto, Jos Kleinjans, Marcel van Herwijnen, Jolanda Piepers, Houman Moteshareie, Daniel Burnside, and Ashkan Golshani. 2021. "Manganese-Induced Neurotoxicity through Impairment of Cross-Talk Pathways in Human Neuroblastoma Cell Line SH-SY5Y Differentiated with Retinoic Acid" Toxics 9, no. 12: 348. https://doi.org/10.3390/toxics9120348
APA StyleHernández, R. B., de Souza-Pinto, N. C., Kleinjans, J., van Herwijnen, M., Piepers, J., Moteshareie, H., Burnside, D., & Golshani, A. (2021). Manganese-Induced Neurotoxicity through Impairment of Cross-Talk Pathways in Human Neuroblastoma Cell Line SH-SY5Y Differentiated with Retinoic Acid. Toxics, 9(12), 348. https://doi.org/10.3390/toxics9120348