Developmental Hazard of Environmentally Persistent Free Radicals and Protective Effect of TEMPOL in Zebrafish Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Zebrafish Embryo
2.3. Zebrafish Embryo Assay
2.3.1. Chemical Exposures
2.3.2. Antioxidant Exposures
2.3.3. EPFRs Plus Antioxidant Exposures
2.4. Embryo Photomotor Response Behavior
2.5. Larval Photomotor Response Behavior
2.6. Statistical Analysis
3. Results and Discussion
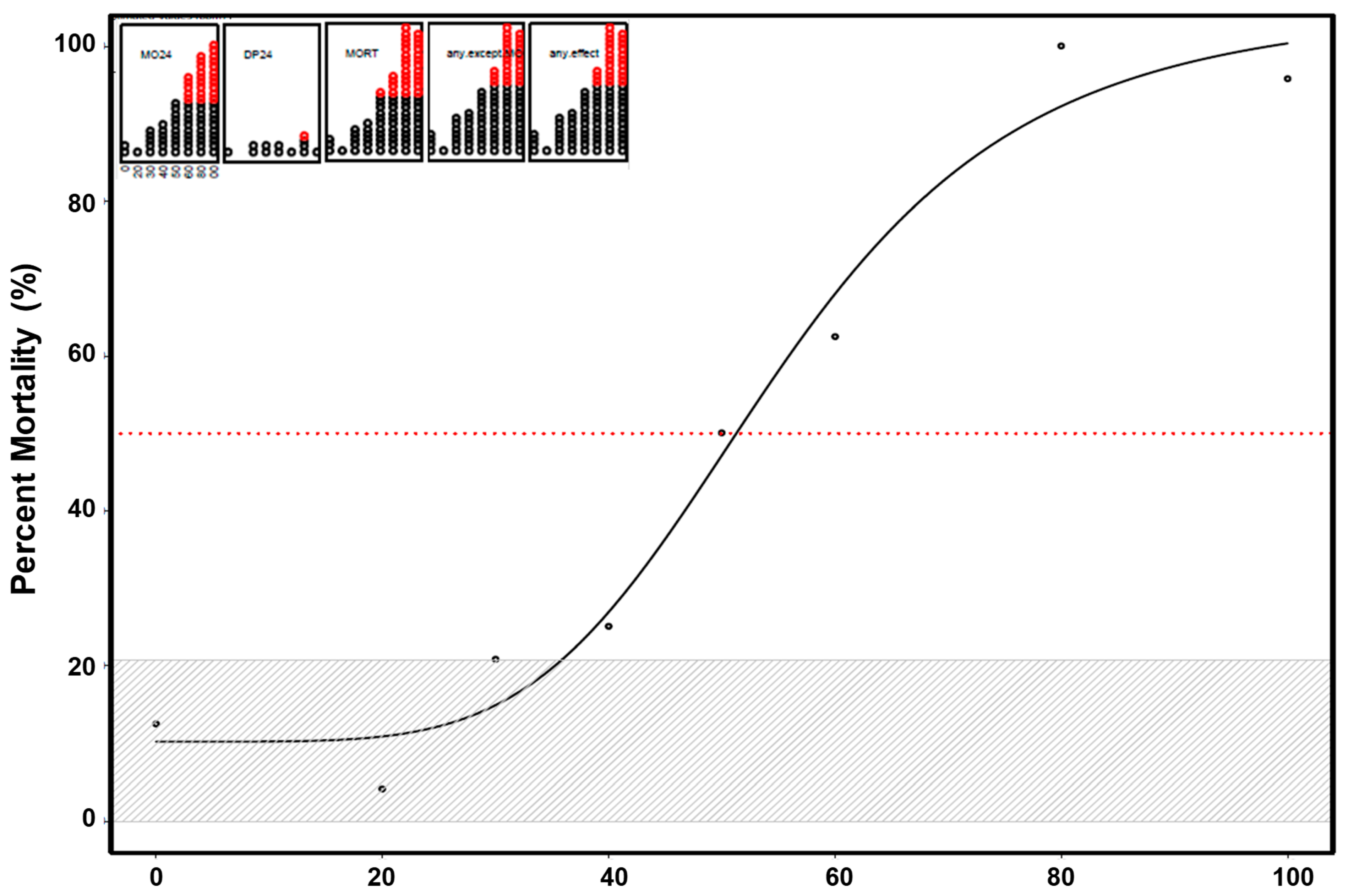
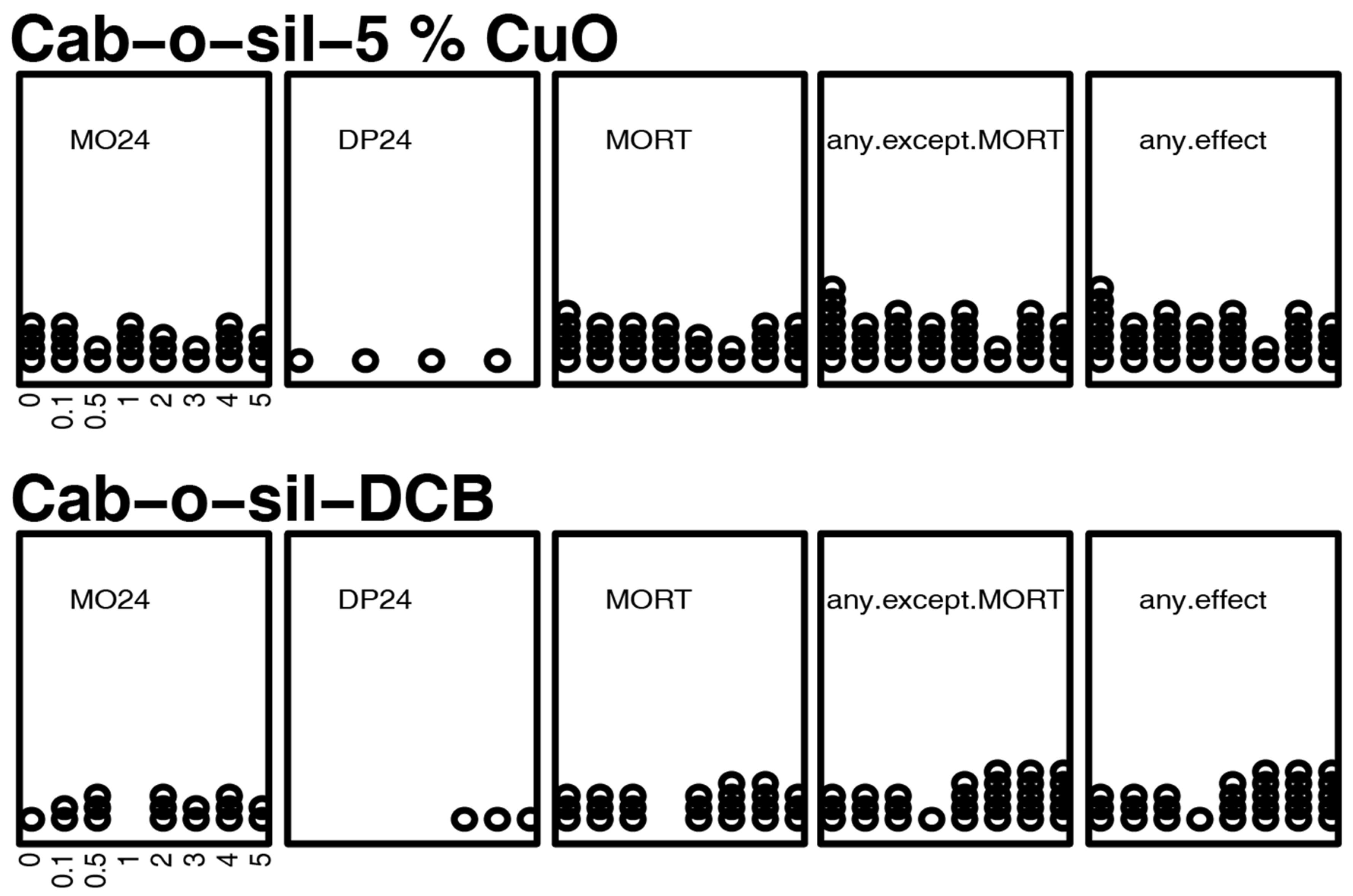
3.1. Mortality and Morphology Responses to EPFRs
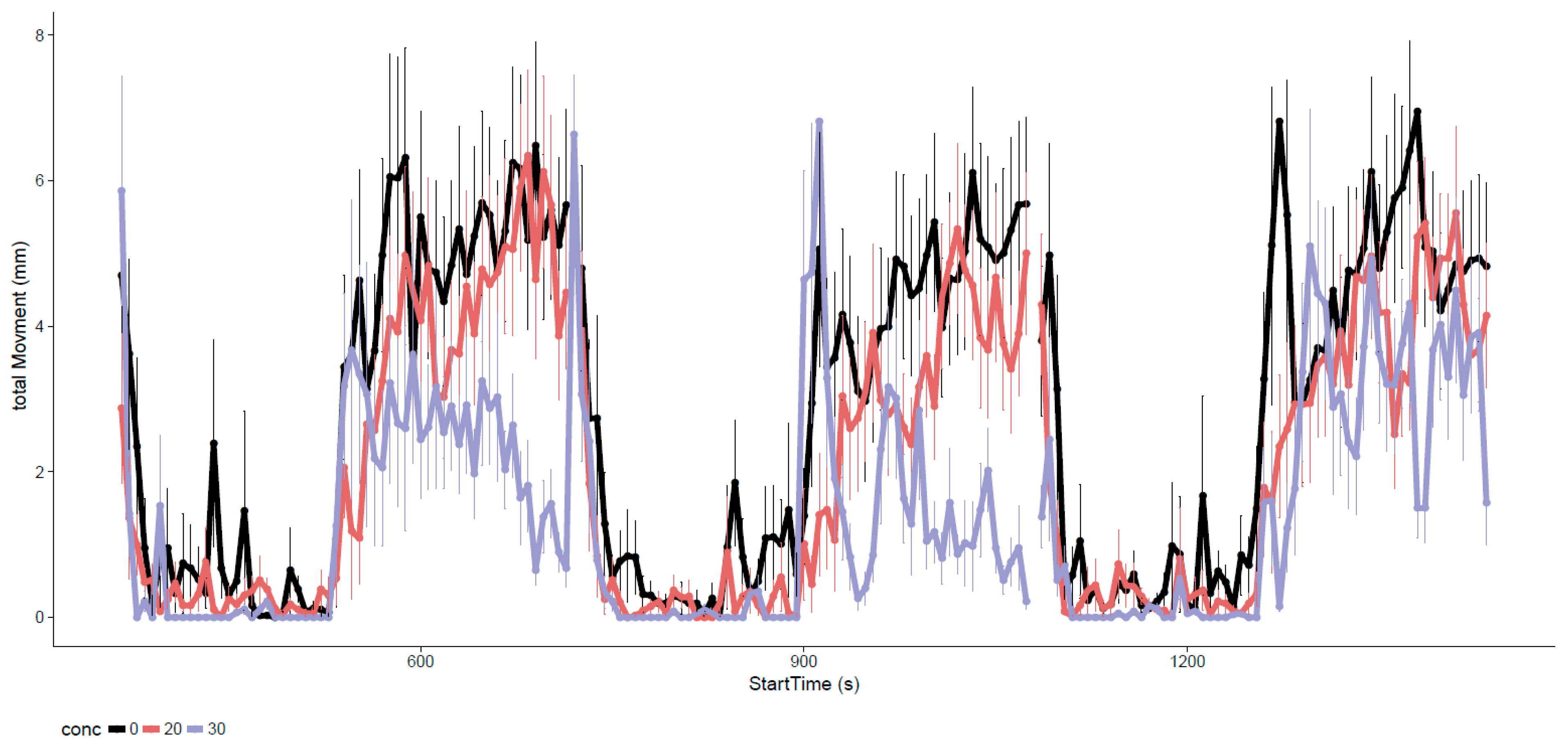
3.2. Neurotoxicity of EPFRs
3.3. Mortality and Morphology Responses to Controls
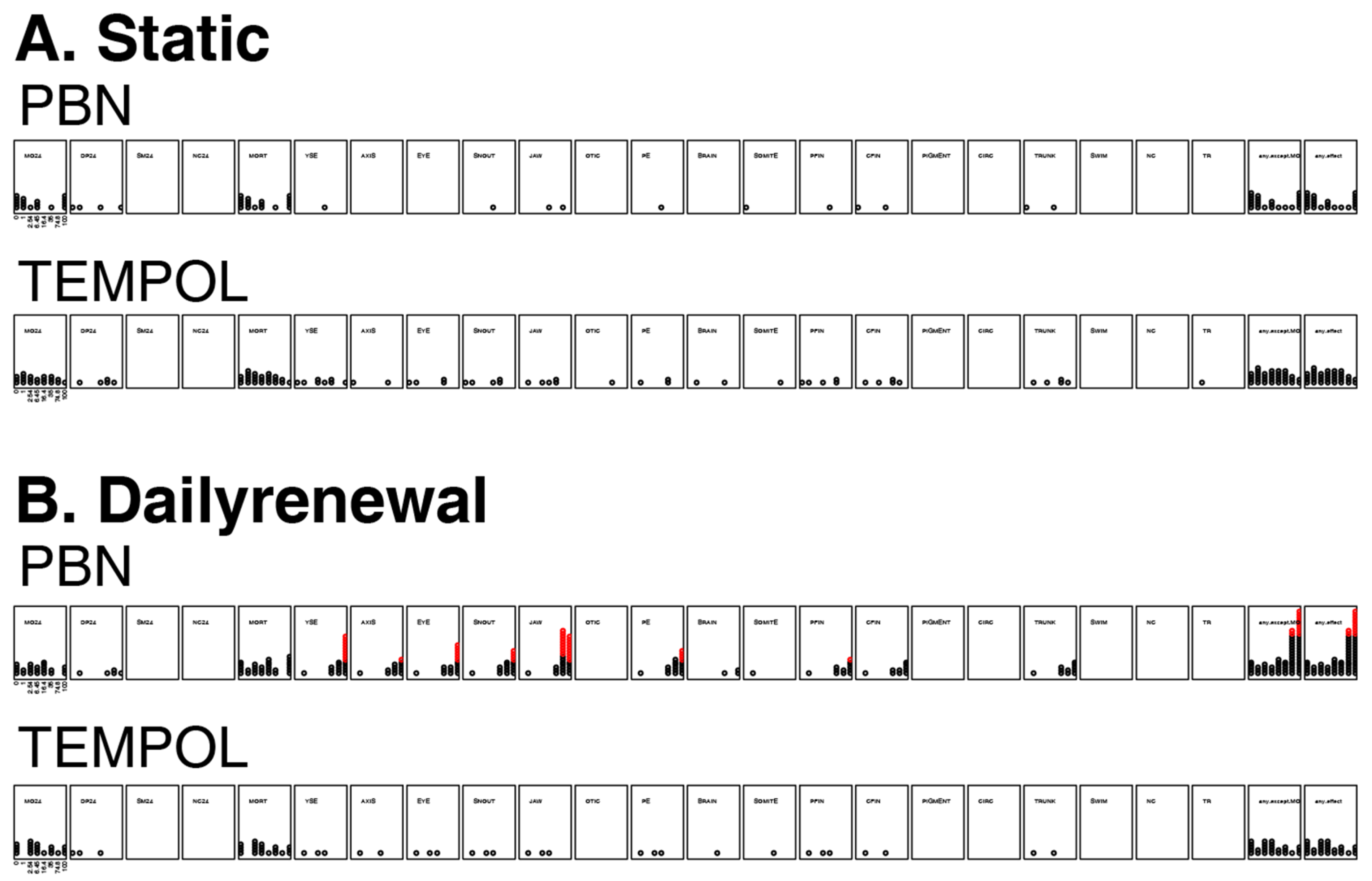
3.4. Mortality and Morphology Responses to Antioxidants (TEMPOL or PBN)
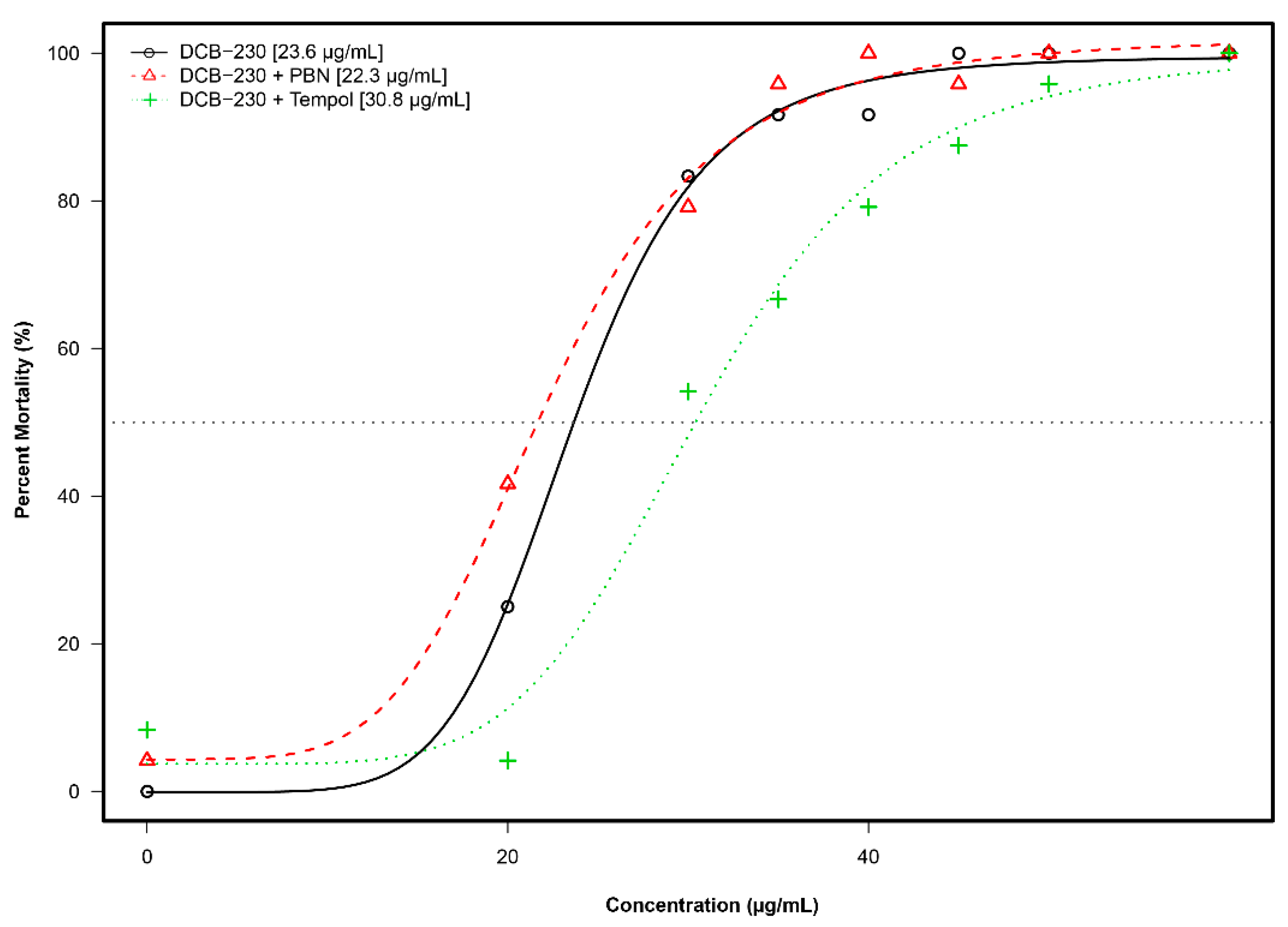
3.5. Mortality and Morphology Responses to EPFRs Plus Antioxidants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomnicki, S.; Truong, H.; Vejerano, E.; Dellinger, B. Copper oxide-based model of persistent free radical formation on combustion-derived particulate matter. Environ. Sci. Technol. 2008, 42, 4982–4988. [Google Scholar] [CrossRef] [PubMed]
- Potter, P.M.; Guan, X.; Lomnicki, S.M. Synergy of iron and copper oxides in the catalytic formation of PCDD/Fs from 2-monochlorophenol. Chemosphere 2018, 203, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Ghimire, A.; Potter, P.M.; Lomnicki, S.M. Role of Fe2O3 in fly ash surrogate on PCDD/Fs formation from 2-monochlorophenol. Chemosphere 2019, 226, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Gehling, W.; Dellinger, B. Environmentally persistent free radicals and their lifetimes in PM2.5. Environ. Sci. Technol. 2013, 47, 8172–8178. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, L.; Wang, X.; Zheng, M.; Li, C.; Zhang, A.; Fu, J.; Yang, Y.; Qin, L.; Liu, X.; et al. Risk evaluation of environmentally persistent free radicals in airborne particulate matter and influence of atmospheric factors. Ecotoxicol. Environ. Saf. 2020, 196, 110571. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, B.; Khachatryan, L.; Masko, S.; Lomnicki, S. Free radicals in tobacco smoke. Mini-Rev. Org. Chem. 2011, 8, 427–433. [Google Scholar] [CrossRef]
- Goel, R.; Bitzer, Z.; Reilly, S.; Trushin, N.; Reinhart, L.; Elias, R.; Richie, J.P. 184-Tobacco smoke free radicals and related biomarkers of oxidative stress. Free Radic. Biol. Med. 2017, 112 (Suppl. 1), 130–131. [Google Scholar] [CrossRef]
- dela Cruz, A.L.N.; Cook, R.L.; Dellinger, B.; Lomnicki, S.M.; Donnelly, K.C.; Kelley, M.A.; Cosgriff, D. Assessment of environmentally persistent free radicals in soils and sediments from three Superfund sites. Environ. Sci. Process. Impacts 2014, 16, 44–52. [Google Scholar] [CrossRef]
- Saravia, J.; Lee, G.I.; Lomnicki, S.; Dellinger, B.; Cormier, S.A. Particulate matter containing environmentally persistent free radicals and adverse infant respiratory health effects: A review. J. Biochem. Mol. Toxicol. 2013, 27, 56–68. [Google Scholar] [CrossRef]
- Harmon, A.C.; Hebert, V.Y.; Cormier, S.A.; Subramanian, B.; Reed, J.R.; Backes, W.L.; Dugas, T.R. Particulate matter containing environmentally persistent free radicals induces AhR-dependent cytokine and reactive oxygen species production in human bronchial epithelial cells. PLoS ONE 2018, 13, e0205412. [Google Scholar] [CrossRef]
- In Lee, G.; Saravia, J.; You, D.; Shrestha, B.; Jaligama, S.; Hebert, V.Y.; Dugas, T.R.; Cormier, S.A. Exposure to combustion generated environmentally persistent free radicals enhances severity of influenza virus infection. Part. Fibre Toxicol. 2014, 11, 1–21. [Google Scholar]
- Balakrishna, S.; Saravia, J.; Thevenot, P.; Ahlert, T.; Lominiki, S.; Dellinger, B.; Cormier, S.A. Environmentally persistent free radicals induce airway hyperresponsiveness in neonatal rat lungs. Part. Fibre Toxicol. 2011, 8, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, P.; Saravia, J.; Giaimo, J.; Happel, K.I.; Dugas, T.R.; Cormier, S.A. Chronic Alcohol Induces M2 Polarization Enhancing Pulmonary Disease Caused by Exposure to Particulate Air Pollution. Alcohol. Clin. Exp. Res. 2013, 37, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Thevenot, P.; Saravia, J.; Ahlert, T.; Cormier, S.A. Radical-Containing Particles Activate Dendritic Cells and Enhance Th17 Inflammation in a Mouse Model of Asthma. Am. J. Respir. Cell Mol. Biol. 2011, 45, 977–983. [Google Scholar] [CrossRef]
- Pingli, W.; You, D.; Saravia, J.; Huahao, S.; Cormier, S.A. Maternal exposure to combustion generated PM inhibits pulmonary Th1 maturation and concomitantly enhances postnatal asthma development in offspring. Part. Fibre Toxicol. 2013, 10, 1–8. [Google Scholar]
- Thevenot, P.T.; Saravia, J.; Jin, N.; Giaimo, J.D.; Chustz, R.E.; Mahne, S.; Kelley, M.A.; Hebert, V.Y.; Dellinger, B.; Dugas, T.R.; et al. Radical-Containing Ultrafine Particulate Matter Initiates Epithelial-to-Mesenchymal Transitions in Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2013, 48, 188–197. [Google Scholar] [CrossRef]
- Khachatryan, L.; Vejerano, E.; Lomnicki, S.; Dellinger, B. Environmentally persistent free radicals (EPFRs). 1. Generation of reactive oxygen species in aqueous solutions. Environ. Sci. Technol. 2011, 45, 8559–8566. [Google Scholar] [CrossRef]
- Lord, K.; Moll, D.; Lindsey, J.K.; Mahne, S.; Raman, G.; Dugas, T.; Cormier, S.; Troxlair, D.; Lomnicki, S.; Dellinger, B. Environmentally persistent free radicals decrease cardiac function before and after ischemia/reperfusion injury in vivo. J. Recept. Signal Transduct. 2011, 31, 157–167. [Google Scholar] [CrossRef]
- Stephenson, E.J.; Ragauskas, A.; Jaligama, S.; Redd, J.R.; Parvathareddy, J.; Peloquin, M.J.; Saravia, J.; Han, J.C.; Cormier, S.A.; Bridges, D. Exposure to environmentally persistent free radicals during gestation lowers energy expenditure and impairs skeletal muscle mitochondrial function in adult mice. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E1003–E1015. [Google Scholar] [CrossRef]
- Zhou, Y.-Q.; Liu, D.-Q.; Chen, S.-P.; Sun, J.; Zhou, X.-R.; Rittner, H.; Mei, W.; Tian, Y.-K.; Zhang, H.-X.; Chen, F.; et al. Reactive oxygen species scavengers ameliorate mechanical allodynia in a rat model of cancer-induced bone pain. Redox Biol. 2018, 14, 391–397. [Google Scholar] [CrossRef]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Tsuhako, M.H.; Augusto, O.; Linares, E.; Chadi, G.; Giorgio, S.; Pereira, C.A. Tempol ameliorates murine viral encephalomyelitis by preserving the blood–brain barrier, reducing viral load, and lessening inflammation. Free Radic. Biol. Med. 2010, 48, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, X.; Qi, X.; Jing, L.; Sun, W.; Jia, Z. Tempol protect against hypoxia induced oxidative stress in PC12 cells. Int. J. Clin. Exp. Med. 2017, 10, 6071–6080. [Google Scholar]
- Wanyong, Y.; Zefeng, T.; Xiufeng, X.; Dawei, D.; Xiaoyan, L.; Ying, Z.; Yaogao, F. Tempol alleviates intracerebral hemorrhage-induced brain injury possibly by attenuating nitrative stress. Neuroreport 2015, 26, 842–849. [Google Scholar] [CrossRef]
- Taye, A.; Abouzied, M.M.; Mohafez, O.M. Tempol ameliorates cardiac fibrosis in streptozotocin-induced diabetic rats: Role of oxidative stress in diabetic cardiomyopathy. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 1071–1080. [Google Scholar] [CrossRef]
- Saito, K.; Yoshioka, H. Protective Effect of Spin Trap Agent, N-tert -butyl-α-phenylnitrone on Hyperoxia-induced Oxidative Stress and Its Potential As a Nitric Oxide Donor. Free Radic. Res. 2002, 36, 143–149. [Google Scholar] [CrossRef]
- Sack, C.A.; Socci, D.J.; Crandall, B.M.; Arendash, G.W. Antioxidant treatment with phenyl-alpha-tert-butyl nitrone (PBN) improves the cognitive performance and survival of aging rats. Neurosci. Lett. 1996, 205, 181–184. [Google Scholar] [CrossRef]
- Sobocan, N.; Sincic, N.; Katusic, A.; Serman, L.; Nikuseva-Martic, T.; Juric-Lekic, G.; Paic, F.; Vlahovic, M.; Bulic-Jakus, F. Antioxidant PBN ameliorates the teratogenic effect of 5-azacytidine in rat. Acta Clin. Croat. 2009, 48, 205–206. [Google Scholar]
- Schmid-Elsaesser, R.; Hungerhuber, E.; Zausinger, S.; Baethmann, A.; Reulen, H.J. Neuroprotective effects of the novel brain-penetrating antioxidant U-101033E and the spin-trapping agent alpha-phenyl-N-tert-butyl nitrone (PBN). Exp. Brain Res. 2000, 30, 60–66. [Google Scholar] [CrossRef]
- Truong, L.; Tanguay, R.L. Evaluation of embryotoxicity using the zebrafish model. Methods Mol. Biol. 2017, 1641, 325–333. [Google Scholar]
- Geier, M.C.; Chlebowski, A.C.; Truong, L.; Simonich, S.L.M.; Anderson, K.A.; Tanguay, R.L. Comparative developmental toxicity of a comprehensive suite of polycyclic aromatic hydrocarbons. Arch. Toxicol. 2018, 92, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.R.; Spitsbergen, J.M.; Hornung, M.W.; Abnet, C.C.; Peterson, R.E. Early life stage toxicity of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 1997, 142, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Gonnerman, G.; Simonich, M.T.; Tanguay, R.L. Assessment of the developmental and neurotoxicity of the mosquito control larvicide, pyriproxyfen, using embryonic zebrafish. Environ. Pollut. 2016, 218, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Reif, D.; Truong, L.; Mandrell, D.; Marvel, S.; Zhang, G.; Tanguay, R. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch. Toxicol. 2016, 90, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Folbergrová, J.; Druga, R.; Otáhal, J.; Haugvicová, R.; Mareš, P.; Kubová, H. Effect of free radical spin trap N- tert-butyl-α-phenylnitrone (PBN) on seizures induced in immature rats by homocysteic acid. Exp. Neurol. 2006, 201, 105–119. [Google Scholar] [CrossRef]
- Erbıs, H.; Aykota, M.R.; Ozturk, B.; Kabay, B.; Sungurtekin, U.; Ozden, A.; Yenisey, C.; Turk, N.S.; Erdem, E. Effects of tempol on experimental acute necrotizing pancreatitis model in rats. J. Investig. Surg. 2015, 28, 268–275. [Google Scholar] [CrossRef]
- Kubova, H.; Folbergrova, J.; Rejchrtova, J.; Tsenov, G.; Parizkova, M.; Burchfiel, J.; Mikulecka, A.; Mares, P. The free radical scavenger N-Tert-Butyl-alpha-Phenylnitrone (PBN) administered to immature rats during status epilepticus alters neurogenesis and has variable effects, both beneficial and detrimental, on long-term outcomes. Front. Cell. Neurosci. 2018, 12, 266. [Google Scholar] [CrossRef]
- Linares, E.; Seixas, L.V.; dos Prazeres, J.N.; Ladd, F.V.L.; Ladd, A.A.B.L.; Coppi, A.A.; Augusto, O. Tempol Moderately Extends Survival in a hSOD1G93A ALS Rat Model by Inhibiting Neuronal Cell Loss, Oxidative Damage and Levels of Non-Native hSOD1G93A Forms. PLoS ONE 2013, 8, e55868. [Google Scholar] [CrossRef]

| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| B | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 |
| C | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| D | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| E | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| F | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| G | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| H | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.; Truong, L.; M. Lomnicki, S.; L. Tanguay, R.; A. Cormier, S. Developmental Hazard of Environmentally Persistent Free Radicals and Protective Effect of TEMPOL in Zebrafish Model. Toxics 2021, 9, 12. https://doi.org/10.3390/toxics9010012
Guan X, Truong L, M. Lomnicki S, L. Tanguay R, A. Cormier S. Developmental Hazard of Environmentally Persistent Free Radicals and Protective Effect of TEMPOL in Zebrafish Model. Toxics. 2021; 9(1):12. https://doi.org/10.3390/toxics9010012
Chicago/Turabian StyleGuan, Xia, Lisa Truong, Slawomir M. Lomnicki, Robyn L. Tanguay, and Stephania A. Cormier. 2021. "Developmental Hazard of Environmentally Persistent Free Radicals and Protective Effect of TEMPOL in Zebrafish Model" Toxics 9, no. 1: 12. https://doi.org/10.3390/toxics9010012
APA StyleGuan, X., Truong, L., M. Lomnicki, S., L. Tanguay, R., & A. Cormier, S. (2021). Developmental Hazard of Environmentally Persistent Free Radicals and Protective Effect of TEMPOL in Zebrafish Model. Toxics, 9(1), 12. https://doi.org/10.3390/toxics9010012







