Morpho-Functional Alterations in the Gills of a Seawater Teleost, the Ornate Wrasse (Thalassoma pavo L.), after Short-Term Exposure to Chlorpyrifos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Maintenance
2.2. Experimental Design
2.3. Light and Transmission Electron Microscopy
2.4. Immunohistochemistry
2.5. Quantification and Statistical Analyses
3. Results
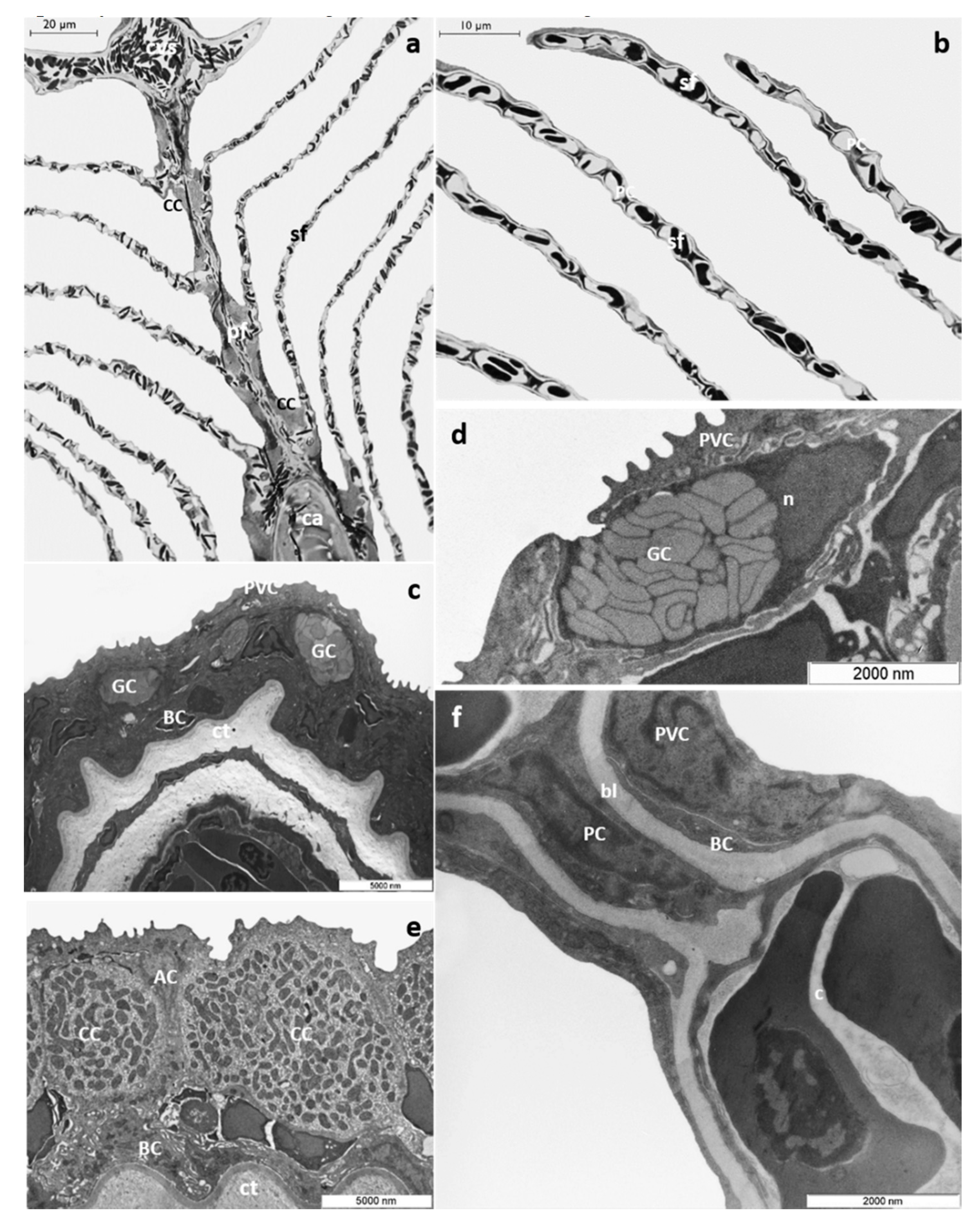
3.1. Histology and Ultrastructure
3.1.1. Control Group
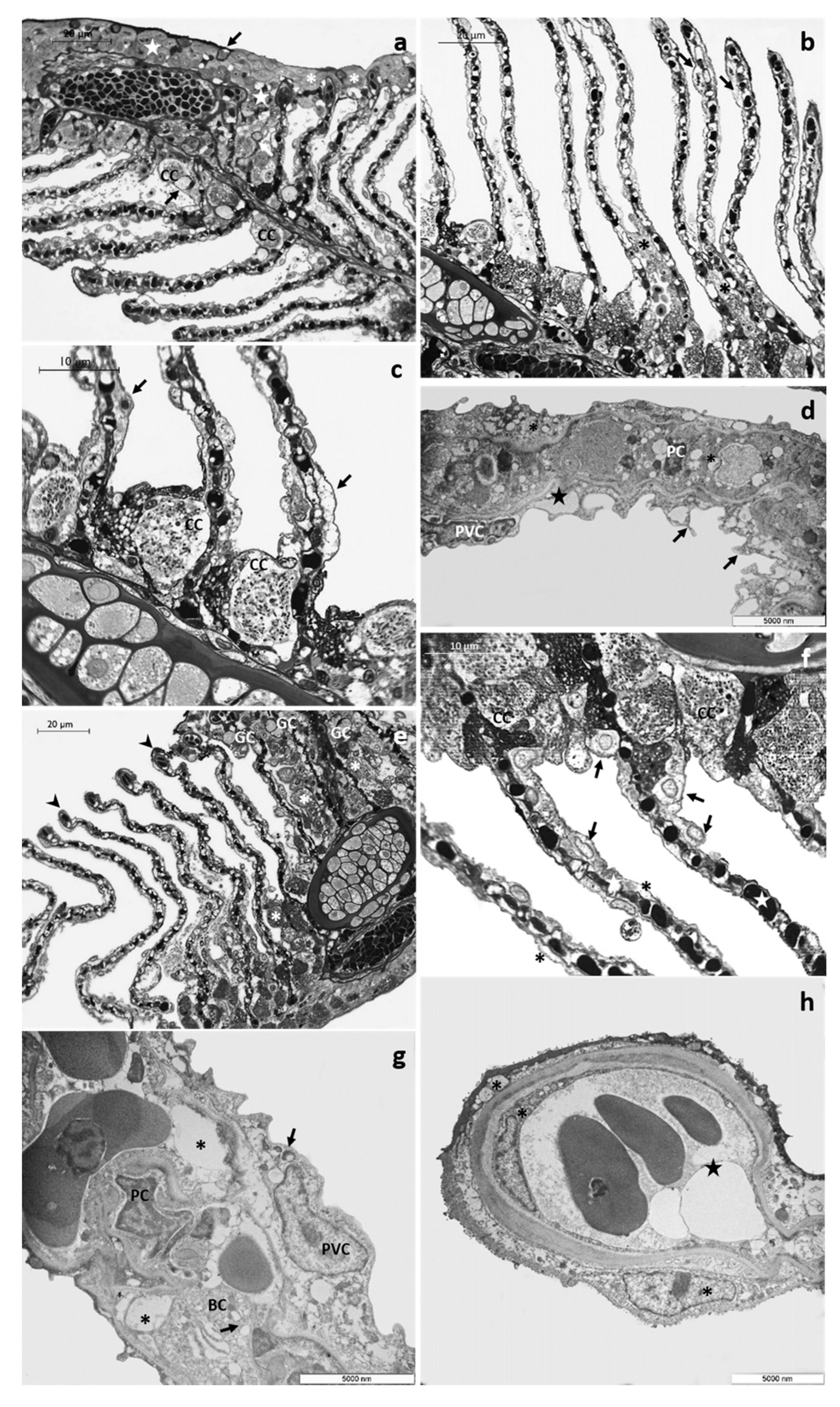
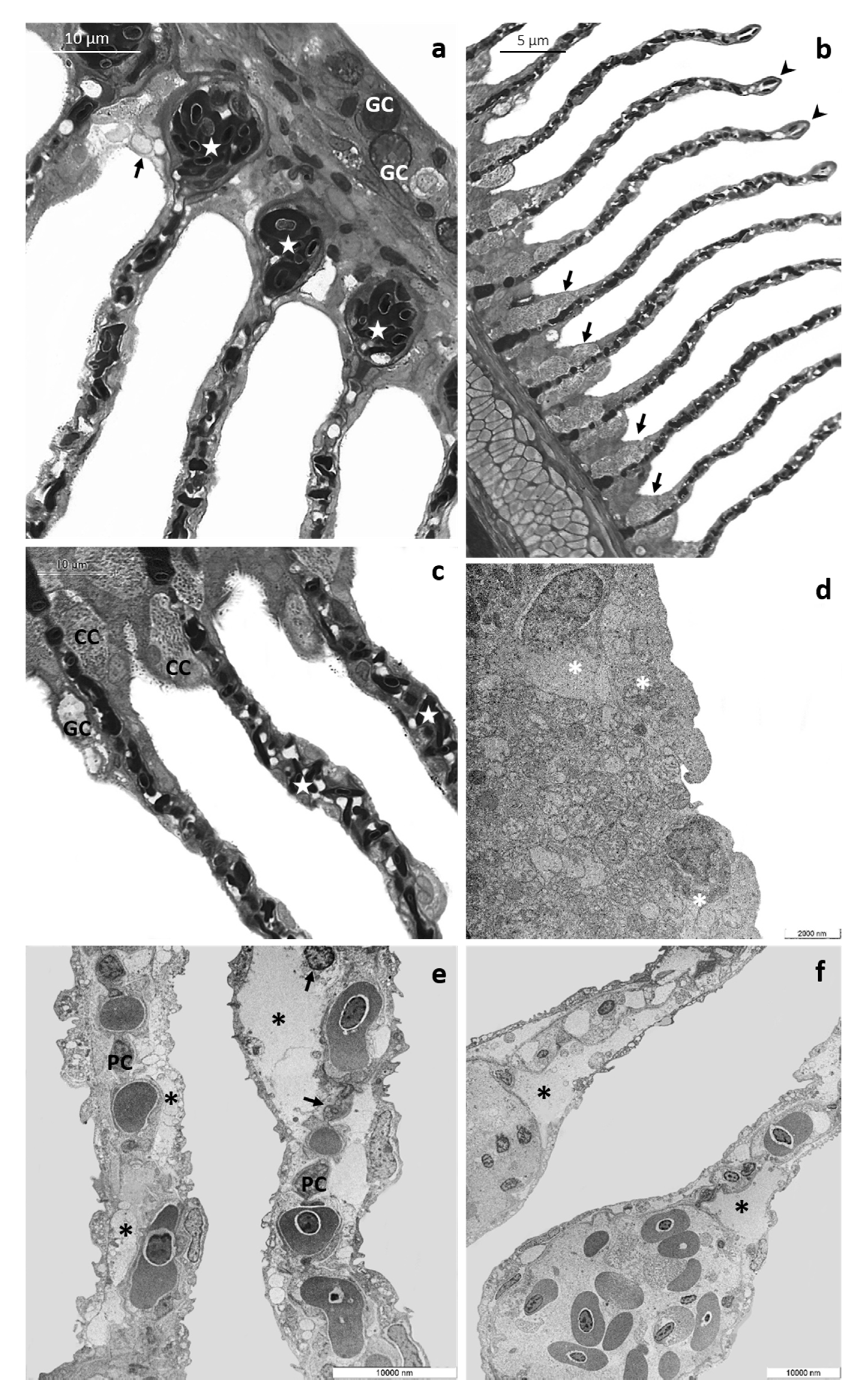
3.1.2. CPF Exposed Group, Low Tested Concentration: 4 µg/L
3.1.3. CPF Exposed Group, High Tested Concentration: 8 µg/L
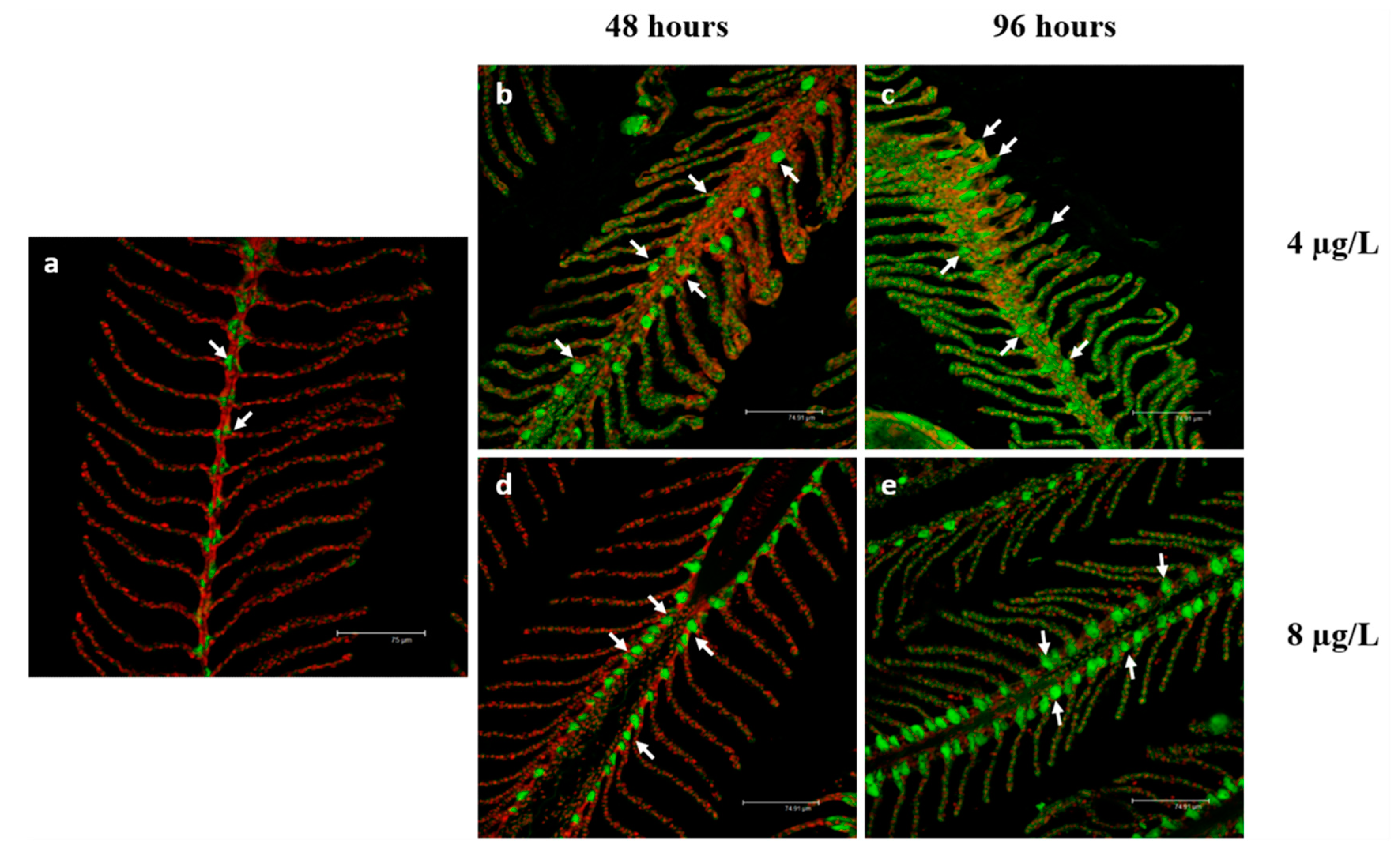
3.2. Na+/K+-ATPase Immunodetection
4. Discussion
4.1. Morphological Alterations
4.2. Na+/K+-ATPase
Author Contributions
Funding
Conflicts of Interest
References
- Global Insecticides Market. 2019–2023: Emerging Trends, Market Dynamics and Strategic Assessments of Leading Suppliers. Available online: https://www.reportbuyer.com/product/5208512/global-insecticides-market-2021-emerging-trends-market-dynamics-and-strategic-assessments-of-leading-suppliers (accessed on 2 September 2020).
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.I.; Williams, M. Feeding 9 billion by 2050–Putting fish back on the menu. J. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Dalmolin, S.P.; Dreon, D.B.; Thiesen, F.V.; Dallegrave, E. Biomarkers of occupational exposure to pesticides: Systematic review of insecticides. Environ. Toxicol. Phar. 2020, 75, 103304. [Google Scholar] [CrossRef] [PubMed]
- Martin-Reina, J.; Duarte, J.A.; Cerrillos, L.; Bautista, J.D.; Moreno, I. Insecticide reproductive toxicity profile: Organophosphate, carbamate and pyrethroids. J. Toxins. 2017, 4, 1–7. [Google Scholar]
- USEPA. Pesticides industry sales and usage 2008–2012 market estimates. 2016. Available online: https://www.epa.gov/sites/production/files/2017–01/documents/pesticides-industry-salesusage-2016_0.pdf (accessed on 25 October 2020).
- Size, E.O. Share & Trends Analysis Report By Application (Cleaning & Home, Medical, Food & Beverages, Spa & Relaxation), By Product, By Sales Channel, And Segment Forecasts, 2019–2025; Report ID. 2019:978–1; Grand View Research: San Francisco, CA, USA, 2019. [Google Scholar]
- Dar, M.A.; Kaushik, G.; Villarreal-Chiu, J.F. Pollution status and bioremediation of chlorpyrifos in environmental matrices by the application of bacterial communities: A review. J. Environ. Manag. 2019, 239, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cui, H.; Duan, W. Ecotoxicity of chlorpyrifos to aquatic organisms: A review. Ecotoxicol. Environ. Saf. 2020, 200, 110731. [Google Scholar] [CrossRef] [PubMed]
- Mamta, R.; Rao, J.; Wani, K.A. Status of organochlorine and organophosphorus pesticides in wetlands and its impact on aquatic organisms. Environ. Claim. J. 2019, 31, 44–78. [Google Scholar] [CrossRef]
- Worthing, C.R. The Pesticide Manual, 6th ed.; The British Crop Protection Council: Croydon, UK, 1979. [Google Scholar]
- Smalling, K.L.; Kuivila, K.M.; Orlando, J.L.; Phillips, B.M.; Anderson, B.S.; Siegler, K.; John, W.H.; Hamilton, M. Environmental fate of fungicides and other current-use pesticides in a central California estuary. Mar. Pollut. Bull. 2013, 73, 144–153. [Google Scholar] [CrossRef]
- Bonansea, R.I.; Marino, D.J.; Bertrand, L.; Wunderlin, D.A.; Amé, M.V. Tissue-specific bioconcentration and biotransformation of cypermethrin and chlorpyrifos in a native fish (Jenynsia multidentate) exposed to these insecticides singly and in mixtures. Environ. Toxicol. Chem. 2017, 36, 1764–1774. [Google Scholar] [CrossRef]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Comm. 2000, 327, 1–73.
- Hongsibsong, S.; Prapamontol, T.; Xu, T.; Hammock, B.D.; Wang, H.; Chen, Z.J.; Xu, Z.L. Monitoring of the organophosphate pesticide chlorpyrifos in vegetable samples from local markets in Northern Thailand by developed Immunoassay. Int. J. Env. Res. Pub. He. 2020, 17, 4723. [Google Scholar] [CrossRef]
- Centner, T.J. Cancelling pesticide registrations and revoking tolerances: The case of chlorpyrifos. Environ. Toxicol. Phar. 2018, 57, 53–61. [Google Scholar] [CrossRef]
- Triassi, M.; Nardone, A.; Giovinetti, M.C.; De Rosa, E.; Canzanella, S.; Sarnacchiaro, P.; Montuori, P. Ecological risk and estimates of organophosphate pesticides loads into the Central Mediterranean Sea from Volturno River, the river of the “Land of Fires” area, southern Italy. Sci. Total Environ. 2019, 678, 741–754. [Google Scholar] [CrossRef]
- Bondarenko, S.; Gan, J.; Haver, D.L.; Kabashima, J.N. Persistence of selected organophosphate and carbamate insecticides in waters from a coastal watershed. Environ. Toxicol. Chem. 2004, 23, 2649–2654. [Google Scholar] [CrossRef]
- Abdel-Halim, K.Y.; Salama, A.K.; El-Khateeb, E.N.; Bakry, N.M. Organophosphorus pollutants (OPP) in aquatic environment at Damietta Governorate, Egypt: Implications for monitoring and biomarker responses. Chemosphere 2006, 63, 1491–1498. [Google Scholar] [CrossRef]
- Moreno-González, R.; Campillo, J.A.; León, V.M. Influence of an intensive agricultural drainage basin on the seasonal distribution of organic pollutants in seawater from a Mediterranean coastal lagoon (Mar Menor, SE Spain). Mar. Pollut. Bull. 2013, 77, 400–411. [Google Scholar] [CrossRef]
- Cabral, H.; Fonseca, V.; Sousa, T.; Costa Leal, M. Synergistic effects of climate change and marine pollution: An overlooked interaction in coastal and estuarine areas. Int. J. Env. Res. Pub. He. 2019, 16, 2737. [Google Scholar] [CrossRef] [Green Version]
- Danovaro, R.; Carugati, L.; Berzano, M.; Cahill, A.E.; Carvalho, S.; Chenuil, A.; Corinaldesi, C.; Cristina, S.; David, R.; Dell’Anno, A.; et al. Implementing and innovating marine monitoring approaches for assessing marine environmental status. Front. Mar. Sci. 2016, 3, 213. [Google Scholar] [CrossRef]
- Directive 2008/56/EC of the European Parliament and of the Council. Off. J. Eur. Comm. 2008, 164, 19.
- Ekebom, J. The long and winding road of the ecosystem approach into marine environmental policies. Aquat. Conserv. 2013, 23, 1–6. [Google Scholar] [CrossRef]
- Covantes-Rosales, C.E.; Trujillo-Lepe, A.M.; Díaz-Reséndiz, K.J.G.; Toledo-Ibarra, G.A.; Ventura-Ramón, G.H.; Ortiz-Lazareno, P.C.; Girón-Pérez, M.I. Phagocytosis and ROS production as biomarkers in Nile tilapia (Oreochromis niloticus) leukocytes by exposure to organophosphorus pesticides. Fish. Shellfish Immun. 2019, 84, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Raibeemol, K.P.; Chitra, K.C. Histopathological alteration in gill of the freshwater fish Pseudetroplus maculatus (Bloch, 1795) under chlorpyrifos toxicity. Int. J. Bio. Biomed. Res. 2016, 3, 141–146. [Google Scholar] [CrossRef]
- Oruç, E.Ö. Oxidative stress, steroid hormone concentrations and acetylcholinesterase activity in Oreochromis niloticus exposed to chlorpyrifos. Pestic. Biochem. Phy. 2010, 96, 160–166. [Google Scholar] [CrossRef]
- Kaur, M.; Jindal, R. Oxidative stress response in liver, kidney and gills of Ctenopharyngodon idellus (Cuvier & Valenciennes) exposed to chlorpyrifos. MOJ Biol. Med. 2017, 1, 103–112. [Google Scholar]
- Khalil, F.; Qiu, X.; Kang, I.J.; Abo-Ghanema, I.; Shimasaki, Y.; Oshima, Y. Comparison of social behavior responses of Japanese medaka (Oryzias latipes) to lethal and sublethal chlorpyrifos concentrations at different exposure times. Ecotoxicol. Environ. Saf. 2017, 145, 78–82. [Google Scholar] [CrossRef]
- Bonifacio, A.F.; Ballesteros, M.L.; Bonansea, R.I.; Filippi, I.; Amé, M.V.; Hued, A.C. Environmental relevant concentrations of a chlorpyrifos commercial formulation affect two neotropical fish species, Cheirodon interruptus and Cnesterodon decemmaculatus. Chemosphere 2017, 188, 486–493. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E.; Awadin, W.; Palić, D. Acute exposure to chlorpyrifos induces reversible changes in health parameters of Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2018, 197, 47–59. [Google Scholar] [CrossRef]
- Jiao, W.; Han, Q.; Xu, Y.; Jiang, H.; Xing, H.; Teng, X. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through oxidative stress and apoptosis. Fish. Shellfish Immun. 2019, 86, 239–245. [Google Scholar] [CrossRef]
- Velmurugan, B.; Cengiz, E.I.; Yolcu, M.; Uğurlu, P.; Selvanayagam, M. Cytological and histological effects of pesticide chlorpyriphos in the gills of Anabas testudineus. Drug Chem. Toxicol. 2018, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marigoudar, S.R.; Mohan, D.; Nagarjuna, A.; Karthikeyan, P. Biomarker and histopathological responses of Lates calcarifer on exposure to sub lethal concentrations of chlorpyrifos. Ecotoxicol. Environ. Saf. 2018, 148, 327–335. [Google Scholar] [CrossRef]
- Marigoudar, S.R.; Nagarjuna, A.; Karthikeyan, P.; Mohan, D.; Sharma, K.V. Comparative toxicity of chlorpyrifos: Sublethal effects on enzyme activities and histopathology of Mugil cephalus and Chanos chanos. Chemosphere 2018, 211, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.B.; Rawat, S.S. Assessment of aquatic pollution using histopathology in fish as a protocol. Int. J. Environ. Sci. 2013, 2, 79–82. [Google Scholar]
- Tse, W.K.F.; Au, D.W.; Wong, C.K. Characterization of ion channel and transporter mRNA expressions in isolated gill chloride and pavement cells of seawater acclimating eels. Biochem. Biophys. Res. Commun. 2006, 346, 1181–1190. [Google Scholar] [CrossRef]
- Macirella, R.; Tripepi, M.; Brunelli, E. Morphological and immunohistochemical modifications in zebrafish (Danio rerio) gills after short-term exposure to the fungicide tebuconazole. Zebrafish 2019, 16, 65–76. [Google Scholar] [CrossRef]
- Macirella, R.; Madeo, G.; Sesti, S.; Tripepi, M.; Bernabò, I.; Godbert, N.; La Russa, D.; Brunelli, E. Exposure and post-exposure effects of chlorpyrifos on Carassius auratus gills: An ultrastructural and morphofunctional investigation. Chemosphere 2020, 251, 126434. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard guide for conducting acute toxicity test with fishes, macroinvertebrates, and amphibians E 729 – 96. In Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 2002. [Google Scholar]
- Georgieva, E.; Stoyanova, S.; Velcheva, I.; Yancheva, V. Histopathological alterations in common carp (Cyprinus carpio L.) gills caused by thiamethoxam. Braz. Arch. Biol. Techn. 2014, 57, 991–996. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, E.; Talarico, E.; Corapi, B.; Perrotta, I.; Tripepi, S. Effects of a sublethal concentration of sodium lauryl sulphate on the morphology and Na+/K+ ATPase activity in the gill of the ornate wrasse (Thalassoma pavo). Ecotoxicol. Environ. Saf. 2008, 71, 436–445. [Google Scholar] [CrossRef]
- Fasulo, S.; Maisano, M.; Sperone, E.; Mauceri, A.; Bernabò, I.; Cappello, T.; D’agata, A.; Tripepi, S.; Brunelli, E. Toxicity of Foroozan crude oil to ornate wrasse (Thalassoma pavo, Osteichthyes, Labridae): Ultrastructure and cellular biomarkers. Ital. J. Zool. 2012, 79, 182–199. [Google Scholar] [CrossRef]
- Velmurugan, B.; Selvanayagam, M.; Cengiz, E.I.; Unlu, E. Histopathological changes in the gill and liver tissues of freshwater fish, Cirrhinus mrigala exposed to dichlorvos. Braz. Arch. Biol. Techn. 2009, 52, 1291–1296. [Google Scholar] [CrossRef]
- Machado, M.R.; Fanta, E. Effects of the organophosphorous methyl parathion on the branchial epithelium of a freshwater fish Metynnis roosevelti. Braz. Arch. Biol. Techn. 2003, 46, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Topal, A.; Atamanalp, M.; Oruç, E.; Demir, Y.; Beydemir, Ş.; Işık, A. In vivo changes in carbonic anhydrase activity and histopathology of gill and liver tissues after acute exposure to chlorpyrifos in rainbow trout. Arh. Hig. Rada Toksikol. 2014, 65, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Barja-Fernández, S.; Míguez, J.M.; Álvarez-Otero, R. Histopathological effects of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) in the gills, intestine and liver of turbot (Psetta maxima). Ecotoxicol. Environ. Saf. 2013, 95, 60–68. [Google Scholar] [CrossRef]
- Brunelli, E.; Mauceri, A.; Maisano, M.; Bernabò, I.; Giannetto, A.; De Domenico, E.; Corapi, B.; Tripepi, S.; Fasulo, S. Ultrastructural and immunohistochemical investigation on the gills of the teleost, Thalassoma pavo L., exposed to cadmium. Acta Histochem. 2011, 113, 201–213. [Google Scholar] [CrossRef]
- Macirella, R.; Sesti, S.; Bernabò, I.; Tripepi, M.; Godbert, N.; Brunelli, E. Lead toxicity in seawater teleosts: A morphofunctional and ultrastructural study on the gills of the Ornate wrasse (Thalassoma pavo L.). Aquat. Toxicol. 2019, 211, 193–201. [Google Scholar] [CrossRef]
- Issa, A.M.; Gawish, A.M.; Esmail, G.M. Histological hazards of chlorpyrifos usage on gills and kidneys of Tilapia nilotica and the role of vitamin E. Life Sci. J. 2011, 8, 113–123. [Google Scholar]
- Ma, J.; Zhu, J.; Wang, W.; Ruan, P.; Rajeshkumar, S.; Li, X. Biochemical and molecular impacts of glyphosate-based herbicide on the gills of common carp. Environ. Pollut. 2019, 252, 1288–1300. [Google Scholar] [CrossRef]
- Li, Z.H.; Zlabek, V.; Grabic, R.; Li, P.; Randak, T. Biochemical responses in gills of rainbow trout exposed to propiconazole. Open Life Sci. 2011, 6, 84–90. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol. 2007, 41, 8178–8186. [Google Scholar] [CrossRef]
- Atli, G.; Canli, M. Enzymatic responses to metal exposures in a freshwater fish Oreochromis niloticus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Fridman, S. Ontogeny of the osmoregulatory capacity of teleosts and the role of ionocytes. Front. Mar. Sci. 2020, 7, 709. [Google Scholar] [CrossRef]
- Perry, S.F. The chloride cell: Structure and function in the gills of freshwater fishes. Ann. Rev. Physiol. 1997, 59, 325–347. [Google Scholar] [CrossRef] [PubMed]

| Fisher’s Exact Probability Tests (Two-Way) | |||
|---|---|---|---|
| Group | Effect | No Effect | p-Value Summary |
| Ctrl 48 h | 0 | 8 | |
| Ctrl 96 h | 0 | 8 | |
| Low concentration 48 h | 8 | 0 | *** |
| Low concentration 96 h | 8 | 0 | *** |
| High concentration 48 h | 8 | 0 | *** |
| High concentration 96 h | 8 | 0 | *** |
| Summary of Histopathological Alterations | Control Group | CPF Exposed Groups | |||
|---|---|---|---|---|---|
| Low Concentration | High Concentration | ||||
| 48 h | 96 h | 48 h | 96 h | ||
| Proliferation of primary epithelium | - | ++ | +++ | + | + |
| Lifting of secondary epithelium | - | + | ++ | ++ | +++ |
| Thinning of epithelial layers | - | - | - | ++ | +++ |
| Increased number of mucous cells | - | + | ++ | ++ | +++ |
| Ectopia of chloride cells and mucous cells | - | + | ++ | ++ | +++ |
| Cell degeneration | - | + | ++ | ++ | +++ |
| Vascular component alterations | - | + | ++ | ++ | +++ |
| Aneurysms’ formation | - | + | ++ | ++ | +++ |
| Exposure Period | ||
|---|---|---|
| Group | 48 h | 96 h |
| Control | 10.71 ± 0.74 | 10.51 ± 0.35 |
| Low concentration | 19.76 ± 0.84 *** | 19.40 ± 0.44 *** |
| High concentration | 20.12 ± 0.58 *** | 19.95 ± 0.69 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macirella, R.; Curcio, V.; Brunelli, E. Morpho-Functional Alterations in the Gills of a Seawater Teleost, the Ornate Wrasse (Thalassoma pavo L.), after Short-Term Exposure to Chlorpyrifos. Toxics 2020, 8, 97. https://doi.org/10.3390/toxics8040097
Macirella R, Curcio V, Brunelli E. Morpho-Functional Alterations in the Gills of a Seawater Teleost, the Ornate Wrasse (Thalassoma pavo L.), after Short-Term Exposure to Chlorpyrifos. Toxics. 2020; 8(4):97. https://doi.org/10.3390/toxics8040097
Chicago/Turabian StyleMacirella, Rachele, Vittoria Curcio, and Elvira Brunelli. 2020. "Morpho-Functional Alterations in the Gills of a Seawater Teleost, the Ornate Wrasse (Thalassoma pavo L.), after Short-Term Exposure to Chlorpyrifos" Toxics 8, no. 4: 97. https://doi.org/10.3390/toxics8040097
APA StyleMacirella, R., Curcio, V., & Brunelli, E. (2020). Morpho-Functional Alterations in the Gills of a Seawater Teleost, the Ornate Wrasse (Thalassoma pavo L.), after Short-Term Exposure to Chlorpyrifos. Toxics, 8(4), 97. https://doi.org/10.3390/toxics8040097






